

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Wee1-like protein kinase (Homo sapiens (Human)) | BDBM50522503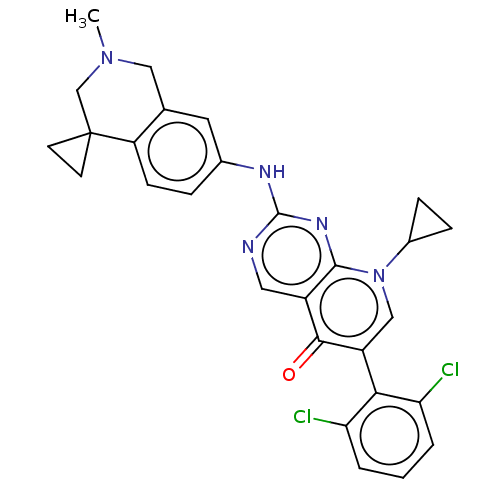 (CHEMBL4454675) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Global Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity to wild type N-terminal GST-tagged human WEE1 catalytic domain (215 to 646 residues) expressed in baculovirus expression system meas... | Bioorg Med Chem Lett 29: 1481-1486 (2019) Article DOI: 10.1016/j.bmcl.2019.04.017 BindingDB Entry DOI: 10.7270/Q2VX0KXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Wee1-like protein kinase (Homo sapiens (Human)) | BDBM50522506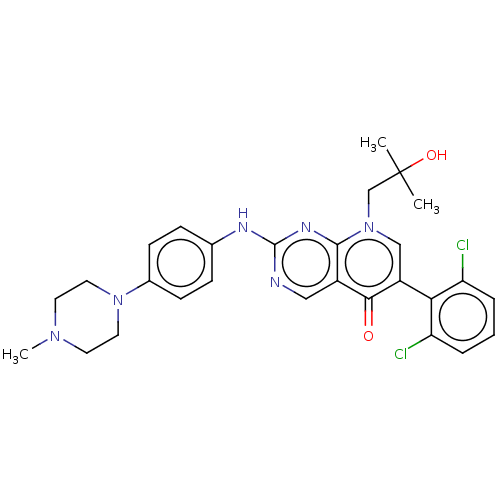 (CHEMBL4562919) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Global Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity to wild type N-terminal GST-tagged human WEE1 catalytic domain (215 to 646 residues) expressed in baculovirus expression system meas... | Bioorg Med Chem Lett 29: 1481-1486 (2019) Article DOI: 10.1016/j.bmcl.2019.04.017 BindingDB Entry DOI: 10.7270/Q2VX0KXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Wee1-like protein kinase (Homo sapiens (Human)) | BDBM50522512 (CHEMBL4544916) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Global Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity to wild type N-terminal GST-tagged human WEE1 catalytic domain (215 to 646 residues) expressed in baculovirus expression system meas... | Bioorg Med Chem Lett 29: 1481-1486 (2019) Article DOI: 10.1016/j.bmcl.2019.04.017 BindingDB Entry DOI: 10.7270/Q2VX0KXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Wee1-like protein kinase (Homo sapiens (Human)) | BDBM50522508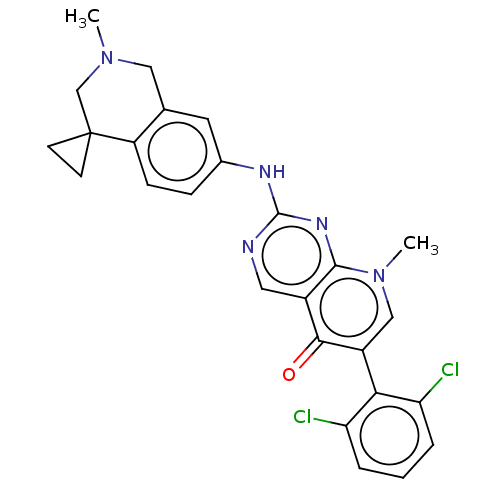 (CHEMBL4441166) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Global Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity to wild type N-terminal GST-tagged human WEE1 catalytic domain (215 to 646 residues) expressed in baculovirus expression system meas... | Bioorg Med Chem Lett 29: 1481-1486 (2019) Article DOI: 10.1016/j.bmcl.2019.04.017 BindingDB Entry DOI: 10.7270/Q2VX0KXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Wee1-like protein kinase (Homo sapiens (Human)) | BDBM50522511 (CHEMBL4443172) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Global Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity to wild type N-terminal GST-tagged human WEE1 catalytic domain (215 to 646 residues) expressed in baculovirus expression system meas... | Bioorg Med Chem Lett 29: 1481-1486 (2019) Article DOI: 10.1016/j.bmcl.2019.04.017 BindingDB Entry DOI: 10.7270/Q2VX0KXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Wee1-like protein kinase (Homo sapiens (Human)) | BDBM50522514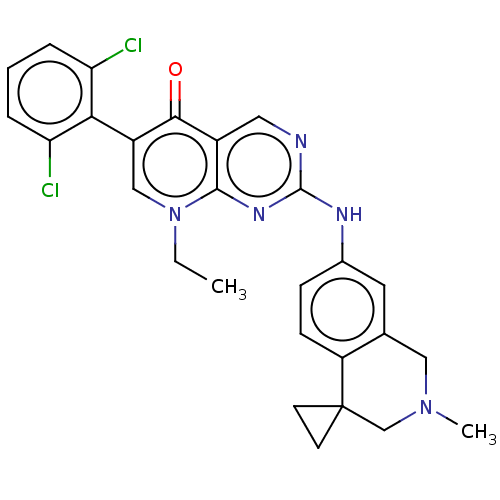 (CHEMBL4554796) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Global Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity