

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50341160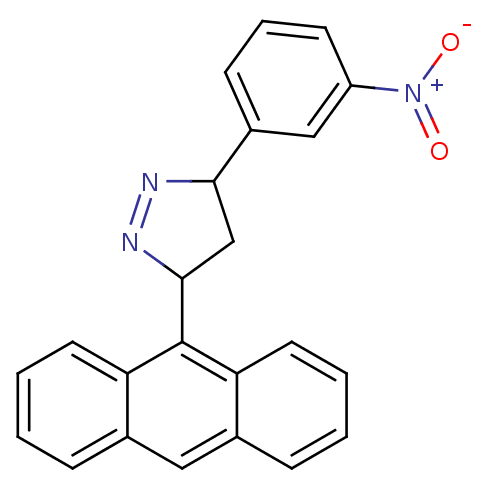 (3-(anthracen-9-yl)-5-(3-nitrophenyl)-4,5-dihydro-1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology Curated by ChEMBL | Assay Description Competitive inhibition of human recombinant MAO-B after 60 mins using p-tyramine as substrate by spectrophotometry | Bioorg Med Chem Lett 21: 1969-73 (2011) Article DOI: 10.1016/j.bmcl.2011.02.030 BindingDB Entry DOI: 10.7270/Q2JM29XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Rattus norvegicus (rat)) | BDBM50341160 (3-(anthracen-9-yl)-5-(3-nitrophenyl)-4,5-dihydro-1...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology Curated by ChEMBL | Assay Description Competitive inhibition of rat liver MAO-B after 60 mins using p-tyramine as substrate by spectrophotometry | Bioorg Med Chem Lett 21: 1969-73 (2011) Article DOI: 10.1016/j.bmcl.2011.02.030 BindingDB Entry DOI: 10.7270/Q2JM29XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Rattus norvegicus (rat)) | BDBM50341161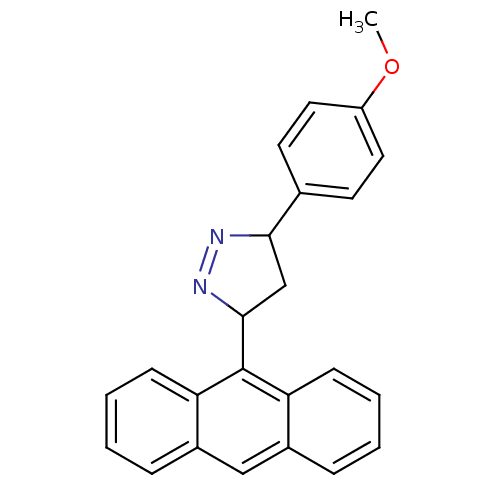 (3-(anthracen-9-yl)-5-(4-methoxyphenyl)-4,5-dihydro...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology Curated by ChEMBL | Assay Description Competitive inhibition of rat liver MAO-B after 60 mins using p-tyramine as substrate by spectrophotometry | Bioorg Med Chem Lett 21: 1969-73 (2011) Article DOI: 10.1016/j.bmcl.2011.02.030 BindingDB Entry DOI: 10.7270/Q2JM29XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50341152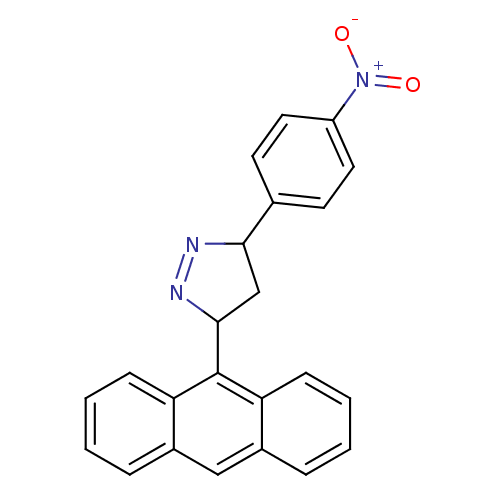 (3-(anthracen-9-yl)-5-(4-nitrophenyl)-4,5-dihydro-1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology Curated by ChEMBL | Assay Description Competitive inhibition of human recombinant MAO-B after 60 mins using p-tyramine as substrate by spectrophotometry | Bioorg Med Chem Lett 21: 1969-73 (2011) Article DOI: 10.1016/j.bmcl.2011.02.030 BindingDB Entry DOI: 10.7270/Q2JM29XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50341161 (3-(anthracen-9-yl)-5-(4-methoxyphenyl)-4,5-dihydro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology Curated by ChEMBL | Assay Description Competitive inhibition of human recombinant MAO-B after 60 mins using p-tyramine as substrate by spectrophotometry | Bioorg Med Chem Lett 21: 1969-73 (2011) Article DOI: 10.1016/j.bmcl.2011.02.030 BindingDB Entry DOI: 10.7270/Q2JM29XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50341154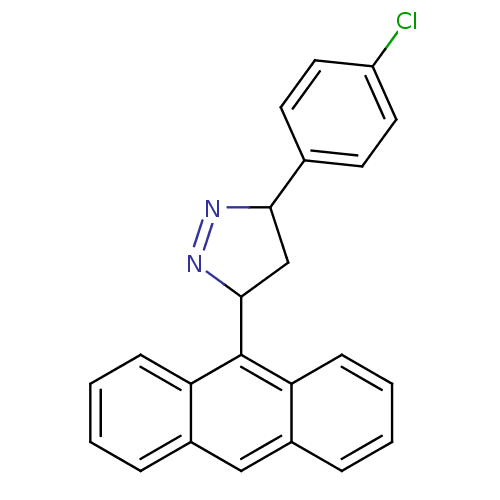 (3-(anthracen-9-yl)-5-(4-chlorophenyl)-4,5-dihydro-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology Curated by ChEMBL | Assay Description Competitive inhibition of human recombinant MAO-B after 60 mins using p-tyramine as substrate by spectrophotometry | Bioorg Med Chem Lett 