

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50180655 (A-157378-0 | A-157378.0 | ABT-378 | CHEBI:31781 | ...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | Bioorg Med Chem 16: 1299-308 (2008) Article DOI: 10.1016/j.bmc.2007.10.062 BindingDB Entry DOI: 10.7270/Q2RJ4N8X | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM580 ((4R)-3-[(2S,3S)-2-hydroxy-3-[(3-hydroxy-2-methylph...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.0350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | Bioorg Med Chem 16: 1299-308 (2008) Article DOI: 10.1016/j.bmc.2007.10.062 BindingDB Entry DOI: 10.7270/Q2RJ4N8X | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM13934 (Atazanavir | BMS 232632 | CGP 73547 | CHEMBL1163 |...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | Bioorg Med Chem 16: 1299-308 (2008) Article DOI: 10.1016/j.bmc.2007.10.062 BindingDB Entry DOI: 10.7270/Q2RJ4N8X | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50478141 (CHEMBL403526) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | Bioorg Med Chem 16: 1299-308 (2008) Article DOI: 10.1016/j.bmc.2007.10.062 BindingDB Entry DOI: 10.7270/Q2RJ4N8X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM719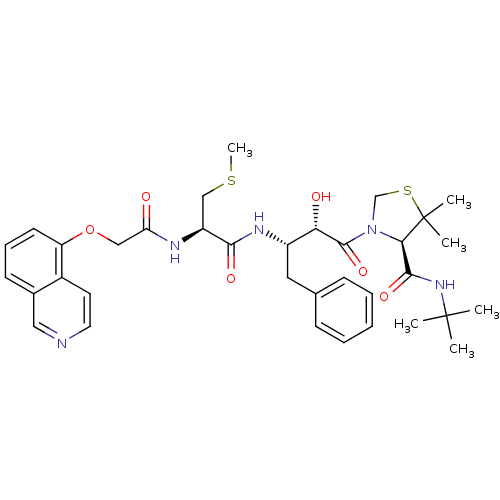 ((4R)-N-tert-butyl-3-[(2S,3S)-2-hydroxy-3-[(2R)-2-[...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0880 | -59.7 | n/a | n/a | n/a | n/a | n/a | 6.0 | 37 |
Japan Energy Corporation | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Substrates and cleavage fragments... | J Med Chem 42: 1789-802 (1999) Article DOI: 10.1021/jm980637h BindingDB Entry DOI: 10.7270/Q2MG7MP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50088504 (A-84538 | ABBOTT-84538 | CHEBI:45409 | Norvir | Ri...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.0980 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | Bioorg Med Chem 16: 1299-308 (2008) Article DOI: 10.1016/j.bmc.2007.10.062 BindingDB Entry DOI: 10.7270/Q2RJ4N8X | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50478138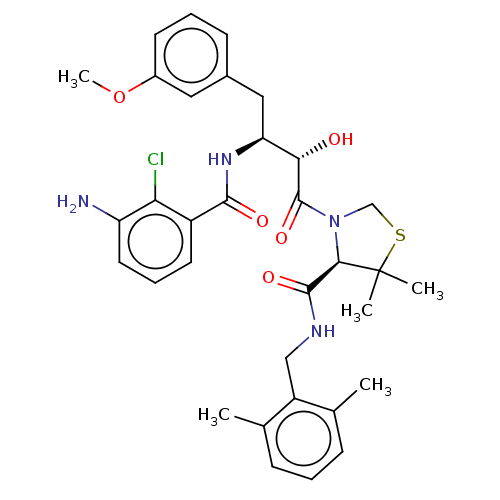 (CHEMBL256934 | SM-309515) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.134 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | Bioorg Med Chem 16: 1299-308 (2008) Article DOI: 10.1016/j.bmc.2007.10.062 BindingDB Entry DOI: 10.7270/Q2RJ4N8X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50213021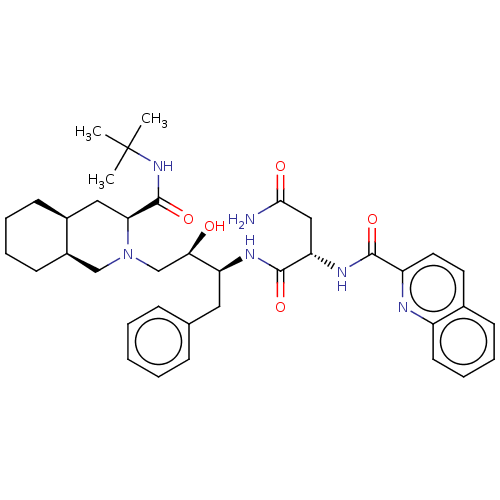 (CHEBI:63621 | Fortovase | Invirase | Ro-31-8959 | ...