 Found 112 hits with Last Name = 'otsubo' and Initial = 't'
Found 112 hits with Last Name = 'otsubo' and Initial = 't' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Plasminogen
(Homo sapiens (Human)) | BDBM50500938
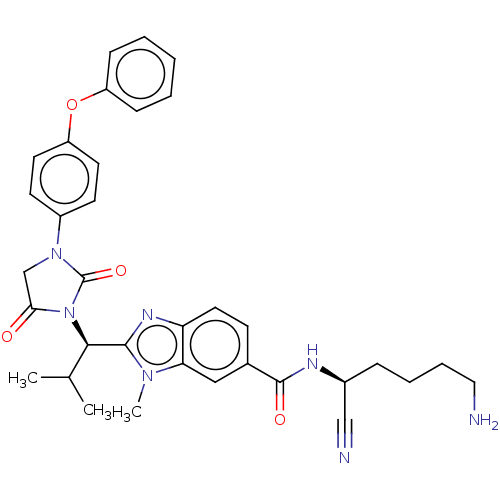
(CHEMBL3799985)Show SMILES CC(C)[C@@H](N1C(=O)CN(C1=O)c1ccc(Oc2ccccc2)cc1)c1nc2ccc(cc2n1C)C(=O)N[C@@H](CCCCN)C#N |r| Show InChI InChI=1S/C34H37N7O4/c1-22(2)31(32-38-28-17-12-23(19-29(28)39(32)3)33(43)37-24(20-36)9-7-8-18-35)41-30(42)21-40(34(41)44)25-13-15-27(16-14-25)45-26-10-5-4-6-11-26/h4-6,10-17,19,22,24,31H,7-9,18,21,35H2,1-3H3,(H,37,43)/t24-,31+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin using Boc-Val-Leu-Lys-MCA as substrate by fluorescence assay |
Bioorg Med Chem Lett 26: 2259-61 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.047
BindingDB Entry DOI: 10.7270/Q2MK6GX7 |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50500939
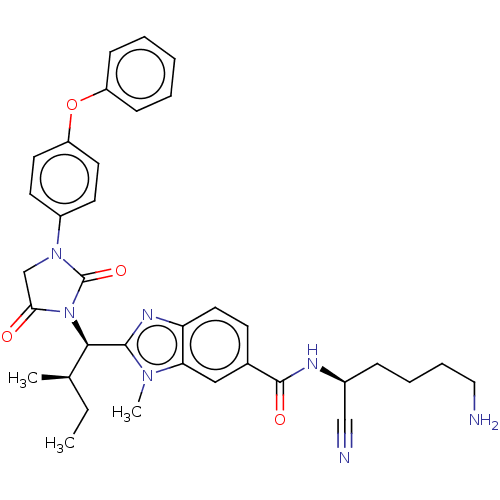
(CHEMBL3798282)Show SMILES CC[C@@H](C)[C@@H](N1C(=O)CN(C1=O)c1ccc(Oc2ccccc2)cc1)c1nc2ccc(cc2n1C)C(=O)N[C@@H](CCCCN)C#N |r| Show InChI InChI=1S/C35H39N7O4/c1-4-23(2)32(33-39-29-18-13-24(20-30(29)40(33)3)34(44)38-25(21-37)10-8-9-19-36)42-31(43)22-41(35(42)45)26-14-16-28(17-15-26)46-27-11-6-5-7-12-27/h5-7,11-18,20,23,25,32H,4,8-10,19,22,36H2,1-3H3,(H,38,44)/t23-,25+,32-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin using Boc-Val-Leu-Lys-MCA as substrate by fluorescence assay |
Bioorg Med Chem Lett 26: 2259-61 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.047
BindingDB Entry DOI: 10.7270/Q2MK6GX7 |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50500937
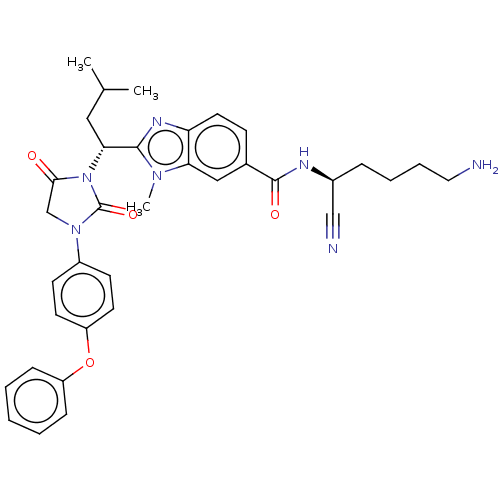
(CHEMBL3800401)Show SMILES CC(C)C[C@@H](N1C(=O)CN(C1=O)c1ccc(Oc2ccccc2)cc1)c1nc2ccc(cc2n1C)C(=O)N[C@@H](CCCCN)C#N |r| Show InChI InChI=1S/C35H39N7O4/c1-23(2)19-31(33-39-29-17-12-24(20-30(29)40(33)3)34(44)38-25(21-37)9-7-8-18-36)42-32(43)22-41(35(42)45)26-13-15-28(16-14-26)46-27-10-5-4-6-11-27/h4-6,10-17,20,23,25,31H,7-9,18-19,22,36H2,1-3H3,(H,38,44)/t25-,31+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin using Boc-Val-Leu-Lys-MCA as substrate by fluorescence assay |
Bioorg Med Chem Lett 26: 2259-61 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.047
BindingDB Entry DOI: 10.7270/Q2MK6GX7 |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50500943
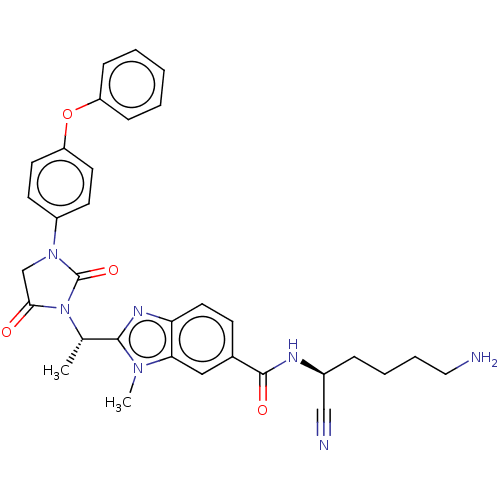
(CHEMBL3799525)Show SMILES C[C@H](N1C(=O)CN(C1=O)c1ccc(Oc2ccccc2)cc1)c1nc2ccc(cc2n1C)C(=O)N[C@@H](CCCCN)C#N |r| Show InChI InChI=1S/C32H33N7O4/c1-21(30-36-27-16-11-22(18-28(27)37(30)2)31(41)35-23(19-34)8-6-7-17-33)39-29(40)20-38(32(39)42)24-12-14-26(15-13-24)43-25-9-4-3-5-10-25/h3-5,9-16,18,21,23H,6-8,17,20,33H2,1-2H3,(H,35,41)/t21-,23-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin using Boc-Val-Leu-Lys-MCA as substrate by fluorescence assay |
Bioorg Med Chem Lett 26: 2259-61 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.047
BindingDB Entry DOI: 10.7270/Q2MK6GX7 |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50500942
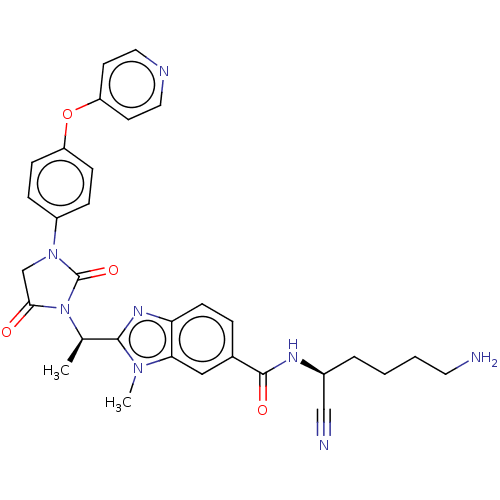
(CHEMBL3799182)Show SMILES C[C@@H](N1C(=O)CN(C1=O)c1ccc(Oc2ccncc2)cc1)c1nc2ccc(cc2n1C)C(=O)N[C@@H](CCCCN)C#N |r| Show InChI InChI=1S/C31H32N8O4/c1-20(29-36-26-11-6-21(17-27(26)37(29)2)30(41)35-22(18-33)5-3-4-14-32)39-28(40)19-38(31(39)42)23-7-9-24(10-8-23)43-25-12-15-34-16-13-25/h6-13,15-17,20,22H,3-5,14,19,32H2,1-2H3,(H,35,41)/t20-,22+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin using Boc-Val-Leu-Lys-MCA as substrate by fluorescence assay |
Bioorg Med Chem Lett 26: 2259-61 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.047
BindingDB Entry DOI: 10.7270/Q2MK6GX7 |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50009200

