

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM10404 ((1S,12S,14R)-9-methoxy-4-methyl-11-oxa-4-azatetrac...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther-Universit£t Halle-Wittenberg Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus acetylcholinesterase using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrat... | Bioorg Med Chem Lett 25: 2654-6 (2015) Article DOI: 10.1016/j.bmcl.2015.04.086 BindingDB Entry DOI: 10.7270/Q2KW5HS8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50094480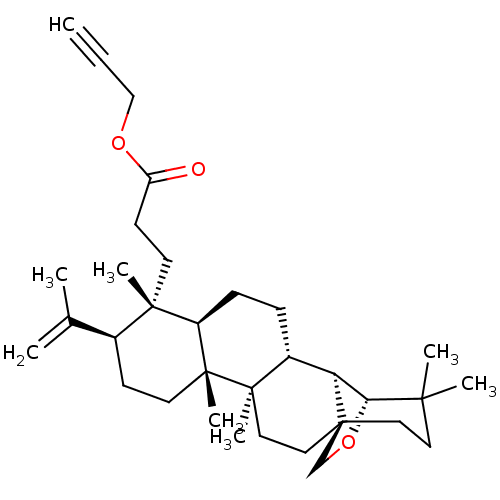 (CHEMBL3586232) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.48E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther-Universit£t Halle-Wittenberg Curated by ChEMBL | Assay Description Uncompetitive Inhibition of Electrophorus electricus acetylcholinesterase using acetylthiocholine iodide as substrate preincubated for 20 mins follow... | Bioorg Med Chem Lett 25: 2654-6 (2015) Article DOI: 10.1016/j.bmcl.2015.04.086 BindingDB Entry DOI: 10.7270/Q2KW5HS8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50094480 (CHEMBL3586232) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.33E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther-Universit£t Halle-Wittenberg Curated by ChEMBL | Assay Description Competitive Inhibition of Electrophorus electricus acetylcholinesterase using acetylthiocholine iodide as substrate preincubated for 20 mins followed... | Bioorg Med Chem Lett 25: 2654-6 (2015) Article DOI: 10.1016/j.bmcl.2015.04.086 BindingDB Entry DOI: 10.7270/Q2KW5HS8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50094483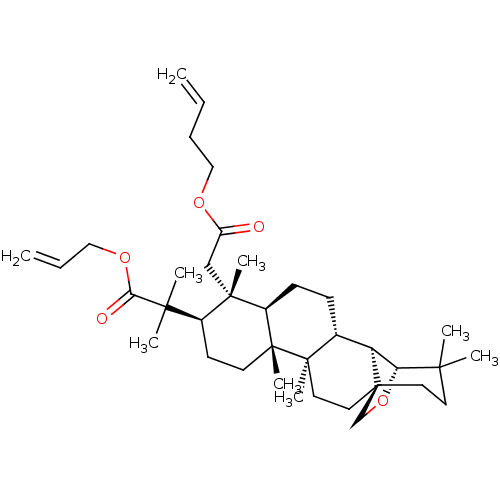 (CHEMBL3590226) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.39E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther-Universit£t Halle-Wittenberg Curated by ChEMBL | Assay Description Competitive Inhibition of Electrophorus electricus acetylcholinesterase using acetylthiocholine iodide as substrate preincubated for 20 mins followed... | Bioorg Med Chem Lett 25: 2654-6 (2015) Article DOI: 10.1016/j.bmcl.2015.04.086 BindingDB Entry DOI: 10.7270/Q2KW5HS8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50094481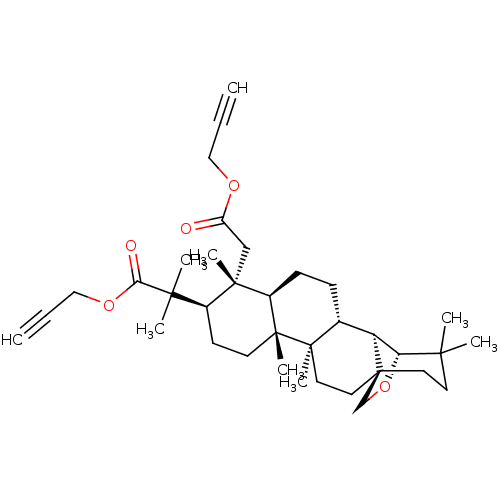 (CHEMBL3586240) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.63E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther-Universit£t Halle-Wittenberg Curated by ChEMBL | Assay Description Uncompetitive Inhibition of Electrophorus electricus acetylcholinesterase using acetylthiocholine iodide as substrate preincubated for 20 mins follow... | Bioorg Med Chem Lett 25: 2654-6 (2015) Article DOI: 10.1016/j.bmcl.2015.04.086 BindingDB Entry DOI: 10.7270/Q2KW5HS8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50094481 (CHEMBL3586240) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.05E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther-Universit£t Halle-Wittenberg Curated by ChEMBL | Assay Description Competitive Inhibition of Electrophorus electricus acetylcholinesterase using acetylthiocholine iodide as substrate preincubated for 20 mins followed... | Bioorg Med Chem Lett 25: 2654-6 (2015) Article DOI: 10.1016/j.bmcl.2015.04.086 BindingDB Entry DOI: 10.7270/Q2KW5HS8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50094482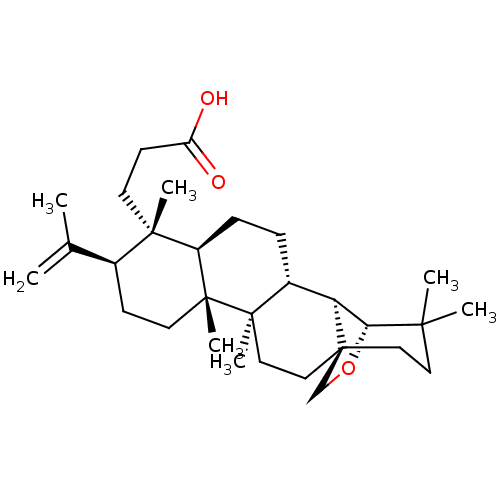 (CHEMBL3586228) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.18E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther-Universit£t