

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lysosomal acid glucosylceramidase (Bos taurus) | BDBM50101405 (CHEMBL3393931) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.3 | n/a |
Universitat Rovira i Virgili Curated by ChEMBL | Assay Description Inhibition of bovine liver beta-glucosidase at pH 7.3 incubated for 10 to 30 mins using beta-D-glycopyranoside substrate by spectrophotometry | Eur J Med Chem 90: 258-66 (2015) Article DOI: 10.1016/j.ejmech.2014.11.002 BindingDB Entry DOI: 10.7270/Q2H996ZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Bos taurus) | BDBM50101398 (CHEMBL3393930) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.11E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.3 | n/a |
Universitat Rovira i Virgili Curated by ChEMBL | Assay Description Inhibition of bovine liver beta-glucosidase at pH 7.3 incubated for 10 to 30 mins using beta-D-glycopyranoside substrate by spectrophotometry | Eur J Med Chem 90: 258-66 (2015) Article DOI: 10.1016/j.ejmech.2014.11.002 BindingDB Entry DOI: 10.7270/Q2H996ZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Bos taurus) | BDBM50101399 (CHEMBL3393929) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.18E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.3 | n/a |
Universitat Rovira i Virgili Curated by ChEMBL | Assay Description Inhibition of bovine liver beta-glucosidase at pH 7.3 incubated for 10 to 30 mins using beta-D-glycopyranoside substrate by spectrophotometry | Eur J Med Chem 90: 258-66 (2015) Article DOI: 10.1016/j.ejmech.2014.11.002 BindingDB Entry DOI: 10.7270/Q2H996ZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Bos taurus) | BDBM50101401 (CHEMBL3393928) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.93E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.3 | n/a |
Universitat Rovira i Virgili Curated by ChEMBL | Assay Description Inhibition of bovine liver beta-glucosidase at pH 7.3 incubated for 10 to 30 mins using beta-D-glycopyranoside substrate by spectrophotometry | Eur J Med Chem 90: 258-66 (2015) Article DOI: 10.1016/j.ejmech.2014.11.002 BindingDB Entry DOI: 10.7270/Q2H996ZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Bos taurus) | BDBM50101403 (CHEMBL3393932) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.26E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.3 | n/a |
Universitat Rovira i Virgili Curated by ChEMBL | Assay Description Inhibition of bovine liver beta-glucosidase at pH 7.3 incubated for 10 to 30 mins using beta-D-glycopyranoside substrate by spectrophotometry | Eur J Med Chem 90: 258-66 (2015) Article DOI: 10.1016/j.ejmech.2014.11.002 BindingDB Entry DOI: 10.7270/Q2H996ZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Bos taurus) | BDBM50101402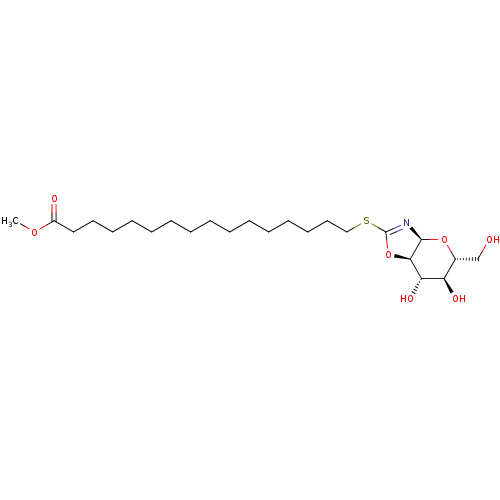 (CHEMBL3393927) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.64E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.3 | n/a |