to wild type N-terminal GST-tagged human WEE1 catalytic domain (215 to 646 residues) expressed in baculovirus expression system meas... | Bioorg Med Chem Lett 29: 1481-1486 (2019) Article DOI: 10.1016/j.bmcl.2019.04.017 BindingDB Entry DOI: 10.7270/Q2VX0KXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Wee1-like protein kinase (Homo sapiens (Human)) | BDBM50522509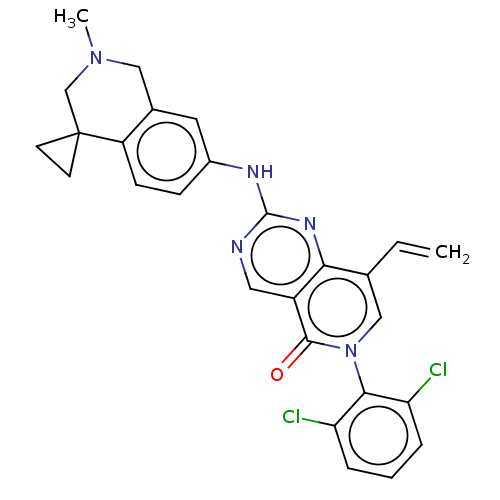 (CHEMBL4444364) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Global Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity to wild type N-terminal GST-tagged human WEE1 catalytic domain (215 to 646 residues) expressed in baculovirus expression system meas... | Bioorg Med Chem Lett 29: 1481-1486 (2019) Article DOI: 10.1016/j.bmcl.2019.04.017 BindingDB Entry DOI: 10.7270/Q2VX0KXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Wee1-like protein kinase (Homo sapiens (Human)) | BDBM50522502 (CHEMBL4529353) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Global Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity to wild type N-terminal GST-tagged human WEE1 catalytic domain (215 to 646 residues) expressed in baculovirus expression system meas... | Bioorg Med Chem Lett 29: 1481-1486 (2019) Article DOI: 10.1016/j.bmcl.2019.04.017 BindingDB Entry DOI: 10.7270/Q2VX0KXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polycomb protein EED (Homo sapiens (Human)) | BDBM223987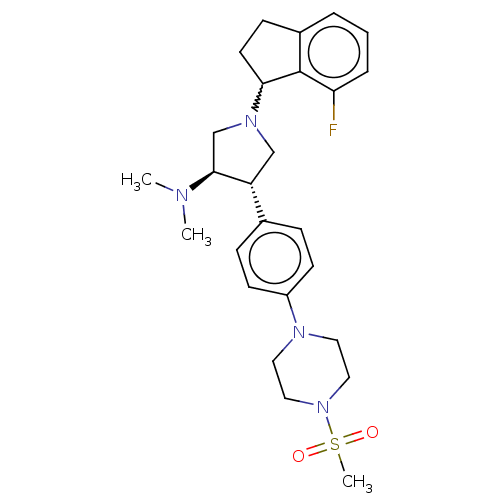 (A-395 (5) | rac-(3R,4S)-1-(7-fluoro-2,3-dihydro-1H...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 0.400 | -53.6 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
AbbVie Inc. | Assay Description For the assay, compounds were dispensed in assay-ready plates using a three-fold serial dilution from 50 μM to ~850 pM using an Echo 550 Acousti... | Nat Chem Biol 13: 389-395 (2017) Article DOI: 10.1038/nchembio.2306 BindingDB Entry DOI: 10.7270/Q2NG4PGD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50229783 (6-[(2-(4-(2,3-dichlorophenyl)piperazin-1-yl)ethyl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from human cloned dopamine D3 receptor expressed in HEK293 cells | J Med Chem 51: 101-17 (2008) Article DOI: 10.1021/jm070860r BindingDB Entry DOI: 10.7270/Q2VQ32FD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50229776 (6-[(2-(4-(2-methoxyphenyl)piperazin-1-yl)ethyl)(pr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from human cloned dopamine D3 receptor expressed in HEK293 cells | J Med Chem 51: 101-17 (2008) Article DOI: 10.1021/jm070860r BindingDB Entry DOI: 10.7270/Q2VQ32FD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50229776 (6-[(2-(4-(2-methoxyphenyl)piperazin-1-yl)ethyl)(pr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from human cloned dopamine D3 receptor expressed in HEK293 cells | J Med Chem 51: 101-17 (2008) Article DOI: 10.1021/jm070860r BindingDB Entry DOI: 10.7270/Q2VQ32FD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polycomb protein EED (Homo sapiens (Human)) | BDBM223987 (A-395 (5) | rac-(3R,4S)-1-(7-fluoro-2,3-dihydro-1H...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 0.5 | -53.1 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