21: 1969-73 (2011) Article DOI: 10.1016/j.bmcl.2011.02.030 BindingDB Entry DOI: 10.7270/Q2JM29XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Rattus norvegicus (rat)) | BDBM50341154 (3-(anthracen-9-yl)-5-(4-chlorophenyl)-4,5-dihydro-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology Curated by ChEMBL | Assay Description Competitive inhibition of rat liver MAO-B after 60 mins using p-tyramine as substrate by spectrophotometry | Bioorg Med Chem Lett 21: 1969-73 (2011) Article DOI: 10.1016/j.bmcl.2011.02.030 BindingDB Entry DOI: 10.7270/Q2JM29XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Rattus norvegicus (rat)) | BDBM50341152 (3-(anthracen-9-yl)-5-(4-nitrophenyl)-4,5-dihydro-1...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology Curated by ChEMBL | Assay Description Competitive inhibition of rat liver MAO-B after 60 mins using p-tyramine as substrate by spectrophotometry | Bioorg Med Chem Lett 21: 1969-73 (2011) Article DOI: 10.1016/j.bmcl.2011.02.030 BindingDB Entry DOI: 10.7270/Q2JM29XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Rattus norvegicus (rat)) | BDBM50341156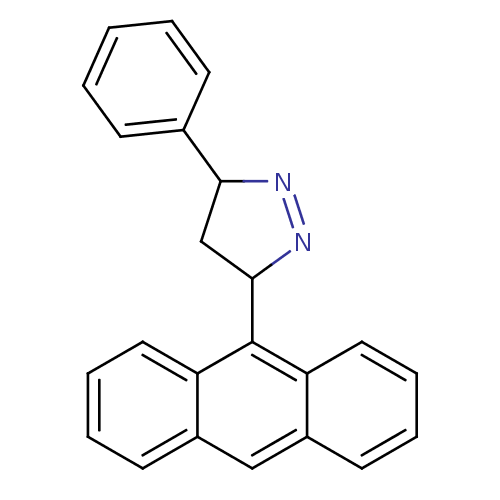 (3-(anthracen-9-yl)-5-phenyl-4,5-dihydro-1H-pyrazol...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology Curated by ChEMBL | Assay Description Competitive inhibition of rat liver MAO-B after 60 mins using p-tyramine as substrate by spectrophotometry | Bioorg Med Chem Lett 21: 1969-73 (2011) Article DOI: 10.1016/j.bmcl.2011.02.030 BindingDB Entry DOI: 10.7270/Q2JM29XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50341155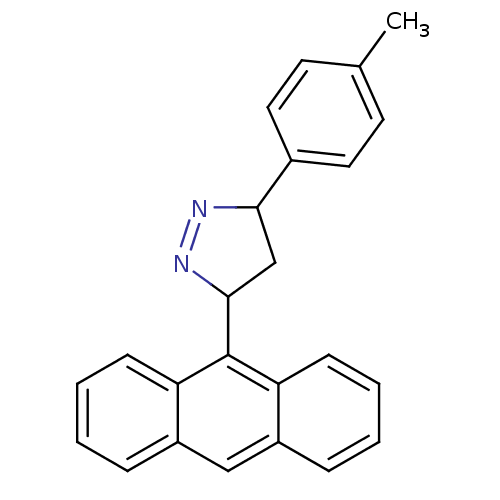 (3-(anthracen-9-yl)-5-p-tolyl-4,5-dihydro-1H-pyrazo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology Curated by ChEMBL | Assay Description Competitive inhibition of human recombinant MAO-B after 60 mins using p-tyramine as substrate by spectrophotometry | Bioorg Med Chem Lett 21: 1969-73 (2011) Article DOI: 10.1016/j.bmcl.2011.02.030 BindingDB Entry DOI: 10.7270/Q2JM29XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Rattus norvegicus (rat)) | BDBM50341155 (3-(anthracen-9-yl)-5-p-tolyl-4,5-dihydro-1H-pyrazo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology Curated by ChEMBL | Assay Description Competitive inhibition of rat liver MAO-B after 60 mins using p-tyramine as substrate by spectrophotometry | Bioorg Med Chem Lett 21: 1969-73 (2011) Article DOI: 10.1016/j.bmcl.2011.02.030 BindingDB Entry DOI: 10.7270/Q2JM29XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50341156 (3-(anthracen-9-yl)-5-phenyl-4,5-dihydro-1H-pyrazol...