

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 1 (Homo sapiens (Human)) | BDBM50019296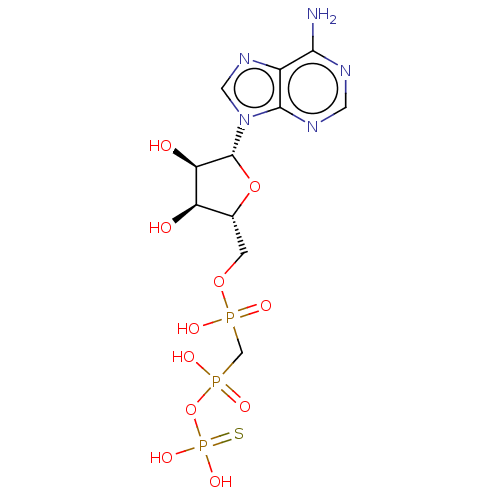 (CHEMBL3289396) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Mixed type inhibition of human NPP1 using pNP-TMP as substrate after 20 mins by Dixon and Cornish-Bowden method | J Med Chem 57: 4677-91 (2014) Article DOI: 10.1021/jm500196c BindingDB Entry DOI: 10.7270/Q2QN68C6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 1 (Homo sapiens (Human)) | BDBM50019294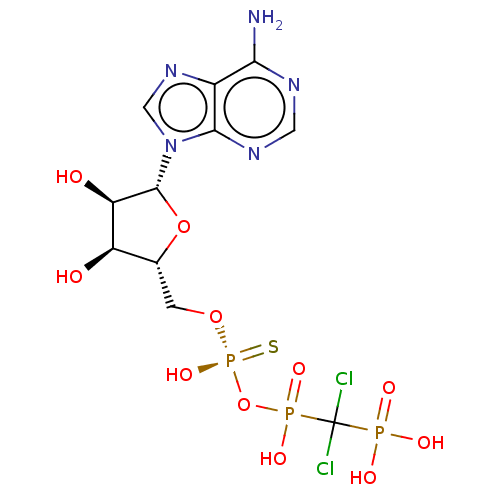 (CHEMBL3289394) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 685 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Mixed type inhibition of human NPP1 using pNP-TMP as substrate after 20 mins by Dixon and Cornish-Bowden method | J Med Chem 57: 4677-91 (2014) Article DOI: 10.1021/jm500196c BindingDB Entry DOI: 10.7270/Q2QN68C6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 1 (Homo sapiens (Human)) | BDBM50019292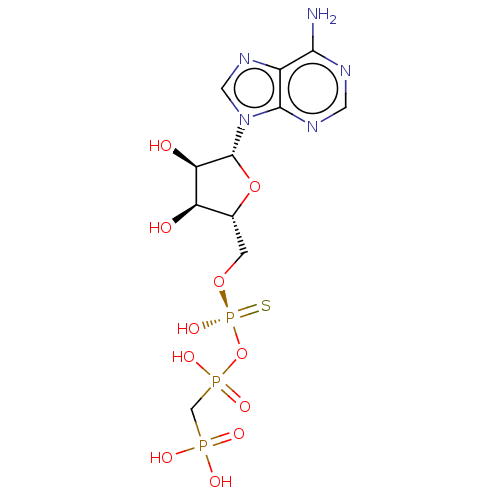 (CHEMBL3289393) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Mixed type inhibition of human NPP1 using pNP-TMP as substrate after 20 mins by Dixon and Cornish-Bowden method | J Med Chem 57: 4677-91 (2014) Article DOI: 10.1021/jm500196c BindingDB Entry DOI: 10.7270/Q2QN68C6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 1 (Homo sapiens (Human)) | BDBM50019291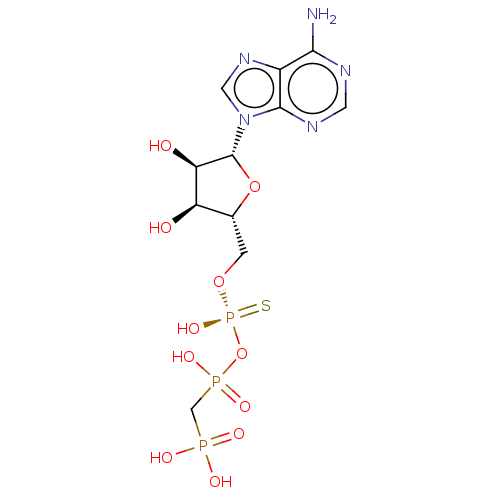 (CHEMBL3289392) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Mixed type inhibition of human NPP1 using pNP-TMP as substrate assessed as dissociation constant for enzyme-inhibitor-substrate complex after 20 mins... | J Med Chem 57: 4677-91 (2014) Article DOI: 10.1021/jm500196c BindingDB Entry DOI: 10.7270/Q2QN68C6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 1 (Homo sapiens (Human)) | BDBM50019291 (CHEMBL3289392) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Mixed type inhibition of human NPP1 using pNP-TMP as substrate after 20 mins by Dixon and Cornish-Bowden method | J Med Chem 57: 4677-91 (2014) Article DOI: 10.1021/jm500196c BindingDB Entry DOI: 10.7270/Q2QN68C6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 1 (Homo sapiens (Human)) | BDBM50019295 (CHEMBL3289395) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.52E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Mixed type inhibition of human NPP1 using pNP-TMP as substrate after 20 mins by Dixon and Cornish-Bowden method | J Med Chem 57: 4677-91 (2014) Article DOI: 10.1021/jm500196c BindingDB Entry DOI: 10.7270/Q2QN68C6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 1 (Homo sapiens (Human)) | BDBM50019295 (CHEMBL3289395) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.92E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Mixed type inhibition of human NPP1 using pNP-TMP as substrate assessed as dissociation