 Found 99 hits with Last Name = 'ohashi' and Initial = 'r'
Found 99 hits with Last Name = 'ohashi' and Initial = 'r' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Extracellular calcium-sensing receptor
(Rattus norvegicus) | BDBM50272596
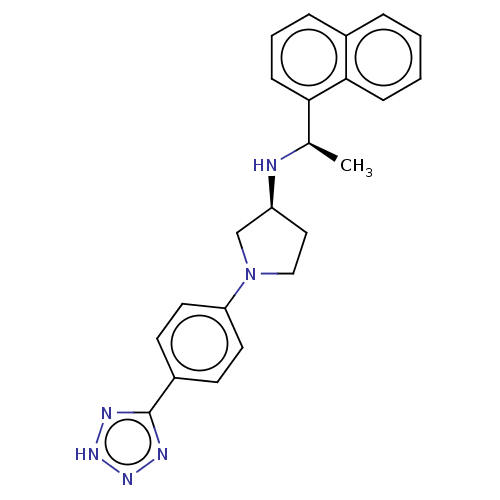
(CHEMBL4126450)Show SMILES Cl.C[C@@H](N[C@H]1CCN(C1)c1ccc(cc1)-c1nn[nH]n1)c1cccc2ccccc12 |r| Show InChI InChI=1S/C23H24N6.ClH/c1-16(21-8-4-6-17-5-2-3-7-22(17)21)24-19-13-14-29(15-19)20-11-9-18(10-12-20)23-25-27-28-26-23;/h2-12,16,19,24H,13-15H2,1H3,(H,25,26,27,28);1H/t16-,19+;/m1./s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
| Assay Description
Agonist activity at CaSR in rat parathyroid cells assessed as inhibition of PTH (1 to 84 residues) production in presence of Cacl2 by ELISA |
Bioorg Med Chem Lett 28: 2055-2060 (2018)
Article DOI: 10.1016/j.bmcl.2018.04.055
BindingDB Entry DOI: 10.7270/Q2DF6TP0 |
More data for this
Ligand-Target Pair | |
Extracellular calcium-sensing receptor
(Rattus norvegicus) | BDBM50272602
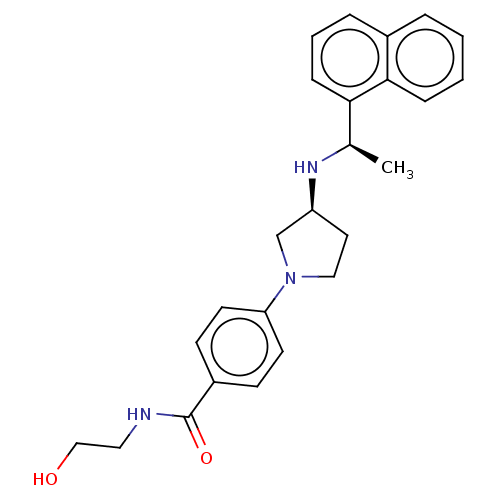
(CHEMBL4126877)Show SMILES Cl.Cl.C[C@@H](N[C@H]1CCN(C1)c1ccc(cc1)C(=O)NCCO)c1cccc2ccccc12 |r| Show InChI InChI=1S/C25H29N3O2.2ClH/c1-18(23-8-4-6-19-5-2-3-7-24(19)23)27-21-13-15-28(17-21)22-11-9-20(10-12-22)25(30)26-14-16-29;;/h2-12,18,21,27,29H,13-17H2,1H3,(H,26,30);2*1H/t18-,21+;;/m1../s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.910 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
| Assay Description
Agonist activity at CaSR in rat parathyroid cells assessed as inhibition of PTH (1 to 84 residues) production in presence of Cacl2 by ELISA |
Bioorg Med Chem Lett 28: 2055-2060 (2018)
Article DOI: 10.1016/j.bmcl.2018.04.055
BindingDB Entry DOI: 10.7270/Q2DF6TP0 |
More data for this
Ligand-Target Pair | |
Extracellular calcium-sensing receptor
(Rattus norvegicus) | BDBM50272605

(CHEMBL4128542)Show SMILES Cl.Cl.C[C@@H](N[C@@H]1CCCN(C1)c1cccc(OC(F)(F)F)c1)c1cccc2ccccc12 |r| Show InChI InChI=1S/C24H25F3N2O.2ClH/c1-17(22-13-4-8-18-7-2-3-12-23(18)22)28-19-9-6-14-29(16-19)20-10-5-11-21(15-20)30-24(25,26)27;;/h2-5,7-8,10-13,15,17,19,28H,6,9,14,16H2,1H3;2*1H/t17-,19-;;/m1../s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
| Assay Description
Agonist activity at CaSR in rat parathyroid cells assessed as inhibition of PTH (1 to 84 residues) production in presence of Cacl2 by ELISA |
Bioorg Med Chem Lett 28: 2055-2060 (2018)
Article DOI: 10.1016/j.bmcl.2018.04.055
BindingDB Entry DOI: 10.7270/Q2DF6TP0 |
More data for this
Ligand-Target Pair | |
Extracellular calcium-sensing receptor
(Rattus norvegicus) | BDBM50272606

(CHEMBL4125917)Show SMILES Cl.Cl.C[C@@H](N[C@@H]1CCCN(C1)c1cccc(c1)C(F)(F)F)c1cccc2ccccc12 |r| Show InChI InChI=1S/C24H25F3N2.2ClH/c1-17(22-13-4-8-18-7-2-3-12-23(18)22)28-20-10-6-14-29(16-20)21-11-5-9-19(15-21)24(25,26)27;;/h2-5,7-9,11-13,15,17,20,28H,6,10,14,16H2,1H3;2*1H/t17-,20-;;/m1../s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
| Assay Description
Agonist activity at CaSR in rat parathyroid cells assessed as inhibition of PTH (1 to 84 residues) production in presence of Cacl2 by ELISA |
Bioorg Med Chem Lett 28: 2055-2060 (2018)
Article DOI: 10.1016/j.bmcl.2018.04.055
BindingDB Entry DOI: 10.7270/Q2DF6TP0 |
More data for this
Ligand-Target Pair | |
Extracellular calcium-sensing receptor
(Rattus norvegicus) | BDBM50272607
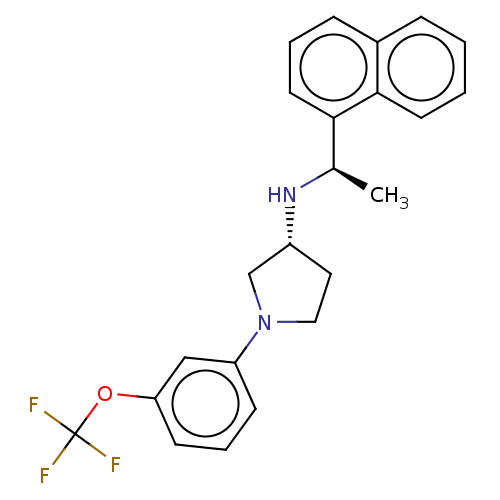
(CHEMBL4126057)Show SMILES Cl.Cl.C[C@@H](N[C@@H]1CCN(C1)c1cccc(OC(F)(F)F)c1)c1cccc2ccccc12 |r| Show InChI InChI=1S/C23H23F3N2O.2ClH/c1-16(21-11-4-7-17-6-2-3-10-22(17)21)27-18-12-13-28(15-18)19-8-5-9-20(14-19)29-23(24,25)26;;/h2-11,14,16,18,27H,12-13,15H2,1H3;2*1H/t16-,18-;;/m1../s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
| Assay Description
Agonist activity at CaSR in rat parathyroid cells assessed as inhibition of PTH (1 to 84 residues) production in presence of Cacl2 by ELISA |
Bioorg Med Chem Lett 28: 2055-2060 (2018)
Article DOI: 10.1016/j.bmcl.2018.04.055
BindingDB Entry DOI: 10.7270/Q2DF6TP0 |
More data for this
Ligand-Target Pair | |
Extracellular calcium-sensing receptor
(Rattus norvegicus) | BDBM50272604