Universitat Rovira i Virgili Curated by ChEMBL | Assay Description Inhibition of bovine liver beta-glucosidase at pH 7.3 incubated for 10 to 30 mins using beta-D-glycopyranoside substrate by spectrophotometry | Eur J Med Chem 90: 258-66 (2015) Article DOI: 10.1016/j.ejmech.2014.11.002 BindingDB Entry DOI: 10.7270/Q2H996ZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Bos taurus) | BDBM50101402 (CHEMBL3393927) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
Universitat Rovira i Virgili Curated by ChEMBL | Assay Description Inhibition of bovine liver beta-glucosidase at pH 5.5 incubated for 10 to 30 mins using beta-D-glycopyranoside substrate by spectrophotometry | Eur J Med Chem 90: 258-66 (2015) Article DOI: 10.1016/j.ejmech.2014.11.002 BindingDB Entry DOI: 10.7270/Q2H996ZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Bos taurus) | BDBM50101403 (CHEMBL3393932) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
Universitat Rovira i Virgili Curated by ChEMBL | Assay Description Inhibition of bovine liver beta-glucosidase at pH 5.5 incubated for 10 to 30 mins using beta-D-glycopyranoside substrate by spectrophotometry | Eur J Med Chem 90: 258-66 (2015) Article DOI: 10.1016/j.ejmech.2014.11.002 BindingDB Entry DOI: 10.7270/Q2H996ZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Bos taurus) | BDBM50101405 (CHEMBL3393931) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
Universitat Rovira i Virgili Curated by ChEMBL | Assay Description Inhibition of bovine liver beta-glucosidase at pH 5.5 incubated for 10 to 30 mins using beta-D-glycopyranoside substrate by spectrophotometry | Eur J Med Chem 90: 258-66 (2015) Article DOI: 10.1016/j.ejmech.2014.11.002 BindingDB Entry DOI: 10.7270/Q2H996ZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Bos taurus) | BDBM50101398 (CHEMBL3393930) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
Universitat Rovira i Virgili Curated by ChEMBL | Assay Description Inhibition of bovine liver beta-glucosidase at pH 5.5 incubated for 10 to 30 mins using beta-D-glycopyranoside substrate by spectrophotometry | Eur J Med Chem 90: 258-66 (2015) Article DOI: 10.1016/j.ejmech.2014.11.002 BindingDB Entry DOI: 10.7270/Q2H996ZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Bos taurus) | BDBM50101401 (CHEMBL3393928) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 9.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
Universitat Rovira i Virgili Curated by ChEMBL | Assay Description Inhibition of bovine liver beta-glucosidase at pH 5.5 incubated for 10 to 30 mins using beta-D-glycopyranoside substrate by spectrophotometry | Eur J Med Chem 90: 258-66 (2015) Article DOI: 10.1016/j.ejmech.2014.11.002 BindingDB Entry DOI: 10.7270/Q2H996ZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Bos taurus) | BDBM50101399 (CHEMBL3393929) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.39E+5 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
