 Found 187 hits with Last Name = 'ohwada' and Initial = 'j'
Found 187 hits with Last Name = 'ohwada' and Initial = 'j' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50362779

(CHEMBL1940181 | US9126931, 346)Show SMILES Cc1cc2C(=O)c3c([nH]c4cc(ccc34)C#N)C(C)(C)c2cc1N1CCC(CC1)N1CCOCC1 Show InChI InChI=1S/C29H32N4O2/c1-18-14-22-23(16-25(18)33-8-6-20(7-9-33)32-10-12-35-13-11-32)29(2,3)28-26(27(22)34)21-5-4-19(17-30)15-24(21)31-28/h4-5,14-16,20,31H,6-13H2,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of ALK using Bio-Gastrintide as substrate by TR-FRET assay in presence of 30 uM of ATP |
Bioorg Med Chem 20: 1271-80 (2012)
Article DOI: 10.1016/j.bmc.2011.12.021
BindingDB Entry DOI: 10.7270/Q23B60KW |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50362778
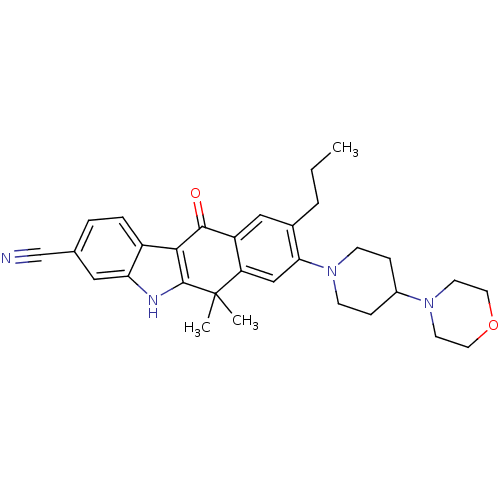
(CHEMBL1940182)Show SMILES CCCc1cc2C(=O)c3c([nH]c4cc(ccc34)C#N)C(C)(C)c2cc1N1CCC(CC1)N1CCOCC1 Show InChI InChI=1S/C31H36N4O2/c1-4-5-21-17-24-25(18-27(21)35-10-8-22(9-11-35)34-12-14-37-15-13-34)31(2,3)30-28(29(24)36)23-7-6-20(19-32)16-26(23)33-30/h6-7,16-18,22,33H,4-5,8-15H2,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of ALK using Bio-Gastrintide as substrate by TR-FRET assay in presence of 30 uM of ATP |
Bioorg Med Chem 20: 1271-80 (2012)
Article DOI: 10.1016/j.bmc.2011.12.021
BindingDB Entry DOI: 10.7270/Q23B60KW |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50344664
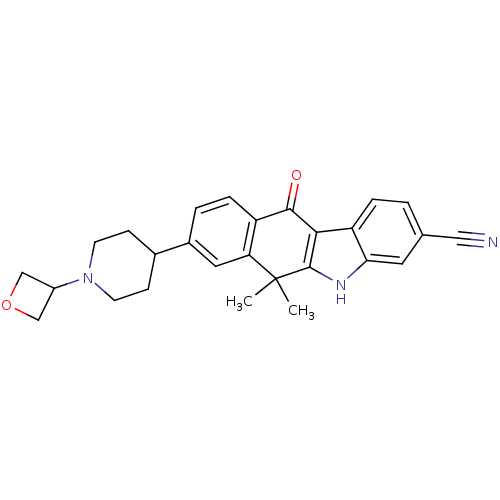
(6,6-dimethyl-8-(1-(oxetan-3-yl)piperidin-4-yl)-11-...)Show SMILES CC1(C)c2[nH]c3cc(ccc3c2C(=O)c2ccc(cc12)C1CCN(CC1)C1COC1)C#N Show InChI InChI=1S/C27H27N3O2/c1-27(2)22-12-18(17-7-9-30(10-8-17)19-14-32-15-19)4-6-20(22)25(31)24-21-5-3-16(13-28)11-23(21)29-26(24)27/h3-6,11-12,17,19,29H,7-10,14-15H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of ALK activity by TR-FRET assay |
J Med Chem 54: 6286-94 (2011)
Article DOI: 10.1021/jm200652u
BindingDB Entry DOI: 10.7270/Q2P55NWZ |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50362781

(1256580-46-7 | AF802 | Alecensa | Alectinib | CH54...)Show SMILES CCc1cc2C(=O)c3c([nH]c4cc(ccc34)C#N)C(C)(C)c2cc1N1CCC(CC1)N1CCOCC1 Show InChI InChI=1S/C30H34N4O2/c1-4-20-16-23-24(17-26(20)34-9-7-21(8-10-34)33-11-13-36-14-12-33)30(2,3)29-27(28(23)35)22-6-5-19(18-31)15-25(22)32-29/h5-6,15-17,21,32H,4,7-14H2,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of ALK using Bio-Gastrintide as substrate by TR-FRET assay in presence of 30 uM of ATP |
Bioorg Med Chem 20: 1271-80 (2012)
Article DOI: 10.1016/j.bmc.2011.12.021
BindingDB Entry DOI: 10.7270/Q23B60KW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50362782
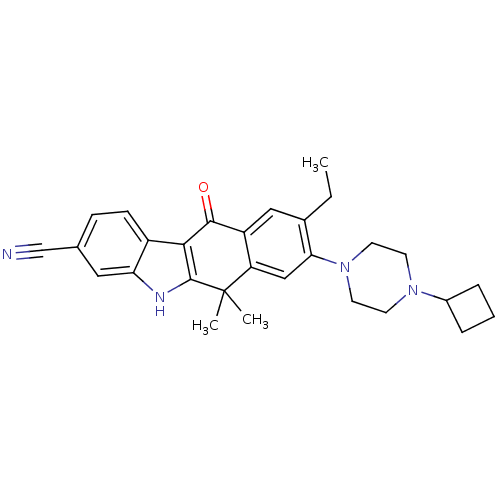
(CHEMBL1940179)Show SMILES CCc1cc2C(=O)c3c([nH]c4cc(ccc34)C#N)C(C)(C)c2cc1N1CCN(CC1)C1CCC1 Show InChI InChI=1S/C29H32N4O/c1-4-19-15-22-23(16-25(19)33-12-10-32(11-13-33)20-6-5-7-20)29(2,3)28-26(27(22)34)21-9-8-18(17-30)14-24(21)31-28/h8-9,14-16,20,31H,4-7,10-13H2,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of ALK using Bio-Gastrintide as substrate by TR-FRET assay in presence of 30 uM of ATP |
Bioorg Med Chem 20: 1271-80 (2012)
Article DOI: 10.1016/j.bmc.2011.12.021
BindingDB Entry DOI: 10.7270/Q23B60KW |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50352764
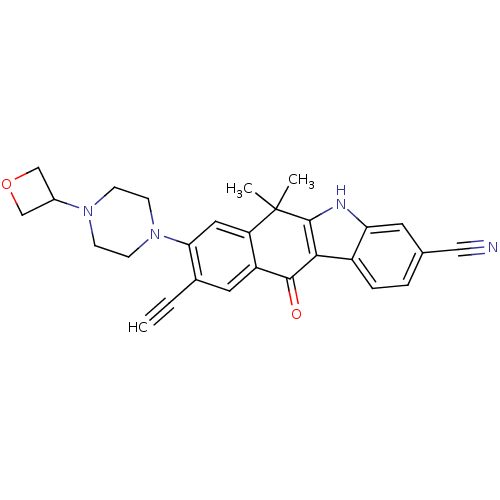
(CHEMBL1823220)Show SMILES CC1(C)c2[nH]c3cc(ccc3c2C(=O)c2cc(C#C)c(cc12)N1CCN(CC1)C1COC1)C#N Show InChI InChI=1S/C28H26N4O2/c1-4-18-12-21-22(13-24(18)32-9-7-31(8-10-32)19-15-34-16-19)28(2,3)27-25(26(21)33)20-6-5-17(14-29)11-23(20)30-27/h1,5-6,11-13,19,30H,7-10,15-16H2,2-3H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of ALK activity by TR-FRET assay |
J Med Chem 54: 6286-94 (2011)
Article DOI: 10.1021/jm200652u
BindingDB Entry DOI: 10.7270/Q2P55NWZ |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50352760