Halle-Wittenberg Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus acetylcholinesterase using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrat... | Bioorg Med Chem Lett 25: 2654-6 (2015) Article DOI: 10.1016/j.bmcl.2015.04.086 BindingDB Entry DOI: 10.7270/Q2KW5HS8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50357212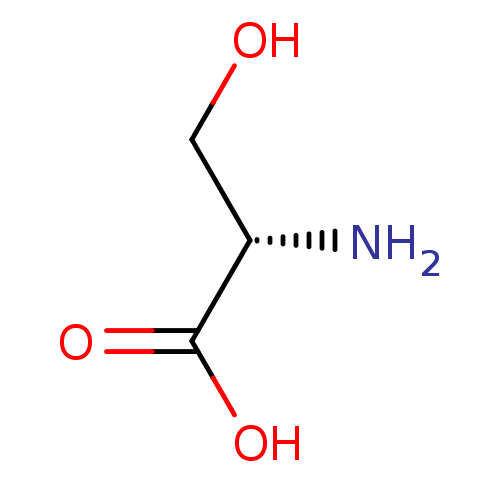 ((L)-SERINE | Serine) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther-Universit£t Halle-Wittenberg Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus acetylcholinesterase using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrat... | Bioorg Med Chem Lett 25: 2654-6 (2015) Article DOI: 10.1016/j.bmcl.2015.04.086 BindingDB Entry DOI: 10.7270/Q2KW5HS8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50357212 ((L)-SERINE | Serine) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther-Universit£t Halle-Wittenberg Curated by ChEMBL | Assay Description Inhibition of equine serum butyrylcholinesterase using butyrylthiocholineidioide as substrate preincubated for 20 mins followed by substrate addition... | Bioorg Med Chem Lett 25: 2654-6 (2015) Article DOI: 10.1016/j.bmcl.2015.04.086 BindingDB Entry DOI: 10.7270/Q2KW5HS8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50089862 (CHEMBL3577700) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
£Philochem AG Curated by ChEMBL | Assay Description Inhibition of full length PARP1 (unknown origin) expressed in Escherichia coli BL21 (DE3) assessed as reduction in ADP-ribosyl transferase activity u... | J Med Chem 58: 5143-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00432 BindingDB Entry DOI: 10.7270/Q2R2134J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50089862 (CHEMBL3577700) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
£Philochem AG Curated by ChEMBL | Assay Description Inhibition of full length PARP1 (unknown origin) expressed in Escherichia coli BL21 (DE3) assessed as reduction in ADP-ribosyl transferase activity u... | J Med Chem 58: 5143-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00432 BindingDB Entry DOI: 10.7270/Q2R2134J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50089863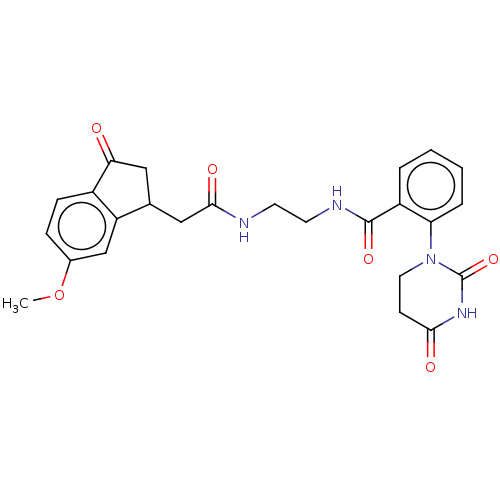 (CHEMBL3577701) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
£Philochem AG Curated by ChEMBL | Assay Description Inhibition of full length PARP1 (unknown origin) expressed in Escherichia coli BL21 (DE3) assessed as reduction in ADP-ribosyl transferase activity u... | J Med Chem 58: 5143-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00432 BindingDB Entry DOI: 10.7270/Q2R2134J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50089863 (CHEMBL3577701) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
£Philochem AG Curated by ChEMBL | Assay Description Inhibition of full length PARP1 (unknown origin) expressed in Escherichia coli BL21 (DE3) assessed as reduction in ADP-ribosyl transferase activity u... | J Med Chem 58: 5143-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00432 BindingDB Entry DOI: 10.7270/Q2R2134J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50089867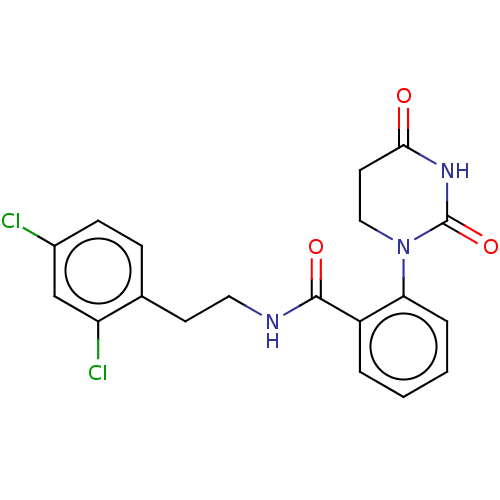 (CHEMBL3577626) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
£Philochem AG Curated by ChEMBL | Assay Description Inhibition of full length PARP1 (unknown origin) expressed in Escherichia coli BL21 (DE3) assessed as reduction in ADP-ribosyl transferase activity u... | J Med Chem 58: 5143-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00432 BindingDB Entry DOI: 10.7270/Q2R2134J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50089867 (CHEMBL3577626) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
