

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM433523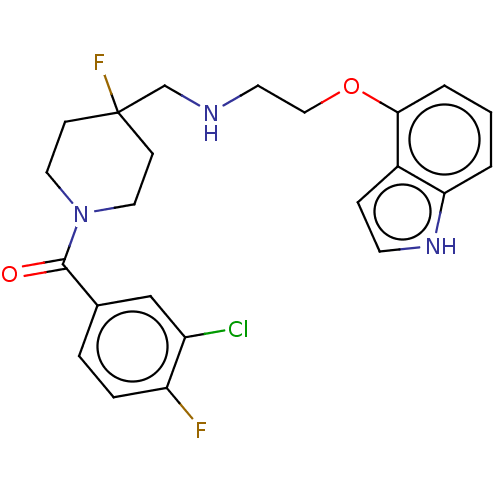 (US10562853, Compound 44) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.000158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]8-OH-DPAT from human 5-HT1A receptor stably expressed in CHO-K1 cell membranes measured after 60 mins by Microbeta2 scintillation... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00814 BindingDB Entry DOI: 10.7270/Q24T6NZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM433523 (US10562853, Compound 44) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.000158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]8-OH-DPAT from human 5-HT1A receptor stably expressed in CHO-K1 cell membranes measured after 60 mins by Microbeta2 scintillation... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00814 BindingDB Entry DOI: 10.7270/Q24T6NZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50469417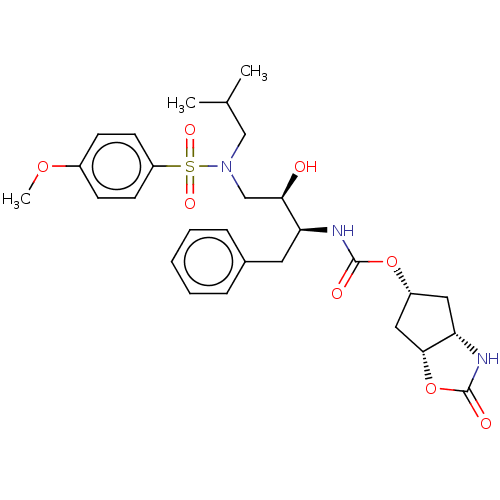 (CHEMBL4293023) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 0.00120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 wild type NL4-3 protease expressed in Escherichia coli Rosetta (DE3) pLysS using Abz-Thr-Ile-Nle-Phe-(pNO2)-Gln-Arg-NH2 as substra... | J Med Chem 61: 9722-9737 (2018) Article DOI: 10.1021/acs.jmedchem.8b01227 BindingDB Entry DOI: 10.7270/Q25T3P6J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50004178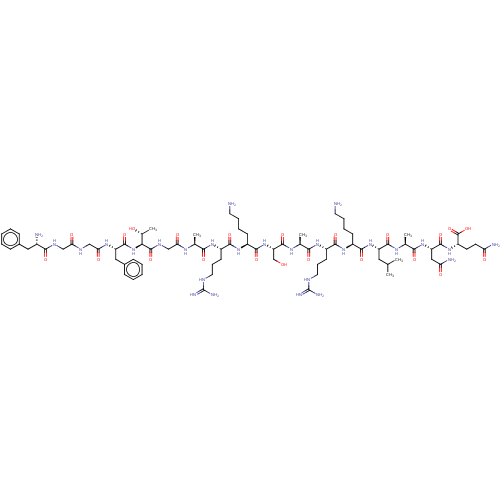 (Nociceptin | Nociceptin/orphanin FQ | ORPHANIN FQ) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.00130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]nociceptin from human recombinant NOP receptor expressed in HEK293 cells by scintillation counting analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00256 BindingDB Entry DOI: 10.7270/Q2VX0MDH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-3 (Homo sapiens (Human)) | BDBM50160957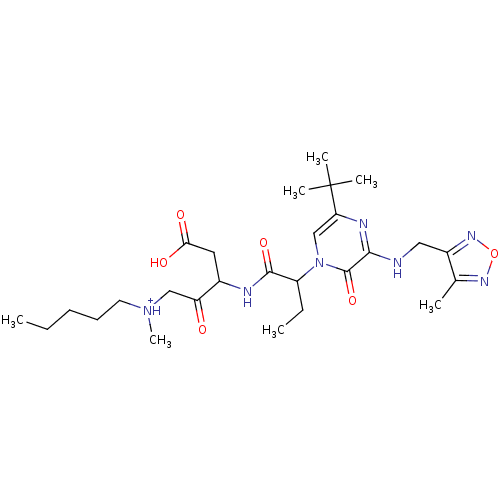 (CHEMBL179503 | [3-(2-{5-tert-Butyl-3-[(4-methyl-fu...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory concentration against recombinant human caspase-3 in neuronal precursor (NT2) cells | Bioorg Med Chem Lett 15: 1173-80 (2005) Article DOI: 10.1016/j.bmcl.2004.12.006 BindingDB Entry DOI: 10.7270/Q2D50MGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50498523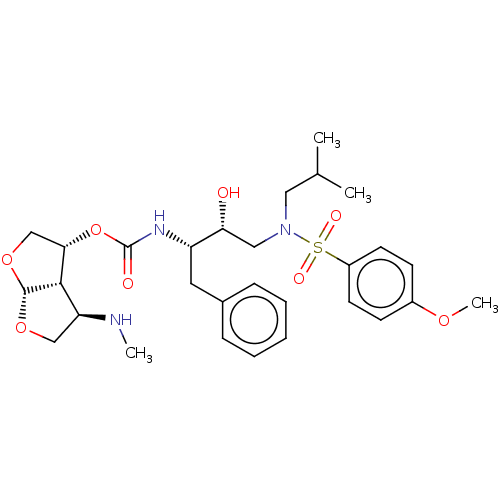 (CHEMBL3605643) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 