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.138 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | Bioorg Med Chem 16: 1299-308 (2008) Article DOI: 10.1016/j.bmc.2007.10.062 BindingDB Entry DOI: 10.7270/Q2RJ4N8X | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50478135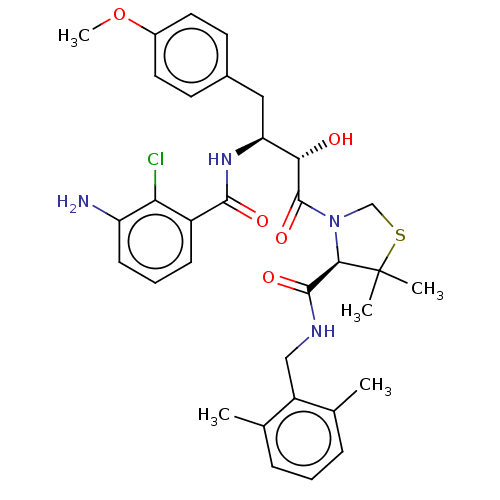 (CHEMBL409007) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.138 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | Bioorg Med Chem 16: 1299-308 (2008) Article DOI: 10.1016/j.bmc.2007.10.062 BindingDB Entry DOI: 10.7270/Q2RJ4N8X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50478136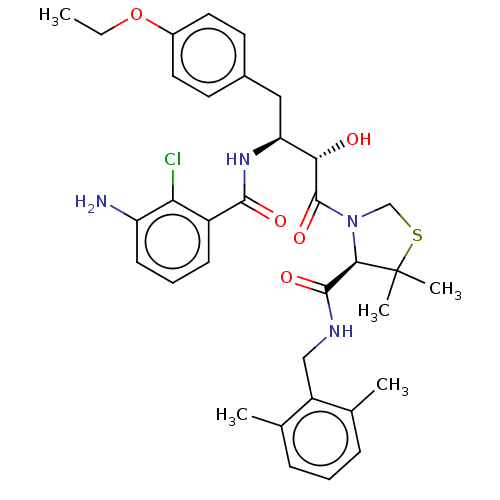 (CHEMBL257257) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.152 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | Bioorg Med Chem 16: 1299-308 (2008) Article DOI: 10.1016/j.bmc.2007.10.062 BindingDB Entry DOI: 10.7270/Q2RJ4N8X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM718 ((4R)-3-[(2S,3S)-3-[(2-ethyl-3-hydroxyphenyl)formam...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.160 | -58.2 | n/a | n/a | n/a | n/a | n/a | 6.0 | 37 |
Japan Energy Corporation | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Substrates and cleavage fragments... | J Med Chem 42: 1789-802 (1999) Article DOI: 10.1021/jm980637h BindingDB Entry DOI: 10.7270/Q2MG7MP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50478137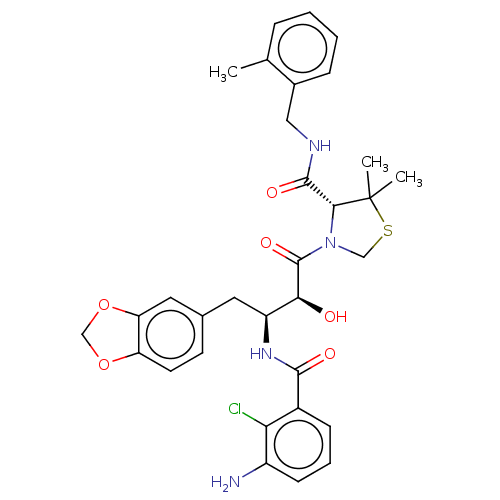 (CHEMBL271391) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.163 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | Bioorg Med Chem 16: 1299-308 (2008) Article DOI: 10.1016/j.bmc.2007.10.062 BindingDB Entry DOI: 10.7270/Q2RJ4N8X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50478143 (CHEMBL269904) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.255 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | Bioorg Med Chem 16: 1299-308 (2008) Article DOI: 10.1016/j.bmc.2007.10.062 BindingDB Entry DOI: 10.7270/Q2RJ4N8X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM717 ((4R)-N-[(2-chlorophenyl)methyl]-3-[(2S,3S)-2-hydro...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.290 | -56.6 | n/a | n/a | n/a | n/a | n/a | 6.0 | 37 |