(CHEMBL4129011)Show SMILES Cl.Cl.C[C@@H](N[C@H]1CCN(C1)c1ccc(OC(F)(F)F)cc1)c1cccc2ccccc12 |r| Show InChI InChI=1S/C23H23F3N2O.2ClH/c1-16(21-8-4-6-17-5-2-3-7-22(17)21)27-18-13-14-28(15-18)19-9-11-20(12-10-19)29-23(24,25)26;;/h2-12,16,18,27H,13-15H2,1H3;2*1H/t16-,18+;;/m1../s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
| Assay Description
Agonist activity at CaSR in rat parathyroid cells assessed as inhibition of PTH (1 to 84 residues) production in presence of Cacl2 by ELISA |
Bioorg Med Chem Lett 28: 2055-2060 (2018)
Article DOI: 10.1016/j.bmcl.2018.04.055
BindingDB Entry DOI: 10.7270/Q2DF6TP0 |
More data for this
Ligand-Target Pair | |
Extracellular calcium-sensing receptor
(Rattus norvegicus) | BDBM50261663

(CHEMBL4079960)Show SMILES C[C@@H](N[C@H]1CCN(C1)c1ccc(cc1)C(O)=O)c1cccc2ccccc12 |r| Show InChI InChI=1S/C23H24N2O2/c1-16(21-8-4-6-17-5-2-3-7-22(17)21)24-19-13-14-25(15-19)20-11-9-18(10-12-20)23(26)27/h2-12,16,19,24H,13-15H2,1H3,(H,26,27)/t16-,19+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
| Assay Description
Agonist activity at CaSR in rat parathyroid cells assessed as inhibition of PTH (1 to 84 residues) production in presence of Cacl2 by ELISA |
Bioorg Med Chem Lett 28: 2055-2060 (2018)
Article DOI: 10.1016/j.bmcl.2018.04.055
BindingDB Entry DOI: 10.7270/Q2DF6TP0 |
More data for this
Ligand-Target Pair | |
Egl nine homolog 1
(Homo sapiens (Human)) | BDBM50543060
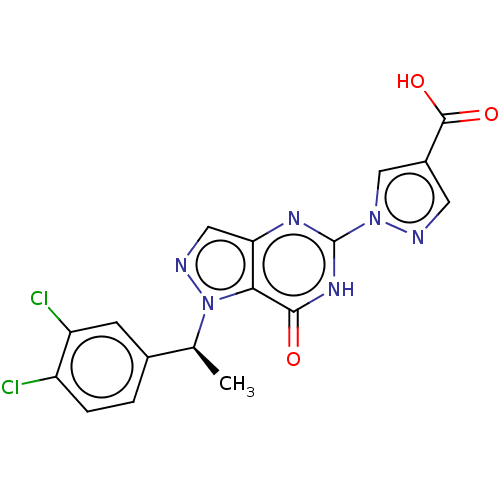
(CHEMBL4642843)Show SMILES C[C@@H](c1ccc(Cl)c(Cl)c1)n1ncc2nc([nH]c(=O)c12)-n1cc(cn1)C(O)=O |r| Show InChI InChI=1S/C17H12Cl2N6O3/c1-8(9-2-3-11(18)12(19)4-9)25-14-13(6-21-25)22-17(23-15(14)26)24-7-10(5-20-24)16(27)28/h2-8H,1H3,(H,27,28)(H,22,23,26)/t8-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of HIF-PHD2 (unknown origin) using FAM-HIF2alpha peptide incubated for 20 to 40 mins by fluorescence polarization assay |
ACS Med Chem Lett 11: 1416-1420 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00108
BindingDB Entry DOI: 10.7270/Q25H7KTS |
More data for this
Ligand-Target Pair | |
Egl nine homolog 1
(Homo sapiens (Human)) | BDBM50543056
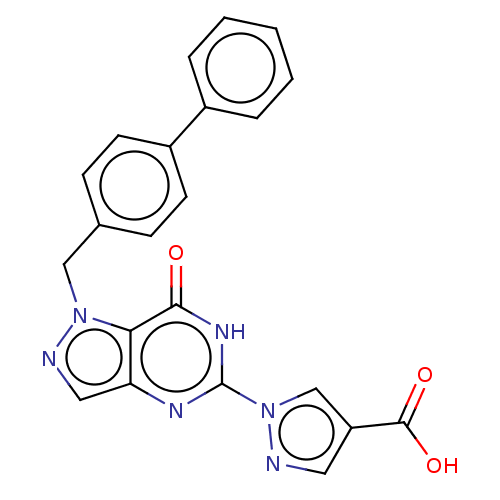
(CHEMBL4647424)Show SMILES OC(=O)c1cnn(c1)-c1nc2cnn(Cc3ccc(cc3)-c3ccccc3)c2c(=O)[nH]1 Show InChI InChI=1S/C22H16N6O3/c29-20-19-18(25-22(26-20)28-13-17(10-23-28)21(30)31)11-24-27(19)12-14-6-8-16(9-7-14)15-4-2-1-3-5-15/h1-11,13H,12H2,(H,30,31)(H,25,26,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of HIF-PHD2 (unknown origin) using FAM-HIF2alpha peptide incubated for 20 to 40 mins by fluorescence polarization assay |
ACS Med Chem Lett 11: 1416-1420 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00108
BindingDB Entry DOI: 10.7270/Q25H7KTS |
More data for this
Ligand-Target Pair | |
Extracellular calcium-sensing receptor
(Rattus norvegicus) | BDBM50272608

(CHEMBL4125688)Show SMILES Cl.Cl.C[C@@H](N[C@@H]1CCN(C1)c1cccc(c1)C(F)(F)F)c1cccc2ccccc12 |r| Show InChI InChI=1S/C23H23F3N2.2ClH/c1-16(21-11-4-7-17-6-2-3-10-22(17)21)27-19-12-13-28(15-19)20-9-5-8-18(14-20)23(24,25)26;;/h2-11,14,16,19,27H,12-13,15H2,1H3;2*1H/t16-,19-;;/m1../s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
| Assay Description
Agonist activity at CaSR in rat parathyroid cells assessed as inhibition of PTH (1 to 84 residues) production in presence of Cacl2 by ELISA |
Bioorg Med Chem Lett 28: 2055-2060 (2018)
Article DOI: 10.1016/j.bmcl.2018.04.055
BindingDB Entry DOI: 10.7270/Q2DF6TP0 |
More data for this
Ligand-Target Pair | |
Extracellular calcium-sensing receptor
(Rattus norvegicus) | BDBM50272592
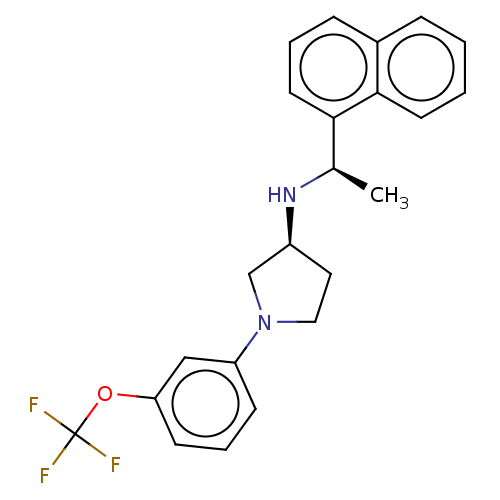
(CHEMBL4125724)Show SMILES Cl.Cl.C[C@@H](N[C@H]1CCN(C1)c1cccc(OC(F)(F)F)c1)c1cccc2ccccc12 |r| Show InChI InChI=1S/C23H23F3N2O.2ClH/c1-16(21-11-4-7-17-6-2-3-10-22(17)21)27-18-12-13-28(15-18)19-8-5-9-20(14-19)29-23(24,25)26;;/h2-11,14,16,18,27H,12-13,15H2,1H3;2*1H/t16-,18+;;/m1../s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
| Assay Description
Agonist activity at CaSR in rat parathyroid cells assessed as inhibition of PTH (1 to 84 residues) production in presence of Cacl2 by ELISA |
Bioorg Med Chem Lett 28: 2055-2060 (2018)
Article DOI: 10.1016/j.bmcl.2018.04.055
BindingDB Entry DOI: 10.7270/Q2DF6TP0 |
More data for this
Ligand-Target Pair | |
Egl nine homolog 1
(Homo sapiens (Human)) | BDBM50543057
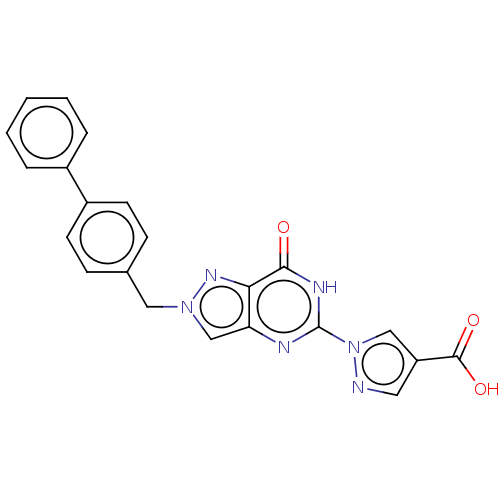
(CHEMBL4645985)Show SMILES OC(=O)c1cnn(c1)-c1nc2cn(Cc3ccc(cc3)-c3ccccc3)nc2c(=O)[nH]1 Show InChI InChI=1S/C22H16N6O3/c29-20-19-18(24-22(25-20)28-12-17(10-23-28)21(30)31)13-27(26-19)11-14-6-8-16(9-7-14)15-4-2-1-3-5-15/h1-10,12-13H,11H2,(H,30,31)(H,24,25,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of HIF-PHD2 (unknown origin) using FAM-HIF2alpha peptide incubated for 20 to 40 mins by fluorescence polarization assay |
ACS Med Chem Lett 11: 1416-1420 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00108
BindingDB Entry DOI: 10.7270/Q25H7KTS |
More data for this
Ligand-Target Pair | |
Extracellular calcium-sensing receptor
(Rattus norvegicus) | BDBM50272595