(CHEMBL1823221 | US9126931, 350)Show SMILES CCc1cc2C(=O)c3c([nH]c4cc(ccc34)C#N)C(C)(C)c2cc1N1CCN(CC1)C1COC1 Show InChI InChI=1S/C28H30N4O2/c1-4-18-12-21-22(13-24(18)32-9-7-31(8-10-32)19-15-34-16-19)28(2,3)27-25(26(21)33)20-6-5-17(14-29)11-23(20)30-27/h5-6,11-13,19,30H,4,7-10,15-16H2,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of ALK using Bio-Gastrintide as substrate by TR-FRET assay in presence of 30 uM of ATP |
Bioorg Med Chem 20: 1271-80 (2012)
Article DOI: 10.1016/j.bmc.2011.12.021
BindingDB Entry DOI: 10.7270/Q23B60KW |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50352760

(CHEMBL1823221 | US9126931, 350)Show SMILES CCc1cc2C(=O)c3c([nH]c4cc(ccc34)C#N)C(C)(C)c2cc1N1CCN(CC1)C1COC1 Show InChI InChI=1S/C28H30N4O2/c1-4-18-12-21-22(13-24(18)32-9-7-31(8-10-32)19-15-34-16-19)28(2,3)27-25(26(21)33)20-6-5-17(14-29)11-23(20)30-27/h5-6,11-13,19,30H,4,7-10,15-16H2,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of ALK activity by TR-FRET assay |
J Med Chem 54: 6286-94 (2011)
Article DOI: 10.1021/jm200652u
BindingDB Entry DOI: 10.7270/Q2P55NWZ |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50362783
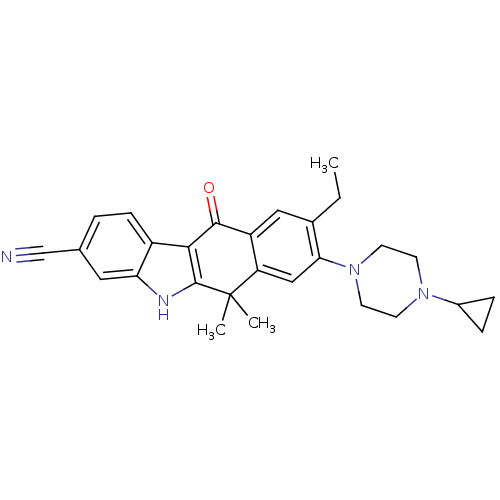
(CHEMBL1940178)Show SMILES CCc1cc2C(=O)c3c([nH]c4cc(ccc34)C#N)C(C)(C)c2cc1N1CCN(CC1)C1CC1 Show InChI InChI=1S/C28H30N4O/c1-4-18-14-21-22(15-24(18)32-11-9-31(10-12-32)19-6-7-19)28(2,3)27-25(26(21)33)20-8-5-17(16-29)13-23(20)30-27/h5,8,13-15,19,30H,4,6-7,9-12H2,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of ALK using Bio-Gastrintide as substrate by TR-FRET assay in presence of 30 uM of ATP |
Bioorg Med Chem 20: 1271-80 (2012)
Article DOI: 10.1016/j.bmc.2011.12.021
BindingDB Entry DOI: 10.7270/Q23B60KW |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50175003
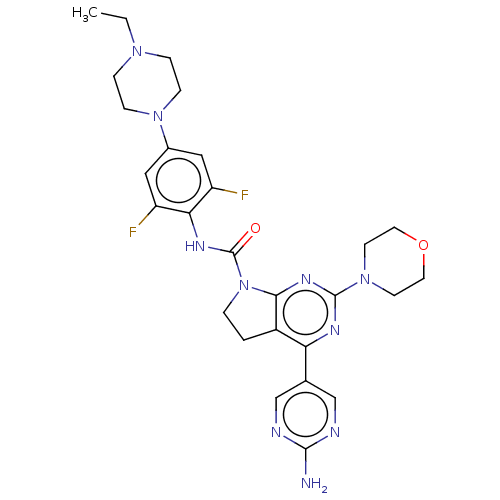
(CHEMBL3808812)Show SMILES CCN1CCN(CC1)c1cc(F)c(NC(=O)N2CCc3c2nc(nc3-c2cnc(N)nc2)N2CCOCC2)c(F)c1 Show InChI InChI=1S/C27H32F2N10O2/c1-2-36-5-7-37(8-6-36)18-13-20(28)23(21(29)14-18)34-27(40)39-4-3-19-22(17-15-31-25(30)32-16-17)33-26(35-24(19)39)38-9-11-41-12-10-38/h13-16H,2-12H2,1H3,(H,34,40)(H2,30,31,32) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human PI3K p110beta/p85alpha expressed in baculovirus preincubated for 20 mins followed by addition of phosphorylate phosphatidylinosit... |
Bioorg Med Chem 24: 2897-2906 (2016)
Article DOI: 10.1016/j.bmc.2016.04.060
BindingDB Entry DOI: 10.7270/Q21J9CQT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50338198

(3-(2-morpholino-7-(pyridin-4-yl)-6,7-dihydro-5H-py...)Show SMILES Oc1cccc(c1)-c1nc(nc2N(CCc12)c1ccncc1)N1CCOCC1 Show InChI InChI=1S/C21H21N5O2/c27-17-3-1-2-15(14-17)19-18-6-9-26(16-4-7-22-8-5-16)20(18)24-21(23-19)25-10-12-28-13-11-25/h1-5,7-8,14,27H,6,9-13H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha |
Bioorg Med Chem Lett 21: 1767-72 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.065
BindingDB Entry DOI: 10.7270/Q23B60F3 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50338198

(3-(2-morpholino-7-(pyridin-4-yl)-6,7-dihydro-5H-py...)Show SMILES Oc1cccc(c1)-c1nc(nc2N(CCc12)c1ccncc1)N1CCOCC1 Show InChI InChI=1S/C21H21N5O2/c27-17-3-1-2-15(14-17)19-18-6-9-26(16-4-7-22-8-5-16)20(18)24-21(23-19)25-10-12-28-13-11-25/h1-5,7-8,14,27H,6,9-13H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using phosphatidylinositol 4,5-bisphosphate as substrate |
Bioorg Med Chem Lett 23: 673-8 (2013)
Article DOI: 10.1016/j.bmcl.2012.11.112
BindingDB Entry DOI: 10.7270/Q2KS6SVT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50175020