0.00150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | J Med Chem 58: 6994-7006 (2015) Article DOI: 10.1021/acs.jmedchem.5b00900 BindingDB Entry DOI: 10.7270/Q2862KFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50484842 (CHEMBL1958482 | GRL-0249A) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 0.00180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease using hexapeptide Ac-Thr-Ile-Nle-Nle-Gln-Arg-NH2 as substrate by fluorimetric assay | Bioorg Med Chem Lett 22: 2308-11 (2012) Article DOI: 10.1016/j.bmcl.2012.01.061 BindingDB Entry DOI: 10.7270/Q2B85C0X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50457611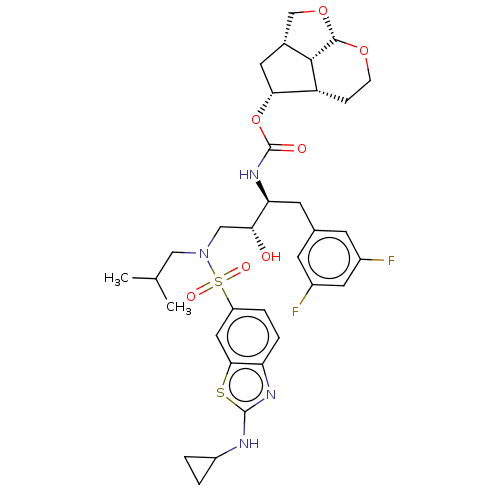 (CHEMBL4214453) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of wild type HIV-1 NL4-3 protease expressed in Escherichia coli Rosetta (DE3)pLysS using Ac-Thr-Ile-Nle-Nle-Gln-Arg-NH2 as substrate by fl... | J Med Chem 61: 4561-4577 (2018) Article DOI: 10.1021/acs.jmedchem.8b00298 BindingDB Entry DOI: 10.7270/Q2445Q35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Rattus norvegicus) | BDBM50470113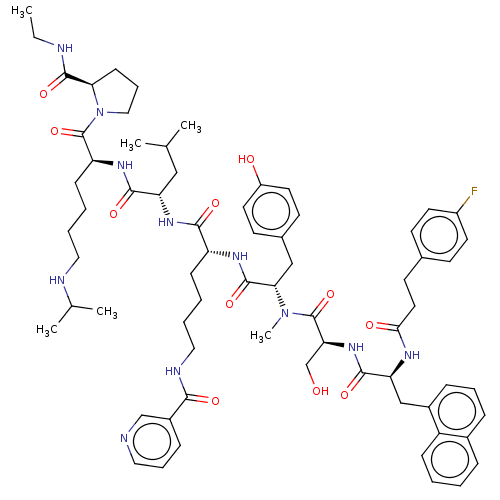 (CHEMBL439134) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TAP Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of the in vitro binding of [125I]-labeled leuprolide to the rat pituitary luteinizing hormone releasing hormone (LHRH) receptor. | J Med Chem 37: 701-5 (1994) Article DOI: 10.1021/jm00031a021 BindingDB Entry DOI: 10.7270/Q2MG7S7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50528152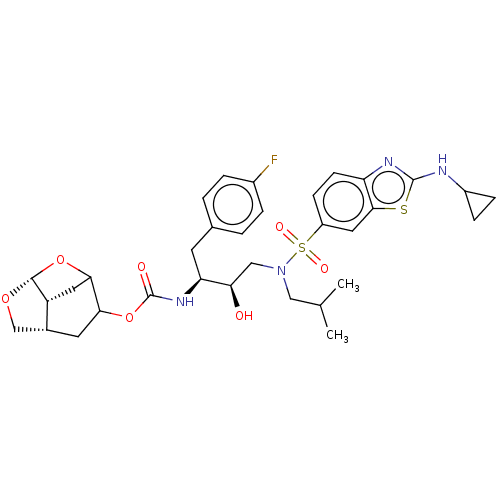 (CHEMBL4532946) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV-1 protease | J Med Chem 63: 4867-4879 (2020) Article DOI: 10.1021/acs.jmedchem.0c00202 BindingDB Entry DOI: 10.7270/Q20V8H82 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Rattus norvegicus) | BDBM50470113 (CHEMBL439134) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TAP Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of the in vitro binding of [125I]-labeled leuprolide to the rat pituitary luteinizing hormone releasing hormone (LHRH) receptor. | J Med Chem 37: 701-5 (1994) Article DOI: 10.1021/jm00031a021 BindingDB Entry DOI: 10.7270/Q2MG7S7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50528147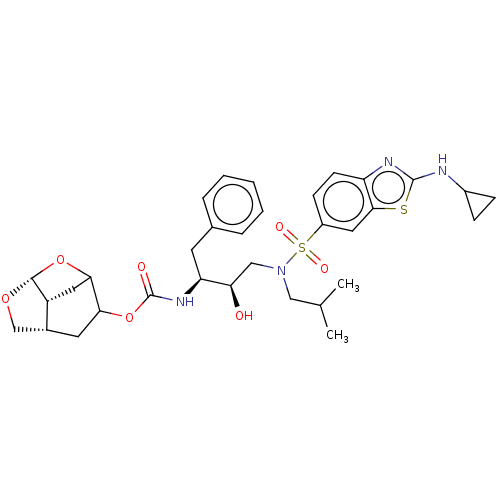 (CHEMBL4514504) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV-1 protease | J Med Chem 63: 4867-4879 (2020) Article DOI: 10.1021/acs.jmedchem.0c00202 BindingDB