

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Replicase polyprotein 1a (Human coronavirus NL63) | BDBM50007789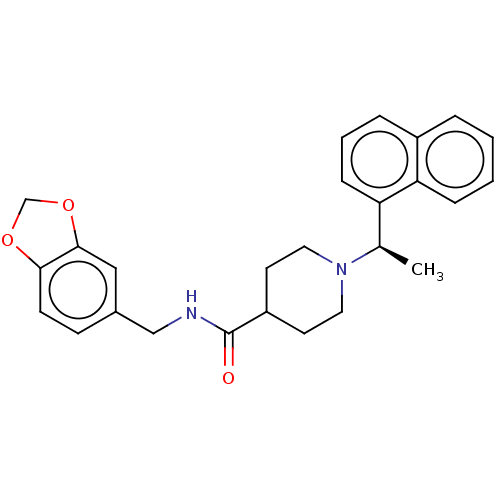 (CHEMBL1173044 | GRL-0667 | N-(2H-1,3-benzodioxol-5...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of human coronavirus NL63 PLP2 (amino acids 1565 to 1894) expressed in Escherichia coli BL21 (DE3) cells assessed as reduction of AMC rele... | J Med Chem 57: 2393-412 (2014) Article DOI: 10.1021/jm401712t BindingDB Entry DOI: 10.7270/Q2KK9DBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ubiquitin carboxyl-terminal hydrolase 21 (Homo sapiens (Human)) | BDBM50007791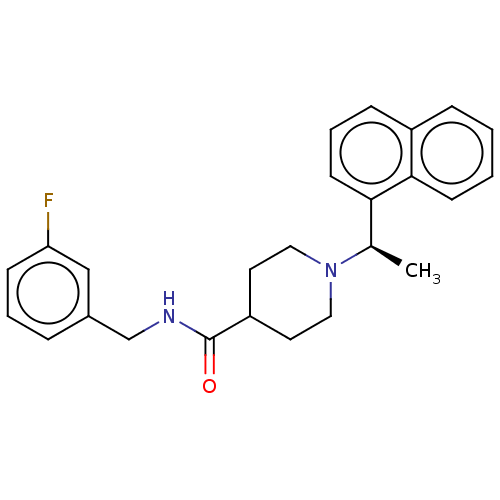 (CHEMBL3233815 | med.21724, Compound 168) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | >3.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of human USP21 using Ub-rhodamine 110 as substrate | J Med Chem 57: 2393-412 (2014) Article DOI: 10.1021/jm401712t BindingDB Entry DOI: 10.7270/Q2KK9DBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ubiquitin carboxyl-terminal hydrolase 21 (Homo sapiens (Human)) | BDBM50007790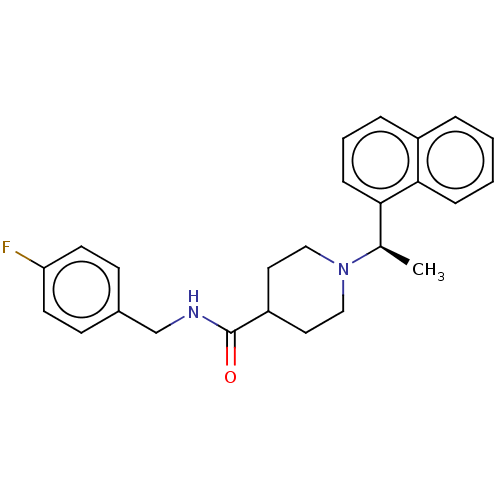 (CHEMBL3233814 | med.21724, Compound 169) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | >3.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of human USP21 using Ub-rhodamine 110 as substrate | J Med Chem 57: 2393-412 (2014) Article DOI: 10.1021/jm401712t BindingDB Entry DOI: 10.7270/Q2KK9DBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ubiquitin carboxyl-terminal hydrolase 21 (Homo sapiens (Human)) | BDBM50007788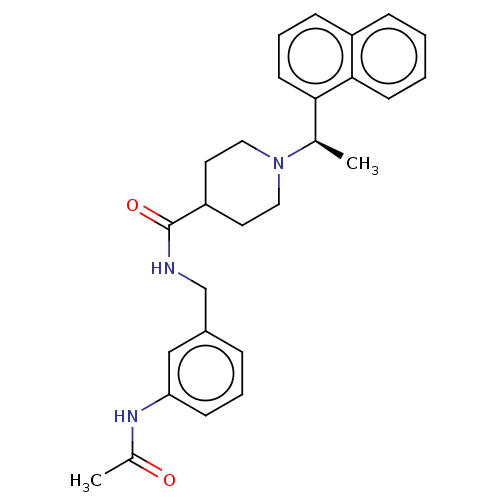 (CHEMBL3233809 | med.21724, Compound 167) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | >3.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of human USP21 using Ub-rhodamine 110 as substrate | J Med Chem 57: 2393-412 (2014) Article DOI: 10.1021/jm401712t BindingDB Entry DOI: 10.7270/Q2KK9DBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ubiquitin carboxyl-terminal hydrolase 21 (Homo sapiens (Human)) | BDBM50007789 (CHEMBL1173044 | GRL-0667 | N-(2H-1,3-benzodioxol-5...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | >3.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of human USP21 using Ub-rhodamine 110 as substrate | J Med Chem 57: 2393-412 (2014) Article DOI: 10.1021/jm401712t BindingDB Entry DOI: 10.7270/Q2KK9DBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1a (Human coronavirus NL63) | BDBM50007791 (CHEMBL3233815 | med.21724, Compound 168) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of human coronavirus NL63 PLP2 (amino acids 1565 to 1894) expressed in Escherichia coli BL21 (DE3) cells assessed as reduction