AbbVie Inc. | Assay Description For the assay, compounds were dispensed in assay-ready plates using a three-fold serial dilution from 50 μM to ~850 pM using an Echo 550 Acousti... | Nat Chem Biol 13: 389-395 (2017) Article DOI: 10.1038/nchembio.2306 BindingDB Entry DOI: 10.7270/Q2NG4PGD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Wee1-like protein kinase (Homo sapiens (Human)) | BDBM50522513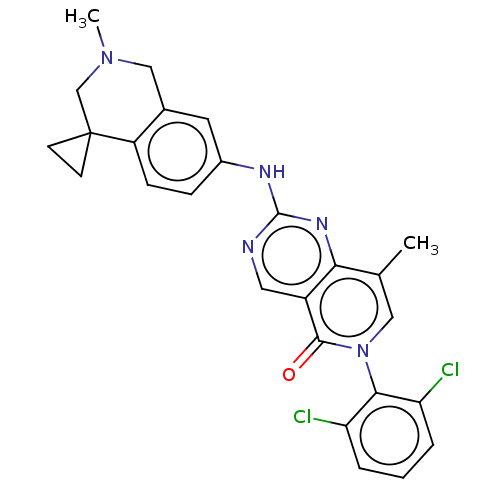 (CHEMBL4526423) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Global Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity to wild type N-terminal GST-tagged human WEE1 catalytic domain (215 to 646 residues) expressed in baculovirus expression system meas... | Bioorg Med Chem Lett 29: 1481-1486 (2019) Article DOI: 10.1016/j.bmcl.2019.04.017 BindingDB Entry DOI: 10.7270/Q2VX0KXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Wee1-like protein kinase (Homo sapiens (Human)) | BDBM50522507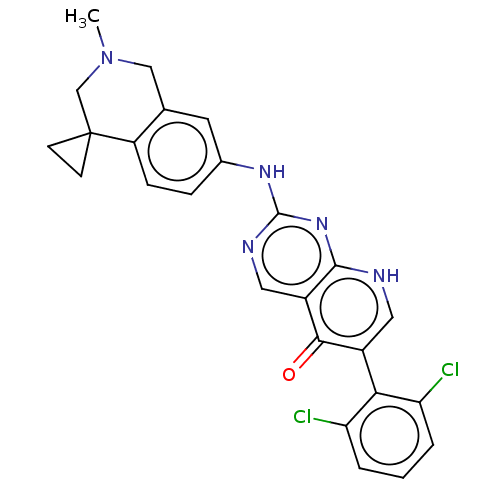 (CHEMBL4470852) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Global Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity to wild type N-terminal GST-tagged human WEE1 catalytic domain (215 to 646 residues) expressed in baculovirus expression system meas... | Bioorg Med Chem Lett 29: 1481-1486 (2019) Article DOI: 10.1016/j.bmcl.2019.04.017 BindingDB Entry DOI: 10.7270/Q2VX0KXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Wee1-like protein kinase (Homo sapiens (Human)) | BDBM50522510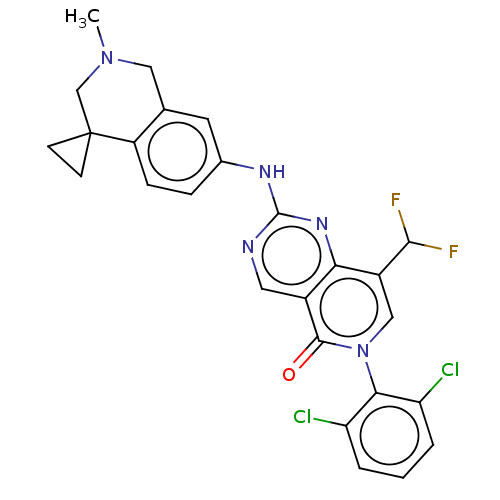 (CHEMBL4447769) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Global Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity to wild type N-terminal GST-tagged human WEE1 catalytic domain (215 to 646 residues) expressed in baculovirus expression system meas... | Bioorg Med Chem Lett 29: 1481-1486 (2019) Article DOI: 10.1016/j.bmcl.2019.04.017 BindingDB Entry DOI: 10.7270/Q2VX0KXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50109927 ((+)-7-[(4-(4-phenylpiperazin-1-yl)butyl)(propyl)am...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from human cloned dopamine D3 receptor expressed in HEK293 cells | J Med Chem 51: 101-17 (2008) Article DOI: 10.1021/jm070860r BindingDB Entry DOI: 10.7270/Q2VQ32FD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50109927 ((+)-7-[(4-(4-phenylpiperazin-1-yl)butyl)(propyl)am...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from human cloned dopamine D3 receptor expressed in HEK293 cells | J Med Chem 51: 101-17 (2008) Article DOI: 10.1021/jm070860r BindingDB Entry DOI: 10.7270/Q2VQ32FD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50109931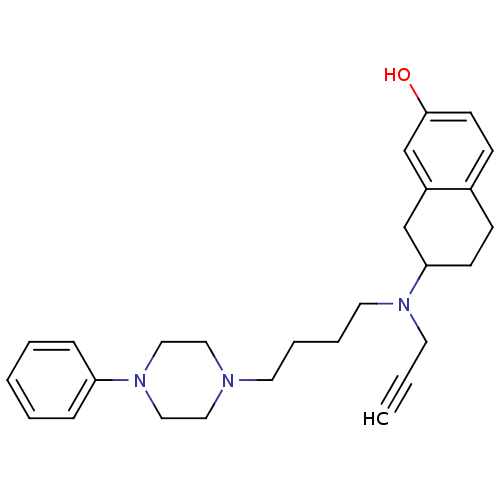 ((+)-7-{[4-(4-phenylpiperazin-1-yl)butyl]prop-2-yny...