

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Deoxyuridine 5'-triphosphate nucleotidohydrolase, mitochondrial (Homo sapiens (Human)) | BDBM50386618 (CHEMBL2048443) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co. Ltd. Curated by ChEMBL | Assay Description Inhibition of human dUTPase | J Med Chem 55: 2960-9 (2012) Article DOI: 10.1021/jm201627n BindingDB Entry DOI: 10.7270/Q2WQ04VS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Deoxyuridine 5'-triphosphate nucleotidohydrolase, mitochondrial (Homo sapiens (Human)) | BDBM50173539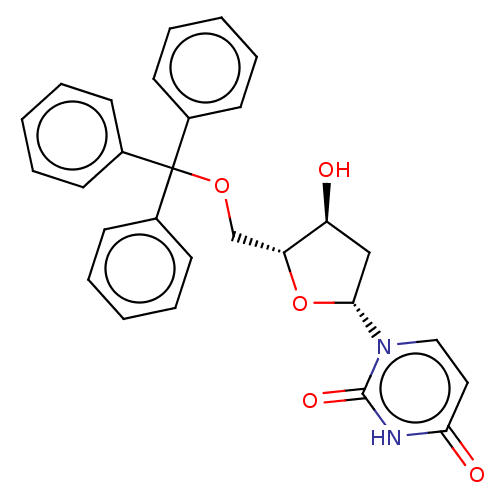 (1-(4-Hydroxy-5-trityloxymethyl-tetrahydro-furan-2-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co. Ltd. Curated by ChEMBL | Assay Description Inhibition of human dUTPase assessed as production of [5-3H]dUMP from [5-3H]dUTP after 15 mins measured by HPLC analysis | J Med Chem 55: 2970-80 (2012) Article DOI: 10.1021/jm201628y BindingDB Entry DOI: 10.7270/Q2C82BB5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribonucleoside-diphosphate reductase large subunit/subunit M2 (Homo sapiens (Human)) | BDBM477482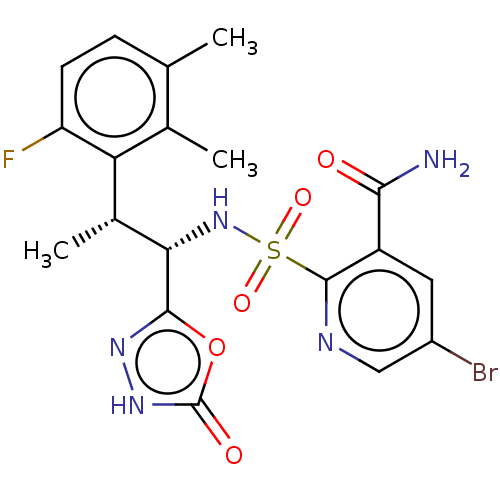 (5-bromo-2-(N-((1S,2R)-2-(6-fluoro-2,3-dimethylphen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2V128RN | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1/M2 (Homo sapiens (Human)) | BDBM477482 (5-bromo-2-(N-((1S,2R)-2-(6-fluoro-2,3-dimethylphen...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description First, test compounds were serially diluted with DMSO. Next, human M1 protein and human M2 protein were added to an aqueous albumin solution derived ... | US Patent US10889555 (2021) BindingDB Entry DOI: 10.7270/Q2RR229N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Deoxyuridine 5'-triphosphate nucleotidohydrolase, mitochondrial (Homo sapiens (Human)) | BDBM101748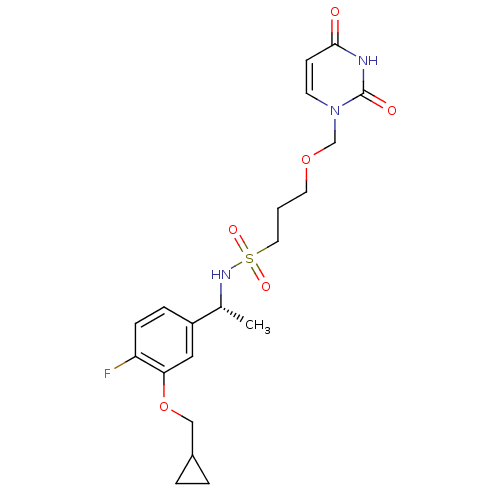 (CHEMBL2057911 | US8530490, 8) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co. Ltd. Curated by ChEMBL | Assay Description Inhibition of human dUTPase assessed as production of [5-3H]dUMP from [5-3H]dUTP after 15 mins measured by HPLC analysis | J Med Chem 55: 2970-80 (2012) Article DOI: 10.1021/jm201628y BindingDB Entry DOI: 10.7270/Q2C82BB5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Deoxyuridine 5'-triphosphate nucleotidohydrolase, mitochondrial (Homo sapiens (Human)) | BDBM50391352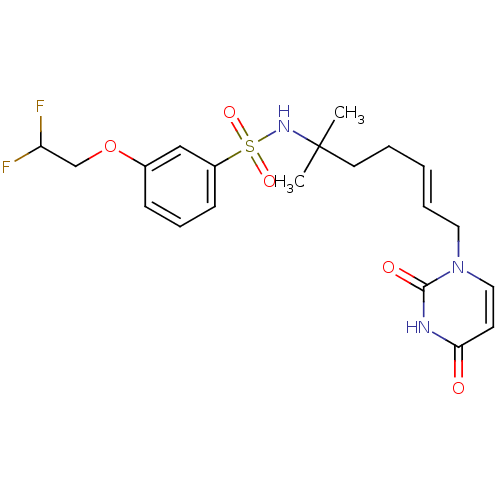 (CHEMBL2147981) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co. Ltd. Curated by ChEMBL | Assay Description Inhibition of human dUTPase-mediated formation of [5-3H]dUMP expressed in Escherichia coli BL21 (DE3) after 15 mins by HPLC analysis | J Med Chem 55: 5483-96 (2012) Article DOI: 10.1021/jm300416h BindingDB Entry DOI: 10.7270/Q2C53MZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Deoxyuridine 