

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Calcium/calmodulin-dependent protein kinase type IV [15-340] (Homo sapiens (Human)) | BDBM223209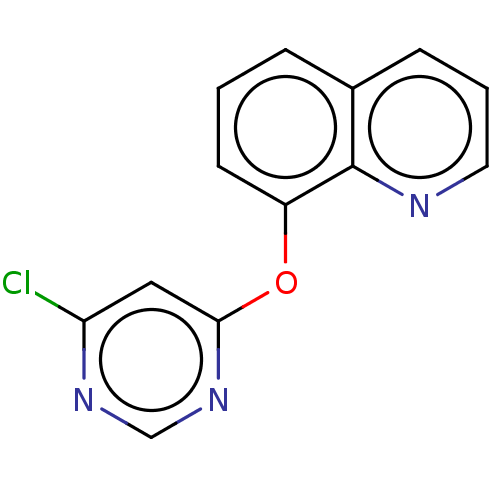 (8-((6-Chloropyrimidin-4-yl)oxy)quinoline (Compound...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 0.0109 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
B.R. Ambedkar Bihar University | Assay Description The docking and scoring of ligands with CAMKIV protein was accomplished using ParDOCK module of Sanjeevini drug design suite, which is based on physi... | Chem Biol Drug Des 89: 741-754 (2017) Article DOI: 10.1111/cbdd.12898 BindingDB Entry DOI: 10.7270/Q28C9V30 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcium/calmodulin-dependent protein kinase type IV [15-340] (Homo sapiens (Human)) | BDBM223208 (7-((6-((5-Methoxy-1H-benzo[d]imidazol-2-yl)thio)py...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.35E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
B.R. Ambedkar Bihar University | Assay Description The docking and scoring of ligands with CAMKIV protein was accomplished using ParDOCK module of Sanjeevini drug design suite, which is based on physi... | Chem Biol Drug Des 89: 741-754 (2017) Article DOI: 10.1111/cbdd.12898 BindingDB Entry DOI: 10.7270/Q28C9V30 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcium/calmodulin-dependent protein kinase type IV [15-340] (Homo sapiens (Human)) | BDBM223207 (4-Methyl-7-((6-(quinolin-8-yloxy)pyrimidin-4-yl)ox...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.45E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
B.R. Ambedkar Bihar University | Assay Description The docking and scoring of ligands with CAMKIV protein was accomplished using ParDOCK module of Sanjeevini drug design suite, which is based on physi... | Chem Biol Drug Des 89: 741-754 (2017) Article DOI: 10.1111/cbdd.12898 BindingDB Entry DOI: 10.7270/Q28C9V30 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcium/calmodulin-dependent protein kinase type IV [15-340] (Homo sapiens (Human)) | BDBM223206 (7-[(6-Chloropyrimidin-4-yl)oxy]-4-methyl-2H-chrome...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.57E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
B.R. Ambedkar Bihar University | Assay Description The docking and scoring of ligands with CAMKIV protein was accomplished using ParDOCK module of Sanjeevini drug design suite, which is based on physi... | Chem Biol Drug Des 89: 741-754 (2017) Article DOI: 10.1111/cbdd.12898 BindingDB Entry DOI: 10.7270/Q28C9V30 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcium/calmodulin-dependent protein kinase type IV [15-340] (Homo sapiens (Human)) | BDBM223205 (6-((5-Methoxy-1H-benzo[d]imidazol-2-yl)thio)-N-(4-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.01E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