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology Curated by ChEMBL | Assay Description Competitive inhibition of human recombinant MAO-B after 60 mins using p-tyramine as substrate by spectrophotometry | Bioorg Med Chem Lett 21: 1969-73 (2011) Article DOI: 10.1016/j.bmcl.2011.02.030 BindingDB Entry DOI: 10.7270/Q2JM29XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Rattus norvegicus (rat)) | BDBM50341158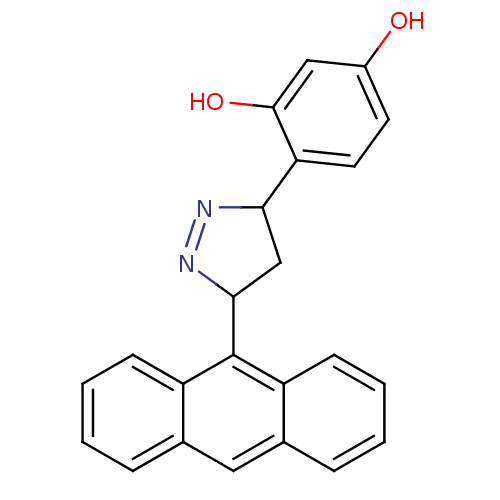 (4-(3-(anthracen-9-yl)-4,5-dihydro-1H-pyrazol-5-yl)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.77 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology Curated by ChEMBL | Assay Description Competitive inhibition of rat liver MAO-B after 60 mins using p-tyramine as substrate by spectrophotometry | Bioorg Med Chem Lett 21: 1969-73 (2011) Article DOI: 10.1016/j.bmcl.2011.02.030 BindingDB Entry DOI: 10.7270/Q2JM29XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50341158 (4-(3-(anthracen-9-yl)-4,5-dihydro-1H-pyrazol-5-yl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.54 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology Curated by ChEMBL | Assay Description Competitive inhibition of human recombinant MAO-B after 60 mins using p-tyramine as substrate by spectrophotometry | Bioorg Med Chem Lett 21: 1969-73 (2011) Article DOI: 10.1016/j.bmcl.2011.02.030 BindingDB Entry DOI: 10.7270/Q2JM29XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50341159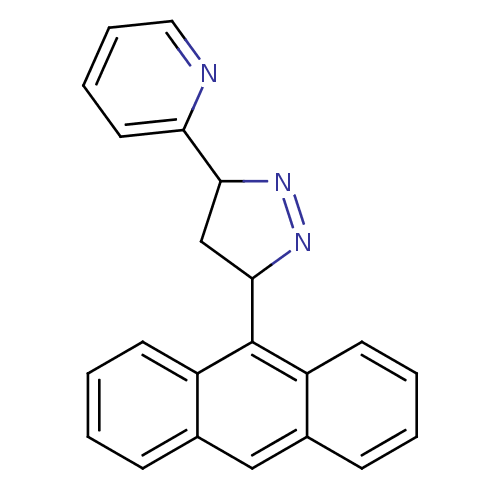 (2-(3-(anthracen-9-yl)-4,5-dihydro-1H-pyrazol-5-yl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology Curated by ChEMBL | Assay Description Competitive inhibition of human recombinant MAO-B after 60 mins using p-tyramine as substrate by spectrophotometry | Bioorg Med Chem Lett 21: 1969-73 (2011) Article DOI: 10.1016/j.bmcl.2011.02.030 BindingDB Entry DOI: 10.7270/Q2JM29XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Rattus norvegicus (rat)) | BDBM50341157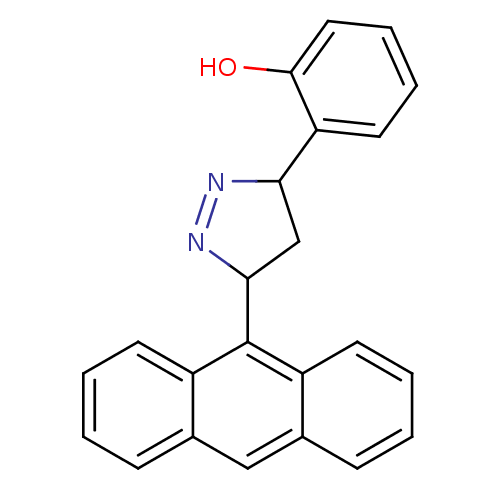 (2-(3-(anthracen-9-yl)-4,5-dihydro-1H-pyrazol-5-yl)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.91 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology Curated by ChEMBL | Assay Description Competitive inhibition of rat liver MAO-B after 60 mins using p-tyramine as substrate by spectrophotometry | Bioorg Med Chem Lett 21: 1969-73 (2011) Article DOI: 10.1016/j.bmcl.2011.02.030 BindingDB Entry DOI: 10.7270/Q2JM29XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Rattus norvegicus (rat)) | BDBM50341153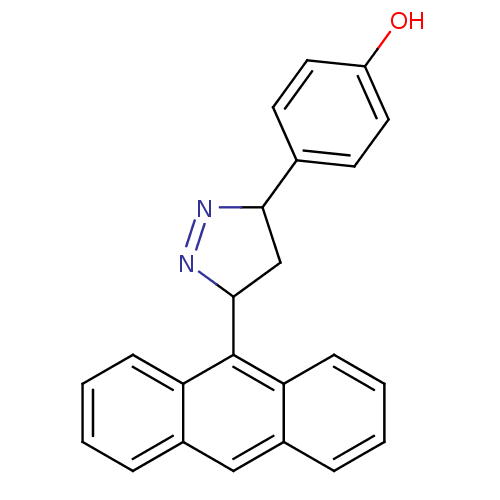 (4-(3-(anthracen-9-yl)-4,5-dihydro-1H-pyrazol-5-yl)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology Curated by ChEMBL | Assay Description Competitive inhibition of rat liver MAO-B after 60 mins using p-tyramine as substrate by spectrophotometry | Bioorg Med Chem Lett 21: 1969-73 (2011) Article DOI: 10.1016/j.bmcl.2011.02.030 BindingDB Entry DOI: 