

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50429069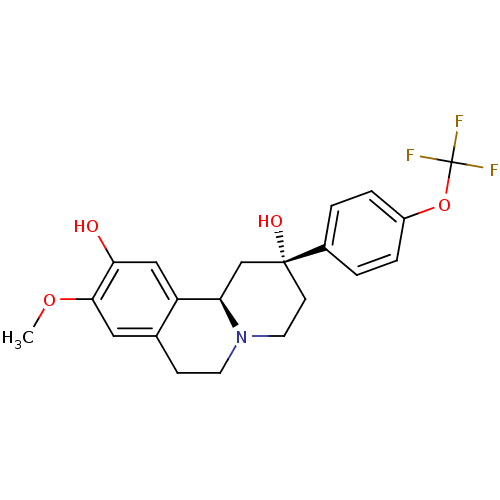 (CHEMBL2335736) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US Curated by ChEMBL | Assay Description Displacement of [3H]SCH23390 from dopamine D1 receptor (unknown origin) expressed in CHO cell membranes after 60 mins | Bioorg Med Chem Lett 23: 1498-501 (2013) Article DOI: 10.1016/j.bmcl.2012.12.046 BindingDB Entry DOI: 10.7270/Q290255G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50429068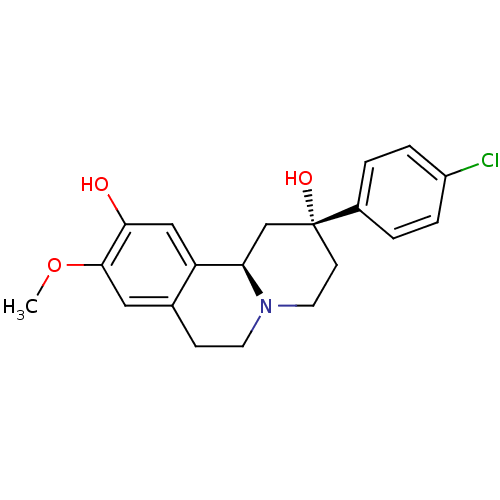 (CHEMBL2335740) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US Curated by ChEMBL | Assay Description Displacement of [3H]SCH23390 from dopamine D1 receptor (unknown origin) expressed in CHO cell membranes after 60 mins | Bioorg Med Chem Lett 23: 1498-501 (2013) Article DOI: 10.1016/j.bmcl.2012.12.046 BindingDB Entry DOI: 10.7270/Q290255G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50429069 (CHEMBL2335736) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US Curated by ChEMBL | Assay Description Displacement of [3H]N-methylspiperone from dopamine D2 receptor (unknown origin) expressed in CHO cell membranes after 60 mins | Bioorg Med Chem Lett 23: 1498-501 (2013) Article DOI: 10.1016/j.bmcl.2012.12.046 BindingDB Entry DOI: 10.7270/Q290255G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50429067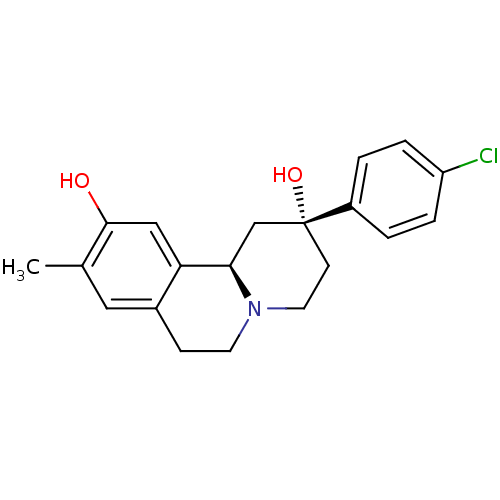 (CHEMBL2335737) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US Curated by ChEMBL | Assay Description Displacement of [3H]SCH23390 from dopamine D1 receptor (unknown origin) expressed in CHO cell membranes after 60 mins | Bioorg Med Chem Lett 23: 1498-501 (2013) Article DOI: 10.1016/j.bmcl.2012.12.046 BindingDB Entry DOI: 10.7270/Q290255G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50429067 (CHEMBL2335737) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US Curated by ChEMBL | Assay Description Displacement of [3H]N-methylspiperone from dopamine D2 receptor (unknown origin) expressed in CHO cell membranes after 60 mins | Bioorg Med Chem Lett 23: 1498-501 (2013) Article DOI: 10.1016/j.bmcl.2012.12.046 BindingDB Entry DOI: 10.7270/Q290255G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50429068 (CHEMBL2335740) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US Curated by ChEMBL | Assay Description Displacement of [3H]N-methylspiperone from dopamine D2 receptor (unknown origin) expressed in CHO cell membranes after 60 mins | Bioorg Med Chem Lett 23: 1498-501 (2013) Article DOI: 10.1016/j.bmcl.2012.12.046 BindingDB Entry DOI: 10.7270/Q290255G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50429065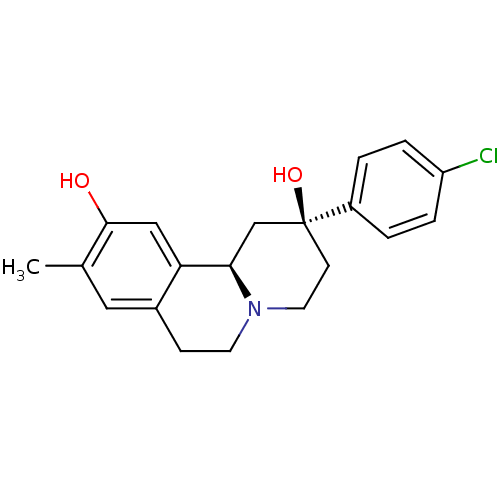 (CHEMBL2331599) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US Curated by ChEMBL | Assay Description Displacement of [3H]SCH23390 from dopamine D1 receptor (unknown origin) expressed in CHO cell membranes after 60 mins | Bioorg Med Chem Lett 23: 1498-501 (2013) Article DOI: 10.1016/j.bmcl.2012.12.046 BindingDB Entry DOI: 10.7270/Q290255G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50429066 (CHEMBL2335741) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 101 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US Curated by ChEMBL | Assay Description Displacement