5'-triphosphate nucleotidohydrolase, mitochondrial (Homo sapiens (Human)) | BDBM50391350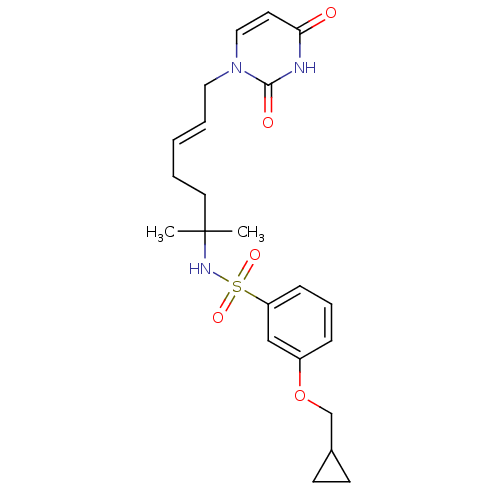 (CHEMBL2147979) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co. Ltd. Curated by ChEMBL | Assay Description Inhibition of human dUTPase-mediated formation of [5-3H]dUMP expressed in Escherichia coli BL21 (DE3) after 15 mins by HPLC analysis | J Med Chem 55: 5483-96 (2012) Article DOI: 10.1021/jm300416h BindingDB Entry DOI: 10.7270/Q2C53MZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Deoxyuridine 5'-triphosphate nucleotidohydrolase, mitochondrial (Homo sapiens (Human)) | BDBM50395031 (CHEMBL2163854) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co. Ltd. Curated by ChEMBL | Assay Description Inhibition of human dUTPase assessed as reduction in [5-3H]dUMP production incubated for 15 mins by HPLC | J Med Chem 55: 6427-37 (2012) Article DOI: 10.1021/jm3004174 BindingDB Entry DOI: 10.7270/Q2XS5WJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1/M2 (Homo sapiens (Human)) | BDBM477358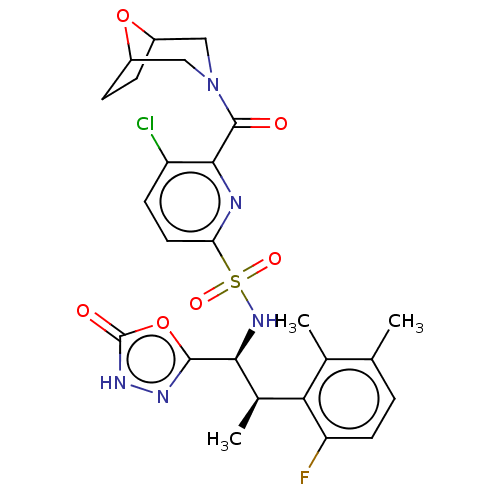 (6-(8-oxa-3-azabicyclo[3.2.1]octane-3-carbonyl)-5-c...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description First, test compounds were serially diluted with DMSO. Next, human M1 protein and human M2 protein were added to an aqueous albumin solution derived ... | US Patent US10889555 (2021) BindingDB Entry DOI: 10.7270/Q2RR229N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Deoxyuridine 5'-triphosphate nucleotidohydrolase, mitochondrial (Homo sapiens (Human)) | BDBM101762 (US8530490, 22) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description The inhibitory activity of the compound against human dUTPase was determined by measuring the production of [5-3H]deoxyuridine monophosphate from [5-... | US Patent US8530490 (2013) BindingDB Entry DOI: 10.7270/Q25H7DX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Deoxyuridine 5'-triphosphate nucleotidohydrolase, mitochondrial (Homo sapiens (Human)) | BDBM101774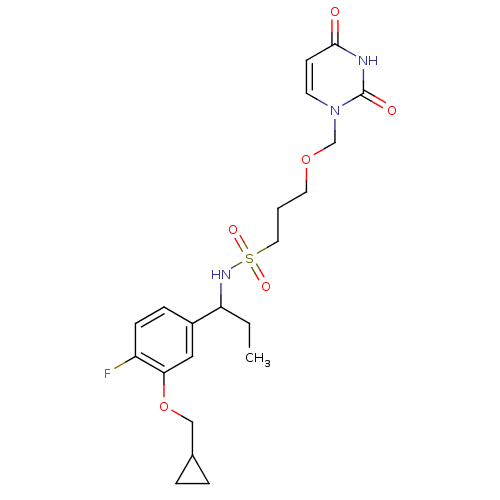 (US8530490, 34) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description The inhibitory activity of the compound against human dUTPase was determined by measuring the production of [5-3H]deoxyuridine monophosphate from [5-... | US Patent US8530490 (2013) BindingDB Entry DOI: 10.7270/Q25H7DX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1/M2 (Homo sapiens (Human)) | BDBM477418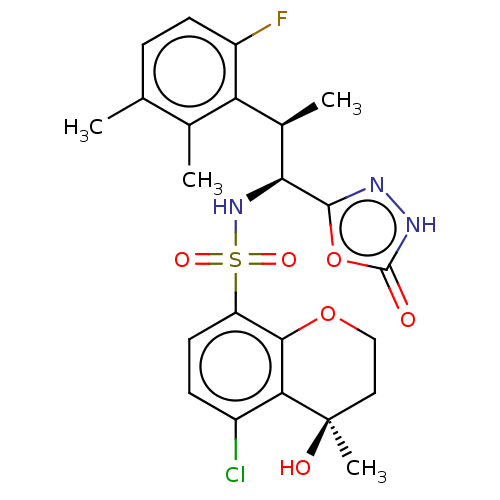 (US10889555, Example 200A | US10889555, Example 207...