(CHEMBL3238374)Show SMILES Cn1c(CN2C(=O)CN(C2=O)c2ccc(Oc3ccccc3)cc2)cc2cnc(nc12)C(=O)N[C@@H](CCCCN)C#N |r| Show InChI InChI=1S/C30H30N8O4/c1-36-23(15-20-17-33-27(35-28(20)36)29(40)34-21(16-32)7-5-6-14-31)18-38-26(39)19-37(30(38)41)22-10-12-25(13-11-22)42-24-8-3-2-4-9-24/h2-4,8-13,15,17,21H,5-7,14,18-19,31H2,1H3,(H,34,40)/t21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University
Curated by ChEMBL
| Assay Description
Inhibition of plasmin (unknown origin) using Boc-Val-Leu-Lys-MCA as substrate by fluorescence analysis |
Bioorg Med Chem 23: 3696-704 (2015)
Article DOI: 10.1016/j.bmc.2015.04.013
BindingDB Entry DOI: 10.7270/Q2X92D2Q |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50500944
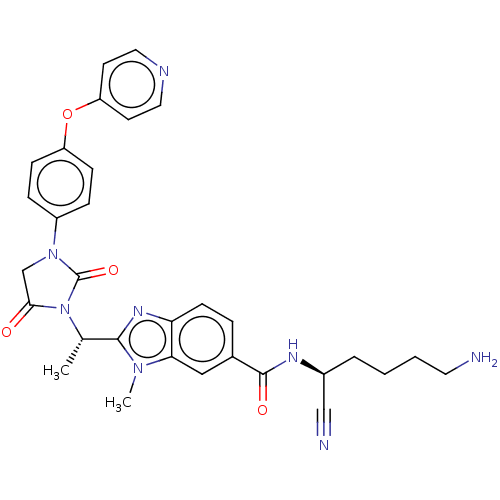
(CHEMBL3800112)Show SMILES C[C@H](N1C(=O)CN(C1=O)c1ccc(Oc2ccncc2)cc1)c1nc2ccc(cc2n1C)C(=O)N[C@@H](CCCCN)C#N |r| Show InChI InChI=1S/C31H32N8O4/c1-20(29-36-26-11-6-21(17-27(26)37(29)2)30(41)35-22(18-33)5-3-4-14-32)39-28(40)19-38(31(39)42)23-7-9-24(10-8-23)43-25-12-15-34-16-13-25/h6-13,15-17,20,22H,3-5,14,19,32H2,1-2H3,(H,35,41)/t20-,22-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin using Boc-Val-Leu-Lys-MCA as substrate by fluorescence assay |
Bioorg Med Chem Lett 26: 2259-61 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.047
BindingDB Entry DOI: 10.7270/Q2MK6GX7 |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50500941
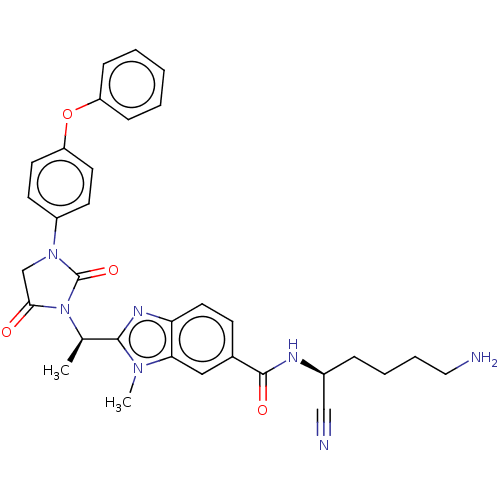
(CHEMBL3800020)Show SMILES C[C@@H](N1C(=O)CN(C1=O)c1ccc(Oc2ccccc2)cc1)c1nc2ccc(cc2n1C)C(=O)N[C@@H](CCCCN)C#N |r| Show InChI InChI=1S/C32H33N7O4/c1-21(30-36-27-16-11-22(18-28(27)37(30)2)31(41)35-23(19-34)8-6-7-17-33)39-29(40)20-38(32(39)42)24-12-14-26(15-13-24)43-25-9-4-3-5-10-25/h3-5,9-16,18,21,23H,6-8,17,20,33H2,1-2H3,(H,35,41)/t21-,23+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin using Boc-Val-Leu-Lys-MCA as substrate by fluorescence assay |
Bioorg Med Chem Lett 26: 2259-61 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.047
BindingDB Entry DOI: 10.7270/Q2MK6GX7 |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50093550
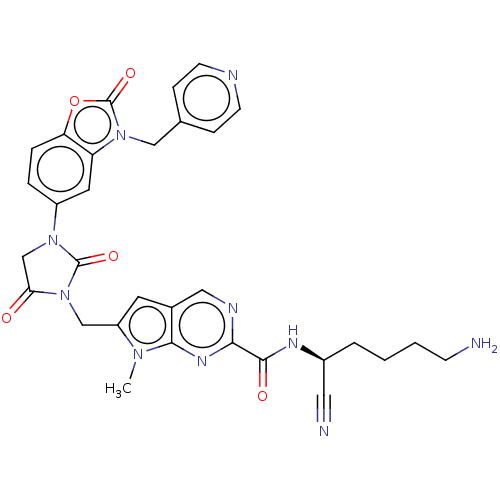
(CHEMBL3585737)Show SMILES Cn1c(CN2C(=O)CN(C2=O)c2ccc3oc(=O)n(Cc4ccncc4)c3c2)cc2cnc(nc12)C(=O)N[C@@H](CCCCN)C#N |r| Show InChI InChI=1S/C31H30N10O5/c1-38-23(12-20-15-35-27(37-28(20)38)29(43)36-21(14-33)4-2-3-9-32)17-41-26(42)18-39(30(41)44)22-5-6-25-24(13-22)40(31(45)46-25)16-19-7-10-34-11-8-19/h5-8,10-13,15,21H,2-4,9,16-18,32H2,1H3,(H,36,43)/t21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University
Curated by ChEMBL
| Assay Description
Inhibition of urokinase (unknown origin) using Pyr-Gly-Arg-MCA as substrate by fluorescence analysis |
Bioorg Med Chem 23: 3696-704 (2015)
Article DOI: 10.1016/j.bmc.2015.04.013
BindingDB Entry DOI: 10.7270/Q2X92D2Q |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50500945
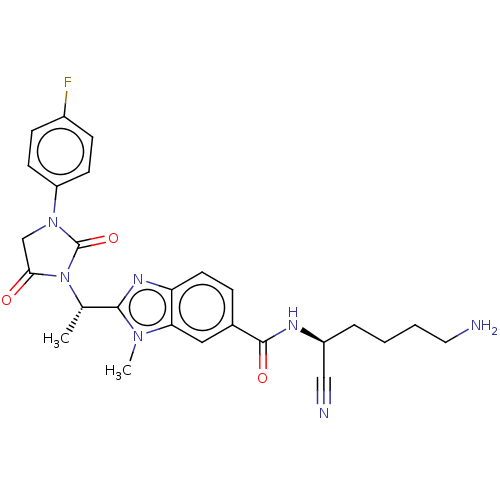
(CHEMBL3799135)Show SMILES C[C@H](N1C(=O)CN(C1=O)c1ccc(F)cc1)c1nc2ccc(cc2n1C)C(=O)N[C@@H](CCCCN)C#N |r| Show InChI InChI=1S/C26H28FN7O3/c1-16(34-23(35)15-33(26(34)37)20-9-7-18(27)8-10-20)24-31-21-11-6-17(13-22(21)32(24)2)25(36)30-19(14-29)5-3-4-12-28/h6-11,13,16,19H,3-5,12,15,28H2,1-2H3,(H,30,36)/t16-,19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin using Boc-Val-Leu-Lys-MCA as substrate by fluorescence assay |
Bioorg Med Chem Lett 26: 2259-61 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.047
BindingDB Entry DOI: 10.7270/Q2MK6GX7 |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50093544
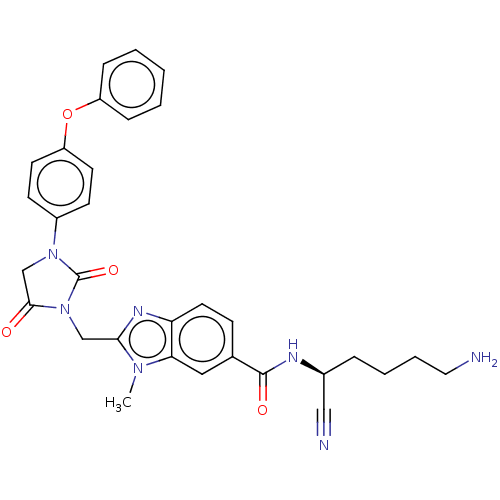
(CHEMBL3585744)Show SMILES Cn1c(CN2C(=O)CN(C2=O)c2ccc(Oc3ccccc3)cc2)nc2ccc(cc12)C(=O)N[C@@H](CCCCN)C#N |r| Show InChI InChI=1S/C31H31N7O4/c1-36-27-17-21(30(40)34-22(18-33)7-5-6-16-32)10-15-26(27)35-28(36)19-38-29(39)20-37(31(38)41)23-11-13-25(14-12-23)42-24-8-3-2-4-9-24/h2-4,8-15,17,22H,5-7,16,19-20,32H2,1H3,(H,34,40)/t22-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University
Curated by ChEMBL
| Assay Description
Inhibition of plasmin (unknown origin) using Boc-Val-Leu-Lys-MCA as substrate by fluorescence analysis |
Bioorg Med Chem 23: 3696-704 (2015)
Article DOI: 10.1016/j.bmc.2015.04.013
BindingDB Entry DOI: 10.7270/Q2X92D2Q |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50093552
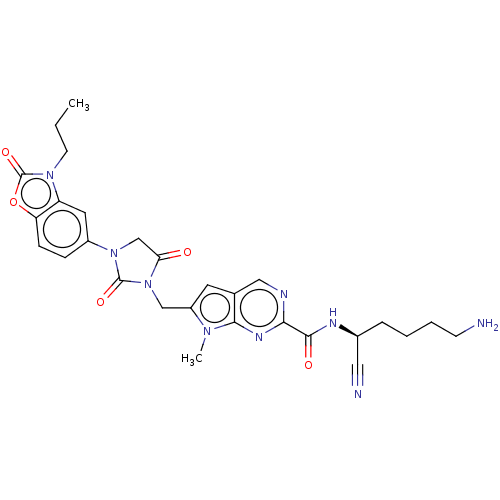
(CHEMBL3585735)Show SMILES CCCn1c2cc(ccc2oc1=O)N1CC(=O)N(Cc2cc3cnc(nc3n2C)C(=O)N[C@@H](CCCCN)C#N)C1=O |r| Show InChI InChI=1S/C28H31N9O5/c1-3-10-35-21-12-19(7-8-22(21)42-28(35)41)36-16-23(38)37(27(36)40)15-20-11-17-14-31-24(33-25(17)34(20)2)26(39)32-18(13-30)6-4-5-9-29/h7-8,11-12,14,18H,3-6,9-10,15-16,29H2,1-2H3,(H,32,39)/t18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University
Curated by ChEMBL
| Assay Description
Inhibition of urokinase (unknown origin) using Pyr-Gly-Arg-MCA as substrate by fluorescence analysis |
Bioorg Med Chem 23: 3696-704 (2015)
Article DOI: 10.1016/j.bmc.2015.04.013
BindingDB Entry DOI: 10.7270/Q2X92D2Q |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50500940
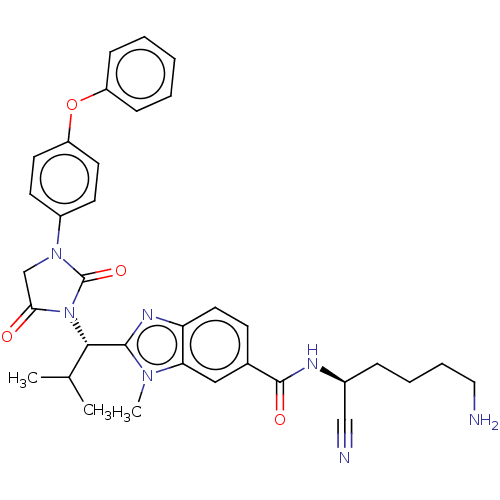
(CHEMBL3797774)Show SMILES CC(C)[C@H](N1C(=O)CN(C1=O)c1ccc(Oc2ccccc2)cc1)c1nc2ccc(cc2n1C)C(=O)N[C@@H](CCCCN)C#N |r| Show InChI InChI=1S/C34H37N7O4/c1-22(2)31(32-38-28-17-12-23(19-29(28)39(32)3)33(43)37-24(20-36)9-7-8-18-35)41-30(42)21-40(34(41)44)25-13-15-27(16-14-25)45-26-10-5-4-6-11-26/h4-6,10-17,19,22,24,31H,7-9,18,21,35H2,1-3H3,(H,37,43)/t24-,31-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin using Boc-Val-Leu-Lys-MCA as substrate by fluorescence assay |
Bioorg Med Chem Lett 26: 2259-61 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.047
BindingDB Entry DOI: 10.7270/Q2MK6GX7 |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50093551
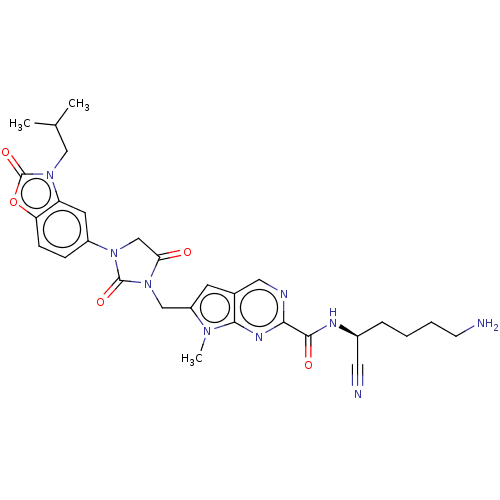
(CHEMBL3585736)Show SMILES CC(C)Cn1c2cc(ccc2oc1=O)N1CC(=O)N(Cc2cc3cnc(nc3n2C)C(=O)N[C@@H](CCCCN)C#N)C1=O |r| Show InChI InChI=1S/C29H33N9O5/c1-17(2)14-37-22-11-20(7-8-23(22)43-29(37)42)36-16-24(39)38(28(36)41)15-21-10-18-13-32-25(34-26(18)35(21)3)27(40)33-19(12-31)6-4-5-9-30/h7-8,10-11,13,17,19H,4-6,9,14-16,30H2,1-3H3,(H,33,40)/t19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University
Curated by ChEMBL
| Assay Description
Inhibition of plasmin (unknown origin) using Boc-Val-Leu-Lys-MCA as substrate by fluorescence analysis |
Bioorg Med Chem 23: 3696-704 (2015)
Article DOI: 10.1016/j.bmc.2015.04.013
BindingDB Entry DOI: 10.7270/Q2X92D2Q |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50093553
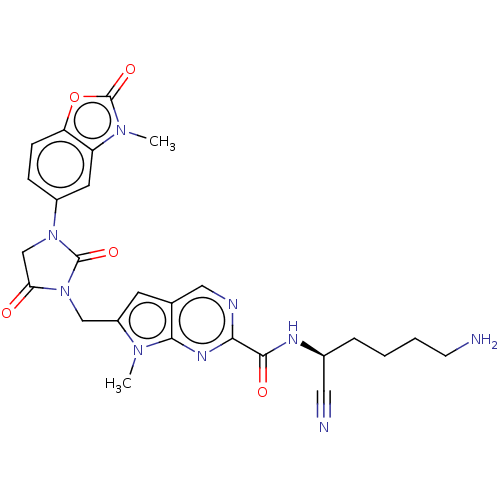
(CHEMBL3585734)Show SMILES Cn1c(CN2C(=O)CN(C2=O)c2ccc3oc(=O)n(C)c3c2)cc2cnc(nc12)C(=O)N[C@@H](CCCCN)C#N |r| Show InChI InChI=1S/C26H27N9O5/c1-32-18(9-15-12-29-22(31-23(15)32)24(37)30-16(11-28)5-3-4-8-27)13-35-21(36)14-34(25(35)38)17-6-7-20-19(10-17)33(2)26(39)40-20/h6-7,9-10,12,16H,3-5,8,13-14,27H2,1-2H3,(H,30,37)/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University
Curated by ChEMBL
| Assay Description
Inhibition of urokinase (unknown origin) using Pyr-Gly-Arg-MCA as substrate by fluorescence analysis |
Bioorg Med Chem 23: 3696-704 (2015)
Article DOI: 10.1016/j.bmc.2015.04.013
BindingDB Entry DOI: 10.7270/Q2X92D2Q |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50009171
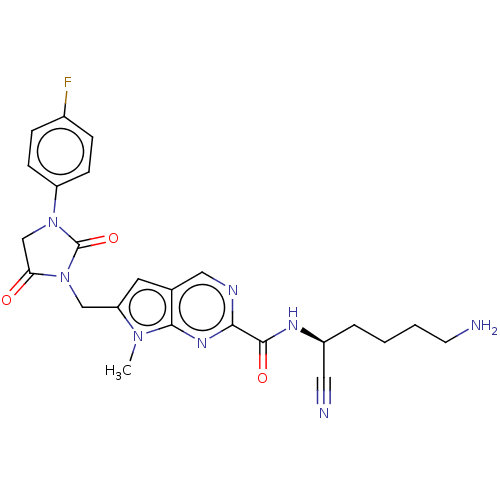
(CHEMBL3238362)Show SMILES Cn1c(CN2C(=O)CN(C2=O)c2ccc(F)cc2)cc2cnc(nc12)C(=O)N[C@@H](CCCCN)C#N |r| Show InChI InChI=1S/C24H25FN8O3/c1-31-19(13-33-20(34)14-32(24(33)36)18-7-5-16(25)6-8-18)10-15-12-28-21(30-22(15)31)23(35)29-17(11-27)4-2-3-9-26/h5-8,10,12,17H,2-4,9,13-14,26H2,1H3,(H,29,35)/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University
Curated by ChEMBL
| Assay Description
Inhibition of plasmin (unknown origin) using Boc-Val-Leu-Lys-MCA as substrate by fluorescence analysis |
Bioorg Med Chem 23: 3696-704 (2015)
Article DOI: 10.1016/j.bmc.2015.04.013
BindingDB Entry DOI: 10.7270/Q2X92D2Q |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50500946
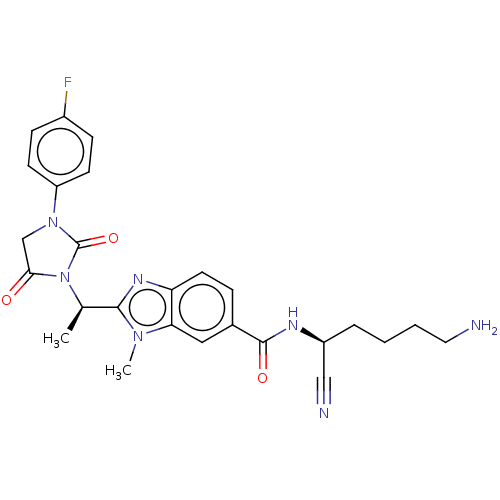
(CHEMBL3799329)Show SMILES C[C@@H](N1C(=O)CN(C1=O)c1ccc(F)cc1)c1nc2ccc(cc2n1C)C(=O)N[C@@H](CCCCN)C#N |r| Show InChI InChI=1S/C26H28FN7O3/c1-16(34-23(35)15-33(26(34)37)20-9-7-18(27)8-10-20)24-31-21-11-6-17(13-22(21)32(24)2)25(36)30-19(14-29)5-3-4-12-28/h6-11,13,16,19H,3-5,12,15,28H2,1-2H3,(H,30,36)/t16-,19+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin using Boc-Val-Leu-Lys-MCA as substrate by fluorescence assay |
Bioorg Med Chem Lett 26: 2259-61 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.047
BindingDB Entry DOI: 10.7270/Q2MK6GX7 |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50009200