of AMC rele... | J Med Chem 57: 2393-412 (2014) Article DOI: 10.1021/jm401712t BindingDB Entry DOI: 10.7270/Q2KK9DBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1a (Human coronavirus NL63) | BDBM50007792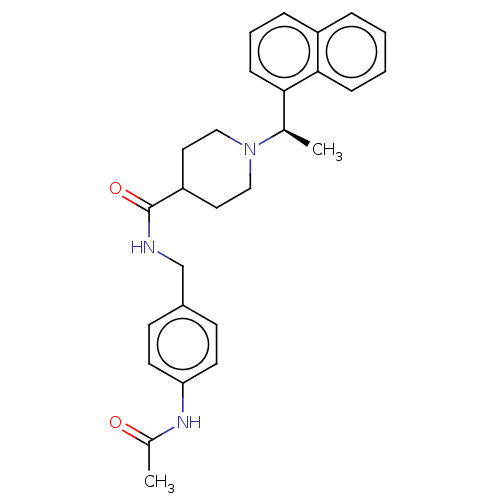 (CHEMBL3233808) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of human coronavirus NL63 PLP2 (amino acids 1565 to 1894) expressed in Escherichia coli BL21 (DE3) cells assessed as reduction of AMC rele... | J Med Chem 57: 2393-412 (2014) Article DOI: 10.1021/jm401712t BindingDB Entry DOI: 10.7270/Q2KK9DBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1a (Human coronavirus NL63) | BDBM50007793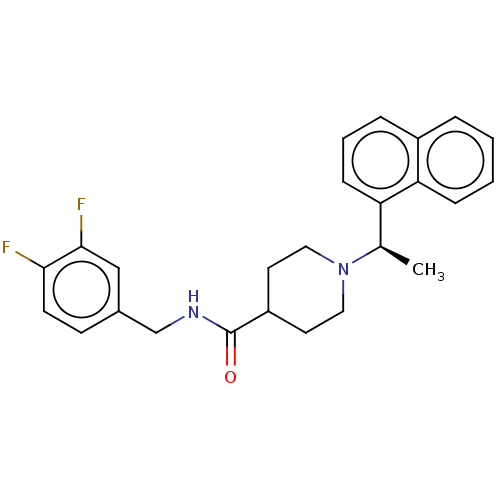 (CHEMBL3233813) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of human coronavirus NL63 PLP2 (amino acids 1565 to 1894) expressed in Escherichia coli BL21 (DE3) cells assessed as reduction of AMC rele... | J Med Chem 57: 2393-412 (2014) Article DOI: 10.1021/jm401712t BindingDB Entry DOI: 10.7270/Q2KK9DBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1a (Human coronavirus NL63) | BDBM50007788 (CHEMBL3233809 | med.21724, Compound 167) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 4.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of human coronavirus NL63 PLP2 (amino acids 1565 to 1894) expressed in Escherichia coli BL21 (DE3) cells assessed as reduction of AMC rele... | J Med Chem 57: 2393-412 (2014) Article DOI: 10.1021/jm401712t BindingDB Entry DOI: 10.7270/Q2KK9DBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1a (Human coronavirus NL63) | BDBM50007794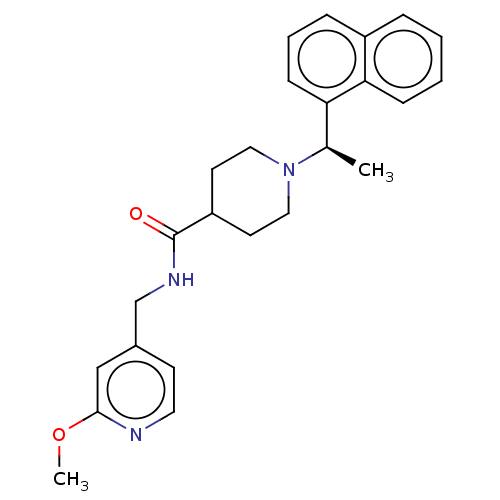 (CHEMBL3233822) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of human coronavirus NL63 PLP2 (amino acids 1565 to 1894) expressed in Escherichia coli BL21 (DE3) cells assessed as reduction of AMC rele... | J Med Chem 57: 2393-412 (2014) Article DOI: 10.1021/jm401712t BindingDB Entry DOI: 10.7270/Q2KK9DBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ubiquitin carboxyl-terminal hydrolase 8 (Homo sapiens (Human)) | BDBM50007788 (CHEMBL3233809 | med.21724, Compound 167) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of human USP8 using Ub-rhodamine 110 as substrate | J Med Chem 57: 2393-412 (2014) Article DOI: 10.1021/jm401712t BindingDB Entry DOI: 10.7270/Q2KK9DBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ubiquitin carboxyl-terminal hydrolase 8 (Homo sapiens (Human)) | BDBM50007789 (CHEMBL1173044 | GRL-0667 | N-(2H-1,3-benzodioxol-5...