of [3H]N-methylspiperone from dopamine D2 receptor (unknown origin) expressed in CHO cell membranes after 60 mins | Bioorg Med Chem Lett 23: 1498-501 (2013) Article DOI: 10.1016/j.bmcl.2012.12.046 BindingDB Entry DOI: 10.7270/Q290255G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50429065 (CHEMBL2331599) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 132 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US Curated by ChEMBL | Assay Description Displacement of [3H]N-methylspiperone from dopamine D2 receptor (unknown origin) expressed in CHO cell membranes after 60 mins | Bioorg Med Chem Lett 23: 1498-501 (2013) Article DOI: 10.1016/j.bmcl.2012.12.046 BindingDB Entry DOI: 10.7270/Q290255G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50429064 (CHEMBL2335738) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 195 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US Curated by ChEMBL | Assay Description Displacement of [3H]N-methylspiperone from dopamine D2 receptor (unknown origin) expressed in CHO cell membranes after 60 mins | Bioorg Med Chem Lett 23: 1498-501 (2013) Article DOI: 10.1016/j.bmcl.2012.12.046 BindingDB Entry DOI: 10.7270/Q290255G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50429066 (CHEMBL2335741) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 244 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US Curated by ChEMBL | Assay Description Displacement of [3H]SCH23390 from dopamine D1 receptor (unknown origin) expressed in CHO cell membranes after 60 mins | Bioorg Med Chem Lett 23: 1498-501 (2013) Article DOI: 10.1016/j.bmcl.2012.12.046 BindingDB Entry DOI: 10.7270/Q290255G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50429064 (CHEMBL2335738) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 252 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US Curated by ChEMBL | Assay Description Displacement of [3H]SCH23390 from dopamine D1 receptor (unknown origin) expressed in CHO cell membranes after 60 mins | Bioorg Med Chem Lett 23: 1498-501 (2013) Article DOI: 10.1016/j.bmcl.2012.12.046 BindingDB Entry DOI: 10.7270/Q290255G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50429063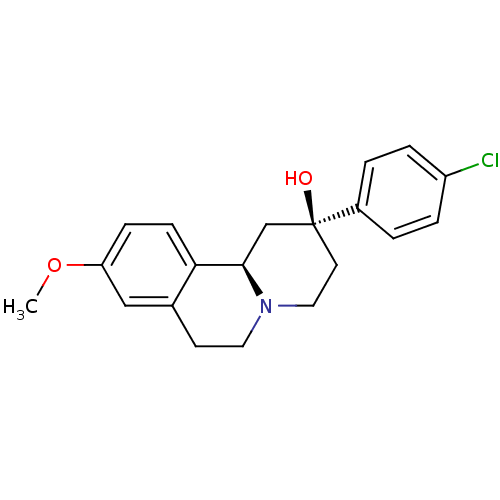 (CHEMBL2335739) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US Curated by ChEMBL | Assay Description Displacement of [3H]SCH23390 from dopamine D1 receptor (unknown origin) expressed in CHO cell membranes after 60 mins | Bioorg Med Chem Lett 23: 1498-501 (2013) Article DOI: 10.1016/j.bmcl.2012.12.046 BindingDB Entry DOI: 10.7270/Q290255G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50429063 (CHEMBL2335739) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US Curated by ChEMBL | Assay Description Displacement of [3H]N-methylspiperone from dopamine D2 receptor (unknown origin) expressed in CHO cell membranes after 60 mins | Bioorg Med Chem Lett 23: 1498-501 (2013) Article DOI: 10.1016/j.bmcl.2012.12.046 BindingDB Entry DOI: 10.7270/Q290255G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50261864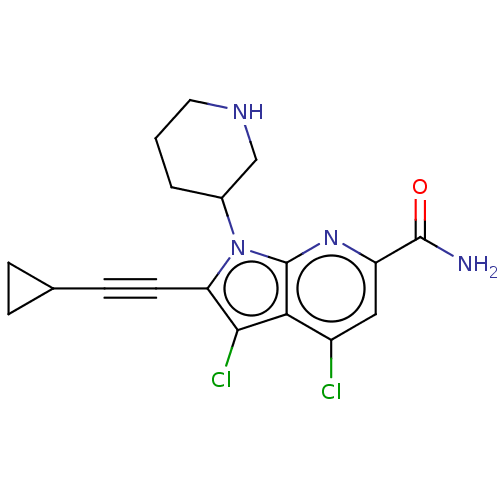 (CHEMBL4100435) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