(CHEMBL3238374)Show SMILES Cn1c(CN2C(=O)CN(C2=O)c2ccc(Oc3ccccc3)cc2)cc2cnc(nc12)C(=O)N[C@@H](CCCCN)C#N |r| Show InChI InChI=1S/C30H30N8O4/c1-36-23(15-20-17-33-27(35-28(20)36)29(40)34-21(16-32)7-5-6-14-31)18-38-26(39)19-37(30(38)41)22-10-12-25(13-11-22)42-24-8-3-2-4-9-24/h2-4,8-13,15,17,21H,5-7,14,18-19,31H2,1H3,(H,34,40)/t21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin using H-D-Val-Leu-LyspNA (S-2251) peptide as substrate assessed as reduction of enzyme hydrolytic activity |
Bioorg Med Chem 22: 2339-52 (2014)
Article DOI: 10.1016/j.bmc.2014.02.002
BindingDB Entry DOI: 10.7270/Q2KH0PVJ |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50093540
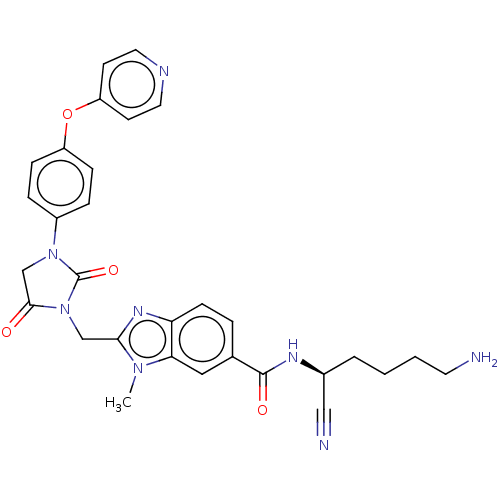
(CHEMBL3585746)Show SMILES Cn1c(CN2C(=O)CN(C2=O)c2ccc(Oc3ccncc3)cc2)nc2ccc(cc12)C(=O)N[C@@H](CCCCN)C#N |r| Show InChI InChI=1S/C30H30N8O4/c1-36-26-16-20(29(40)34-21(17-32)4-2-3-13-31)5-10-25(26)35-27(36)18-38-28(39)19-37(30(38)41)22-6-8-23(9-7-22)42-24-11-14-33-15-12-24/h5-12,14-16,21H,2-4,13,18-19,31H2,1H3,(H,34,40)/t21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University
Curated by ChEMBL
| Assay Description
Inhibition of urokinase (unknown origin) using Pyr-Gly-Arg-MCA as substrate by fluorescence analysis |
Bioorg Med Chem 23: 3696-704 (2015)
Article DOI: 10.1016/j.bmc.2015.04.013
BindingDB Entry DOI: 10.7270/Q2X92D2Q |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50009201

