 Found 1344 hits with Last Name = 'prat' and Initial = 'm'
Found 1344 hits with Last Name = 'prat' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50296336
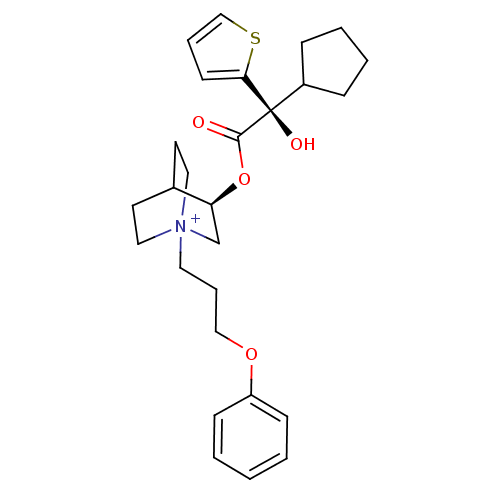
((3R)-3-{[(2S)-2-Cyclopentyl-2-hydroxy-2-(2-thienyl...)Show SMILES O[C@@](C1CCCC1)(C(=O)O[C@H]1C[N+]2(CCCOc3ccccc3)CCC1CC2)c1cccs1 |r,wU:1.31,wD:10.10,1.0,(16.18,-14.66,;15.09,-15.75,;13.76,-16.53,;12.35,-15.91,;11.32,-17.05,;12.1,-18.39,;13.6,-18.06,;16.43,-16.52,;16.43,-18.06,;17.76,-15.75,;19.09,-16.51,;19.09,-18.05,;20.42,-18.81,;20.41,-20.35,;21.74,-21.14,;23.08,-20.38,;24.4,-21.16,;25.74,-20.41,;27.07,-21.19,;28.41,-20.43,;28.42,-18.89,;27.09,-18.11,;25.75,-18.87,;21.75,-18.05,;21.75,-16.51,;20.42,-15.73,;20.84,-16.98,;19.79,-17.33,;15.09,-14.21,;13.83,-13.31,;14.3,-11.85,;15.84,-11.84,;16.32,-13.31,)| Show InChI InChI=1S/C27H36NO4S/c29-26(27(30,22-8-4-5-9-22)25-12-6-19-33-25)32-24-20-28(16-13-21(24)14-17-28)15-7-18-31-23-10-2-1-3-11-23/h1-3,6,10-12,19,21-22,24,30H,4-5,7-9,13-18,20H2/q+1/t21?,24-,27+,28?/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Binding affinity to muscarinic M2 receptor |
J Med Chem 52: 5076-92 (2010)
Article DOI: 10.1021/jm900132z
BindingDB Entry DOI: 10.7270/Q2SX6F5Z |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50296331
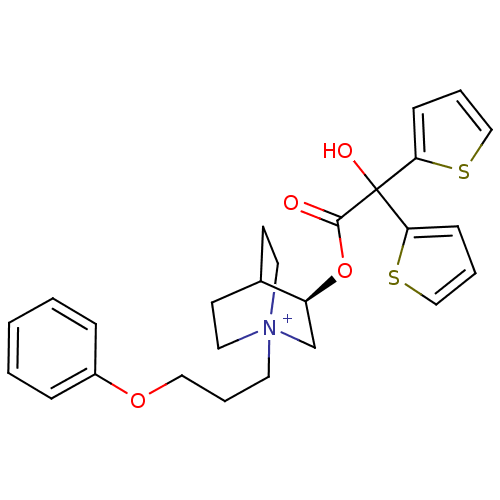
((3R)-3-{[Hydroxy(di-2-thienyl)acetyl]oxy}-1-(3-phe...)Show SMILES OC(C(=O)O[C@H]1C[N+]2(CCCOc3ccccc3)CCC1CC2)(c1cccs1)c1cccs1 |r,wD:5.4,(-4.87,-33.87,;-5.96,-34.96,;-4.62,-35.72,;-4.61,-37.26,;-3.29,-34.95,;-1.95,-35.72,;-1.95,-37.26,;-.62,-38.02,;-.64,-39.56,;.69,-40.34,;2.03,-39.58,;3.36,-40.37,;4.7,-39.61,;6.02,-40.39,;7.36,-39.64,;7.37,-38.1,;6.04,-37.31,;4.7,-38.07,;.71,-37.26,;.71,-35.72,;-.62,-34.94,;-.2,-36.18,;-1.25,-36.53,;-5.96,-33.42,;-7.21,-32.51,;-6.74,-31.04,;-5.2,-31.04,;-4.72,-32.5,;-7.29,-35.73,;-8.7,-35.11,;-9.73,-36.25,;-8.96,-37.59,;-7.45,-37.26,)| Show InChI InChI=1S/C26H30NO4S2/c28-25(26(29,23-9-4-17-32-23)24-10-5-18-33-24)31-22-19-27(14-11-20(22)12-15-27)13-6-16-30-21-7-2-1-3-8-21/h1-5,7-10,17-18,20,22,29H,6,11-16,19H2/q+1/t20?,22-,27?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Similars
| DrugBank
Article
PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Binding affinity to muscarinic M3 receptor |
J Med Chem 52: 5076-92 (2010)
Article DOI: 10.1021/jm900132z
BindingDB Entry DOI: 10.7270/Q2SX6F5Z |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50296336

((3R)-3-{[(2S)-2-Cyclopentyl-2-hydroxy-2-(2-thienyl...)Show SMILES O[C@@](C1CCCC1)(C(=O)O[C@H]1C[N+]2(CCCOc3ccccc3)CCC1CC2)c1cccs1 |r,wU:1.31,wD:10.10,1.0,(16.18,-14.66,;15.09,-15.75,;13.76,-16.53,;12.35,-15.91,;11.32,-17.05,;12.1,-18.39,;13.6,-18.06,;16.43,-16.52,;16.43,-18.06,;17.76,-15.75,;19.09,-16.51,;19.09,-18.05,;20.42,-18.81,;20.41,-20.35,;21.74,-21.14,;23.08,-20.38,;24.4,-21.16,;25.74,-20.41,;27.07,-21.19,;28.41,-20.43,;28.42,-18.89,;27.09,-18.11,;25.75,-18.87,;21.75,-18.05,;21.75,-16.51,;20.42,-15.73,;20.84,-16.98,;19.79,-17.33,;15.09,-14.21,;13.83,-13.31,;14.3,-11.85,;15.84,-11.84,;16.32,-13.31,)| Show InChI InChI=1S/C27H36NO4S/c29-26(27(30,22-8-4-5-9-22)25-12-6-19-33-25)32-24-20-28(16-13-21(24)14-17-28)15-7-18-31-23-10-2-1-3-11-23/h1-3,6,10-12,19,21-22,24,30H,4-5,7-9,13-18,20H2/q+1/t21?,24-,27+,28?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Binding affinity to muscarinic M3 receptor |
J Med Chem 52: 5076-92 (2010)
Article DOI: 10.1021/jm900132z
BindingDB Entry DOI: 10.7270/Q2SX6F5Z |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50296329
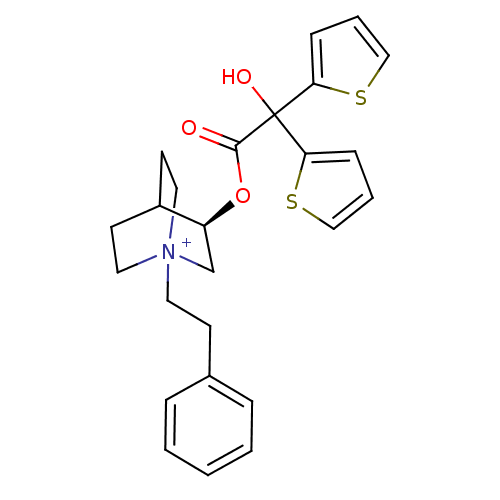
((3R)-3-{[Hydroxy(di-2-thienyl)acetyl]oxy}-1-(2-phe...)Show SMILES OC(C(=O)O[C@H]1C[N+]2(CCc3ccccc3)CCC1CC2)(c1cccs1)c1cccs1 |r,wD:5.4,(12.26,-17.08,;11.17,-18.17,;12.51,-18.94,;12.51,-20.48,;13.84,-18.17,;15.17,-18.93,;15.17,-20.47,;16.5,-21.23,;16.49,-22.77,;17.82,-23.56,;19.16,-22.8,;20.48,-23.59,;21.82,-22.83,;21.84,-21.29,;20.5,-20.51,;19.17,-21.27,;17.83,-20.47,;17.83,-18.93,;16.5,-18.15,;16.92,-19.4,;15.87,-19.75,;11.17,-16.63,;9.92,-15.73,;10.39,-14.26,;11.93,-14.26,;12.41,-15.72,;9.84,-18.95,;8.42,-18.32,;7.4,-19.47,;8.17,-20.8,;9.68,-20.48,)| Show InChI InChI=1S/C25H28NO3S2/c27-24(25(28,22-8-4-16-30-22)23-9-5-17-31-23)29-21-18-26(14-11-20(21)12-15-26)13-10-19-6-2-1-3-7-19/h1-9,16-17,20-21,28H,10-15,18H2/q+1/t20?,21-,26?/m0/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Binding affinity to muscarinic M1 receptor |
J Med Chem 52: 5076-92 (2010)
Article DOI: 10.1021/jm900132z
BindingDB Entry DOI: 10.7270/Q2SX6F5Z |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50296331