constant for enzyme-inhibitor-substrate complex after 20 mins... | J Med Chem 57: 4677-91 (2014) Article DOI: 10.1021/jm500196c BindingDB Entry DOI: 10.7270/Q2QN68C6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 1 (Homo sapiens (Human)) | BDBM50019296 (CHEMBL3289396) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Mixed type inhibition of human NPP1 using pNP-TMP as substrate after 20 mins by Dixon and Cornish-Bowden method | J Med Chem 57: 4677-91 (2014) Article DOI: 10.1021/jm500196c BindingDB Entry DOI: 10.7270/Q2QN68C6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 1 (Homo sapiens (Human)) | BDBM50019294 (CHEMBL3289394) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Mixed type inhibition of human NPP1 using pNP-TMP as substrate after 20 mins by Dixon and Cornish-Bowden method | J Med Chem 57: 4677-91 (2014) Article DOI: 10.1021/jm500196c BindingDB Entry DOI: 10.7270/Q2QN68C6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 1 (Homo sapiens (Human)) | BDBM50019291 (CHEMBL3289392) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.63E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Mixed type inhibition of human NPP1 using pNP-TMP as substrate after 20 mins by Dixon and Cornish-Bowden method | J Med Chem 57: 4677-91 (2014) Article DOI: 10.1021/jm500196c BindingDB Entry DOI: 10.7270/Q2QN68C6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 1 (Homo sapiens (Human)) | BDBM50019292 (CHEMBL3289393) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.87E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Mixed type inhibition of human NPP1 using pNP-TMP as substrate after 20 mins by Dixon and Cornish-Bowden method | J Med Chem 57: 4677-91 (2014) Article DOI: 10.1021/jm500196c BindingDB Entry DOI: 10.7270/Q2QN68C6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 1 (Homo sapiens (Human)) | BDBM50019295 (CHEMBL3289395) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.12E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Mixed type inhibition of human NPP1 using pNP-TMP as substrate after 20 mins by Dixon and Cornish-Bowden method | J Med Chem 57: 4677-91 (2014) Article DOI: 10.1021/jm500196c BindingDB Entry DOI: 10.7270/Q2QN68C6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 6 (Homo sapiens (Human)) | BDBM50114076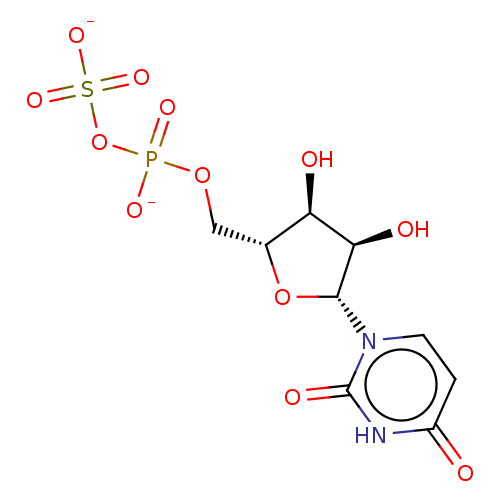 (CHEMBL3604024) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.12E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bar Ilan University Curated by ChEMBL | Assay Description Antagonist activity at human recombinant P2Y6 receptor expressed in human 1321N1 cells assessed as inhibition of UDP-induced increase in intracellula... | Bioorg Med Chem 23: 5764-73 (2015) Article DOI: 10.1016/j.bmc.2015.07.004 BindingDB Entry DOI: 10.7270/Q2PR7XR3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50368125 (ADENOSINE DIPHOSPHATE | ADP) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 24 | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Agonist activity at human GFP-tagged P2Y1R transfected in human 1321N1 cells assessed as induction of intracellular calcium mobilization by fluoresce... | J Med Chem 56: 4938-52 (2013) Article DOI: 10.1021/jm400197m BindingDB Entry DOI: 10.7270/Q2Q52R0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 6 (Homo sapiens (Human)) | BDBM50421169 (CHEMBL2086770) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 28 | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Agonist activity at human P2Y6 receptor | Bioorg Med Chem 20: 5483-95 (2012) Article DOI: 10.1016/j.bmc.2012.07.042 BindingDB Entry DOI: 10.7270/Q21837S1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 6 (Homo sapiens (Human)) | BDBM50403871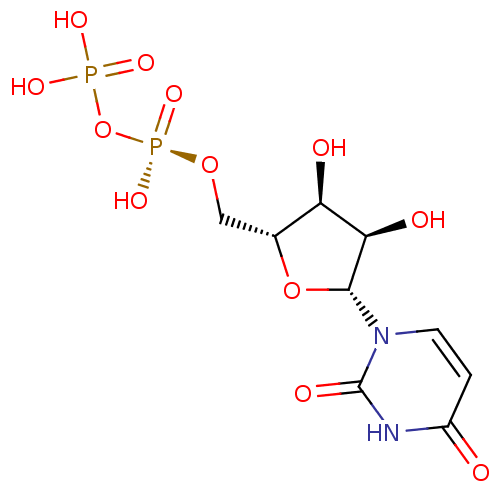 (URIDINE_DIPHOSPHATE) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 15 | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Agonist activity at P2Y6 receptor expressed in human 1321N1 cells assessed as inositol phosphate accumulation | Bioorg Med Chem 20: 5483-95 (2012) Article DOI: 10.1016/j.bmc.2012.07.042 BindingDB Entry DOI: 10.7270/Q21837S1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 6 (Homo sapiens (Human)) | BDBM50306710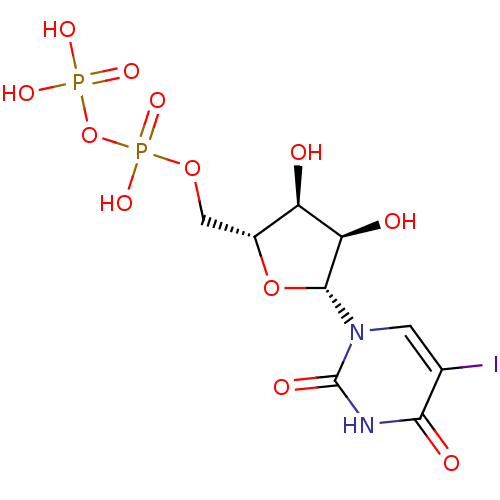 (((2R,3S,4R,5R)-3,4-dihydroxy-5-(5-iodo-2,4-dioxo-3...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 13 | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Agonist activity at P2Y6 receptor expressed in human 1321N1 cells assessed as inositol phosphate accumulation | Bioorg Med Chem 20: 5483-95 (2012) Article DOI: 10.1016/j.bmc.2012.07.042 BindingDB Entry DOI: 10.7270/Q21837S1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Rattus norvegicus) | BDBM50435007 (CHEMBL2386493) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Agonist activity at rat brain P2Y1R transfected in HEK293 cells assessed as induction of intracellular calcium mobilization by fluorescence assay | J Med Chem 56: 4938-52 (2013) Article DOI: 10.1021/jm400197m BindingDB Entry DOI: 10.7270/Q2Q52R0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 11 (Homo sapiens (Human)) | BDBM50435008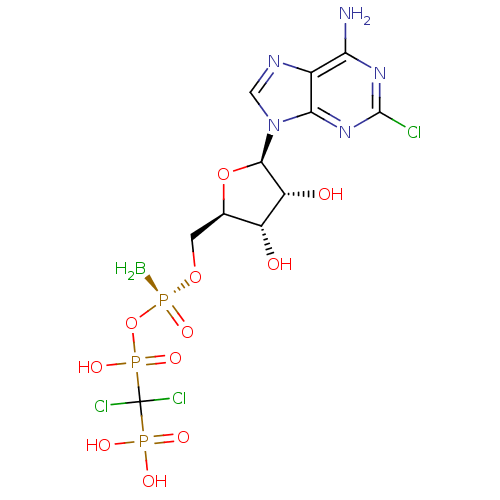 (CHEMBL2386496) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 130 | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Agonist activity at human GFP-tagged P2Y11R transfected in human 1321N1 cells assessed as induction of intracellular calcium mobilization by fluoresc... | J Med Chem 56: 4938-52 (2013) Article DOI: 10.1021/jm400197m BindingDB Entry DOI: 10.7270/Q2Q52R0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 11 (Homo sapiens (Human)) | BDBM50435009 (CHEMBL2386495) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Agonist activity at human GFP-tagged P2Y11R