IDD Medicinal Chemistry, Sanofi Genzyme, 153 Second Avenue, Waltham, MA 02451, USA. Electronic address: claude.barberis2@sanofi.com. Curated by ChEMBL | Assay Description Inhibition of PIM1 (unknown origin) assessed as reduction in BAD phosphorylation at Ser112 residues by TR-FRET assay | Bioorg Med Chem Lett 27: 4735-4740 (2017) Article DOI: 10.1016/j.bmcl.2017.08.068 BindingDB Entry DOI: 10.7270/Q2NG4T34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50261828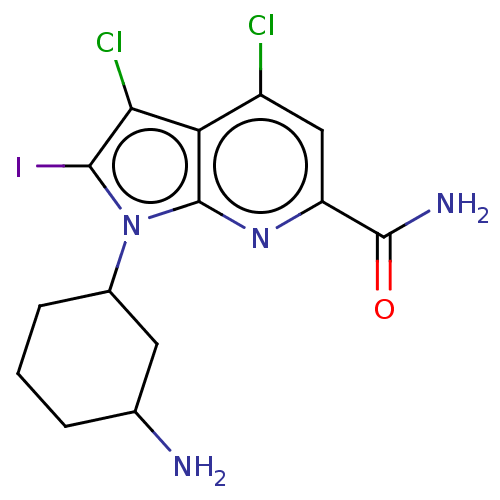 (CHEMBL4095220) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
IDD Medicinal Chemistry, Sanofi Genzyme, 153 Second Avenue, Waltham, MA 02451, USA. Electronic address: claude.barberis2@sanofi.com. Curated by ChEMBL | Assay Description Inhibition of PIM1 (unknown origin) assessed as reduction in BAD phosphorylation at Ser112 residues by TR-FRET assay | Bioorg Med Chem Lett 27: 4735-4740 (2017) Article DOI: 10.1016/j.bmcl.2017.08.068 BindingDB Entry DOI: 10.7270/Q2NG4T34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50261857 (CHEMBL4082422) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
IDD Medicinal Chemistry, Sanofi Genzyme, 153 Second Avenue, Waltham, MA 02451, USA. Electronic address: claude.barberis2@sanofi.com. Curated by ChEMBL | Assay Description Inhibition of PIM1 (unknown origin) assessed as reduction in BAD phosphorylation at Ser112 residues by TR-FRET assay | Bioorg Med Chem Lett 27: 4735-4740 (2017) Article DOI: 10.1016/j.bmcl.2017.08.068 BindingDB Entry DOI: 10.7270/Q2NG4T34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50261808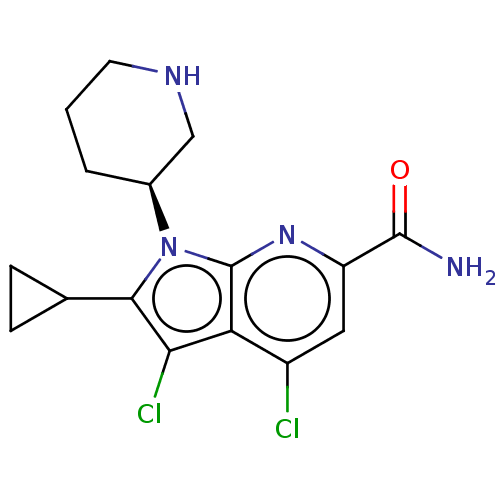 (CHEMBL4104355) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
IDD Medicinal Chemistry, Sanofi Genzyme, 153 Second Avenue, Waltham, MA 02451, USA. Electronic address: claude.barberis2@sanofi.com. Curated by ChEMBL | Assay Description Inhibition of PIM1 (unknown origin) assessed as reduction in BAD phosphorylation at Ser112 residues by TR-FRET assay | Bioorg Med Chem Lett 27: 4735-4740 (2017) Article DOI: 10.1016/j.bmcl.2017.08.068 BindingDB Entry DOI: 10.7270/Q2NG4T34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50261822 (CHEMBL4093592) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
IDD Medicinal Chemistry, Sanofi Genzyme, 153 Second Avenue, Waltham, MA 02451, USA. Electronic address: claude.barberis2@sanofi.com. Curated by ChEMBL | Assay Description Inhibition of PIM1 (unknown origin) assessed as reduction in BAD phosphorylation at Ser112 residues by TR-FRET assay | Bioorg Med Chem Lett 27: 4735-4740 (2017) Article DOI: 10.1016/j.bmcl.2017.08.068 BindingDB Entry DOI: 10.7270/Q2NG4T34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50261827 (CHEMBL4070441) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
IDD Medicinal Chemistry, Sanofi Genzyme, 153 Second Avenue, Waltham, MA 02451, USA. Electronic address: claude.barberis2@sanofi.com. Curated by ChEMBL | Assay Description Inhibition of PIM1 (unknown origin) assessed as reduction in BAD phosphorylation at Ser112 residues by TR-FRET assay | Bioorg Med Chem Lett 27: 4735-4740 (2017) Article DOI: 10.1016/j.bmcl.2017.08.068 BindingDB Entry DOI: 10.7270/Q2NG4T34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-3 (Homo sapiens (Human)) | BDBM50261822 (CHEMBL4093592) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