Universitat Rovira i Virgili Curated by ChEMBL | Assay Description Inhibition of bovine liver beta-glucosidase at pH 5.5 incubated for 10 to 30 mins using beta-D-glycopyranoside substrate by spectrophotometry | Eur J Med Chem 90: 258-66 (2015) Article DOI: 10.1016/j.ejmech.2014.11.002 BindingDB Entry DOI: 10.7270/Q2H996ZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Signal transducer and activator of transcription 6 (Homo sapiens (Human)) | BDBM50304425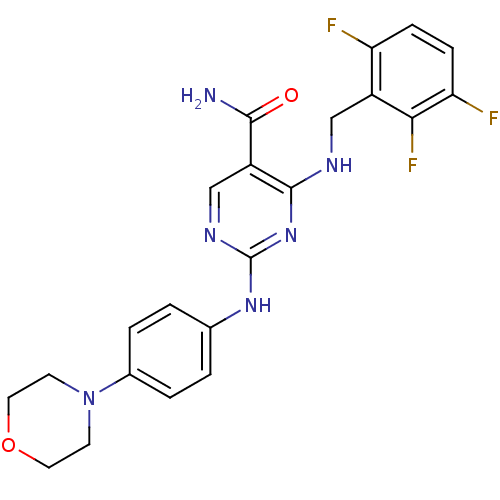 (2-(4-morpholinophenylamino)-4-(2,3,6-trifluorobenz...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Inhibition of STAT6 in IL4-stimulated human FW4 reporter cells by luciferase assay | Bioorg Med Chem 17: 6926-36 (2009) Article DOI: 10.1016/j.bmc.2009.08.021 BindingDB Entry DOI: 10.7270/Q2RV0NS7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM264699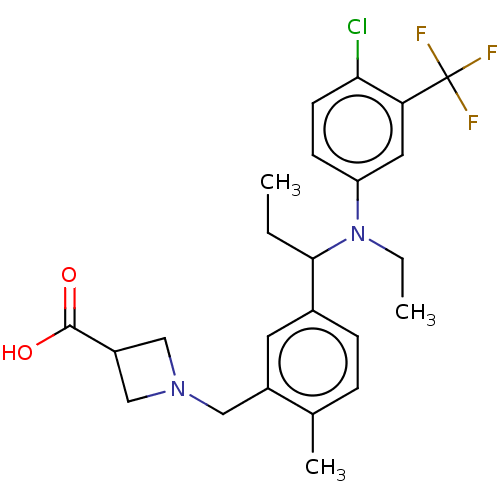 (US9718771, 2-45) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | 7.4 | 25 |
KISSEI PHARMACEUTICAL CO., LTD. US Patent | Assay Description Inhibitory activity evaluation was performed by multi-screen (registered trademark) HTS 96-well plate (Millipore) using the cell membrane fraction st... | US Patent US9718771 (2017) BindingDB Entry DOI: 10.7270/Q2FJ2JSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM264702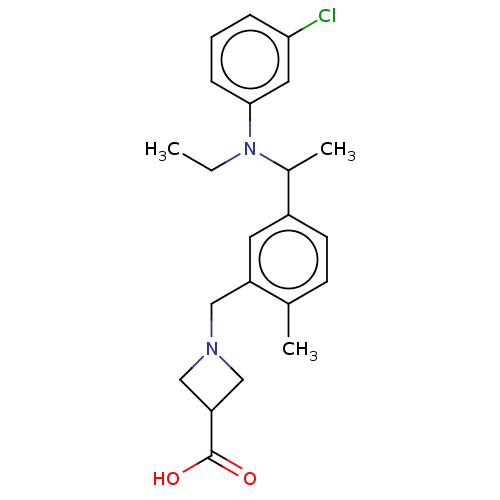 (US9718771, 2-49) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | 7.4 | 25 |
KISSEI PHARMACEUTICAL CO., LTD. US Patent | Assay Description Inhibitory activity evaluation was performed by multi-screen (registered trademark) HTS 96-well plate (Millipore) using the cell membrane fraction st... | US Patent US9718771 (2017) BindingDB Entry DOI: 10.7270/Q2FJ2JSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM261482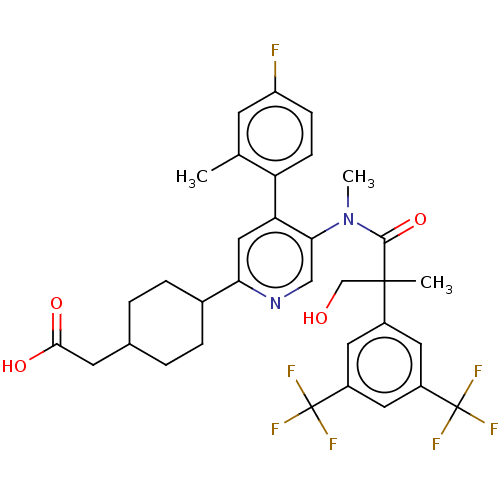 (US10011568, Ex. No. 5 | US9708266, 5) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.980 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research | Assay Description The buffer solution for the receptor binding test was dispensed to the wells of a 96-well assay plate (Greiner) at 22.5 μL/well. DMSO solutions ... | J Med Chem 52: 5013-6 (2009) BindingDB Entry DOI: 10.7270/Q2HT2RQT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50297172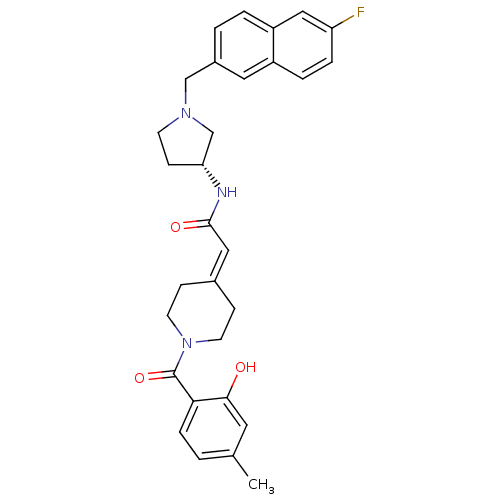 (CHEMBL560275 | N-{(3R)-1-[(6-Fluoro-2-naphthyl)met...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.980 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Antagonist activity at human CCR3 expressed in mouse B300-19 cells assessed as inhibition of eotaxin-induced calcium flux | Bioorg Med Chem 17: 5989-6002 (2009) Article DOI: 10.1016/j.bmc.2009.06.066 BindingDB Entry DOI: 10.7270/Q2WD40NV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM261482 (US10011568, Ex. No. 5 | US9708266, 5) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.980 | n/a | n/a | n/a | n/a | 7.4 | 25 |