£Philochem AG Curated by ChEMBL | Assay Description Inhibition of full length PARP1 (unknown origin) expressed in Escherichia coli BL21 (DE3) assessed as reduction in ADP-ribosyl transferase activity u... | J Med Chem 58: 5143-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00432 BindingDB Entry DOI: 10.7270/Q2R2134J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50089864 (CHEMBL3577702) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 195 | n/a | n/a | n/a | n/a | n/a | n/a |
£Philochem AG Curated by ChEMBL | Assay Description Inhibition of full length PARP1 (unknown origin) expressed in Escherichia coli BL21 (DE3) assessed as reduction in ADP-ribosyl transferase activity u... | J Med Chem 58: 5143-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00432 BindingDB Entry DOI: 10.7270/Q2R2134J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50089864 (CHEMBL3577702) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
£Philochem AG Curated by ChEMBL | Assay Description Inhibition of full length PARP1 (unknown origin) expressed in Escherichia coli BL21 (DE3) assessed as reduction in ADP-ribosyl transferase activity u... | J Med Chem 58: 5143-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00432 BindingDB Entry DOI: 10.7270/Q2R2134J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50089866 (CHEMBL3577625) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
£Philochem AG Curated by ChEMBL | Assay Description Inhibition of full length PARP1 (unknown origin) expressed in Escherichia coli BL21 (DE3) assessed as reduction in ADP-ribosyl transferase activity u... | J Med Chem 58: 5143-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00432 BindingDB Entry DOI: 10.7270/Q2R2134J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50089866 (CHEMBL3577625) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 224 | n/a | n/a | n/a | n/a | n/a | n/a |
£Philochem AG Curated by ChEMBL | Assay Description Inhibition of full length PARP1 (unknown origin) expressed in Escherichia coli BL21 (DE3) assessed as reduction in ADP-ribosyl transferase activity u... | J Med Chem 58: 5143-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00432 BindingDB Entry DOI: 10.7270/Q2R2134J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-1 (Homo sapiens (Human)) | BDBM50089865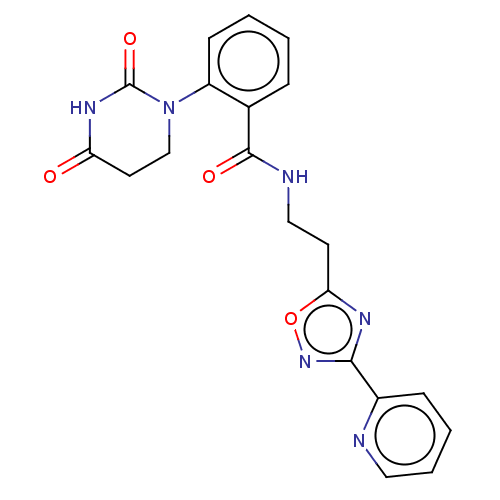 (CHEMBL3577624) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 245 | n/a | n/a | n/a | n/a | n/a | n/a |
£Philochem AG Curated by ChEMBL | Assay Description Inhibition of hexa-histidine tagged recombinant tankyrase 1 biotinylated catalytic domain (amino acids 1106 to 1325) (unknown origin) expressed in Es... | J Med Chem 58: 5143-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00432 BindingDB Entry DOI: 10.7270/Q2R2134J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-1 (Homo sapiens (Human)) | BDBM50089865 (CHEMBL3577624) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
£Philochem AG Curated by ChEMBL | Assay Description Inhibition of hexa-histidine tagged recombinant tankyrase 1 biotinylated catalytic domain (amino acids 1106 to 1325) (unknown origin) expressed in Es... | J Med Chem 58: 5143-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00432 BindingDB Entry DOI: 10.7270/Q2R2134J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50089865 (CHEMBL3577624) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 257 | n/a | n/a | n/a | n/a | n/a | n/a |