IDD Medicinal Chemistry, Sanofi Genzyme, 153 Second Avenue, Waltham, MA 02451, USA. Electronic address: claude.barberis2@sanofi.com. Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis H37RV InhA | Bioorg Med Chem Lett 27: 4735-4740 (2017) Article DOI: 10.1016/j.bmcl.2017.08.068 BindingDB Entry DOI: 10.7270/Q2NG4T34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50261826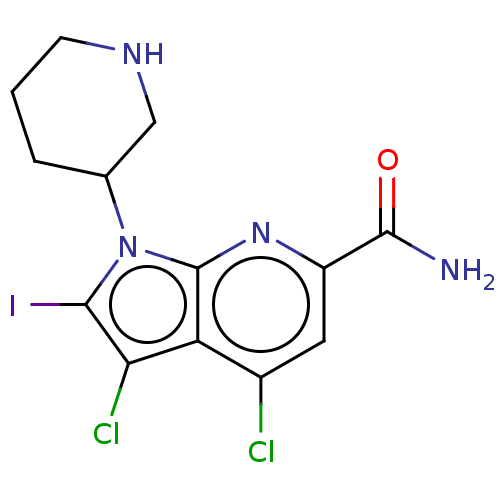 (CHEMBL4084988) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
IDD Medicinal Chemistry, Sanofi Genzyme, 153 Second Avenue, Waltham, MA 02451, USA. Electronic address: claude.barberis2@sanofi.com. Curated by ChEMBL | Assay Description Inhibition of PIM1 (unknown origin) assessed as reduction in BAD phosphorylation at Ser112 residues by TR-FRET assay | Bioorg Med Chem Lett 27: 4735-4740 (2017) Article DOI: 10.1016/j.bmcl.2017.08.068 BindingDB Entry DOI: 10.7270/Q2NG4T34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50261829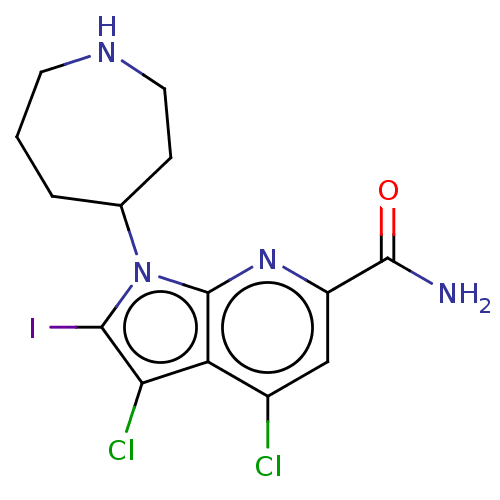 (CHEMBL4067797) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
IDD Medicinal Chemistry, Sanofi Genzyme, 153 Second Avenue, Waltham, MA 02451, USA. Electronic address: claude.barberis2@sanofi.com. Curated by ChEMBL | Assay Description Inhibition of PIM1 (unknown origin) assessed as reduction in BAD phosphorylation at Ser112 residues by TR-FRET assay | Bioorg Med Chem Lett 27: 4735-4740 (2017) Article DOI: 10.1016/j.bmcl.2017.08.068 BindingDB Entry DOI: 10.7270/Q2NG4T34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50261858 (CHEMBL4062258) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
IDD Medicinal Chemistry, Sanofi Genzyme, 153 Second Avenue, Waltham, MA 02451, USA. Electronic address: claude.barberis2@sanofi.com. Curated by ChEMBL | Assay Description Inhibition of PIM1 (unknown origin) assessed as reduction in BAD phosphorylation at Ser112 residues by TR-FRET assay | Bioorg Med Chem Lett 27: 4735-4740 (2017) Article DOI: 10.1016/j.bmcl.2017.08.068 BindingDB Entry DOI: 10.7270/Q2NG4T34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-3 (Homo sapiens (Human)) | BDBM50261826 (CHEMBL4084988) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
IDD Medicinal Chemistry, Sanofi Genzyme, 153 Second Avenue, Waltham, MA 02451, USA. Electronic address: claude.barberis2@sanofi.com. Curated by ChEMBL | Assay Description Inhibition of PIM3 (unknown origin) assessed as reduction in BAD phosphorylation at Ser112 residues by TR-FRET assay | Bioorg Med Chem Lett 27: 4735-4740 (2017) Article DOI: 10.1016/j.bmcl.2017.08.068 BindingDB Entry DOI: 10.7270/Q2NG4T34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50261873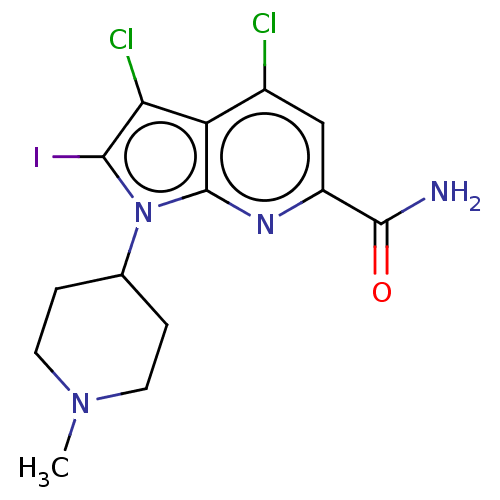 (CHEMBL4070019) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
IDD Medicinal Chemistry, Sanofi Genzyme, 153 Second Avenue, Waltham, MA 02451, USA. Electronic address: claude.barberis2@sanofi.com. Curated by ChEMBL | Assay Description Inhibition of PIM1 (unknown origin) assessed as reduction in BAD phosphorylation at Ser112 residues by TR-FRET assay | Bioorg Med Chem Lett 27: 4735-4740 (2017) Article DOI: 10.1016/j.bmcl.2017.08.068 BindingDB Entry DOI: 10.7270/Q2NG4T34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-3 (Homo sapiens (Human)) | BDBM50261828 (CHEMBL4095220) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