transfected in human 1321N1 cells assessed as induction of intracellular calcium mobilization by fluoresc... | J Med Chem 56: 4938-52 (2013) Article DOI: 10.1021/jm400197m BindingDB Entry DOI: 10.7270/Q2Q52R0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 11 (Homo sapiens (Human)) | BDBM50435010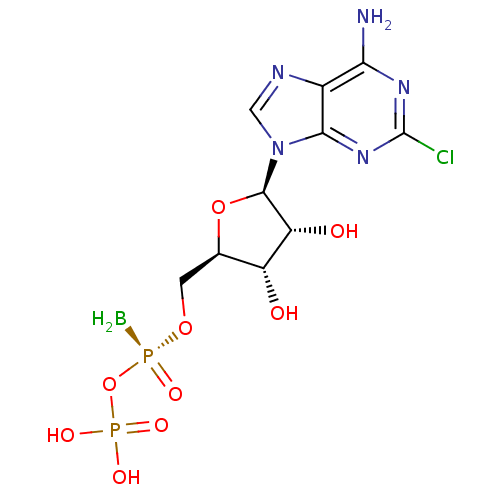 (CHEMBL2386492) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Agonist activity at human GFP-tagged P2Y11R transfected in human 1321N1 cells assessed as induction of intracellular calcium mobilization by fluoresc... | J Med Chem 56: 4938-52 (2013) Article DOI: 10.1021/jm400197m BindingDB Entry DOI: 10.7270/Q2Q52R0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 11 (Homo sapiens (Human)) | BDBM50435011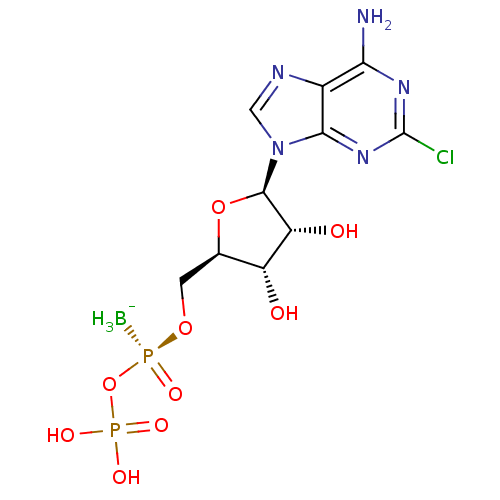 (CHEMBL2386491) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.68E+3 | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Agonist activity at human GFP-tagged P2Y11R transfected in human 1321N1 cells assessed as induction of intracellular calcium mobilization by fluoresc... | J Med Chem 56: 4938-52 (2013) Article DOI: 10.1021/jm400197m BindingDB Entry DOI: 10.7270/Q2Q52R0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 11 (Homo sapiens (Human)) | BDBM50398070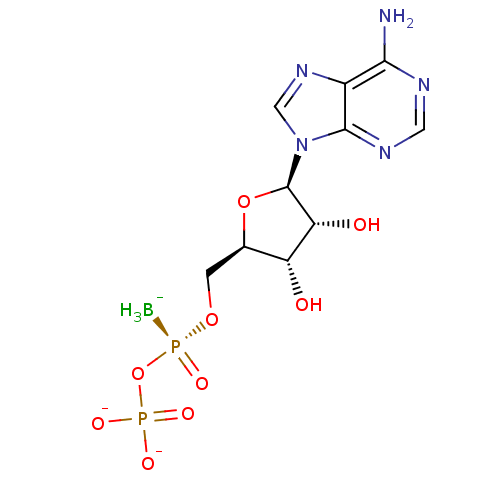 (CHEMBL2181938) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Agonist activity at human GFP-tagged P2Y11R transfected in human 1321N1 cells assessed as induction of intracellular calcium mobilization by fluoresc... | J Med Chem 56: 4938-52 (2013) Article DOI: 10.1021/jm400197m BindingDB Entry DOI: 10.7270/Q2Q52R0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 11 (Homo sapiens (Human)) | BDBM50435012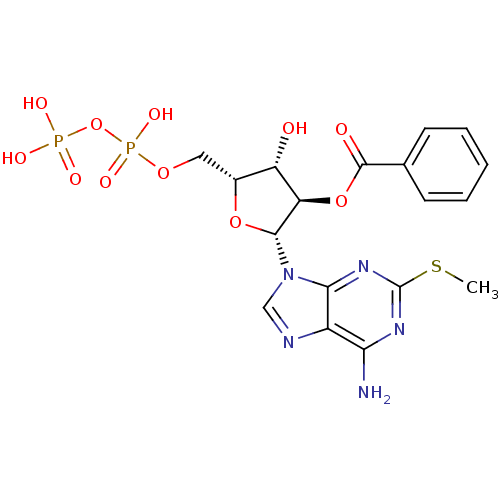 (CHEMBL2386490) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Agonist activity at human GFP-tagged P2Y11R transfected in human 1321N1 cells assessed as induction of intracellular calcium mobilization by fluoresc... | J Med Chem 56: 4938-52 (2013) Article DOI: 10.1021/jm400197m BindingDB Entry DOI: 10.7270/Q2Q52R0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 11 (Homo sapiens (Human)) | BDBM50368125 (ADENOSINE DIPHOSPHATE | ADP) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Agonist activity at human GFP-tagged P2Y11R transfected in human 1321N1 cells assessed as induction of intracellular calcium mobilization by fluoresc... | J Med Chem 56: 4938-52 (2013) Article DOI: 10.1021/jm400197m BindingDB Entry DOI: 10.7270/Q2Q52R0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 11 (Homo sapiens (Human)) | BDBM50366480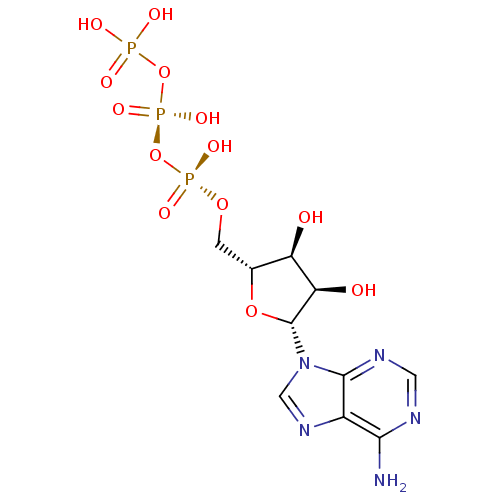 (ADENOSINE TRIPHOSPHATE | ATP) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 6.70E+3 | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Agonist activity at human GFP-tagged P2Y11R transfected in human 1321N1 