Japan Energy Corporation | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Substrates and cleavage fragments... | J Med Chem 42: 1789-802 (1999) Article DOI: 10.1021/jm980637h BindingDB Entry DOI: 10.7270/Q2MG7MP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50478144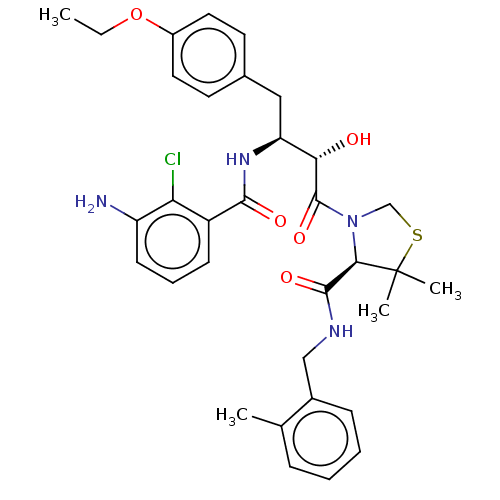 (CHEMBL272025) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | Bioorg Med Chem 16: 1299-308 (2008) Article DOI: 10.1016/j.bmc.2007.10.062 BindingDB Entry DOI: 10.7270/Q2RJ4N8X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50478142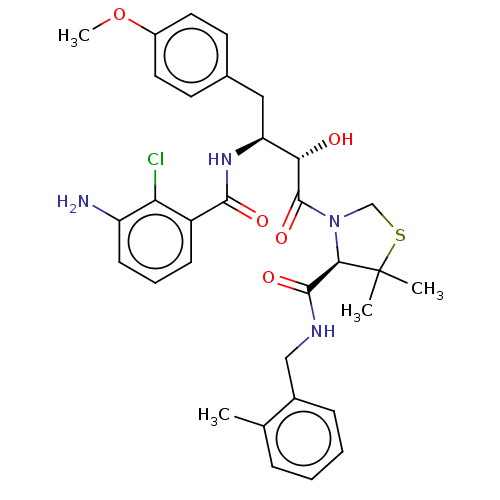 (CHEMBL272797) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.311 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | Bioorg Med Chem 16: 1299-308 (2008) Article DOI: 10.1016/j.bmc.2007.10.062 BindingDB Entry DOI: 10.7270/Q2RJ4N8X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM580 ((4R)-3-[(2S,3S)-2-hydroxy-3-[(3-hydroxy-2-methylph...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.330 | -56.3 | n/a | n/a | n/a | n/a | n/a | 6.0 | 37 |
Japan Energy Corporation | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Substrates and cleavage fragments... | J Med Chem 42: 1789-802 (1999) Article DOI: 10.1021/jm980637h BindingDB Entry DOI: 10.7270/Q2MG7MP3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50478140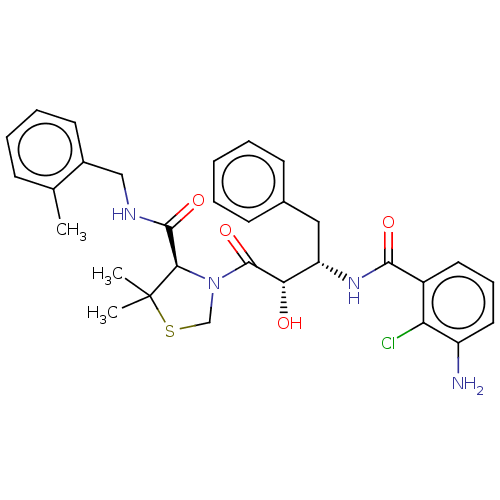 (CHEMBL404154) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.353 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | Bioorg Med Chem 16: 1299-308 (2008) Article DOI: 10.1016/j.bmc.2007.10.062 BindingDB Entry DOI: 10.7270/Q2RJ4N8X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM577 ((3S)-oxolan-3-yl N-[(2S,3R)-4-[(4-aminobenzene)(2-...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.359 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | Bioorg Med Chem 16: 1299-308 (2008) Article DOI: 10.1016/j.bmc.2007.10.062 BindingDB Entry DOI: 10.7270/Q2RJ4N8X | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50067593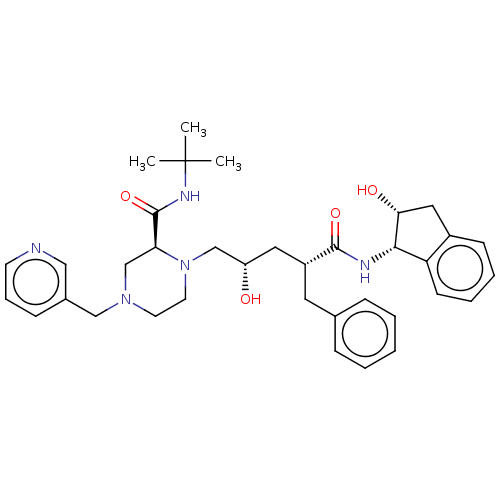 (CHEBI:44032 | Crixivan | Indinavir | L-735524 | MK...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.739 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | Bioorg Med Chem 16: 1299-308 (2008) Article DOI: 10.1016/j.bmc.2007.10.062 BindingDB Entry DOI: 10.7270/Q2RJ4N8X | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM579 ((4R)-N-tert-butyl-3-[(2S,3S)-2-hydroxy-3-[(2R)-2-[...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.740 | -54.2 | n/a | n/a | n/a | n/a | n/a | 6.0 | 37 |