Entry DOI: 10.7270/Q20V8H82 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50498230 (CHEMBL3577576) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia State University Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 protease | J Med Chem 58: 5088-95 (2015) Article DOI: 10.1021/acs.jmedchem.5b00474 BindingDB Entry DOI: 10.7270/Q27D2Z42 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50498524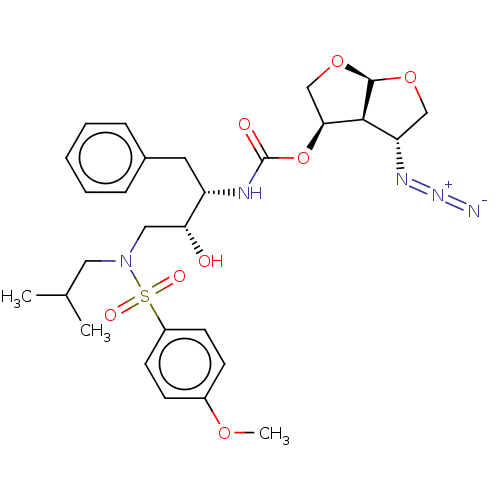 (CHEMBL3605638) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | J Med Chem 58: 6994-7006 (2015) Article DOI: 10.1021/acs.jmedchem.5b00900 BindingDB Entry DOI: 10.7270/Q2862KFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50484847 (CHEMBL1958483 | GRL-0289A) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease using hexapeptide Ac-Thr-Ile-Nle-Nle-Gln-Arg-NH2 as substrate by fluorimetric assay | Bioorg Med Chem Lett 22: 2308-11 (2012) Article DOI: 10.1016/j.bmcl.2012.01.061 BindingDB Entry DOI: 10.7270/Q2B85C0X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50457604 (CHEMBL4213229) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of wild type HIV-1 NL4-3 protease expressed in Escherichia coli Rosetta (DE3)pLysS using Ac-Thr-Ile-Nle-Nle-Gln-Arg-NH2 as substrate by fl... | J Med Chem 61: 4561-4577 (2018) Article DOI: 10.1021/acs.jmedchem.8b00298 BindingDB Entry DOI: 10.7270/Q2445Q35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50484190 (CHEMBL1817686) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of Human immunodeficiency virus 1 protease assessed as release of fluorescent N-terminal tripeptide using fluorogenic substrate by continu... | J Med Chem 54: 5890-901 (2011) Article DOI: 10.1021/jm200649p BindingDB Entry DOI: 10.7270/Q2DB84QW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-3 (Homo sapiens (Human)) | BDBM50160974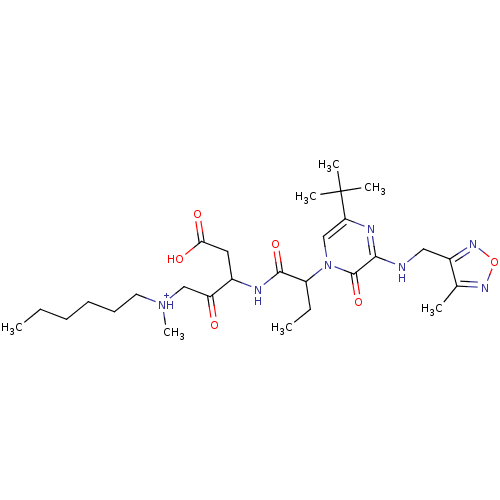 (CHEMBL366927 | [3-(2-{5-tert-Butyl-3-[(4-methyl-fu...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory concentration against recombinant human caspase-3 in neuronal precursor (NT2) cells | Bioorg Med Chem Lett 15: 1173-80 (2005) Article DOI: 10.1016/j.bmcl.2004.12.006 BindingDB Entry DOI: 10.7270/Q2D50MGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM8125 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia State University Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 protease | J Med Chem 58: 5088-95 (2015) Article DOI: 10.1021/acs.jmedchem.5b00474 BindingDB Entry DOI: 10.7270/Q27D2Z42 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM194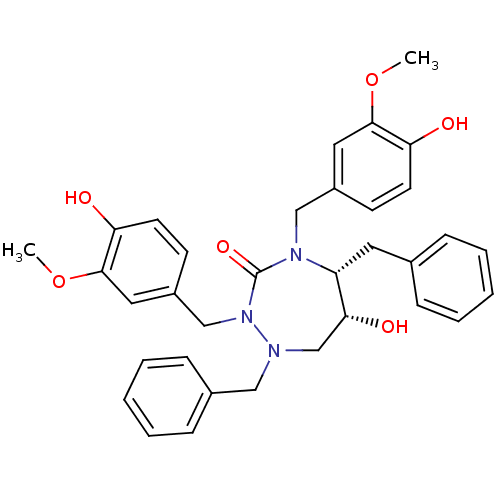 ((5R,6R)-1,5-dibenzyl-6-hydroxy-2,4-bis[(4-hydroxy-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.00500 | -65.6 | n/a | n/a | n/a | n/a | n/a | 4.7 | 30 |