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.760 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from human cloned dopamine D3 receptor expressed in HEK293 cells | J Med Chem 51: 101-17 (2008) Article DOI: 10.1021/jm070860r BindingDB Entry DOI: 10.7270/Q2VQ32FD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50109931 ((+)-7-{[4-(4-phenylpiperazin-1-yl)butyl]prop-2-yny...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.760 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from human cloned dopamine D3 receptor expressed in HEK293 cells | J Med Chem 51: 101-17 (2008) Article DOI: 10.1021/jm070860r BindingDB Entry DOI: 10.7270/Q2VQ32FD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50229775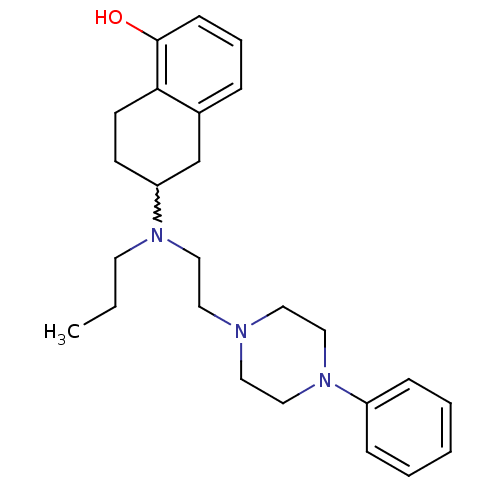 ((+)-6-[(2-(4-phenylpiperazin-1-yl)ethyl)(propyl)am...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from human cloned dopamine D3 receptor expressed in HEK293 cells | J Med Chem 51: 101-17 (2008) Article DOI: 10.1021/jm070860r BindingDB Entry DOI: 10.7270/Q2VQ32FD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50229775 ((+)-6-[(2-(4-phenylpiperazin-1-yl)ethyl)(propyl)am...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.820 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from human cloned dopamine D3 receptor expressed in HEK293 cells | J Med Chem 51: 101-17 (2008) Article DOI: 10.1021/jm070860r BindingDB Entry DOI: 10.7270/Q2VQ32FD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50229775 ((+)-6-[(2-(4-phenylpiperazin-1-yl)ethyl)(propyl)am...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.820 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from human cloned dopamine D3 receptor expressed in HEK293 cells | J Med Chem 51: 101-17 (2008) Article DOI: 10.1021/jm070860r BindingDB Entry DOI: 10.7270/Q2VQ32FD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acetylated-alpha-linked acidic dipeptidase 2 (Homo sapiens (Human)) | BDBM50143073 ((S)-2-{3-[(S)-1-Carboxy-3-(1H-tetrazol-5-yl)-propy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description In vitro inhibitory activity against human Glutamate carboxypeptidase II by using fluorescent assay | J Med Chem 47: 1729-38 (2004) Article DOI: 10.1021/jm0306226 BindingDB Entry DOI: 10.7270/Q2DZ07RR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Wee1-like protein kinase (Homo sapiens (Human)) | BDBM50043651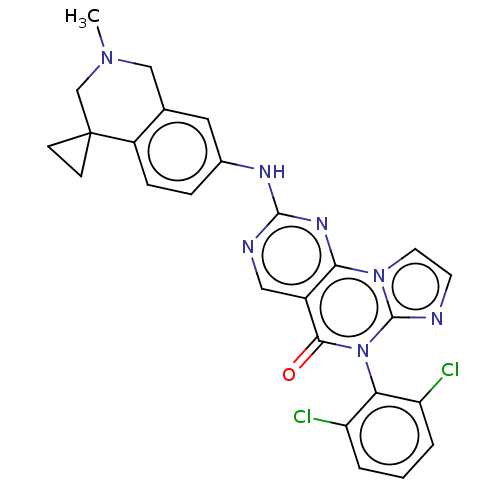 (CHEMBL3355536) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Curated by ChEMBL | Assay Description Inhibition of GST-tagged human Wee1 | ACS Med Chem Lett 6: 58-62 (2015) Article DOI: 10.1021/ml5002745 BindingDB Entry DOI: 10.7270/Q2NZ8972 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Wee1-like protein kinase (Homo sapiens (Human)) | BDBM50043652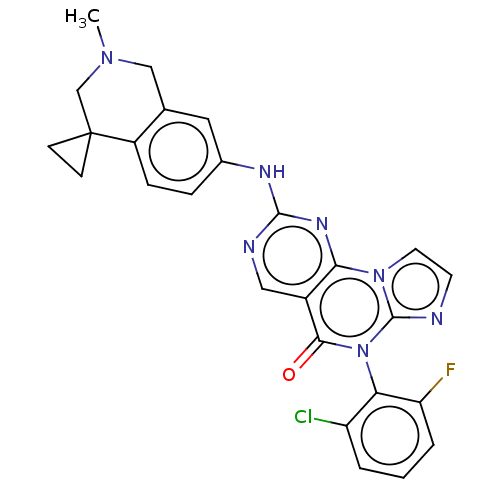 (CHEMBL3355537) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Curated by ChEMBL | Assay Description Inhibition of GST-tagged human Wee1 | ACS Med Chem Lett 6: 58-62 (2015) Article DOI: 10.1021/ml5002745 BindingDB Entry DOI: 10.7270/Q2NZ8972 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50300015 (2-(4-(Pyridin-3-yl)phenyl)-1H-benzo[d]imidazole-4-...