(CHEMBL4127153)Show SMILES Cl.Cl.C[C@@H](N[C@H]1CCN(C1)c1cccc(c1)C(F)(F)F)c1cccc2ccccc12 |r| Show InChI InChI=1S/C23H23F3N2.2ClH/c1-16(21-11-4-7-17-6-2-3-10-22(17)21)27-19-12-13-28(15-19)20-9-5-8-18(14-20)23(24,25)26;;/h2-11,14,16,19,27H,12-13,15H2,1H3;2*1H/t16-,19+;;/m1../s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
| Assay Description
Agonist activity at CaSR in rat parathyroid cells assessed as inhibition of PTH (1 to 84 residues) production in presence of Cacl2 by ELISA |
Bioorg Med Chem Lett 28: 2055-2060 (2018)
Article DOI: 10.1016/j.bmcl.2018.04.055
BindingDB Entry DOI: 10.7270/Q2DF6TP0 |
More data for this
Ligand-Target Pair | |
Egl nine homolog 1
(Homo sapiens (Human)) | BDBM50543054
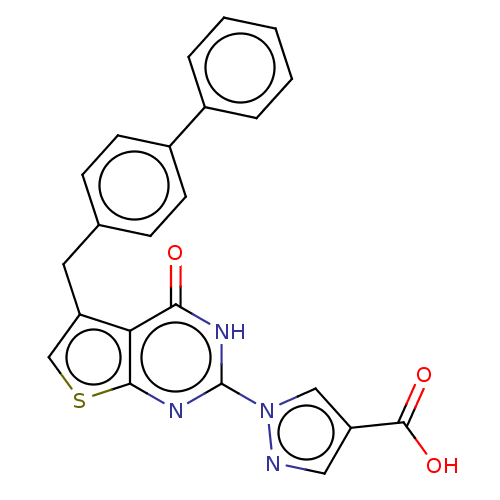
(CHEMBL4648388)Show SMILES OC(=O)c1cnn(c1)-c1nc2scc(Cc3ccc(cc3)-c3ccccc3)c2c(=O)[nH]1 Show InChI InChI=1S/C23H16N4O3S/c28-20-19-17(10-14-6-8-16(9-7-14)15-4-2-1-3-5-15)13-31-21(19)26-23(25-20)27-12-18(11-24-27)22(29)30/h1-9,11-13H,10H2,(H,29,30)(H,25,26,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of HIF-PHD2 (unknown origin) using FAM-HIF2alpha peptide incubated for 20 to 40 mins by fluorescence polarization assay |
ACS Med Chem Lett 11: 1416-1420 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00108
BindingDB Entry DOI: 10.7270/Q25H7KTS |
More data for this
Ligand-Target Pair | |
Egl nine homolog 1
(Homo sapiens (Human)) | BDBM50543059
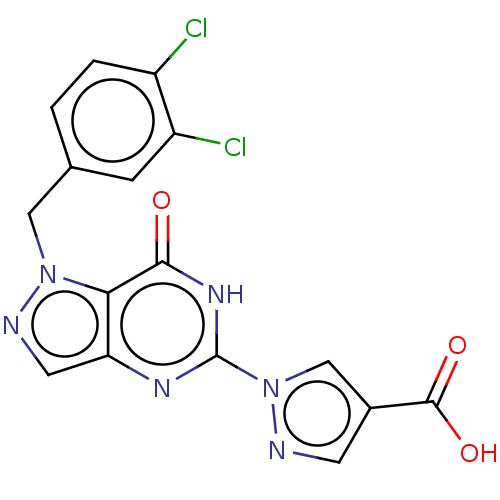
(CHEMBL4643092)Show SMILES OC(=O)c1cnn(c1)-c1nc2cnn(Cc3ccc(Cl)c(Cl)c3)c2c(=O)[nH]1 Show InChI InChI=1S/C16H10Cl2N6O3/c17-10-2-1-8(3-11(10)18)6-23-13-12(5-20-23)21-16(22-14(13)25)24-7-9(4-19-24)15(26)27/h1-5,7H,6H2,(H,26,27)(H,21,22,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of HIF-PHD2 (unknown origin) using FAM-HIF2alpha peptide incubated for 20 to 40 mins by fluorescence polarization assay |
ACS Med Chem Lett 11: 1416-1420 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00108
BindingDB Entry DOI: 10.7270/Q25H7KTS |
More data for this
Ligand-Target Pair | |
Egl nine homolog 1
(Homo sapiens (Human)) | BDBM50543058
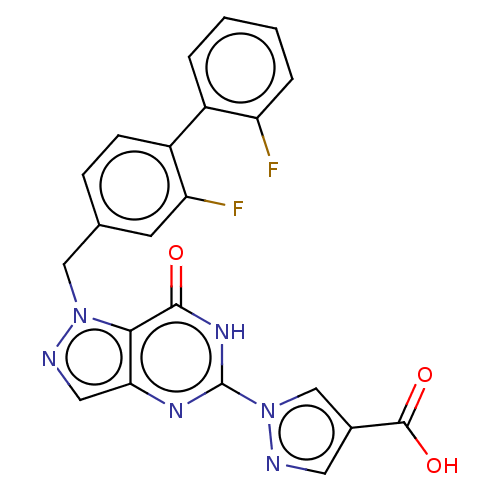
(CHEMBL4648359)Show SMILES OC(=O)c1cnn(c1)-c1nc2cnn(Cc3ccc(c(F)c3)-c3ccccc3F)c2c(=O)[nH]1 Show InChI InChI=1S/C22H14F2N6O3/c23-16-4-2-1-3-14(16)15-6-5-12(7-17(15)24)10-29-19-18(9-26-29)27-22(28-20(19)31)30-11-13(8-25-30)21(32)33/h1-9,11H,10H2,(H,32,33)(H,27,28,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of HIF-PHD2 (unknown origin) using FAM-HIF2alpha peptide incubated for 20 to 40 mins by fluorescence polarization assay |
ACS Med Chem Lett 11: 1416-1420 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00108
BindingDB Entry DOI: 10.7270/Q25H7KTS |
More data for this
Ligand-Target Pair | |
Extracellular calcium-sensing receptor
(Rattus norvegicus) | BDBM50272591
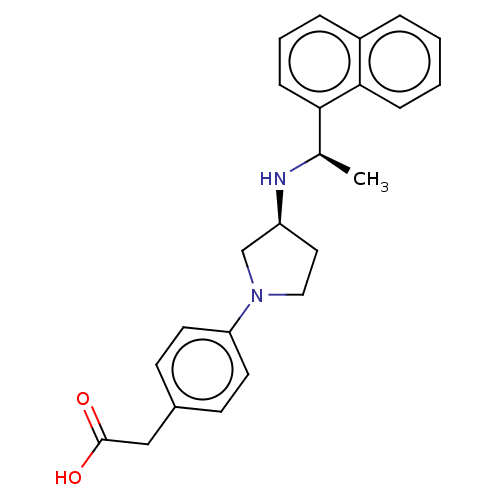
(CHEMBL4128835)Show SMILES Cl.Cl.C[C@@H](N[C@H]1CCN(C1)c1ccc(CC(O)=O)cc1)c1cccc2ccccc12 |r| Show InChI InChI=1S/C24H26N2O2.2ClH/c1-17(22-8-4-6-19-5-2-3-7-23(19)22)25-20-13-14-26(16-20)21-11-9-18(10-12-21)15-24(27)28;;/h2-12,17,20,25H,13-16H2,1H3,(H,27,28);2*1H/t17-,20+;;/m1../s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
| Assay Description
Agonist activity at CaSR in rat parathyroid cells assessed as inhibition of PTH (1 to 84 residues) production in presence of Cacl2 by ELISA |
Bioorg Med Chem Lett 28: 2055-2060 (2018)
Article DOI: 10.1016/j.bmcl.2018.04.055
BindingDB Entry DOI: 10.7270/Q2DF6TP0 |
More data for this
Ligand-Target Pair | |
Extracellular calcium-sensing receptor
(Rattus norvegicus) | BDBM50272594