(CHEMBL3810369)Show SMILES CCN1CCN(CC1)c1ccc(NC(=O)N2CCc3c2nc(nc3-c2cnc(N)nc2)N2CCOCC2)c(C)c1 Show InChI InChI=1S/C19H13Cl2F3N2OS/c20-14-2-1-3-15(21)13(14)8-9-17-26-16(10-28-17)18(27)25-12-6-4-11(5-7-12)19(22,23)24/h1-7,10H,8-9H2,(H,25,27) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human PI3K p110alpha/p85alpha expressed in baculovirus preincubated for 20 mins followed by addition of phosphorylate phosphatidylinosi... |
Bioorg Med Chem 24: 2897-2906 (2016)
Article DOI: 10.1016/j.bmc.2016.04.060
BindingDB Entry DOI: 10.7270/Q21J9CQT |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50352765
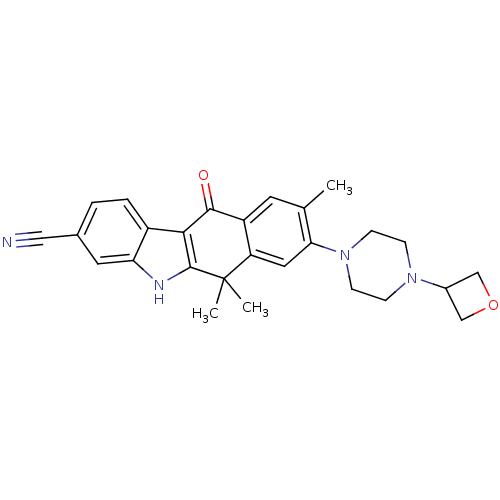
(CHEMBL1823219)Show SMILES Cc1cc2C(=O)c3c([nH]c4cc(ccc34)C#N)C(C)(C)c2cc1N1CCN(CC1)C1COC1 Show InChI InChI=1S/C27H28N4O2/c1-16-10-20-21(12-23(16)31-8-6-30(7-9-31)18-14-33-15-18)27(2,3)26-24(25(20)32)19-5-4-17(13-28)11-22(19)29-26/h4-5,10-12,18,29H,6-9,14-15H2,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of ALK activity by TR-FRET assay |
J Med Chem 54: 6286-94 (2011)
Article DOI: 10.1021/jm200652u
BindingDB Entry DOI: 10.7270/Q2P55NWZ |
More data for this
Ligand-Target Pair | |
Lactoylglutathione lyase
(Homo sapiens (Human)) | BDBM50402202
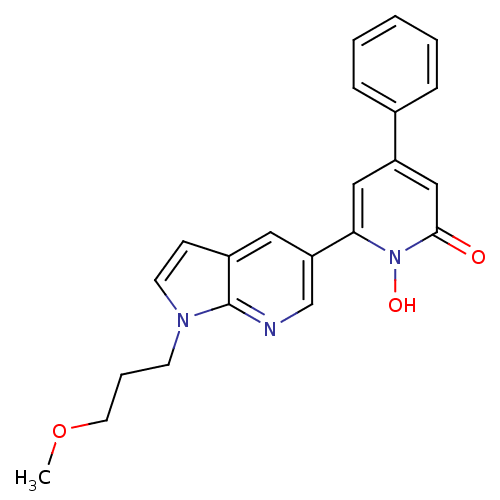
(CHEMBL2203964)Show SMILES COCCCn1ccc2cc(cnc12)-c1cc(cc(=O)n1O)-c1ccccc1 Show InChI InChI=1S/C22H21N3O3/c1-28-11-5-9-24-10-8-17-12-19(15-23-22(17)24)20-13-18(14-21(26)25(20)27)16-6-3-2-4-7-16/h2-4,6-8,10,12-15,27H,5,9,11H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GLO1 expressed in Escherichia coli BL21 assessed as decrease in reduced glutathione level after 1 hr by Ellman's meth... |
Bioorg Med Chem Lett 22: 7486-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.045
BindingDB Entry DOI: 10.7270/Q2RX9D8Q |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50174998
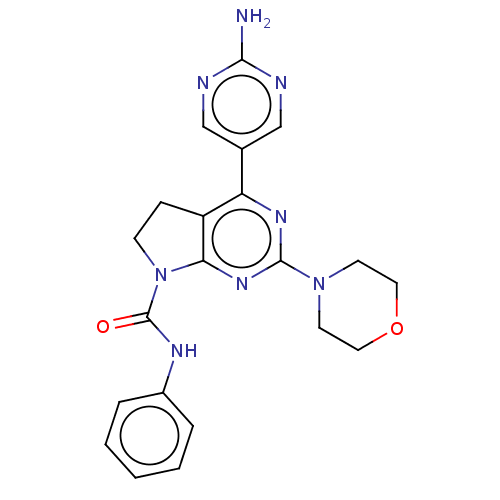
(CHEMBL3808691)Show SMILES Nc1ncc(cn1)-c1nc(nc2N(CCc12)C(=O)Nc1ccccc1)N1CCOCC1 Show InChI InChI=1S/C21H22N8O2/c22-19-23-12-14(13-24-19)17-16-6-7-29(21(30)25-15-4-2-1-3-5-15)18(16)27-20(26-17)28-8-10-31-11-9-28/h1-5,12-13H,6-11H2,(H,25,30)(H2,22,23,24) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human PI3K p110alpha/p85alpha expressed in baculovirus preincubated for 20 mins followed by addition of phosphorylate phosphatidylinosi... |
Bioorg Med Chem 24: 2897-2906 (2016)
Article DOI: 10.1016/j.bmc.2016.04.060
BindingDB Entry DOI: 10.7270/Q21J9CQT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50499395
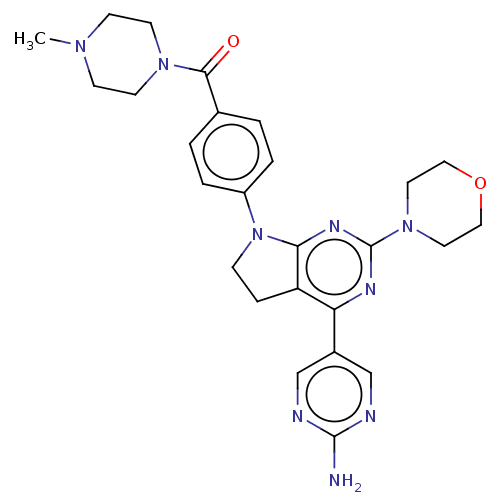
(CHEMBL3735840)Show SMILES CN1CCN(CC1)C(=O)c1ccc(cc1)N1CCc2c1nc(nc2-c1cnc(N)nc1)N1CCOCC1 Show InChI InChI=1S/C26H31N9O2/c1-32-8-10-33(11-9-32)24(36)18-2-4-20(5-3-18)35-7-6-21-22(19-16-28-25(27)29-17-19)30-26(31-23(21)35)34-12-14-37-15-13-34/h2-5,16-17H,6-15H2,1H3,(H2,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co. Ltd
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) by adapta universal kinase assay |
Bioorg Med Chem 23: 7650-60 (2015)
Article DOI: 10.1016/j.bmc.2015.11.009
BindingDB Entry DOI: 10.7270/Q2CN76XT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50338197
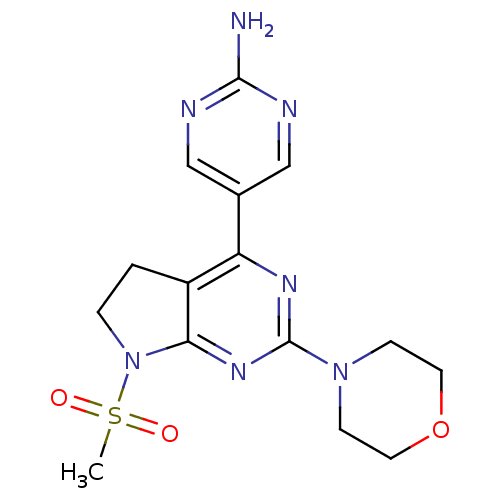
(5-(7-(methylsulfonyl)-2-morpholino-6,7-dihydro-5H-...)Show SMILES CS(=O)(=O)N1CCc2c1nc(nc2-c1cnc(N)nc1)N1CCOCC1 Show InChI InChI=1S/C15H19N7O3S/c1-26(23,24)22-3-2-11-12(10-8-17-14(16)18-9-10)19-15(20-13(11)22)21-4-6-25-7-5-21/h8-9H,2-7H2,1H3,(H2,16,17,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha |
Bioorg Med Chem Lett 21: 1767-72 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.065
BindingDB Entry DOI: 10.7270/Q23B60F3 |
More data for this
Ligand-Target Pair | |
Lactoylglutathione lyase
(Homo sapiens (Human)) | BDBM50402204
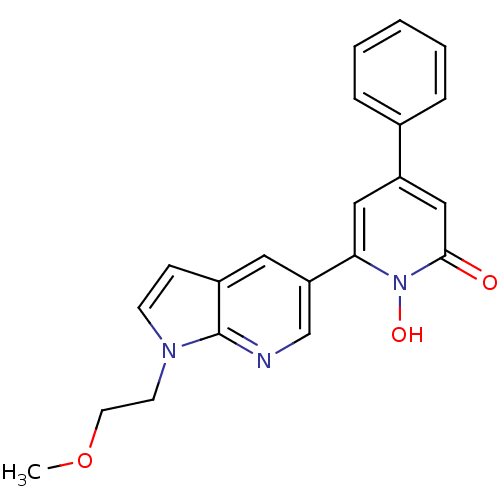
(CHEMBL2203978)Show SMILES COCCn1ccc2cc(cnc12)-c1cc(cc(=O)n1O)-c1ccccc1 Show InChI InChI=1S/C21H19N3O3/c1-27-10-9-23-8-7-16-11-18(14-22-21(16)23)19-12-17(13-20(25)24(19)26)15-5-3-2-4-6-15/h2-8,11-14,26H,9-10H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GLO1 expressed in Escherichia coli BL21 assessed as decrease in reduced glutathione level after 1 hr by Ellman's meth... |
Bioorg Med Chem Lett 22: 7486-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.045
BindingDB Entry DOI: 10.7270/Q2RX9D8Q |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50352763
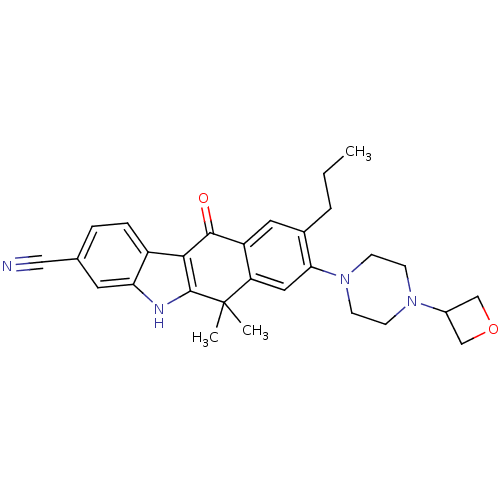
(CHEMBL1823222)Show SMILES CCCc1cc2C(=O)c3c([nH]c4cc(ccc34)C#N)C(C)(C)c2cc1N1CCN(CC1)C1COC1 Show InChI InChI=1S/C29H32N4O2/c1-4-5-19-13-22-23(14-25(19)33-10-8-32(9-11-33)20-16-35-17-20)29(2,3)28-26(27(22)34)21-7-6-18(15-30)12-24(21)31-28/h6-7,12-14,20,31H,4-5,8-11,16-17H2,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of ALK activity by TR-FRET assay |
J Med Chem 54: 6286-94 (2011)
Article DOI: 10.1021/jm200652u
BindingDB Entry DOI: 10.7270/Q2P55NWZ |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50362780
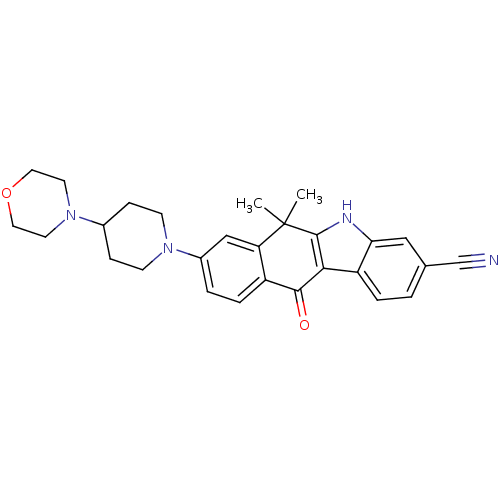
(CHEMBL1940180)Show SMILES CC1(C)c2[nH]c3cc(ccc3c2C(=O)c2ccc(cc12)N1CCC(CC1)N1CCOCC1)C#N Show InChI InChI=1S/C28H30N4O2/c1-28(2)23-16-20(31-9-7-19(8-10-31)32-11-13-34-14-12-32)4-6-21(23)26(33)25-22-5-3-18(17-29)15-24(22)30-27(25)28/h3-6,15-16,19,30H,7-14H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of ALK using Bio-Gastrintide as substrate by TR-FRET assay in presence of 30 uM of ATP |
Bioorg Med Chem 20: 1271-80 (2012)
Article DOI: 10.1016/j.bmc.2011.12.021
BindingDB Entry DOI: 10.7270/Q23B60KW |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50175003

