

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50005711 (CHEBI:46024 | GNF-Pf-1011 | TRICHOSTATIN | Trichos...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lille Curated by ChEMBL | Assay Description Inhibition of recombinant human HDAC6 using RHK-K(Ac) as substrate by homogeneous fluorescence release assay | Eur J Med Chem 161: 277-291 (2019) Article DOI: 10.1016/j.ejmech.2018.10.041 BindingDB Entry DOI: 10.7270/Q21V5J76 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50506775 (CHEMBL4593437) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lille Curated by ChEMBL | Assay Description Inhibition of recombinant human HDAC6 using RHK-K(Ac) as substrate by homogeneous fluorescence release assay | Eur J Med Chem 161: 277-291 (2019) Article DOI: 10.1016/j.ejmech.2018.10.041 BindingDB Entry DOI: 10.7270/Q21V5J76 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50005711 (CHEBI:46024 | GNF-Pf-1011 | TRICHOSTATIN | Trichos...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lille Curated by ChEMBL | Assay Description Inhibition of recombinant human HDAC1 using RHK-K(Ac) as substrate by homogeneous fluorescence release assay | Eur J Med Chem 161: 277-291 (2019) Article DOI: 10.1016/j.ejmech.2018.10.041 BindingDB Entry DOI: 10.7270/Q21V5J76 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50506777 (CHEMBL4476057) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lille Curated by ChEMBL | Assay Description Inhibition of recombinant human HDAC6 using RHK-K(Ac) as substrate by homogeneous fluorescence release assay | Eur J Med Chem 161: 277-291 (2019) Article DOI: 10.1016/j.ejmech.2018.10.041 BindingDB Entry DOI: 10.7270/Q21V5J76 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50506780 (CHEMBL4550522) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lille Curated by ChEMBL | Assay Description Inhibition of recombinant human HDAC6 using RHK-K(Ac) as substrate by homogeneous fluorescence release assay | Eur J Med Chem 161: 277-291 (2019) Article DOI: 10.1016/j.ejmech.2018.10.041 BindingDB Entry DOI: 10.7270/Q21V5J76 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50506778 (CHEMBL4469596) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lille Curated by ChEMBL | Assay Description Inhibition of recombinant human HDAC6 using RHK-K(Ac) as substrate by homogeneous fluorescence release assay | Eur J Med Chem 161: 277-291 (2019) Article DOI: 10.1016/j.ejmech.2018.10.041 BindingDB Entry DOI: 10.7270/Q21V5J76 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50506779 (CHEMBL4521590) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lille Curated by ChEMBL | Assay Description Inhibition of recombinant human HDAC6 using RHK-K(Ac) as substrate by homogeneous fluorescence release assay | Eur J Med Chem 161: 277-291 (2019) Article DOI: 10.1016/j.ejmech.2018.10.041 BindingDB Entry DOI: 10.7270/Q21V5J76 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50506781 (CHEMBL4435005) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lille Curated by ChEMBL | Assay Description Inhibition of recombinant human HDAC6 using