Abbott Laboratories | Assay Description HIV-1 protease activity was measured by a continuous fluorometric assay using the internally quenched fluorogenic substrate DABCYL-GABA-Ser-Gln-Tyr-P... | J Med Chem 39: 392-7 (1996) Article DOI: 10.1021/jm9507183 BindingDB Entry DOI: 10.7270/Q2V40SC6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 5'-nucleotidase (Homo sapiens (Human)) | BDBM50527134 (CHEMBL4471306 | US20230295213, Compound a) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arcus Biosciences, Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant human C-terminal His-tagged CD73 (27 to 549 residues) expressed in HEK293 cells using AMP as substrate preincubated for 1 h... | J Med Chem 63: 3935-3955 (2020) Article DOI: 10.1021/acs.jmedchem.9b01713 BindingDB Entry DOI: 10.7270/Q2G1648T | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50489369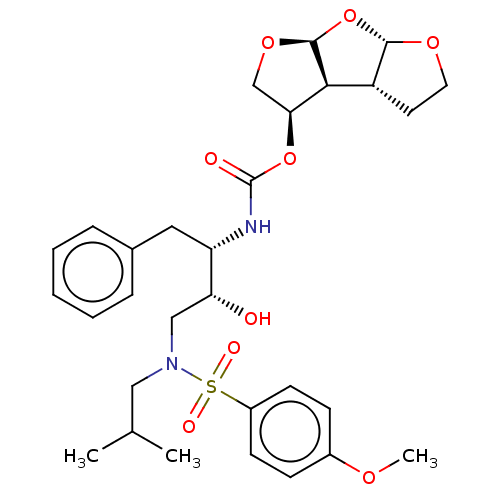 (CHEMBL1232930 | GRL-0519) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | J Med Chem 56: 6792-802 (2013) Article DOI: 10.1021/jm400768f BindingDB Entry DOI: 10.7270/Q2K07764 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50528144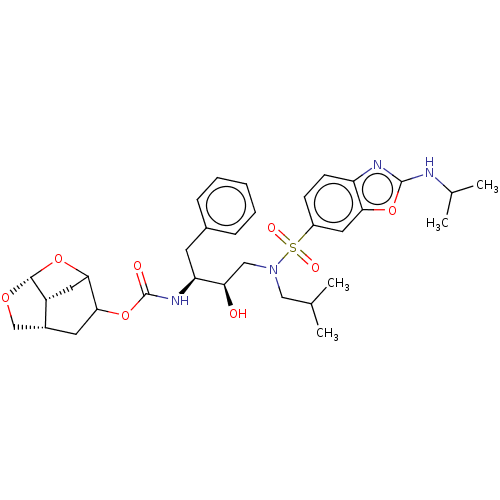 (CHEMBL4435411) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV-1 protease | J Med Chem 63: 4867-4879 (2020) Article DOI: 10.1021/acs.jmedchem.0c00202 BindingDB Entry DOI: 10.7270/Q20V8H82 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50498229 (CHEMBL3577575) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia State University Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 protease | J Med Chem 58: 5088-95 (2015) Article DOI: 10.1021/acs.jmedchem.5b00474 BindingDB Entry DOI: 10.7270/Q27D2Z42 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocyte-stimulating hormone receptor (Homo sapiens (Human)) | BDBM50131251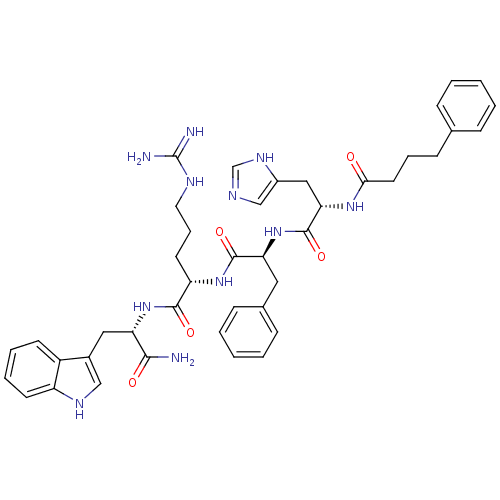 ((S)-5-Guanidino-2-{(S)-2-[(S)-3-(3H-imidazol-4-yl)...