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of PARP1 using [3H]NAD+ by top count scintillation counting | J Med Chem 53: 3142-53 (2010) Article DOI: 10.1021/jm901775y BindingDB Entry DOI: 10.7270/Q2N879XR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50315779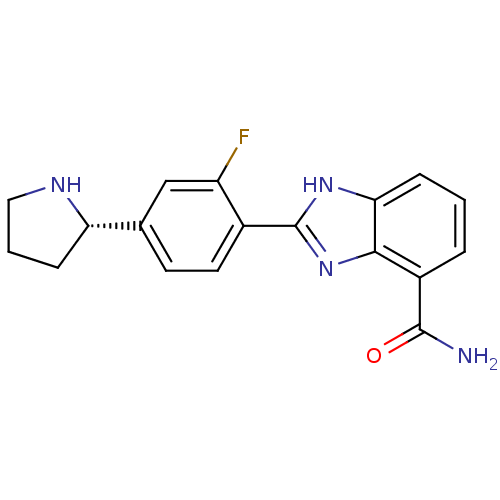 ((S)-2-(2-Fluoro-4-(pyrrolidin-2-yl)phenyl)-1H-benz...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of PARP1 using [3H]NAD+ by top count scintillation counting | J Med Chem 53: 3142-53 (2010) Article DOI: 10.1021/jm901775y BindingDB Entry DOI: 10.7270/Q2N879XR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 2 (Homo sapiens (Human)) | BDBM50300014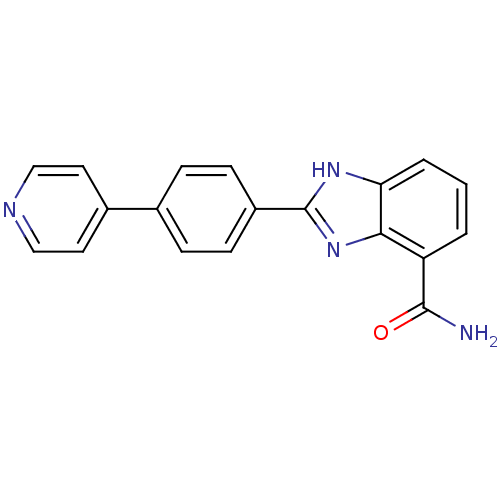 (2-(4-(Pyridin-4-yl)phenyl)-1H-benzimidazole-4-carb...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of PARP2 by top count scintillation counting | J Med Chem 53: 3142-53 (2010) Article DOI: 10.1021/jm901775y BindingDB Entry DOI: 10.7270/Q2N879XR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Wee1-like protein kinase (Homo sapiens (Human)) | BDBM50522504 (CHEMBL4551299) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Global Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity to wild type N-terminal GST-tagged human WEE1 catalytic domain (215 to 646 residues) expressed in baculovirus expression system meas... | Bioorg Med Chem Lett 29: 1481-1486 (2019) Article DOI: 10.1016/j.bmcl.2019.04.017 BindingDB Entry DOI: 10.7270/Q2VX0KXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide phosphoribosyltransferase (Homo sapiens (Human)) | BDBM217112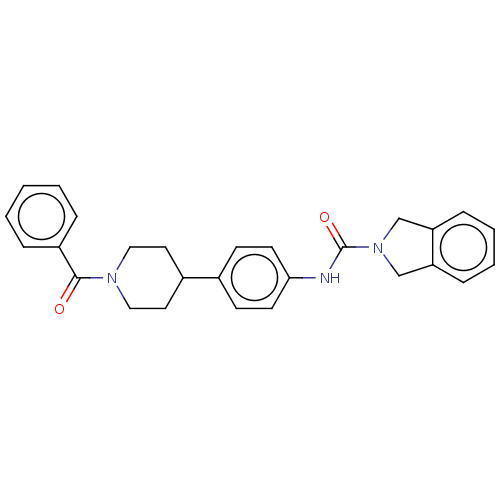 (US9302989, 407) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc, 1 North Waukegan Rd., North Chicago, IL 60064, United States. Electronic address: mike.curtin@abbvie.com. Curated by ChEMBL | Assay Description Inhibition of C-terminal His-tagged human recombinant NAMPT using FK866 or isoindoline urea-based Oregon green (488) probe incubated for 3 hrs by TR-... | Bioorg Med Chem Lett 27: 3317-3325 (2017) Article DOI: 10.1016/j.bmcl.2017.06.018 BindingDB Entry DOI: 10.7270/Q2D22123 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 2 (Homo sapiens (Human)) | BDBM50315783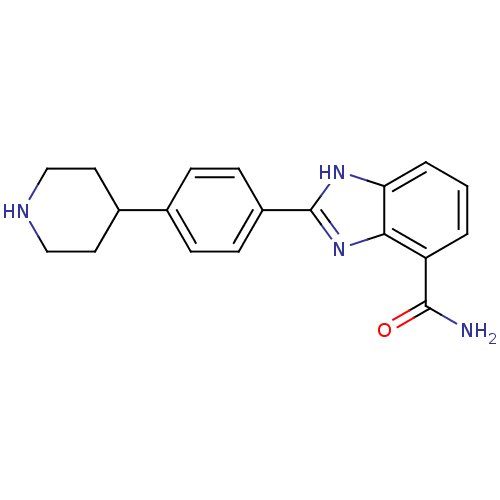 (2-(4-Piperidin-4-ylphenyl)-1H-benzimidazole-4-carb...