(CHEMBL3238375)Show SMILES COc1ccc(Oc2ccc(cc2)N2CC(=O)N(Cc3cc4cnc(nc4n3C)C(=O)N[C@@H](CCCCN)C#N)C2=O)cc1 |r| Show InChI InChI=1S/C31H32N8O5/c1-37-23(15-20-17-34-28(36-29(20)37)30(41)35-21(16-33)5-3-4-14-32)18-39-27(40)19-38(31(39)42)22-6-8-25(9-7-22)44-26-12-10-24(43-2)11-13-26/h6-13,15,17,21H,3-5,14,18-19,32H2,1-2H3,(H,35,41)/t21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin using H-D-Val-Leu-LyspNA (S-2251) peptide as substrate assessed as reduction of enzyme hydrolytic activity |
Bioorg Med Chem 22: 2339-52 (2014)
Article DOI: 10.1016/j.bmc.2014.02.002
BindingDB Entry DOI: 10.7270/Q2KH0PVJ |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50093541
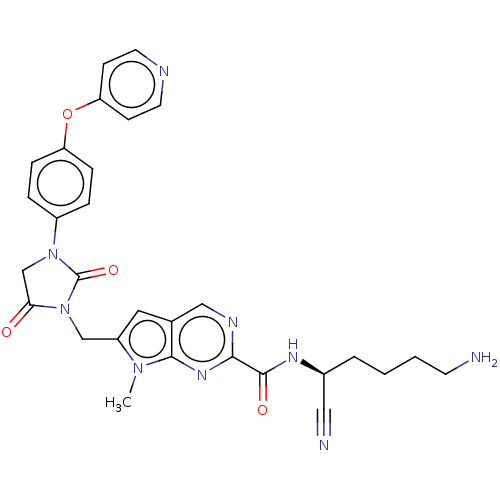
(CHEMBL3585745)Show SMILES Cn1c(CN2C(=O)CN(C2=O)c2ccc(Oc3ccncc3)cc2)cc2cnc(nc12)C(=O)N[C@@H](CCCCN)C#N |r| Show InChI InChI=1S/C29H29N9O4/c1-36-22(14-19-16-33-26(35-27(19)36)28(40)34-20(15-31)4-2-3-11-30)17-38-25(39)18-37(29(38)41)21-5-7-23(8-6-21)42-24-9-12-32-13-10-24/h5-10,12-14,16,20H,2-4,11,17-18,30H2,1H3,(H,34,40)/t20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University
Curated by ChEMBL
| Assay Description
Inhibition of urokinase (unknown origin) using Pyr-Gly-Arg-MCA as substrate by fluorescence analysis |
Bioorg Med Chem 23: 3696-704 (2015)
Article DOI: 10.1016/j.bmc.2015.04.013
BindingDB Entry DOI: 10.7270/Q2X92D2Q |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50009202
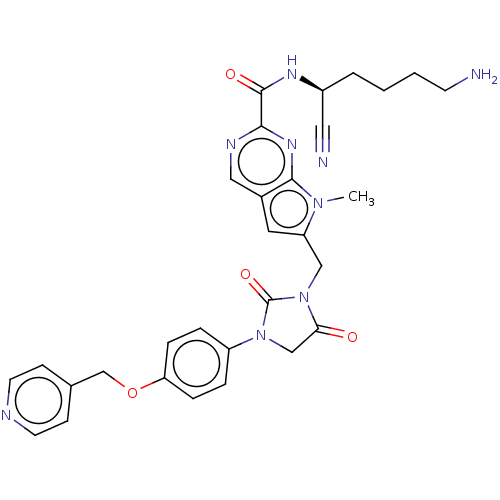
(CHEMBL3238376)Show SMILES Cn1c(CN2C(=O)CN(C2=O)c2ccc(OCc3ccncc3)cc2)cc2cnc(nc12)C(=O)N[C@@H](CCCCN)C#N |r| Show InChI InChI=1S/C30H31N9O4/c1-37-24(14-21-16-34-27(36-28(21)37)29(41)35-22(15-32)4-2-3-11-31)17-39-26(40)18-38(30(39)42)23-5-7-25(8-6-23)43-19-20-9-12-33-13-10-20/h5-10,12-14,16,22H,2-4,11,17-19,31H2,1H3,(H,35,41)/t22-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin using H-D-Val-Leu-LyspNA (S-2251) peptide as substrate assessed as reduction of enzyme hydrolytic activity |
Bioorg Med Chem 22: 2339-52 (2014)
Article DOI: 10.1016/j.bmc.2014.02.002
BindingDB Entry DOI: 10.7270/Q2KH0PVJ |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50009204
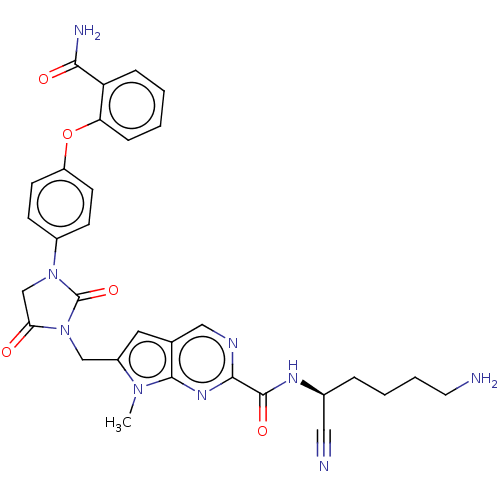
(CHEMBL3238378)Show SMILES Cn1c(CN2C(=O)CN(C2=O)c2ccc(Oc3ccccc3C(N)=O)cc2)cc2cnc(nc12)C(=O)N[C@@H](CCCCN)C#N |r| Show InChI InChI=1S/C31H31N9O5/c1-38-22(14-19-16-35-28(37-29(19)38)30(43)36-20(15-33)6-4-5-13-32)17-40-26(41)18-39(31(40)44)21-9-11-23(12-10-21)45-25-8-3-2-7-24(25)27(34)42/h2-3,7-12,14,16,20H,4-6,13,17-18,32H2,1H3,(H2,34,42)(H,36,43)/t20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin using H-D-Val-Leu-LyspNA (S-2251) peptide as substrate assessed as reduction of enzyme hydrolytic activity |
Bioorg Med Chem 22: 2339-52 (2014)
Article DOI: 10.1016/j.bmc.2014.02.002
BindingDB Entry DOI: 10.7270/Q2KH0PVJ |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50093542
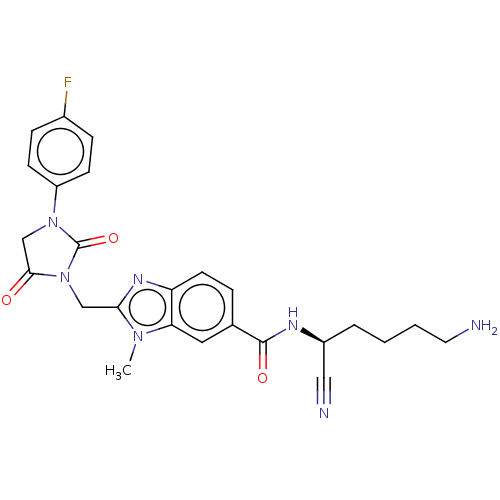
(CHEMBL3585742)Show SMILES Cn1c(CN2C(=O)CN(C2=O)c2ccc(F)cc2)nc2ccc(cc12)C(=O)N[C@@H](CCCCN)C#N |r| Show InChI InChI=1S/C25H26FN7O3/c1-31-21-12-16(24(35)29-18(13-28)4-2-3-11-27)5-10-20(21)30-22(31)14-33-23(34)15-32(25(33)36)19-8-6-17(26)7-9-19/h5-10,12,18H,2-4,11,14-15,27H2,1H3,(H,29,35)/t18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin using Boc-Val-Leu-Lys-MCA as substrate by fluorescence assay |
Bioorg Med Chem Lett 26: 2259-61 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.047
BindingDB Entry DOI: 10.7270/Q2MK6GX7 |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50093542