IDD Medicinal Chemistry, Sanofi Genzyme, 153 Second Avenue, Waltham, MA 02451, USA. Electronic address: claude.barberis2@sanofi.com. Curated by ChEMBL | Assay Description Inhibition of PIM3 (unknown origin) assessed as reduction in BAD phosphorylation at Ser112 residues by TR-FRET assay | Bioorg Med Chem Lett 27: 4735-4740 (2017) Article DOI: 10.1016/j.bmcl.2017.08.068 BindingDB Entry DOI: 10.7270/Q2NG4T34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50261825 (CHEMBL4077977) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
IDD Medicinal Chemistry, Sanofi Genzyme, 153 Second Avenue, Waltham, MA 02451, USA. Electronic address: claude.barberis2@sanofi.com. Curated by ChEMBL | Assay Description Inhibition of PIM1 (unknown origin) assessed as reduction in BAD phosphorylation at Ser112 residues by TR-FRET assay | Bioorg Med Chem Lett 27: 4735-4740 (2017) Article DOI: 10.1016/j.bmcl.2017.08.068 BindingDB Entry DOI: 10.7270/Q2NG4T34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50261866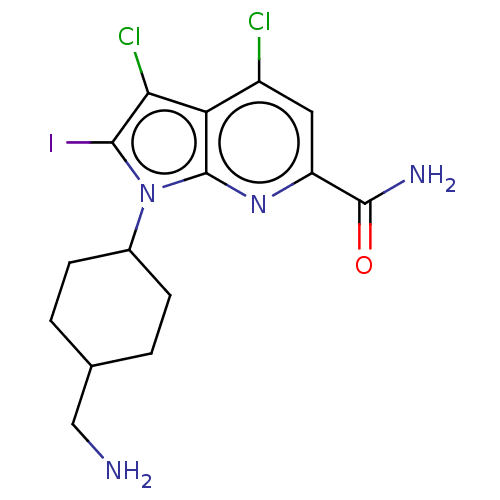 (CHEMBL4087517) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
IDD Medicinal Chemistry, Sanofi Genzyme, 153 Second Avenue, Waltham, MA 02451, USA. Electronic address: claude.barberis2@sanofi.com. Curated by ChEMBL | Assay Description Inhibition of PIM1 (unknown origin) assessed as reduction in BAD phosphorylation at Ser112 residues by TR-FRET assay | Bioorg Med Chem Lett 27: 4735-4740 (2017) Article DOI: 10.1016/j.bmcl.2017.08.068 BindingDB Entry DOI: 10.7270/Q2NG4T34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50261874 (CHEMBL4064302) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
IDD Medicinal Chemistry, Sanofi Genzyme, 153 Second Avenue, Waltham, MA 02451, USA. Electronic address: claude.barberis2@sanofi.com. Curated by ChEMBL | Assay Description Inhibition of PIM1 (unknown origin) assessed as reduction in BAD phosphorylation at Ser112 residues by TR-FRET assay | Bioorg Med Chem Lett 27: 4735-4740 (2017) Article DOI: 10.1016/j.bmcl.2017.08.068 BindingDB Entry DOI: 10.7270/Q2NG4T34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50261854 (CHEMBL4075702) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
IDD Medicinal Chemistry, Sanofi Genzyme, 153 Second Avenue, Waltham, MA 02451, USA. Electronic address: claude.barberis2@sanofi.com. Curated by ChEMBL | Assay Description Inhibition of PIM1 (unknown origin) assessed as reduction in BAD phosphorylation at Ser112 residues by TR-FRET assay | Bioorg Med Chem Lett 27: 4735-4740 (2017) Article DOI: 10.1016/j.bmcl.2017.08.068 BindingDB Entry DOI: 10.7270/Q2NG4T34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-3 (Homo sapiens (Human)) | BDBM50261829 (CHEMBL4067797) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
IDD Medicinal Chemistry, Sanofi Genzyme, 153 Second Avenue, Waltham, MA 02451, USA. Electronic address: claude.barberis2@sanofi.com. Curated by ChEMBL | Assay Description Inhibition of PIM3 (unknown origin) assessed as reduction in BAD phosphorylation at Ser112 residues by TR-FRET assay | Bioorg Med Chem Lett 27: 4735-4740 (2017) Article DOI: 10.1016/j.bmcl.2017.08.068 BindingDB Entry DOI: 10.7270/Q2NG4T34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-3 (Homo sapiens (Human)) | BDBM50261827 (CHEMBL4070441) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
