 Found 38 hits with Last Name = 'pendley' and Initial = 'ce'
Found 38 hits with Last Name = 'pendley' and Initial = 'ce' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
5-hydroxytryptamine receptor 3A/3B
(Rattus norvegicus-RAT) | BDBM50000485
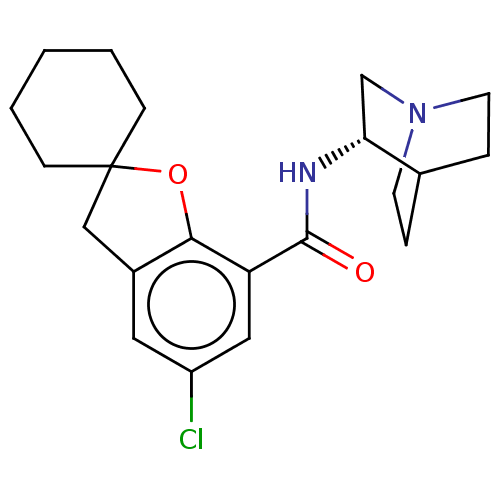
((S)-2-(5-chlorospiro[2,3-dihydrobenzo[b]furan-2,1'...)Show SMILES Clc1cc2CC3(CCCCC3)Oc2c(c1)C(=O)N[C@@H]1CN2CCC1CC2 |wU:18.20,(5.27,-4.79,;6.6,-5.56,;6.6,-7.11,;7.93,-7.88,;8.26,-9.38,;9.8,-9.54,;9.03,-10.87,;9.8,-12.21,;11.35,-12.21,;12.12,-10.87,;11.35,-9.52,;10.41,-8.14,;9.27,-7.11,;9.27,-5.55,;7.93,-4.79,;10.6,-4.77,;10.04,-3.34,;12.12,-5,;13.08,-3.79,;14.6,-4.1,;15.63,-2.95,;15.14,-1.5,;13.64,-1.18,;12.61,-2.34,;13.71,-3.05,;14.57,-2.32,)| Show InChI InChI=1S/C21H27ClN2O2/c22-16-10-15-12-21(6-2-1-3-7-21)26-19(15)17(11-16)20(25)23-18-13-24-8-4-14(18)5-9-24/h10-11,14,18H,1-9,12-13H2,(H,23,25)/t18-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Central Research
Curated by ChEMBL
| Assay Description
Displacement of the 5-hydroxytryptamine 3 receptor ligand [3H]GR-65630 from rat brain cortical membranes. |
J Med Chem 35: 895-903 (1992)
BindingDB Entry DOI: 10.7270/Q2SJ1M77 |
More data for this
Ligand-Target Pair | |
Cholecystokinin
(GUINEA PIG) | BDBM21147

((3S)-3-[(2S)-2-[(2S)-2-{2-[(2S)-2-[(2S)-2-[(3S)-3-...)Show SMILES CSCC[C@H](NC(=O)[C@H](Cc1ccc(OS(O)(=O)=O)cc1)NC(=O)[C@@H](N)CC(O)=O)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C49H62N10O16S3/c1-76-18-16-34(55-47(69)37(58-44(66)32(50)23-41(61)62)21-28-12-14-30(15-13-28)75-78(72,73)74)45(67)53-26-40(60)54-38(22-29-25-52-33-11-7-6-10-31(29)33)48(70)56-35(17-19-77-2)46(68)59-39(24-42(63)64)49(71)57-36(43(51)65)20-27-8-4-3-5-9-27/h3-15,25,32,34-39,52H,16-24,26,50H2,1-2H3,(H2,51,65)(H,53,67)(H,54,60)(H,55,69)(H,56,70)(H,57,71)(H,58,66)(H,59,68)(H,61,62)(H,63,64)(H,72,73,74)/t32-,34-,35-,36-,37-,38-,39-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Rorer Central Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 273: 1015-22 (1995)
BindingDB Entry DOI: 10.7270/Q2QV3K1C |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(GUINEA PIG) | BDBM50005463

((R)-1H-Indole-2-carboxylic acid (1-methyl-2-oxo-5-...)Show SMILES CN1c2ccccc2C(=N[C@H](NC(=O)c2cc3ccccc3[nH]2)C1=O)c1ccccc1 |r,c:9| Show InChI InChI=1S/C25H20N4O2/c1-29-21-14-8-6-12-18(21)22(16-9-3-2-4-10-16)27-23(25(29)31)28-24(30)20-15-17-11-5-7-13-19(17)26-20/h2-15,23,26H,1H3,(H,28,30)/t23-/m1/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Rorer Central Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 273: 1015-22 (1995)
BindingDB Entry DOI: 10.7270/Q2QV3K1C |
More data for this
Ligand-Target Pair | |
Cholecystokinin
(GUINEA PIG) | BDBM82403
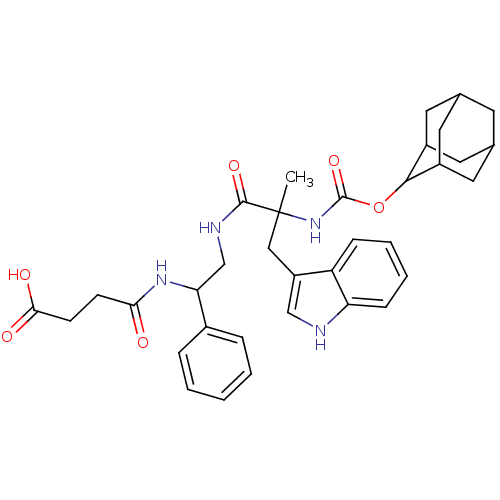
(CAS_108186 | CI-988 | NSC_108186)Show SMILES CC(Cc1c[nH]c2ccccc12)(NC(=O)OC1C2CC3CC(C2)CC1C3)C(=O)NCC(NC(=O)CCC(O)=O)c1ccccc1 |TLB:25:24:18.19.20:22,THB:15:16:18.19.20:22,20:19:16:21.22.23,20:21:16:18.19.25,25:19:22:16.23.24,(.76,2.29,;.32,.82,;-.11,-.66,;.95,-1.78,;.68,-3.29,;2.03,-4.02,;3.15,-2.96,;4.68,-3.07,;5.55,-1.8,;4.88,-.42,;3.35,-.3,;2.48,-1.57,;1.8,.38,;2.92,1.45,;2.55,2.94,;4.39,1.01,;5.51,2.08,;6.88,2.77,;6.88,4.17,;7.72,5.64,;9.09,4.95,;9.09,3.55,;8.25,2.08,;7.72,2.85,;6.35,3.55,;6.35,4.95,;-1.15,1.25,;-1.52,2.75,;-2.27,.19,;-3.75,.62,;-4.86,-.44,;-6.34,-.01,;-7.45,-1.07,;-7.09,-2.57,;-8.93,-.64,;-10.04,-1.7,;-11.52,-1.27,;-11.89,.23,;-12.64,-2.33,;-4.5,-1.94,;-3.02,-2.37,;-2.65,-3.87,;-3.77,-4.93,;-5.25,-4.5,;-5.61,-3,)| Show InChI InChI=1S/C35H42N4O6/c1-35(18-26-19-36-28-10-6-5-9-27(26)28,39-34(44)45-32-24-14-21-13-22(16-24)17-25(32)15-21)33(43)37-20-29(23-7-3-2-4-8-23)38-30(40)11-12-31(41)42/h2-10,19,21-22,24-25,29,32,36H,11-18,20H2,1H3,(H,37,43)(H,38,40)(H,39,44)(H,41,42) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Rorer Central Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 273: 1015-22 (1995)
BindingDB Entry DOI: 10.7270/Q2QV3K1C |
More data for this
Ligand-Target Pair | |
Cholecystokinin
(GUINEA PIG) | BDBM82404
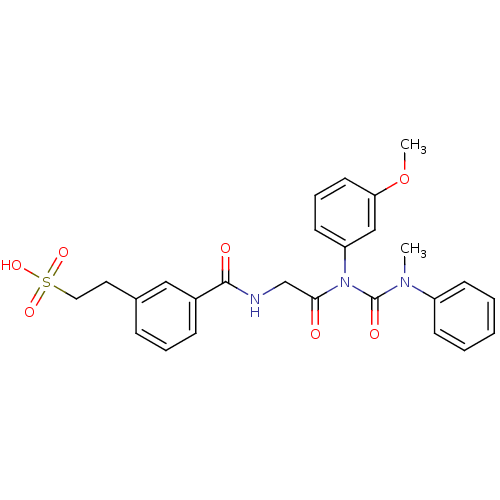
(Glycinamide,N-[[[3-(2-sulfoethyl)phenyl]amino]carb...)Show SMILES COc1cccc(c1)N(C(=O)CNC(=O)c1cccc(CCS(O)(=O)=O)c1)C(=O)N(C)c1ccccc1 Show InChI InChI=1S/C26H27N3O7S/c1-28(21-10-4-3-5-11-21)26(32)29(22-12-7-13-23(17-22)36-2)24(30)18-27-25(31)20-9-6-8-19(16-20)14-15-37(33,34)35/h3-13,16-17H,14-15,18H2,1-2H3,(H,27,31)(H,33,34,35) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| PubMed
| 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Rorer Central Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 273: 1015-22 (1995)
BindingDB Entry DOI: 10.7270/Q2QV3K1C |
More data for this
Ligand-Target Pair | |
Cholecystokinin
(GUINEA PIG) | BDBM82404