£Philochem AG Curated by ChEMBL | Assay Description Inhibition of full length PARP1 (unknown origin) expressed in Escherichia coli BL21 (DE3) assessed as reduction in ADP-ribosyl transferase activity u... | J Med Chem 58: 5143-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00432 BindingDB Entry DOI: 10.7270/Q2R2134J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50089865 (CHEMBL3577624) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
£Philochem AG Curated by ChEMBL | Assay Description Inhibition of full length PARP1 (unknown origin) expressed in Escherichia coli BL21 (DE3) assessed as reduction in ADP-ribosyl transferase activity u... | J Med Chem 58: 5143-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00432 BindingDB Entry DOI: 10.7270/Q2R2134J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-1 (Homo sapiens (Human)) | BDBM50089864 (CHEMBL3577702) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 288 | n/a | n/a | n/a | n/a | n/a | n/a |
£Philochem AG Curated by ChEMBL | Assay Description Inhibition of hexa-histidine tagged recombinant tankyrase 1 biotinylated catalytic domain (amino acids 1106 to 1325) (unknown origin) expressed in Es... | J Med Chem 58: 5143-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00432 BindingDB Entry DOI: 10.7270/Q2R2134J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-1 (Homo sapiens (Human)) | BDBM50089863 (CHEMBL3577701) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 288 | n/a | n/a | n/a | n/a | n/a | n/a |
£Philochem AG Curated by ChEMBL | Assay Description Inhibition of hexa-histidine tagged recombinant tankyrase 1 biotinylated catalytic domain (amino acids 1106 to 1325) (unknown origin) expressed in Es... | J Med Chem 58: 5143-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00432 BindingDB Entry DOI: 10.7270/Q2R2134J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-1 (Homo sapiens (Human)) | BDBM50089863 (CHEMBL3577701) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
£Philochem AG Curated by ChEMBL | Assay Description Inhibition of hexa-histidine tagged recombinant tankyrase 1 biotinylated catalytic domain (amino acids 1106 to 1325) (unknown origin) expressed in Es... | J Med Chem 58: 5143-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00432 BindingDB Entry DOI: 10.7270/Q2R2134J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-1 (Homo sapiens (Human)) | BDBM50089864 (CHEMBL3577702) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
£Philochem AG Curated by ChEMBL | Assay Description Inhibition of hexa-histidine tagged recombinant tankyrase 1 biotinylated catalytic domain (amino acids 1106 to 1325) (unknown origin) expressed in Es... | J Med Chem 58: 5143-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00432 BindingDB Entry DOI: 10.7270/Q2R2134J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-2 (Homo sapiens (Human)) | BDBM50089864 (CHEMBL3577702) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
£Philochem AG Curated by ChEMBL | Assay Description Inhibition of tankyrase 2 catalytic domain (unknown origin) expressed in Escherichia coli BL21 (DE3) assessed as reduction in ADP-ribosyl transferase... | J Med Chem 58: 5143-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00432 BindingDB Entry DOI: 10.7270/Q2R2134J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-1 (Homo sapiens (Human)) | BDBM50089866 (CHEMBL3577625) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