(CHEMBL3808812)Show SMILES CCN1CCN(CC1)c1cc(F)c(NC(=O)N2CCc3c2nc(nc3-c2cnc(N)nc2)N2CCOCC2)c(F)c1 Show InChI InChI=1S/C27H32F2N10O2/c1-2-36-5-7-37(8-6-36)18-13-20(28)23(21(29)14-18)34-27(40)39-4-3-19-22(17-15-31-25(30)32-16-17)33-26(35-24(19)39)38-9-11-41-12-10-38/h13-16H,2-12H2,1H3,(H,34,40)(H2,30,31,32) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human PI3K p110alpha/p85alpha expressed in baculovirus preincubated for 20 mins followed by addition of phosphorylate phosphatidylinosi... |
Bioorg Med Chem 24: 2897-2906 (2016)
Article DOI: 10.1016/j.bmc.2016.04.060
BindingDB Entry DOI: 10.7270/Q21J9CQT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50499397
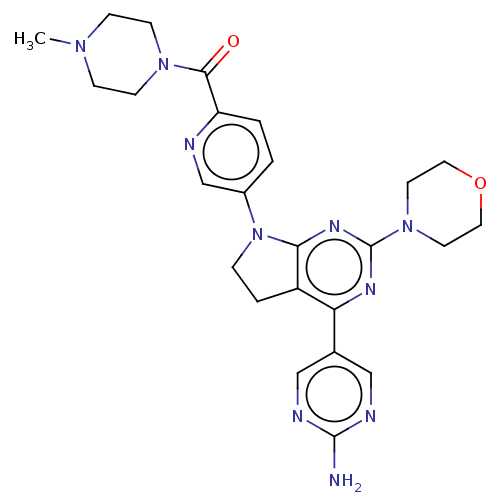
(CHEMBL3734954)Show SMILES CN1CCN(CC1)C(=O)c1ccc(cn1)N1CCc2c1nc(nc2-c1cnc(N)nc1)N1CCOCC1 Show InChI InChI=1S/C25H30N10O2/c1-32-6-8-33(9-7-32)23(36)20-3-2-18(16-27-20)35-5-4-19-21(17-14-28-24(26)29-15-17)30-25(31-22(19)35)34-10-12-37-13-11-34/h2-3,14-16H,4-13H2,1H3,(H2,26,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co. Ltd
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) by adapta universal kinase assay |
Bioorg Med Chem 23: 7650-60 (2015)
Article DOI: 10.1016/j.bmc.2015.11.009
BindingDB Entry DOI: 10.7270/Q2CN76XT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50425230
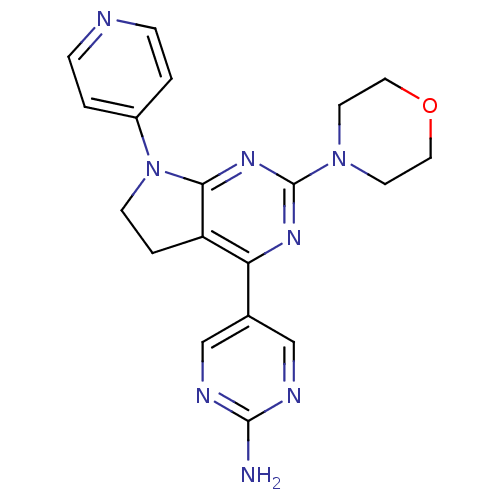
(CHEMBL2314287)Show SMILES Nc1ncc(cn1)-c1nc(nc2N(CCc12)c1ccncc1)N1CCOCC1 Show InChI InChI=1S/C19H20N8O/c20-18-22-11-13(12-23-18)16-15-3-6-27(14-1-4-21-5-2-14)17(15)25-19(24-16)26-7-9-28-10-8-26/h1-2,4-5,11-12H,3,6-10H2,(H2,20,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using phosphatidylinositol 4,5-bisphosphate as substrate |
Bioorg Med Chem Lett 23: 673-8 (2013)
Article DOI: 10.1016/j.bmcl.2012.11.112
BindingDB Entry DOI: 10.7270/Q2KS6SVT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50175021