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of PARP2 by top count scintillation counting | J Med Chem 53: 3142-53 (2010) Article DOI: 10.1021/jm901775y BindingDB Entry DOI: 10.7270/Q2N879XR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Wee1-like protein kinase (Homo sapiens (Human)) | BDBM50043653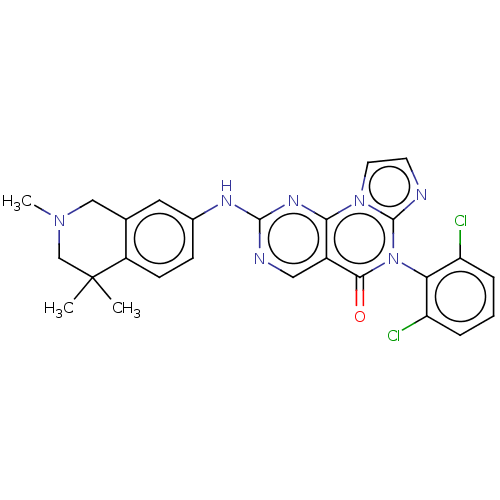 (CHEMBL3355538) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Curated by ChEMBL | Assay Description Inhibition of GST-tagged human Wee1 | ACS Med Chem Lett 6: 58-62 (2015) Article DOI: 10.1021/ml5002745 BindingDB Entry DOI: 10.7270/Q2NZ8972 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Wee1-like protein kinase (Homo sapiens (Human)) | BDBM50043654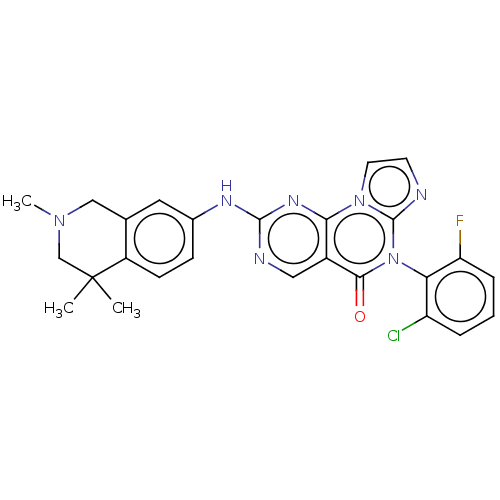 (CHEMBL3355539) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Curated by ChEMBL | Assay Description Inhibition of GST-tagged human Wee1 | ACS Med Chem Lett 6: 58-62 (2015) Article DOI: 10.1021/ml5002745 BindingDB Entry DOI: 10.7270/Q2NZ8972 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polycomb protein EED (Homo sapiens (Human)) | BDBM223986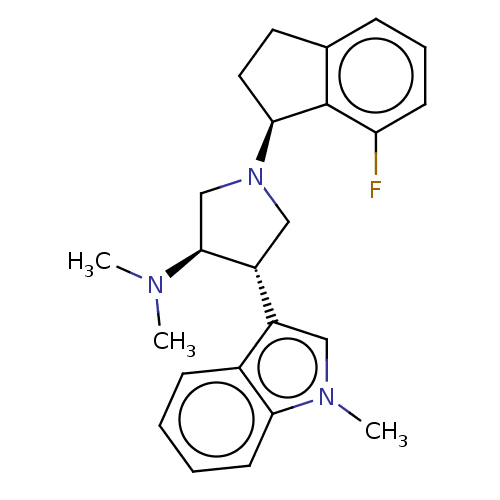 ((3R,4S)-1-[(1S)-7-fluoroindan-1-yl]-N,N-dimethyl-4...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 1 | -51.4 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
AbbVie Inc. | Assay Description For the assay, compounds were dispensed in assay-ready plates using a three-fold serial dilution from 50 μM to ~850 pM using an Echo 550 Acousti... | Nat Chem Biol 13: 389-395 (2017) Article DOI: 10.1038/nchembio.2306 BindingDB Entry DOI: 10.7270/Q2NG4PGD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Wee1-like protein kinase (Homo sapiens (Human)) | BDBM50043648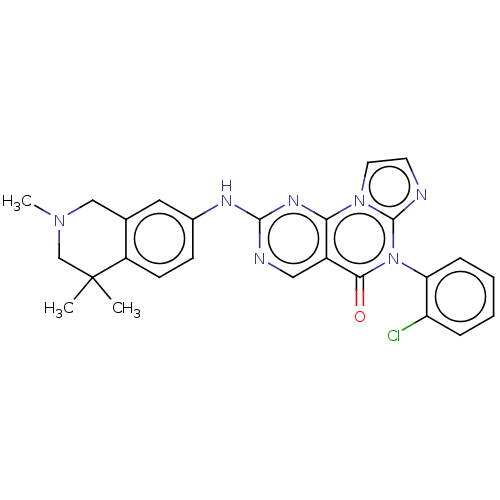 (CHEMBL3355533) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Curated by ChEMBL | Assay Description Inhibition of GST-tagged human Wee1 | ACS Med Chem Lett 6: 58-62 (2015) Article DOI: 10.1021/ml5002745 BindingDB Entry DOI: 10.7270/Q2NZ8972 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Wee1-like protein kinase (Homo sapiens (Human)) | BDBM50043649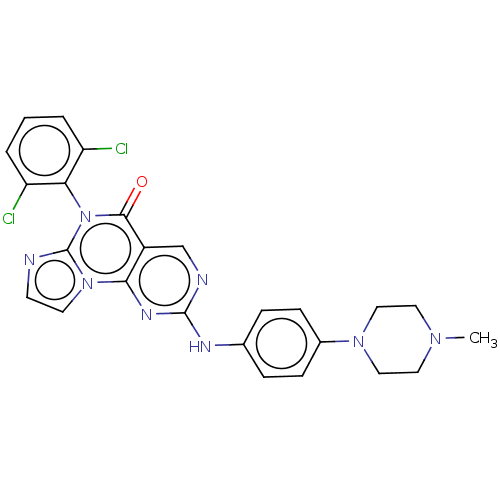 (CHEMBL3355534) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Curated by ChEMBL | Assay Description Inhibition of GST-tagged human Wee1 | ACS Med Chem Lett 6: 58-62 (2015) Article DOI: 10.1021/ml5002745 BindingDB Entry DOI: 10.7270/Q2NZ8972 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Wee1-like protein kinase (Homo sapiens (Human)) | BDBM50043654 (CHEMBL3355539) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Global Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity to wild type N-terminal GST-tagged human WEE1 catalytic domain (215 to 646 residues) expressed in baculovirus expression system meas... | Bioorg Med Chem Lett 29: 1481-1486 (2019) Article DOI: 10.1016/j.bmcl.2019.04.017 BindingDB Entry DOI: 10.7270/Q2VX0KXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Wee1-like protein kinase (Homo sapiens (Human)) | BDBM50043646 (CHEMBL3355531) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Curated by