(Glycinamide,N-[[[3-(2-sulfoethyl)phenyl]amino]carb...)Show SMILES COc1cccc(c1)N(C(=O)CNC(=O)c1cccc(CCS(O)(=O)=O)c1)C(=O)N(C)c1ccccc1 Show InChI InChI=1S/C26H27N3O7S/c1-28(21-10-4-3-5-11-21)26(32)29(22-12-7-13-23(17-22)36-2)24(30)18-27-25(31)20-9-6-8-19(16-20)14-15-37(33,34)35/h3-13,16-17H,14-15,18H2,1-2H3,(H,27,31)(H,33,34,35) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Rorer Central Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 273: 1015-22 (1995)
BindingDB Entry DOI: 10.7270/Q2QV3K1C |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A/3B
(Rattus norvegicus-RAT) | BDBM50000482
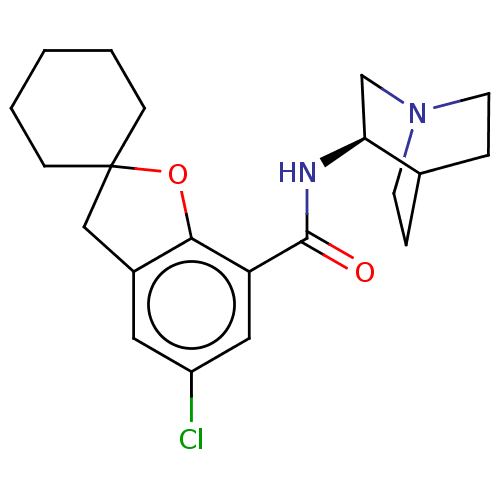
((R)-2-(5-chlorospiro[2,3-dihydrobenzo[b]furan-2,1'...)Show SMILES Clc1cc2CC3(CCCCC3)Oc2c(c1)C(=O)N[C@H]1CN2CCC1CC2 |wD:18.20,(5.27,-4.79,;6.6,-5.56,;6.6,-7.11,;7.93,-7.88,;8.26,-9.38,;9.8,-9.54,;9.03,-10.87,;9.8,-12.21,;11.35,-12.21,;12.12,-10.87,;11.35,-9.52,;10.41,-8.14,;9.27,-7.11,;9.27,-5.55,;7.93,-4.79,;10.6,-4.77,;10.04,-3.34,;12.12,-5,;13.08,-3.79,;14.6,-4.1,;15.63,-2.95,;15.14,-1.5,;13.64,-1.18,;12.61,-2.34,;13.71,-3.05,;14.57,-2.32,)| Show InChI InChI=1S/C21H27ClN2O2/c22-16-10-15-12-21(6-2-1-3-7-21)26-19(15)17(11-16)20(25)23-18-13-24-8-4-14(18)5-9-24/h10-11,14,18H,1-9,12-13H2,(H,23,25)/t18-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.890 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Central Research
Curated by ChEMBL
| Assay Description
Displacement of the 5-hydroxytryptamine 3 receptor ligand [3H]GR-65630 from rat brain cortical membranes. |
J Med Chem 35: 895-903 (1992)
BindingDB Entry DOI: 10.7270/Q2SJ1M77 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A/3B
(Rattus norvegicus-RAT) | BDBM50000480
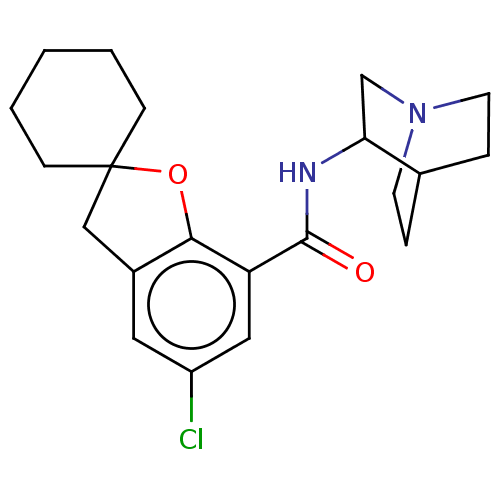
(2-(5-chlorospiro[2,3-dihydrobenzo[b]furan-2,1'-cyc...)Show SMILES Clc1cc2CC3(CCCCC3)Oc2c(c1)C(=O)NC1CN2CCC1CC2 |(5.27,-4.79,;6.6,-5.56,;6.6,-7.11,;7.93,-7.88,;8.26,-9.38,;9.8,-9.54,;9.03,-10.87,;9.8,-12.21,;11.35,-12.21,;12.12,-10.87,;11.35,-9.52,;10.41,-8.14,;9.27,-7.11,;9.27,-5.55,;7.93,-4.79,;10.6,-4.77,;10.04,-3.34,;12.12,-5,;13.08,-3.79,;14.6,-4.1,;15.63,-2.95,;15.14,-1.5,;13.64,-1.18,;12.61,-2.34,;13.71,-3.05,;14.57,-2.32,)| Show InChI InChI=1S/C21H27ClN2O2/c22-16-10-15-12-21(6-2-1-3-7-21)26-19(15)17(11-16)20(25)23-18-13-24-8-4-14(18)5-9-24/h10-11,14,18H,1-9,12-13H2,(H,23,25) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Central Research
Curated by ChEMBL
| Assay Description
Displacement of the 5-hydroxytryptamine 3 receptor ligand [3H]GR-65630 from rat brain cortical membranes. |
J Med Chem 35: 895-903 (1992)
BindingDB Entry DOI: 10.7270/Q2SJ1M77 |
More data for this
Ligand-Target Pair | |
Cholecystokinin
(GUINEA PIG) | BDBM21147

((3S)-3-[(2S)-2-[(2S)-2-{2-[(2S)-2-[(2S)-2-[(3S)-3-...)Show SMILES CSCC[C@H](NC(=O)[C@H](Cc1ccc(OS(O)(=O)=O)cc1)NC(=O)[C@@H](N)CC(O)=O)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C49H62N10O16S3/c1-76-18-16-34(55-47(69)37(58-44(66)32(50)23-41(61)62)21-28-12-14-30(15-13-28)75-78(72,73)74)45(67)53-26-40(60)54-38(22-29-25-52-33-11-7-6-10-31(29)33)48(70)56-35(17-19-77-2)46(68)59-39(24-42(63)64)49(71)57-36(43(51)65)20-27-8-4-3-5-9-27/h3-15,25,32,34-39,52H,16-24,26,50H2,1-2H3,(H2,51,65)(H,53,67)(H,54,60)(H,55,69)(H,56,70)(H,57,71)(H,58,66)(H,59,68)(H,61,62)(H,63,64)(H,72,73,74)/t32-,34-,35-,36-,37-,38-,39-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Rorer Central Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 273: 1015-22 (1995)
BindingDB Entry DOI: 10.7270/Q2QV3K1C |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A/3B
(Rattus norvegicus-RAT) | BDBM50000479
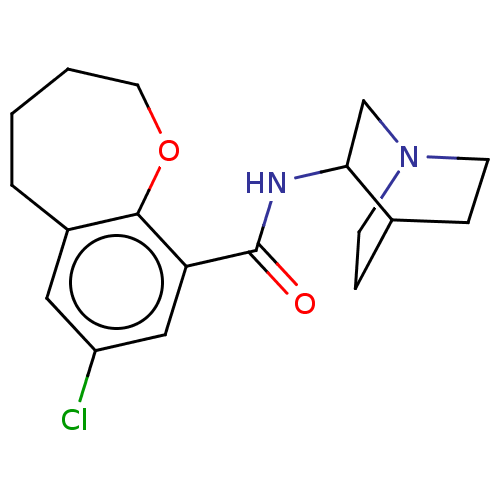
(7-Chloro-2,3,4,5-tetrahydro-benzo[b]oxepine-9-carb...)Show SMILES Clc1cc2CCCCOc2c(c1)C(=O)NC1CN2CCC1CC2 |(3.81,-7.51,;5.15,-6.74,;6.48,-7.51,;7.82,-6.74,;9.03,-7.7,;10.52,-7.35,;11.18,-5.96,;10.52,-4.59,;9.03,-4.24,;7.82,-5.2,;6.48,-4.43,;5.15,-5.2,;6.48,-2.88,;5.15,-2.11,;7.82,-2.11,;7.82,-.57,;9.21,.13,;9.29,1.65,;8,2.52,;6.62,1.82,;6.53,.27,;7.84,.39,;8.07,1.5,)| Show InChI InChI=1S/C18H23ClN2O2/c19-14-9-13-3-1-2-8-23-17(13)15(10-14)18(22)20-16-11-21-6-4-12(16)5-7-21/h9-10,12,16H,1-8,11H2,(H,20,22) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Central Research
Curated by ChEMBL
| Assay Description
Displacement of the 5-hydroxytryptamine 3 receptor ligand [3H]GR-65630 from rat brain cortical membranes. |
J Med Chem 35: 895-903 (1992)
BindingDB Entry DOI: 10.7270/Q2SJ1M77 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A/3B
(Rattus norvegicus-RAT) | BDBM50000492