B.R. Ambedkar Bihar University | Assay Description The docking and scoring of ligands with CAMKIV protein was accomplished using ParDOCK module of Sanjeevini drug design suite, which is based on physi... | Chem Biol Drug Des 89: 741-754 (2017) Article DOI: 10.1111/cbdd.12898 BindingDB Entry DOI: 10.7270/Q28C9V30 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcium/calmodulin-dependent protein kinase type IV [15-340] (Homo sapiens (Human)) | BDBM223204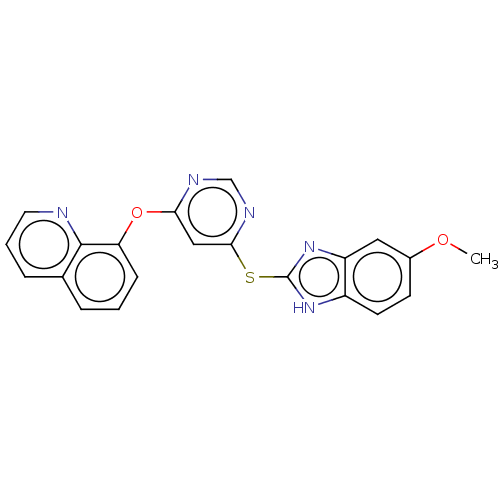 (8-((6-((5-Methoxy-1H-benzo[d]imidazol-2-yl)thio)py...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.44E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
B.R. Ambedkar Bihar University | Assay Description The docking and scoring of ligands with CAMKIV protein was accomplished using ParDOCK module of Sanjeevini drug design suite, which is based on physi... | Chem Biol Drug Des 89: 741-754 (2017) Article DOI: 10.1111/cbdd.12898 BindingDB Entry DOI: 10.7270/Q28C9V30 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcium/calmodulin-dependent protein kinase type IV [15-340] (Homo sapiens (Human)) | BDBM223203 (8-((6-(Naphthalen-2-yloxy)pyrimidin-4-yl)oxy)quino...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.67E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
B.R. Ambedkar Bihar University | Assay Description The docking and scoring of ligands with CAMKIV protein was accomplished using ParDOCK module of Sanjeevini drug design suite, which is based on physi... | Chem Biol Drug Des 89: 741-754 (2017) Article DOI: 10.1111/cbdd.12898 BindingDB Entry DOI: 10.7270/Q28C9V30 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM50062351 ((R)-N*4*-Hydroxy-N*1*-[(S)-2-(1H-indol-3-yl)-1-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 0.177 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human MMP-9 by fluorometric assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01215 BindingDB Entry DOI: 10.7270/Q2Q81HPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50062351 ((R)-N*4*-Hydroxy-N*1*-[(S)-2-(1H-indol-3-yl)-1-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 0.422 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human MMP-2 by fluorometric assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01215 BindingDB Entry DOI: 10.7270/Q2Q81HPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Pseudomonas aeruginosa) | BDBM50549085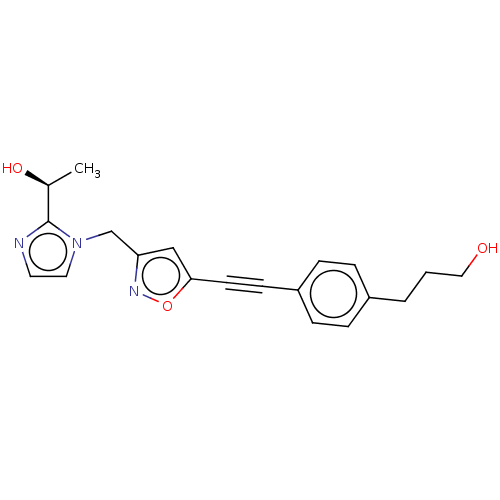 (CHEMBL4752810) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of histidine-tagged Pseudomonas aeruginosa LpxC (1-303) expressed in Escherichia coli BL21 (DE3) using UDP-3-O-(R-3-hydroxydecanoyl)-N-ace... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01215 BindingDB Entry DOI: 10.7270/Q2Q81HPF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Pseudomonas aeruginosa) | BDBM50549082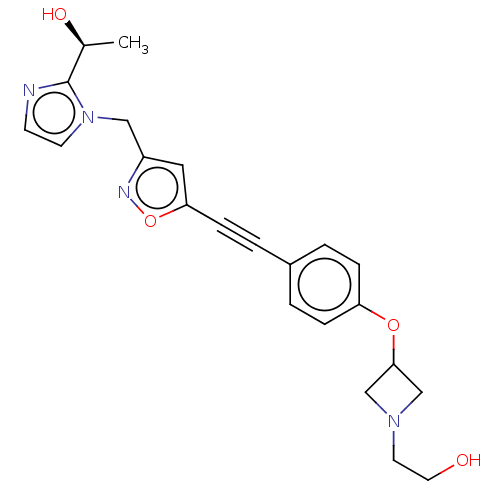 (CHEMBL4763699) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of histidine-tagged Pseudomonas aeruginosa LpxC (1-303) expressed in Escherichia coli BL21 (DE3) using UDP-3-O-(R-3-hydroxydecanoyl)-N-ace... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01215 BindingDB Entry DOI: 10.7270/Q2Q81HPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Rattus norvegicus (rat)) | BDBM15581 (CHEMBL8706 | CLG | CLORGILINE | Clorgyline | N-[3-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Government College University Curated by ChEMBL | Assay Description Inhibition of MAO-A in rat liver mitochondria using p-tyramine as substrate preincubated for 10 mins followed by substrate addition measured after 30... | Eur J Med Chem 143: 1373-1386 (2018) Article DOI: 10.1016/j.ejmech.2017.10.036 BindingDB Entry DOI: 10.7270/Q21V5HHC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Pseudomonas aeruginosa) | BDBM50549081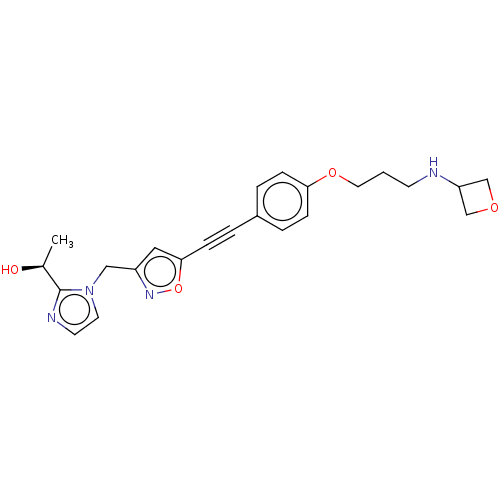 (CHEMBL4779477) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of histidine-tagged Pseudomonas aeruginosa LpxC (1-303) expressed in Escherichia coli BL21 (DE3) using UDP-3-O-(R-3-hydroxydecanoyl)-N-ace... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01215 BindingDB Entry DOI: 10.7270/Q2Q81HPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Pseudomonas aeruginosa) | BDBM50549083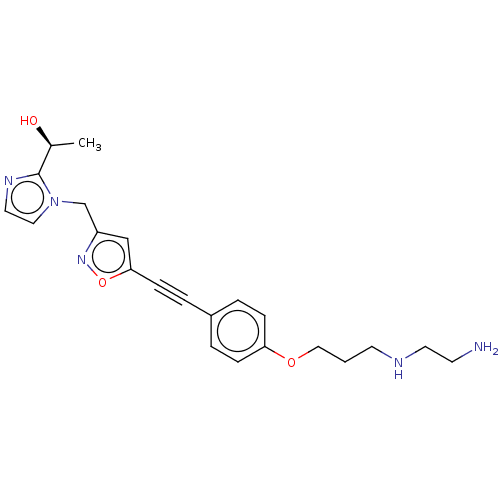 (CHEMBL4798463) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of histidine-tagged Pseudomonas aeruginosa LpxC (1-303) expressed in Escherichia coli BL21 (DE3) using UDP-3-O-(R-3-hydroxydecanoyl)-N-ace... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01215 BindingDB Entry DOI: 10.7270/Q2Q81HPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Pseudomonas aeruginosa) | BDBM50549086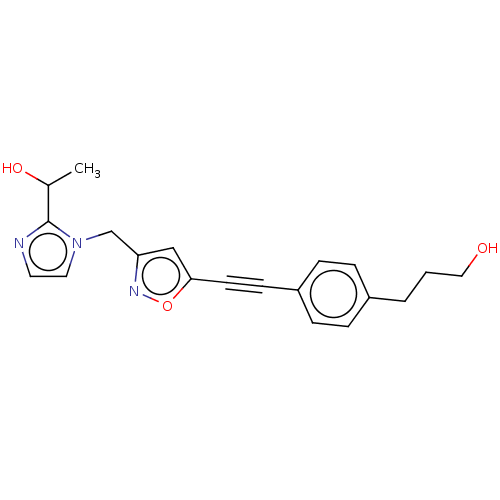 (CHEMBL4795002) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of histidine-tagged Pseudomonas aeruginosa LpxC (1-303) expressed in Escherichia coli BL21 (DE3) using UDP-3-O-(R-3-hydroxydecanoyl)-N-ace... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01215 BindingDB Entry DOI: 10.7270/Q2Q81HPF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Matrilysin (Homo sapiens (Human)) | BDBM50589859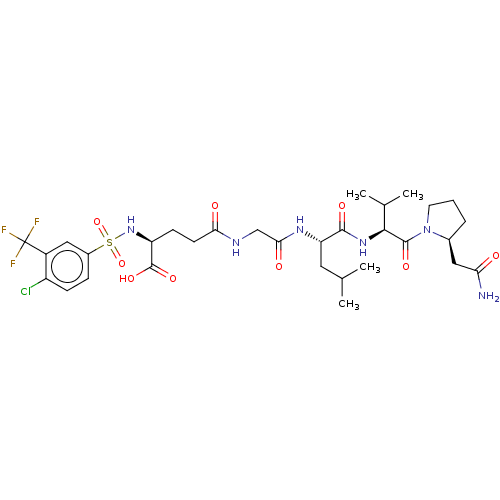 (CHEMBL5204457) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c01088 BindingDB Entry DOI: 10.7270/Q2542SJ0 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50062351 ((R)-N*4*-Hydroxy-N*1*-[(S)-2-(1H-indol-3-yl)-1-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human MMP-3 by fluorometric assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01215 BindingDB Entry DOI: 10.7270/Q2Q81HPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrilysin (Homo sapiens (Human)) | BDBM50589860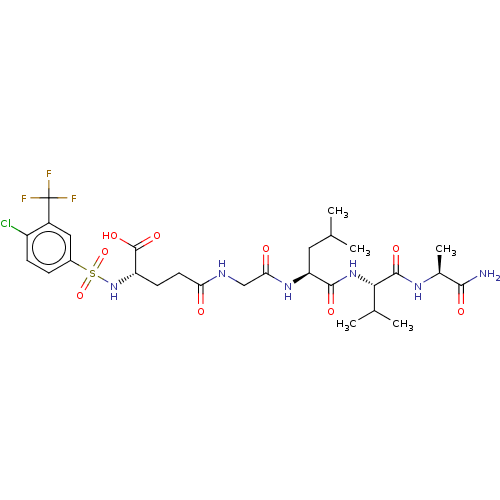 (CHEMBL5183142) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c01088 BindingDB Entry DOI: 10.7270/Q2542SJ0 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50478376 (BB-78485 | CHEMBL261713) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human MMP-2 by fluorometric assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01215 BindingDB Entry DOI: 10.7270/Q2Q81HPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrilysin (Homo sapiens (Human)) | BDBM50589865 (CHEMBL5169356) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c01088 BindingDB Entry DOI: 10.7270/Q2542SJ0 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Pseudomonas aeruginosa) | BDBM50549079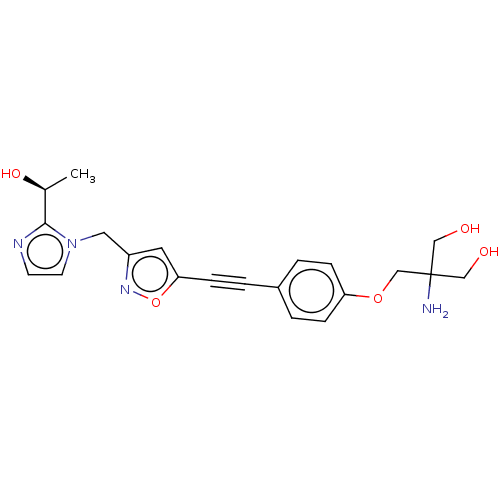 (CHEMBL4747965) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of histidine-tagged Pseudomonas aeruginosa LpxC (1-303) expressed in Escherichia coli BL21 (DE3) using UDP-3-O-(R-3-hydroxydecanoyl)-N-ace... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01215 BindingDB Entry DOI: 10.7270/Q2Q81HPF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Amine oxidase [flavin-containing] B (Rattus norvegicus (rat)) | BDBM39862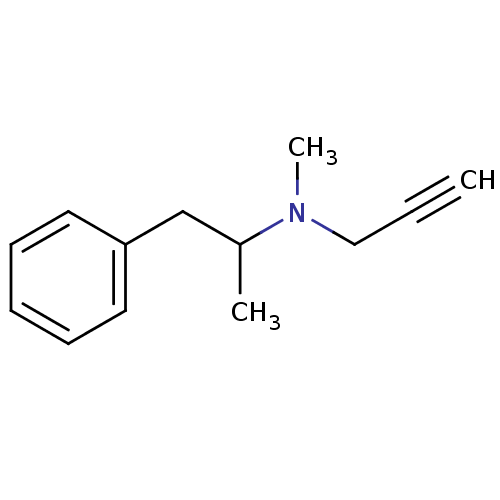 (Deprenyl | METHYL-(1-METHYL-2-PHENYL-ETHYL)-PROP-2...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Government College University Curated by ChEMBL | Assay Description Inhibition of MAO-B in rat liver mitochondria using p-tyramine as substrate preincubated for 10 mins followed by substrate addition measured after 30... | Eur J Med Chem 143: 1373-1386 (2018) Article DOI: 10.1016/j.ejmech.2017.10.036 BindingDB Entry DOI: 10.7270/Q21V5HHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Pseudomonas aeruginosa) | BDBM50549087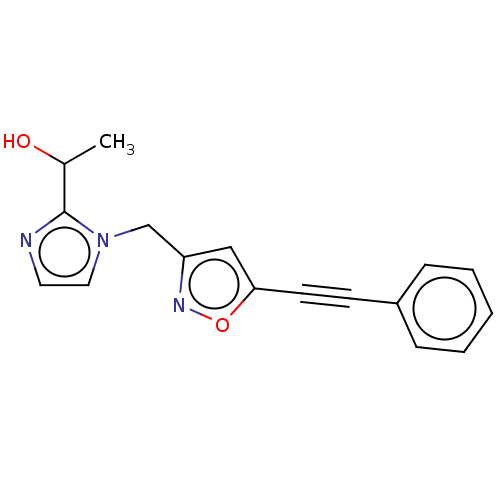 (CHEMBL4750316) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of (S)-N-(2-(N-(3-biphenyl-4-ylcarboxamido-4-(hydroxyamino)-4-oxobutyl)sulfamoyl)ethyl)-3',6'-dihydroxy-3-oxo-3H-spiro[isobenzofuran-1,9... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01215 BindingDB Entry DOI: 10.7270/Q2Q81HPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Pseudomonas aeruginosa) | BDBM50549102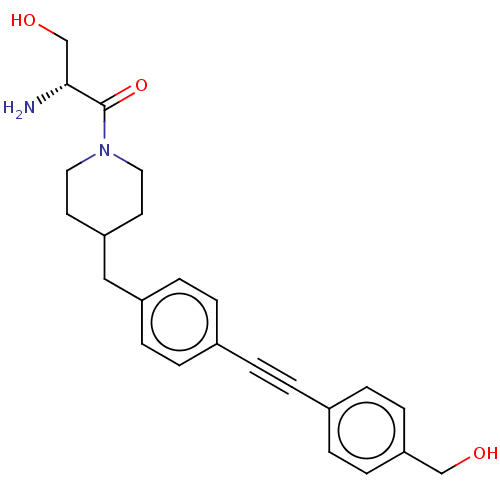 (CHEMBL4743193) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of histidine-tagged Pseudomonas aeruginosa LpxC (1-303) expressed in Escherichia coli BL21 (DE3) using UDP-3-O-(R-3-hydroxydecanoyl)-N-ace... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01215 BindingDB Entry DOI: 10.7270/Q2Q81HPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Pseudomonas aeruginosa) | BDBM50549092 (CHEMBL4793599) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of (S)-N-(2-(N-(3-biphenyl-4-ylcarboxamido-4-(hydroxyamino)-4-oxobutyl)sulfamoyl)ethyl)-3',6'-dihydroxy-3-oxo-3H-spiro[isobenzofuran-1,9... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01215 BindingDB Entry DOI: 10.7270/Q2Q81HPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Pseudomonas aeruginosa) | BDBM50549103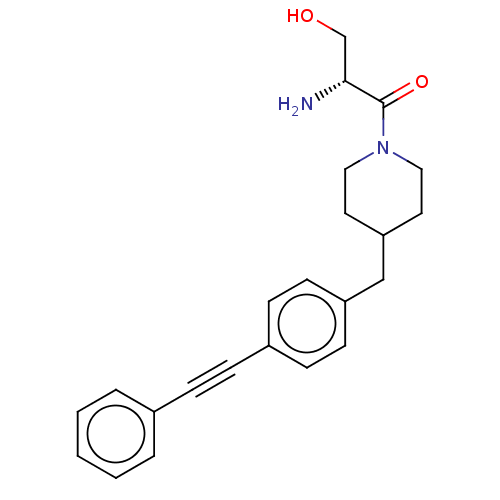 (CHEMBL4786856) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of histidine-tagged Pseudomonas aeruginosa LpxC (1-303) expressed in Escherichia coli BL21 (DE3) using UDP-3-O-(R-3-hydroxydecanoyl)-N-ace... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01215 BindingDB Entry DOI: 10.7270/Q2Q81HPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Pseudomonas aeruginosa) | BDBM50549087 (CHEMBL4750316) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of histidine-tagged Pseudomonas aeruginosa LpxC (1-303) expressed in Escherichia coli BL21 (DE3) using UDP-3-O-(R-3-hydroxydecanoyl)-N-ace... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01215 BindingDB Entry DOI: 10.7270/Q2Q81HPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Pseudomonas aeruginosa) | BDBM50549089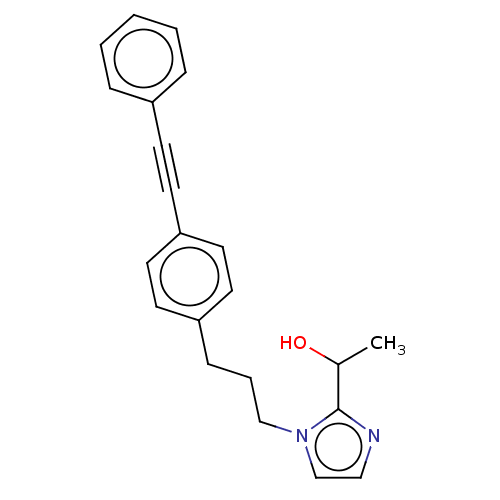 (CHEMBL4794374) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of (S)-N-(2-(N-(3-biphenyl-4-ylcarboxamido-4-(hydroxyamino)-4-oxobutyl)sulfamoyl)ethyl)-3',6'-dihydroxy-3-oxo-3H-spiro[isobenzofuran-1,9... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01215 BindingDB Entry DOI: 10.7270/Q2Q81HPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrilysin (Homo sapiens (Human)) | BDBM50589866 (CHEMBL5192674) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c01088 BindingDB Entry DOI: 10.7270/Q2542SJ0 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Pseudomonas aeruginosa) | BDBM50549091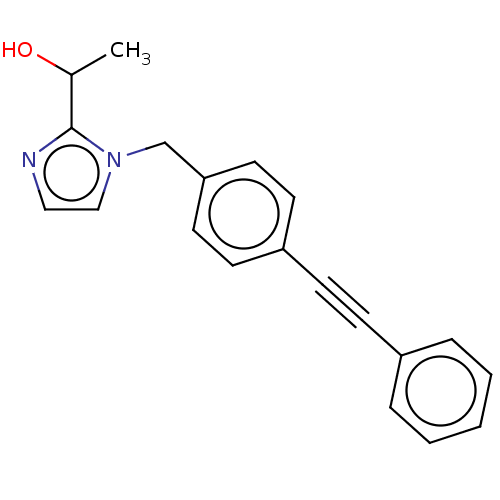 (CHEMBL4791421) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 93 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of (S)-N-(2-(N-(3-biphenyl-4-ylcarboxamido-4-(hydroxyamino)-4-oxobutyl)sulfamoyl)ethyl)-3',6'-dihydroxy-3-oxo-3H-spiro[isobenzofuran-1,9... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01215 BindingDB Entry DOI: 10.7270/Q2Q81HPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM50478376 (BB-78485 | CHEMBL261713) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 97 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human MMP-9 by fluorometric assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01215 BindingDB Entry DOI: 10.7270/Q2Q81HPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrilysin (Homo sapiens (Human)) | BDBM50589869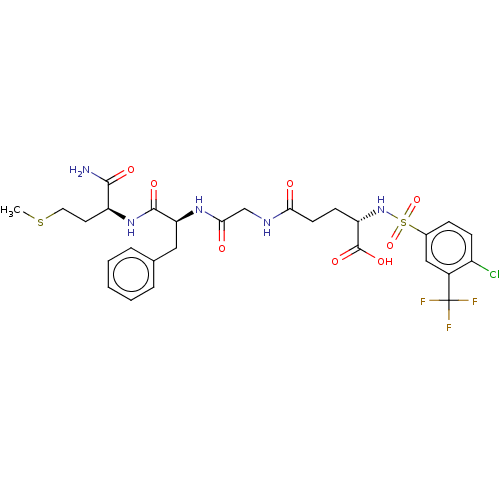 (CHEMBL5170327) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c01088 BindingDB Entry DOI: 10.7270/Q2542SJ0 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Rattus norvegicus (rat)) | BDBM50283860 (CHEMBL4169543) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Government College University Curated by ChEMBL | Assay Description Inhibition of MAO-A in rat liver