((3R)-3-{[Hydroxy(di-2-thienyl)acetyl]oxy}-1-(3-phe...)Show SMILES OC(C(=O)O[C@H]1C[N+]2(CCCOc3ccccc3)CCC1CC2)(c1cccs1)c1cccs1 |r,wD:5.4,(-4.87,-33.87,;-5.96,-34.96,;-4.62,-35.72,;-4.61,-37.26,;-3.29,-34.95,;-1.95,-35.72,;-1.95,-37.26,;-.62,-38.02,;-.64,-39.56,;.69,-40.34,;2.03,-39.58,;3.36,-40.37,;4.7,-39.61,;6.02,-40.39,;7.36,-39.64,;7.37,-38.1,;6.04,-37.31,;4.7,-38.07,;.71,-37.26,;.71,-35.72,;-.62,-34.94,;-.2,-36.18,;-1.25,-36.53,;-5.96,-33.42,;-7.21,-32.51,;-6.74,-31.04,;-5.2,-31.04,;-4.72,-32.5,;-7.29,-35.73,;-8.7,-35.11,;-9.73,-36.25,;-8.96,-37.59,;-7.45,-37.26,)| Show InChI InChI=1S/C26H30NO4S2/c28-25(26(29,23-9-4-17-32-23)24-10-5-18-33-24)31-22-19-27(14-11-20(22)12-15-27)13-6-16-30-21-7-2-1-3-8-21/h1-5,7-10,17-18,20,22,29H,6,11-16,19H2/q+1/t20?,22-,27?/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Binding affinity to muscarinic M2 receptor |
J Med Chem 52: 5076-92 (2010)
Article DOI: 10.1021/jm900132z
BindingDB Entry DOI: 10.7270/Q2SX6F5Z |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50296329

((3R)-3-{[Hydroxy(di-2-thienyl)acetyl]oxy}-1-(2-phe...)Show SMILES OC(C(=O)O[C@H]1C[N+]2(CCc3ccccc3)CCC1CC2)(c1cccs1)c1cccs1 |r,wD:5.4,(12.26,-17.08,;11.17,-18.17,;12.51,-18.94,;12.51,-20.48,;13.84,-18.17,;15.17,-18.93,;15.17,-20.47,;16.5,-21.23,;16.49,-22.77,;17.82,-23.56,;19.16,-22.8,;20.48,-23.59,;21.82,-22.83,;21.84,-21.29,;20.5,-20.51,;19.17,-21.27,;17.83,-20.47,;17.83,-18.93,;16.5,-18.15,;16.92,-19.4,;15.87,-19.75,;11.17,-16.63,;9.92,-15.73,;10.39,-14.26,;11.93,-14.26,;12.41,-15.72,;9.84,-18.95,;8.42,-18.32,;7.4,-19.47,;8.17,-20.8,;9.68,-20.48,)| Show InChI InChI=1S/C25H28NO3S2/c27-24(25(28,22-8-4-16-30-22)23-9-5-17-31-23)29-21-18-26(14-11-20(21)12-15-26)13-10-19-6-2-1-3-7-19/h1-9,16-17,20-21,28H,10-15,18H2/q+1/t20?,21-,26?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Binding affinity to muscarinic M3 receptor |
J Med Chem 52: 5076-92 (2010)
Article DOI: 10.1021/jm900132z
BindingDB Entry DOI: 10.7270/Q2SX6F5Z |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50296329

((3R)-3-{[Hydroxy(di-2-thienyl)acetyl]oxy}-1-(2-phe...)Show SMILES OC(C(=O)O[C@H]1C[N+]2(CCc3ccccc3)CCC1CC2)(c1cccs1)c1cccs1 |r,wD:5.4,(12.26,-17.08,;11.17,-18.17,;12.51,-18.94,;12.51,-20.48,;13.84,-18.17,;15.17,-18.93,;15.17,-20.47,;16.5,-21.23,;16.49,-22.77,;17.82,-23.56,;19.16,-22.8,;20.48,-23.59,;21.82,-22.83,;21.84,-21.29,;20.5,-20.51,;19.17,-21.27,;17.83,-20.47,;17.83,-18.93,;16.5,-18.15,;16.92,-19.4,;15.87,-19.75,;11.17,-16.63,;9.92,-15.73,;10.39,-14.26,;11.93,-14.26,;12.41,-15.72,;9.84,-18.95,;8.42,-18.32,;7.4,-19.47,;8.17,-20.8,;9.68,-20.48,)| Show InChI InChI=1S/C25H28NO3S2/c27-24(25(28,22-8-4-16-30-22)23-9-5-17-31-23)29-21-18-26(14-11-20(21)12-15-26)13-10-19-6-2-1-3-7-19/h1-9,16-17,20-21,28H,10-15,18H2/q+1/t20?,21-,26?/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Binding affinity to muscarinic M2 receptor |
J Med Chem 52: 5076-92 (2010)
Article DOI: 10.1021/jm900132z
BindingDB Entry DOI: 10.7270/Q2SX6F5Z |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50296331

((3R)-3-{[Hydroxy(di-2-thienyl)acetyl]oxy}-1-(3-phe...)Show SMILES OC(C(=O)O[C@H]1C[N+]2(CCCOc3ccccc3)CCC1CC2)(c1cccs1)c1cccs1 |r,wD:5.4,(-4.87,-33.87,;-5.96,-34.96,;-4.62,-35.72,;-4.61,-37.26,;-3.29,-34.95,;-1.95,-35.72,;-1.95,-37.26,;-.62,-38.02,;-.64,-39.56,;.69,-40.34,;2.03,-39.58,;3.36,-40.37,;4.7,-39.61,;6.02,-40.39,;7.36,-39.64,;7.37,-38.1,;6.04,-37.31,;4.7,-38.07,;.71,-37.26,;.71,-35.72,;-.62,-34.94,;-.2,-36.18,;-1.25,-36.53,;-5.96,-33.42,;-7.21,-32.51,;-6.74,-31.04,;-5.2,-31.04,;-4.72,-32.5,;-7.29,-35.73,;-8.7,-35.11,;-9.73,-36.25,;-8.96,-37.59,;-7.45,-37.26,)| Show InChI InChI=1S/C26H30NO4S2/c28-25(26(29,23-9-4-17-32-23)24-10-5-18-33-24)31-22-19-27(14-11-20(22)12-15-27)13-6-16-30-21-7-2-1-3-8-21/h1-5,7-10,17-18,20,22,29H,6,11-16,19H2/q+1/t20?,22-,27?/m0/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Binding affinity to muscarinic M1 receptor |
J Med Chem 52: 5076-92 (2010)
Article DOI: 10.1021/jm900132z
BindingDB Entry DOI: 10.7270/Q2SX6F5Z |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50296336