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description First, test compounds were serially diluted with DMSO. Next, human M1 protein and human M2 protein were added to an aqueous albumin solution derived ... | US Patent US10889555 (2021) BindingDB Entry DOI: 10.7270/Q2RR229N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Deoxyuridine 5'-triphosphate nucleotidohydrolase, mitochondrial (Homo sapiens (Human)) | BDBM101762 (US8530490, 22) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 30 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description The inhibitory activity of these compounds of the present invention against human dUTPase was determined by measuring the production of [5-3H]deoxyur... | US Patent US8883759 (2014) BindingDB Entry DOI: 10.7270/Q27943CK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribonucleoside-diphosphate reductase large subunit/subunit M2 (Homo sapiens (Human)) | BDBM477358 (6-(8-oxa-3-azabicyclo[3.2.1]octane-3-carbonyl)-5-c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2V128RN | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribonucleoside-diphosphate reductase large subunit/subunit M2 (Homo sapiens (Human)) | BDBM601172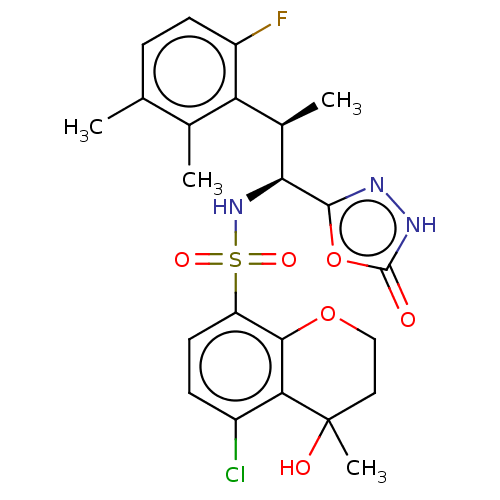 (US11634395, Example 200A | US11634395, Example 200...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2V128RN | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Deoxyuridine 5'-triphosphate nucleotidohydrolase, mitochondrial (Homo sapiens (Human)) | BDBM101778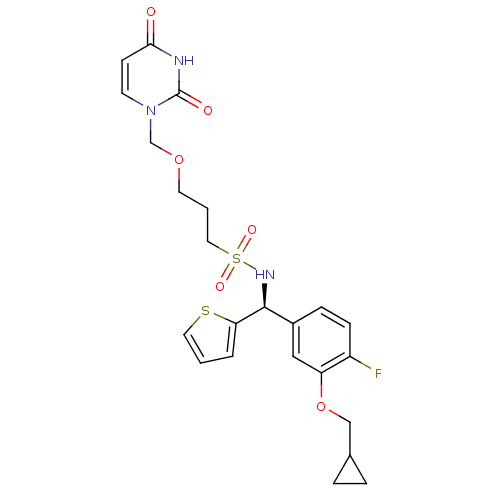 (US8530490, 38) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description The inhibitory activity of the compound against human dUTPase was determined by measuring the production of [5-3H]deoxyuridine monophosphate from [5-... | US Patent US8530490 (2013) BindingDB Entry DOI: 10.7270/Q25H7DX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Deoxyuridine 5'-triphosphate nucleotidohydrolase, mitochondrial (Homo sapiens (Human)) | BDBM50391347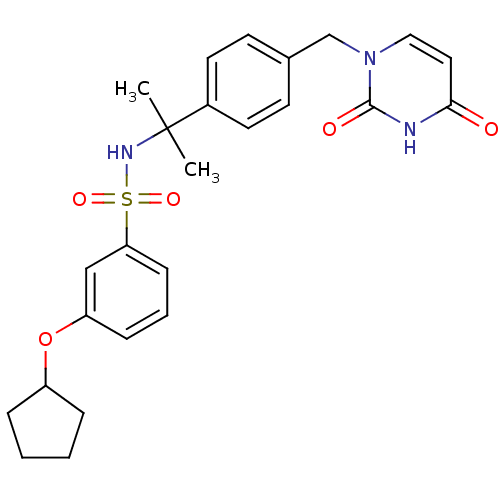 (CHEMBL2147976) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co. Ltd. Curated by ChEMBL | Assay Description Inhibition of human dUTPase-mediated formation of [5-3H]dUMP expressed in Escherichia coli BL21 (DE3) after 15 mins by HPLC analysis | J Med Chem 55: 5483-96 (2012) Article DOI: 10.1021/jm300416h BindingDB Entry DOI: 10.7270/Q2C53MZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Deoxyuridine 5'-triphosphate nucleotidohydrolase, mitochondrial (Homo sapiens (Human)) | BDBM50391351 (CHEMBL2147980) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co. Ltd. Curated by ChEMBL | Assay Description Inhibition of human dUTPase-mediated formation of [5-3H]dUMP expressed in Escherichia coli BL21 (DE3) after 15 mins by HPLC analysis | J Med Chem 55: 5483-96 (2012) Article DOI: 10.1021/jm300416h BindingDB Entry DOI: 10.7270/Q2C53MZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Deoxyuridine 5'-triphosphate nucleotidohydrolase, mitochondrial (Homo sapiens (Human)) | BDBM101889 (CHEMBL2057599 | US8530490, 159) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co. Ltd. Curated by ChEMBL | Assay Description Inhibition of human dUTPase-mediated formation of [5-3H]dUMP expressed in Escherichia coli BL21 (DE3) after 15 mins by HPLC analysis | J Med Chem 55: 5483-96 (2012) Article DOI: 10.1021/jm300416h BindingDB Entry DOI: 10.7270/Q2C53MZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Deoxyuridine 5'-triphosphate nucleotidohydrolase, mitochondrial (Homo sapiens (Human)) | BDBM101889 (CHEMBL2057599 | US8530490, 159) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co. Ltd. Curated by ChEMBL | Assay Description Inhibition of human dUTPase assessed as production of [5-3H]dUMP from [5-3H]dUTP after 15 mins measured by HPLC analysis | J Med Chem 55: 2970-80 (2012) Article DOI: 10.1021/jm201628y BindingDB Entry DOI: 10.7270/Q2C82BB5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Deoxyuridine 5'-triphosphate nucleotidohydrolase, mitochondrial (Homo sapiens (Human)) | BDBM101847 (CHEMBL2147985 | US8530490, 110) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co. Ltd. Curated by ChEMBL | Assay Description Inhibition of human dUTPase-mediated formation of [5-3H]dUMP expressed in Escherichia coli BL21 (DE3) after 15 mins by HPLC analysis | J Med Chem 55: 5483-96 (2012) Article DOI: 10.1021/jm300416h BindingDB Entry DOI: 10.7270/Q2C53MZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Deoxyuridine 5'-triphosphate nucleotidohydrolase, mitochondrial (Homo sapiens (Human)) | BDBM101763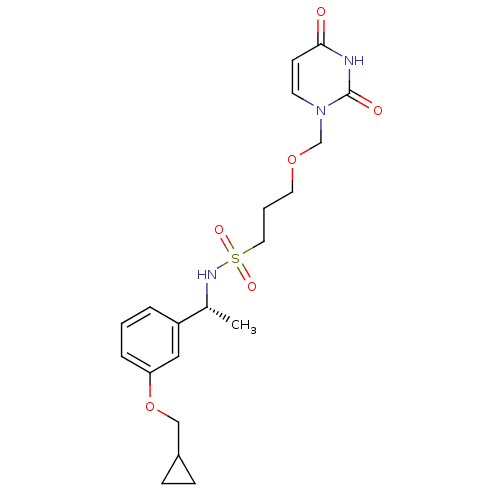 (CHEMBL2057909 | US8530490, 23) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co. Ltd. Curated by ChEMBL | Assay Description Inhibition of human dUTPase assessed as production of [5-3H]dUMP from [5-3H]dUTP after 15 mins measured by HPLC analysis | J Med Chem 55: 2970-80 (2012) Article DOI: 10.1021/jm201628y BindingDB Entry DOI: 10.7270/Q2C82BB5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1/M2 (Homo sapiens (Human)) | BDBM477305 (US10889555, Example 39 | US11634395, Example 39) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description First, test compounds were serially diluted with DMSO. Next, human M1 protein and human M2 protein were added to an aqueous albumin solution derived ... | US Patent US10889555 (2021) BindingDB Entry DOI: 10.7270/Q2RR229N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1/M2 (Homo sapiens (Human)) | BDBM477477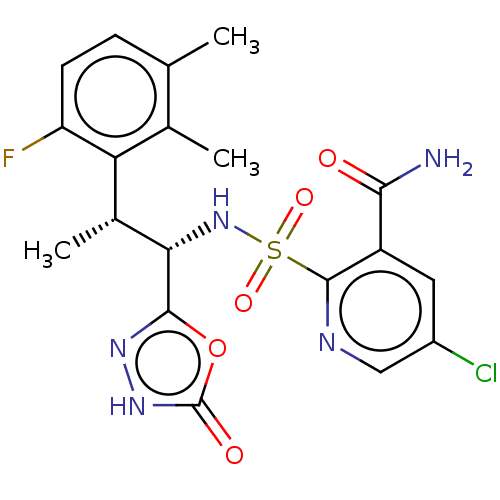 (US10889555, Example 245 | US11634395, Example 245) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description First, test compounds were serially diluted with DMSO. Next, human M1 protein and human M2 protein were added to an aqueous albumin solution derived ... | US Patent US10889555 (2021) BindingDB Entry DOI: 10.7270/Q2RR229N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Deoxyuridine 