mitochondria using p-tyramine as substrate preincubated for 10 mins followed by substrate addition measured after 30... | Eur J Med Chem 143: 1373-1386 (2018) Article DOI: 10.1016/j.ejmech.2017.10.036 BindingDB Entry DOI: 10.7270/Q21V5HHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Rattus norvegicus (rat)) | BDBM50283916 (CHEMBL1782842) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Government College University Curated by ChEMBL | Assay Description Inhibition of MAO-A in rat liver mitochondria using p-tyramine as substrate preincubated for 10 mins followed by substrate addition measured after 30... | Eur J Med Chem 143: 1373-1386 (2018) Article DOI: 10.1016/j.ejmech.2017.10.036 BindingDB Entry DOI: 10.7270/Q21V5HHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrilysin (Homo sapiens (Human)) | BDBM50589870 (CHEMBL5202455) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c01088 BindingDB Entry DOI: 10.7270/Q2542SJ0 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Pseudomonas aeruginosa) | BDBM50549088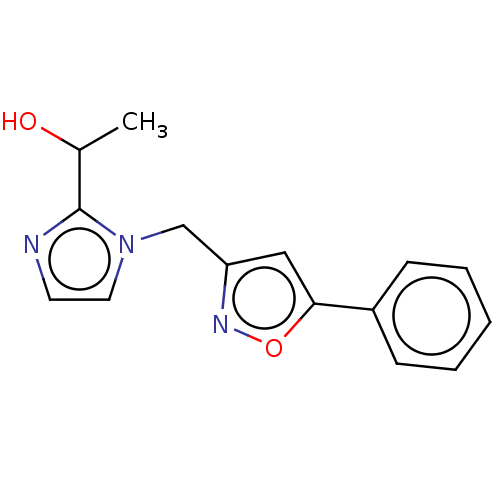 (CHEMBL4795750) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 125 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of (S)-N-(2-(N-(3-biphenyl-4-ylcarboxamido-4-(hydroxyamino)-4-oxobutyl)sulfamoyl)ethyl)-3',6'-dihydroxy-3-oxo-3H-spiro[isobenzofuran-1,9... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01215 BindingDB Entry DOI: 10.7270/Q2Q81HPF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Pseudomonas aeruginosa) | BDBM50549092 (CHEMBL4793599) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 133 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of histidine-tagged Pseudomonas aeruginosa LpxC (1-303) expressed in Escherichia coli BL21 (DE3) using UDP-3-O-(R-3-hydroxydecanoyl)-N-ace... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01215 BindingDB Entry DOI: 10.7270/Q2Q81HPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Pseudomonas aeruginosa) | BDBM50549089 (CHEMBL4794374) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 161 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of histidine-tagged Pseudomonas aeruginosa LpxC (1-303) expressed in Escherichia coli BL21 (DE3) using UDP-3-O-(R-3-hydroxydecanoyl)-N-ace... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01215 BindingDB Entry DOI: 10.7270/Q2Q81HPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Rattus norvegicus (rat)) | BDBM50283866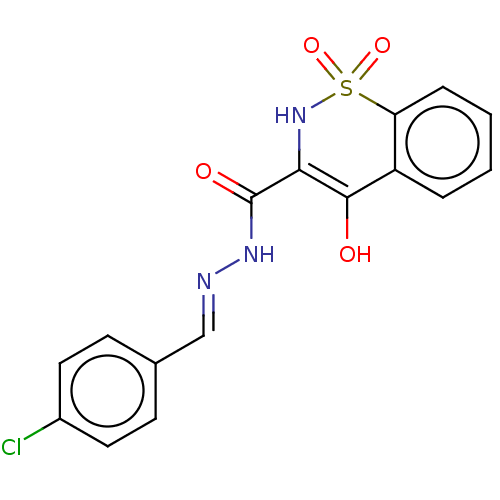 (CHEMBL475017) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 177 | n/a | n/a | n/a | n/a | n/a | n/a |