£Philochem AG Curated by ChEMBL | Assay Description Inhibition of hexa-histidine tagged recombinant tankyrase 1 biotinylated catalytic domain (amino acids 1106 to 1325) (unknown origin) expressed in Es... | J Med Chem 58: 5143-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00432 BindingDB Entry DOI: 10.7270/Q2R2134J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-1 (Homo sapiens (Human)) | BDBM50089866 (CHEMBL3577625) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 331 | n/a | n/a | n/a | n/a | n/a | n/a |
£Philochem AG Curated by ChEMBL | Assay Description Inhibition of hexa-histidine tagged recombinant tankyrase 1 biotinylated catalytic domain (amino acids 1106 to 1325) (unknown origin) expressed in Es... | J Med Chem 58: 5143-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00432 BindingDB Entry DOI: 10.7270/Q2R2134J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-2 (Homo sapiens (Human)) | BDBM50089864 (CHEMBL3577702) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 331 | n/a | n/a | n/a | n/a | n/a | n/a |
£Philochem AG Curated by ChEMBL | Assay Description Inhibition of tankyrase 2 catalytic domain (unknown origin) expressed in Escherichia coli BL21 (DE3) assessed as reduction in ADP-ribosyl transferase... | J Med Chem 58: 5143-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00432 BindingDB Entry DOI: 10.7270/Q2R2134J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-1 (Homo sapiens (Human)) | BDBM50089858 (CHEMBL3577627) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 347 | n/a | n/a | n/a | n/a | n/a | n/a |
£Philochem AG Curated by ChEMBL | Assay Description Inhibition of hexa-histidine tagged recombinant tankyrase 1 biotinylated catalytic domain (amino acids 1106 to 1325) (unknown origin) expressed in Es... | J Med Chem 58: 5143-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00432 BindingDB Entry DOI: 10.7270/Q2R2134J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-1 (Homo sapiens (Human)) | BDBM50089858 (CHEMBL3577627) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
£Philochem AG Curated by ChEMBL | Assay Description Inhibition of hexa-histidine tagged recombinant tankyrase 1 biotinylated catalytic domain (amino acids 1106 to 1325) (unknown origin) expressed in Es... | J Med Chem 58: 5143-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00432 BindingDB Entry DOI: 10.7270/Q2R2134J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-1 (Homo sapiens (Human)) | BDBM50089862 (CHEMBL3577700) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
£Philochem AG Curated by ChEMBL | Assay Description Inhibition of hexa-histidine tagged recombinant tankyrase 1 biotinylated catalytic domain (amino acids 1106 to 1325) (unknown origin) expressed in Es... | J Med Chem 58: 5143-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00432 BindingDB Entry DOI: 10.7270/Q2R2134J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-1 (Homo sapiens (Human)) | BDBM50089862 (CHEMBL3577700) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 372 | n/a | n/a | n/a | n/a | n/a | n/a |