((3R)-3-{[(2S)-2-Cyclopentyl-2-hydroxy-2-(2-thienyl...)Show SMILES O[C@@](C1CCCC1)(C(=O)O[C@H]1C[N+]2(CCCOc3ccccc3)CCC1CC2)c1cccs1 |r,wU:1.31,wD:10.10,1.0,(16.18,-14.66,;15.09,-15.75,;13.76,-16.53,;12.35,-15.91,;11.32,-17.05,;12.1,-18.39,;13.6,-18.06,;16.43,-16.52,;16.43,-18.06,;17.76,-15.75,;19.09,-16.51,;19.09,-18.05,;20.42,-18.81,;20.41,-20.35,;21.74,-21.14,;23.08,-20.38,;24.4,-21.16,;25.74,-20.41,;27.07,-21.19,;28.41,-20.43,;28.42,-18.89,;27.09,-18.11,;25.75,-18.87,;21.75,-18.05,;21.75,-16.51,;20.42,-15.73,;20.84,-16.98,;19.79,-17.33,;15.09,-14.21,;13.83,-13.31,;14.3,-11.85,;15.84,-11.84,;16.32,-13.31,)| Show InChI InChI=1S/C27H36NO4S/c29-26(27(30,22-8-4-5-9-22)25-12-6-19-33-25)32-24-20-28(16-13-21(24)14-17-28)15-7-18-31-23-10-2-1-3-11-23/h1-3,6,10-12,19,21-22,24,30H,4-5,7-9,13-18,20H2/q+1/t21?,24-,27+,28?/m0/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Binding affinity to muscarinic M1 receptor |
J Med Chem 52: 5076-92 (2010)
Article DOI: 10.1021/jm900132z
BindingDB Entry DOI: 10.7270/Q2SX6F5Z |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50296345

((1R,2R,4S,5R,7S)-7-(2-hydroxy-2,2-di-thiophen-2-yl...)Show SMILES C[N+]1(C)[C@@H]2C[C@H](C[C@@H]1[C@@H]1O[C@H]21)OC(=O)C(O)(c1cccs1)c1cccs1 |TLB:11:5:1:8.10| Show InChI InChI=1S/C19H22NO4S2/c1-20(2)12-9-11(10-13(20)17-16(12)24-17)23-18(21)19(22,14-5-3-7-25-14)15-6-4-8-26-15/h3-8,11-13,16-17,22H,9-10H2,1-2H3/q+1/t11-,12-,13-,16-,17+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Binding affinity to muscarinic M2 receptor |
J Med Chem 52: 5076-92 (2010)
Article DOI: 10.1021/jm900132z
BindingDB Entry DOI: 10.7270/Q2SX6F5Z |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50296345

((1R,2R,4S,5R,7S)-7-(2-hydroxy-2,2-di-thiophen-2-yl...)Show SMILES C[N+]1(C)[C@@H]2C[C@H](C[C@@H]1[C@@H]1O[C@H]21)OC(=O)C(O)(c1cccs1)c1cccs1 |TLB:11:5:1:8.10| Show InChI InChI=1S/C19H22NO4S2/c1-20(2)12-9-11(10-13(20)17-16(12)24-17)23-18(21)19(22,14-5-3-7-25-14)15-6-4-8-26-15/h3-8,11-13,16-17,22H,9-10H2,1-2H3/q+1/t11-,12-,13-,16-,17+/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Binding affinity to muscarinic M1 receptor |
J Med Chem 52: 5076-92 (2010)
Article DOI: 10.1021/jm900132z
BindingDB Entry DOI: 10.7270/Q2SX6F5Z |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50296345

((1R,2R,4S,5R,7S)-7-(2-hydroxy-2,2-di-thiophen-2-yl...)Show SMILES C[N+]1(C)[C@@H]2C[C@H](C[C@@H]1[C@@H]1O[C@H]21)OC(=O)C(O)(c1cccs1)c1cccs1 |TLB:11:5:1:8.10| Show InChI InChI=1S/C19H22NO4S2/c1-20(2)12-9-11(10-13(20)17-16(12)24-17)23-18(21)19(22,14-5-3-7-25-14)15-6-4-8-26-15/h3-8,11-13,16-17,22H,9-10H2,1-2H3/q+1/t11-,12-,13-,16-,17+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Binding affinity to muscarinic M3 receptor |
J Med Chem 52: 5076-92 (2010)
Article DOI: 10.1021/jm900132z
BindingDB Entry DOI: 10.7270/Q2SX6F5Z |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50237067
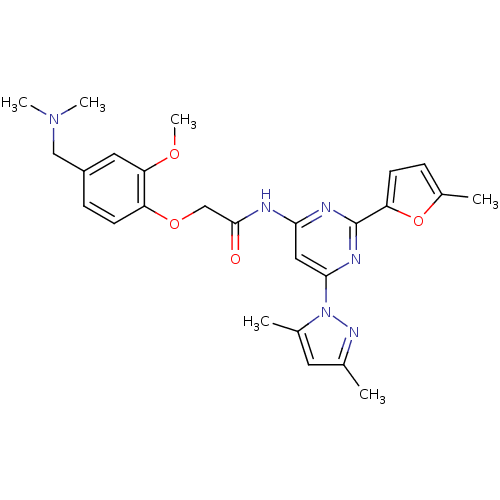
(CHEMBL429125 | N-(6-(3,5-dimethyl-1H-pyrazol-1-yl)...)Show SMILES COc1cc(CN(C)C)ccc1OCC(=O)Nc1cc(nc(n1)-c1ccc(C)o1)-n1nc(C)cc1C Show InChI InChI=1S/C26H30N6O4/c1-16-11-17(2)32(30-16)24-13-23(28-26(29-24)21-9-7-18(3)36-21)27-25(33)15-35-20-10-8-19(14-31(4)5)12-22(20)34-6/h7-13H,14-15H2,1-6H3,(H,27,28,29,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]-ZM241385 from human adenosine A2A receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 1778-83 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.032
BindingDB Entry DOI: 10.7270/Q2VD6Z62 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50372562
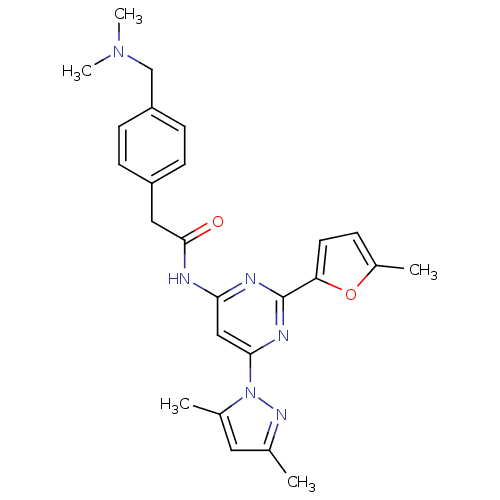
(CHEMBL255739)Show SMILES CN(C)Cc1ccc(CC(=O)Nc2cc(nc(n2)-c2ccc(C)o2)-n2nc(C)cc2C)cc1 Show InChI InChI=1S/C25H28N6O2/c1-16-12-17(2)31(29-16)23-14-22(27-25(28-23)21-11-6-18(3)33-21)26-24(32)13-19-7-9-20(10-8-19)15-30(4)5/h6-12,14H,13,15H2,1-5H3,(H,26,27,28,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]ZM241385 from human adenosine A2A receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 1269-73 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.036
BindingDB Entry DOI: 10.7270/Q2K35VGF |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50237078
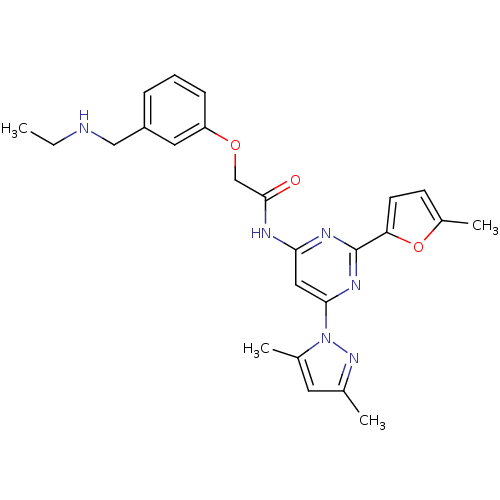
(CHEMBL256123 | N-(6-(3,5-dimethyl-1H-pyrazol-1-yl)...)Show SMILES CCNCc1cccc(OCC(=O)Nc2cc(nc(n2)-c2ccc(C)o2)-n2nc(C)cc2C)c1 Show InChI InChI=1S/C25H28N6O3/c1-5-26-14-19-7-6-8-20(12-19)33-15-24(32)27-22-13-23(31-17(3)11-16(2)30-31)29-25(28-22)21-10-9-18(4)34-21/h6-13,26H,5,14-15H2,1-4H3,(H,27,28,29,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]-ZM241385 from human adenosine A2A receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 1778-83 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.032
BindingDB Entry DOI: 10.7270/Q2VD6Z62 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50237083
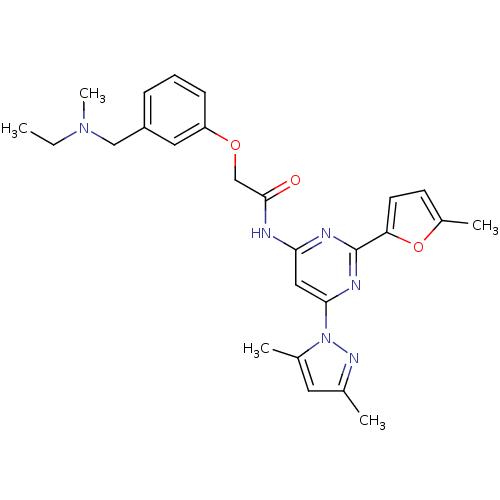
(CHEMBL256124 | N-(6-(3,5-dimethyl-1H-pyrazol-1-yl)...)Show SMILES CCN(C)Cc1cccc(OCC(=O)Nc2cc(nc(n2)-c2ccc(C)o2)-n2nc(C)cc2C)c1 Show InChI InChI=1S/C26H30N6O3/c1-6-31(5)15-20-8-7-9-21(13-20)34-16-25(33)27-23-14-24(32-18(3)12-17(2)30-32)29-26(28-23)22-11-10-19(4)35-22/h7-14H,6,15-16H2,1-5H3,(H,27,28,29,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Binding affinity at adenosine A2A receptor |
Bioorg Med Chem Lett 18: 1778-83 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.032
BindingDB Entry DOI: 10.7270/Q2VD6Z62 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50237078