10.7270/Q2JM29XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate after 60 mins by Ellman's method | Bioorg Med Chem Lett 23: 702-5 (2013) Article DOI: 10.1016/j.bmcl.2012.11.100 BindingDB Entry DOI: 10.7270/Q2F19118 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50425411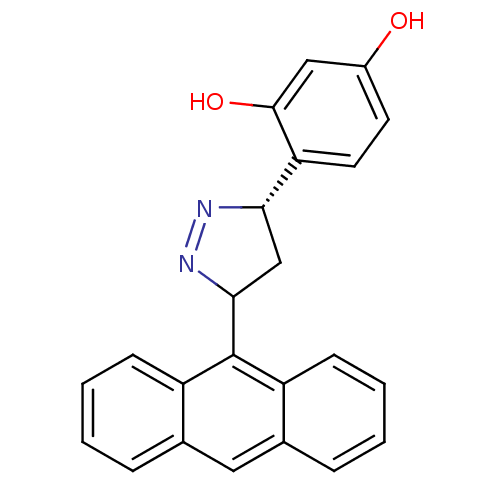 (CHEMBL2312573) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate after 60 mins by Ellman's method | Bioorg Med Chem Lett 23: 702-5 (2013) Article DOI: 10.1016/j.bmcl.2012.11.100 BindingDB Entry DOI: 10.7270/Q2F19118 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50341157 (2-(3-(anthracen-9-yl)-4,5-dihydro-1H-pyrazol-5-yl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology Curated by ChEMBL | Assay Description Competitive inhibition of human recombinant MAO-B after 60 mins using p-tyramine as substrate by spectrophotometry | Bioorg Med Chem Lett 21: 1969-73 (2011) Article DOI: 10.1016/j.bmcl.2011.02.030 BindingDB Entry DOI: 10.7270/Q2JM29XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50341153 (4-(3-(anthracen-9-yl)-4,5-dihydro-1H-pyrazol-5-yl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology Curated by ChEMBL | Assay Description Competitive inhibition of human recombinant MAO-B after 60 mins using p-tyramine as substrate by spectrophotometry | Bioorg Med Chem Lett 21: 1969-73 (2011) Article DOI: 10.1016/j.bmcl.2011.02.030 BindingDB Entry DOI: 10.7270/Q2JM29XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Rattus norvegicus (rat)) | BDBM50341159 (2-(3-(anthracen-9-yl)-4,5-dihydro-1H-pyrazol-5-yl)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.91 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology Curated by ChEMBL | Assay Description Competitive inhibition of rat liver MAO-B after 60 mins using p-tyramine as substrate by spectrophotometry | Bioorg Med Chem Lett 21: 1969-73 (2011) Article DOI: 10.1016/j.bmcl.2011.02.030 BindingDB Entry DOI: 10.7270/Q2JM29XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50341152 (3-(anthracen-9-yl)-5-(4-nitrophenyl)-4,5-dihydro-1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 17.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology Curated by ChEMBL | Assay Description Competitive inhibition of human recombinant MAO-A after 60 mins using p-tyramine as substrate by spectrophotometry | Bioorg Med Chem Lett 21: 1969-73 (2011) Article DOI: 10.1016/j.bmcl.2011.02.030 BindingDB Entry DOI: 10.7270/Q2JM29XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Rattus norvegicus (rat)) | BDBM50341152 (3-(anthracen-9-yl)-5-(4-nitrophenyl)-4,5-dihydro-1...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 20.