Japan Energy Corporation | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Substrates and cleavage fragments... | J Med Chem 42: 1789-802 (1999) Article DOI: 10.1021/jm980637h BindingDB Entry DOI: 10.7270/Q2MG7MP3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50478133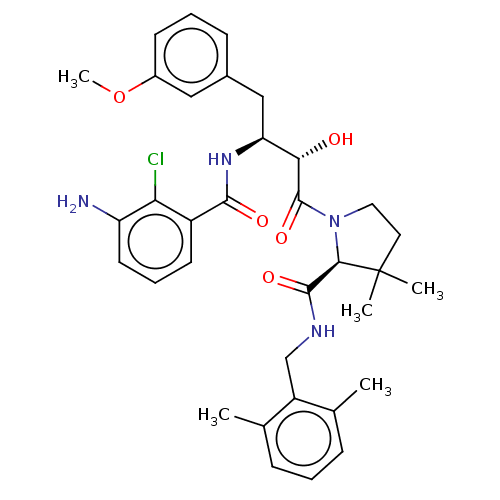 (CHEMBL272796) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | Bioorg Med Chem 16: 1299-308 (2008) Article DOI: 10.1016/j.bmc.2007.10.062 BindingDB Entry DOI: 10.7270/Q2RJ4N8X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50478134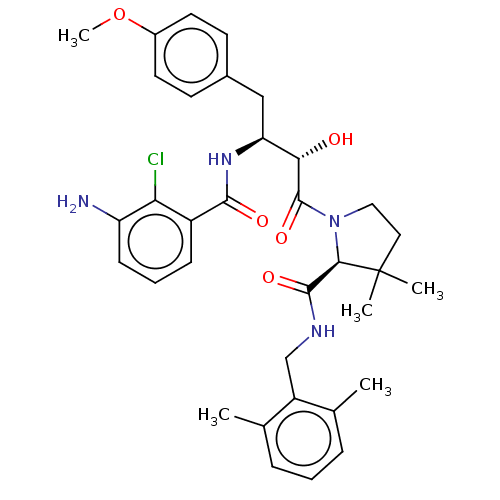 (CHEMBL408620) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.804 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | Bioorg Med Chem 16: 1299-308 (2008) Article DOI: 10.1016/j.bmc.2007.10.062 BindingDB Entry DOI: 10.7270/Q2RJ4N8X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50478139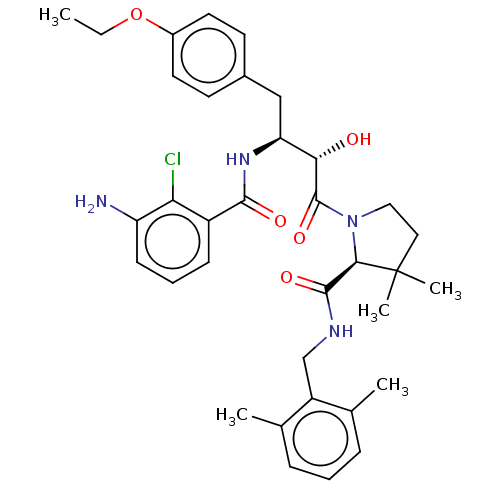 (CHEMBL437457) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.861 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | Bioorg Med Chem 16: 1299-308 (2008) Article DOI: 10.1016/j.bmc.2007.10.062 BindingDB Entry DOI: 10.7270/Q2RJ4N8X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50061306 ((3S,4aS,8aS)-2-[(2R,3R)-2-Hydroxy-3-(3-hydroxy-2-m...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.931 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | Bioorg Med Chem 16: 1299-308 (2008) Article DOI: 10.1016/j.bmc.2007.10.062 BindingDB Entry DOI: 10.7270/Q2RJ4N8X | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM712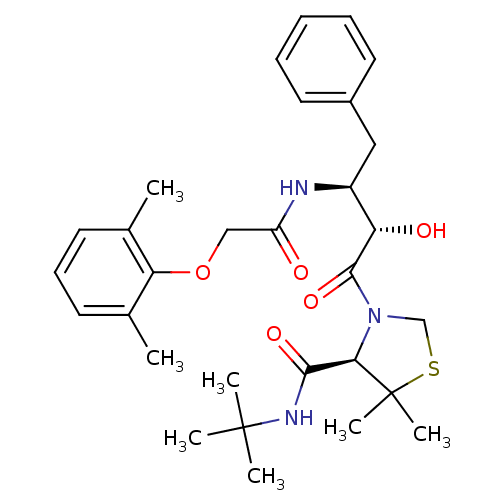 ((4R)-N-tert-butyl-3-[(2S,3S)-3-[2-(2,6-dimethylphe...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.40 | -52.6 | n/a | n/a | n/a | n/a | n/a | 6.0 | 37 |
Japan Energy Corporation | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Substrates and cleavage fragments... | J Med Chem 42: 1789-802 (1999) Article DOI: 10.1021/jm980637h BindingDB Entry DOI: 10.7270/Q2MG7MP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM715 ((4R)-N-tert-butyl-3-[(2S,3S)-3-[(2-ethyl-3-hydroxy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.24 | -51.4 | n/a | n/a | n/a | n/a | n/a | 6.0 | 37 |
Japan Energy Corporation | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Substrates and cleavage fragments... | J Med Chem 42: 1789-802 (1999) Article DOI: 10.1021/jm980637h BindingDB Entry DOI: 10.7270/Q2MG7MP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM714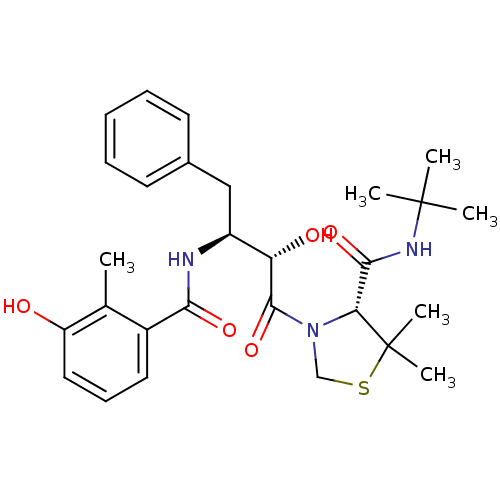 ((4R)-N-tert-butyl-3-[(2S,3S)-2-hydroxy-3-[(3-hydro...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 5.14 | -49.2 | n/a | n/a | n/a | n/a | n/a | 6.0 | 37 |