(CHEMBL256123 | N-(6-(3,5-dimethyl-1H-pyrazol-1-yl)...)Show SMILES CCNCc1cccc(OCC(=O)Nc2cc(nc(n2)-c2ccc(C)o2)-n2nc(C)cc2C)c1 Show InChI InChI=1S/C25H28N6O3/c1-5-26-14-19-7-6-8-20(12-19)33-15-24(32)27-22-13-23(31-17(3)11-16(2)30-31)29-25(28-22)21-10-9-18(4)34-21/h6-13,26H,5,14-15H2,1-4H3,(H,27,28,29,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Binding affinity at adenosine A2A receptor |
Bioorg Med Chem Lett 18: 1778-83 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.032
BindingDB Entry DOI: 10.7270/Q2VD6Z62 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50237082
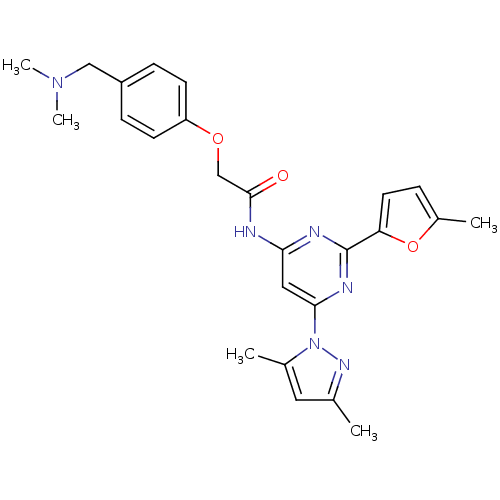
(CHEMBL404863 | N-(6-(3,5-dimethyl-1H-pyrazol-1-yl)...)Show SMILES CN(C)Cc1ccc(OCC(=O)Nc2cc(nc(n2)-c2ccc(C)o2)-n2nc(C)cc2C)cc1 Show InChI InChI=1S/C25H28N6O3/c1-16-12-17(2)31(29-16)23-13-22(27-25(28-23)21-11-6-18(3)34-21)26-24(32)15-33-20-9-7-19(8-10-20)14-30(4)5/h6-13H,14-15H2,1-5H3,(H,26,27,28,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]-ZM241385 from human adenosine A2A receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 1778-83 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.032
BindingDB Entry DOI: 10.7270/Q2VD6Z62 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50237065
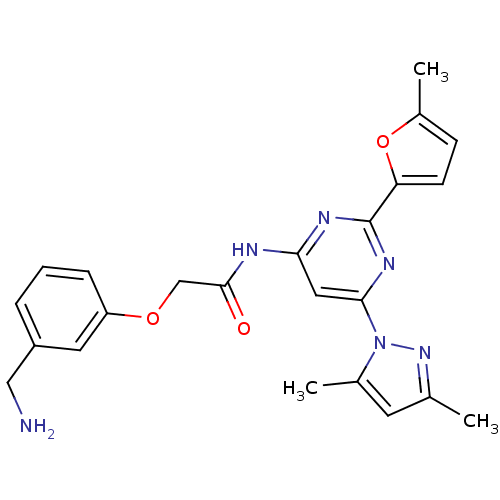
(2-(3-(aminomethyl)phenoxy)-N-(6-(3,5-dimethyl-1H-p...)Show SMILES Cc1cc(C)n(n1)-c1cc(NC(=O)COc2cccc(CN)c2)nc(n1)-c1ccc(C)o1 Show InChI InChI=1S/C23H24N6O3/c1-14-9-15(2)29(28-14)21-11-20(26-23(27-21)19-8-7-16(3)32-19)25-22(30)13-31-18-6-4-5-17(10-18)12-24/h4-11H,12-13,24H2,1-3H3,(H,25,26,27,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Binding affinity at adenosine A2A receptor |
Bioorg Med Chem Lett 18: 1778-83 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.032
BindingDB Entry DOI: 10.7270/Q2VD6Z62 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50237083

(CHEMBL256124 | N-(6-(3,5-dimethyl-1H-pyrazol-1-yl)...)Show SMILES CCN(C)Cc1cccc(OCC(=O)Nc2cc(nc(n2)-c2ccc(C)o2)-n2nc(C)cc2C)c1 Show InChI InChI=1S/C26H30N6O3/c1-6-31(5)15-20-8-7-9-21(13-20)34-16-25(33)27-23-14-24(32-18(3)12-17(2)30-32)29-26(28-23)22-11-10-19(4)35-22/h7-14H,6,15-16H2,1-5H3,(H,27,28,29,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]-ZM241385 from human adenosine A2A receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 1778-83 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.032
BindingDB Entry DOI: 10.7270/Q2VD6Z62 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50237075