((zacopride)4-Amino-N-(1-aza-bicyclo[2.2.2]oct-3-yl...)Show SMILES COc1cc(N)c(Cl)cc1C(=O)NC1CN2CCC1CC2 |(27.19,-33.96,;28.52,-34.73,;28.53,-36.27,;27.2,-37.04,;27.2,-38.58,;25.86,-39.35,;28.53,-39.36,;28.53,-40.89,;29.87,-38.58,;29.86,-37.03,;31.19,-36.26,;31.19,-34.72,;32.53,-37.02,;33.86,-36.25,;35.2,-37.02,;36.52,-36.25,;36.52,-34.71,;35.19,-33.94,;33.85,-34.71,;34.61,-36.04,;35.74,-34.91,)| Show InChI InChI=1S/C15H20ClN3O2/c1-21-14-7-12(17)11(16)6-10(14)15(20)18-13-8-19-4-2-9(13)3-5-19/h6-7,9,13H,2-5,8,17H2,1H3,(H,18,20) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Central Research
Curated by ChEMBL
| Assay Description
Displacement of the 5-hydroxytryptamine 3 receptor ligand [3H]GR-65630 from rat brain cortical membranes. |
J Med Chem 35: 895-903 (1992)
BindingDB Entry DOI: 10.7270/Q2SJ1M77 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A/3B
(Rattus norvegicus-RAT) | BDBM50000495
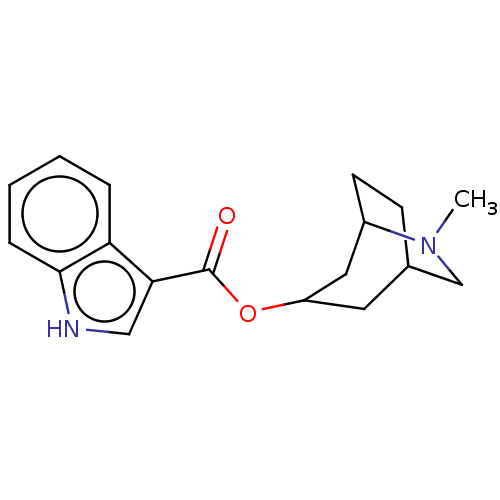
((ICS 205-930)1H-Indole-3-carboxylic acid 6-methyl-...)Show SMILES CN1CC2CCC1CC(C2)OC(=O)c1c[nH]c2ccccc12 |THB:0:1:8.7.9:5.4,10:8:1.2:5.4| Show InChI InChI=1S/C18H22N2O2/c1-20-11-12-6-7-13(20)9-14(8-12)22-18(21)16-10-19-17-5-3-2-4-15(16)17/h2-5,10,12-14,19H,6-9,11H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Central Research
Curated by ChEMBL
| Assay Description
Displacement of the 5-hydroxytryptamine 3 receptor ligand [3H]GR-65630 from rat brain cortical membranes. |
J Med Chem 35: 895-903 (1992)
BindingDB Entry DOI: 10.7270/Q2SJ1M77 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A/3B
(Rattus norvegicus-RAT) | BDBM50000483
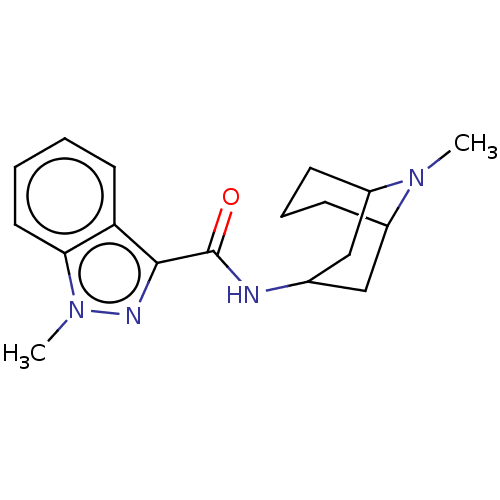
((BRL 43694)1-Methyl-1H-indazole-3-carboxylic acid ...)Show SMILES CN1C2CCCC1CC(C2)NC(=O)c1nn(C)c2ccccc12 |THB:10:8:1:3.5.4| Show InChI InChI=1S/C18H24N4O/c1-21-13-6-5-7-14(21)11-12(10-13)19-18(23)17-15-8-3-4-9-16(15)22(2)20-17/h3-4,8-9,12-14H,5-7,10-11H2,1-2H3,(H,19,23) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Central Research
Curated by ChEMBL
| Assay Description
Displacement of the 5-hydroxytryptamine 3 receptor ligand [3H]GR-65630 from rat brain cortical membranes. |
J Med Chem 35: 895-903 (1992)
BindingDB Entry DOI: 10.7270/Q2SJ1M77 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cholecystokinin
(GUINEA PIG) | BDBM82403

(CAS_108186 | CI-988 | NSC_108186)Show SMILES CC(Cc1c[nH]c2ccccc12)(NC(=O)OC1C2CC3CC(C2)CC1C3)C(=O)NCC(NC(=O)CCC(O)=O)c1ccccc1 |TLB:25:24:18.19.20:22,THB:15:16:18.19.20:22,20:19:16:21.22.23,20:21:16:18.19.25,25:19:22:16.23.24,(.76,2.29,;.32,.82,;-.11,-.66,;.95,-1.78,;.68,-3.29,;2.03,-4.02,;3.15,-2.96,;4.68,-3.07,;5.55,-1.8,;4.88,-.42,;3.35,-.3,;2.48,-1.57,;1.8,.38,;2.92,1.45,;2.55,2.94,;4.39,1.01,;5.51,2.08,;6.88,2.77,;6.88,4.17,;7.72,5.64,;9.09,4.95,;9.09,3.55,;8.25,2.08,;7.72,2.85,;6.35,3.55,;6.35,4.95,;-1.15,1.25,;-1.52,2.75,;-2.27,.19,;-3.75,.62,;-4.86,-.44,;-6.34,-.01,;-7.45,-1.07,;-7.09,-2.57,;-8.93,-.64,;-10.04,-1.7,;-11.52,-1.27,;-11.89,.23,;-12.64,-2.33,;-4.5,-1.94,;-3.02,-2.37,;-2.65,-3.87,;-3.77,-4.93,;-5.25,-4.5,;-5.61,-3,)| Show InChI InChI=1S/C35H42N4O6/c1-35(18-26-19-36-28-10-6-5-9-27(26)28,39-34(44)45-32-24-14-21-13-22(16-24)17-25(32)15-21)33(43)37-20-29(23-7-3-2-4-8-23)38-30(40)11-12-31(41)42/h2-10,19,21-22,24-25,29,32,36H,11-18,20H2,1H3,(H,37,43)(H,38,40)(H,39,44)(H,41,42) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Rorer Central Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 273: 1015-22 (1995)
BindingDB Entry DOI: 10.7270/Q2QV3K1C |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A/3B
(Rattus norvegicus-RAT) | BDBM50000494