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cincinnati Curated by ChEMBL | Assay Description Binding affinity towards human Melanocortin 1 receptor (hMC1R) | Bioorg Med Chem Lett 13: 2647-50 (2003) BindingDB Entry DOI: 10.7270/Q2474BD9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50484193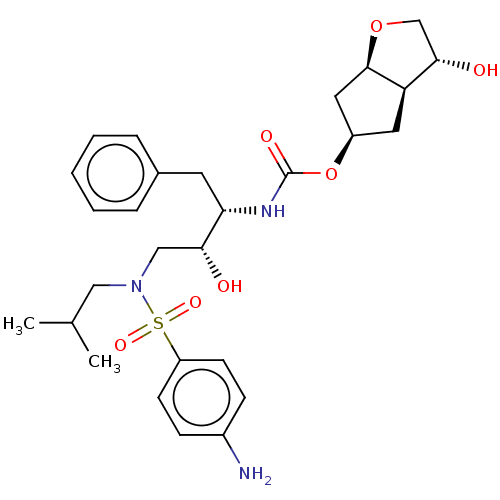 (CHEMBL1819294) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of Human immunodeficiency virus 1 protease assessed as release of fluorescent N-terminal tripeptide using fluorogenic substrate by continu... | J Med Chem 54: 5890-901 (2011) Article DOI: 10.1021/jm200649p BindingDB Entry DOI: 10.7270/Q2DB84QW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50484191 (CHEMBL1819295) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of Human immunodeficiency virus 1 protease assessed as release of fluorescent N-terminal tripeptide using fluorogenic substrate by continu... | J Med Chem 54: 5890-901 (2011) Article DOI: 10.1021/jm200649p BindingDB Entry DOI: 10.7270/Q2DB84QW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50498534 (CHEMBL3605635) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | J Med Chem 58: 6994-7006 (2015) Article DOI: 10.1021/acs.jmedchem.5b00900 BindingDB Entry DOI: 10.7270/Q2862KFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50528145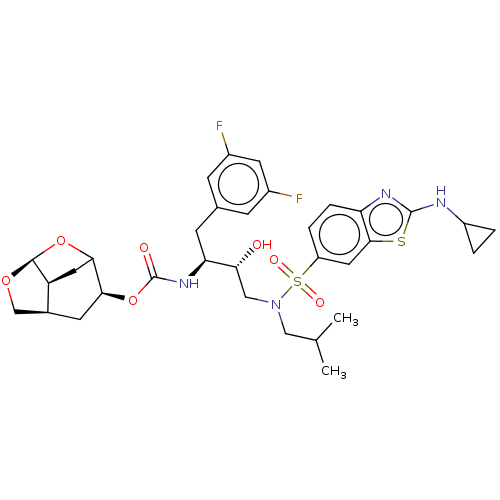 (CHEMBL4586218) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV-1 protease | J Med Chem 63: 4867-4879 (2020) Article DOI: 10.1021/acs.jmedchem.0c00202 BindingDB Entry DOI: 10.7270/Q20V8H82 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50484845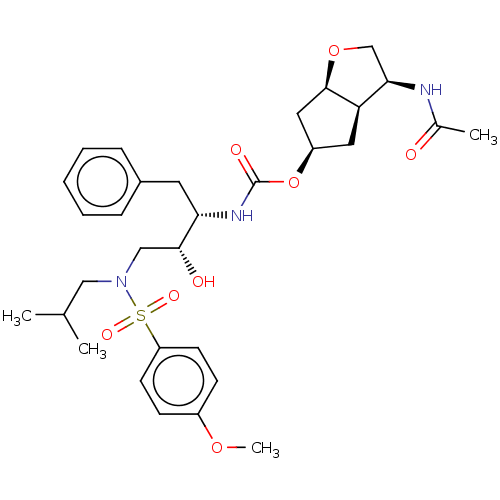 (CHEMBL1958480) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease using hexapeptide Ac-Thr-Ile-Nle-Nle-Gln-Arg-NH2 as substrate by fluorimetric assay | Bioorg Med Chem Lett 22: 2308-11 (2012) Article DOI: 10.1016/j.bmcl.2012.01.061 BindingDB Entry DOI: 10.7270/Q2B85C0X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50484841 (CHEMBL1958481) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00750 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease using hexapeptide Ac-Thr-Ile-Nle-Nle-Gln-Arg-NH2 as substrate by fluorimetric assay | Bioorg Med Chem Lett 22: 2308-11 (2012) Article DOI: 10.1016/j.bmcl.2012.01.061 BindingDB Entry DOI: 10.7270/Q2B85C0X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1A (Homo sapiens (Human)) | BDBM50093262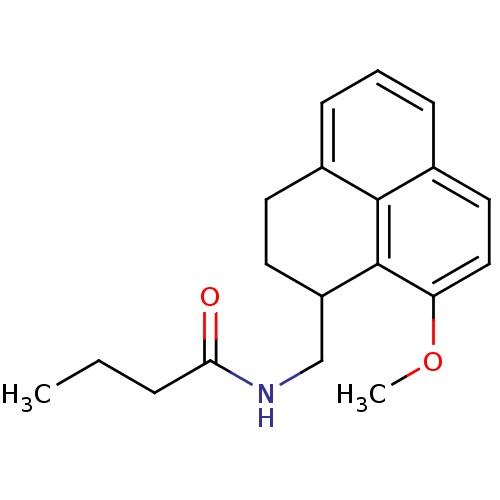 (CHEMBL131252 | N-(9-Methoxy-2,3-dihydro-1H-phenale...