Japan Energy Corporation | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Substrates and cleavage fragments... | J Med Chem 42: 1789-802 (1999) Article DOI: 10.1021/jm980637h BindingDB Entry DOI: 10.7270/Q2MG7MP3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM716 ((4R)-3-[(2S,3S)-2-hydroxy-3-[(3-hydroxy-2-methylph...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 8.91 | -47.8 | n/a | n/a | n/a | n/a | n/a | 6.0 | 37 |
Japan Energy Corporation | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Substrates and cleavage fragments... | J Med Chem 42: 1789-802 (1999) Article DOI: 10.1021/jm980637h BindingDB Entry DOI: 10.7270/Q2MG7MP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM708 ((4R)-N-tert-butyl-3-[(2S,3S)-3-[2-(2,6-dimethylphe...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 21.7 | -45.5 | n/a | n/a | n/a | n/a | n/a | 6.0 | 37 |
Japan Energy Corporation | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Substrates and cleavage fragments... | J Med Chem 42: 1789-802 (1999) Article DOI: 10.1021/jm980637h BindingDB Entry DOI: 10.7270/Q2MG7MP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM713 ((4R)-N-tert-butyl-3-[(2S,3S)-2-hydroxy-3-[(3-hydro...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 24.9 | -45.1 | n/a | n/a | n/a | n/a | n/a | 6.0 | 37 |
Japan Energy Corporation | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Substrates and cleavage fragments... | J Med Chem 42: 1789-802 (1999) Article DOI: 10.1021/jm980637h BindingDB Entry DOI: 10.7270/Q2MG7MP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM109863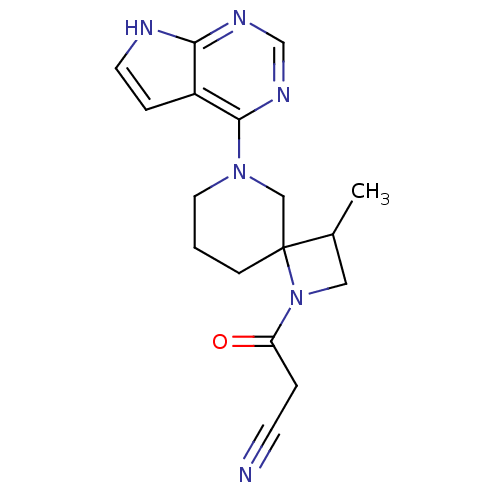 (US8609647, 1 | US8609647, 15 | US8609647, 2 | US86...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Japan Tobacco Inc. US Patent | Assay Description In the kinase reactions, fused proteins (6His tag-fused hJAK2 kinase domain (aa808-end)) which were coexpressed in Sf21 cells and purified by Ni2+/NT... | US Patent US8609647 (2013) BindingDB Entry DOI: 10.7270/Q29C6W24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM50193995 (3-((3R,4R)-4-methyl-3-(methyl(7H-pyrrolo[2,3-d]pyr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Japan Tobacco Inc. Curated by ChEMBL | Assay Description Inhibition of human JAK3 (780 to end residues) expressed in baculovirus infected Sf9 cells using TK-substrate-biotin as substrate incubated for 60 mi... | J Med Chem 63: 7163-7185 (2020) Article DOI: 10.1021/acs.jmedchem.0c00450 BindingDB Entry DOI: 10.7270/Q2FX7F2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM50193995 (3-((3R,4R)-4-methyl-3-(methyl(7H-pyrrolo[2,3-d]pyr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Japan Tobacco Inc. Curated by ChEMBL | Assay Description