(7-Bromo-2,3,4,5-tetrahydro-benzo[b]oxepine-9-carbo...)Show SMILES Brc1cc2CCCCOc2c(c1)C(=O)NC1CN2CCC1CC2 |(3.81,-7.51,;5.15,-6.74,;6.48,-7.51,;7.82,-6.74,;9.03,-7.7,;10.52,-7.35,;11.18,-5.96,;10.52,-4.59,;9.03,-4.24,;7.82,-5.2,;6.48,-4.43,;5.15,-5.2,;6.48,-2.88,;5.15,-2.11,;7.82,-2.11,;7.82,-.57,;9.21,.13,;9.29,1.65,;8,2.52,;6.62,1.82,;6.53,.27,;7.84,.39,;8.07,1.5,)| Show InChI InChI=1S/C18H23BrN2O2/c19-14-9-13-3-1-2-8-23-17(13)15(10-14)18(22)20-16-11-21-6-4-12(16)5-7-21/h9-10,12,16H,1-8,11H2,(H,20,22) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Central Research
Curated by ChEMBL
| Assay Description
Displacement of the 5-hydroxytryptamine 3 receptor ligand [3H]GR-65630 from rat brain cortical membranes. |
J Med Chem 35: 895-903 (1992)
BindingDB Entry DOI: 10.7270/Q2SJ1M77 |
More data for this
Ligand-Target Pair | |
Cholecystokinin
(GUINEA PIG) | BDBM82241
(CAS_5534-95-2 | NSC_444007 | Pentagastrin)Show SMILES CSCCC(NC(=O)C(Cc1c[nH]c2ccccc12)NC(=O)CCNC(=O)OCC(C)C)C(=O)NC(CC(O)=O)C(=O)NC(Cc1ccccc1)C(N)=O Show InChI InChI=1S/C37H49N7O9S/c1-22(2)21-53-37(52)39-15-13-31(45)41-29(18-24-20-40-26-12-8-7-11-25(24)26)35(50)42-27(14-16-54-3)34(49)44-30(19-32(46)47)36(51)43-28(33(38)48)17-23-9-5-4-6-10-23/h4-12,20,22,27-30,40H,13-19,21H2,1-3H3,(H2,38,48)(H,39,52)(H,41,45)(H,42,50)(H,43,51)(H,44,49)(H,46,47) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Rorer Central Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 273: 1015-22 (1995)
BindingDB Entry DOI: 10.7270/Q2QV3K1C |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A/3B
(Rattus norvegicus-RAT) | BDBM50000487

(8-Chloro-3,4,5,6-tetrahydro-2H-benzo[b]oxocine-10-...)Show SMILES Clc1cc2CCCCCOc2c(c1)C(=O)NC1CN2CCC1CC2 |(3.8,-7.5,;5.14,-6.73,;6.47,-7.5,;7.8,-6.73,;8.9,-7.82,;10.45,-7.82,;11.55,-6.73,;11.55,-5.19,;10.46,-4.09,;8.91,-4.08,;7.8,-5.18,;6.47,-4.42,;5.14,-5.19,;6.47,-2.88,;5.14,-2.11,;7.8,-2.11,;7.8,-.57,;9.19,.13,;9.27,1.65,;7.99,2.51,;6.61,1.81,;6.52,.27,;7.82,.39,;8.06,1.49,)| Show InChI InChI=1S/C19H25ClN2O2/c20-15-10-14-4-2-1-3-9-24-18(14)16(11-15)19(23)21-17-12-22-7-5-13(17)6-8-22/h10-11,13,17H,1-9,12H2,(H,21,23) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Central Research
Curated by ChEMBL
| Assay Description
Displacement of the 5-hydroxytryptamine 3 receptor ligand [3H]GR-65630 from rat brain cortical membranes. |
J Med Chem 35: 895-903 (1992)
BindingDB Entry DOI: 10.7270/Q2SJ1M77 |
More data for this
Ligand-Target Pair | |
Cholecystokinin
(GUINEA PIG) | BDBM50061220
(1-((S)-1-Methyl-2-oxo-5-phenyl-2,3-dihydro-1H-benz...)Show SMILES CN1c2ccccc2C(=N[C@H](NC(=O)Nc2cccc(C)c2)C1=O)c1ccccc1 |c:9| Show InChI InChI=1S/C24H22N4O2/c1-16-9-8-12-18(15-16)25-24(30)27-22-23(29)28(2)20-14-7-6-13-19(20)21(26-22)17-10-4-3-5-11-17/h3-15,22H,1-2H3,(H2,25,27,30)/t22-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Rorer Central Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 273: 1015-22 (1995)
BindingDB Entry DOI: 10.7270/Q2QV3K1C |
More data for this
Ligand-Target Pair | |
Cholecystokinin
(GUINEA PIG) | BDBM82235
(Gastrin I | Gastrin-17 | Gastrin-I-(1-17))Show SMILES CSCC[C@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)CNC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](C)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCSC)NC(=O)[C@@H]1CCCN1C(=O)[C@@H]1CCCN1C(=O)[C@H](CCCN=C(N)N)NC(=O)[C@@H]1CCC(=O)N1)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r,wU:4.4,138.143,38.41,52.60,70.78,88.95,96.98,wD:8.20,130.135,26.37,43.51,61.69,79.87,103.106,110.122,121.125,(26,6.14,;24.67,5.35,;24.67,3.8,;23.32,3.01,;23.32,1.49,;22,.73,;20.68,1.49,;20.68,3.04,;19.35,.73,;19.35,-.76,;20.68,-1.52,;21.94,-.7,;23.15,-1.69,;22.59,-3.1,;23.29,-4.48,;22.45,-5.77,;20.9,-5.71,;20.2,-4.31,;21.04,-2.98,;18,1.49,;16.68,.7,;16.68,-.76,;15.36,1.49,;14.03,.7,;12.68,1.46,;12.68,3.01,;11.36,.68,;11.36,-.79,;12.71,-1.55,;12.71,-3.1,;14.03,-3.86,;15.36,-3.07,;16.71,-3.86,;15.36,-1.55,;14.03,-.79,;10.01,1.44,;8.66,.68,;8.66,-.82,;7.33,1.44,;7.3,2.98,;6.01,.65,;4.66,1.44,;4.66,2.98,;3.34,.65,;3.34,-.84,;4.66,-1.6,;4.66,-3.15,;6.01,-3.91,;3.34,-3.91,;2.01,1.44,;.66,.65,;.66,-.84,;-.61,1.44,;-.61,2.96,;.66,3.74,;.66,5.32,;2.01,6.11,;-.61,6.11,;-1.93,.65,;-3.28,1.41,;-3.28,2.96,;-4.6,.65,;-4.6,-.84,;-3.28,-1.6,;-3.28,-3.15,;-1.93,-3.94,;-4.6,-3.94,;-5.93,1.41,;-7.28,.65,;-7.28,-.84,;-8.6,1.44,;-8.6,2.96,;-7.28,3.74,;-7.28,5.32,;-5.93,6.11,;-8.6,6.11,;-9.95,.65,;-11.33,1.44,;-11.33,2.98,;-12.65,.65,;-12.65,-.82,;-11.33,-1.6,;-11.33,-3.15,;-9.95,-3.94,;-12.65,-3.91,;-13.98,1.44,;-15.33,.68,;-15.33,-.79,;-16.65,1.44,;-16.65,2.96,;-15.3,3.77,;-15.3,5.29,;-13.98,6.08,;-17.97,.68,;-19.32,1.44,;-19.32,2.98,;-20.62,.68,;-19.69,1.83,;-20.39,3.26,;-21.91,2.96,;-21.91,1.44,;-23.24,.68,;-23.24,-.96,;-24.7,1.52,;-23.97,.34,;-24.56,-.98,;-26.16,-.7,;-26.16,.68,;-27.52,1.44,;-27.52,2.98,;-28.84,.68,;-28.84,-.82,;-27.52,-1.55,;-27.52,-3.1,;-26.14,-3.86,;-26.11,-5.4,;-27.52,-6.16,;-24.78,-6.16,;-30.19,1.44,;-31.51,.68,;-31.51,-.82,;-32.84,1.41,;-31.85,.28,;-32.55,-1.1,;-34.07,-.79,;-35.37,-1.58,;-34.07,.68,;24.67,.73,;24.67,-.73,;26,1.49,;27.35,.7,;27.35,-.76,;28.7,-1.55,;30.02,-.76,;28.7,-3.07,;28.7,1.49,;28.7,3.04,;30.02,.7,;31.34,1.49,;31.34,3.01,;32.7,3.83,;32.7,5.38,;34.02,6.16,;35.37,5.38,;35.37,3.83,;34.02,3.01,;32.7,.7,;34.02,1.49,;32.7,-.76,)| Show InChI InChI=1S/C94H128N22O31S2/c1-48(79(133)113-65(43-50-19-21-52(117)22-20-50)80(134)100-47-71(119)103-66(44-51-46-99-54-15-8-7-14-53(51)54)89(143)109-61(35-40-148-2)88(142)114-67(45-77(130)131)90(144)112-64(78(95)132)42-49-12-5-4-6-13-49)101-81(135)56(24-30-72(120)121)104-83(137)57(25-31-73(122)123)105-84(138)58(26-32-74(124)125)106-85(139)59(27-33-75(126)127)107-86(140)60(28-34-76(128)129)108-87(141)62(36-41-149-3)110-91(145)68-17-10-38-115(68)93(147)69-18-11-39-116(69)92(146)63(16-9-37-98-94(96)97)111-82(136)55-23-29-70(118)102-55/h4-8,12-15,19-22,46,48,55-69,99,117H,9-11,16-18,23-45,47H2,1-3H3,(H2,95,132)(H,100,134)(H,101,135)(H,102,118)(H,103,119)(H,104,137)(H,105,138)(H,106,139)(H,107,140)(H,108,141)(H,109,143)(H,110,145)(H,111,136)(H,112,144)(H,113,133)(H,114,142)(H,120,121)(H,122,123)(H,124,125)(H,126,127)(H,128,129)(H,130,131)(H4,96,97,98)/t48-,55-,56-,57-,58-,59-,60-,61-,62-,63-,64-,65-,66-,67-,68-,69-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 5.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Rorer Central Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 273: 1015-22 (1995)
BindingDB Entry DOI: 10.7270/Q2QV3K1C |
More data for this
Ligand-Target Pair | |
Cholecystokinin
(GUINEA PIG) | BDBM82235