(CHEMBL253317 | N-(6-(3,5-dimethyl-1H-pyrazol-1-yl)...)Show SMILES COc1cc(OCC(=O)Nc2cc(nc(n2)-c2ccc(C)o2)-n2nc(C)cc2C)ccc1CN(C)C Show InChI InChI=1S/C26H30N6O4/c1-16-11-17(2)32(30-16)24-13-23(28-26(29-24)21-10-7-18(3)36-21)27-25(33)15-35-20-9-8-19(14-31(4)5)22(12-20)34-6/h7-13H,14-15H2,1-6H3,(H,27,28,29,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]-ZM241385 from human adenosine A2A receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 1778-83 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.032
BindingDB Entry DOI: 10.7270/Q2VD6Z62 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50237064
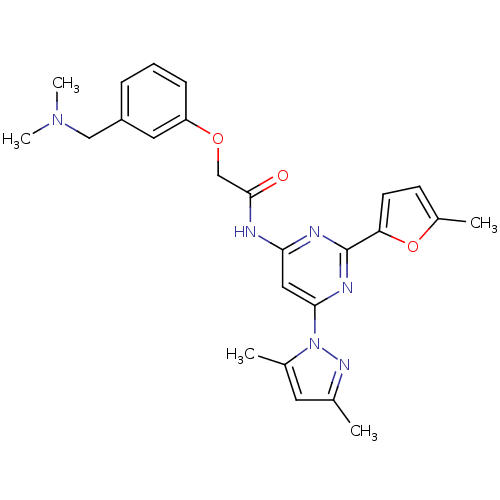
(CHEMBL401895 | N-(6-(3,5-dimethyl-1H-pyrazol-1-yl)...)Show SMILES CN(C)Cc1cccc(OCC(=O)Nc2cc(nc(n2)-c2ccc(C)o2)-n2nc(C)cc2C)c1 Show InChI InChI=1S/C25H28N6O3/c1-16-11-17(2)31(29-16)23-13-22(27-25(28-23)21-10-9-18(3)34-21)26-24(32)15-33-20-8-6-7-19(12-20)14-30(4)5/h6-13H,14-15H2,1-5H3,(H,26,27,28,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]-ZM241385 from human adenosine A2A receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 1778-83 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.032
BindingDB Entry DOI: 10.7270/Q2VD6Z62 |
More data for this
Ligand-Target Pair | |
Neutrophil collagenase
(Homo sapiens (Human)) | BDBM50104967
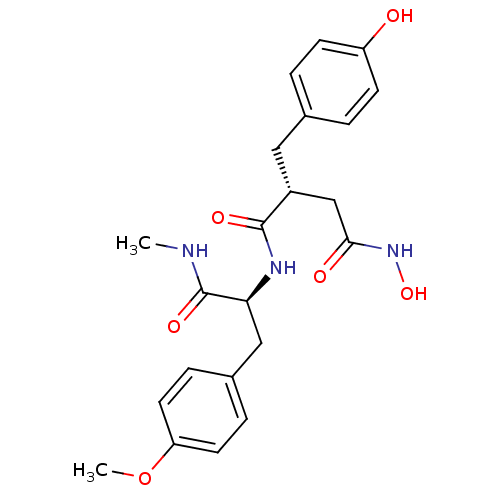
(CHEMBL419751 | N*4*-Hydroxy-2-(4-hydroxy-benzyl)-N...)Show SMILES CNC(=O)[C@H](Cc1ccc(OC)cc1)NC(=O)[C@@H](CC(=O)NO)Cc1ccc(O)cc1 Show InChI InChI=1S/C22H27N3O6/c1-23-22(29)19(12-15-5-9-18(31-2)10-6-15)24-21(28)16(13-20(27)25-30)11-14-3-7-17(26)8-4-14/h3-10,16,19,26,30H,11-13H2,1-2H3,(H,23,29)(H,24,28)(H,25,27)/t16-,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloprotease-8 |
J Med Chem 44: 3347-50 (2001)
BindingDB Entry DOI: 10.7270/Q2057F7H |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50237084
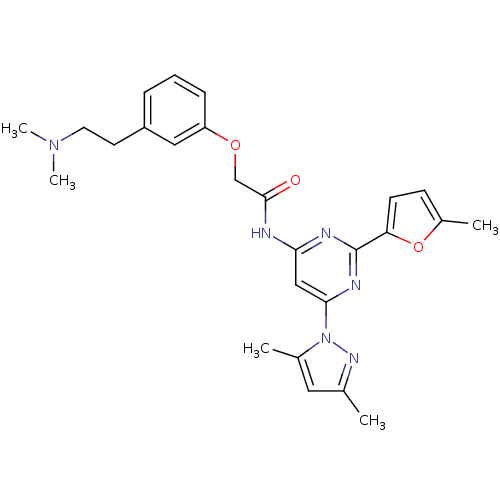
(CHEMBL403846 | N-(6-(3,5-dimethyl-1H-pyrazol-1-yl)...)Show SMILES CN(C)CCc1cccc(OCC(=O)Nc2cc(nc(n2)-c2ccc(C)o2)-n2nc(C)cc2C)c1 Show InChI InChI=1S/C26H30N6O3/c1-17-13-18(2)32(30-17)24-15-23(28-26(29-24)22-10-9-19(3)35-22)27-25(33)16-34-21-8-6-7-20(14-21)11-12-31(4)5/h6-10,13-15H,11-12,16H2,1-5H3,(H,27,28,29,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]-ZM241385 from human adenosine A2A receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 1778-83 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.032
BindingDB Entry DOI: 10.7270/Q2VD6Z62 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50237076
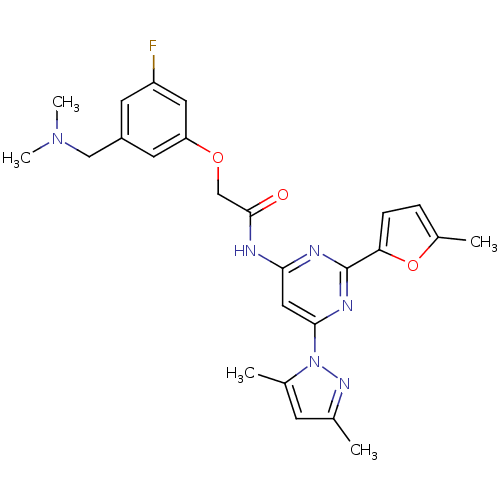
(CHEMBL256331 | N-(6-(3,5-dimethyl-1H-pyrazol-1-yl)...)Show SMILES CN(C)Cc1cc(F)cc(OCC(=O)Nc2cc(nc(n2)-c2ccc(C)o2)-n2nc(C)cc2C)c1 Show InChI InChI=1S/C25H27FN6O3/c1-15-8-16(2)32(30-15)23-12-22(28-25(29-23)21-7-6-17(3)35-21)27-24(33)14-34-20-10-18(13-31(4)5)9-19(26)11-20/h6-12H,13-14H2,1-5H3,(H,27,28,29,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]-ZM241385 from human adenosine A2A receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 1778-83 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.032
BindingDB Entry DOI: 10.7270/Q2VD6Z62 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50237094
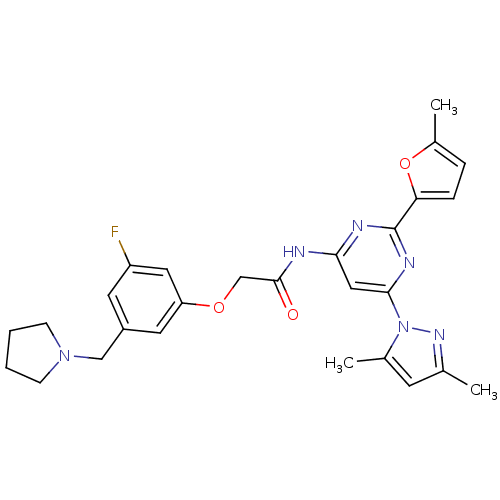
(CHEMBL256332 | N-(6-(3,5-dimethyl-1H-pyrazol-1-yl)...)Show SMILES Cc1cc(C)n(n1)-c1cc(NC(=O)COc2cc(F)cc(CN3CCCC3)c2)nc(n1)-c1ccc(C)o1 Show InChI InChI=1S/C27H29FN6O3/c1-17-10-18(2)34(32-17)25-14-24(30-27(31-25)23-7-6-19(3)37-23)29-26(35)16-36-22-12-20(11-21(28)13-22)15-33-8-4-5-9-33/h6-7,10-14H,4-5,8-9,15-16H2,1-3H3,(H,29,30,31,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]-ZM241385 from human adenosine A2A receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 1778-83 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.032
BindingDB Entry DOI: 10.7270/Q2VD6Z62 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50232154
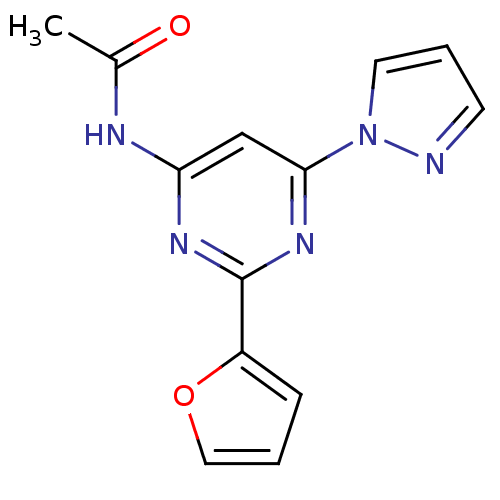
(CHEMBL401321 | N-(2-(furan-2-yl)-6-(1H-pyrazol-1-y...)Show InChI InChI=1S/C13H11N5O2/c1-9(19)15-11-8-12(18-6-3-5-14-18)17-13(16-11)10-4-2-7-20-10/h2-8H,1H3,(H,15,16,17,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Binding affinity at adenosine A2A receptor |
Bioorg Med Chem Lett 18: 1778-83 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.032
BindingDB Entry DOI: 10.7270/Q2VD6Z62 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50237065