(CHEMBL3810395)Show SMILES CCN1CCN(CC1)C(=O)c1ccc(NC(=O)N2CCc3c2nc(nc3-c2cnc(N)nc2)N2CCOCC2)c(C)c1 Show InChI InChI=1S/C16H9Cl2F3N4OS/c17-10-5-22-6-11(18)13(10)25-15-24-12(7-27-15)14(26)23-9-3-1-8(2-4-9)16(19,20)21/h1-7H,(H,23,26)(H,22,24,25) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human PI3K p110alpha/p85alpha expressed in baculovirus preincubated for 20 mins followed by addition of phosphorylate phosphatidylinosi... |
Bioorg Med Chem 24: 2897-2906 (2016)
Article DOI: 10.1016/j.bmc.2016.04.060
BindingDB Entry DOI: 10.7270/Q21J9CQT |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50344663

(6,6-dimethyl-8-(4-(oxetan-3-yl)piperazin-1-yl)-11-...)Show SMILES CC1(C)c2[nH]c3cc(ccc3c2C(=O)c2ccc(cc12)N1CCN(CC1)C1COC1)C#N Show InChI InChI=1S/C26H26N4O2/c1-26(2)21-12-17(29-7-9-30(10-8-29)18-14-32-15-18)4-6-19(21)24(31)23-20-5-3-16(13-27)11-22(20)28-25(23)26/h3-6,11-12,18,28H,7-10,14-15H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of ALK activity by TR-FRET assay |
J Med Chem 54: 6286-94 (2011)
Article DOI: 10.1021/jm200652u
BindingDB Entry DOI: 10.7270/Q2P55NWZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50499396

(CHEMBL3736189)Show SMILES CN1CCN(CC1)C(=O)c1cncc(c1)N1CCc2c1nc(nc2-c1cnc(N)nc1)N1CCOCC1 Show InChI InChI=1S/C25H30N10O2/c1-32-4-6-33(7-5-32)23(36)17-12-19(16-27-13-17)35-3-2-20-21(18-14-28-24(26)29-15-18)30-25(31-22(20)35)34-8-10-37-11-9-34/h12-16H,2-11H2,1H3,(H2,26,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co. Ltd
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) by adapta universal kinase assay |
Bioorg Med Chem 23: 7650-60 (2015)
Article DOI: 10.1016/j.bmc.2015.11.009
BindingDB Entry DOI: 10.7270/Q2CN76XT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50338199

(5-(2-Morpholin-4-yl-7-pyridin-3-yl-6,7-dihydro-5H-...)Show SMILES Nc1ncc(cn1)-c1nc(nc2N(CCc12)c1cccnc1)N1CCOCC1 Show InChI InChI=1S/C19H20N8O/c20-18-22-10-13(11-23-18)16-15-3-5-27(14-2-1-4-21-12-14)17(15)25-19(24-16)26-6-8-28-9-7-26/h1-2,4,10-12H,3,5-9H2,(H2,20,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using phosphatidylinositol 4,5-bisphosphate as substrate |
Bioorg Med Chem Lett 23: 673-8 (2013)
Article DOI: 10.1016/j.bmcl.2012.11.112
BindingDB Entry DOI: 10.7270/Q2KS6SVT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50338199

(5-(2-Morpholin-4-yl-7-pyridin-3-yl-6,7-dihydro-5H-...)Show SMILES Nc1ncc(cn1)-c1nc(nc2N(CCc12)c1cccnc1)N1CCOCC1 Show InChI InChI=1S/C19H20N8O/c20-18-22-10-13(11-23-18)16-15-3-5-27(14-2-1-4-21-12-14)17(15)25-19(24-16)26-6-8-28-9-7-26/h1-2,4,10-12H,3,5-9H2,(H2,20,22,23) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human PI3K p110alpha/p85alpha expressed in baculovirus preincubated for 20 mins followed by addition of phosphorylate phosphatidylinosi... |
Bioorg Med Chem 24: 2897-2906 (2016)
Article DOI: 10.1016/j.bmc.2016.04.060
BindingDB Entry DOI: 10.7270/Q21J9CQT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50338199

(5-(2-Morpholin-4-yl-7-pyridin-3-yl-6,7-dihydro-5H-...)Show SMILES Nc1ncc(cn1)-c1nc(nc2N(CCc12)c1cccnc1)N1CCOCC1 Show InChI InChI=1S/C19H20N8O/c20-18-22-10-13(11-23-18)16-15-3-5-27(14-2-1-4-21-12-14)17(15)25-19(24-16)26-6-8-28-9-7-26/h1-2,4,10-12H,3,5-9H2,(H2,20,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha |
Bioorg Med Chem Lett 21: 1767-72 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.065
BindingDB Entry DOI: 10.7270/Q23B60F3 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50338199