RHK-K(Ac) as substrate by homogeneous fluorescence release assay | Eur J Med Chem 161: 277-291 (2019) Article DOI: 10.1016/j.ejmech.2018.10.041 BindingDB Entry DOI: 10.7270/Q21V5J76 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50506775 (CHEMBL4593437) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lille Curated by ChEMBL | Assay Description Inhibition of recombinant human HDAC1 using RHK-K(Ac) as substrate by homogeneous fluorescence release assay | Eur J Med Chem 161: 277-291 (2019) Article DOI: 10.1016/j.ejmech.2018.10.041 BindingDB Entry DOI: 10.7270/Q21V5J76 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50506780 (CHEMBL4550522) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lille Curated by ChEMBL | Assay Description Inhibition of recombinant human HDAC1 using RHK-K(Ac) as substrate by homogeneous fluorescence release assay | Eur J Med Chem 161: 277-291 (2019) Article DOI: 10.1016/j.ejmech.2018.10.041 BindingDB Entry DOI: 10.7270/Q21V5J76 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50506781 (CHEMBL4435005) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lille Curated by ChEMBL | Assay Description Inhibition of recombinant human HDAC1 using RHK-K(Ac) as substrate by homogeneous fluorescence release assay | Eur J Med Chem 161: 277-291 (2019) Article DOI: 10.1016/j.ejmech.2018.10.041 BindingDB Entry DOI: 10.7270/Q21V5J76 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM50446481 (CHEMBL3110004 | US10011611, TMP269 | US10722597, C...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lille Curated by ChEMBL | Assay Description Inhibition of recombinant human HDAC4 using Boc-Lys(trifluoroacetyl)-AMC as substrate by homogeneous fluorescence release assay | Eur J Med Chem 161: 277-291 (2019) Article DOI: 10.1016/j.ejmech.2018.10.041 BindingDB Entry DOI: 10.7270/Q21V5J76 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50506779 (CHEMBL4521590) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lille Curated by ChEMBL | Assay Description Inhibition of recombinant human HDAC1 using RHK-K(Ac) as substrate by homogeneous fluorescence release assay | Eur J Med Chem 161: 277-291 (2019) Article DOI: 10.1016/j.ejmech.2018.10.041 BindingDB Entry DOI: 10.7270/Q21V5J76 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50506778 (CHEMBL4469596) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lille Curated by ChEMBL | Assay Description Inhibition of recombinant human HDAC1 using RHK-K(Ac) as substrate by homogeneous fluorescence release assay | Eur J Med Chem 161: 277-291 (2019) Article DOI: 10.1016/j.ejmech.2018.10.041 BindingDB Entry DOI: 10.7270/Q21V5J76 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50506777 (CHEMBL4476057) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lille Curated by ChEMBL | Assay Description Inhibition of recombinant human HDAC1 using RHK-K(Ac) as substrate by homogeneous fluorescence release assay | Eur J Med Chem 161: 277-291 (2019) Article DOI: 10.1016/j.ejmech.2018.10.041 BindingDB Entry DOI: 10.7270/Q21V5J76 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM50506780 (CHEMBL4550522) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lille Curated by ChEMBL | Assay Description Inhibition of recombinant human HDAC4 using Boc-Lys(trifluoroacetyl)-AMC as substrate by homogeneous fluorescence release assay | Eur J Med Chem 161: 277-291 (2019) Article DOI: 10.1016/j.ejmech.2018.10.041 BindingDB Entry DOI: 10.7270/Q21V5J76 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM50005711 (CHEBI:46024 | GNF-Pf-1011 | TRICHOSTATIN | Trichos...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lille Curated by ChEMBL | Assay Description Inhibition of recombinant human HDAC4 using Boc-Lys(trifluoroacetyl)-AMC as substrate by homogeneous fluorescence release assay | Eur J Med Chem 161: 277-291 (2019) Article DOI: 10.1016/j.ejmech.2018.10.041 BindingDB Entry DOI: 10.7270/Q21V5J76 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM50506781 (CHEMBL4435005) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lille Curated by ChEMBL | Assay Description Inhibition of recombinant human HDAC4 using Boc-Lys(trifluoroacetyl)-AMC as substrate by homogeneous fluorescence release assay | Eur J Med Chem 161: 277-291 (2019) Article DOI: 10.1016/j.ejmech.2018.10.041 BindingDB Entry DOI: 10.7270/Q21V5J76 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM50506775 (CHEMBL4593437) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lille Curated by ChEMBL | Assay Description Inhibition of recombinant human HDAC4 using Boc-Lys(trifluoroacetyl)-AMC as substrate by homogeneous fluorescence release assay | Eur J Med Chem 161: 277-291 (2019) Article DOI: 10.1016/j.ejmech.2018.10.041 BindingDB Entry DOI: 10.7270/Q21V5J76 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM50506779 (CHEMBL4521590) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lille Curated by ChEMBL | Assay Description Inhibition of recombinant human HDAC4 using Boc-Lys(trifluoroacetyl)-AMC as substrate by homogeneous fluorescence release assay | Eur J Med Chem 161: 277-291 (2019) Article DOI: 10.1016/j.ejmech.2018.10.041 BindingDB Entry DOI: 10.7270/Q21V5J76 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM50506778 (CHEMBL4469596) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lille Curated by ChEMBL | Assay Description Inhibition of recombinant human HDAC4 using Boc-Lys(trifluoroacetyl)-AMC as substrate by homogeneous fluorescence release assay | Eur J Med Chem 161: 277-291 (2019) Article DOI: 10.1016/j.ejmech.2018.10.041 BindingDB Entry DOI: 10.7270/Q21V5J76 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM50506777 (CHEMBL4476057) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lille Curated by ChEMBL | Assay Description Inhibition of recombinant human HDAC4 using Boc-Lys(trifluoroacetyl)-AMC as substrate by homogeneous fluorescence release assay | Eur J Med Chem 161: 277-291 (2019) Article DOI: 10.1016/j.ejmech.2018.10.041 BindingDB Entry DOI: 10.7270/Q21V5J76 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA (cytosine-5)-methyltransferase 3A (Homo sapiens (Human)) | BDBM50239284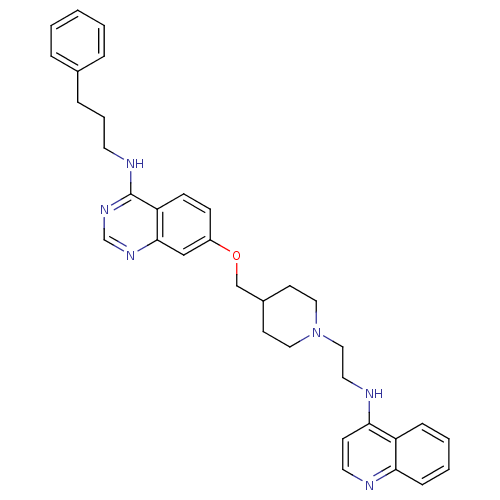 (CHEMBL4096360) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 4.40E+3 | n/a | n/a | n/a | n/a |