Government College University Curated by ChEMBL | Assay Description Inhibition of MAO-A in rat liver mitochondria using p-tyramine as substrate preincubated for 10 mins followed by substrate addition measured after 30... | Eur J Med Chem 143: 1373-1386 (2018) Article DOI: 10.1016/j.ejmech.2017.10.036 BindingDB Entry DOI: 10.7270/Q21V5HHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Rattus norvegicus (rat)) | BDBM50283933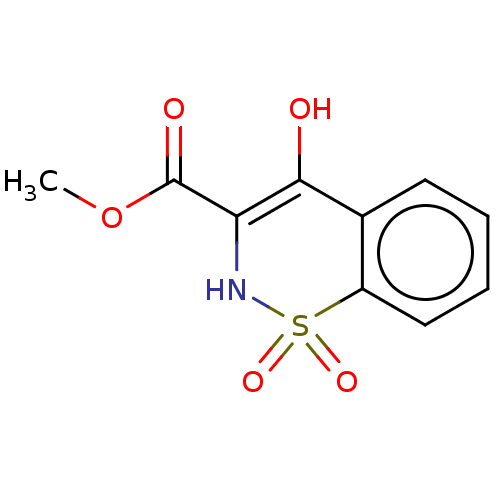 (CHEMBL4163328) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Government College University Curated by ChEMBL | Assay Description Inhibition of MAO-B in rat liver mitochondria using p-tyramine as substrate preincubated for 10 mins followed by substrate addition measured after 30... | Eur J Med Chem 143: 1373-1386 (2018) Article DOI: 10.1016/j.ejmech.2017.10.036 BindingDB Entry DOI: 10.7270/Q21V5HHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Rattus norvegicus (rat)) | BDBM50283915 (CHEMBL4167042) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
Government College University Curated by ChEMBL | Assay Description Inhibition of MAO-A in rat liver mitochondria using p-tyramine as substrate preincubated for 10 mins followed by substrate addition measured after 30... | Eur J Med Chem 143: 1373-1386 (2018) Article DOI: 10.1016/j.ejmech.2017.10.036 BindingDB Entry DOI: 10.7270/Q21V5HHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Rattus norvegicus (rat)) | BDBM50283855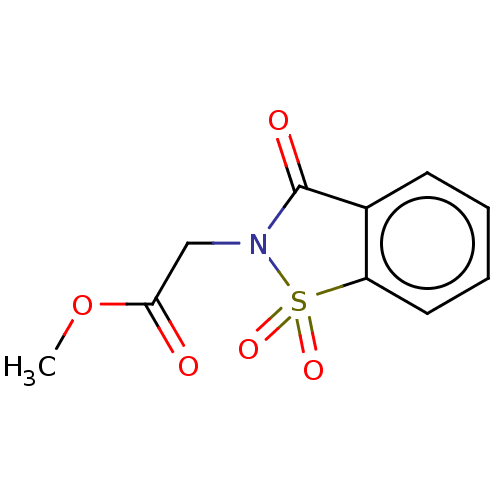 (CHEMBL4167829) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Government College University Curated by ChEMBL | Assay Description Inhibition of MAO-B in rat liver mitochondria using p-tyramine as substrate preincubated for 10 mins followed by substrate addition measured after 30... | Eur J Med Chem 143: 1373-1386 (2018) Article DOI: 10.1016/j.ejmech.2017.10.036 BindingDB Entry DOI: 10.7270/Q21V5HHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Rattus norvegicus (rat)) | BDBM50283851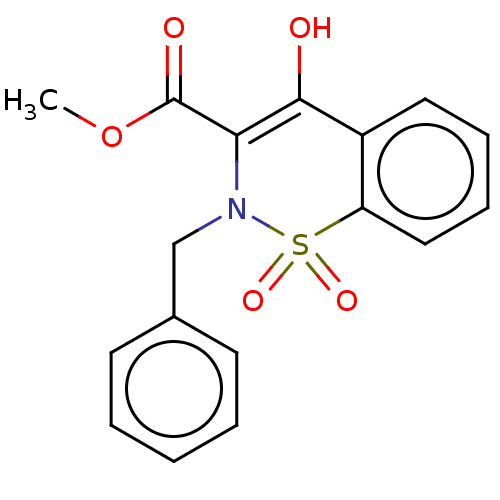 (CHEMBL4167325) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
Government College University Curated by ChEMBL | Assay Description Inhibition of MAO-B in rat liver mitochondria using p-tyramine as substrate preincubated for 10 mins followed by substrate addition measured after 30... | Eur J Med Chem 143: 1373-1386 (2018) Article DOI: 10.1016/j.ejmech.2017.10.036 BindingDB Entry DOI: 10.7270/Q21V5HHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Rattus norvegicus (rat)) | BDBM50283915 (CHEMBL4167042) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
Government College University Curated by ChEMBL | Assay Description Inhibition of MAO-B in rat liver mitochondria using p-tyramine as substrate preincubated for 10 mins followed by substrate addition measured after 30... | Eur J Med Chem 143: 1373-1386 (2018) Article DOI: 10.1016/j.ejmech.2017.10.036 BindingDB Entry DOI: 10.7270/Q21V5HHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Rattus norvegicus (rat)) | BDBM50283866 (CHEMBL475017) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