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology Curated by ChEMBL | Assay Description Competitive inhibition of rat liver MAO-A after 60 mins using p-tyramine as substrate by spectrophotometry | Bioorg Med Chem Lett 21: 1969-73 (2011) Article DOI: 10.1016/j.bmcl.2011.02.030 BindingDB Entry DOI: 10.7270/Q2JM29XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50425409 (CHEMBL2312575) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate after 60 mins by Ellman's method | Bioorg Med Chem Lett 23: 702-5 (2013) Article DOI: 10.1016/j.bmcl.2012.11.100 BindingDB Entry DOI: 10.7270/Q2F19118 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50425412 (CHEMBL2312285) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate after 60 mins by Ellman's method | Bioorg Med Chem Lett 23: 702-5 (2013) Article DOI: 10.1016/j.bmcl.2012.11.100 BindingDB Entry DOI: 10.7270/Q2F19118 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50425408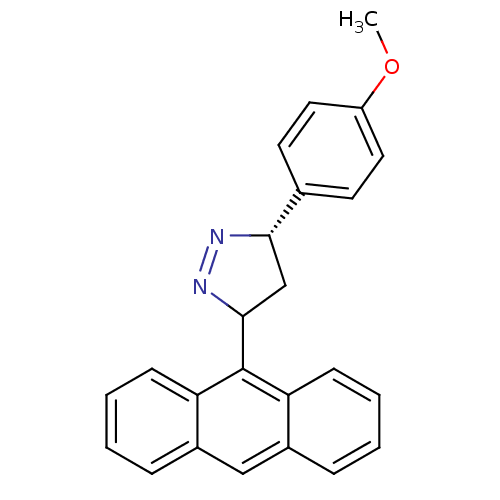 (CHEMBL2312576) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate after 60 mins by Ellman's method | Bioorg Med Chem Lett 23: 702-5 (2013) Article DOI: 10.1016/j.bmcl.2012.11.100 BindingDB Entry DOI: 10.7270/Q2F19118 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50425413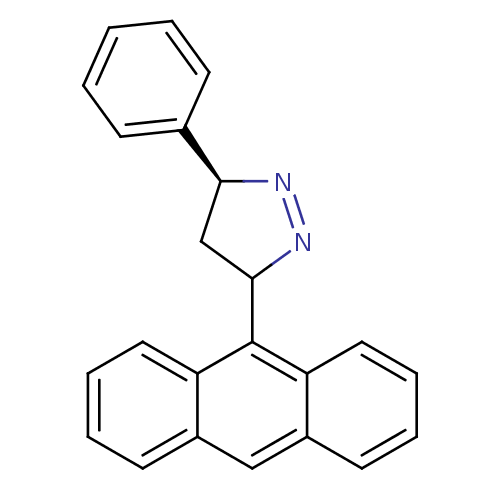 (CHEMBL2312284) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate after 60 mins by Ellman's method | Bioorg Med Chem Lett 23: 702-5 (2013) Article DOI: 10.1016/j.bmcl.2012.11.100 BindingDB Entry DOI: 10.7270/Q2F19118 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50425417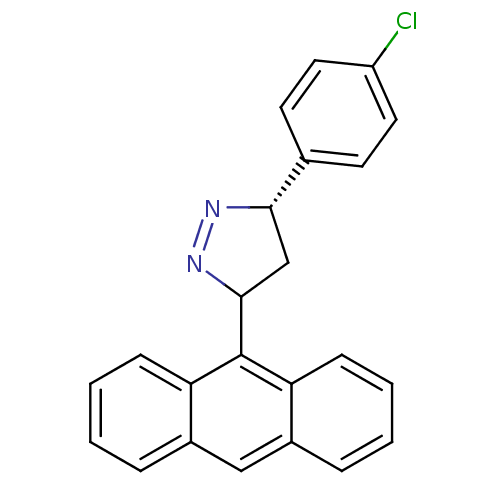 (CHEMBL2312282) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate after 60 mins by Ellman's method | Bioorg Med Chem Lett 23: 702-5 (2013) Article DOI: 10.1016/j.bmcl.2012.11.100 BindingDB Entry DOI: 10.7270/Q2F19118 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50341160 (3-(anthracen-9-yl)-5-(3-nitrophenyl)-4,5-dihydro-1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 32.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology Curated by ChEMBL | Assay Description Competitive inhibition of human recombinant MAO-A after 60 mins using p-tyramine as substrate by spectrophotometry | Bioorg Med Chem Lett 21: 1969-73 (2011) Article DOI: 10.1016/j.bmcl.2011.02.030 BindingDB Entry DOI: 10.7270/Q2JM29XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50425410 (CHEMBL2312574) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate after 60 mins by Ellman's method | Bioorg Med Chem Lett 23: 702-5 (2013) Article DOI: 10.1016/j.bmcl.2012.11.100 BindingDB Entry DOI: 10.7270/Q2F19118 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Rattus norvegicus (rat)) | BDBM50341160 (3-(anthracen-9-yl)-5-(3-nitrophenyl)-4,5-dihydro-1...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 43.