KISSEI PHARMACEUTICAL CO., LTD. US Patent | Assay Description The buffer solution for the receptor binding test was dispensed to the wells of a 96-well assay plate (Greiner) at 22.5 μL/well. DMSO solutions ... | US Patent US9708266 (2017) BindingDB Entry DOI: 10.7270/Q2HM5BGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1/2 (RAT) | BDBM50472616 (CHEMBL65325) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory effect on production of prostaglandin E2 (PGE2) in rat synovial cells. | J Med Chem 43: 2040-8 (2000) Article DOI: 10.1021/jm9906015 BindingDB Entry DOI: 10.7270/Q2H134R8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1/2 (RAT) | BDBM50472623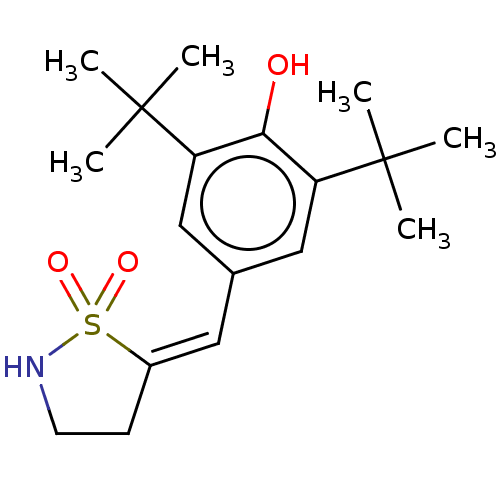 (CHEMBL294100) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory effect on production of prostaglandin E2 (PGE2) in rat synovial cells. | J Med Chem 43: 2040-8 (2000) Article DOI: 10.1021/jm9906015 BindingDB Entry DOI: 10.7270/Q2H134R8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM261483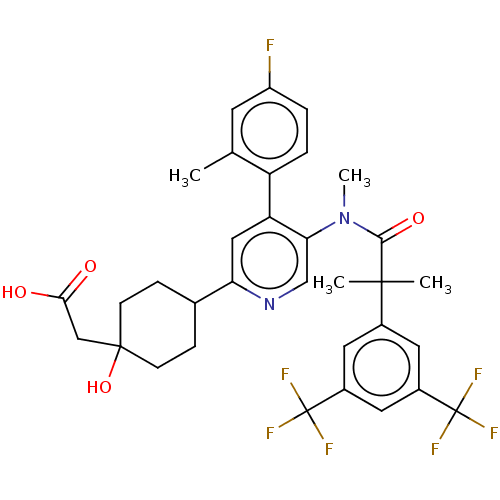 (US10011568, Ex. No. 6 | US9708266, 6) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research | Assay Description The buffer solution for the receptor binding test was dispensed to the wells of a 96-well assay plate (Greiner) at 22.5 μL/well. DMSO solutions ... | J Med Chem 52: 5013-6 (2009) BindingDB Entry DOI: 10.7270/Q2HT2RQT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1/2 (RAT) | BDBM50472618 (CHEMBL303092) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory effect on production of prostaglandin E2 (PGE2) in rat synovial cells. | J Med Chem 43: 2040-8 (2000) Article DOI: 10.1021/jm9906015 BindingDB Entry DOI: 10.7270/Q2H134R8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1/2 (RAT) | BDBM50472624 (CHEMBL62439) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory effect on production of prostaglandin E2 (PGE2) in rat synovial cells. | J Med Chem 43: 2040-8 (2000) Article DOI: 10.1021/jm9906015 BindingDB Entry DOI: 10.7270/Q2H134R8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1/2 (RAT) | BDBM50472632 (CHEMBL65140) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory effect on production of prostaglandin E2 (PGE2) in rat synovial cells. | J Med Chem 43: 2040-8 (2000) Article DOI: 10.1021/jm9906015 BindingDB Entry DOI: 10.7270/Q2H134R8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM264671 (US9718771, 2-6) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.4 | 25 |