cells assessed as induction of intracellular calcium mobilization by fluoresc... | J Med Chem 56: 4938-52 (2013) Article DOI: 10.1021/jm400197m BindingDB Entry DOI: 10.7270/Q2Q52R0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50435013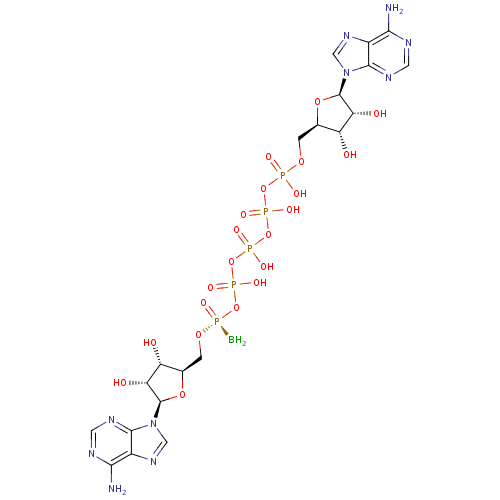 (CHEMBL2386499) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Agonist activity at human GFP-tagged P2Y1R transfected in human 1321N1 cells assessed as induction of intracellular calcium mobilization by fluoresce... | J Med Chem 56: 4938-52 (2013) Article DOI: 10.1021/jm400197m BindingDB Entry DOI: 10.7270/Q2Q52R0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50435014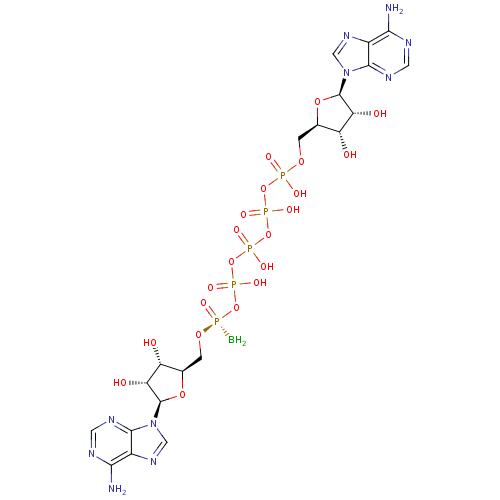 (CHEMBL2386498) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 200 | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Agonist activity at human GFP-tagged P2Y1R transfected in human 1321N1 cells assessed as induction of intracellular calcium mobilization by fluoresce... | J Med Chem 56: 4938-52 (2013) Article DOI: 10.1021/jm400197m BindingDB Entry DOI: 10.7270/Q2Q52R0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50435015 (CHEMBL2386497) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Agonist activity at human GFP-tagged P2Y1R transfected in human 1321N1 cells assessed as induction of intracellular calcium mobilization by fluoresce... | J Med Chem 56: 4938-52 (2013) Article DOI: 10.1021/jm400197m BindingDB Entry DOI: 10.7270/Q2Q52R0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50435008 (CHEMBL2386496) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 950 | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Agonist activity at human GFP-tagged P2Y1R transfected in human 1321N1 cells assessed as induction of intracellular calcium mobilization by fluoresce... | J Med Chem 56: 4938-52 (2013) Article DOI: 10.1021/jm400197m BindingDB Entry DOI: 10.7270/Q2Q52R0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50435009 (CHEMBL2386495) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 150 | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Agonist activity at human GFP-tagged P2Y1R transfected in human 1321N1 cells assessed as induction of intracellular calcium mobilization by fluoresce... | J Med Chem 56: 4938-52 (2013) Article DOI: 10.1021/jm400197m BindingDB Entry DOI: 10.7270/Q2Q52R0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50435016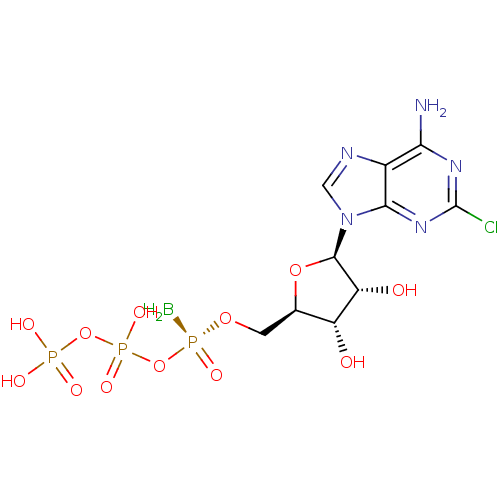 (CHEMBL2386494) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Agonist activity at human GFP-tagged P2Y1R transfected in human 1321N1 cells assessed as induction of intracellular calcium mobilization by fluoresce... | J Med Chem 56: 4938-52 (2013) Article DOI: 10.1021/jm400197m BindingDB Entry DOI: 10.7270/Q2Q52R0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50435007 (CHEMBL2386493) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 