(CHEMBL4130093)Show SMILES Cl.Cl.C[C@@H](N[C@H]1CCCN(C1)c1cccc(OC(F)(F)F)c1)c1cccc2ccccc12 |r| Show InChI InChI=1S/C24H25F3N2O.2ClH/c1-17(22-13-4-8-18-7-2-3-12-23(18)22)28-19-9-6-14-29(16-19)20-10-5-11-21(15-20)30-24(25,26)27;;/h2-5,7-8,10-13,15,17,19,28H,6,9,14,16H2,1H3;2*1H/t17-,19+;;/m1../s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
| Assay Description
Agonist activity at CaSR in rat parathyroid cells assessed as inhibition of PTH (1 to 84 residues) production in presence of Cacl2 by ELISA |
Bioorg Med Chem Lett 28: 2055-2060 (2018)
Article DOI: 10.1016/j.bmcl.2018.04.055
BindingDB Entry DOI: 10.7270/Q2DF6TP0 |
More data for this
Ligand-Target Pair | |
Egl nine homolog 1
(Homo sapiens (Human)) | BDBM50543055
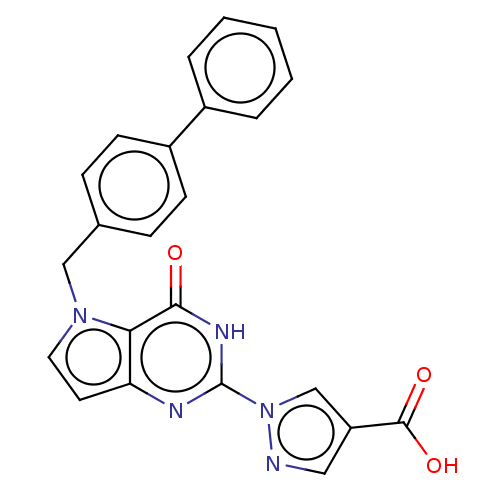
(CHEMBL4647772)Show SMILES OC(=O)c1cnn(c1)-c1nc2ccn(Cc3ccc(cc3)-c3ccccc3)c2c(=O)[nH]1 Show InChI InChI=1S/C23H17N5O3/c29-21-20-19(25-23(26-21)28-14-18(12-24-28)22(30)31)10-11-27(20)13-15-6-8-17(9-7-15)16-4-2-1-3-5-16/h1-12,14H,13H2,(H,30,31)(H,25,26,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of HIF-PHD2 (unknown origin) using FAM-HIF2alpha peptide incubated for 20 to 40 mins by fluorescence polarization assay |
ACS Med Chem Lett 11: 1416-1420 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00108
BindingDB Entry DOI: 10.7270/Q25H7KTS |
More data for this
Ligand-Target Pair | |
Egl nine homolog 1
(Homo sapiens (Human)) | BDBM50543062
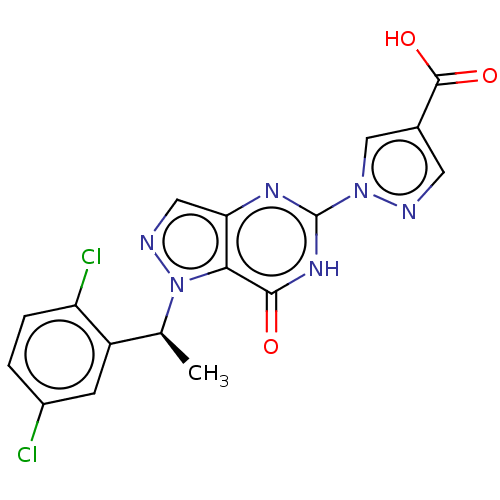
(CHEMBL4646935)Show SMILES C[C@@H](c1cc(Cl)ccc1Cl)n1ncc2nc([nH]c(=O)c12)-n1cc(cn1)C(O)=O |r| Show InChI InChI=1S/C17H12Cl2N6O3/c1-8(11-4-10(18)2-3-12(11)19)25-14-13(6-21-25)22-17(23-15(14)26)24-7-9(5-20-24)16(27)28/h2-8H,1H3,(H,27,28)(H,22,23,26)/t8-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of HIF-PHD2 (unknown origin) using FAM-HIF2alpha peptide incubated for 20 to 40 mins by fluorescence polarization assay |
ACS Med Chem Lett 11: 1416-1420 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00108
BindingDB Entry DOI: 10.7270/Q25H7KTS |
More data for this
Ligand-Target Pair | |
Egl nine homolog 1
(Homo sapiens (Human)) | BDBM50543061
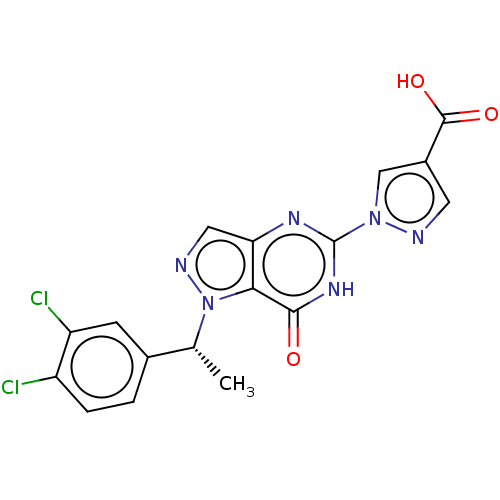
(CHEMBL4638332)Show SMILES C[C@H](c1ccc(Cl)c(Cl)c1)n1ncc2nc([nH]c(=O)c12)-n1cc(cn1)C(O)=O |r| Show InChI InChI=1S/C17H12Cl2N6O3/c1-8(9-2-3-11(18)12(19)4-9)25-14-13(6-21-25)22-17(23-15(14)26)24-7-10(5-20-24)16(27)28/h2-8H,1H3,(H,27,28)(H,22,23,26)/t8-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of HIF-PHD2 (unknown origin) using FAM-HIF2alpha peptide incubated for 20 to 40 mins by fluorescence polarization assay |
ACS Med Chem Lett 11: 1416-1420 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00108
BindingDB Entry DOI: 10.7270/Q25H7KTS |
More data for this
Ligand-Target Pair | |
Egl nine homolog 1
(Homo sapiens (Human)) | BDBM50543053
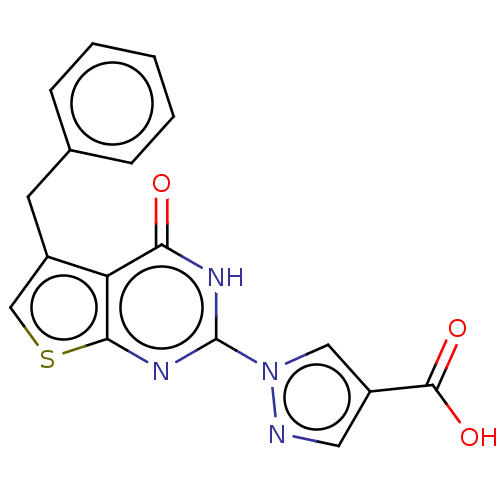
(CHEMBL4649343)Show SMILES OC(=O)c1cnn(c1)-c1nc2scc(Cc3ccccc3)c2c(=O)[nH]1 Show InChI InChI=1S/C17H12N4O3S/c22-14-13-11(6-10-4-2-1-3-5-10)9-25-15(13)20-17(19-14)21-8-12(7-18-21)16(23)24/h1-5,7-9H,6H2,(H,23,24)(H,19,20,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 78 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of HIF-PHD2 (unknown origin) using FAM-HIF2alpha peptide incubated for 20 to 40 mins by fluorescence polarization assay |
ACS Med Chem Lett 11: 1416-1420 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00108
BindingDB Entry DOI: 10.7270/Q25H7KTS |
More data for this
Ligand-Target Pair | |
Extracellular calcium-sensing receptor
(Rattus norvegicus) | BDBM50272603