(2-(3-(aminomethyl)phenoxy)-N-(6-(3,5-dimethyl-1H-p...)Show SMILES Cc1cc(C)n(n1)-c1cc(NC(=O)COc2cccc(CN)c2)nc(n1)-c1ccc(C)o1 Show InChI InChI=1S/C23H24N6O3/c1-14-9-15(2)29(28-14)21-11-20(26-23(27-21)19-8-7-16(3)32-19)25-22(30)13-31-18-6-4-5-17(10-18)12-24/h4-11H,12-13,24H2,1-3H3,(H,25,26,27,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]-ZM241385 from human adenosine A2A receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 1778-83 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.032
BindingDB Entry DOI: 10.7270/Q2VD6Z62 |
More data for this
Ligand-Target Pair | |
Neutrophil collagenase
(Homo sapiens (Human)) | BDBM50104969
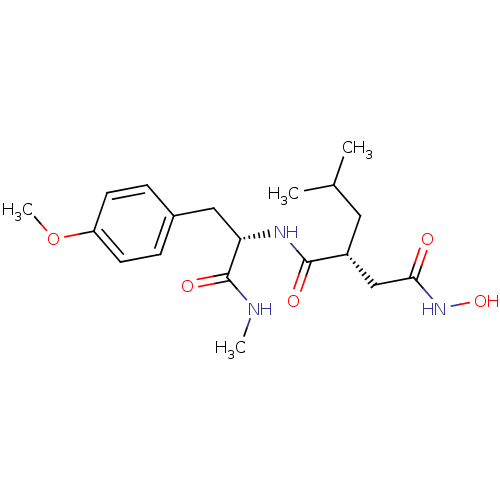
((R)-N*4*-Hydroxy-2-isobutyl-N*1*-[(S)-2-(4-methoxy...)Show SMILES CNC(=O)[C@H](Cc1ccc(OC)cc1)NC(=O)[C@H](CC(C)C)CC(=O)NO Show InChI InChI=1S/C19H29N3O5/c1-12(2)9-14(11-17(23)22-26)18(24)21-16(19(25)20-3)10-13-5-7-15(27-4)8-6-13/h5-8,12,14,16,26H,9-11H2,1-4H3,(H,20,25)(H,21,24)(H,22,23)/t14-,16+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloprotease-8 |
J Med Chem 44: 3347-50 (2001)
BindingDB Entry DOI: 10.7270/Q2057F7H |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50237072
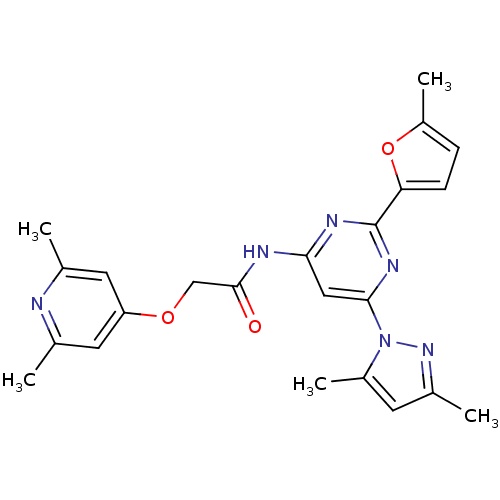
(CHEMBL253528 | N-(6-(3,5-dimethyl-1H-pyrazol-1-yl)...)Show SMILES Cc1cc(C)n(n1)-c1cc(NC(=O)COc2cc(C)nc(C)c2)nc(n1)-c1ccc(C)o1 Show InChI InChI=1S/C23H24N6O3/c1-13-9-18(10-14(2)24-13)31-12-22(30)25-20-11-21(29-16(4)8-15(3)28-29)27-23(26-20)19-7-6-17(5)32-19/h6-11H,12H2,1-5H3,(H,25,26,27,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]-ZM241385 from human adenosine A2A receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 1778-83 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.032
BindingDB Entry DOI: 10.7270/Q2VD6Z62 |
More data for this
Ligand-Target Pair | |
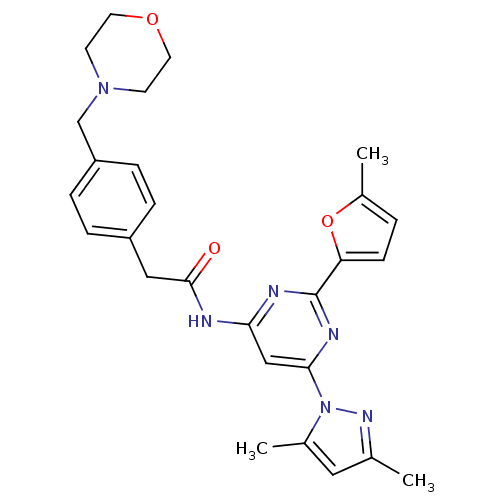
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50372561
(CHEMBL255951)Show SMILES Cc1cc(C)n(n1)-c1cc(NC(=O)Cc2ccc(CN3CCOCC3)cc2)nc(n1)-c1ccc(C)o1 Show InChI InChI=1S/C27H30N6O3/c1-18-14-19(2)33(31-18)25-16-24(29-27(30-25)23-9-4-20(3)36-23)28-26(34)15-21-5-7-22(8-6-21)17-32-10-12-35-13-11-32/h4-9,14,16H,10-13,15,17H2,1-3H3,(H,28,29,30,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]ZM241385 from human adenosine A2A receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 1269-73 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.036
BindingDB Entry DOI: 10.7270/Q2K35VGF |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50378083
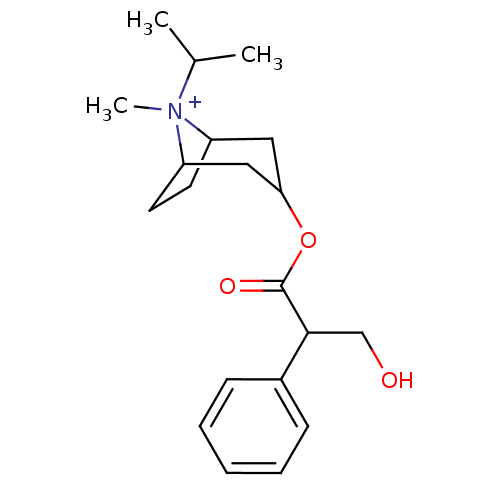
(Atrovent HFA | IPRATROPIUM BROMIDE)Show SMILES CC(C)[N+]1(C)C2CCC1CC(C2)OC(=O)C(CO)c1ccccc1 |TLB:4:3:10.11.9:6.7,THB:12:10:3:6.7,1:3:10.11.9:6.7,(8.93,-5.03,;7.4,-5.04,;6.62,-3.72,;6.64,-6.39,;5.29,-5.61,;6.91,-7.92,;5.92,-9.27,;7.1,-8.56,;7.69,-7.22,;9.45,-7.19,;9.72,-8.74,;8.74,-7.92,;9.71,-10.27,;11.04,-11.06,;11.03,-12.6,;12.38,-10.29,;13.71,-11.07,;15.04,-10.31,;12.39,-8.75,;13.74,-8,;13.74,-6.46,;12.41,-5.67,;11.07,-6.44,;11.06,-7.98,)| Show InChI InChI=1S/C20H30NO3/c1-14(2)21(3)16-9-10-17(21)12-18(11-16)24-20(23)19(13-22)15-7-5-4-6-8-15/h4-8,14,16-19,22H,9-13H2,1-3H3/q+1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.830 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Binding affinity to muscarinic M3 receptor |
J Med Chem 52: 5076-92 (2010)
Article DOI: 10.1021/jm900132z
BindingDB Entry DOI: 10.7270/Q2SX6F5Z |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM21174