KISSEI PHARMACEUTICAL CO., LTD. US Patent | Assay Description Inhibitory activity evaluation was performed by multi-screen (registered trademark) HTS 96-well plate (Millipore) using the cell membrane fraction st... | US Patent US9718771 (2017) BindingDB Entry DOI: 10.7270/Q2FJ2JSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50297171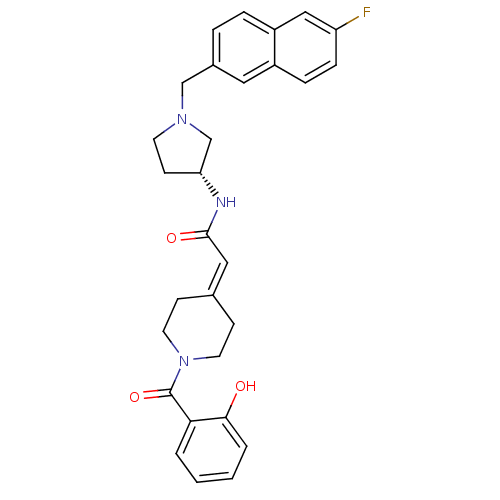 (CHEMBL551735 | N-{(3R)-1-[(6-Fluoro-2-naphthyl)met...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Antagonist activity at human CCR3 expressed in mouse B300-19 cells by functional inhibition curve analysis | Bioorg Med Chem 17: 5989-6002 (2009) Article DOI: 10.1016/j.bmc.2009.06.066 BindingDB Entry DOI: 10.7270/Q2WD40NV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM261483 (US10011568, Ex. No. 6 | US9708266, 6) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.4 | 25 |
KISSEI PHARMACEUTICAL CO., LTD. US Patent | Assay Description The buffer solution for the receptor binding test was dispensed to the wells of a 96-well assay plate (Greiner) at 22.5 μL/well. DMSO solutions ... | US Patent US9708266 (2017) BindingDB Entry DOI: 10.7270/Q2HM5BGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1/2 (RAT) | BDBM50472627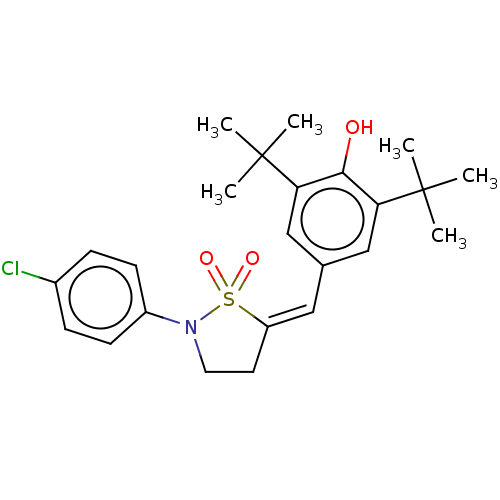 (CHEMBL304489) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory effect on production of prostaglandin E2 (PGE2) in rat synovial cells. | J Med Chem 43: 2040-8 (2000) Article DOI: 10.1021/jm9906015 BindingDB Entry DOI: 10.7270/Q2H134R8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM264691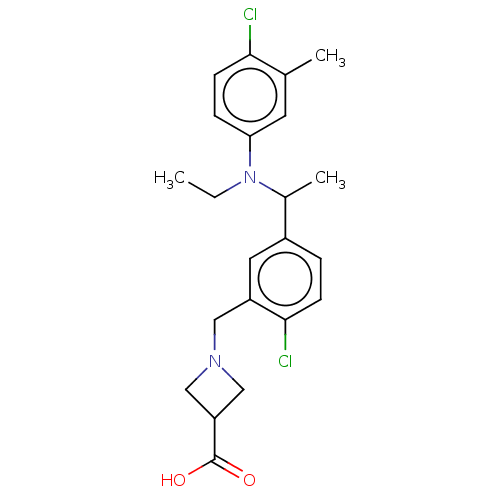 (US9718771, 2-32) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | 7.4 | 25 |
KISSEI PHARMACEUTICAL CO., LTD. US Patent | Assay Description Inhibitory activity evaluation was performed by multi-screen (registered trademark) HTS 96-well plate (Millipore) using the cell membrane fraction st... | US Patent US9718771 (2017) BindingDB Entry DOI: 10.7270/Q2FJ2JSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM264676 (US9718771, 2-11) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | 7.4 | 25 |
KISSEI PHARMACEUTICAL CO., LTD. US Patent | Assay Description Inhibitory activity evaluation was performed by multi-screen (registered trademark) HTS 96-well plate (Millipore) using the cell membrane fraction st... | US Patent US9718771 (2017) BindingDB Entry DOI: 10.7270/Q2FJ2JSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM264706 (US9718771, 4-1) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | 7.4 | 25 |
KISSEI PHARMACEUTICAL CO., LTD. US Patent | Assay Description Inhibitory activity evaluation was performed by multi-screen (registered trademark) HTS 96-well plate (Millipore) using the cell membrane fraction st... | US Patent US9718771 (2017) BindingDB Entry DOI: 10.7270/Q2FJ2JSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM264688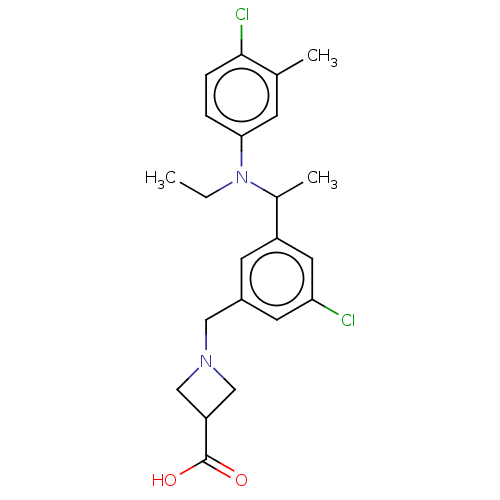 (US9718771, 2-29) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | 7.4 | 25 |