(CHEMBL4130141)Show SMILES Cl.Cl.C[C@@H](N[C@H]1CCCN(C1)c1ccc(cc1)C(O)=O)c1cccc2ccccc12 |r| Show InChI InChI=1S/C24H26N2O2.2ClH/c1-17(22-10-4-7-18-6-2-3-9-23(18)22)25-20-8-5-15-26(16-20)21-13-11-19(12-14-21)24(27)28;;/h2-4,6-7,9-14,17,20,25H,5,8,15-16H2,1H3,(H,27,28);2*1H/t17-,20+;;/m1../s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
| Assay Description
Agonist activity at CaSR in rat parathyroid cells assessed as inhibition of PTH (1 to 84 residues) production in presence of Cacl2 by ELISA |
Bioorg Med Chem Lett 28: 2055-2060 (2018)
Article DOI: 10.1016/j.bmcl.2018.04.055
BindingDB Entry DOI: 10.7270/Q2DF6TP0 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50416875
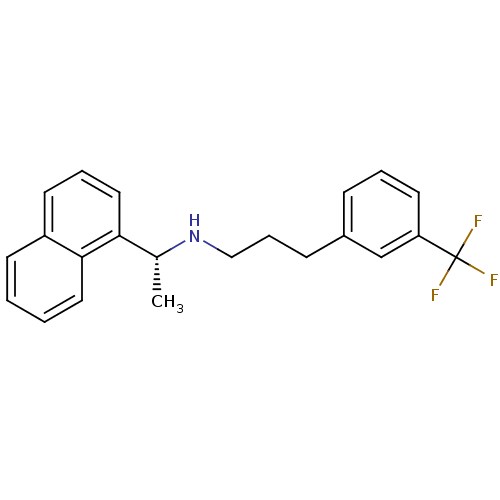
(AMG-073 | AMG073 HCL | CINACALCET | CINACALCET HYD...)Show SMILES C[C@@H](NCCCc1cccc(c1)C(F)(F)F)c1cccc2ccccc12 |r| Show InChI InChI=1S/C22H22F3N/c1-16(20-13-5-10-18-9-2-3-12-21(18)20)26-14-6-8-17-7-4-11-19(15-17)22(23,24)25/h2-5,7,9-13,15-16,26H,6,8,14H2,1H3/t16-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human CYP2D6 expressed in bacterial membranes using AMMC as substrate preincubated for 10 mins followed by NADPH addition m... |
Bioorg Med Chem Lett 28: 2055-2060 (2018)
Article DOI: 10.1016/j.bmcl.2018.04.055
BindingDB Entry DOI: 10.7270/Q2DF6TP0 |
More data for this
Ligand-Target Pair | |
Extracellular calcium-sensing receptor
(Rattus norvegicus) | BDBM50272593
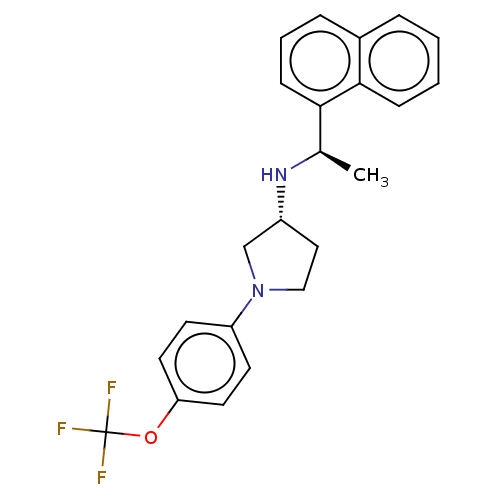
(CHEMBL4129371)Show SMILES Cl.Cl.C[C@@H](N[C@@H]1CCN(C1)c1ccc(OC(F)(F)F)cc1)c1cccc2ccccc12 |r| Show InChI InChI=1S/C23H23F3N2O.2ClH/c1-16(21-8-4-6-17-5-2-3-7-22(17)21)27-18-13-14-28(15-18)19-9-11-20(12-10-19)29-23(24,25)26;;/h2-12,16,18,27H,13-15H2,1H3;2*1H/t16-,18-;;/m1../s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 116 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
| Assay Description
Agonist activity at CaSR in rat parathyroid cells assessed as inhibition of PTH (1 to 84 residues) production in presence of Cacl2 by ELISA |
Bioorg Med Chem Lett 28: 2055-2060 (2018)
Article DOI: 10.1016/j.bmcl.2018.04.055
BindingDB Entry DOI: 10.7270/Q2DF6TP0 |
More data for this
Ligand-Target Pair | |
Egl nine homolog 1
(Homo sapiens (Human)) | BDBM50543051
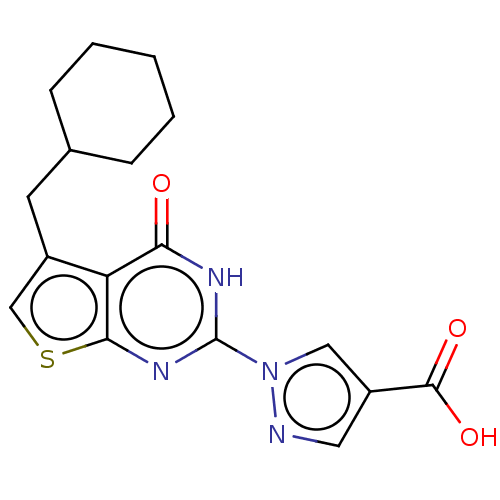
(CHEMBL4638096)Show SMILES OC(=O)c1cnn(c1)-c1nc2scc(CC3CCCCC3)c2c(=O)[nH]1 Show InChI InChI=1S/C17H18N4O3S/c22-14-13-11(6-10-4-2-1-3-5-10)9-25-15(13)20-17(19-14)21-8-12(7-18-21)16(23)24/h7-10H,1-6H2,(H,23,24)(H,19,20,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of HIF-PHD2 (unknown origin) using FAM-HIF2alpha peptide incubated for 20 to 40 mins by fluorescence polarization assay |
ACS Med Chem Lett 11: 1416-1420 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00108
BindingDB Entry DOI: 10.7270/Q25H7KTS |
More data for this
Ligand-Target Pair | |
Egl nine homolog 1
(Homo sapiens (Human)) | BDBM50543052
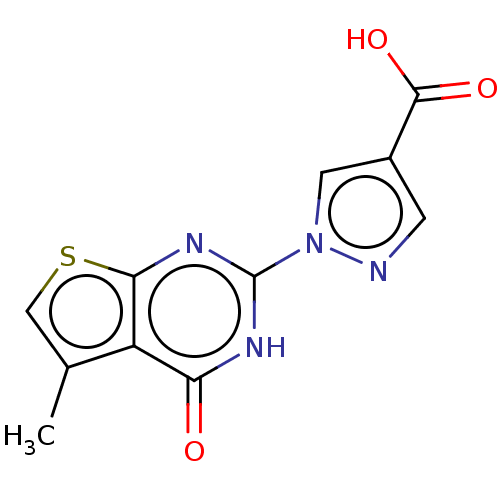
(CHEMBL4644023)Show InChI InChI=1S/C11H8N4O3S/c1-5-4-19-9-7(5)8(16)13-11(14-9)15-3-6(2-12-15)10(17)18/h2-4H,1H3,(H,17,18)(H,13,14,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of HIF-PHD2 (unknown origin) using FAM-HIF2alpha peptide incubated for 20 to 40 mins by fluorescence polarization assay |
ACS Med Chem Lett 11: 1416-1420 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00108
BindingDB Entry DOI: 10.7270/Q25H7KTS |
More data for this
Ligand-Target Pair | |
Egl nine homolog 1
(Homo sapiens (Human)) | BDBM50193145