CNRS-Pierre Fabre USR3388 Curated by ChEMBL | Assay Description Inhibition of human DNMT3A catalytic domain using AdoMet assessed as reduction of methylation in 5'-biotin/3' 6-carboxyfluorescein labeled DNA duplex... | J Med Chem 60: 4665-4679 (2017) Article DOI: 10.1021/acs.jmedchem.7b00176 BindingDB Entry DOI: 10.7270/Q2MK6G1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA (cytosine-5)-methyltransferase 3A (Homo sapiens (Human)) | BDBM50239285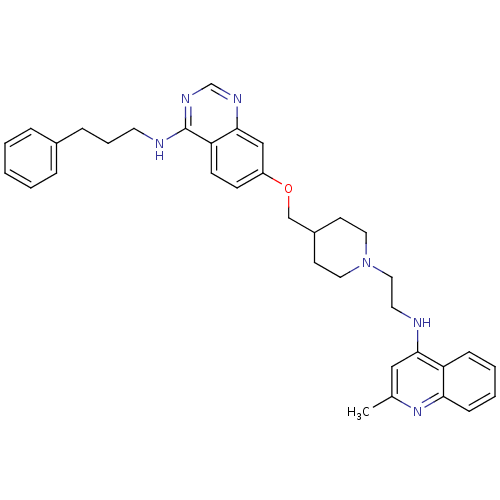 (CHEMBL4096869) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a |
CNRS-Pierre Fabre USR3388 Curated by ChEMBL | Assay Description Inhibition of human DNMT3A catalytic domain using AdoMet assessed as reduction of methylation in 5'-biotin/3' 6-carboxyfluorescein labeled DNA duplex... | J Med Chem 60: 4665-4679 (2017) Article DOI: 10.1021/acs.jmedchem.7b00176 BindingDB Entry DOI: 10.7270/Q2MK6G1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA (cytosine-5)-methyltransferase 3A (Homo sapiens (Human)) | BDBM50239286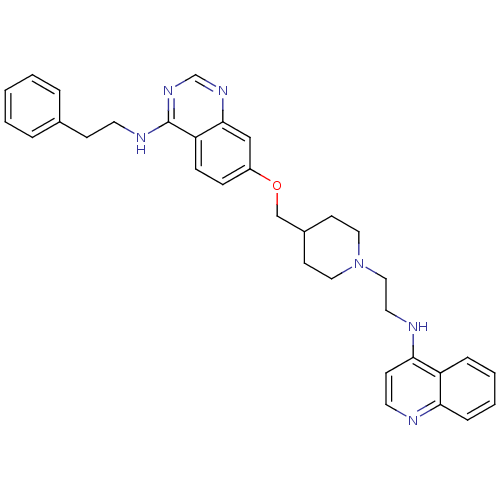 (CHEMBL4084601) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 4.90E+3 | n/a | n/a | n/a | n/a |
CNRS-Pierre Fabre USR3388 Curated by ChEMBL | Assay Description Inhibition of human DNMT3A catalytic domain using AdoMet assessed as reduction of methylation in 5'-biotin/3' 6-carboxyfluorescein labeled DNA duplex... | J Med Chem 60: 4665-4679 (2017) Article DOI: 10.1021/acs.jmedchem.7b00176 BindingDB Entry DOI: 10.7270/Q2MK6G1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA (cytosine-5)-methyltransferase 3A (Homo sapiens (Human)) | BDBM50239287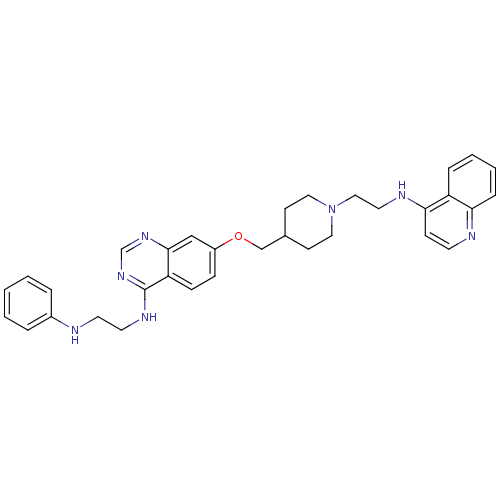 (CHEMBL4100095) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 6.80E+3 | n/a | n/a | n/a | n/a |
CNRS-Pierre Fabre USR3388 Curated by ChEMBL | Assay Description Inhibition of human DNMT3A catalytic domain using AdoMet assessed as reduction of methylation in 5'-biotin/3' 6-carboxyfluorescein labeled DNA duplex... | J Med Chem 60: 4665-4679 (2017) Article DOI: 10.1021/acs.jmedchem.7b00176 BindingDB Entry DOI: 10.7270/Q2MK6G1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA (cytosine-5)-methyltransferase 3A (Homo sapiens (Human)) | BDBM50239288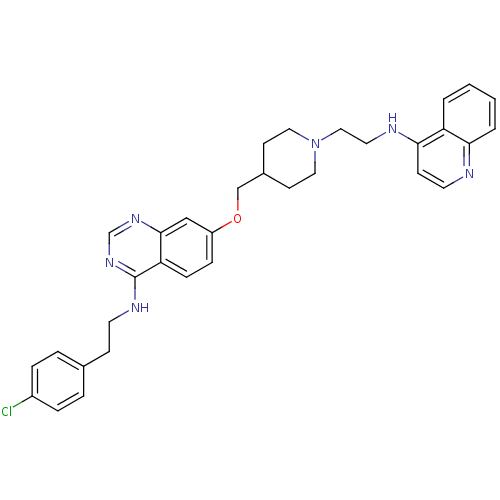 (CHEMBL4097685) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a |
CNRS-Pierre Fabre USR3388 Curated by ChEMBL | Assay Description Inhibition of human DNMT3A catalytic domain using AdoMet assessed as reduction of methylation in 5'-biotin/3' 6-carboxyfluorescein labeled DNA duplex... | J Med Chem 60: 4665-4679 (2017) Article DOI: 10.1021/acs.jmedchem.7b00176 BindingDB Entry DOI: 10.7270/Q2MK6G1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA (cytosine-5)-methyltransferase 3A (Homo sapiens (Human)) | BDBM50239289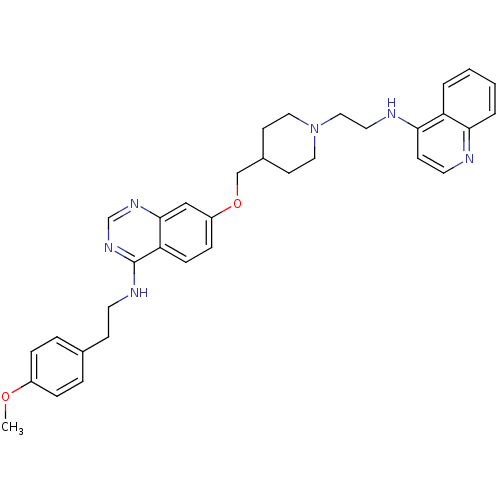 (CHEMBL4096752) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a |