(Gastrin I | Gastrin-17 | Gastrin-I-(1-17))Show SMILES CSCC[C@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)CNC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](C)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCSC)NC(=O)[C@@H]1CCCN1C(=O)[C@@H]1CCCN1C(=O)[C@H](CCCN=C(N)N)NC(=O)[C@@H]1CCC(=O)N1)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r,wU:4.4,138.143,38.41,52.60,70.78,88.95,96.98,wD:8.20,130.135,26.37,43.51,61.69,79.87,103.106,110.122,121.125,(26,6.14,;24.67,5.35,;24.67,3.8,;23.32,3.01,;23.32,1.49,;22,.73,;20.68,1.49,;20.68,3.04,;19.35,.73,;19.35,-.76,;20.68,-1.52,;21.94,-.7,;23.15,-1.69,;22.59,-3.1,;23.29,-4.48,;22.45,-5.77,;20.9,-5.71,;20.2,-4.31,;21.04,-2.98,;18,1.49,;16.68,.7,;16.68,-.76,;15.36,1.49,;14.03,.7,;12.68,1.46,;12.68,3.01,;11.36,.68,;11.36,-.79,;12.71,-1.55,;12.71,-3.1,;14.03,-3.86,;15.36,-3.07,;16.71,-3.86,;15.36,-1.55,;14.03,-.79,;10.01,1.44,;8.66,.68,;8.66,-.82,;7.33,1.44,;7.3,2.98,;6.01,.65,;4.66,1.44,;4.66,2.98,;3.34,.65,;3.34,-.84,;4.66,-1.6,;4.66,-3.15,;6.01,-3.91,;3.34,-3.91,;2.01,1.44,;.66,.65,;.66,-.84,;-.61,1.44,;-.61,2.96,;.66,3.74,;.66,5.32,;2.01,6.11,;-.61,6.11,;-1.93,.65,;-3.28,1.41,;-3.28,2.96,;-4.6,.65,;-4.6,-.84,;-3.28,-1.6,;-3.28,-3.15,;-1.93,-3.94,;-4.6,-3.94,;-5.93,1.41,;-7.28,.65,;-7.28,-.84,;-8.6,1.44,;-8.6,2.96,;-7.28,3.74,;-7.28,5.32,;-5.93,6.11,;-8.6,6.11,;-9.95,.65,;-11.33,1.44,;-11.33,2.98,;-12.65,.65,;-12.65,-.82,;-11.33,-1.6,;-11.33,-3.15,;-9.95,-3.94,;-12.65,-3.91,;-13.98,1.44,;-15.33,.68,;-15.33,-.79,;-16.65,1.44,;-16.65,2.96,;-15.3,3.77,;-15.3,5.29,;-13.98,6.08,;-17.97,.68,;-19.32,1.44,;-19.32,2.98,;-20.62,.68,;-19.69,1.83,;-20.39,3.26,;-21.91,2.96,;-21.91,1.44,;-23.24,.68,;-23.24,-.96,;-24.7,1.52,;-23.97,.34,;-24.56,-.98,;-26.16,-.7,;-26.16,.68,;-27.52,1.44,;-27.52,2.98,;-28.84,.68,;-28.84,-.82,;-27.52,-1.55,;-27.52,-3.1,;-26.14,-3.86,;-26.11,-5.4,;-27.52,-6.16,;-24.78,-6.16,;-30.19,1.44,;-31.51,.68,;-31.51,-.82,;-32.84,1.41,;-31.85,.28,;-32.55,-1.1,;-34.07,-.79,;-35.37,-1.58,;-34.07,.68,;24.67,.73,;24.67,-.73,;26,1.49,;27.35,.7,;27.35,-.76,;28.7,-1.55,;30.02,-.76,;28.7,-3.07,;28.7,1.49,;28.7,3.04,;30.02,.7,;31.34,1.49,;31.34,3.01,;32.7,3.83,;32.7,5.38,;34.02,6.16,;35.37,5.38,;35.37,3.83,;34.02,3.01,;32.7,.7,;34.02,1.49,;32.7,-.76,)| Show InChI InChI=1S/C94H128N22O31S2/c1-48(79(133)113-65(43-50-19-21-52(117)22-20-50)80(134)100-47-71(119)103-66(44-51-46-99-54-15-8-7-14-53(51)54)89(143)109-61(35-40-148-2)88(142)114-67(45-77(130)131)90(144)112-64(78(95)132)42-49-12-5-4-6-13-49)101-81(135)56(24-30-72(120)121)104-83(137)57(25-31-73(122)123)105-84(138)58(26-32-74(124)125)106-85(139)59(27-33-75(126)127)107-86(140)60(28-34-76(128)129)108-87(141)62(36-41-149-3)110-91(145)68-17-10-38-115(68)93(147)69-18-11-39-116(69)92(146)63(16-9-37-98-94(96)97)111-82(136)55-23-29-70(118)102-55/h4-8,12-15,19-22,46,48,55-69,99,117H,9-11,16-18,23-45,47H2,1-3H3,(H2,95,132)(H,100,134)(H,101,135)(H,102,118)(H,103,119)(H,104,137)(H,105,138)(H,106,139)(H,107,140)(H,108,141)(H,109,143)(H,110,145)(H,111,136)(H,112,144)(H,113,133)(H,114,142)(H,120,121)(H,122,123)(H,124,125)(H,126,127)(H,128,129)(H,130,131)(H4,96,97,98)/t48-,55-,56-,57-,58-,59-,60-,61-,62-,63-,64-,65-,66-,67-,68-,69-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 5.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Rorer Central Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 273: 1015-22 (1995)
BindingDB Entry DOI: 10.7270/Q2QV3K1C |
More data for this
Ligand-Target Pair | |
Cholecystokinin
(GUINEA PIG) | BDBM82241

(CAS_5534-95-2 | NSC_444007 | Pentagastrin)Show SMILES CSCCC(NC(=O)C(Cc1c[nH]c2ccccc12)NC(=O)CCNC(=O)OCC(C)C)C(=O)NC(CC(O)=O)C(=O)NC(Cc1ccccc1)C(N)=O Show InChI InChI=1S/C37H49N7O9S/c1-22(2)21-53-37(52)39-15-13-31(45)41-29(18-24-20-40-26-12-8-7-11-25(24)26)35(50)42-27(14-16-54-3)34(49)44-30(19-32(46)47)36(51)43-28(33(38)48)17-23-9-5-4-6-10-23/h4-12,20,22,27-30,40H,13-19,21H2,1-3H3,(H2,38,48)(H,39,52)(H,41,45)(H,42,50)(H,43,51)(H,44,49)(H,46,47) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Rorer Central Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 273: 1015-22 (1995)
BindingDB Entry DOI: 10.7270/Q2QV3K1C |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A/3B
(Rattus norvegicus-RAT) | BDBM50000489
(CHEMBL353993 | N-(1-Aza-bicyclo[2.2.2]oct-3-yl)-5-...)Show SMILES COc1c(cc(Cl)cc1C(=O)NC1CN2CCC1CC2)C1CCCCC1 |(13.13,-7.36,;11.79,-8.15,;10.46,-7.38,;9.14,-8.16,;7.78,-7.38,;7.78,-5.84,;6.45,-5.07,;9.13,-5.07,;10.46,-5.82,;11.79,-5.05,;11.19,-3.63,;13.32,-5.24,;14.25,-4.02,;15.77,-4.3,;16.77,-3.14,;16.26,-1.68,;14.74,-1.39,;13.74,-2.57,;14.85,-3.27,;15.7,-2.53,;9.14,-9.7,;10.49,-10.45,;10.49,-12,;9.14,-12.77,;7.81,-12,;7.81,-10.47,)| Show InChI InChI=1S/C21H29ClN2O2/c1-26-20-17(14-5-3-2-4-6-14)11-16(22)12-18(20)21(25)23-19-13-24-9-7-15(19)8-10-24/h11-12,14-15,19H,2-10,13H2,1H3,(H,23,25) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 6.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Central Research
Curated by ChEMBL
| Assay Description
Displacement of the 5-hydroxytryptamine 3 receptor ligand [3H]GR-65630 from rat brain cortical membranes. |
J Med Chem 35: 895-903 (1992)
BindingDB Entry DOI: 10.7270/Q2SJ1M77 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A/3B
(Rattus norvegicus-RAT) | BDBM85330