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Paris-Sud Curated by ChEMBL | Assay Description Binding affinity for human melatonin receptor type 1A, expressed in HEK-293 cells (2-[125I]-Iodomelatonin is used as radioligand) | J Med Chem 43: 4051-62 (2000) BindingDB Entry DOI: 10.7270/Q20Z72JX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50528150 (CHEMBL4449179) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV-1 protease | J Med Chem 63: 4867-4879 (2020) Article DOI: 10.1021/acs.jmedchem.0c00202 BindingDB Entry DOI: 10.7270/Q20V8H82 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50528154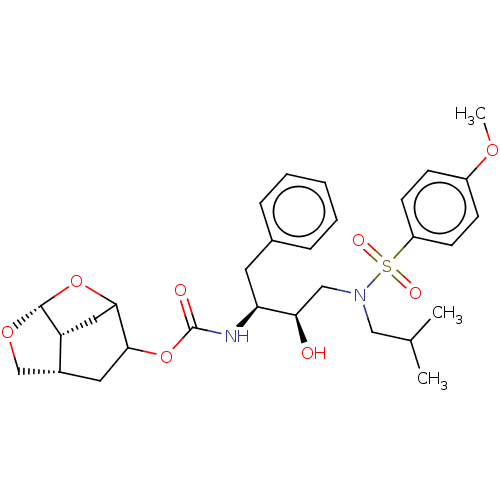 (CHEMBL4545005) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV-1 protease | J Med Chem 63: 4867-4879 (2020) Article DOI: 10.1021/acs.jmedchem.0c00202 BindingDB Entry DOI: 10.7270/Q20V8H82 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50520752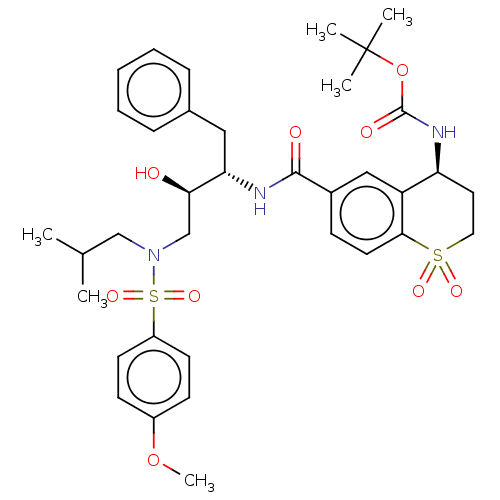 (CHEMBL4474261) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease using fluorogenic substrate by continuous fluorometric assay | Eur J Med Chem 160: 171-182 (2018) Article DOI: 10.1016/j.ejmech.2018.09.046 BindingDB Entry DOI: 10.7270/Q2320098 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50457612 (CHEMBL4218164) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of wild type HIV-1 NL4-3 protease expressed in Escherichia coli Rosetta (DE3)pLysS using Ac-Thr-Ile-Nle-Nle-Gln-Arg-NH2 as substrate by fl... | J Med Chem 61: 4561-4577 (2018) Article DOI: 10.1021/acs.jmedchem.8b00298 BindingDB Entry DOI: 10.7270/Q2445Q35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM433537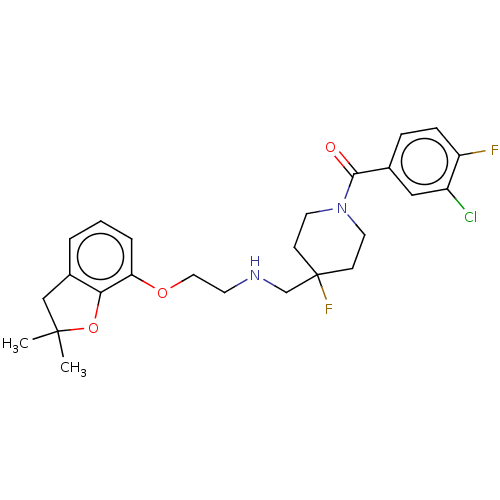 (US10562853, Compound 60) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00851 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]8-OH-DPAT from human 5-HT1A receptor stably expressed in CHO-K1 cell membranes measured after 60 mins by Microbeta2 scintillation... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00814 BindingDB Entry DOI: 10.7270/Q24T6NZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM433537 (US10562853, Compound 60) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00851 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]8-OH-DPAT from human 5-HT1A receptor stably expressed in CHO-K1 cell membranes measured after 60 mins by Microbeta2 scintillation... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00814 BindingDB Entry DOI: 10.7270/Q24T6NZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50498528 (CHEMBL3605644) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 0.00990 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | J Med Chem 58: 6994-7006 (2015) Article DOI: 10.1021/acs.jmedchem.5b00900 BindingDB Entry DOI: 10.7270/Q2862KFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Rattus norvegicus) | BDBM50470098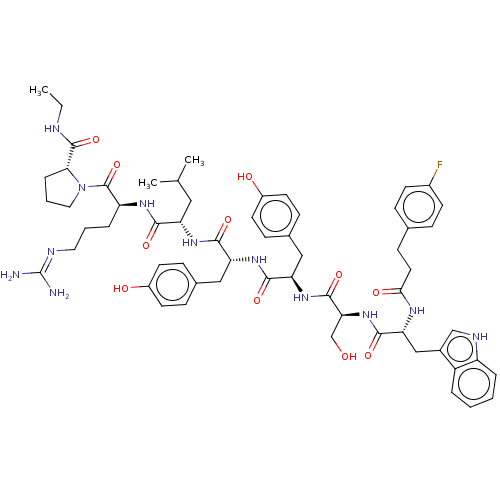 (CHEMBL2371296) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TAP Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of the in vitro binding of [125I]-labeled leuprolide to the rat pituitary luteinizing hormone releasing hormone (LHRH) receptor. | J Med Chem 37: 701-5 (1994) Article DOI: 10.1021/jm00031a021 BindingDB Entry DOI: 10.7270/Q2MG7S7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM433537 (US10562853, Compound 60) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NEUROLIXIS; UNIVERSITE JAGELLONE US Patent | Assay Description 5-HT1A: Radioligand binding was performed using membranes from CHO-K1 cells stably transfected with the human 5-HT1A receptor. All assays were carrie... | US Patent US10562853 (2020) BindingDB Entry DOI: 10.7270/Q29Z979D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide FF receptor 1 (Homo sapiens (Human)) | BDBM50029188 (CHEMBL2165920) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [125I]-1DMeNPFF from recombinant human NPFF1 receptor expressed in CHO cells by TopCount scintillation counting method | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00256 BindingDB Entry DOI: 10.7270/Q2VX0MDH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progonadoliberin-1 (RAT) | BDBM84726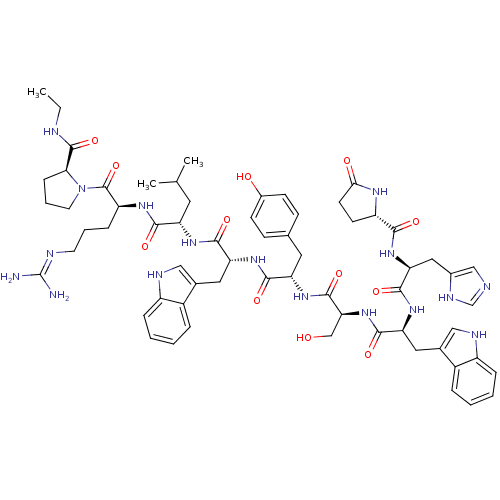 (deslorelin) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by PDSP Ki Database | J Med Chem 36: 363-9 (1993) Article DOI: 10.1021/jm00055a007 BindingDB Entry DOI: 10.7270/Q2GQ6W8T | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progonadoliberin-1 (RAT) | BDBM84707 (nafarelin) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by PDSP Ki Database | J Med Chem 36: 363-9 (1993) Article DOI: 10.1021/jm00055a007 BindingDB Entry DOI: 10.7270/Q2GQ6W8T | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1A (Homo sapiens (Human)) | BDBM50093275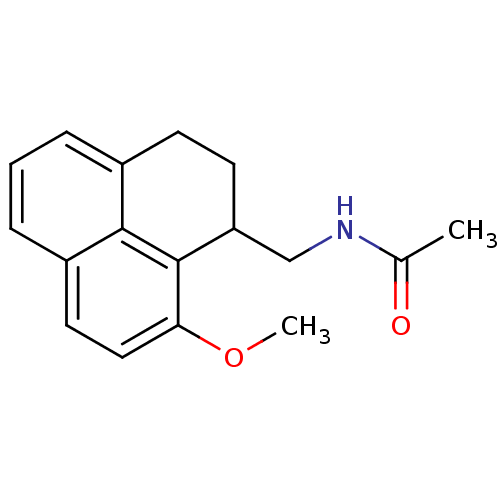 (CHEMBL132802 | N-(9-Methoxy-2,3-dihydro-1H-phenale...