(CHEMBL3585742)Show SMILES Cn1c(CN2C(=O)CN(C2=O)c2ccc(F)cc2)nc2ccc(cc12)C(=O)N[C@@H](CCCCN)C#N |r| Show InChI InChI=1S/C25H26FN7O3/c1-31-21-12-16(24(35)29-18(13-28)4-2-3-11-27)5-10-20(21)30-22(31)14-33-23(34)15-32(25(33)36)19-8-6-17(26)7-9-19/h5-10,12,18H,2-4,11,14-15,27H2,1H3,(H,29,35)/t18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University
Curated by ChEMBL
| Assay Description
Inhibition of plasmin (unknown origin) using Boc-Val-Leu-Lys-MCA as substrate by fluorescence analysis |
Bioorg Med Chem 23: 3696-704 (2015)
Article DOI: 10.1016/j.bmc.2015.04.013
BindingDB Entry DOI: 10.7270/Q2X92D2Q |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50009178
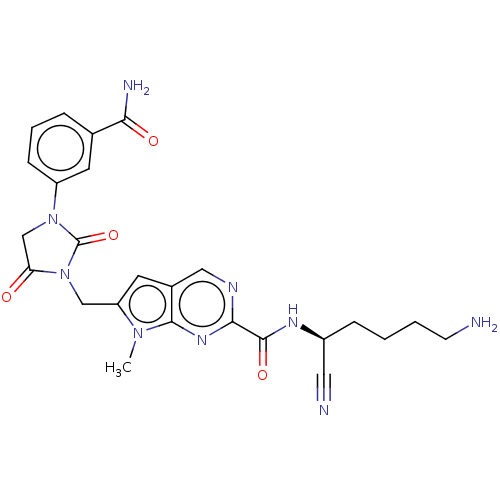
(CHEMBL3238369)Show SMILES Cn1c(CN2C(=O)CN(C2=O)c2cccc(c2)C(N)=O)cc2cnc(nc12)C(=O)N[C@@H](CCCCN)C#N |r| Show InChI InChI=1S/C25H27N9O4/c1-32-19(13-34-20(35)14-33(25(34)38)18-7-4-5-15(9-18)21(28)36)10-16-12-29-22(31-23(16)32)24(37)30-17(11-27)6-2-3-8-26/h4-5,7,9-10,12,17H,2-3,6,8,13-14,26H2,1H3,(H2,28,36)(H,30,37)/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin using H-D-Val-Leu-LyspNA (S-2251) peptide as substrate assessed as reduction of enzyme hydrolytic activity |
Bioorg Med Chem 22: 2339-52 (2014)
Article DOI: 10.1016/j.bmc.2014.02.002
BindingDB Entry DOI: 10.7270/Q2KH0PVJ |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50009199
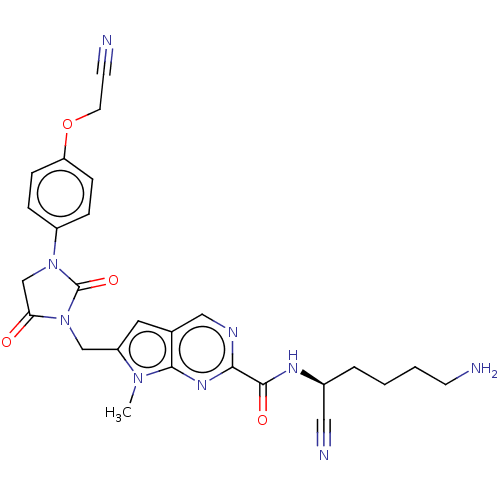
(CHEMBL3238373)Show SMILES Cn1c(CN2C(=O)CN(C2=O)c2ccc(OCC#N)cc2)cc2cnc(nc12)C(=O)N[C@@H](CCCCN)C#N |r| Show InChI InChI=1S/C26H27N9O4/c1-33-20(12-17-14-30-23(32-24(17)33)25(37)31-18(13-29)4-2-3-9-27)15-35-22(36)16-34(26(35)38)19-5-7-21(8-6-19)39-11-10-28/h5-8,12,14,18H,2-4,9,11,15-16,27H2,1H3,(H,31,37)/t18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin using H-D-Val-Leu-LyspNA (S-2251) peptide as substrate assessed as reduction of enzyme hydrolytic activity |
Bioorg Med Chem 22: 2339-52 (2014)
Article DOI: 10.1016/j.bmc.2014.02.002
BindingDB Entry DOI: 10.7270/Q2KH0PVJ |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50009171