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of human USP8 using Ub-rhodamine 110 as substrate | J Med Chem 57: 2393-412 (2014) Article DOI: 10.1021/jm401712t BindingDB Entry DOI: 10.7270/Q2KK9DBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ubiquitin carboxyl-terminal hydrolase 8 (Homo sapiens (Human)) | BDBM50007791 (CHEMBL3233815 | med.21724, Compound 168) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of human USP8 using Ub-rhodamine 110 as substrate | J Med Chem 57: 2393-412 (2014) Article DOI: 10.1021/jm401712t BindingDB Entry DOI: 10.7270/Q2KK9DBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ubiquitin carboxyl-terminal hydrolase 2 (Homo sapiens (Human)) | BDBM50007788 (CHEMBL3233809 | med.21724, Compound 167) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of human USP2 using Ub-rhodamine 110 as substrate | J Med Chem 57: 2393-412 (2014) Article DOI: 10.1021/jm401712t BindingDB Entry DOI: 10.7270/Q2KK9DBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ubiquitin carboxyl-terminal hydrolase 8 (Homo sapiens (Human)) | BDBM50007790 (CHEMBL3233814 | med.21724, Compound 169) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of human USP8 using Ub-rhodamine 110 as substrate | J Med Chem 57: 2393-412 (2014) Article DOI: 10.1021/jm401712t BindingDB Entry DOI: 10.7270/Q2KK9DBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ubiquitin carboxyl-terminal hydrolase 20 (Homo sapiens (Human)) | BDBM50007791 (CHEMBL3233815 | med.21724, Compound 168) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of human USP20 using Ub-rhodamine 110 as substrate | J Med Chem 57: 2393-412 (2014) Article DOI: 10.1021/jm401712t BindingDB Entry DOI: 10.7270/Q2KK9DBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ubiquitin carboxyl-terminal hydrolase 20 (Homo sapiens (Human)) | BDBM50007790 (CHEMBL3233814 | med.21724, Compound 169) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of human USP20 using Ub-rhodamine 110 as substrate | J Med Chem 57: 2393-412 (2014) Article DOI: 10.1021/jm401712t BindingDB Entry DOI: 10.7270/Q2KK9DBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ubiquitin carboxyl-terminal hydrolase 20 (Homo sapiens (Human)) | BDBM50007788 (CHEMBL3233809 | med.21724, Compound 167) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of human USP20 using Ub-rhodamine 110 as substrate | J Med Chem 57: 2393-412 (2014) Article DOI: 10.1021/jm401712t BindingDB Entry DOI: 10.7270/Q2KK9DBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ubiquitin carboxyl-terminal hydrolase 7 (Homo sapiens (Human)) | BDBM50007788 (CHEMBL3233809 | med.21724, Compound 167) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of human USP7 using Ub-rhodamine 110 as substrate | J Med Chem 57: 2393-412 (2014) Article DOI: 10.1021/jm401712t BindingDB Entry DOI: 10.7270/Q2KK9DBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||