KISSEI PHARMACEUTICAL CO., LTD. US Patent | Assay Description Inhibitory activity evaluation was performed by multi-screen (registered trademark) HTS 96-well plate (Millipore) using the cell membrane fraction st... | US Patent US9718771 (2017) BindingDB Entry DOI: 10.7270/Q2FJ2JSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM264712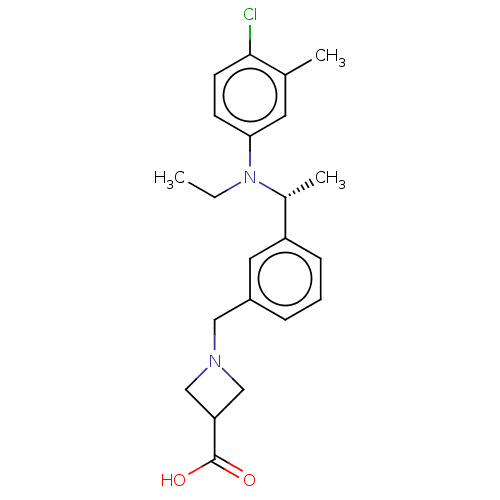 (US9718771, 6-5) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | 7.4 | 25 |
KISSEI PHARMACEUTICAL CO., LTD. US Patent | Assay Description Inhibitory activity evaluation was performed by multi-screen (registered trademark) HTS 96-well plate (Millipore) using the cell membrane fraction st... | US Patent US9718771 (2017) BindingDB Entry DOI: 10.7270/Q2FJ2JSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM264672 (US9718771, 2-7) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | 7.4 | 25 |
KISSEI PHARMACEUTICAL CO., LTD. US Patent | Assay Description Inhibitory activity evaluation was performed by multi-screen (registered trademark) HTS 96-well plate (Millipore) using the cell membrane fraction st... | US Patent US9718771 (2017) BindingDB Entry DOI: 10.7270/Q2FJ2JSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM292016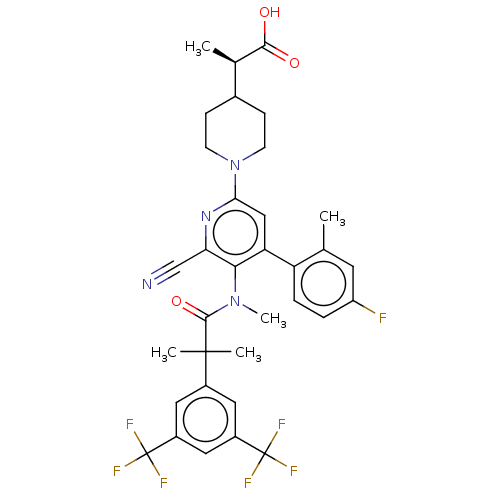 (US10100030, Example 11) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.32 | n/a | n/a | n/a | n/a | 7.4 | 60 |
KISSEI PHARMACEUTICAL CO., LTD. US Patent | Assay Description The buffer solution for the receptor binding test was dispensed to the wells of a 96-well assay plate (Greiner) at 22.5 uL/well. DMSO solutions of a ... | US Patent US10100030 (2018) BindingDB Entry DOI: 10.7270/Q2W95C7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM261478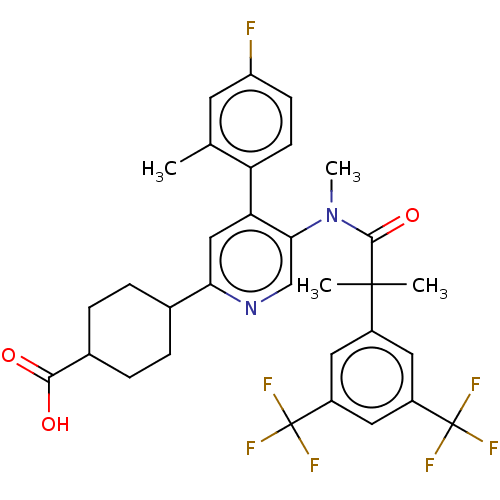 (US10011568, Ex. No. 1 | US9708266, 1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.45 | n/a | n/a | n/a | n/a | 7.4 | 25 |
KISSEI PHARMACEUTICAL CO., LTD. US Patent | Assay Description The buffer solution for the receptor binding test was dispensed to the wells of a 96-well assay plate (Greiner) at 22.5 μL/well. DMSO solutions ... | US Patent US9708266 (2017) BindingDB Entry DOI: 10.7270/Q2HM5BGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM261478 (US10011568, Ex. No. 1 | US9708266, 1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.45 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research | Assay Description The buffer solution for the receptor binding test was dispensed to the wells of a 96-well assay plate (Greiner) at 22.5 μL/well. DMSO solutions ... | J Med Chem 52: 5013-6 (2009) BindingDB Entry DOI: 10.7270/Q2HT2RQT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM264693 (US9718771, 2-34) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | 7.4 | 25 |