IDD Medicinal Chemistry, Sanofi Genzyme, 153 Second Avenue, Waltham, MA 02451, USA. Electronic address: claude.barberis2@sanofi.com. Curated by ChEMBL | Assay Description Inhibition of PIM3 (unknown origin) assessed as reduction in BAD phosphorylation at Ser112 residues by TR-FRET assay | Bioorg Med Chem Lett 27: 4735-4740 (2017) Article DOI: 10.1016/j.bmcl.2017.08.068 BindingDB Entry DOI: 10.7270/Q2NG4T34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50261856 (CHEMBL4071926) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
IDD Medicinal Chemistry, Sanofi Genzyme, 153 Second Avenue, Waltham, MA 02451, USA. Electronic address: claude.barberis2@sanofi.com. Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis H37RV InhA | Bioorg Med Chem Lett 27: 4735-4740 (2017) Article DOI: 10.1016/j.bmcl.2017.08.068 BindingDB Entry DOI: 10.7270/Q2NG4T34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50261823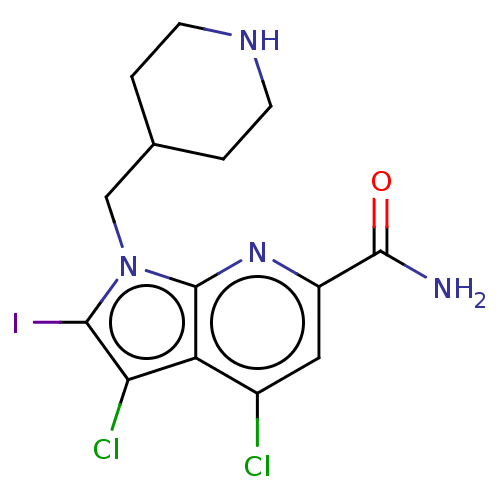 (CHEMBL4072887) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
IDD Medicinal Chemistry, Sanofi Genzyme, 153 Second Avenue, Waltham, MA 02451, USA. Electronic address: claude.barberis2@sanofi.com. Curated by ChEMBL | Assay Description Inhibition of PIM1 (unknown origin) assessed as reduction in BAD phosphorylation at Ser112 residues by TR-FRET assay | Bioorg Med Chem Lett 27: 4735-4740 (2017) Article DOI: 10.1016/j.bmcl.2017.08.068 BindingDB Entry DOI: 10.7270/Q2NG4T34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-3 (Homo sapiens (Human)) | BDBM50261825 (CHEMBL4077977) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
IDD Medicinal Chemistry, Sanofi Genzyme, 153 Second Avenue, Waltham, MA 02451, USA. Electronic address: claude.barberis2@sanofi.com. Curated by ChEMBL | Assay Description Inhibition of PIM3 (unknown origin) assessed as reduction in BAD phosphorylation at Ser112 residues by TR-FRET assay | Bioorg Med Chem Lett 27: 4735-4740 (2017) Article DOI: 10.1016/j.bmcl.2017.08.068 BindingDB Entry DOI: 10.7270/Q2NG4T34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50261807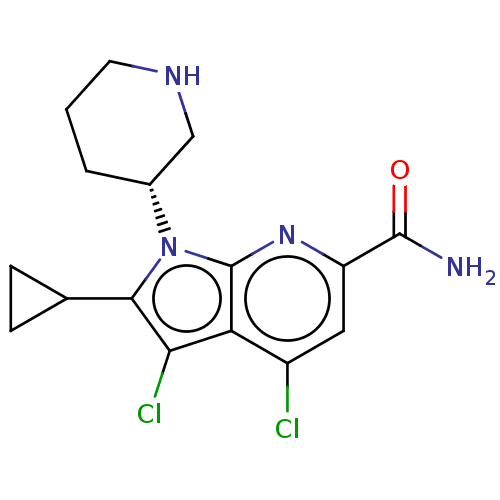 (CHEMBL4086461) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
IDD Medicinal Chemistry, Sanofi Genzyme, 153 Second Avenue, Waltham, MA 02451, USA. Electronic address: claude.barberis2@sanofi.com. Curated by ChEMBL | Assay Description Inhibition of PIM1 (unknown origin) assessed as reduction in BAD phosphorylation at Ser112 residues by TR-FRET assay | Bioorg Med Chem Lett 27: 4735-4740 (2017) Article DOI: 10.1016/j.bmcl.2017.08.068 BindingDB Entry DOI: 10.7270/Q2NG4T34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50429069 (CHEMBL2335736) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US Curated by ChEMBL | Assay Description Antagonist activity at dopamine D2 receptor (unknown origin) transfected in CHO cell membranes assessed as inhibition of forskolin-stimulated cAMP le... | Bioorg Med Chem Lett 23: 1498-501 (2013) Article DOI: 10.1016/j.bmcl.2012.12.046 BindingDB Entry DOI: 10.7270/Q290255G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-2 (Homo sapiens (Human)) | BDBM50261864 (CHEMBL4100435) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