(5-(2-Morpholin-4-yl-7-pyridin-3-yl-6,7-dihydro-5H-...)Show SMILES Nc1ncc(cn1)-c1nc(nc2N(CCc12)c1cccnc1)N1CCOCC1 Show InChI InChI=1S/C19H20N8O/c20-18-22-10-13(11-23-18)16-15-3-5-27(14-2-1-4-21-12-14)17(15)25-19(24-16)26-6-8-28-9-7-26/h1-2,4,10-12H,3,5-9H2,(H2,20,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co. Ltd
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) by adapta universal kinase assay |
Bioorg Med Chem 23: 7650-60 (2015)
Article DOI: 10.1016/j.bmc.2015.11.009
BindingDB Entry DOI: 10.7270/Q2CN76XT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50499390

(CHEMBL3736165)Show SMILES Nc1ncc(cn1)-c1nc(nc2N(CCc12)c1ccccc1)N1CCOCC1 Show InChI InChI=1S/C20H21N7O/c21-19-22-12-14(13-23-19)17-16-6-7-27(15-4-2-1-3-5-15)18(16)25-20(24-17)26-8-10-28-11-9-26/h1-5,12-13H,6-11H2,(H2,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co. Ltd
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) by adapta universal kinase assay |
Bioorg Med Chem 23: 7650-60 (2015)
Article DOI: 10.1016/j.bmc.2015.11.009
BindingDB Entry DOI: 10.7270/Q2CN76XT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50338197

(5-(7-(methylsulfonyl)-2-morpholino-6,7-dihydro-5H-...)Show SMILES CS(=O)(=O)N1CCc2c1nc(nc2-c1cnc(N)nc1)N1CCOCC1 Show InChI InChI=1S/C15H19N7O3S/c1-26(23,24)22-3-2-11-12(10-8-17-14(16)18-9-10)19-15(20-13(11)22)21-4-6-25-7-5-21/h8-9H,2-7H2,1H3,(H2,16,17,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
| PDB
Article
PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma |
Bioorg Med Chem Lett 21: 1767-72 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.065
BindingDB Entry DOI: 10.7270/Q23B60F3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Lactoylglutathione lyase
(Homo sapiens (Human)) | BDBM50402206
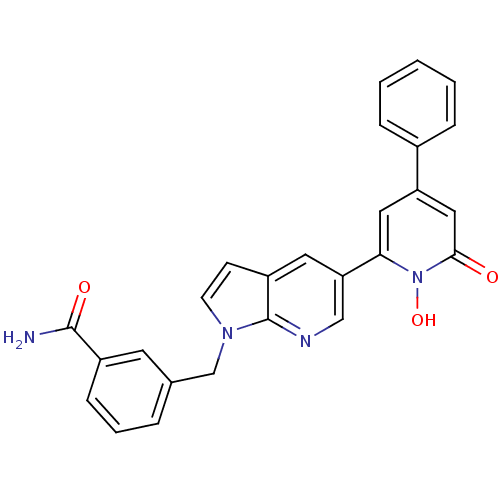
(CHEMBL2203976)Show SMILES NC(=O)c1cccc(Cn2ccc3cc(cnc23)-c2cc(cc(=O)n2O)-c2ccccc2)c1 Show InChI InChI=1S/C26H20N4O3/c27-25(32)19-8-4-5-17(11-19)16-29-10-9-20-12-22(15-28-26(20)29)23-13-21(14-24(31)30(23)33)18-6-2-1-3-7-18/h1-15,33H,16H2,(H2,27,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GLO1 expressed in Escherichia coli BL21 assessed as decrease in reduced glutathione level after 1 hr by Ellman's meth... |
Bioorg Med Chem Lett 22: 7486-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.045
BindingDB Entry DOI: 10.7270/Q2RX9D8Q |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50499394

(CHEMBL3736509)Show SMILES CN1CCN(CC1)C(=O)c1cc(ccn1)N1CCc2c1nc(nc2-c1cnc(N)nc1)N1CCOCC1 Show InChI InChI=1S/C25H30N10O2/c1-32-6-8-33(9-7-32)23(36)20-14-18(2-4-27-20)35-5-3-19-21(17-15-28-24(26)29-16-17)30-25(31-22(19)35)34-10-12-37-13-11-34/h2,4,14-16H,3,5-13H2,1H3,(H2,26,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co. Ltd
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) by adapta universal kinase assay |
Bioorg Med Chem 23: 7650-60 (2015)
Article DOI: 10.1016/j.bmc.2015.11.009
BindingDB Entry DOI: 10.7270/Q2CN76XT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50338200

(3-(2-morpholino-7-(pyridin-3-yl)-6,7-dihydro-5H-py...)Show SMILES Oc1cccc(c1)-c1nc(nc2N(CCc12)c1cccnc1)N1CCOCC1 Show InChI InChI=1S/C21H21N5O2/c27-17-5-1-3-15(13-17)19-18-6-8-26(16-4-2-7-22-14-16)20(18)24-21(23-19)25-9-11-28-12-10-25/h1-5,7,13-14,27H,6,8-12H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using phosphatidylinositol 4,5-bisphosphate as substrate |
Bioorg Med Chem Lett 23: 673-8 (2013)
Article DOI: 10.1016/j.bmcl.2012.11.112
BindingDB Entry DOI: 10.7270/Q2KS6SVT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50338200