CNRS-Pierre Fabre USR3388 Curated by ChEMBL | Assay Description Inhibition of human DNMT3A catalytic domain using AdoMet assessed as reduction of methylation in 5'-biotin/3' 6-carboxyfluorescein labeled DNA duplex... | J Med Chem 60: 4665-4679 (2017) Article DOI: 10.1021/acs.jmedchem.7b00176 BindingDB Entry DOI: 10.7270/Q2MK6G1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA (cytosine-5)-methyltransferase 3A (Homo sapiens (Human)) | BDBM50239290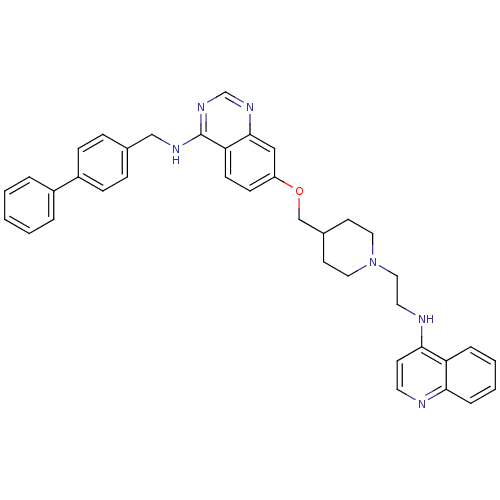 (CHEMBL4099197) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a |
CNRS-Pierre Fabre USR3388 Curated by ChEMBL | Assay Description Inhibition of human DNMT3A catalytic domain using AdoMet assessed as reduction of methylation in 5'-biotin/3' 6-carboxyfluorescein labeled DNA duplex... | J Med Chem 60: 4665-4679 (2017) Article DOI: 10.1021/acs.jmedchem.7b00176 BindingDB Entry DOI: 10.7270/Q2MK6G1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA (cytosine-5)-methyltransferase 3A (Homo sapiens (Human)) | BDBM50239291 (CHEMBL4091463) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 300 | n/a | n/a | n/a | n/a |
CNRS-Pierre Fabre USR3388 Curated by ChEMBL | Assay Description Inhibition of human DNMT3A catalytic domain using AdoMet assessed as reduction of methylation in 5'-biotin/3' 6-carboxyfluorescein labeled DNA duplex... | J Med Chem 60: 4665-4679 (2017) Article DOI: 10.1021/acs.jmedchem.7b00176 BindingDB Entry DOI: 10.7270/Q2MK6G1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA (cytosine-5)-methyltransferase 1 (Homo sapiens (Human)) | BDBM50239284 (CHEMBL4096360) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 4.60E+4 | n/a | n/a | n/a | n/a |
CNRS-Pierre Fabre USR3388 Curated by ChEMBL | Assay Description Inhibition of full length His-tagged human DNMT1 using SAM/[methyl-3H]SAM assessed as reduction of methylation in biotinylated hemimethylated DNA dup... | J Med Chem 60: 4665-4679 (2017) Article DOI: 10.1021/acs.jmedchem.7b00176 BindingDB Entry DOI: 10.7270/Q2MK6G1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA (cytosine-5)-methyltransferase 1 (Homo sapiens (Human)) | BDBM50239285 (CHEMBL4096869) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.40E+4 | n/a | n/a | n/a | n/a |
CNRS-Pierre Fabre USR3388 Curated by ChEMBL | Assay Description Inhibition of full length His-tagged human DNMT1 using SAM/[methyl-3H]SAM assessed as reduction of methylation in biotinylated hemimethylated DNA dup... | J Med Chem 60: 4665-4679 (2017) Article DOI: 10.1021/acs.jmedchem.7b00176 BindingDB Entry DOI: 10.7270/Q2MK6G1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA (cytosine-5)-methyltransferase 1 (Homo sapiens (Human)) | BDBM50239286 (CHEMBL4084601) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.20E+4 | n/a | n/a | n/a | n/a |
CNRS-Pierre Fabre USR3388 Curated by ChEMBL | Assay Description Inhibition of full length His-tagged human DNMT1 using SAM/[methyl-3H]SAM assessed as reduction of methylation in biotinylated hemimethylated DNA dup... | J Med Chem 60: 4665-4679 (2017) Article DOI: 10.1021/acs.jmedchem.7b00176 BindingDB Entry DOI: 10.7270/Q2MK6G1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA (cytosine-5)-methyltransferase 1 (Homo sapiens (Human)) | BDBM50239287 (CHEMBL4100095) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 7.30E+4 | n/a | n/a | n/a | n/a |