(CHEMBL3238362)Show SMILES Cn1c(CN2C(=O)CN(C2=O)c2ccc(F)cc2)cc2cnc(nc12)C(=O)N[C@@H](CCCCN)C#N |r| Show InChI InChI=1S/C24H25FN8O3/c1-31-19(13-33-20(34)14-32(24(33)36)18-7-5-16(25)6-8-18)10-15-12-28-21(30-22(15)31)23(35)29-17(11-27)4-2-3-9-26/h5-8,10,12,17H,2-4,9,13-14,26H2,1H3,(H,29,35)/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin using H-D-Val-Leu-LyspNA (S-2251) peptide as substrate assessed as reduction of enzyme hydrolytic activity |
Bioorg Med Chem 22: 2339-52 (2014)
Article DOI: 10.1016/j.bmc.2014.02.002
BindingDB Entry DOI: 10.7270/Q2KH0PVJ |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50093549
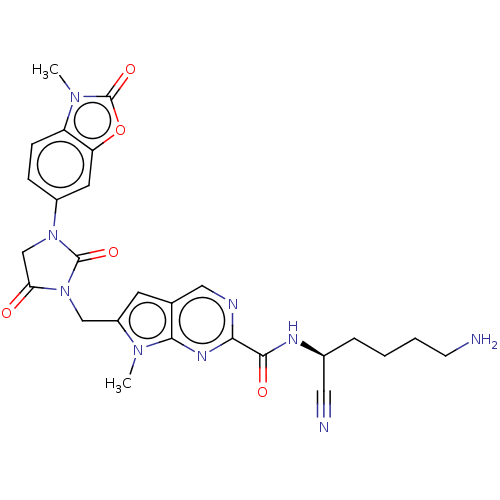
(CHEMBL3585738)Show SMILES Cn1c(CN2C(=O)CN(C2=O)c2ccc3n(C)c(=O)oc3c2)cc2cnc(nc12)C(=O)N[C@@H](CCCCN)C#N |r| Show InChI InChI=1S/C26H27N9O5/c1-32-18(9-15-12-29-22(31-23(15)32)24(37)30-16(11-28)5-3-4-8-27)13-35-21(36)14-34(25(35)38)17-6-7-19-20(10-17)40-26(39)33(19)2/h6-7,9-10,12,16H,3-5,8,13-14,27H2,1-2H3,(H,30,37)/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University
Curated by ChEMBL
| Assay Description
Inhibition of plasmin (unknown origin) using Boc-Val-Leu-Lys-MCA as substrate by fluorescence analysis |
Bioorg Med Chem 23: 3696-704 (2015)
Article DOI: 10.1016/j.bmc.2015.04.013
BindingDB Entry DOI: 10.7270/Q2X92D2Q |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50500937

(CHEMBL3800401)Show SMILES CC(C)C[C@@H](N1C(=O)CN(C1=O)c1ccc(Oc2ccccc2)cc1)c1nc2ccc(cc2n1C)C(=O)N[C@@H](CCCCN)C#N |r| Show InChI InChI=1S/C35H39N7O4/c1-23(2)19-31(33-39-29-17-12-24(20-30(29)40(33)3)34(44)38-25(21-37)9-7-8-18-36)42-32(43)22-41(35(42)45)26-13-15-28(16-14-26)46-27-10-5-4-6-11-27/h4-6,10-17,20,23,25,31H,7-9,18-19,22,36H2,1-3H3,(H,38,44)/t25-,31+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University
Curated by ChEMBL
| Assay Description
Inhibition of human urokinase using Pyr-Gly-Arg-MCA as substrate by fluorescence assay |
Bioorg Med Chem Lett 26: 2259-61 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.047
BindingDB Entry DOI: 10.7270/Q2MK6GX7 |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50009175
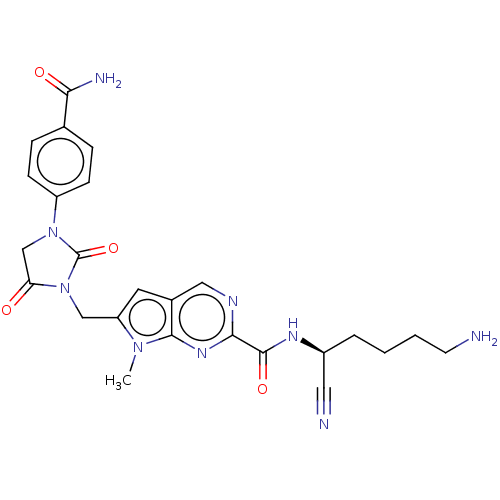
(CHEMBL3238366)Show SMILES Cn1c(CN2C(=O)CN(C2=O)c2ccc(cc2)C(N)=O)cc2cnc(nc12)C(=O)N[C@@H](CCCCN)C#N |r| Show InChI InChI=1S/C25H27N9O4/c1-32-19(13-34-20(35)14-33(25(34)38)18-7-5-15(6-8-18)21(28)36)10-16-12-29-22(31-23(16)32)24(37)30-17(11-27)4-2-3-9-26/h5-8,10,12,17H,2-4,9,13-14,26H2,1H3,(H2,28,36)(H,30,37)/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin using H-D-Val-Leu-LyspNA (S-2251) peptide as substrate assessed as reduction of enzyme hydrolytic activity |
Bioorg Med Chem 22: 2339-52 (2014)
Article DOI: 10.1016/j.bmc.2014.02.002
BindingDB Entry DOI: 10.7270/Q2KH0PVJ |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50500941

(CHEMBL3800020)Show SMILES C[C@@H](N1C(=O)CN(C1=O)c1ccc(Oc2ccccc2)cc1)c1nc2ccc(cc2n1C)C(=O)N[C@@H](CCCCN)C#N |r| Show InChI InChI=1S/C32H33N7O4/c1-21(30-36-27-16-11-22(18-28(27)37(30)2)31(41)35-23(19-34)8-6-7-17-33)39-29(40)20-38(32(39)42)24-12-14-26(15-13-24)43-25-9-4-3-5-10-25/h3-5,9-16,18,21,23H,6-8,17,20,33H2,1-2H3,(H,35,41)/t21-,23+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University
Curated by ChEMBL
| Assay Description
Inhibition of human urokinase using Pyr-Gly-Arg-MCA as substrate by fluorescence assay |
Bioorg Med Chem Lett 26: 2259-61 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.047
BindingDB Entry DOI: 10.7270/Q2MK6GX7 |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50093548
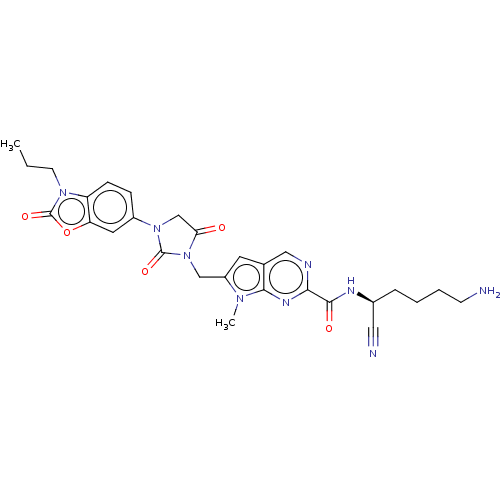
(CHEMBL3585739)Show SMILES CCCn1c2ccc(cc2oc1=O)N1CC(=O)N(Cc2cc3cnc(nc3n2C)C(=O)N[C@@H](CCCCN)C#N)C1=O |r| Show InChI InChI=1S/C28H31N9O5/c1-3-10-35-21-8-7-19(12-22(21)42-28(35)41)36-16-23(38)37(27(36)40)15-20-11-17-14-31-24(33-25(17)34(20)2)26(39)32-18(13-30)6-4-5-9-29/h7-8,11-12,14,18H,3-6,9-10,15-16,29H2,1-2H3,(H,32,39)/t18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University
Curated by ChEMBL
| Assay Description
Inhibition of plasmin (unknown origin) using Boc-Val-Leu-Lys-MCA as substrate by fluorescence analysis |
Bioorg Med Chem 23: 3696-704 (2015)
Article DOI: 10.1016/j.bmc.2015.04.013
BindingDB Entry DOI: 10.7270/Q2X92D2Q |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50009206
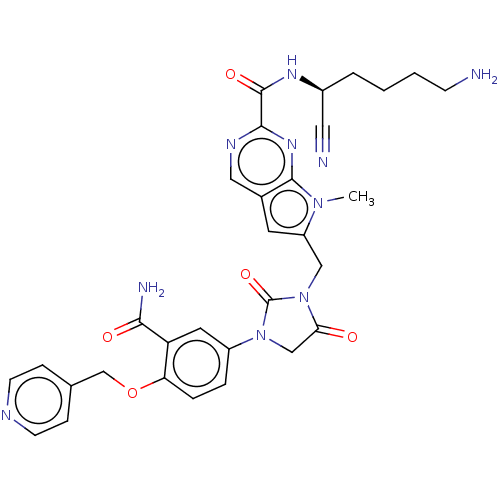
(CHEMBL3233150)Show SMILES Cn1c(CN2C(=O)CN(C2=O)c2ccc(OCc3ccncc3)c(c2)C(N)=O)cc2cnc(nc12)C(=O)N[C@@H](CCCCN)C#N |r| Show InChI InChI=1S/C31H32N10O5/c1-39-23(12-20-15-36-28(38-29(20)39)30(44)37-21(14-33)4-2-3-9-32)16-41-26(42)17-40(31(41)45)22-5-6-25(24(13-22)27(34)43)46-18-19-7-10-35-11-8-19/h5-8,10-13,15,21H,2-4,9,16-18,32H2,1H3,(H2,34,43)(H,37,44)/t21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin using H-D-Val-Leu-LyspNA (S-2251) peptide as substrate assessed as reduction of enzyme hydrolytic activity |
Bioorg Med Chem 22: 2339-52 (2014)
Article DOI: 10.1016/j.bmc.2014.02.002
BindingDB Entry DOI: 10.7270/Q2KH0PVJ |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50009172