(3-(2-morpholino-7-(pyridin-3-yl)-6,7-dihydro-5H-py...)Show SMILES Oc1cccc(c1)-c1nc(nc2N(CCc12)c1cccnc1)N1CCOCC1 Show InChI InChI=1S/C21H21N5O2/c27-17-5-1-3-15(13-17)19-18-6-8-26(16-4-2-7-22-14-16)20(18)24-21(23-19)25-9-11-28-12-10-25/h1-5,7,13-14,27H,6,8-12H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha |
Bioorg Med Chem Lett 21: 1767-72 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.065
BindingDB Entry DOI: 10.7270/Q23B60F3 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50499389
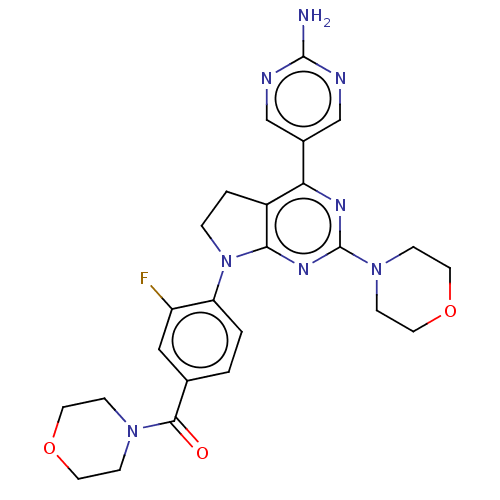
(CHEMBL3736206)Show SMILES Nc1ncc(cn1)-c1nc(nc2N(CCc12)c1ccc(cc1F)C(=O)N1CCOCC1)N1CCOCC1 Show InChI InChI=1S/C25H27FN8O3/c26-19-13-16(23(35)32-5-9-36-10-6-32)1-2-20(19)34-4-3-18-21(17-14-28-24(27)29-15-17)30-25(31-22(18)34)33-7-11-37-12-8-33/h1-2,13-15H,3-12H2,(H2,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co. Ltd
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) by adapta universal kinase assay |
Bioorg Med Chem 23: 7650-60 (2015)
Article DOI: 10.1016/j.bmc.2015.11.009
BindingDB Entry DOI: 10.7270/Q2CN76XT |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50362787
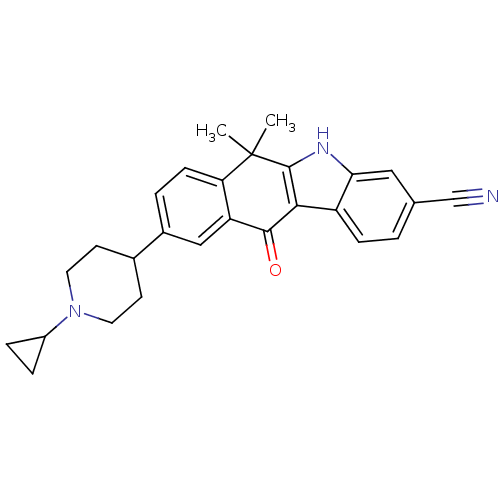
(CHEMBL1940174)Show SMILES CC1(C)c2[nH]c3cc(ccc3c2C(=O)c2cc(ccc12)C1CCN(CC1)C1CC1)C#N Show InChI InChI=1S/C27H27N3O/c1-27(2)22-8-4-18(17-9-11-30(12-10-17)19-5-6-19)14-21(22)25(31)24-20-7-3-16(15-28)13-23(20)29-26(24)27/h3-4,7-8,13-14,17,19,29H,5-6,9-12H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of ALK using Bio-Gastrintide as substrate by TR-FRET assay in presence of 30 uM of ATP |
Bioorg Med Chem 20: 1271-80 (2012)
Article DOI: 10.1016/j.bmc.2011.12.021
BindingDB Entry DOI: 10.7270/Q23B60KW |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50499392

(CHEMBL3736186)Show SMILES Nc1ncc(cn1)-c1nc(nc2N(CCc12)c1ccc(CN2CCOCC2)cc1)N1CCOCC1 Show InChI InChI=1S/C25H30N8O2/c26-24-27-15-19(16-28-24)22-21-5-6-33(23(21)30-25(29-22)32-9-13-35-14-10-32)20-3-1-18(2-4-20)17-31-7-11-34-12-8-31/h1-4,15-16H,5-14,17H2,(H2,26,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co. Ltd
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) by adapta universal kinase assay |
Bioorg Med Chem 23: 7650-60 (2015)
Article DOI: 10.1016/j.bmc.2015.11.009
BindingDB Entry DOI: 10.7270/Q2CN76XT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50499393
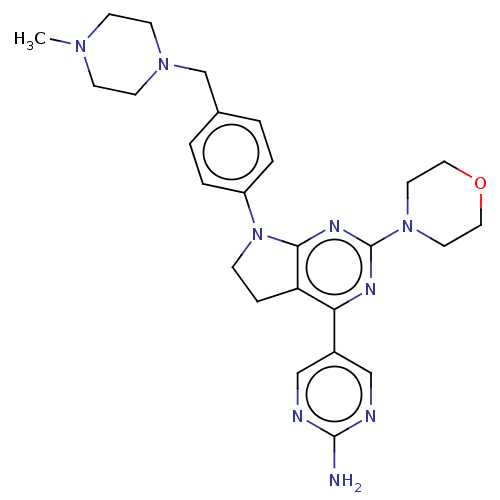
(CHEMBL3735088)Show SMILES CN1CCN(Cc2ccc(cc2)N2CCc3c2nc(nc3-c2cnc(N)nc2)N2CCOCC2)CC1 Show InChI InChI=1S/C26H33N9O/c1-32-8-10-33(11-9-32)18-19-2-4-21(5-3-19)35-7-6-22-23(20-16-28-25(27)29-17-20)30-26(31-24(22)35)34-12-14-36-15-13-34/h2-5,16-17H,6-15,18H2,1H3,(H2,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co. Ltd
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) by adapta universal kinase assay |
Bioorg Med Chem 23: 7650-60 (2015)
Article DOI: 10.1016/j.bmc.2015.11.009
BindingDB Entry DOI: 10.7270/Q2CN76XT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50425224
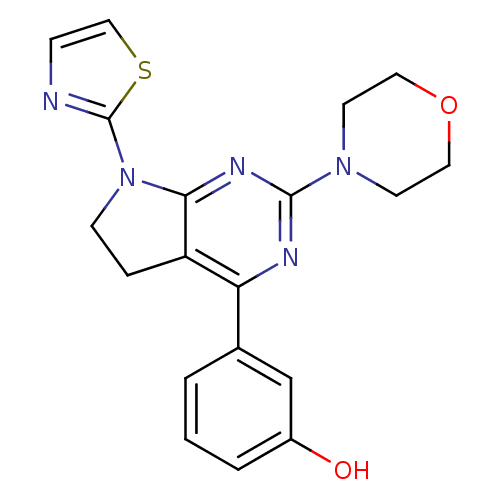
(CHEMBL2314582)Show SMILES Oc1cccc(c1)-c1nc(nc2N(CCc12)c1nccs1)N1CCOCC1 Show InChI InChI=1S/C19H19N5O2S/c25-14-3-1-2-13(12-14)16-15-4-6-24(19-20-5-11-27-19)17(15)22-18(21-16)23-7-9-26-10-8-23/h1-3,5,11-12,25H,4,6-10H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using phosphatidylinositol 4,5-bisphosphate as substrate |
Bioorg Med Chem Lett 23: 673-8 (2013)
Article DOI: 10.1016/j.bmcl.2012.11.112
BindingDB Entry DOI: 10.7270/Q2KS6SVT |
More data for this
Ligand-Target Pair | |
Mast/stem cell growth factor receptor Kit
(Homo sapiens (Human)) | BDBM50352765

(CHEMBL1823219)Show SMILES Cc1cc2C(=O)c3c([nH]c4cc(ccc34)C#N)C(C)(C)c2cc1N1CCN(CC1)C1COC1 Show InChI InChI=1S/C27H28N4O2/c1-16-10-20-21(12-23(16)31-8-6-30(7-9-31)18-14-33-15-18)27(2,3)26-24(25(20)32)19-5-4-17(13-28)11-22(19)29-26/h4-5,10-12,18,29H,6-9,14-15H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of KIT activity by TR-FRET assay |
J Med Chem 54: 6286-94 (2011)
Article DOI: 10.1021/jm200652u
BindingDB Entry DOI: 10.7270/Q2P55NWZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50175004

(CHEMBL3808686)Show SMILES Cc1ccccc1NC(=O)N1CCc2c1nc(nc2-c1cnc(N)nc1)N1CCOCC1 Show InChI InChI=1S/C22H24N8O2/c1-14-4-2-3-5-17(14)26-22(31)30-7-6-16-18(15-12-24-20(23)25-13-15)27-21(28-19(16)30)29-8-10-32-11-9-29/h2-5,12-13H,6-11H2,1H3,(H,26,31)(H2,23,24,25) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human PI3K p110alpha/p85alpha expressed in baculovirus preincubated for 20 mins followed by addition of phosphorylate phosphatidylinosi... |
Bioorg Med Chem 24: 2897-2906 (2016)
Article DOI: 10.1016/j.bmc.2016.04.060
BindingDB Entry DOI: 10.7270/Q21J9CQT |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50352765