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology Curated by ChEMBL | Assay Description Competitive inhibition of rat liver MAO-A after 60 mins using p-tyramine as substrate by spectrophotometry | Bioorg Med Chem Lett 21: 1969-73 (2011) Article DOI: 10.1016/j.bmcl.2011.02.030 BindingDB Entry DOI: 10.7270/Q2JM29XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50341157 (2-(3-(anthracen-9-yl)-4,5-dihydro-1H-pyrazol-5-yl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 69.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology Curated by ChEMBL | Assay Description Competitive inhibition of human recombinant MAO-A after 60 mins using p-tyramine as substrate by spectrophotometry | Bioorg Med Chem Lett 21: 1969-73 (2011) Article DOI: 10.1016/j.bmcl.2011.02.030 BindingDB Entry DOI: 10.7270/Q2JM29XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Rattus norvegicus (rat)) | BDBM50341157 (2-(3-(anthracen-9-yl)-4,5-dihydro-1H-pyrazol-5-yl)...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 77.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology Curated by ChEMBL | Assay Description Competitive inhibition of rat liver MAO-A after 60 mins using p-tyramine as substrate by spectrophotometry | Bioorg Med Chem Lett 21: 1969-73 (2011) Article DOI: 10.1016/j.bmcl.2011.02.030 BindingDB Entry DOI: 10.7270/Q2JM29XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50341158 (4-(3-(anthracen-9-yl)-4,5-dihydro-1H-pyrazol-5-yl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 81.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology Curated by ChEMBL | Assay Description Competitive inhibition of human recombinant MAO-A after 60 mins using p-tyramine as substrate by spectrophotometry | Bioorg Med Chem Lett 21: 1969-73 (2011) Article DOI: 10.1016/j.bmcl.2011.02.030 BindingDB Entry DOI: 10.7270/Q2JM29XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50443232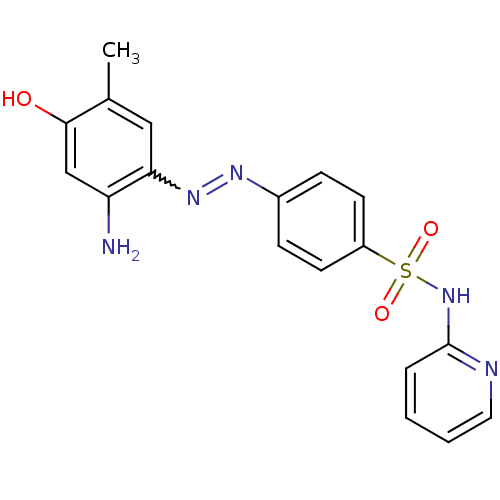 (CHEMBL3086883) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | <85 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of human 6x-His-tagged BRD4 bromodomain 1 expressed in Escherichia coli | J Med Chem 61: 9316-9334 (2018) Article DOI: 10.1021/acs.jmedchem.8b01248 BindingDB Entry DOI: 10.7270/Q25T3PRQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Amine oxidase [flavin-containing] A (Rattus norvegicus (rat)) | BDBM50341158 (4-(3-(anthracen-9-yl)-4,5-dihydro-1H-pyrazol-5-yl)...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 95.