Inhibition of N-terminal GST-fused human JAK2 (880 to end residues) expressed in baculovirus infected Sf21 cells using TK-substrate-biotin as substra... | J Med Chem 63: 7163-7185 (2020) Article DOI: 10.1021/acs.jmedchem.0c00450 BindingDB Entry DOI: 10.7270/Q2FX7F2W | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM109863 (US8609647, 1 | US8609647, 15 | US8609647, 2 | US86...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Japan Tobacco Inc. US Patent | Assay Description In the kinase reactions, fused proteins (6His tag-fused hJAK2 kinase domain (aa808-end)) which were coexpressed in Sf21 cells and purified by Ni2+/NT... | US Patent US8609647 (2013) BindingDB Entry DOI: 10.7270/Q29C6W24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM109866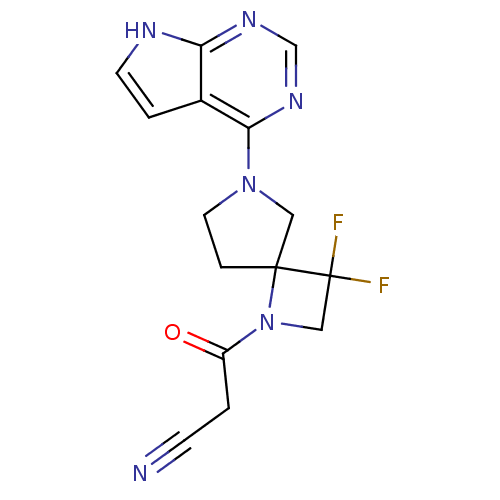 (US8609647, 26 | US8609647, 4) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Japan Tobacco Inc. US Patent | Assay Description In the kinase reactions, fused proteins (6His tag-fused hJAK2 kinase domain (aa808-end)) which were coexpressed in Sf21 cells and purified by Ni2+/NT... | US Patent US8609647 (2013) BindingDB Entry DOI: 10.7270/Q29C6W24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM109867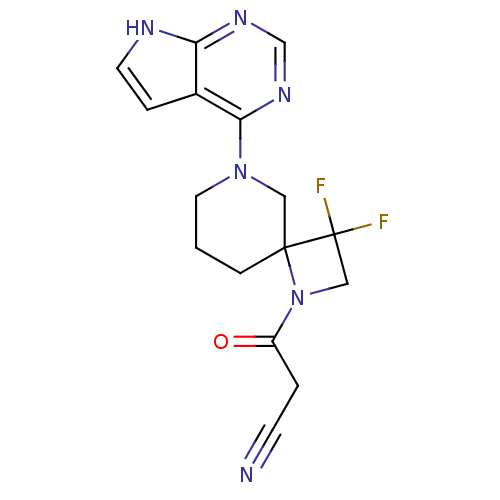 (US8609647, 27 | US8609647, 37 | US8609647, 5) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Japan Tobacco Inc. US Patent | Assay Description In the kinase reactions, fused proteins (6His tag-fused hJAK2 kinase domain (aa808-end)) which were coexpressed in Sf21 cells and purified by Ni2+/NT... | US Patent US8609647 (2013) BindingDB Entry DOI: 10.7270/Q29C6W24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM109868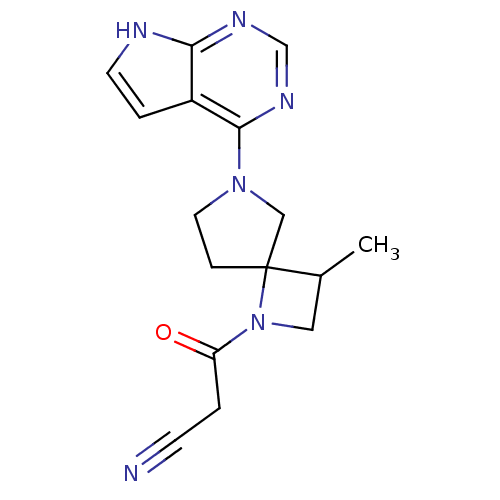 (US8609647, 19 | US8609647, 21 | US8609647, 6) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | US Patent | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Japan Tobacco Inc. US Patent | Assay Description In the kinase reactions, fused proteins (6His tag-fused hJAK2 kinase domain (aa808-end)) which were coexpressed in Sf21 cells and purified by Ni2+/NT... | US Patent US8609647 (2013) BindingDB Entry DOI: 10.7270/Q29C6W24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM109865 (US8609647, 25 | US8609647, 3 | US8609647, 59) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Japan Tobacco Inc. US Patent | Assay Description In the kinase reactions, fused proteins (6His tag-fused hJAK2 kinase domain (aa808-end)) which were coexpressed in Sf21 cells and purified by Ni2+/NT... | US Patent US8609647 (2013) BindingDB Entry DOI: 10.7270/Q29C6W24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM50545650 (Delgocitinib | JTE-052 | JTE-052A | LEO 124249 | L...