IDD Medicinal Chemistry, Sanofi Genzyme, 153 Second Avenue, Waltham, MA 02451, USA. Electronic address: claude.barberis2@sanofi.com. Curated by ChEMBL | Assay Description Inhibition of PIM2 (unknown origin) assessed as reduction in BAD phosphorylation at Ser112 residues by TR-FRET assay | Bioorg Med Chem Lett 27: 4735-4740 (2017) Article DOI: 10.1016/j.bmcl.2017.08.068 BindingDB Entry DOI: 10.7270/Q2NG4T34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-2 (Homo sapiens (Human)) | BDBM50261808 (CHEMBL4104355) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
IDD Medicinal Chemistry, Sanofi Genzyme, 153 Second Avenue, Waltham, MA 02451, USA. Electronic address: claude.barberis2@sanofi.com. Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis H37RV InhA | Bioorg Med Chem Lett 27: 4735-4740 (2017) Article DOI: 10.1016/j.bmcl.2017.08.068 BindingDB Entry DOI: 10.7270/Q2NG4T34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-3 (Homo sapiens (Human)) | BDBM50261873 (CHEMBL4070019) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
IDD Medicinal Chemistry, Sanofi Genzyme, 153 Second Avenue, Waltham, MA 02451, USA. Electronic address: claude.barberis2@sanofi.com. Curated by ChEMBL | Assay Description Inhibition of PIM3 (unknown origin) assessed as reduction in BAD phosphorylation at Ser112 residues by TR-FRET assay | Bioorg Med Chem Lett 27: 4735-4740 (2017) Article DOI: 10.1016/j.bmcl.2017.08.068 BindingDB Entry DOI: 10.7270/Q2NG4T34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50261830 (CHEMBL4105379) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
IDD Medicinal Chemistry, Sanofi Genzyme, 153 Second Avenue, Waltham, MA 02451, USA. Electronic address: claude.barberis2@sanofi.com. Curated by ChEMBL | Assay Description Inhibition of PIM1 (unknown origin) assessed as reduction in BAD phosphorylation at Ser112 residues by TR-FRET assay | Bioorg Med Chem Lett 27: 4735-4740 (2017) Article DOI: 10.1016/j.bmcl.2017.08.068 BindingDB Entry DOI: 10.7270/Q2NG4T34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-2 (Homo sapiens (Human)) | BDBM50261826 (CHEMBL4084988) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
IDD Medicinal Chemistry, Sanofi Genzyme, 153 Second Avenue, Waltham, MA 02451, USA. Electronic address: claude.barberis2@sanofi.com. Curated by ChEMBL | Assay Description Inhibition of PIM2 (unknown origin) assessed as reduction in BAD phosphorylation at Ser112 residues by TR-FRET assay | Bioorg Med Chem Lett 27: 4735-4740 (2017) Article DOI: 10.1016/j.bmcl.2017.08.068 BindingDB Entry DOI: 10.7270/Q2NG4T34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-2 (Homo sapiens (Human)) | BDBM50261827 (CHEMBL4070441) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
IDD Medicinal Chemistry, Sanofi Genzyme, 153 Second Avenue, Waltham, MA 02451, USA. Electronic address: claude.barberis2@sanofi.com. Curated by ChEMBL | Assay Description Inhibition of PIM2 (unknown origin) assessed as reduction in BAD phosphorylation at Ser112 residues by TR-FRET assay | Bioorg Med Chem Lett 27: 4735-4740 (2017) Article DOI: 10.1016/j.bmcl.2017.08.068 BindingDB Entry DOI: 10.7270/Q2NG4T34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50261831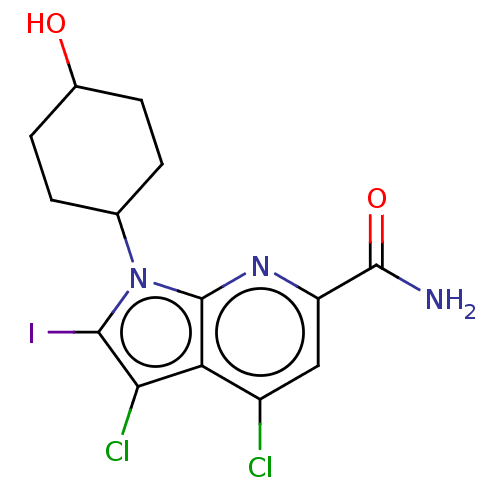 (CHEMBL4065094) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