5'-triphosphate nucleotidohydrolase, mitochondrial (Homo sapiens (Human)) | BDBM101749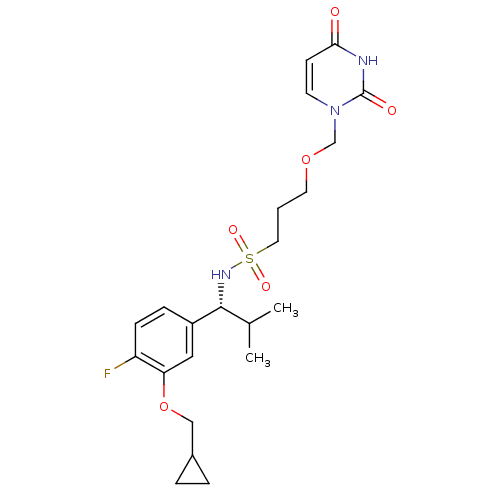 (US8530490, 9) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description The inhibitory activity of the compound against human dUTPase was determined by measuring the production of [5-3H]deoxyuridine monophosphate from [5-... | US Patent US8530490 (2013) BindingDB Entry DOI: 10.7270/Q25H7DX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Deoxyuridine 5'-triphosphate nucleotidohydrolase, mitochondrial (Homo sapiens (Human)) | BDBM101764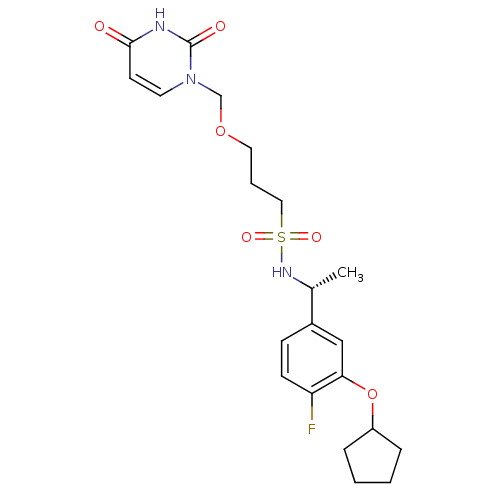 (US8530490, 24) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description The inhibitory activity of the compound against human dUTPase was determined by measuring the production of [5-3H]deoxyuridine monophosphate from [5-... | US Patent US8530490 (2013) BindingDB Entry DOI: 10.7270/Q25H7DX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Deoxyuridine 5'-triphosphate nucleotidohydrolase, mitochondrial (Homo sapiens (Human)) | BDBM101767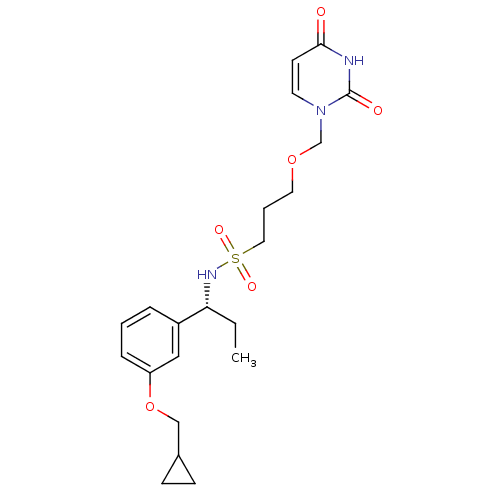 (US8530490, 27) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description The inhibitory activity of the compound against human dUTPase was determined by measuring the production of [5-3H]deoxyuridine monophosphate from [5-... | US Patent US8530490 (2013) BindingDB Entry DOI: 10.7270/Q25H7DX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribonucleoside-diphosphate reductase large subunit/subunit M2 (Homo sapiens (Human)) | BDBM477305 (US10889555, Example 39 | US11634395, Example 39) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2V128RN | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Deoxyuridine 5'-triphosphate nucleotidohydrolase, mitochondrial (Homo sapiens (Human)) | BDBM101837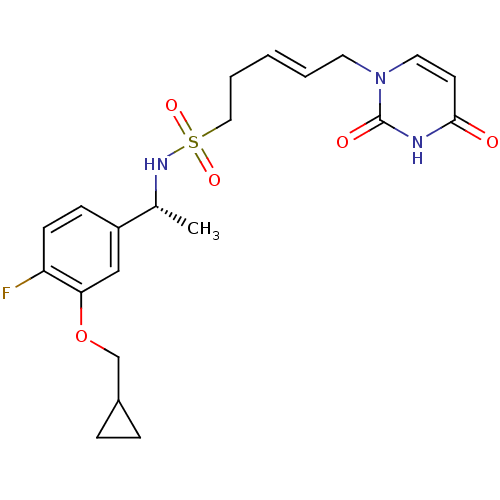 (US8530490, 100) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description The inhibitory activity of the compound against human dUTPase was determined by measuring the production of [5-3H]deoxyuridine monophosphate from [5-... | US Patent US8530490 (2013) BindingDB Entry DOI: 10.7270/Q25H7DX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Deoxyuridine 5'-triphosphate nucleotidohydrolase, mitochondrial (Homo sapiens (Human)) | BDBM5563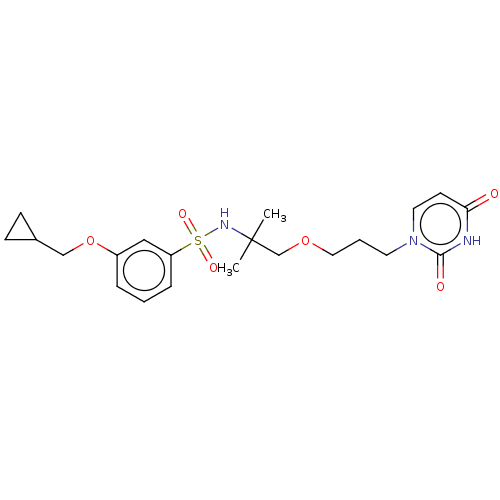 (US8530490, 173 | US8530490, 175) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description The inhibitory activity of the compound against human dUTPase was determined by measuring the production of [5-3H]deoxyuridine monophosphate from [5-... | US Patent US8530490 (2013) BindingDB Entry DOI: 10.7270/Q25H7DX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Deoxyuridine 5'-triphosphate nucleotidohydrolase, mitochondrial (Homo sapiens (Human)) | BDBM101764 (US8530490, 24) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 40 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description The inhibitory activity of these compounds of the present invention against human dUTPase was determined by measuring the production of [5-3H]deoxyur... | US Patent US8883759 (2014) BindingDB Entry DOI: 10.7270/Q27943CK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Deoxyuridine 5'-triphosphate nucleotidohydrolase, mitochondrial (Homo sapiens (Human)) | BDBM101763 (CHEMBL2057909 | US8530490, 23) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co. Ltd. Curated by ChEMBL | Assay Description Inhibition of human dUTPase-mediated formation of [5-3H]dUMP expressed in Escherichia coli BL21 (DE3) after 15 mins by HPLC analysis | J Med Chem 55: 5483-96 (2012) Article DOI: 10.1021/jm300416h BindingDB Entry DOI: 10.7270/Q2C53MZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribonucleoside-diphosphate reductase large subunit/subunit M2 (Homo sapiens (Human)) | BDBM477477 (US10889555, Example 245 | US11634395, Example 245) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2V128RN | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Deoxyuridine 5'-triphosphate nucleotidohydrolase, mitochondrial (Homo sapiens (Human)) | BDBM101781 (US8530490, 41) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description The inhibitory activity of the compound against human dUTPase was determined by measuring the production of [5-3H]deoxyuridine monophosphate from [5-... | US Patent US8530490 (2013) BindingDB Entry DOI: 10.7270/Q25H7DX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Deoxyuridine 5'-triphosphate nucleotidohydrolase, mitochondrial (Homo sapiens (Human)) | BDBM101842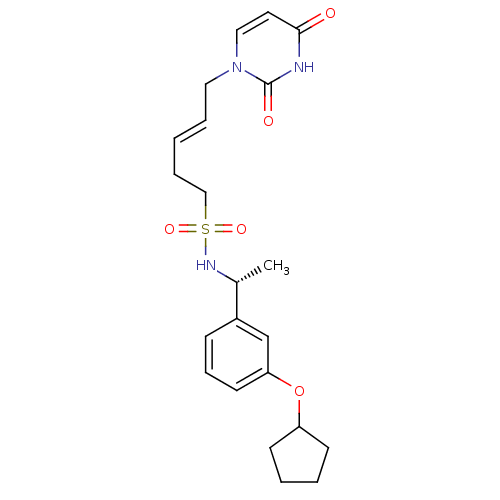 (CHEMBL2147986 | US8530490, 105) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co. Ltd. Curated by ChEMBL | Assay Description Inhibition of human dUTPase-mediated formation of [5-3H]dUMP expressed in Escherichia coli BL21 (DE3) after 15 mins by HPLC analysis | J Med Chem 55: 5483-96 (2012) Article DOI: 10.1021/jm300416h BindingDB Entry DOI: 10.7270/Q2C53MZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1/M2 (Homo sapiens (Human)) | BDBM477445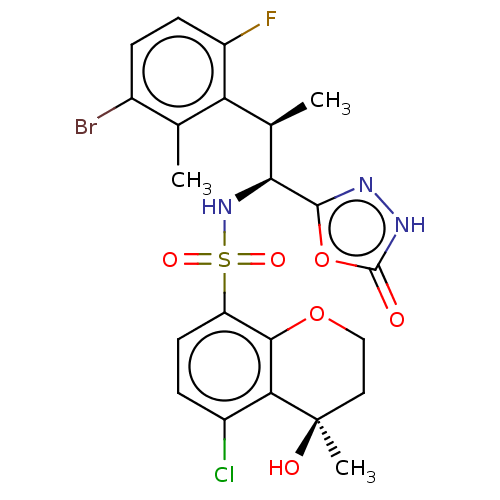 (US10889555, Example 222B | US10889555, Example 226...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description First, test compounds were serially diluted with DMSO. Next, human M1 protein and human M2 protein were added to an aqueous albumin solution derived ... | US Patent US10889555 (2021) BindingDB Entry DOI: 10.7270/Q2RR229N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1/M2 (Homo sapiens (Human)) | BDBM477447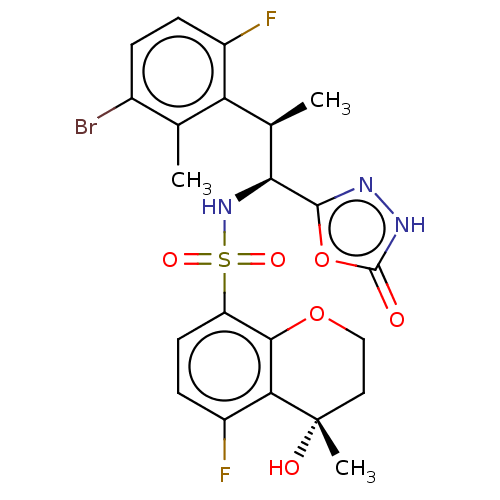 (5-fluoro-N-((1S,2R)-2-(6-fluoro-2,3-dimethylphenyl...