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology Curated by ChEMBL | Assay Description Competitive inhibition of rat liver MAO-A after 60 mins using p-tyramine as substrate by spectrophotometry | Bioorg Med Chem Lett 21: 1969-73 (2011) Article DOI: 10.1016/j.bmcl.2011.02.030 BindingDB Entry DOI: 10.7270/Q2JM29XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50425415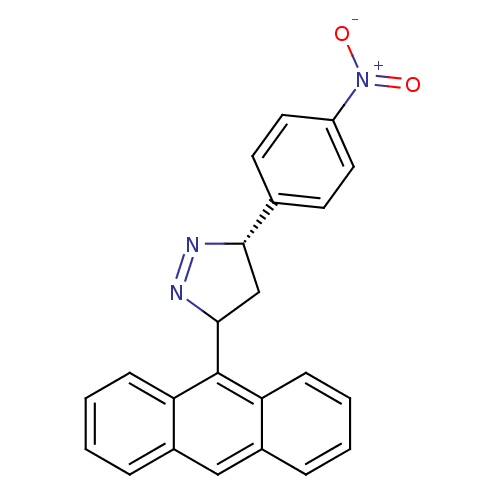 (CHEMBL2312577) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate after 60 mins by Ellman's method | Bioorg Med Chem Lett 23: 702-5 (2013) Article DOI: 10.1016/j.bmcl.2012.11.100 BindingDB Entry DOI: 10.7270/Q2F19118 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50425416 (CHEMBL2312578) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate after 60 mins by Ellman's method | Bioorg Med Chem Lett 23: 702-5 (2013) Article DOI: 10.1016/j.bmcl.2012.11.100 BindingDB Entry DOI: 10.7270/Q2F19118 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 3 (Homo sapiens (Human)) | BDBM50443232 (CHEMBL3086883) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of human 6x-His-tagged BRD3 bromodomain 1 expressed in Escherichia coli | J Med Chem 61: 9316-9334 (2018) Article DOI: 10.1021/acs.jmedchem.8b01248 BindingDB Entry DOI: 10.7270/Q25T3PRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50425414 (CHEMBL2312283) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate after 60 mins by Ellman's method | Bioorg Med Chem Lett 23: 702-5 (2013) Article DOI: 10.1016/j.bmcl.2012.11.100 BindingDB Entry DOI: 10.7270/Q2F19118 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 3 (Homo sapiens (Human)) | BDBM50443232 (CHEMBL3086883) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of human 6x-His-tagged BRD3 bromodomain 2 expressed in Escherichia coli | J Med Chem 61: 9316-9334 (2018) Article DOI: 10.1021/acs.jmedchem.8b01248 BindingDB Entry DOI: 10.7270/Q25T3PRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50341159 (2-(3-(anthracen-9-yl)-4,5-dihydro-1H-pyrazol-5-yl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 205 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology Curated by ChEMBL | Assay Description Competitive inhibition of human recombinant MAO-A after 60 mins using p-tyramine as substrate by spectrophotometry | Bioorg Med Chem Lett 21: 1969-73 (2011) Article DOI: 10.1016/j.bmcl.2011.02.030 BindingDB Entry DOI: 10.7270/Q2JM29XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50341153 (4-(3-(anthracen-9-yl)-4,5-dihydro-1H-pyrazol-5-yl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 225 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology Curated by ChEMBL | Assay Description Competitive inhibition of human recombinant MAO-A after 60 mins using p-tyramine as substrate by spectrophotometry | Bioorg Med Chem Lett 21: 1969-73 (2011) Article DOI: 10.1016/j.bmcl.2011.02.030 BindingDB Entry DOI: 10.7270/Q2JM29XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Rattus norvegicus (rat)) | BDBM50341161 (3-(anthracen-9-yl)-5-(4-methoxyphenyl)-4,5-dihydro...