IDD Medicinal Chemistry, Sanofi Genzyme, 153 Second Avenue, Waltham, MA 02451, USA. Electronic address: claude.barberis2@sanofi.com. Curated by ChEMBL | Assay Description Inhibition of PIM1 (unknown origin) assessed as reduction in BAD phosphorylation at Ser112 residues by TR-FRET assay | Bioorg Med Chem Lett 27: 4735-4740 (2017) Article DOI: 10.1016/j.bmcl.2017.08.068 BindingDB Entry DOI: 10.7270/Q2NG4T34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50261805 (CHEMBL4096630) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
IDD Medicinal Chemistry, Sanofi Genzyme, 153 Second Avenue, Waltham, MA 02451, USA. Electronic address: claude.barberis2@sanofi.com. Curated by ChEMBL | Assay Description Inhibition of PIM1 (unknown origin) assessed as reduction in BAD phosphorylation at Ser112 residues by TR-FRET assay | Bioorg Med Chem Lett 27: 4735-4740 (2017) Article DOI: 10.1016/j.bmcl.2017.08.068 BindingDB Entry DOI: 10.7270/Q2NG4T34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50261832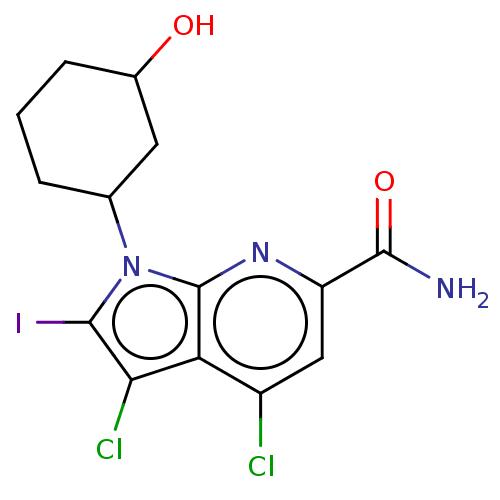 (CHEMBL4086532) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
IDD Medicinal Chemistry, Sanofi Genzyme, 153 Second Avenue, Waltham, MA 02451, USA. Electronic address: claude.barberis2@sanofi.com. Curated by ChEMBL | Assay Description Inhibition of PIM1 (unknown origin) assessed as reduction in BAD phosphorylation at Ser112 residues by TR-FRET assay | Bioorg Med Chem Lett 27: 4735-4740 (2017) Article DOI: 10.1016/j.bmcl.2017.08.068 BindingDB Entry DOI: 10.7270/Q2NG4T34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-2 (Homo sapiens (Human)) | BDBM50261866 (CHEMBL4087517) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
IDD Medicinal Chemistry, Sanofi Genzyme, 153 Second Avenue, Waltham, MA 02451, USA. Electronic address: claude.barberis2@sanofi.com. Curated by ChEMBL | Assay Description Inhibition of PIM2 (unknown origin) assessed as reduction in BAD phosphorylation at Ser112 residues by TR-FRET assay | Bioorg Med Chem Lett 27: 4735-4740 (2017) Article DOI: 10.1016/j.bmcl.2017.08.068 BindingDB Entry DOI: 10.7270/Q2NG4T34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-2 (Homo sapiens (Human)) | BDBM50261828 (CHEMBL4095220) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
IDD Medicinal Chemistry, Sanofi Genzyme, 153 Second Avenue, Waltham, MA 02451, USA. Electronic address: claude.barberis2@sanofi.com. Curated by ChEMBL | Assay Description Inhibition of PIM2 (unknown origin) assessed as reduction in BAD phosphorylation at Ser112 residues by TR-FRET assay | Bioorg Med Chem Lett 27: 4735-4740 (2017) Article DOI: 10.1016/j.bmcl.2017.08.068 BindingDB Entry DOI: 10.7270/Q2NG4T34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-3 (Homo sapiens (Human)) | BDBM50261823 (CHEMBL4072887) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
IDD Medicinal Chemistry, Sanofi Genzyme, 153 Second Avenue, Waltham, MA 02451, USA. Electronic address: claude.barberis2@sanofi.com. Curated by ChEMBL | Assay Description Inhibition of PIM3 (unknown origin) assessed as reduction in BAD phosphorylation at Ser112 residues by TR-FRET assay | Bioorg Med Chem Lett 27: 4735-4740 (2017) Article DOI: 10.1016/j.bmcl.2017.08.068 BindingDB Entry DOI: 10.7270/Q2NG4T34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 173 total ) | Next | Last >> |