£Philochem AG Curated by ChEMBL | Assay Description Inhibition of hexa-histidine tagged recombinant tankyrase 1 biotinylated catalytic domain (amino acids 1106 to 1325) (unknown origin) expressed in Es... | J Med Chem 58: 5143-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00432 BindingDB Entry DOI: 10.7270/Q2R2134J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-2 (Homo sapiens (Human)) | BDBM50089862 (CHEMBL3577700) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 468 | n/a | n/a | n/a | n/a | n/a | n/a |
£Philochem AG Curated by ChEMBL | Assay Description Inhibition of tankyrase 2 catalytic domain (unknown origin) expressed in Escherichia coli BL21 (DE3) assessed as reduction in ADP-ribosyl transferase... | J Med Chem 58: 5143-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00432 BindingDB Entry DOI: 10.7270/Q2R2134J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-2 (Homo sapiens (Human)) | BDBM50089862 (CHEMBL3577700) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 470 | n/a | n/a | n/a | n/a | n/a | n/a |
£Philochem AG Curated by ChEMBL | Assay Description Inhibition of tankyrase 2 catalytic domain (unknown origin) expressed in Escherichia coli BL21 (DE3) assessed as reduction in ADP-ribosyl transferase... | J Med Chem 58: 5143-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00432 BindingDB Entry DOI: 10.7270/Q2R2134J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-1 (Homo sapiens (Human)) | BDBM50089859 (CHEMBL3577628) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 570 | n/a | n/a | n/a | n/a | n/a | n/a |
£Philochem AG Curated by ChEMBL | Assay Description Inhibition of hexa-histidine tagged recombinant tankyrase 1 biotinylated catalytic domain (amino acids 1106 to 1325) (unknown origin) expressed in Es... | J Med Chem 58: 5143-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00432 BindingDB Entry DOI: 10.7270/Q2R2134J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-1 (Homo sapiens (Human)) | BDBM50089859 (CHEMBL3577628) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 575 | n/a | n/a | n/a | n/a | n/a | n/a |
£Philochem AG Curated by ChEMBL | Assay Description Inhibition of hexa-histidine tagged recombinant tankyrase 1 biotinylated catalytic domain (amino acids 1106 to 1325) (unknown origin) expressed in Es... | J Med Chem 58: 5143-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00432 BindingDB Entry DOI: 10.7270/Q2R2134J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-1 (Homo sapiens (Human)) | BDBM50089867 (CHEMBL3577626) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 676 | n/a | n/a | n/a | n/a | n/a | n/a |
£Philochem AG Curated by ChEMBL | Assay Description Inhibition of hexa-histidine tagged recombinant tankyrase 1 biotinylated catalytic domain (amino acids 1106 to 1325) (unknown origin) expressed in Es... | J Med Chem 58: 5143-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00432 BindingDB Entry DOI: 10.7270/Q2R2134J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-1 (Homo sapiens (Human)) | BDBM50089867 (CHEMBL3577626) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
£Philochem AG Curated by ChEMBL | Assay Description Inhibition of hexa-histidine tagged recombinant tankyrase 1 biotinylated catalytic domain (amino acids 1106 to 1325) (unknown origin) expressed in Es... | J Med Chem 58: 5143-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00432 BindingDB Entry DOI: 10.7270/Q2R2134J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-2 (Homo sapiens (Human)) | BDBM50089858 (CHEMBL3577627) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 910 | n/a | n/a | n/a | n/a | n/a | n/a |