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology Curated by ChEMBL | Assay Description Competitive inhibition of rat liver MAO-A after 60 mins using p-tyramine as substrate by spectrophotometry | Bioorg Med Chem Lett 21: 1969-73 (2011) Article DOI: 10.1016/j.bmcl.2011.02.030 BindingDB Entry DOI: 10.7270/Q2JM29XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Rattus norvegicus (rat)) | BDBM50341159 (2-(3-(anthracen-9-yl)-4,5-dihydro-1H-pyrazol-5-yl)...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 242 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology Curated by ChEMBL | Assay Description Competitive inhibition of rat liver MAO-A after 60 mins using p-tyramine as substrate by spectrophotometry | Bioorg Med Chem Lett 21: 1969-73 (2011) Article DOI: 10.1016/j.bmcl.2011.02.030 BindingDB Entry DOI: 10.7270/Q2JM29XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50341155 (3-(anthracen-9-yl)-5-p-tolyl-4,5-dihydro-1H-pyrazo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology Curated by ChEMBL | Assay Description Competitive inhibition of human recombinant MAO-A after 60 mins using p-tyramine as substrate by spectrophotometry | Bioorg Med Chem Lett 21: 1969-73 (2011) Article DOI: 10.1016/j.bmcl.2011.02.030 BindingDB Entry DOI: 10.7270/Q2JM29XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50341154 (3-(anthracen-9-yl)-5-(4-chlorophenyl)-4,5-dihydro-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 281 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology Curated by ChEMBL | Assay Description Competitive inhibition of human recombinant MAO-A after 60 mins using p-tyramine as substrate by spectrophotometry | Bioorg Med Chem Lett 21: 1969-73 (2011) Article DOI: 10.1016/j.bmcl.2011.02.030 BindingDB Entry DOI: 10.7270/Q2JM29XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50341161 (3-(anthracen-9-yl)-5-(4-methoxyphenyl)-4,5-dihydro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 301 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology Curated by ChEMBL | Assay Description Competitive inhibition of human recombinant MAO-A after 60 mins using p-tyramine as substrate by spectrophotometry | Bioorg Med Chem Lett 21: 1969-73 (2011) Article DOI: 10.1016/j.bmcl.2011.02.030 BindingDB Entry DOI: 10.7270/Q2JM29XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Rattus norvegicus (rat)) | BDBM50341154 (3-(anthracen-9-yl)-5-(4-chlorophenyl)-4,5-dihydro-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology Curated by ChEMBL | Assay Description Competitive inhibition of rat liver MAO-A after 60 mins using p-tyramine as substrate by spectrophotometry | Bioorg Med Chem Lett 21: 1969-73 (2011) Article DOI: 10.1016/j.bmcl.2011.02.030 BindingDB Entry DOI: 10.7270/Q2JM29XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 296 total ) | Next | Last >> |