CNRS-Pierre Fabre USR3388 Curated by ChEMBL | Assay Description Inhibition of full length His-tagged human DNMT1 using SAM/[methyl-3H]SAM assessed as reduction of methylation in biotinylated hemimethylated DNA dup... | J Med Chem 60: 4665-4679 (2017) Article DOI: 10.1021/acs.jmedchem.7b00176 BindingDB Entry DOI: 10.7270/Q2MK6G1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA (cytosine-5)-methyltransferase 1 (Homo sapiens (Human)) | BDBM50239288 (CHEMBL4097685) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.60E+4 | n/a | n/a | n/a | n/a |
CNRS-Pierre Fabre USR3388 Curated by ChEMBL | Assay Description Inhibition of full length His-tagged human DNMT1 using SAM/[methyl-3H]SAM assessed as reduction of methylation in biotinylated hemimethylated DNA dup... | J Med Chem 60: 4665-4679 (2017) Article DOI: 10.1021/acs.jmedchem.7b00176 BindingDB Entry DOI: 10.7270/Q2MK6G1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA (cytosine-5)-methyltransferase 1 (Homo sapiens (Human)) | BDBM50239290 (CHEMBL4099197) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a |
CNRS-Pierre Fabre USR3388 Curated by ChEMBL | Assay Description Inhibition of full length His-tagged human DNMT1 using SAM/[methyl-3H]SAM assessed as reduction of methylation in biotinylated hemimethylated DNA dup... | J Med Chem 60: 4665-4679 (2017) Article DOI: 10.1021/acs.jmedchem.7b00176 BindingDB Entry DOI: 10.7270/Q2MK6G1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA (cytosine-5)-methyltransferase 1 (Homo sapiens (Human)) | BDBM50239292 (CHEMBL4070549) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a |
CNRS-Pierre Fabre USR3388 Curated by ChEMBL | Assay Description Inhibition of full length His-tagged human DNMT1 using SAM/[methyl-3H]SAM assessed as reduction of methylation in biotinylated hemimethylated DNA dup... | J Med Chem 60: 4665-4679 (2017) Article DOI: 10.1021/acs.jmedchem.7b00176 BindingDB Entry DOI: 10.7270/Q2MK6G1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA (cytosine-5)-methyltransferase 1 (Homo sapiens (Human)) | BDBM50239291 (CHEMBL4091463) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a |
CNRS-Pierre Fabre USR3388 Curated by ChEMBL | Assay Description Inhibition of full length His-tagged human DNMT1 using SAM/[methyl-3H]SAM assessed as reduction of methylation in biotinylated hemimethylated DNA dup... | J Med Chem 60: 4665-4679 (2017) Article DOI: 10.1021/acs.jmedchem.7b00176 BindingDB Entry DOI: 10.7270/Q2MK6G1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA (cytosine-5)-methyltransferase 3A (Homo sapiens (Human)) | BDBM50239293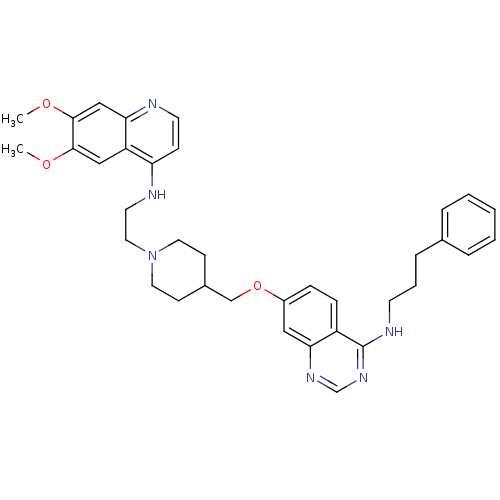 (CHEMBL4076088) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a |