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description First, test compounds were serially diluted with DMSO. Next, human M1 protein and human M2 protein were added to an aqueous albumin solution derived ... | US Patent US10889555 (2021) BindingDB Entry DOI: 10.7270/Q2RR229N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1/M2 (Homo sapiens (Human)) | BDBM477459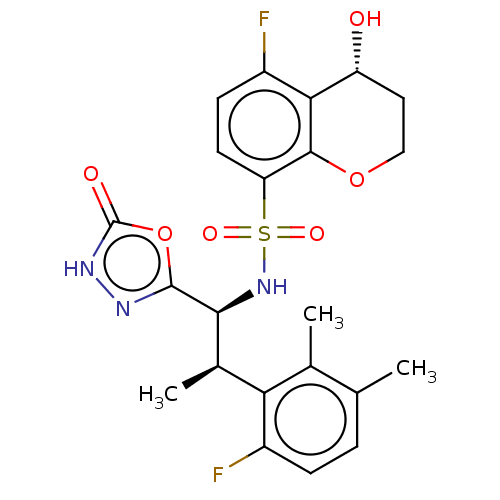 (US10889555, Example 230B | US11634395, Example 230...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description First, test compounds were serially diluted with DMSO. Next, human M1 protein and human M2 protein were added to an aqueous albumin solution derived ... | US Patent US10889555 (2021) BindingDB Entry DOI: 10.7270/Q2RR229N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Deoxyuridine 5'-triphosphate nucleotidohydrolase, mitochondrial (Homo sapiens (Human)) | BDBM101763 (CHEMBL2057909 | US8530490, 23) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description The inhibitory activity of the compound against human dUTPase was determined by measuring the production of [5-3H]deoxyuridine monophosphate from [5-... | US Patent US8530490 (2013) BindingDB Entry DOI: 10.7270/Q25H7DX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Deoxyuridine 5'-triphosphate nucleotidohydrolase, mitochondrial (Homo sapiens (Human)) | BDBM101847 (CHEMBL2147985 | US8530490, 110) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description The inhibitory activity of the compound against human dUTPase was determined by measuring the production of [5-3H]deoxyuridine monophosphate from [5-... | US Patent US8530490 (2013) BindingDB Entry DOI: 10.7270/Q25H7DX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribonucleoside-diphosphate reductase large subunit/subunit M2 (Homo sapiens (Human)) | BDBM477519 (US10889555, Example 287 | US11634395, Example 287) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2V128RN | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribonucleoside-diphosphate reductase large subunit/subunit M2 (Homo sapiens (Human)) | BDBM601197 (N-((1S,2R)-2-(3-bromo-6-fluoro-2-methylphenyl)-1-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2V128RN | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribonucleoside-diphosphate reductase large subunit/subunit M2 (Homo sapiens (Human)) | BDBM601200 (5-fluoro-N-((1S,2R)-2-(6-fluoro-2,3-dimethylphenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2V128RN | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribonucleoside-diphosphate reductase large subunit/subunit M2 (Homo sapiens (Human)) | BDBM477459 (US10889555, Example 230B | US11634395, Example 230...