KISSEI PHARMACEUTICAL CO., LTD. US Patent | Assay Description Inhibitory activity evaluation was performed by multi-screen (registered trademark) HTS 96-well plate (Millipore) using the cell membrane fraction st... | US Patent US9718771 (2017) BindingDB Entry DOI: 10.7270/Q2FJ2JSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50297181 (2-[1-(1,3-Benzodioxol-5-ylcarbonyl)piperidin-4-yli...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Antagonist activity at human CCR3 expressed in mouse B300-19 cells assessed as inhibition of eotaxin-induced calcium flux | Bioorg Med Chem 17: 5989-6002 (2009) Article DOI: 10.1016/j.bmc.2009.06.066 BindingDB Entry DOI: 10.7270/Q2WD40NV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM261490 (US10011568, Ex. No. 13 | US9708266, 13) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.62 | n/a | n/a | n/a | n/a | 7.4 | 25 |
KISSEI PHARMACEUTICAL CO., LTD. US Patent | Assay Description The buffer solution for the receptor binding test was dispensed to the wells of a 96-well assay plate (Greiner) at 22.5 μL/well. DMSO solutions ... | US Patent US9708266 (2017) BindingDB Entry DOI: 10.7270/Q2HM5BGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM261490 (US10011568, Ex. No. 13 | US9708266, 13) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.62 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research | Assay Description The buffer solution for the receptor binding test was dispensed to the wells of a 96-well assay plate (Greiner) at 22.5 μL/well. DMSO solutions ... | J Med Chem 52: 5013-6 (2009) BindingDB Entry DOI: 10.7270/Q2HT2RQT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM261484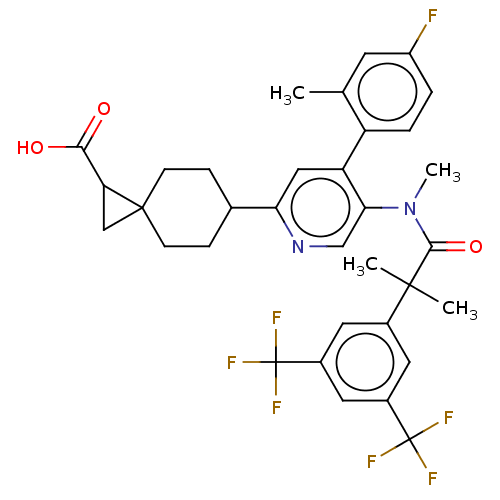 (US10011568, Ex. No. 7 | US9708266, 7) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.63 | n/a | n/a | n/a | n/a | 7.4 | 25 |
KISSEI PHARMACEUTICAL CO., LTD. US Patent | Assay Description The buffer solution for the receptor binding test was dispensed to the wells of a 96-well assay plate (Greiner) at 22.5 μL/well. DMSO solutions ... | US Patent US9708266 (2017) BindingDB Entry DOI: 10.7270/Q2HM5BGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM261484 (US10011568, Ex. No. 7 | US9708266, 7) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.63 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research | Assay Description The buffer solution for the receptor binding test was dispensed to the wells of a 96-well assay plate (Greiner) at 22.5 μL/well. DMSO solutions ... | J Med Chem 52: 5013-6 (2009) BindingDB Entry DOI: 10.7270/Q2HT2RQT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM264679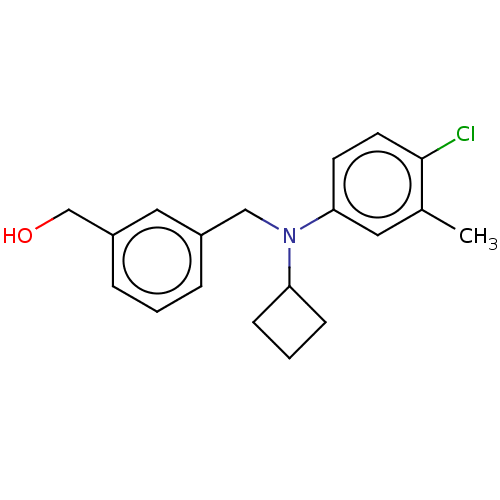 (US9718771, 2-14) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | 7.4 | 25 |