(CHEMBL1823219)Show SMILES Cc1cc2C(=O)c3c([nH]c4cc(ccc34)C#N)C(C)(C)c2cc1N1CCN(CC1)C1COC1 Show InChI InChI=1S/C27H28N4O2/c1-16-10-20-21(12-23(16)31-8-6-30(7-9-31)18-14-33-15-18)27(2,3)26-24(25(20)32)19-5-4-17(13-28)11-22(19)29-26/h4-5,10-12,18,29H,6-9,14-15H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 61 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of KDR activity by TR-FRET assay |
J Med Chem 54: 6286-94 (2011)
Article DOI: 10.1021/jm200652u
BindingDB Entry DOI: 10.7270/Q2P55NWZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50175023
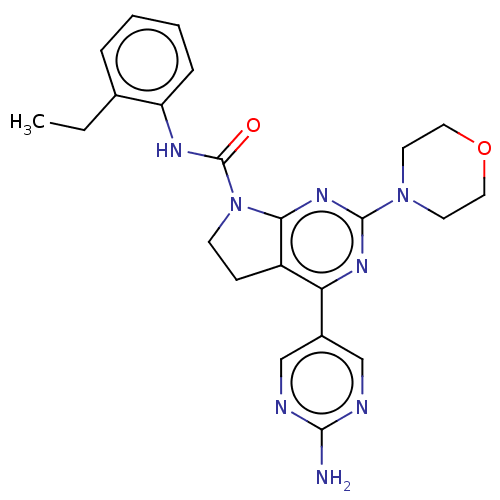
(CHEMBL3810130)Show SMILES CCc1ccccc1NC(=O)N1CCc2c1nc(nc2-c1cnc(N)nc1)N1CCOCC1 Show InChI InChI=1S/C19H13Cl2F3N2OS/c1-10-17(18(27)26-12-7-5-11(6-8-12)19(22,23)24)28-16(25-10)9-13-14(20)3-2-4-15(13)21/h2-8H,9H2,1H3,(H,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human PI3K p110alpha/p85alpha expressed in baculovirus preincubated for 20 mins followed by addition of phosphorylate phosphatidylinosi... |
Bioorg Med Chem 24: 2897-2906 (2016)
Article DOI: 10.1016/j.bmc.2016.04.060
BindingDB Entry DOI: 10.7270/Q21J9CQT |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50352762

(CHEMBL1823223)Show SMILES CC(C)c1cc2C(=O)c3c([nH]c4cc(ccc34)C#N)C(C)(C)c2cc1N1CCN(CC1)C1COC1 Show InChI InChI=1S/C29H32N4O2/c1-17(2)21-12-22-23(13-25(21)33-9-7-32(8-10-33)19-15-35-16-19)29(3,4)28-26(27(22)34)20-6-5-18(14-30)11-24(20)31-28/h5-6,11-13,17,19,31H,7-10,15-16H2,1-4H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 66 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of ALK activity by TR-FRET assay |
J Med Chem 54: 6286-94 (2011)
Article DOI: 10.1021/jm200652u
BindingDB Entry DOI: 10.7270/Q2P55NWZ |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50362786
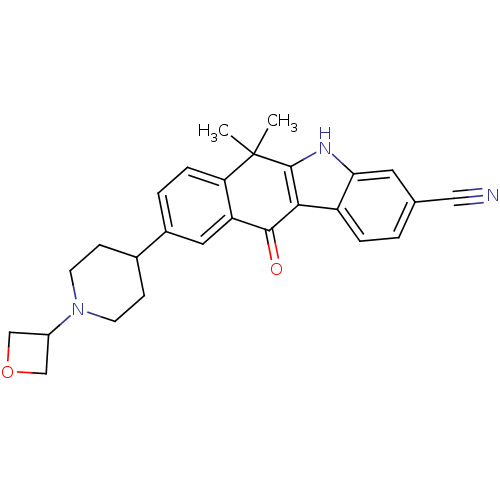
(CHEMBL1940175)Show SMILES CC1(C)c2[nH]c3cc(ccc3c2C(=O)c2cc(ccc12)C1CCN(CC1)C1COC1)C#N Show InChI InChI=1S/C27H27N3O2/c1-27(2)22-6-4-18(17-7-9-30(10-8-17)19-14-32-15-19)12-21(22)25(31)24-20-5-3-16(13-28)11-23(20)29-26(24)27/h3-6,11-12,17,19,29H,7-10,14-15H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of ALK using Bio-Gastrintide as substrate by TR-FRET assay in presence of 30 uM of ATP |
Bioorg Med Chem 20: 1271-80 (2012)
Article DOI: 10.1016/j.bmc.2011.12.021
BindingDB Entry DOI: 10.7270/Q23B60KW |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50362784
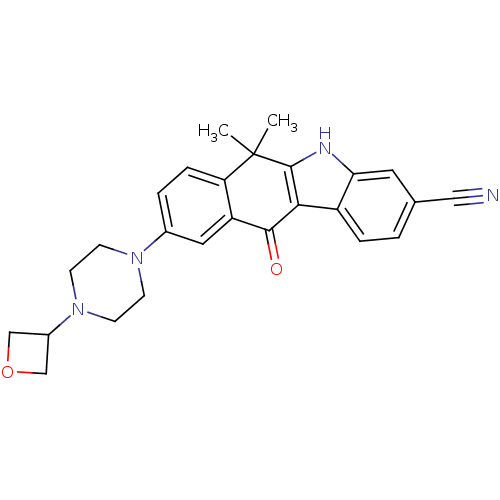
(CHEMBL1940177 | US9126931, 438)Show SMILES CC1(C)c2[nH]c3cc(ccc3c2C(=O)c2cc(ccc12)N1CCN(CC1)C1COC1)C#N Show InChI InChI=1S/C26H26N4O2/c1-26(2)21-6-4-17(29-7-9-30(10-8-29)18-14-32-15-18)12-20(21)24(31)23-19-5-3-16(13-27)11-22(19)28-25(23)26/h3-6,11-12,18,28H,7-10,14-15H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 69.3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of ALK using Bio-Gastrintide as substrate by TR-FRET assay in presence of 30 uM of ATP |
Bioorg Med Chem 20: 1271-80 (2012)
Article DOI: 10.1016/j.bmc.2011.12.021
BindingDB Entry DOI: 10.7270/Q23B60KW |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50338196

(3-(7-(1H-benzo[d]imidazol-6-yl)-2-morpholino-6,7-d...)Show SMILES Oc1cccc(c1)-c1nc(nc2N(CCc12)c1ccc2nc[nH]c2c1)N1CCOCC1 Show InChI InChI=1S/C23H22N6O2/c30-17-3-1-2-15(12-17)21-18-6-7-29(16-4-5-19-20(13-16)25-14-24-19)22(18)27-23(26-21)28-8-10-31-11-9-28/h1-5,12-14,30H,6-11H2,(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 77 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using phosphatidylinositol 4,5-bisphosphate as substrate |
Bioorg Med Chem Lett 23: 673-8 (2013)
Article DOI: 10.1016/j.bmcl.2012.11.112
BindingDB Entry DOI: 10.7270/Q2KS6SVT |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data