(CAS_68647 | NSC_68647 | ONDANSETRON | Ondansetron ...)Show InChI InChI=1S/C18H19N3O/c1-12-19-9-10-21(12)11-13-7-8-16-17(18(13)22)14-5-3-4-6-15(14)20(16)2/h3-6,9-10,13H,7-8,11H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PDB
PubMed
| 6.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Central Research
Curated by ChEMBL
| Assay Description
Displacement of the 5-hydroxytryptamine 3 receptor ligand [3H]GR-65630 from rat brain cortical membranes. |
J Med Chem 35: 895-903 (1992)
BindingDB Entry DOI: 10.7270/Q2SJ1M77 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cholecystokinin
(GUINEA PIG) | BDBM50061220

(1-((S)-1-Methyl-2-oxo-5-phenyl-2,3-dihydro-1H-benz...)Show SMILES CN1c2ccccc2C(=N[C@H](NC(=O)Nc2cccc(C)c2)C1=O)c1ccccc1 |c:9| Show InChI InChI=1S/C24H22N4O2/c1-16-9-8-12-18(15-16)25-24(30)27-22-23(29)28(2)20-14-7-6-13-19(20)21(26-22)17-10-4-3-5-11-17/h3-15,22H,1-2H3,(H2,25,27,30)/t22-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 7.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Rorer Central Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 273: 1015-22 (1995)
BindingDB Entry DOI: 10.7270/Q2QV3K1C |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A/3B
(Rattus norvegicus-RAT) | BDBM50000478

(2,3,4,5-Tetrahydro-benzo[b]oxepine-9-carboxylic ac...)Show SMILES O=C(NC1CN2CCC1CC2)c1cccc2CCCCOc12 |(5.15,-2.11,;6.48,-2.88,;7.82,-2.11,;7.82,-.57,;9.21,.13,;9.29,1.65,;8.07,1.5,;7.84,.39,;6.53,.27,;6.62,1.82,;8,2.52,;6.48,-4.43,;5.15,-5.2,;5.15,-6.74,;6.48,-7.51,;7.82,-6.74,;9.03,-7.7,;10.52,-7.35,;11.18,-5.96,;10.52,-4.59,;9.03,-4.24,;7.82,-5.2,)| Show InChI InChI=1S/C18H24N2O2/c21-18(19-16-12-20-9-7-13(16)8-10-20)15-6-3-5-14-4-1-2-11-22-17(14)15/h3,5-6,13,16H,1-2,4,7-12H2,(H,19,21) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Central Research
Curated by ChEMBL
| Assay Description
Displacement of the 5-hydroxytryptamine 3 receptor ligand [3H]GR-65630 from rat brain cortical membranes. |
J Med Chem 35: 895-903 (1992)
BindingDB Entry DOI: 10.7270/Q2SJ1M77 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A/3B
(Rattus norvegicus-RAT) | BDBM50000484

(7-Chloro-2,2-dimethyl-2,3,4,5-tetrahydro-benzo[b]o...)Show SMILES CC1(C)CCCc2cc(Cl)cc(C(=O)NC3CN4CCC3CC4)c2O1 |(13.36,-9.45,;12.03,-10.23,;12.42,-11.72,;11.13,-11.54,;9.56,-11.63,;8.61,-10.46,;8.93,-9.22,;7.58,-8.45,;7.58,-6.91,;6.25,-6.13,;8.92,-6.13,;10.25,-6.9,;11.58,-6.13,;11.04,-4.68,;13.1,-6.37,;14.08,-5.19,;15.6,-5.53,;16.65,-4.4,;15.6,-3.75,;14.71,-4.45,;13.63,-3.72,;14.68,-2.58,;16.19,-2.92,;10.25,-8.45,;11.54,-8.81,)| Show InChI InChI=1S/C20H27ClN2O2/c1-20(2)7-3-4-14-10-15(21)11-16(18(14)25-20)19(24)22-17-12-23-8-5-13(17)6-9-23/h10-11,13,17H,3-9,12H2,1-2H3,(H,22,24) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Central Research
Curated by ChEMBL
| Assay Description
Displacement of the 5-hydroxytryptamine 3 receptor ligand [3H]GR-65630 from rat brain cortical membranes. |
J Med Chem 35: 895-903 (1992)
BindingDB Entry DOI: 10.7270/Q2SJ1M77 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A/3B
(Rattus norvegicus-RAT) | BDBM50000486

(CHEMBL368992 | N-(1-Aza-bicyclo[2.2.2]oct-3-yl)-2-...)Show SMILES CCC(C)Oc1c(cc(Cl)cc1C(=O)NC1CN2CCC1CC2)C1CCCCC1 |(9.13,-5.17,;8.18,-5.01,;7.85,-4.11,;8.31,-3.27,;6.58,-4.11,;5.23,-3.35,;3.93,-4.14,;2.57,-3.35,;2.57,-1.81,;1.24,-1.03,;3.91,-1.03,;5.23,-1.79,;6.02,-.61,;5.51,.79,;7.67,-.72,;8.65,.66,;10.18,.85,;10.81,2.23,;9.61,2.53,;9,1.57,;7.73,1.9,;8.36,3.32,;9.9,3.5,;3.93,-5.68,;2.58,-6.44,;2.6,-7.97,;3.93,-8.76,;5.26,-7.97,;5.26,-6.43,)| Show InChI InChI=1S/C24H35ClN2O2/c1-3-16(2)29-23-20(17-7-5-4-6-8-17)13-19(25)14-21(23)24(28)26-22-15-27-11-9-18(22)10-12-27/h13-14,16-18,22H,3-12,15H2,1-2H3,(H,26,28) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Central Research
Curated by ChEMBL
| Assay Description
Displacement of the 5-hydroxytryptamine 3 receptor ligand [3H]GR-65630 from rat brain cortical membranes. |
J Med Chem 35: 895-903 (1992)
BindingDB Entry DOI: 10.7270/Q2SJ1M77 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A/3B
(Rattus norvegicus-RAT) | BDBM50000488
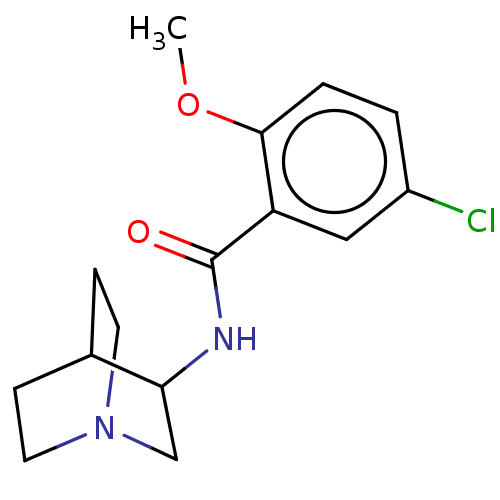
(CHEMBL171070 | N-(1-Aza-bicyclo[2.2.2]oct-3-yl)-5-...)Show SMILES COc1ccc(Cl)cc1C(=O)NC1CN2CCC1CC2 |(11.79,-11.86,;11.78,-10.32,;10.45,-9.55,;9.13,-10.32,;7.78,-9.55,;7.78,-8.01,;6.45,-7.24,;9.12,-7.24,;10.45,-8,;11.78,-7.22,;11.55,-5.7,;13.21,-7.78,;14.42,-6.81,;15.83,-7.45,;17.06,-6.56,;16.19,-5.72,;15.17,-6.24,;14.26,-5.28,;15.52,-4.39,;16.92,-5.02,)| Show InChI InChI=1S/C15H19ClN2O2/c1-20-14-3-2-11(16)8-12(14)15(19)17-13-9-18-6-4-10(13)5-7-18/h2-3,8,10,13H,4-7,9H2,1H3,(H,17,19) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Central Research
Curated by ChEMBL
| Assay Description
Displacement of the 5-hydroxytryptamine 3 receptor ligand [3H]GR-65630 from rat brain cortical membranes. |
J Med Chem 35: 895-903 (1992)
BindingDB Entry DOI: 10.7270/Q2SJ1M77 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A/3B
(Rattus norvegicus-RAT) | BDBM50000490
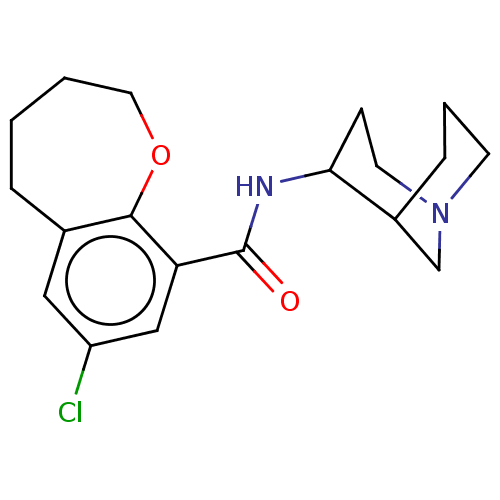
(7-Chloro-2,3,4,5-tetrahydro-benzo[b]oxepine-9-carb...)Show InChI InChI=1S/C19H25ClN2O2/c20-15-10-13-4-1-2-9-24-18(13)16(11-15)19(23)21-17-6-8-22-7-3-5-14(17)12-22/h10-11,14,17H,1-9,12H2,(H,21,23) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 63 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Central Research
Curated by ChEMBL
| Assay Description
Displacement of the 5-hydroxytryptamine 3 receptor ligand [3H]GR-65630 from rat brain cortical membranes. |
J Med Chem 35: 895-903 (1992)
BindingDB Entry DOI: 10.7270/Q2SJ1M77 |
More data for this
Ligand-Target Pair | |
Cholecystokinin
(GUINEA PIG) | BDBM50005463