ChEMBL | Assay Description Inhibition of GST-tagged human Wee1 | ACS Med Chem Lett 6: 58-62 (2015) Article DOI: 10.1021/ml5002745 BindingDB Entry DOI: 10.7270/Q2NZ8972 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Wee1-like protein kinase (Homo sapiens (Human)) | BDBM50522505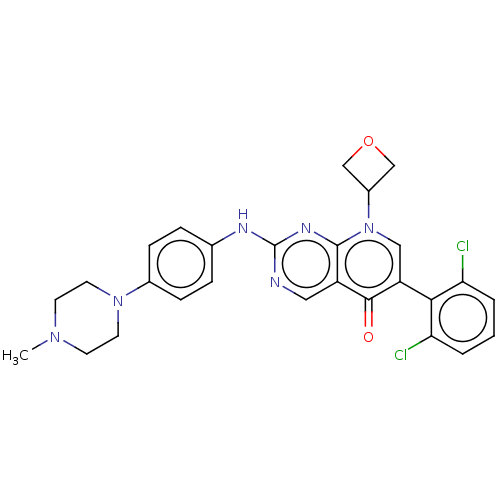 (CHEMBL4469678) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Global Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity to wild type N-terminal GST-tagged human WEE1 catalytic domain (215 to 646 residues) expressed in baculovirus expression system meas... | Bioorg Med Chem Lett 29: 1481-1486 (2019) Article DOI: 10.1016/j.bmcl.2019.04.017 BindingDB Entry DOI: 10.7270/Q2VX0KXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50229778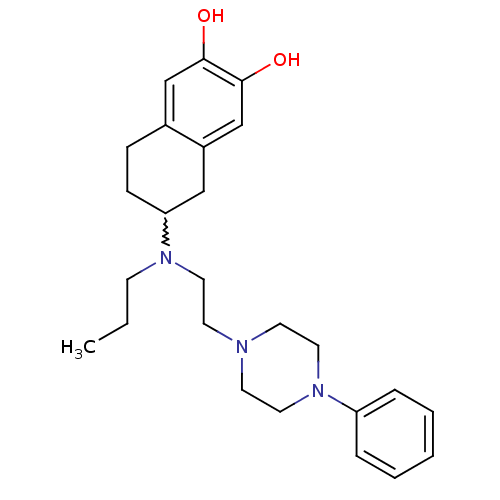 (6-[(2-(4-phenylpiperazin-1-yl)ethyl)(propyl)amino]...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from human cloned dopamine D3 receptor expressed in HEK293 cells | J Med Chem 51: 101-17 (2008) Article DOI: 10.1021/jm070860r BindingDB Entry DOI: 10.7270/Q2VQ32FD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acetylated-alpha-linked acidic dipeptidase 2 (Homo sapiens (Human)) | BDBM17659 ((R,S)-2-phosphonomethylpentanedioic acid | 2-(phos...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description In vitro inhibitory activity against human Glutamate carboxypeptidase II by using fluorescent assay | J Med Chem 47: 1729-38 (2004) Article DOI: 10.1021/jm0306226 BindingDB Entry DOI: 10.7270/Q2DZ07RR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide phosphoribosyltransferase (Homo sapiens (Human)) | BDBM50254198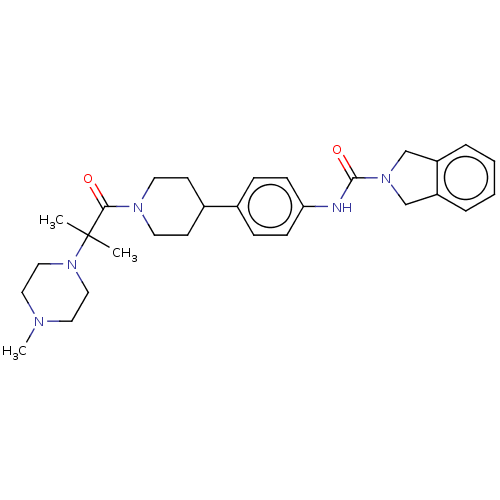 (CHEMBL4060799) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc, 1 North Waukegan Rd., North Chicago, IL 60064, United States. Electronic address: mike.curtin@abbvie.com. Curated by ChEMBL | Assay Description Inhibition of C-terminal His-tagged human recombinant NAMPT using FK866 or isoindoline urea-based Oregon green (488) probe incubated for 3 hrs by TR-... | Bioorg Med Chem Lett 27: 3317-3325 (2017) Article DOI: 10.1016/j.bmcl.2017.06.018 BindingDB Entry DOI: 10.7270/Q2D22123 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 2 (Homo sapiens (Human)) | BDBM50315779 ((S)-2-(2-Fluoro-4-(pyrrolidin-2-yl)phenyl)-1H-benz...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of PARP2 by top count scintillation counting | J Med Chem 53: 3142-53 (2010) Article DOI: 10.1021/jm901775y BindingDB Entry DOI: 10.7270/Q2N879XR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50109926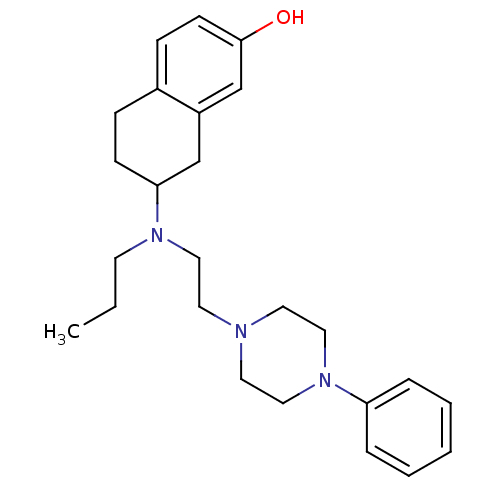 (7-((2-(4-phenylpiperazin-1-yl)ethyl)(propyl)amino)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from human cloned dopamine D3 receptor expressed in HEK293 cells | J Med Chem 51: 101-17 (2008) Article DOI: 10.1021/jm070860r BindingDB Entry DOI: 