KISSEI PHARMACEUTICAL CO., LTD. US Patent | Assay Description Inhibitory activity evaluation was performed by multi-screen (registered trademark) HTS 96-well plate (Millipore) using the cell membrane fraction st... | US Patent US9718771 (2017) BindingDB Entry DOI: 10.7270/Q2FJ2JSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM261479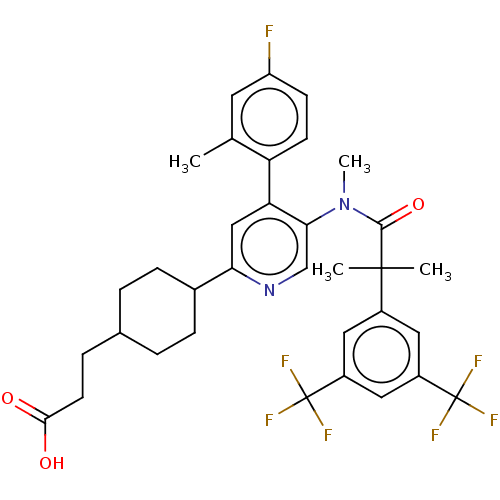 (US10011568, Ex. No. 2 | US9708266, 2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.78 | n/a | n/a | n/a | n/a | 7.4 | 25 |
KISSEI PHARMACEUTICAL CO., LTD. US Patent | Assay Description The buffer solution for the receptor binding test was dispensed to the wells of a 96-well assay plate (Greiner) at 22.5 μL/well. DMSO solutions ... | US Patent US9708266 (2017) BindingDB Entry DOI: 10.7270/Q2HM5BGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM261479 (US10011568, Ex. No. 2 | US9708266, 2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.78 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research | Assay Description The buffer solution for the receptor binding test was dispensed to the wells of a 96-well assay plate (Greiner) at 22.5 μL/well. DMSO solutions ... | J Med Chem 52: 5013-6 (2009) BindingDB Entry DOI: 10.7270/Q2HT2RQT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50297183 (CHEMBL551738 | N-{(3R)-1-[(6-Fluoro-2-naphthyl)met...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Antagonist activity at human CCR3 expressed in mouse B300-19 cells assessed as inhibition of eotaxin-induced calcium flux | Bioorg Med Chem 17: 5989-6002 (2009) Article DOI: 10.1016/j.bmc.2009.06.066 BindingDB Entry DOI: 10.7270/Q2WD40NV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM264701 (US9718771, 2-47) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | 7.4 | 25 |
KISSEI PHARMACEUTICAL CO., LTD. US Patent | Assay Description Inhibitory activity evaluation was performed by multi-screen (registered trademark) HTS 96-well plate (Millipore) using the cell membrane fraction st... | US Patent US9718771 (2017) BindingDB Entry DOI: 10.7270/Q2FJ2JSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM264684 (US9718771, 2-21) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | 7.4 | 25 |
KISSEI PHARMACEUTICAL CO., LTD. US Patent | Assay Description Inhibitory activity evaluation was performed by multi-screen (registered trademark) HTS 96-well plate (Millipore) using the cell membrane fraction st... | US Patent US9718771 (2017) BindingDB Entry DOI: 10.7270/Q2FJ2JSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM264700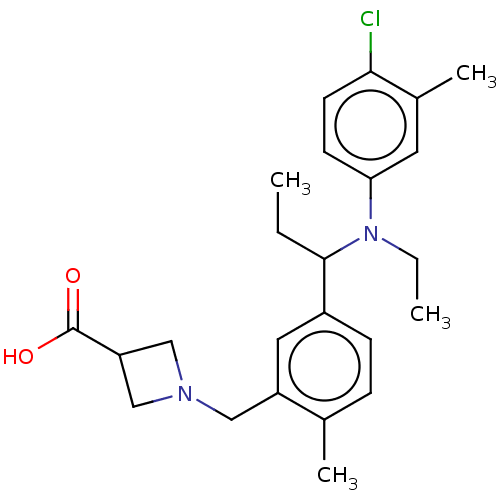 (US9718771, 2-46) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | 7.4 | 25 |
KISSEI PHARMACEUTICAL CO., LTD. US Patent | Assay Description Inhibitory activity evaluation was performed by multi-screen (registered trademark) HTS 96-well plate (Millipore) using the cell membrane fraction st... | US Patent US9718771 (2017) BindingDB Entry DOI: 10.7270/Q2FJ2JSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 442 total ) | Next | Last >> |