((R)-1H-Indole-2-carboxylic acid (1-methyl-2-oxo-5-...)Show SMILES CN1c2ccccc2C(=N[C@H](NC(=O)c2cc3ccccc3[nH]2)C1=O)c1ccccc1 |r,c:9| Show InChI InChI=1S/C25H20N4O2/c1-29-21-14-8-6-12-18(21)22(16-9-3-2-4-10-16)27-23(25(29)31)28-24(30)20-15-17-11-5-7-13-19(17)26-20/h2-15,23,26H,1H3,(H,28,30)/t23-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 87 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Rorer Central Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 273: 1015-22 (1995)
BindingDB Entry DOI: 10.7270/Q2QV3K1C |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A/3B
(Rattus norvegicus-RAT) | BDBM50000481
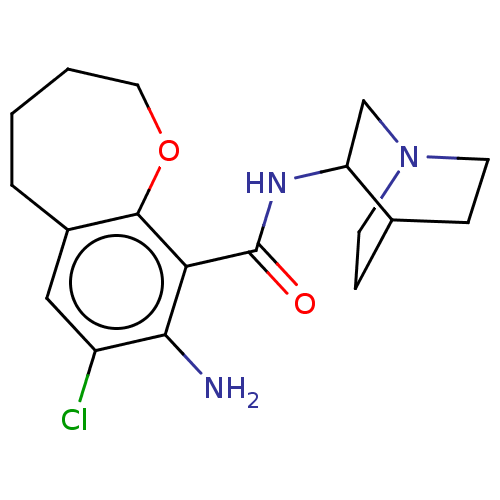
(8-Amino-7-chloro-2,3,4,5-tetrahydro-benzo[b]oxepin...)Show SMILES Nc1c(Cl)cc2CCCCOc2c1C(=O)NC1CN2CCC1CC2 |(4.17,-2.82,;5.51,-3.6,;5.51,-5.14,;4.17,-5.91,;6.86,-5.92,;8.2,-5.13,;9.11,-6.03,;10.6,-5.78,;11.31,-4.39,;10.64,-2.94,;9.16,-2.66,;8.18,-3.59,;6.85,-2.82,;6.85,-1.28,;5.51,-.52,;8.18,-.51,;8.18,1.03,;9.56,1.72,;9.65,3.25,;8.45,3.09,;8.21,1.99,;6.89,1.87,;7,3.41,;8.36,4.11,)| Show InChI InChI=1S/C18H24ClN3O2/c19-13-9-12-3-1-2-8-24-17(12)15(16(13)20)18(23)21-14-10-22-6-4-11(14)5-7-22/h9,11,14H,1-8,10,20H2,(H,21,23) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Central Research
Curated by ChEMBL
| Assay Description
Displacement of the 5-hydroxytryptamine 3 receptor ligand [3H]GR-65630 from rat brain cortical membranes. |
J Med Chem 35: 895-903 (1992)
BindingDB Entry DOI: 10.7270/Q2SJ1M77 |
More data for this
Ligand-Target Pair | |
Cholecystokinin
(GUINEA PIG) | BDBM50005463

((R)-1H-Indole-2-carboxylic acid (1-methyl-2-oxo-5-...)Show SMILES CN1c2ccccc2C(=N[C@H](NC(=O)c2cc3ccccc3[nH]2)C1=O)c1ccccc1 |r,c:9| Show InChI InChI=1S/C25H20N4O2/c1-29-21-14-8-6-12-18(21)22(16-9-3-2-4-10-16)27-23(25(29)31)28-24(30)20-15-17-11-5-7-13-19(17)26-20/h2-15,23,26H,1H3,(H,28,30)/t23-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 141 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Rorer Central Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 273: 1015-22 (1995)
BindingDB Entry DOI: 10.7270/Q2QV3K1C |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(GUINEA PIG) | BDBM82235