(2-(1-chloro-4-hydroxyisoquinoline-3-carboxamido)ac...)Show InChI InChI=1S/C12H9ClN2O4/c13-11-7-4-2-1-3-6(7)10(18)9(15-11)12(19)14-5-8(16)17/h1-4,18H,5H2,(H,14,19)(H,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 640 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of HIF-PHD2 (unknown origin) using FAM-HIF2alpha peptide incubated for 20 to 40 mins by fluorescence polarization assay |
ACS Med Chem Lett 11: 1416-1420 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00108
BindingDB Entry DOI: 10.7270/Q25H7KTS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50543892
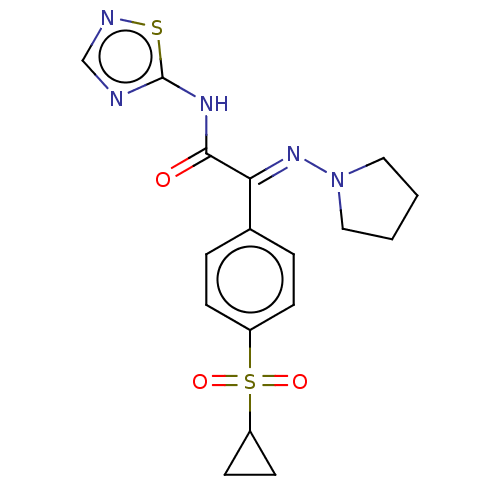
(CHEMBL4649482)Show SMILES O=C(Nc1ncns1)C(=N\N1CCCC1)\c1ccc(cc1)S(=O)(=O)C1CC1 Show InChI InChI=1S/C17H19N5O3S2/c23-16(20-17-18-11-19-26-17)15(21-22-9-1-2-10-22)12-3-5-13(6-4-12)27(24,25)14-7-8-14/h3-6,11,14H,1-2,7-10H2,(H,18,19,20,23)/b21-15+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chiba University
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.127249
BindingDB Entry DOI: 10.7270/Q2P84GG5 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50543890

(CHEMBL4641678)Show SMILES Clc1cnc(NC(=O)C(=N\N2CCCC2)\c2ccc(cc2)S(=O)(=O)C2CC2)s1 Show InChI InChI=1S/C18H19ClN4O3S2/c19-15-11-20-18(27-15)21-17(24)16(22-23-9-1-2-10-23)12-3-5-13(6-4-12)28(25,26)14-7-8-14/h3-6,11,14H,1-2,7-10H2,(H,20,21,24)/b22-16+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.67E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chiba University
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.127249
BindingDB Entry DOI: 10.7270/Q2P84GG5 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50543895
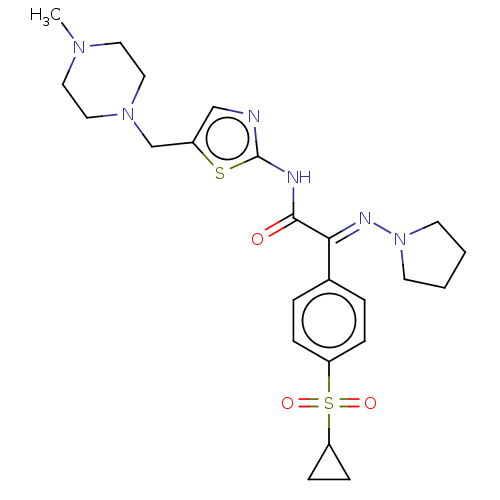
(CHEMBL4646396)Show SMILES CN1CCN(Cc2cnc(NC(=O)C(=N\N3CCCC3)\c3ccc(cc3)S(=O)(=O)C3CC3)s2)CC1 Show InChI InChI=1S/C24H32N6O3S2/c1-28-12-14-29(15-13-28)17-19-16-25-24(34-19)26-23(31)22(27-30-10-2-3-11-30)18-4-6-20(7-5-18)35(32,33)21-8-9-21/h4-7,16,21H,2-3,8-15,17H2,1H3,(H,25,26,31)/b27-22+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.69E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chiba University
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.127249
BindingDB Entry DOI: 10.7270/Q2P84GG5 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50543889
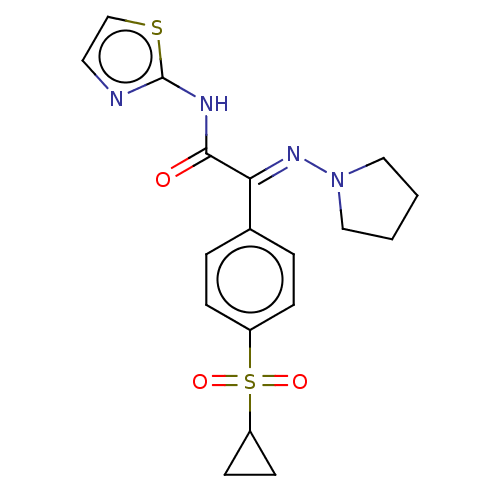
(CHEMBL4645293)Show SMILES O=C(Nc1nccs1)C(=N\N1CCCC1)\c1ccc(cc1)S(=O)(=O)C1CC1 Show InChI InChI=1S/C18H20N4O3S2/c23-17(20-18-19-9-12-26-18)16(21-22-10-1-2-11-22)13-3-5-14(6-4-13)27(24,25)15-7-8-15/h3-6,9,12,15H,1-2,7-8,10-11H2,(H,19,20,23)/b21-16+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.75E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chiba University
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.127249
BindingDB Entry DOI: 10.7270/Q2P84GG5 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50272591

(CHEMBL4128835)Show SMILES Cl.Cl.C[C@@H](N[C@H]1CCN(C1)c1ccc(CC(O)=O)cc1)c1cccc2ccccc12 |r| Show InChI InChI=1S/C24H26N2O2.2ClH/c1-17(22-8-4-6-19-5-2-3-7-23(19)22)25-20-13-14-26(16-20)21-11-9-18(10-12-21)15-24(27)28;;/h2-12,17,20,25H,13-16H2,1H3,(H,27,28);2*1H/t17-,20+;;/m1../s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 (unknown origin) |
Bioorg Med Chem Lett 28: 2055-2060 (2018)
Article DOI: 10.1016/j.bmcl.2018.04.055
BindingDB Entry DOI: 10.7270/Q2DF6TP0 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50272591

(CHEMBL4128835)Show SMILES Cl.Cl.C[C@@H](N[C@H]1CCN(C1)c1ccc(CC(O)=O)cc1)c1cccc2ccccc12 |r| Show InChI InChI=1S/C24H26N2O2.2ClH/c1-17(22-8-4-6-19-5-2-3-7-23(19)22)25-20-13-14-26(16-20)21-11-9-18(10-12-21)15-24(27)28;;/h2-12,17,20,25H,13-16H2,1H3,(H,27,28);2*1H/t17-,20+;;/m1../s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 (unknown origin) |
Bioorg Med Chem Lett 28: 2055-2060 (2018)
Article DOI: 10.1016/j.bmcl.2018.04.055
BindingDB Entry DOI: 10.7270/Q2DF6TP0 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2E1
(Homo sapiens (Human)) | BDBM50272591

(CHEMBL4128835)Show SMILES Cl.Cl.C[C@@H](N[C@H]1CCN(C1)c1ccc(CC(O)=O)cc1)c1cccc2ccccc12 |r| Show InChI InChI=1S/C24H26N2O2.2ClH/c1-17(22-8-4-6-19-5-2-3-7-23(19)22)25-20-13-14-26(16-20)21-11-9-18(10-12-21)15-24(27)28;;/h2-12,17,20,25H,13-16H2,1H3,(H,27,28);2*1H/t17-,20+;;/m1../s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of CYP2E1 (unknown origin) |
Bioorg Med Chem Lett 28: 2055-2060 (2018)
Article DOI: 10.1016/j.bmcl.2018.04.055
BindingDB Entry DOI: 10.7270/Q2DF6TP0 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4/3A5
(Homo sapiens (Human)) | BDBM50272591

(CHEMBL4128835)Show SMILES Cl.Cl.C[C@@H](N[C@H]1CCN(C1)c1ccc(CC(O)=O)cc1)c1cccc2ccccc12 |r| Show InChI InChI=1S/C24H26N2O2.2ClH/c1-17(22-8-4-6-19-5-2-3-7-23(19)22)25-20-13-14-26(16-20)21-11-9-18(10-12-21)15-24(27)28;;/h2-12,17,20,25H,13-16H2,1H3,(H,27,28);2*1H/t17-,20+;;/m1../s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4/5 (unknown origin) |
Bioorg Med Chem Lett 28: 2055-2060 (2018)
Article DOI: 10.1016/j.bmcl.2018.04.055
BindingDB Entry DOI: 10.7270/Q2DF6TP0 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2B6
(Homo sapiens (Human)) | BDBM50272591

(CHEMBL4128835)Show SMILES Cl.Cl.C[C@@H](N[C@H]1CCN(C1)c1ccc(CC(O)=O)cc1)c1cccc2ccccc12 |r| Show InChI InChI=1S/C24H26N2O2.2ClH/c1-17(22-8-4-6-19-5-2-3-7-23(19)22)25-20-13-14-26(16-20)21-11-9-18(10-12-21)15-24(27)28;;/h2-12,17,20,25H,13-16H2,1H3,(H,27,28);2*1H/t17-,20+;;/m1../s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of CYP2B6 (unknown origin) |
Bioorg Med Chem Lett 28: 2055-2060 (2018)
Article DOI: 10.1016/j.bmcl.2018.04.055
BindingDB Entry DOI: 10.7270/Q2DF6TP0 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2A6
(Homo sapiens (Human)) | BDBM50272591