Government College University Curated by ChEMBL | Assay Description Inhibition of MAO-B in rat liver mitochondria using p-tyramine as substrate preincubated for 10 mins followed by substrate addition measured after 30... | Eur J Med Chem 143: 1373-1386 (2018) Article DOI: 10.1016/j.ejmech.2017.10.036 BindingDB Entry DOI: 10.7270/Q21V5HHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Rattus norvegicus (rat)) | BDBM50283853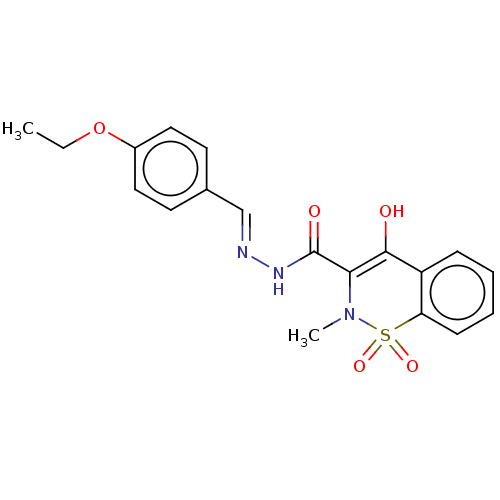 (CHEMBL4172849) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
Government College University Curated by ChEMBL | Assay Description Inhibition of MAO-A in rat liver mitochondria using p-tyramine as substrate preincubated for 10 mins followed by substrate addition measured after 30... | Eur J Med Chem 143: 1373-1386 (2018) Article DOI: 10.1016/j.ejmech.2017.10.036 BindingDB Entry DOI: 10.7270/Q21V5HHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Rattus norvegicus (rat)) | BDBM50283802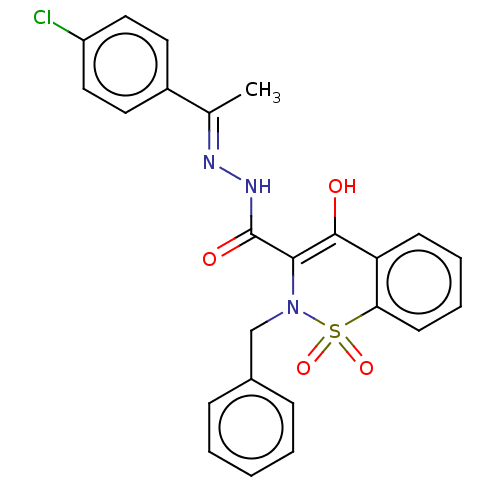 (CHEMBL4176805) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
Government College University Curated by ChEMBL | Assay Description Inhibition of MAO-A in rat liver mitochondria using p-tyramine as substrate preincubated for 10 mins followed by substrate addition measured after 30... | Eur J Med Chem 143: 1373-1386 (2018) Article DOI: 10.1016/j.ejmech.2017.10.036 BindingDB Entry DOI: 10.7270/Q21V5HHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Rattus norvegicus (rat)) | BDBM50283801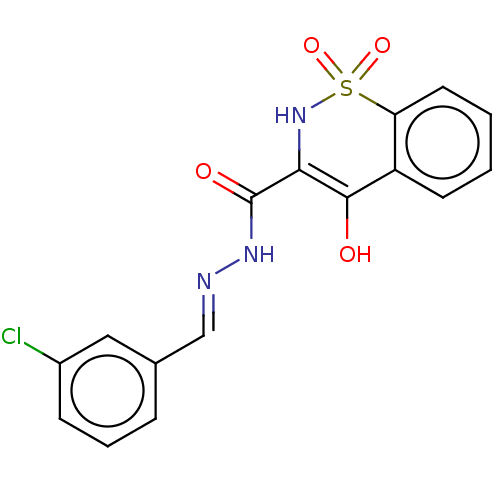 (CHEMBL475188) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
Government College University Curated by ChEMBL | Assay Description Inhibition of MAO-A in rat liver mitochondria using p-tyramine as substrate preincubated for 10 mins followed by substrate addition measured after 30... | Eur J Med Chem 143: 1373-1386 (2018) Article DOI: 10.1016/j.ejmech.2017.10.036 BindingDB Entry DOI: 10.7270/Q21V5HHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Rattus norvegicus (rat)) | BDBM50283934 (CHEMBL4165825) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
Government College University Curated by ChEMBL | Assay Description Inhibition of MAO-A in rat liver mitochondria using p-tyramine as substrate preincubated for 10 mins followed by substrate addition measured after 30... | Eur J Med Chem 143: 1373-1386 (2018) Article DOI: 10.1016/j.ejmech.2017.10.036 BindingDB Entry DOI: 10.7270/Q21V5HHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50478376 (BB-78485 | CHEMBL261713) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 416 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human MMP-3 by fluorometric assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01215 BindingDB Entry DOI: 10.7270/Q2Q81HPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 266 total ) | Next | Last >> |