CNRS-Pierre Fabre USR3388 Curated by ChEMBL | Assay Description Inhibition of human DNMT3A catalytic domain using AdoMet assessed as reduction of methylation in 5'-biotin/3' 6-carboxyfluorescein labeled DNA duplex... | J Med Chem 60: 4665-4679 (2017) Article DOI: 10.1021/acs.jmedchem.7b00176 BindingDB Entry DOI: 10.7270/Q2MK6G1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA (cytosine-5)-methyltransferase 3A (Homo sapiens (Human)) | BDBM50239294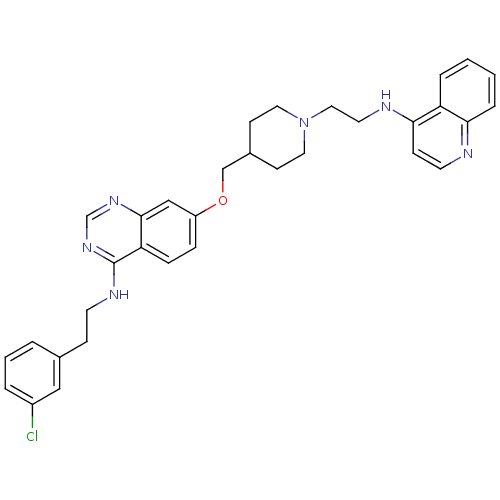 (CHEMBL4070517) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a |
CNRS-Pierre Fabre USR3388 Curated by ChEMBL | Assay Description Inhibition of human DNMT3A catalytic domain using AdoMet assessed as reduction of methylation in 5'-biotin/3' 6-carboxyfluorescein labeled DNA duplex... | J Med Chem 60: 4665-4679 (2017) Article DOI: 10.1021/acs.jmedchem.7b00176 BindingDB Entry DOI: 10.7270/Q2MK6G1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA (cytosine-5)-methyltransferase 3A (Homo sapiens (Human)) | BDBM50239292 (CHEMBL4070549) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a |
CNRS-Pierre Fabre USR3388 Curated by ChEMBL | Assay Description Inhibition of human DNMT3A catalytic domain using AdoMet assessed as reduction of methylation in 5'-biotin/3' 6-carboxyfluorescein labeled DNA duplex... | J Med Chem 60: 4665-4679 (2017) Article DOI: 10.1021/acs.jmedchem.7b00176 BindingDB Entry DOI: 10.7270/Q2MK6G1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA (cytosine-5)-methyltransferase 1 (Homo sapiens (Human)) | BDBM50239293 (CHEMBL4076088) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a |
CNRS-Pierre Fabre USR3388 Curated by ChEMBL | Assay Description Inhibition of full length His-tagged human DNMT1 using SAM/[methyl-3H]SAM assessed as reduction of methylation in biotinylated hemimethylated DNA dup... | J Med Chem 60: 4665-4679 (2017) Article DOI: 10.1021/acs.jmedchem.7b00176 BindingDB Entry DOI: 10.7270/Q2MK6G1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA (cytosine-5)-methyltransferase 1 (Homo sapiens (Human)) | BDBM50239289 (CHEMBL4096752) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.70E+4 | n/a | n/a | n/a | n/a |
CNRS-Pierre Fabre USR3388 Curated by ChEMBL | Assay Description Inhibition of full length His-tagged human DNMT1 using SAM/[methyl-3H]SAM assessed as reduction of methylation in biotinylated hemimethylated DNA dup... | J Med Chem 60: 4665-4679 (2017) Article DOI: 10.1021/acs.jmedchem.7b00176 BindingDB Entry DOI: 10.7270/Q2MK6G1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA (cytosine-5)-methyltransferase 3A (Homo sapiens (Human)) | BDBM50506774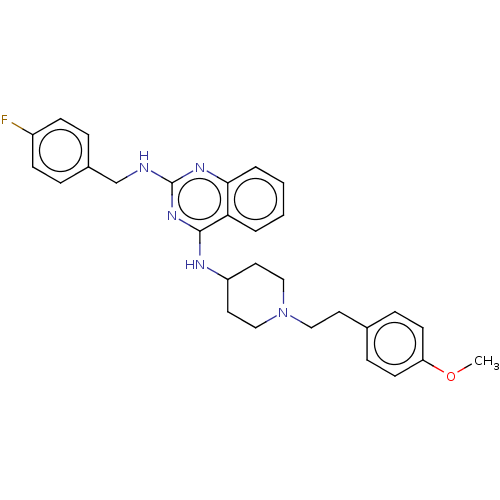 (CHEMBL4470149) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 4.80E+3 | n/a | n/a | n/a | n/a |
Universit£ de Lille Curated by ChEMBL | Assay Description Inhibition of recombinant human DNMT3a C-terminal catalytic domain (623 to 908 residues) using 5'-biotinylated/3'-FAM-oligonucleotide as substrate me... | Eur J Med Chem 161: 277-291 (2019) Article DOI: 10.1016/j.ejmech.2018.10.041 BindingDB Entry DOI: 10.7270/Q21V5J76 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EHMT2 (Homo sapiens (Human)) | BDBM50506774 (CHEMBL4470149) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 5.09E+4 | n/a | n/a | n/a | n/a |