74 | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Agonist activity at human GFP-tagged P2Y1R transfected in human 1321N1 cells assessed as induction of intracellular calcium mobilization by fluoresce... | J Med Chem 56: 4938-52 (2013) Article DOI: 10.1021/jm400197m BindingDB Entry DOI: 10.7270/Q2Q52R0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50435010 (CHEMBL2386492) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 600 | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Agonist activity at human GFP-tagged P2Y1R transfected in human 1321N1 cells assessed as induction of intracellular calcium mobilization by fluoresce... | J Med Chem 56: 4938-52 (2013) Article DOI: 10.1021/jm400197m BindingDB Entry DOI: 10.7270/Q2Q52R0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50435011 (CHEMBL2386491) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 7 | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Agonist activity at human GFP-tagged P2Y1R transfected in human 1321N1 cells assessed as induction of intracellular calcium mobilization by fluoresce... | J Med Chem 56: 4938-52 (2013) Article DOI: 10.1021/jm400197m BindingDB Entry DOI: 10.7270/Q2Q52R0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50398070 (CHEMBL2181938) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Agonist activity at human GFP-tagged P2Y1R transfected in human 1321N1 cells assessed as induction of intracellular calcium mobilization by fluoresce... | J Med Chem 56: 4938-52 (2013) Article DOI: 10.1021/jm400197m BindingDB Entry DOI: 10.7270/Q2Q52R0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50398071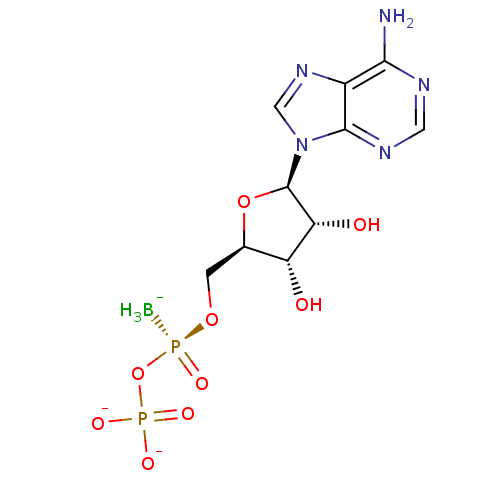 (CHEMBL2181939) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 300 | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Agonist activity at human GFP-tagged P2Y1R transfected in human 1321N1 cells assessed as induction of intracellular calcium mobilization by fluoresce... | J Med Chem 56: 4938-52 (2013) Article DOI: 10.1021/jm400197m BindingDB Entry DOI: 10.7270/Q2Q52R0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50435017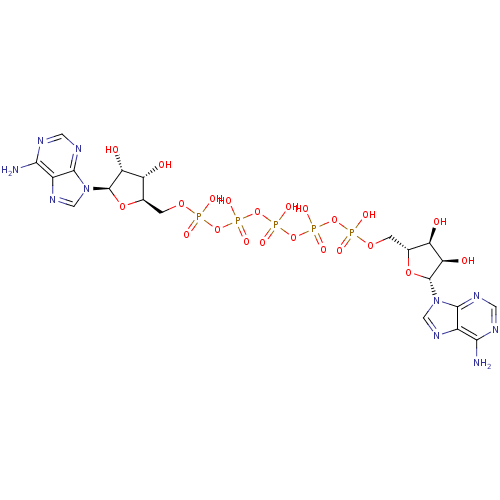 (CHEMBL437508) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 760 | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Agonist activity at human GFP-tagged P2Y1R transfected in human 1321N1 cells assessed as induction of intracellular calcium mobilization by fluoresce... | J Med Chem 56: 4938-52 (2013) Article DOI: 10.1021/jm400197m BindingDB Entry DOI: 10.7270/Q2Q52R0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50118242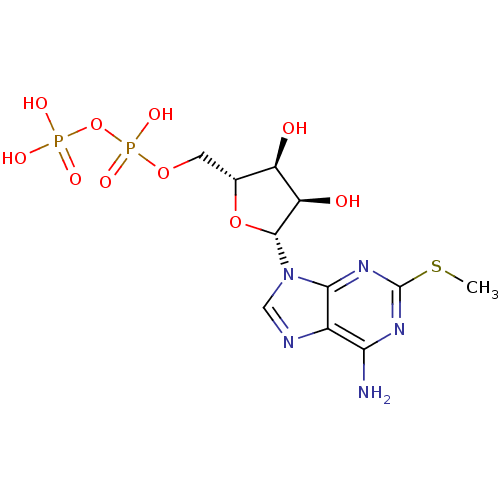 (((2R,3S,4R,5R)-5-(6-amino-2-(methylthio)-9H-purin-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 13 | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Agonist activity at human GFP-tagged P2Y1R transfected