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2V128RN | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1/M2 (Homo sapiens (Human)) | BDBM477519 (US10889555, Example 287 | US11634395, Example 287) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description First, test compounds were serially diluted with DMSO. Next, human M1 protein and human M2 protein were added to an aqueous albumin solution derived ... | US Patent US10889555 (2021) BindingDB Entry DOI: 10.7270/Q2RR229N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Deoxyuridine 5'-triphosphate nucleotidohydrolase, mitochondrial (Homo sapiens (Human)) | BDBM101763 (CHEMBL2057909 | US8530490, 23) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 50 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description The inhibitory activity of these compounds of the present invention against human dUTPase was determined by measuring the production of [5-3H]deoxyur... | US Patent US8883759 (2014) BindingDB Entry DOI: 10.7270/Q27943CK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Deoxyuridine 5'-triphosphate nucleotidohydrolase, mitochondrial (Homo sapiens (Human)) | BDBM50395047 (CHEMBL2163852) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co. Ltd. Curated by ChEMBL | Assay Description Inhibition of human dUTPase assessed as reduction in [5-3H]dUMP production incubated for 15 mins by HPLC | J Med Chem 55: 6427-37 (2012) Article DOI: 10.1021/jm3004174 BindingDB Entry DOI: 10.7270/Q2XS5WJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1/M2 (Homo sapiens (Human)) | BDBM477350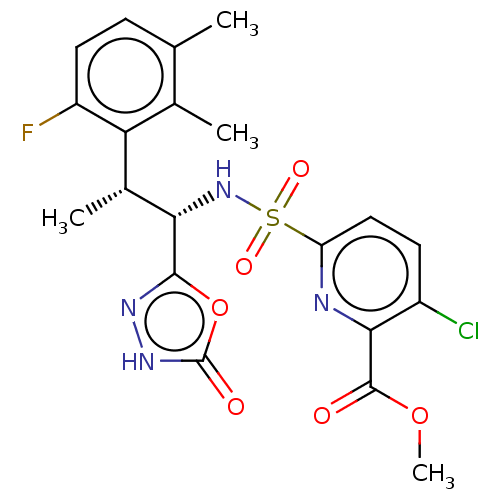 (US10889555, Example 109 | US11634395, Example 109 ...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description First, test compounds were serially diluted with DMSO. Next, human M1 protein and human M2 protein were added to an aqueous albumin solution derived ... | US Patent US10889555 (2021) BindingDB Entry DOI: 10.7270/Q2RR229N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1/M2 (Homo sapiens (Human)) | BDBM477415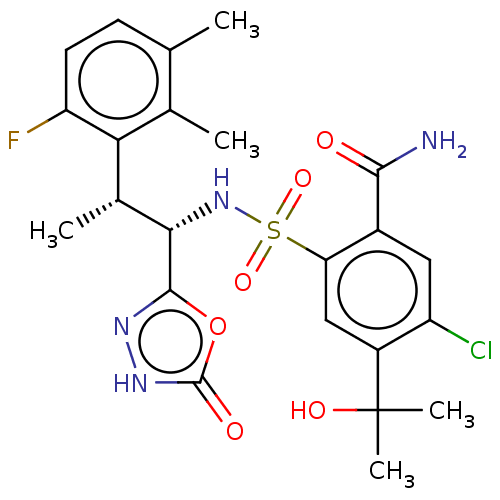 (5-chloro-2-(N-((1S,2R)-2-(6-fluoro-2,3-dimethylphe...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description First, test compounds were serially diluted with DMSO. Next, human M1 protein and human M2 protein were added to an aqueous albumin solution derived ... | US Patent US10889555 (2021) BindingDB Entry DOI: 10.7270/Q2RR229N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1/M2 (Homo sapiens (Human)) | BDBM477355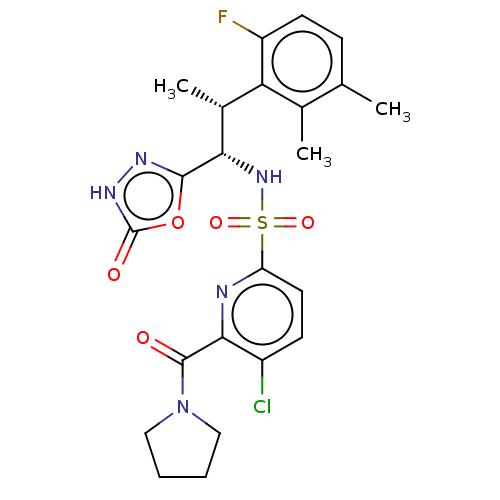 (5-chloro-N-((1S,2R) 2-(6-fluoro-2,3-dimethylphenyl...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description First, test compounds were serially diluted with DMSO. Next, human M1 protein and human M2 protein were added to an aqueous albumin solution derived ... | US Patent US10889555 (2021) BindingDB Entry DOI: 10.7270/Q2RR229N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 855 total ) | Next | Last >> |