Universit£ de Lille Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal GST-fused G9a (786 to 1210 residues) expressed in Escherichia coli using biotinylated H3 (1 to 21 residues... | Eur J Med Chem 161: 277-291 (2019) Article DOI: 10.1016/j.ejmech.2018.10.041 BindingDB Entry DOI: 10.7270/Q21V5J76 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA (cytosine-5)-methyltransferase 1 (Homo sapiens (Human)) | BDBM50506776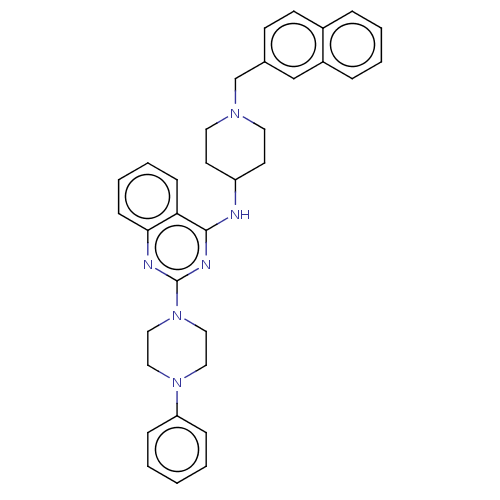 (CHEMBL4442279) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
Universit£ de Lille Curated by ChEMBL | Assay Description Inhibition of recombinant human DNMT1 using biotinylated DNA duplex as substrate measured after 2 hrs in presence of Adomet/[methyl-3H]Adomet by topc... | Eur J Med Chem 161: 277-291 (2019) Article DOI: 10.1016/j.ejmech.2018.10.041 BindingDB Entry DOI: 10.7270/Q21V5J76 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA (cytosine-5)-methyltransferase 1 (Homo sapiens (Human)) | BDBM50506774 (CHEMBL4470149) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
Universit£ de Lille Curated by ChEMBL | Assay Description Inhibition of recombinant human DNMT1 using biotinylated DNA duplex as substrate measured after 2 hrs in presence of Adomet/[methyl-3H]Adomet by topc... | Eur J Med Chem 161: 277-291 (2019) Article DOI: 10.1016/j.ejmech.2018.10.041 BindingDB Entry DOI: 10.7270/Q21V5J76 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA (cytosine-5)-methyltransferase 1 (Homo sapiens (Human)) | BDBM50506782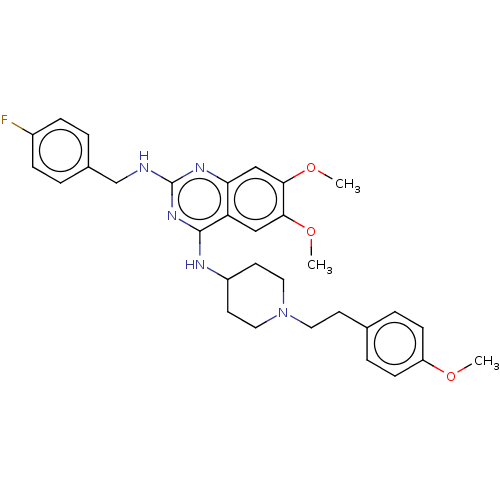 (CHEMBL4461581) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
Universit£ de Lille Curated by ChEMBL | Assay Description Inhibition of recombinant human DNMT1 using biotinylated DNA duplex as substrate measured after 2 hrs in presence of Adomet/[methyl-3H]Adomet by topc... | Eur J Med Chem 161: 277-291 (2019) Article DOI: 10.1016/j.ejmech.2018.10.041 BindingDB Entry DOI: 10.7270/Q21V5J76 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA (cytosine-5)-methyltransferase 1 (Homo sapiens (Human)) | BDBM50506783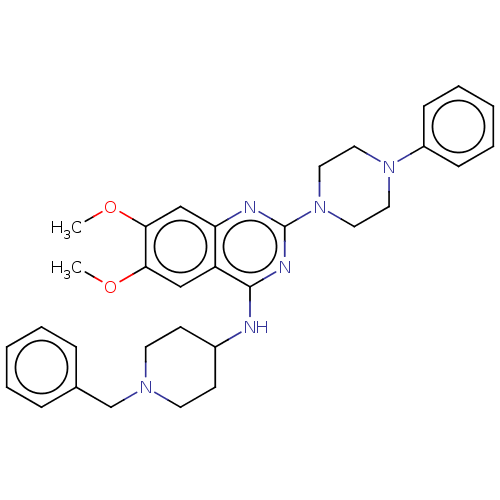 (CHEMBL4582042) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
Universit£ de Lille Curated by ChEMBL | Assay Description Inhibition of recombinant human DNMT1 using biotinylated DNA duplex as substrate measured after 2 hrs in presence of Adomet/[methyl-3H]Adomet by topc... | Eur J Med Chem 161: 277-291 (2019) Article DOI: 10.1016/j.ejmech.2018.10.041 BindingDB Entry DOI: 10.7270/Q21V5J76 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA (cytosine-5)-methyltransferase 1 (Homo sapiens (Human)) | BDBM50506784 (CHEMBL4527662) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
Universit£ de Lille Curated by ChEMBL | Assay Description Inhibition of recombinant human DNMT1 using biotinylated DNA duplex as substrate measured after 2 hrs in presence of Adomet/[methyl-3H]Adomet by topc... | Eur J Med Chem 161: 277-291 (2019) Article DOI: 10.1016/j.ejmech.2018.10.041 BindingDB Entry DOI: 10.7270/Q21V5J76 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 81 total ) | Next | Last >> |