in human 1321N1 cells assessed as induction of intracellular calcium mobilization by fluoresce... | J Med Chem 56: 4938-52 (2013) Article DOI: 10.1021/jm400197m BindingDB Entry DOI: 10.7270/Q2Q52R0Q | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50366480 (ADENOSINE TRIPHOSPHATE | ATP) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 850 | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Agonist activity at human GFP-tagged P2Y1R transfected in human 1321N1 cells assessed as induction of intracellular calcium mobilization by fluoresce... | J Med Chem 56: 4938-52 (2013) Article DOI: 10.1021/jm400197m BindingDB Entry DOI: 10.7270/Q2Q52R0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 6 (Homo sapiens (Human)) | BDBM50421168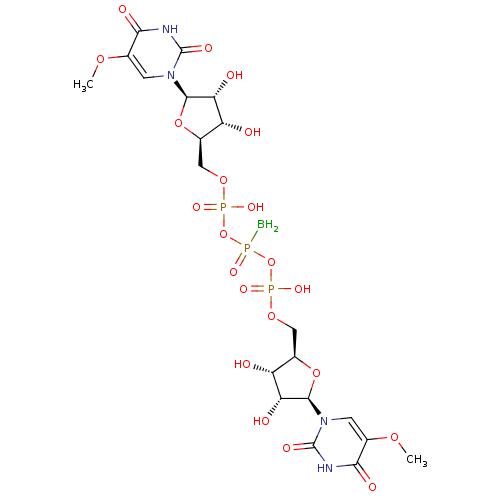 (CHEMBL2086768) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 200 | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Agonist activity at human GFP-tagged P2Y6 receptor expressed in human 1321N1 cells assessed as intracellular calcium mobilization by fura2/AM-based f... | Bioorg Med Chem 20: 5483-95 (2012) Article DOI: 10.1016/j.bmc.2012.07.042 BindingDB Entry DOI: 10.7270/Q21837S1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 6 (Homo sapiens (Human)) | BDBM50421167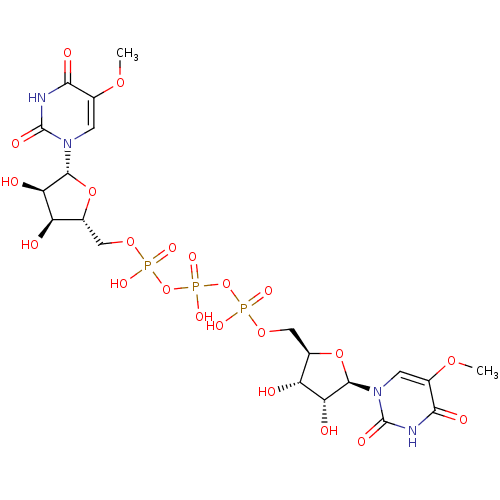 (CHEMBL2086767) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Agonist activity at human GFP-tagged P2Y6 receptor expressed in human 1321N1 cells assessed as intracellular calcium mobilization by fura2/AM-based f... | Bioorg Med Chem 20: 5483-95 (2012) Article DOI: 10.1016/j.bmc.2012.07.042 BindingDB Entry DOI: 10.7270/Q21837S1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 6 (Homo sapiens (Human)) | BDBM50421166 (CHEMBL2086766) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Agonist activity at human GFP-tagged P2Y6 receptor expressed in human 1321N1 cells assessed as intracellular calcium mobilization by fura2/AM-based f... | Bioorg Med Chem 20: 5483-95 (2012) Article DOI: 10.1016/j.bmc.2012.07.042 BindingDB Entry DOI: 10.7270/Q21837S1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 6 (Homo sapiens (Human)) | BDBM50421165 (CHEMBL2086765) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 60 | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Agonist activity at human GFP-tagged P2Y6 receptor expressed in human 1321N1 cells assessed as intracellular calcium mobilization by fura2/AM-based f... | Bioorg Med Chem 20: 5483-95 (2012) Article DOI: 10.1016/j.bmc.2012.07.042 BindingDB Entry DOI: 10.7270/Q21837S1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 6 (Homo sapiens (Human)) | BDBM50421164 (CHEMBL2086764) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.01E+4 | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Agonist activity at human GFP-tagged P2Y6 receptor expressed in human 1321N1 cells