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Japan Tobacco Inc. Curated by ChEMBL | Assay Description Inhibition of N-terminal GST-fused human JAK2 (880 to end residues) expressed in baculovirus infected Sf21 cells using TK-substrate-biotin as substra... | J Med Chem 63: 7163-7185 (2020) Article DOI: 10.1021/acs.jmedchem.0c00450 BindingDB Entry DOI: 10.7270/Q2FX7F2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM50545650 (Delgocitinib | JTE-052 | JTE-052A | LEO 124249 | L...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Japan Tobacco Inc. Curated by ChEMBL | Assay Description Inhibition of N-terminal GST-fused human JAK1 (850 to 1154 residues) expressed in baculovirus expression system using TK-substrate-biotin as substrat... | J Med Chem 63: 7163-7185 (2020) Article DOI: 10.1021/acs.jmedchem.0c00450 BindingDB Entry DOI: 10.7270/Q2FX7F2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM50193995 (3-((3R,4R)-4-methyl-3-(methyl(7H-pyrrolo[2,3-d]pyr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Japan Tobacco Inc. Curated by ChEMBL | Assay Description Inhibition of N-terminal GST-fused human JAK1 (850 to 1154 residues) expressed in baculovirus expression system using TK-substrate-biotin as substrat... | J Med Chem 63: 7163-7185 (2020) Article DOI: 10.1021/acs.jmedchem.0c00450 BindingDB Entry DOI: 10.7270/Q2FX7F2W | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM109867 (US8609647, 27 | US8609647, 37 | US8609647, 5) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Japan Tobacco Inc. US Patent | Assay Description In the kinase reactions, fused proteins (6His tag-fused hJAK3 kinase domain (aa781-end)) which were coexpressed in Sf21 cells and purified by Ni2+/NT... | US Patent US8609647 (2013) BindingDB Entry DOI: 10.7270/Q29C6W24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM109863 (US8609647, 1 | US8609647, 15 | US8609647, 2 | US86...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Japan Tobacco Inc. US Patent | Assay Description In the kinase reactions, fused proteins (6His tag-fused hJAK3 kinase domain (aa781-end)) which were coexpressed in Sf21 cells and purified by Ni2+/NT... | US Patent US8609647 (2013) BindingDB Entry DOI: 10.7270/Q29C6W24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM109867 (US8609647, 27 | US8609647, 37 | US8609647, 5) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Japan