(CHEMBL4128835)Show SMILES Cl.Cl.C[C@@H](N[C@H]1CCN(C1)c1ccc(CC(O)=O)cc1)c1cccc2ccccc12 |r| Show InChI InChI=1S/C24H26N2O2.2ClH/c1-17(22-8-4-6-19-5-2-3-7-23(19)22)25-20-13-14-26(16-20)21-11-9-18(10-12-21)15-24(27)28;;/h2-12,17,20,25H,13-16H2,1H3,(H,27,28);2*1H/t17-,20+;;/m1../s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of CYP2A6 (unknown origin) |
Bioorg Med Chem Lett 28: 2055-2060 (2018)
Article DOI: 10.1016/j.bmcl.2018.04.055
BindingDB Entry DOI: 10.7270/Q2DF6TP0 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50272591

(CHEMBL4128835)Show SMILES Cl.Cl.C[C@@H](N[C@H]1CCN(C1)c1ccc(CC(O)=O)cc1)c1cccc2ccccc12 |r| Show InChI InChI=1S/C24H26N2O2.2ClH/c1-17(22-8-4-6-19-5-2-3-7-23(19)22)25-20-13-14-26(16-20)21-11-9-18(10-12-21)15-24(27)28;;/h2-12,17,20,25H,13-16H2,1H3,(H,27,28);2*1H/t17-,20+;;/m1../s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 (unknown origin) |
Bioorg Med Chem Lett 28: 2055-2060 (2018)
Article DOI: 10.1016/j.bmcl.2018.04.055
BindingDB Entry DOI: 10.7270/Q2DF6TP0 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM50272591

(CHEMBL4128835)Show SMILES Cl.Cl.C[C@@H](N[C@H]1CCN(C1)c1ccc(CC(O)=O)cc1)c1cccc2ccccc12 |r| Show InChI InChI=1S/C24H26N2O2.2ClH/c1-17(22-8-4-6-19-5-2-3-7-23(19)22)25-20-13-14-26(16-20)21-11-9-18(10-12-21)15-24(27)28;;/h2-12,17,20,25H,13-16H2,1H3,(H,27,28);2*1H/t17-,20+;;/m1../s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C8 (unknown origin) |
Bioorg Med Chem Lett 28: 2055-2060 (2018)
Article DOI: 10.1016/j.bmcl.2018.04.055
BindingDB Entry DOI: 10.7270/Q2DF6TP0 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50272591