assessed as intracellular calcium mobilization by fura2/AM-based f... | Bioorg Med Chem 20: 5483-95 (2012) Article DOI: 10.1016/j.bmc.2012.07.042 BindingDB Entry DOI: 10.7270/Q21837S1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 6 (Homo sapiens (Human)) | BDBM50421163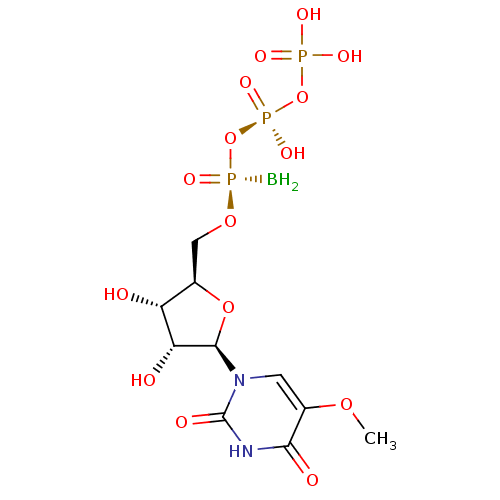 (CHEMBL2086763) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.12E+4 | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Agonist activity at human GFP-tagged P2Y6 receptor expressed in human 1321N1 cells assessed as intracellular calcium mobilization by fura2/AM-based f... | Bioorg Med Chem 20: 5483-95 (2012) Article DOI: 10.1016/j.bmc.2012.07.042 BindingDB Entry DOI: 10.7270/Q21837S1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 6 (Homo sapiens (Human)) | BDBM50421162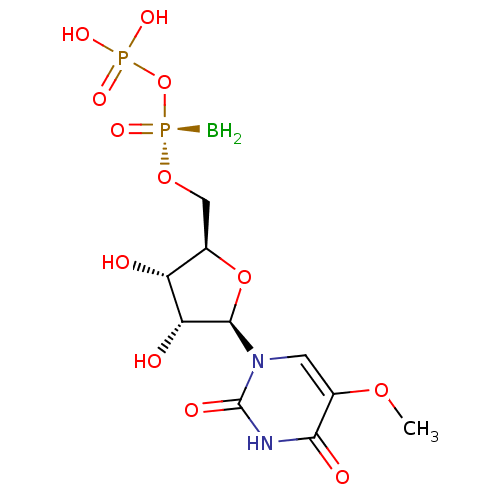 (CHEMBL2086762) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 4.30E+3 | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Agonist activity at human GFP-tagged P2Y6 receptor expressed in human 1321N1 cells assessed as intracellular calcium mobilization by fura2/AM-based f... | Bioorg Med Chem 20: 5483-95 (2012) Article DOI: 10.1016/j.bmc.2012.07.042 BindingDB Entry DOI: 10.7270/Q21837S1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 6 (Homo sapiens (Human)) | BDBM50421161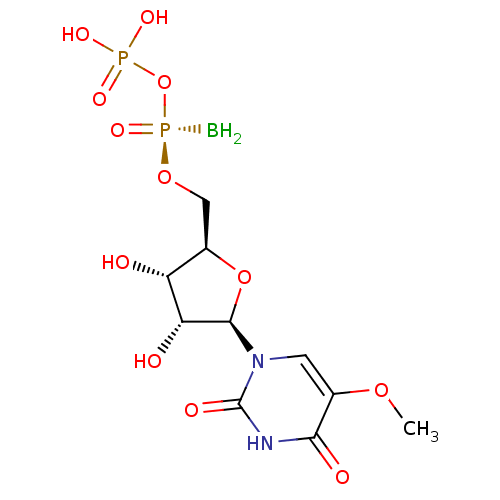 (CHEMBL2086761) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 8 | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Agonist activity at human GFP-tagged P2Y6 receptor expressed in human 1321N1 cells assessed as intracellular calcium mobilization by fura2/AM-based f... | Bioorg Med Chem 20: 5483-95 (2012) Article DOI: 10.1016/j.bmc.2012.07.042 BindingDB Entry DOI: 10.7270/Q21837S1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 6 (Homo sapiens (Human)) | BDBM50403871 (URIDINE_DIPHOSPHATE) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 150 | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Agonist activity at human GFP-tagged P2Y6 receptor expressed in human 1321N1 cells assessed as intracellular calcium mobilization by fura2/AM-based f... | Bioorg Med Chem 20: 5483-95 (2012) Article DOI: 10.1016/j.bmc.2012.07.042 BindingDB Entry DOI: 10.7270/Q21837S1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 4 (Homo sapiens (Human)) | BDBM50118213 (5'-UTP | CHEMBL336296 | H4utp | UTP | uridine 5'-(...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 900 | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Agonist activity at human GFP-tagged P2Y4 receptor expressed in human 1321N1 cells assessed as intracellular calcium mobilization by fura2/AM-based f... | Bioorg Med Chem 20: 5483-95 (2012) Article DOI: 10.1016/j.bmc.2012.07.042 BindingDB Entry DOI: 10.7270/Q21837S1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 54 total ) | Next | Last >> |