(CHEMBL3238363)Show SMILES COc1ccc(cc1)N1CC(=O)N(Cc2cc3cnc(nc3n2C)C(=O)N[C@@H](CCCCN)C#N)C1=O |r| Show InChI InChI=1S/C25H28N8O4/c1-31-19(14-33-21(34)15-32(25(33)36)18-6-8-20(37-2)9-7-18)11-16-13-28-22(30-23(16)31)24(35)29-17(12-27)5-3-4-10-26/h6-9,11,13,17H,3-5,10,14-15,26H2,1-2H3,(H,29,35)/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.80E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin using H-D-Val-Leu-LyspNA (S-2251) peptide as substrate assessed as reduction of enzyme hydrolytic activity |
Bioorg Med Chem 22: 2339-52 (2014)
Article DOI: 10.1016/j.bmc.2014.02.002
BindingDB Entry DOI: 10.7270/Q2KH0PVJ |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50500944

(CHEMBL3800112)Show SMILES C[C@H](N1C(=O)CN(C1=O)c1ccc(Oc2ccncc2)cc1)c1nc2ccc(cc2n1C)C(=O)N[C@@H](CCCCN)C#N |r| Show InChI InChI=1S/C31H32N8O4/c1-20(29-36-26-11-6-21(17-27(26)37(29)2)30(41)35-22(18-33)5-3-4-14-32)39-28(40)19-38(31(39)42)23-7-9-24(10-8-23)43-25-12-15-34-16-13-25/h6-13,15-17,20,22H,3-5,14,19,32H2,1-2H3,(H,35,41)/t20-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University
Curated by ChEMBL
| Assay Description
Inhibition of human urokinase using Pyr-Gly-Arg-MCA as substrate by fluorescence assay |
Bioorg Med Chem Lett 26: 2259-61 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.047
BindingDB Entry DOI: 10.7270/Q2MK6GX7 |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50500945

(CHEMBL3799135)Show SMILES C[C@H](N1C(=O)CN(C1=O)c1ccc(F)cc1)c1nc2ccc(cc2n1C)C(=O)N[C@@H](CCCCN)C#N |r| Show InChI InChI=1S/C26H28FN7O3/c1-16(34-23(35)15-33(26(34)37)20-9-7-18(27)8-10-20)24-31-21-11-6-17(13-22(21)32(24)2)25(36)30-19(14-29)5-3-4-12-28/h6-11,13,16,19H,3-5,12,15,28H2,1-2H3,(H,30,36)/t16-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University
Curated by ChEMBL
| Assay Description
Inhibition of human urokinase using Pyr-Gly-Arg-MCA as substrate by fluorescence assay |
Bioorg Med Chem Lett 26: 2259-61 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.047
BindingDB Entry DOI: 10.7270/Q2MK6GX7 |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50500946

(CHEMBL3799329)Show SMILES C[C@@H](N1C(=O)CN(C1=O)c1ccc(F)cc1)c1nc2ccc(cc2n1C)C(=O)N[C@@H](CCCCN)C#N |r| Show InChI InChI=1S/C26H28FN7O3/c1-16(34-23(35)15-33(26(34)37)20-9-7-18(27)8-10-20)24-31-21-11-6-17(13-22(21)32(24)2)25(36)30-19(14-29)5-3-4-12-28/h6-11,13,16,19H,3-5,12,15,28H2,1-2H3,(H,30,36)/t16-,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University
Curated by ChEMBL
| Assay Description
Inhibition of human urokinase using Pyr-Gly-Arg-MCA as substrate by fluorescence assay |
Bioorg Med Chem Lett 26: 2259-61 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.047
BindingDB Entry DOI: 10.7270/Q2MK6GX7 |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50500938

(CHEMBL3799985)Show SMILES CC(C)[C@@H](N1C(=O)CN(C1=O)c1ccc(Oc2ccccc2)cc1)c1nc2ccc(cc2n1C)C(=O)N[C@@H](CCCCN)C#N |r| Show InChI InChI=1S/C34H37N7O4/c1-22(2)31(32-38-28-17-12-23(19-29(28)39(32)3)33(43)37-24(20-36)9-7-8-18-35)41-30(42)21-40(34(41)44)25-13-15-27(16-14-25)45-26-10-5-4-6-11-26/h4-6,10-17,19,22,24,31H,7-9,18,21,35H2,1-3H3,(H,37,43)/t24-,31+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University
Curated by ChEMBL
| Assay Description
Inhibition of human urokinase using Pyr-Gly-Arg-MCA as substrate by fluorescence assay |
Bioorg Med Chem Lett 26: 2259-61 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.047
BindingDB Entry DOI: 10.7270/Q2MK6GX7 |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50500943

(CHEMBL3799525)Show SMILES C[C@H](N1C(=O)CN(C1=O)c1ccc(Oc2ccccc2)cc1)c1nc2ccc(cc2n1C)C(=O)N[C@@H](CCCCN)C#N |r| Show InChI InChI=1S/C32H33N7O4/c1-21(30-36-27-16-11-22(18-28(27)37(30)2)31(41)35-23(19-34)8-6-7-17-33)39-29(40)20-38(32(39)42)24-12-14-26(15-13-24)43-25-9-4-3-5-10-25/h3-5,9-16,18,21,23H,6-8,17,20,33H2,1-2H3,(H,35,41)/t21-,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University
Curated by ChEMBL
| Assay Description
Inhibition of human urokinase using Pyr-Gly-Arg-MCA as substrate by fluorescence assay |
Bioorg Med Chem Lett 26: 2259-61 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.047
BindingDB Entry DOI: 10.7270/Q2MK6GX7 |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50093542

(CHEMBL3585742)Show SMILES Cn1c(CN2C(=O)CN(C2=O)c2ccc(F)cc2)nc2ccc(cc12)C(=O)N[C@@H](CCCCN)C#N |r| Show InChI InChI=1S/C25H26FN7O3/c1-31-21-12-16(24(35)29-18(13-28)4-2-3-11-27)5-10-20(21)30-22(31)14-33-23(34)15-32(25(33)36)19-8-6-17(26)7-9-19/h5-10,12,18H,2-4,11,14-15,27H2,1H3,(H,29,35)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University
Curated by ChEMBL
| Assay Description
Inhibition of human urokinase using Pyr-Gly-Arg-MCA as substrate by fluorescence assay |
Bioorg Med Chem Lett 26: 2259-61 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.047
BindingDB Entry DOI: 10.7270/Q2MK6GX7 |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50500942

(CHEMBL3799182)Show SMILES C[C@@H](N1C(=O)CN(C1=O)c1ccc(Oc2ccncc2)cc1)c1nc2ccc(cc2n1C)C(=O)N[C@@H](CCCCN)C#N |r| Show InChI InChI=1S/C31H32N8O4/c1-20(29-36-26-11-6-21(17-27(26)37(29)2)30(41)35-22(18-33)5-3-4-14-32)39-28(40)19-38(31(39)42)23-7-9-24(10-8-23)43-25-12-15-34-16-13-25/h6-13,15-17,20,22H,3-5,14,19,32H2,1-2H3,(H,35,41)/t20-,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University
Curated by ChEMBL
| Assay Description
Inhibition of human urokinase using Pyr-Gly-Arg-MCA as substrate by fluorescence assay |
Bioorg Med Chem Lett 26: 2259-61 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.047
BindingDB Entry DOI: 10.7270/Q2MK6GX7 |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50500940