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Paris-Sud Curated by ChEMBL | Assay Description Binding affinity for human melatonin receptor type 1A, expressed in HEK-293 cells (2-[125I]-Iodomelatonin is used as radioligand) | J Med Chem 43: 4051-62 (2000) BindingDB Entry DOI: 10.7270/Q20Z72JX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Rattus norvegicus) | BDBM50470098 (CHEMBL2371296) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TAP Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of the in vitro binding of [125I]-labeled leuprolide to the rat pituitary luteinizing hormone releasing hormone (LHRH) receptor. | J Med Chem 37: 701-5 (1994) Article DOI: 10.1021/jm00031a021 BindingDB Entry DOI: 10.7270/Q2MG7S7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1B (Homo sapiens (Human)) | BDBM50114725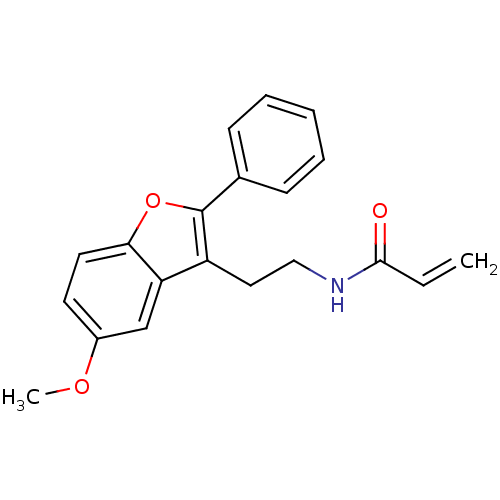 (CHEMBL314512 | N-[2-(5-Methoxy-2-phenyl-benzofuran...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Chimie Pharmaceutique Albert Lespagnol Curated by ChEMBL | Assay Description Binding affinity on human melatonin receptor type 1B stably transfected in human embryonic kidney (HEK 293) cells using 2-[125I]-iodomelatonin as rad... | J Med Chem 45: 2788-800 (2002) BindingDB Entry DOI: 10.7270/Q2Q52P0W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1A (Homo sapiens (Human)) | BDBM50114703 (CHEMBL287560 | N-[2-(5-Methoxy-2-phenyl-benzofuran...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Chimie Pharmaceutique Albert Lespagnol Curated by ChEMBL | Assay Description Binding affinity on human melatonin receptor type 1A stably transfected in human embryonic kidney (HEK 293) using 2-[125I]-iodomelatonin as radioliga... | J Med Chem 45: 2788-800 (2002) BindingDB Entry DOI: 10.7270/Q2Q52P0W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1A (Homo sapiens (Human)) | BDBM50114718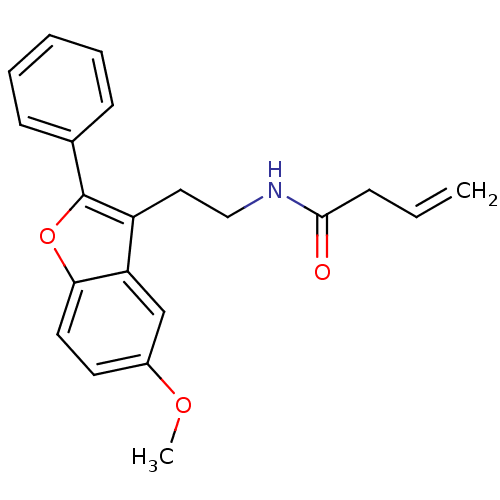 (But-3-enoic acid [2-(5-methoxy-2-phenyl-benzofuran...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Chimie Pharmaceutique Albert Lespagnol Curated by ChEMBL | Assay Description Binding affinity on human melatonin receptor type 1A stably transfected in human embryonic kidney (HEK 293) using 2-[125I]-iodomelatonin as radioliga... | J Med Chem 45: 2788-800 (2002) BindingDB Entry DOI: 10.7270/Q2Q52P0W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50353092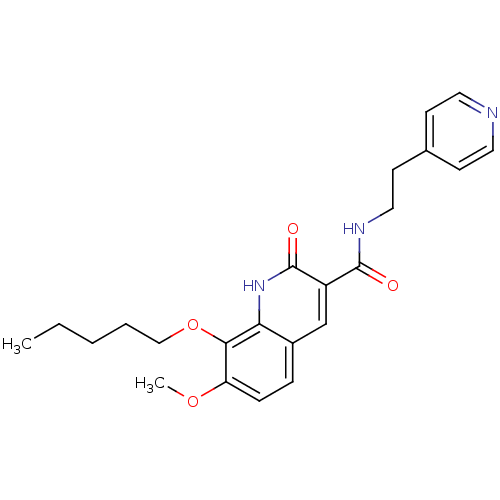 (CHEMBL1822945) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Texas Curated by ChEMBL | Assay Description Binding affinity to CB2 receptor | Bioorg Med Chem 19: 5698-707 (2011) Article DOI: 10.1016/j.bmc.2011.07.062 BindingDB Entry DOI: 10.7270/Q2GH9JC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 72153 total ) | Next | Last >> |