(CHEMBL398272 | N-[2-(furan-2-yl)-6-(1,3-thiazol-2-...)Show SMILES CN1CCN(CC(=O)Nc2cc(nc(n2)-c2ccco2)-c2nccs2)CC1 Show InChI InChI=1S/C18H20N6O2S/c1-23-5-7-24(8-6-23)12-16(25)21-15-11-13(18-19-4-10-27-18)20-17(22-15)14-3-2-9-26-14/h2-4,9-11H,5-8,12H2,1H3,(H,20,21,22,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.900 | -51.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Neuroscience
| Assay Description
The membranes prepared from Flp-In HEK cells transfected with adenosine receptors were used in binding assays. Nonspecific binding was determined in ... |
J Med Chem 51: 1719-29 (2008)
Article DOI: 10.1021/jm701185v
BindingDB Entry DOI: 10.7270/Q2W66J2J |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM21174

(CHEMBL398272 | N-[2-(furan-2-yl)-6-(1,3-thiazol-2-...)Show SMILES CN1CCN(CC(=O)Nc2cc(nc(n2)-c2ccco2)-c2nccs2)CC1 Show InChI InChI=1S/C18H20N6O2S/c1-23-5-7-24(8-6-23)12-16(25)21-15-11-13(18-19-4-10-27-18)20-17(22-15)14-3-2-9-26-14/h2-4,9-11H,5-8,12H2,1H3,(H,20,21,22,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]ZM241385 from human recombinant adenosine A2A receptor expressed in HEK293 cells |
J Med Chem 51: 400-6 (2008)
Article DOI: 10.1021/jm070623o
BindingDB Entry DOI: 10.7270/Q2WH2PQ9 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50237068
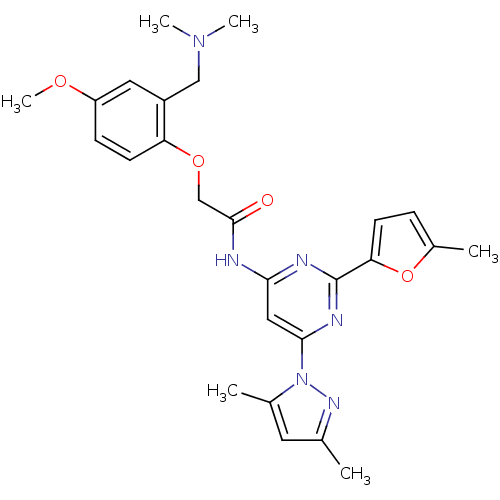
(CHEMBL429850 | N-(6-(3,5-dimethyl-1H-pyrazol-1-yl)...)Show SMILES COc1ccc(OCC(=O)Nc2cc(nc(n2)-c2ccc(C)o2)-n2nc(C)cc2C)c(CN(C)C)c1 Show InChI InChI=1S/C26H30N6O4/c1-16-11-17(2)32(30-16)24-13-23(28-26(29-24)22-9-7-18(3)36-22)27-25(33)15-35-21-10-8-20(34-6)12-19(21)14-31(4)5/h7-13H,14-15H2,1-6H3,(H,27,28,29,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]-ZM241385 from human adenosine A2A receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 1778-83 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.032
BindingDB Entry DOI: 10.7270/Q2VD6Z62 |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50104969

((R)-N*4*-Hydroxy-2-isobutyl-N*1*-[(S)-2-(4-methoxy...)Show SMILES CNC(=O)[C@H](Cc1ccc(OC)cc1)NC(=O)[C@H](CC(C)C)CC(=O)NO Show InChI InChI=1S/C19H29N3O5/c1-12(2)9-14(11-17(23)22-26)18(24)21-16(19(25)20-3)10-13-5-7-15(27-4)8-6-13/h5-8,12,14,16,26H,9-11H2,1-4H3,(H,20,25)(H,21,24)(H,22,23)/t14-,16+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
PubMed
| <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloprotease-1 |
J Med Chem 44: 3347-50 (2001)
BindingDB Entry DOI: 10.7270/Q2057F7H |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50377558
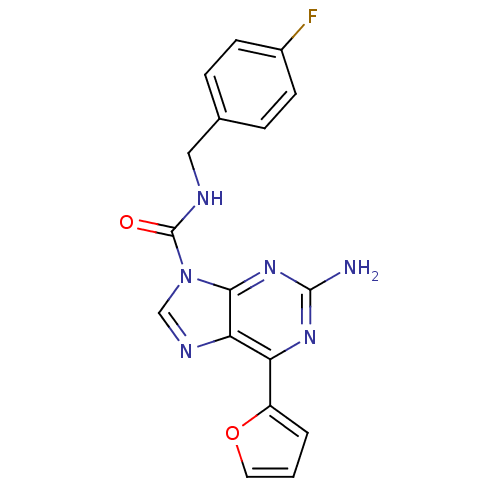
(CHEMBL429144)Show SMILES Nc1nc(-c2ccco2)c2ncn(C(=O)NCc3ccc(F)cc3)c2n1 Show InChI InChI=1S/C17H13FN6O2/c18-11-5-3-10(4-6-11)8-20-17(25)24-9-21-14-13(12-2-1-7-26-12)22-16(19)23-15(14)24/h1-7,9H,8H2,(H,20,25)(H2,19,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd
Curated by ChEMBL
| Assay Description
Binding affinity at human adenosine A2A receptor |
Bioorg Med Chem Lett 18: 2924-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.072
BindingDB Entry DOI: 10.7270/Q23779KZ |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50377557

(CHEMBL264432)Show SMILES Cc1ccc(CNC(=O)n2cnc3c(nc(N)nc23)-c2ccco2)cc1 Show InChI InChI=1S/C18H16N6O2/c1-11-4-6-12(7-5-11)9-20-18(25)24-10-21-15-14(13-3-2-8-26-13)22-17(19)23-16(15)24/h2-8,10H,9H2,1H3,(H,20,25)(H2,19,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd
Curated by ChEMBL
| Assay Description
Binding affinity at human adenosine A2A receptor |
Bioorg Med Chem Lett 18: 2924-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.072
BindingDB Entry DOI: 10.7270/Q23779KZ |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50377556