(CHEMBL3797774)Show SMILES CC(C)[C@H](N1C(=O)CN(C1=O)c1ccc(Oc2ccccc2)cc1)c1nc2ccc(cc2n1C)C(=O)N[C@@H](CCCCN)C#N |r| Show InChI InChI=1S/C34H37N7O4/c1-22(2)31(32-38-28-17-12-23(19-29(28)39(32)3)33(43)37-24(20-36)9-7-8-18-35)41-30(42)21-40(34(41)44)25-13-15-27(16-14-25)45-26-10-5-4-6-11-26/h4-6,10-17,19,22,24,31H,7-9,18,21,35H2,1-3H3,(H,37,43)/t24-,31-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University
Curated by ChEMBL
| Assay Description
Inhibition of human urokinase using Pyr-Gly-Arg-MCA as substrate by fluorescence assay |
Bioorg Med Chem Lett 26: 2259-61 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.047
BindingDB Entry DOI: 10.7270/Q2MK6GX7 |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50500939

(CHEMBL3798282)Show SMILES CC[C@@H](C)[C@@H](N1C(=O)CN(C1=O)c1ccc(Oc2ccccc2)cc1)c1nc2ccc(cc2n1C)C(=O)N[C@@H](CCCCN)C#N |r| Show InChI InChI=1S/C35H39N7O4/c1-4-23(2)32(33-39-29-18-13-24(20-30(29)40(33)3)34(44)38-25(21-37)10-8-9-19-36)42-31(43)22-41(35(42)45)26-14-16-28(17-15-26)46-27-11-6-5-7-12-27/h5-7,11-18,20,23,25,32H,4,8-10,19,22,36H2,1-3H3,(H,38,44)/t23-,25+,32-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University
Curated by ChEMBL
| Assay Description
Inhibition of human urokinase using Pyr-Gly-Arg-MCA as substrate by fluorescence assay |
Bioorg Med Chem Lett 26: 2259-61 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.047
BindingDB Entry DOI: 10.7270/Q2MK6GX7 |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50093541

(CHEMBL3585745)Show SMILES Cn1c(CN2C(=O)CN(C2=O)c2ccc(Oc3ccncc3)cc2)cc2cnc(nc12)C(=O)N[C@@H](CCCCN)C#N |r| Show InChI InChI=1S/C29H29N9O4/c1-36-22(14-19-16-33-26(35-27(19)36)28(40)34-20(15-31)4-2-3-11-30)17-38-25(39)18-37(29(38)41)21-5-7-23(8-6-21)42-24-9-12-32-13-10-24/h5-10,12-14,16,20H,2-4,11,17-18,30H2,1H3,(H,34,40)/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University
Curated by ChEMBL
| Assay Description
Inhibition of plasmin (unknown origin) using Boc-Val-Leu-Lys-MCA as substrate by fluorescence analysis |
Bioorg Med Chem 23: 3696-704 (2015)
Article DOI: 10.1016/j.bmc.2015.04.013
BindingDB Entry DOI: 10.7270/Q2X92D2Q |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50093540

(CHEMBL3585746)Show SMILES Cn1c(CN2C(=O)CN(C2=O)c2ccc(Oc3ccncc3)cc2)nc2ccc(cc12)C(=O)N[C@@H](CCCCN)C#N |r| Show InChI InChI=1S/C30H30N8O4/c1-36-26-16-20(29(40)34-21(17-32)4-2-3-13-31)5-10-25(26)35-27(36)18-38-28(39)19-37(30(38)41)22-6-8-23(9-7-22)42-24-11-14-33-15-12-24/h5-12,14-16,21H,2-4,13,18-19,31H2,1H3,(H,34,40)/t21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University
Curated by ChEMBL
| Assay Description
Inhibition of plasmin (unknown origin) using Boc-Val-Leu-Lys-MCA as substrate by fluorescence analysis |
Bioorg Med Chem 23: 3696-704 (2015)
Article DOI: 10.1016/j.bmc.2015.04.013
BindingDB Entry DOI: 10.7270/Q2X92D2Q |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50009171

(CHEMBL3238362)Show SMILES Cn1c(CN2C(=O)CN(C2=O)c2ccc(F)cc2)cc2cnc(nc12)C(=O)N[C@@H](CCCCN)C#N |r| Show InChI InChI=1S/C24H25FN8O3/c1-31-19(13-33-20(34)14-32(24(33)36)18-7-5-16(25)6-8-18)10-15-12-28-21(30-22(15)31)23(35)29-17(11-27)4-2-3-9-26/h5-8,10,12,17H,2-4,9,13-14,26H2,1H3,(H,29,35)/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University
Curated by ChEMBL
| Assay Description
Inhibition of plasmin (unknown origin) using Boc-Val-Leu-Lys-MCA as substrate by fluorescence analysis |
Bioorg Med Chem 23: 3696-704 (2015)
Article DOI: 10.1016/j.bmc.2015.04.013
BindingDB Entry DOI: 10.7270/Q2X92D2Q |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50009200

(CHEMBL3238374)Show SMILES Cn1c(CN2C(=O)CN(C2=O)c2ccc(Oc3ccccc3)cc2)cc2cnc(nc12)C(=O)N[C@@H](CCCCN)C#N |r| Show InChI InChI=1S/C30H30N8O4/c1-36-23(15-20-17-33-27(35-28(20)36)29(40)34-21(16-32)7-5-6-14-31)18-38-26(39)19-37(30(38)41)22-10-12-25(13-11-22)42-24-8-3-2-4-9-24/h2-4,8-13,15,17,21H,5-7,14,18-19,31H2,1H3,(H,34,40)/t21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University
Curated by ChEMBL
| Assay Description
Inhibition of plasmin (unknown origin) using Boc-Val-Leu-Lys-MCA as substrate by fluorescence analysis |
Bioorg Med Chem 23: 3696-704 (2015)
Article DOI: 10.1016/j.bmc.2015.04.013
BindingDB Entry DOI: 10.7270/Q2X92D2Q |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50093542

(CHEMBL3585742)Show SMILES Cn1c(CN2C(=O)CN(C2=O)c2ccc(F)cc2)nc2ccc(cc12)C(=O)N[C@@H](CCCCN)C#N |r| Show InChI InChI=1S/C25H26FN7O3/c1-31-21-12-16(24(35)29-18(13-28)4-2-3-11-27)5-10-20(21)30-22(31)14-33-23(34)15-32(25(33)36)19-8-6-17(26)7-9-19/h5-10,12,18H,2-4,11,14-15,27H2,1H3,(H,29,35)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University
Curated by ChEMBL
| Assay Description
Inhibition of plasmin (unknown origin) using Boc-Val-Leu-Lys-MCA as substrate by fluorescence analysis |
Bioorg Med Chem 23: 3696-704 (2015)
Article DOI: 10.1016/j.bmc.2015.04.013
BindingDB Entry DOI: 10.7270/Q2X92D2Q |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50093547
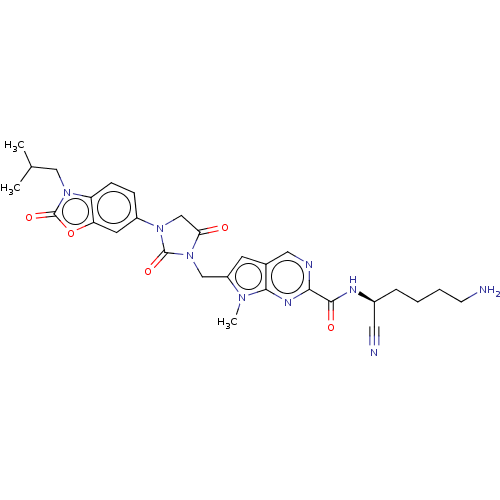
(CHEMBL3585740)Show SMILES CC(C)Cn1c2ccc(cc2oc1=O)N1CC(=O)N(Cc2cc3cnc(nc3n2C)C(=O)N[C@@H](CCCCN)C#N)C1=O |r| Show InChI InChI=1S/C29H33N9O5/c1-17(2)14-37-22-8-7-20(11-23(22)43-29(37)42)36-16-24(39)38(28(36)41)15-21-10-18-13-32-25(34-26(18)35(21)3)27(40)33-19(12-31)6-4-5-9-30/h7-8,10-11,13,17,19H,4-6,9,14-16,30H2,1-3H3,(H,33,40)/t19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University
Curated by ChEMBL
| Assay Description
Inhibition of plasmin (unknown origin) using Boc-Val-Leu-Lys-MCA as substrate by fluorescence analysis |
Bioorg Med Chem 23: 3696-704 (2015)
Article DOI: 10.1016/j.bmc.2015.04.013
BindingDB Entry DOI: 10.7270/Q2X92D2Q |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data