10.7270/Q2VQ32FD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Wee1-like protein kinase (Homo sapiens (Human)) | BDBM50043643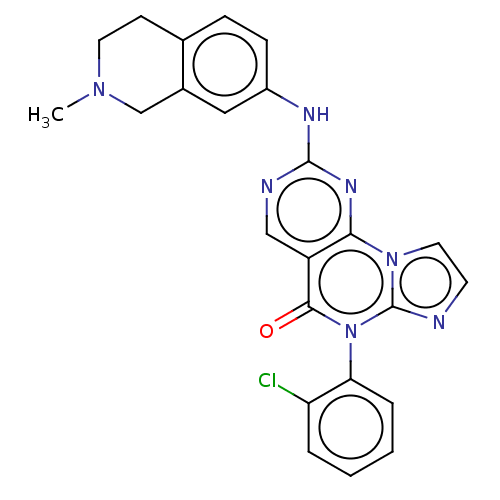 (CHEMBL3355528) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Curated by ChEMBL | Assay Description Inhibition of GST-tagged human Wee1 | ACS Med Chem Lett 6: 58-62 (2015) Article DOI: 10.1021/ml5002745 BindingDB Entry DOI: 10.7270/Q2NZ8972 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide phosphoribosyltransferase (Homo sapiens (Human)) | BDBM217057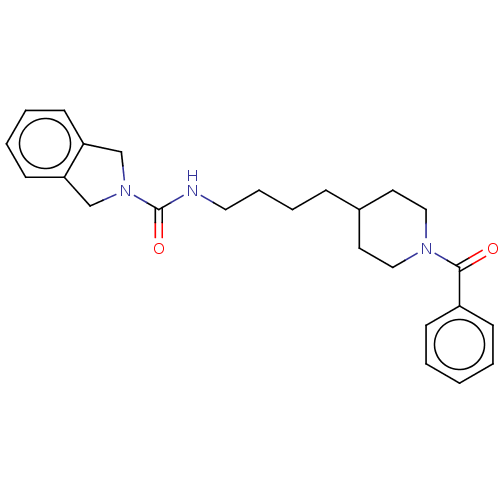 (US9302989, 349) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc, 1 North Waukegan Rd., North Chicago, IL 60064, United States. Electronic address: mike.curtin@abbvie.com. Curated by ChEMBL | Assay Description Inhibition of C-terminal His-tagged human recombinant NAMPT using FK866 or isoindoline urea-based Oregon green (488) probe incubated for 3 hrs by TR-... | Bioorg Med Chem Lett 27: 3317-3325 (2017) Article DOI: 10.1016/j.bmcl.2017.06.018 BindingDB Entry DOI: 10.7270/Q2D22123 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide phosphoribosyltransferase (Homo sapiens (Human)) | BDBM50254199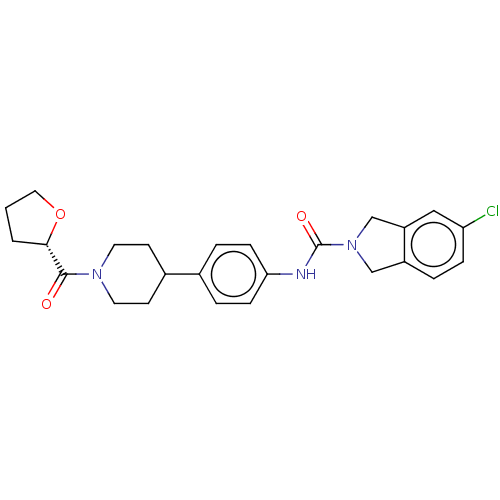 (CHEMBL4083505) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc, 1 North Waukegan Rd., North Chicago, IL 60064, United States. Electronic address: mike.curtin@abbvie.com. Curated by ChEMBL | Assay Description Inhibition of C-terminal His-tagged human recombinant NAMPT using FK866 or isoindoline urea-based Oregon green (488) probe incubated for 3 hrs by TR-... | Bioorg Med Chem Lett 27: 3317-3325 (2017) Article DOI: 10.1016/j.bmcl.2017.06.018 BindingDB Entry DOI: 10.7270/Q2D22123 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor (Mus musculus) | BDBM50152513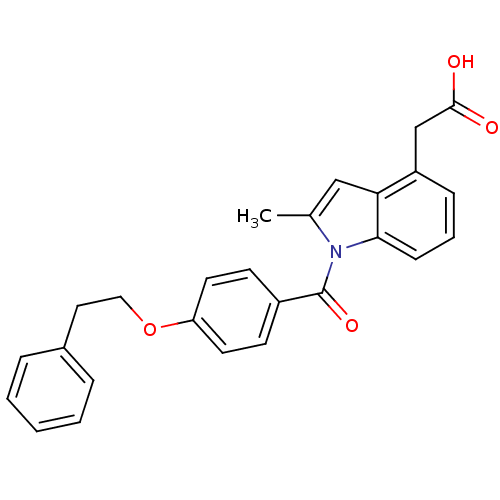 (CHEMBL361457 | [2-Methyl-1-(4-phenethyloxy-benzoyl...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Ability to inhibit the binding of [3H]-PGD-2 radioligand to membranes of CHO cells stably expressing mouse Prostaglandin D2 receptor | Bioorg Med Chem Lett 14: 4891-5 (2004) Article DOI: 10.1016/j.bmcl.2004.07.039 BindingDB Entry DOI: 10.7270/Q2BK1BTV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide phosphoribosyltransferase (Homo sapiens (Human)) | BDBM50254216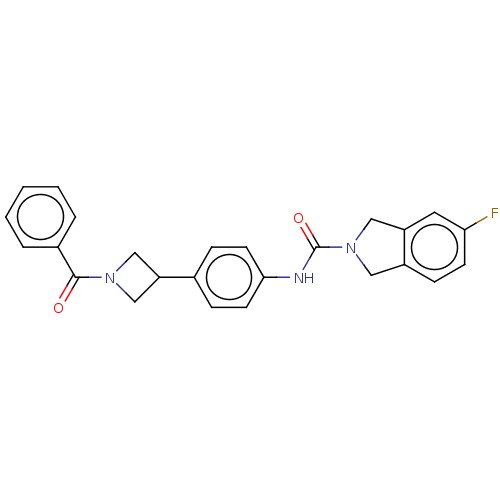 (CHEMBL4096471) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc, 1 North Waukegan Rd., North Chicago, IL 60064, United States. Electronic address: mike.curtin@abbvie.com. Curated by ChEMBL | Assay Description Inhibition of C-terminal His-tagged human recombinant NAMPT using FK866 or isoindoline urea-based Oregon green (488) probe incubated for 3 hrs by TR-... | Bioorg Med Chem Lett 27: 3317-3325 (2017) Article DOI: 10.1016/j.bmcl.2017.06.018 BindingDB Entry DOI: 10.7270/Q2D22123 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 2811 total ) | Next | Last >> |