£Philochem AG Curated by ChEMBL | Assay Description Inhibition of tankyrase 2 catalytic domain (unknown origin) expressed in Escherichia coli BL21 (DE3) assessed as reduction in ADP-ribosyl transferase... | J Med Chem 58: 5143-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00432 BindingDB Entry DOI: 10.7270/Q2R2134J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-2 (Homo sapiens (Human)) | BDBM50089858 (CHEMBL3577627) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 912 | n/a | n/a | n/a | n/a | n/a | n/a |
£Philochem AG Curated by ChEMBL | Assay Description Inhibition of tankyrase 2 catalytic domain (unknown origin) expressed in Escherichia coli BL21 (DE3) assessed as reduction in ADP-ribosyl transferase... | J Med Chem 58: 5143-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00432 BindingDB Entry DOI: 10.7270/Q2R2134J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A/1B/1D/1F (RAT-Rattus norvegicus (rat)-Rattus norvegicus (Rat...) | BDBM50226062 (CHEMBL78593) | PDB MMDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity towards 5-hydroxytryptamine 1 receptor in brain cortical membranes of rat | J Med Chem 29: 2415-8 (1986) BindingDB Entry DOI: 10.7270/Q2V69MT2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A/1B/1D/1F (RAT-Rattus norvegicus (rat)-Rattus norvegicus (Rat...) | BDBM50226061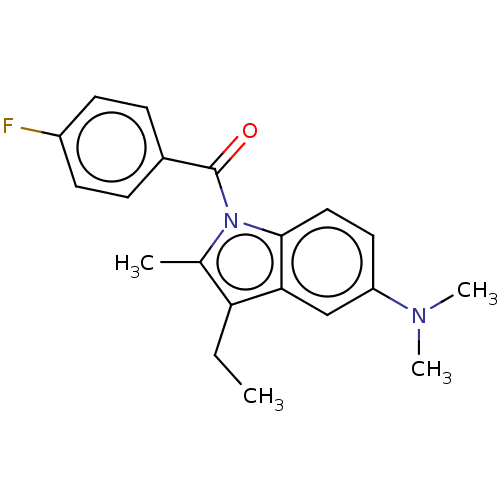 (CHEMBL312405) | PDB MMDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for its binding affinity towards 5-hydroxytryptamine 1 receptor in rat | J Med Chem 29: 2415-8 (1986) BindingDB Entry DOI: 10.7270/Q2V69MT2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serotonin 2 (5-HT2) receptor (RAT-Rattus norvegicus (Rat)-Rattus norvegicus (rat...) | BDBM50226105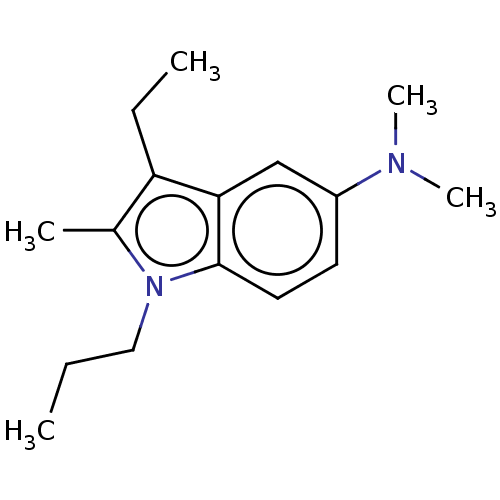 (CHEMBL81187) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity towards 5-hydroxytryptamine 2 receptor in rat brain cortical membranes | J Med Chem 29: 2415-8 (1986) BindingDB Entry DOI: 10.7270/Q2V69MT2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A/1B/1D/1F (RAT-Rattus norvegicus (rat)-Rattus norvegicus (Rat...) | BDBM50226125 (CHEMBL52816) | PDB MMDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity towards 5-hydroxytryptamine 1 receptor in brain cortical membranes of rat | J Med Chem 29: 2415-8 (1986) BindingDB Entry DOI: 10.7270/Q2V69MT2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serotonin 2 (5-HT2) receptor (RAT-Rattus norvegicus (Rat)-Rattus norvegicus (rat...) | BDBM50226061 (CHEMBL312405) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity towards 5-hydroxytryptamine 2 receptor in rat brain cortical membranes | J Med Chem 29: 2415-8 (1986) BindingDB Entry DOI: 10.7270/Q2V69MT2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A/1B/1D/1F (RAT-Rattus norvegicus (rat)-Rattus norvegicus (Rat...) | BDBM50226105 (CHEMBL81187) | PDB MMDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity towards 5-hydroxytryptamine 1 receptor in brain cortical membranes of rat | J Med Chem 29: 2415-8 (1986) BindingDB Entry DOI: 10.7270/Q2V69MT2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A/1B/1D/1F (RAT-Rattus norvegicus (rat)-Rattus norvegicus (Rat...) | BDBM50226057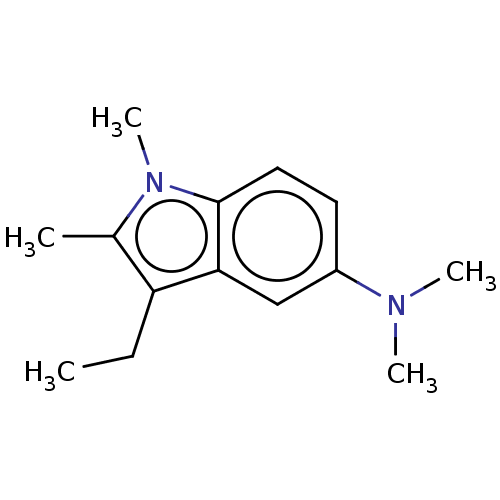 (CHEMBL298780) | PDB MMDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity towards 5-hydroxytryptamine 1 receptor in brain cortical membranes of rat | J Med Chem 29: 2415-8 (1986) BindingDB Entry DOI: 10.7270/Q2V69MT2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 86 total ) | Next | Last >> |