Tobacco Inc. US Patent | Assay Description In the kinase reactions, fused proteins (6His tag-fused hJAK3 kinase domain (aa781-end)) which were coexpressed in Sf21 cells and purified by Ni2+/NT... | US Patent US8609647 (2013) BindingDB Entry DOI: 10.7270/Q29C6W24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM109863 (US8609647, 1 | US8609647, 15 | US8609647, 2 | US86...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Japan Tobacco Inc. US Patent | Assay Description In the kinase reactions, fused proteins (6His tag-fused hJAK3 kinase domain (aa781-end)) which were coexpressed in Sf21 cells and purified by Ni2+/NT... | US Patent US8609647 (2013) BindingDB Entry DOI: 10.7270/Q29C6W24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM109869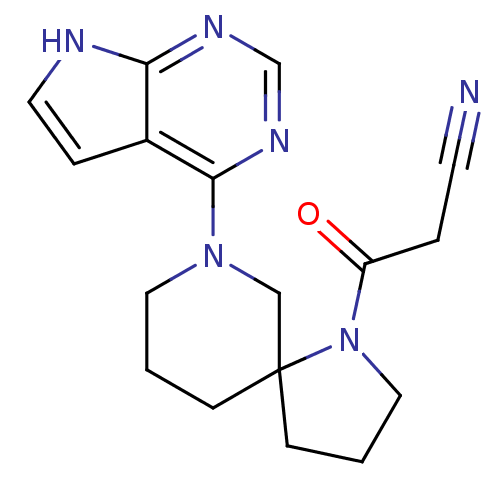 (US8609647, 7 | US8609647, 84) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Japan Tobacco Inc. US Patent | Assay Description In the kinase reactions, fused proteins (6His tag-fused hJAK2 kinase domain (aa808-end)) which were coexpressed in Sf21 cells and purified by Ni2+/NT... | US Patent US8609647 (2013) BindingDB Entry DOI: 10.7270/Q29C6W24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM109866 (US8609647, 26 | US8609647, 4) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Japan Tobacco Inc. US Patent | Assay Description In the kinase reactions, fused proteins (6His tag-fused hJAK3 kinase domain (aa781-end)) which were coexpressed in Sf21 cells and purified by Ni2+/NT... | US Patent US8609647 (2013) BindingDB Entry DOI: 10.7270/Q29C6W24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM109869 (US8609647, 7 | US8609647, 84) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Japan Tobacco Inc. US Patent | Assay Description In the kinase reactions, fused proteins (6His tag-fused hJAK3 kinase domain (aa781-end)) which were coexpressed in Sf21 cells and purified by Ni2+/NT... | US Patent US8609647 (2013) BindingDB Entry DOI: 10.7270/Q29C6W24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM109909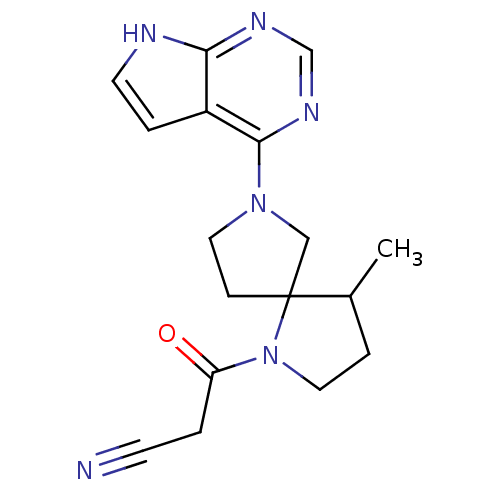 (US8609647, 54 | US8609647, 66) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Japan Tobacco Inc. US Patent | Assay Description In the kinase reactions, fused proteins (6His tag-fused hJAK3 kinase domain (aa781-end)) which were coexpressed in Sf21 cells and purified by Ni2+/NT... | US Patent US8609647 (2013) BindingDB Entry DOI: 10.7270/Q29C6W24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 354 total ) | Next | Last >> |