(CHEMBL4128835)Show SMILES Cl.Cl.C[C@@H](N[C@H]1CCN(C1)c1ccc(CC(O)=O)cc1)c1cccc2ccccc12 |r| Show InChI InChI=1S/C24H26N2O2.2ClH/c1-17(22-8-4-6-19-5-2-3-7-23(19)22)25-20-13-14-26(16-20)21-11-9-18(10-12-21)15-24(27)28;;/h2-12,17,20,25H,13-16H2,1H3,(H,27,28);2*1H/t17-,20+;;/m1../s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 (unknown origin) |
Bioorg Med Chem Lett 28: 2055-2060 (2018)
Article DOI: 10.1016/j.bmcl.2018.04.055
BindingDB Entry DOI: 10.7270/Q2DF6TP0 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50543894
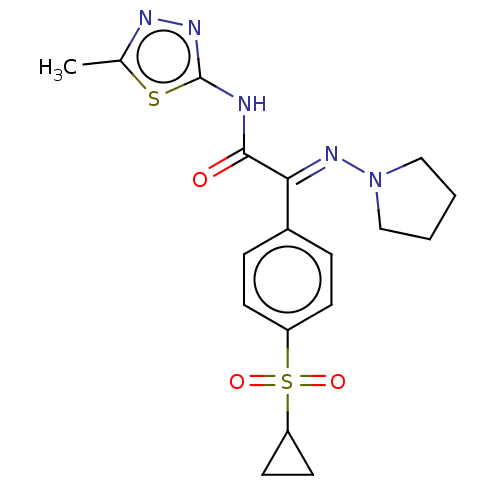
(CHEMBL4637653)Show SMILES Cc1nnc(NC(=O)C(=N\N2CCCC2)\c2ccc(cc2)S(=O)(=O)C2CC2)s1 Show InChI InChI=1S/C18H21N5O3S2/c1-12-20-21-18(27-12)19-17(24)16(22-23-10-2-3-11-23)13-4-6-14(7-5-13)28(25,26)15-8-9-15/h4-7,15H,2-3,8-11H2,1H3,(H,19,21,24)/b22-16+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Chiba University
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.127249
BindingDB Entry DOI: 10.7270/Q2P84GG5 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50543896
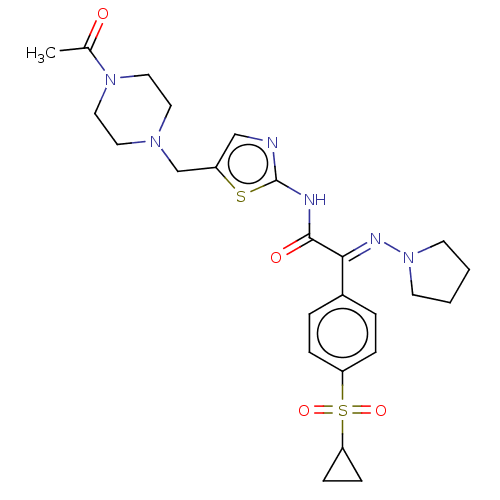
(CHEMBL4644080)Show SMILES CC(=O)N1CCN(Cc2cnc(NC(=O)C(=N\N3CCCC3)\c3ccc(cc3)S(=O)(=O)C3CC3)s2)CC1 Show InChI InChI=1S/C25H32N6O4S2/c1-18(32)30-14-12-29(13-15-30)17-20-16-26-25(36-20)27-24(33)23(28-31-10-2-3-11-31)19-4-6-21(7-5-19)37(34,35)22-8-9-22/h4-7,16,22H,2-3,8-15,17H2,1H3,(H,26,27,33)/b28-23+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Chiba University
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.127249
BindingDB Entry DOI: 10.7270/Q2P84GG5 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50543893
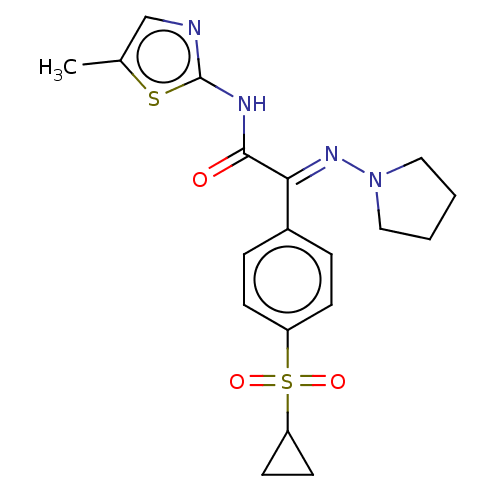
(CHEMBL4632835)Show SMILES Cc1cnc(NC(=O)C(=N\N2CCCC2)\c2ccc(cc2)S(=O)(=O)C2CC2)s1 Show InChI InChI=1S/C19H22N4O3S2/c1-13-12-20-19(27-13)21-18(24)17(22-23-10-2-3-11-23)14-4-6-15(7-5-14)28(25,26)16-8-9-16/h4-7,12,16H,2-3,8-11H2,1H3,(H,20,21,24)/b22-17+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Chiba University
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.127249
BindingDB Entry DOI: 10.7270/Q2P84GG5 |
More data for this
Ligand-Target Pair | |
Hexokinase-4
(Rattus norvegicus) | BDBM50161668
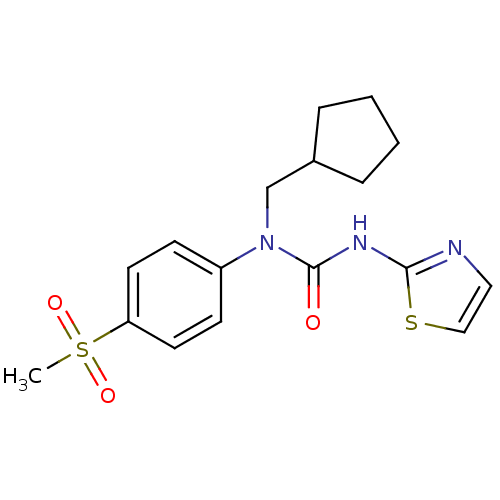
(1-Cyclopentylmethyl-1-(4-methanesulfonyl-phenyl)-3...)Show SMILES CS(=O)(=O)c1ccc(cc1)N(CC1CCCC1)C(=O)Nc1nccs1 Show InChI InChI=1S/C17H21N3O3S2/c1-25(22,23)15-8-6-14(7-9-15)20(12-13-4-2-3-5-13)17(21)19-16-18-10-11-24-16/h6-11,13H,2-5,12H2,1H3,(H,18,19,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals
Curated by ChEMBL
| Assay Description
Effective concentration for glucokinase activation with 5 mM glucose |
Bioorg Med Chem Lett 15: 1501-4 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.083
BindingDB Entry DOI: 10.7270/Q2HT2NVC |
More data for this
Ligand-Target Pair | |
Hexokinase-4
(Rattus norvegicus) | BDBM50161669
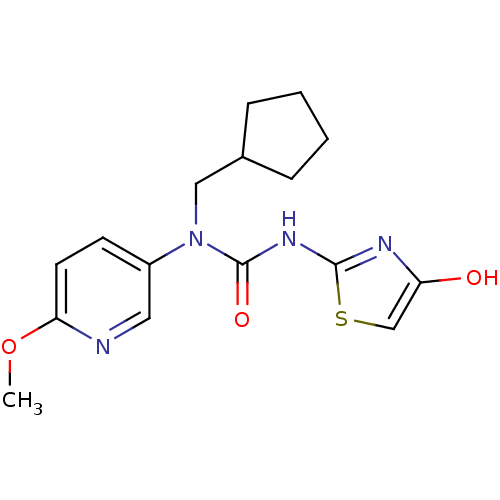
(1-Cyclopentylmethyl-1-(6-methoxy-pyridin-3-yl)-3-(...)Show InChI InChI=1S/C16H20N4O3S/c1-23-14-7-6-12(8-17-14)20(9-11-4-2-3-5-11)16(22)19-15-18-13(21)10-24-15/h6-8,10-11,21H,2-5,9H2,1H3,(H,18,19,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.91E+4 | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals
Curated by ChEMBL
| Assay Description
Effective concentration for glucokinase activation with 5 mM glucose |
Bioorg Med Chem Lett 15: 1501-4 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.083
BindingDB Entry DOI: 10.7270/Q2HT2NVC |
More data for this
Ligand-Target Pair | |
Hexokinase-4
(Rattus norvegicus) | BDBM50161670
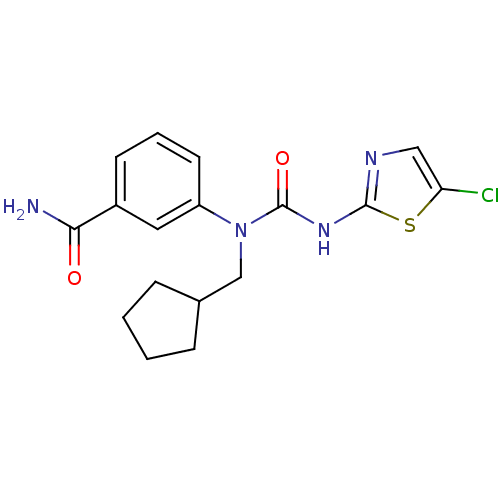
(3-[3-(5-Chloro-thiazol-2-yl)-1-cyclopentylmethyl-u...)Show SMILES NC(=O)c1cccc(c1)N(CC1CCCC1)C(=O)Nc1ncc(Cl)s1 Show InChI InChI=1S/C17H19ClN4O2S/c18-14-9-20-16(25-14)21-17(24)22(10-11-4-1-2-5-11)13-7-3-6-12(8-13)15(19)23/h3,6-9,11H,1-2,4-5,10H2,(H2,19,23)(H,20,21,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals
Curated by ChEMBL
| Assay Description
Effective concentration for glucokinase activation with 5 mM glucose |
Bioorg Med Chem Lett 15: 1501-4 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.083
BindingDB Entry DOI: 10.7270/Q2HT2NVC |
More data for this
Ligand-Target Pair | |
Hexokinase-4
(Rattus norvegicus) | BDBM50161671
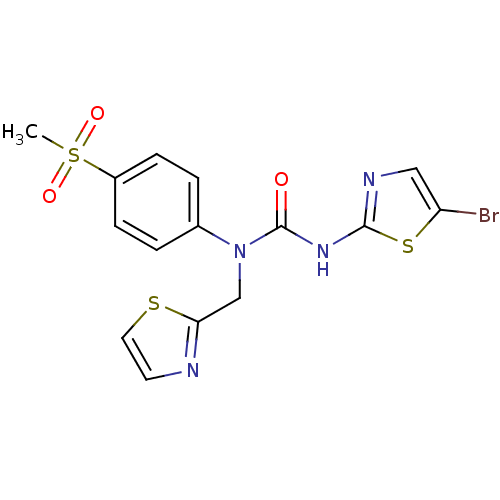
(3-(5-Bromo-thiazol-2-yl)-1-(4-methanesulfonyl-phen...)Show SMILES CS(=O)(=O)c1ccc(cc1)N(Cc1nccs1)C(=O)Nc1ncc(Br)s1 Show InChI InChI=1S/C15H13BrN4O3S3/c1-26(22,23)11-4-2-10(3-5-11)20(9-13-17-6-7-24-13)15(21)19-14-18-8-12(16)25-14/h2-8H,9H2,1H3,(H,18,19,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.41E+4 | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals
Curated by ChEMBL
| Assay Description
Effective concentration for glucokinase activation with 5 mM glucose |
Bioorg Med Chem Lett 15: 1501-4 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.083
BindingDB Entry DOI: 10.7270/Q2HT2NVC |
More data for this
Ligand-Target Pair | |
Hexokinase-4
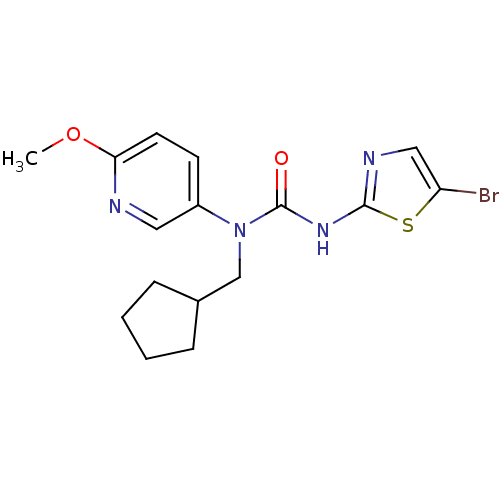
(Rattus norvegicus) | BDBM50161672
(3-(5-Bromo-thiazol-2-yl)-1-cyclopentylmethyl-1-(6-...)Show InChI InChI=1S/C16H19BrN4O2S/c1-23-14-7-6-12(8-18-14)21(10-11-4-2-3-5-11)16(22)20-15-19-9-13(17)24-15/h6-9,11H,2-5,10H2,1H3,(H,19,20,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals
Curated by ChEMBL
| Assay Description
Effective concentration for glucokinase activation with 5 mM glucose |
Bioorg Med Chem Lett 15: 1501-4 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.083
BindingDB Entry DOI: 10.7270/Q2HT2NVC |
More data for this
Ligand-Target Pair | |
Hexokinase-4
(Rattus norvegicus) | BDBM50161673

(1-Cyclopentylmethyl-1-(4-methanesulfonyl-phenyl)-3...)Show SMILES CS(=O)(=O)c1ccc(cc1)N(CC1CCCC1)C(=O)Nc1nnc(s1)C(F)(F)F Show InChI InChI=1S/C17H19F3N4O3S2/c1-29(26,27)13-8-6-12(7-9-13)24(10-11-4-2-3-5-11)16(25)21-15-23-22-14(28-15)17(18,19)20/h6-9,11H,2-5,10H2,1H3,(H,21,23,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 3.42E+4 | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals
Curated by ChEMBL
| Assay Description
Effective concentration for glucokinase activation with 5 mM glucose |
Bioorg Med Chem Lett 15: 1501-4 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.083
BindingDB Entry DOI: 10.7270/Q2HT2NVC |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data