(Gastrin I | Gastrin-17 | Gastrin-I-(1-17))Show SMILES CSCC[C@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)CNC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](C)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCSC)NC(=O)[C@@H]1CCCN1C(=O)[C@@H]1CCCN1C(=O)[C@H](CCCN=C(N)N)NC(=O)[C@@H]1CCC(=O)N1)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r,wU:4.4,138.143,38.41,52.60,70.78,88.95,96.98,wD:8.20,130.135,26.37,43.51,61.69,79.87,103.106,110.122,121.125,(26,6.14,;24.67,5.35,;24.67,3.8,;23.32,3.01,;23.32,1.49,;22,.73,;20.68,1.49,;20.68,3.04,;19.35,.73,;19.35,-.76,;20.68,-1.52,;21.94,-.7,;23.15,-1.69,;22.59,-3.1,;23.29,-4.48,;22.45,-5.77,;20.9,-5.71,;20.2,-4.31,;21.04,-2.98,;18,1.49,;16.68,.7,;16.68,-.76,;15.36,1.49,;14.03,.7,;12.68,1.46,;12.68,3.01,;11.36,.68,;11.36,-.79,;12.71,-1.55,;12.71,-3.1,;14.03,-3.86,;15.36,-3.07,;16.71,-3.86,;15.36,-1.55,;14.03,-.79,;10.01,1.44,;8.66,.68,;8.66,-.82,;7.33,1.44,;7.3,2.98,;6.01,.65,;4.66,1.44,;4.66,2.98,;3.34,.65,;3.34,-.84,;4.66,-1.6,;4.66,-3.15,;6.01,-3.91,;3.34,-3.91,;2.01,1.44,;.66,.65,;.66,-.84,;-.61,1.44,;-.61,2.96,;.66,3.74,;.66,5.32,;2.01,6.11,;-.61,6.11,;-1.93,.65,;-3.28,1.41,;-3.28,2.96,;-4.6,.65,;-4.6,-.84,;-3.28,-1.6,;-3.28,-3.15,;-1.93,-3.94,;-4.6,-3.94,;-5.93,1.41,;-7.28,.65,;-7.28,-.84,;-8.6,1.44,;-8.6,2.96,;-7.28,3.74,;-7.28,5.32,;-5.93,6.11,;-8.6,6.11,;-9.95,.65,;-11.33,1.44,;-11.33,2.98,;-12.65,.65,;-12.65,-.82,;-11.33,-1.6,;-11.33,-3.15,;-9.95,-3.94,;-12.65,-3.91,;-13.98,1.44,;-15.33,.68,;-15.33,-.79,;-16.65,1.44,;-16.65,2.96,;-15.3,3.77,;-15.3,5.29,;-13.98,6.08,;-17.97,.68,;-19.32,1.44,;-19.32,2.98,;-20.62,.68,;-19.69,1.83,;-20.39,3.26,;-21.91,2.96,;-21.91,1.44,;-23.24,.68,;-23.24,-.96,;-24.7,1.52,;-23.97,.34,;-24.56,-.98,;-26.16,-.7,;-26.16,.68,;-27.52,1.44,;-27.52,2.98,;-28.84,.68,;-28.84,-.82,;-27.52,-1.55,;-27.52,-3.1,;-26.14,-3.86,;-26.11,-5.4,;-27.52,-6.16,;-24.78,-6.16,;-30.19,1.44,;-31.51,.68,;-31.51,-.82,;-32.84,1.41,;-31.85,.28,;-32.55,-1.1,;-34.07,-.79,;-35.37,-1.58,;-34.07,.68,;24.67,.73,;24.67,-.73,;26,1.49,;27.35,.7,;27.35,-.76,;28.7,-1.55,;30.02,-.76,;28.7,-3.07,;28.7,1.49,;28.7,3.04,;30.02,.7,;31.34,1.49,;31.34,3.01,;32.7,3.83,;32.7,5.38,;34.02,6.16,;35.37,5.38,;35.37,3.83,;34.02,3.01,;32.7,.7,;34.02,1.49,;32.7,-.76,)| Show InChI InChI=1S/C94H128N22O31S2/c1-48(79(133)113-65(43-50-19-21-52(117)22-20-50)80(134)100-47-71(119)103-66(44-51-46-99-54-15-8-7-14-53(51)54)89(143)109-61(35-40-148-2)88(142)114-67(45-77(130)131)90(144)112-64(78(95)132)42-49-12-5-4-6-13-49)101-81(135)56(24-30-72(120)121)104-83(137)57(25-31-73(122)123)105-84(138)58(26-32-74(124)125)106-85(139)59(27-33-75(126)127)107-86(140)60(28-34-76(128)129)108-87(141)62(36-41-149-3)110-91(145)68-17-10-38-115(68)93(147)69-18-11-39-116(69)92(146)63(16-9-37-98-94(96)97)111-82(136)55-23-29-70(118)102-55/h4-8,12-15,19-22,46,48,55-69,99,117H,9-11,16-18,23-45,47H2,1-3H3,(H2,95,132)(H,100,134)(H,101,135)(H,102,118)(H,103,119)(H,104,137)(H,105,138)(H,106,139)(H,107,140)(H,108,141)(H,109,143)(H,110,145)(H,111,136)(H,112,144)(H,113,133)(H,114,142)(H,120,121)(H,122,123)(H,124,125)(H,126,127)(H,128,129)(H,130,131)(H4,96,97,98)/t48-,55-,56-,57-,58-,59-,60-,61-,62-,63-,64-,65-,66-,67-,68-,69-/m0/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 883 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Rorer Central Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 273: 1015-22 (1995)
BindingDB Entry DOI: 10.7270/Q2QV3K1C |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A/3B
(Rattus norvegicus-RAT) | BDBM48320
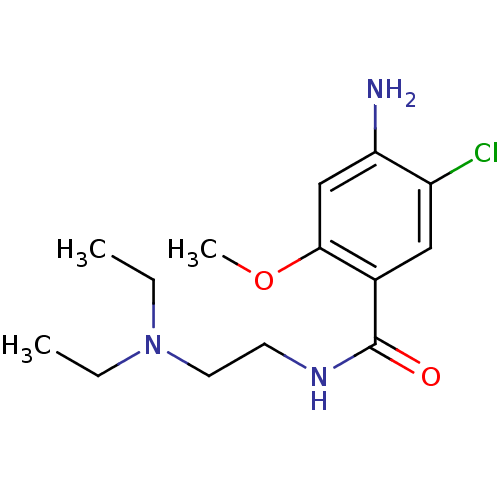
(4-amino-5-chloro-N-[2-(diethylamino)ethyl]-2-metho...)Show InChI InChI=1S/C14H22ClN3O2/c1-4-18(5-2)7-6-17-14(19)10-8-11(15)12(16)9-13(10)20-3/h8-9H,4-7,16H2,1-3H3,(H,17,19) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 995 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Central Research
Curated by ChEMBL
| Assay Description
Displacement of the 5-hydroxytryptamine 3 receptor ligand [3H]GR-65630 from rat brain cortical membranes. |
J Med Chem 35: 895-903 (1992)
BindingDB Entry DOI: 10.7270/Q2SJ1M77 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(GUINEA PIG) | BDBM82403

(CAS_108186 | CI-988 | NSC_108186)Show SMILES CC(Cc1c[nH]c2ccccc12)(NC(=O)OC1C2CC3CC(C2)CC1C3)C(=O)NCC(NC(=O)CCC(O)=O)c1ccccc1 |TLB:25:24:18.19.20:22,THB:15:16:18.19.20:22,20:19:16:21.22.23,20:21:16:18.19.25,25:19:22:16.23.24,(.76,2.29,;.32,.82,;-.11,-.66,;.95,-1.78,;.68,-3.29,;2.03,-4.02,;3.15,-2.96,;4.68,-3.07,;5.55,-1.8,;4.88,-.42,;3.35,-.3,;2.48,-1.57,;1.8,.38,;2.92,1.45,;2.55,2.94,;4.39,1.01,;5.51,2.08,;6.88,2.77,;6.88,4.17,;7.72,5.64,;9.09,4.95,;9.09,3.55,;8.25,2.08,;7.72,2.85,;6.35,3.55,;6.35,4.95,;-1.15,1.25,;-1.52,2.75,;-2.27,.19,;-3.75,.62,;-4.86,-.44,;-6.34,-.01,;-7.45,-1.07,;-7.09,-2.57,;-8.93,-.64,;-10.04,-1.7,;-11.52,-1.27,;-11.89,.23,;-12.64,-2.33,;-4.5,-1.94,;-3.02,-2.37,;-2.65,-3.87,;-3.77,-4.93,;-5.25,-4.5,;-5.61,-3,)| Show InChI InChI=1S/C35H42N4O6/c1-35(18-26-19-36-28-10-6-5-9-27(26)28,39-34(44)45-32-24-14-21-13-22(16-24)17-25(32)15-21)33(43)37-20-29(23-7-3-2-4-8-23)38-30(40)11-12-31(41)42/h2-10,19,21-22,24-25,29,32,36H,11-18,20H2,1H3,(H,37,43)(H,38,40)(H,39,44)(H,41,42) | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 1.44E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Rorer Central Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 273: 1015-22 (1995)
BindingDB Entry DOI: 10.7270/Q2QV3K1C |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(GUINEA PIG) | BDBM82404

(Glycinamide,N-[[[3-(2-sulfoethyl)phenyl]amino]carb...)Show SMILES COc1cccc(c1)N(C(=O)CNC(=O)c1cccc(CCS(O)(=O)=O)c1)C(=O)N(C)c1ccccc1 Show InChI InChI=1S/C26H27N3O7S/c1-28(21-10-4-3-5-11-21)26(32)29(22-12-7-13-23(17-22)36-2)24(30)18-27-25(31)20-9-6-8-19(16-20)14-15-37(33,34)35/h3-13,16-17H,14-15,18H2,1-2H3,(H,27,31)(H,33,34,35) | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| PubMed
| 1.63E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Rorer Central Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 273: 1015-22 (1995)
BindingDB Entry DOI: 10.7270/Q2QV3K1C |
More data for this
Ligand-Target Pair | |
Cholecystokinin
(GUINEA PIG) | BDBM50008673

(2-(4-Chloro-benzoylamino)-3-(1H-indol-3-yl)-propio...)Show SMILES OC(=O)C(Cc1c[nH]c2ccccc12)NC(=O)c1ccc(Cl)cc1 Show InChI InChI=1S/C18H15ClN2O3/c19-13-7-5-11(6-8-13)17(22)21-16(18(23)24)9-12-10-20-15-4-2-1-3-14(12)15/h1-8,10,16,20H,9H2,(H,21,22)(H,23,24) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Rorer Central Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 273: 1015-22 (1995)
BindingDB Entry DOI: 10.7270/Q2QV3K1C |
More data for this
Ligand-Target Pair | |
Cholecystokinin
(GUINEA PIG) | BDBM50008673

(2-(4-Chloro-benzoylamino)-3-(1H-indol-3-yl)-propio...)Show SMILES OC(=O)C(Cc1c[nH]c2ccccc12)NC(=O)c1ccc(Cl)cc1 Show InChI InChI=1S/C18H15ClN2O3/c19-13-7-5-11(6-8-13)17(22)21-16(18(23)24)9-12-10-20-15-4-2-1-3-14(12)15/h1-8,10,16,20H,9H2,(H,21,22)(H,23,24) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Rorer Central Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 273: 1015-22 (1995)
BindingDB Entry DOI: 10.7270/Q2QV3K1C |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data







