(CHEMBL411034)Show SMILES Nc1nc(-c2ccco2)c2ncn(C(=O)NCc3ccccc3Cl)c2n1 Show InChI InChI=1S/C17H13ClN6O2/c18-11-5-2-1-4-10(11)8-20-17(25)24-9-21-14-13(12-6-3-7-26-12)22-16(19)23-15(14)24/h1-7,9H,8H2,(H,20,25)(H2,19,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd
Curated by ChEMBL
| Assay Description
Binding affinity at human adenosine A2A receptor |
Bioorg Med Chem Lett 18: 2924-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.072
BindingDB Entry DOI: 10.7270/Q23779KZ |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50377567
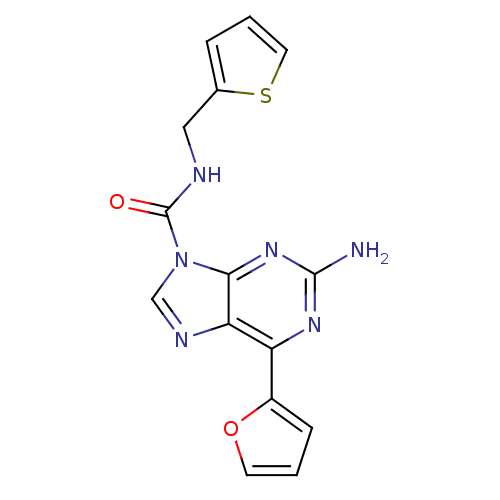
(CHEMBL409915)Show InChI InChI=1S/C15H12N6O2S/c16-14-19-11(10-4-1-5-23-10)12-13(20-14)21(8-18-12)15(22)17-7-9-3-2-6-24-9/h1-6,8H,7H2,(H,17,22)(H2,16,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd
Curated by ChEMBL
| Assay Description
Binding affinity at human adenosine A2A receptor |
Bioorg Med Chem Lett 18: 2924-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.072
BindingDB Entry DOI: 10.7270/Q23779KZ |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50377566

(CHEMBL259049)Show SMILES Cc1cccc(CNC(=O)n2cnc3c(nc(N)nc23)-c2ccco2)c1 Show InChI InChI=1S/C18H16N6O2/c1-11-4-2-5-12(8-11)9-20-18(25)24-10-21-15-14(13-6-3-7-26-13)22-17(19)23-16(15)24/h2-8,10H,9H2,1H3,(H,20,25)(H2,19,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd
Curated by ChEMBL
| Assay Description
Binding affinity at human adenosine A2A receptor |
Bioorg Med Chem Lett 18: 2924-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.072
BindingDB Entry DOI: 10.7270/Q23779KZ |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50372576
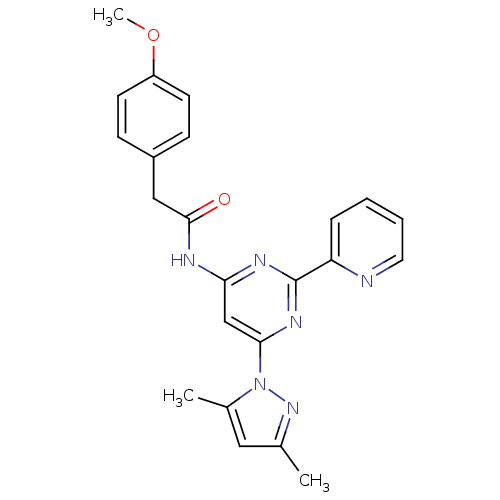
(CHEMBL402071)Show SMILES COc1ccc(CC(=O)Nc2cc(nc(n2)-c2ccccn2)-n2nc(C)cc2C)cc1 Show InChI InChI=1S/C23H22N6O2/c1-15-12-16(2)29(28-15)21-14-20(26-23(27-21)19-6-4-5-11-24-19)25-22(30)13-17-7-9-18(31-3)10-8-17/h4-12,14H,13H2,1-3H3,(H,25,26,27,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]ZM241385 from human adenosine A2A receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 1269-73 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.036
BindingDB Entry DOI: 10.7270/Q2K35VGF |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50372575
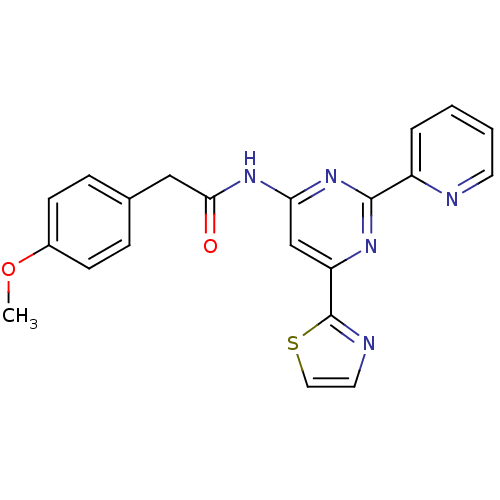
(CHEMBL256382)Show SMILES COc1ccc(CC(=O)Nc2cc(nc(n2)-c2ccccn2)-c2nccs2)cc1 Show InChI InChI=1S/C21H17N5O2S/c1-28-15-7-5-14(6-8-15)12-19(27)25-18-13-17(21-23-10-11-29-21)24-20(26-18)16-4-2-3-9-22-16/h2-11,13H,12H2,1H3,(H,24,25,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]ZM241385 from human adenosine A2A receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 1269-73 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.036
BindingDB Entry DOI: 10.7270/Q2K35VGF |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
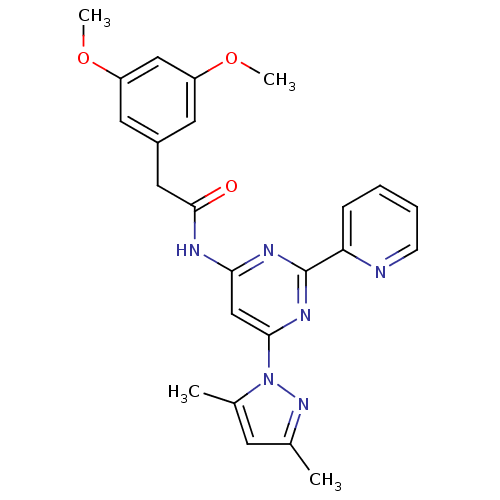
(Homo sapiens (Human)) | BDBM50372574
(CHEMBL403525)Show SMILES COc1cc(CC(=O)Nc2cc(nc(n2)-c2ccccn2)-n2nc(C)cc2C)cc(OC)c1 Show InChI InChI=1S/C24H24N6O3/c1-15-9-16(2)30(29-15)22-14-21(27-24(28-22)20-7-5-6-8-25-20)26-23(31)12-17-10-18(32-3)13-19(11-17)33-4/h5-11,13-14H,12H2,1-4H3,(H,26,27,28,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]ZM241385 from human adenosine A2A receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 1269-73 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.036
BindingDB Entry DOI: 10.7270/Q2K35VGF |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
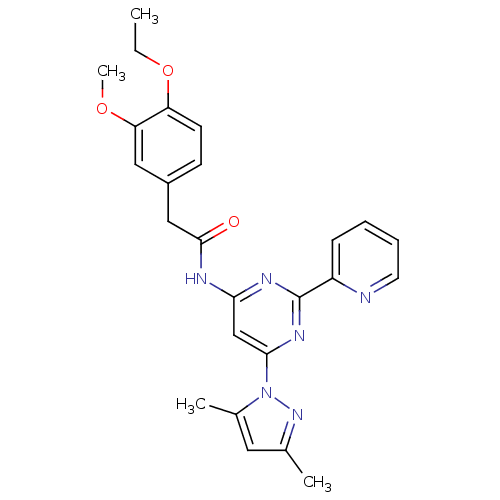
(Homo sapiens (Human)) | BDBM50372573
(CHEMBL270655)Show SMILES CCOc1ccc(CC(=O)Nc2cc(nc(n2)-c2ccccn2)-n2nc(C)cc2C)cc1OC Show InChI InChI=1S/C25H26N6O3/c1-5-34-20-10-9-18(13-21(20)33-4)14-24(32)27-22-15-23(31-17(3)12-16(2)30-31)29-25(28-22)19-8-6-7-11-26-19/h6-13,15H,5,14H2,1-4H3,(H,27,28,29,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]ZM241385 from human adenosine A2A receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 1269-73 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.036
BindingDB Entry DOI: 10.7270/Q2K35VGF |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50372571
(CHEMBL270654)Show SMILES Cc1cc(C)n(n1)-c1cc(NC(=O)Cc2ccc(cc2)S(C)(=O)=O)nc(n1)-c1ccccn1 Show InChI InChI=1S/C23H22N6O3S/c1-15-12-16(2)29(28-15)21-14-20(26-23(27-21)19-6-4-5-11-24-19)25-22(30)13-17-7-9-18(10-8-17)33(3,31)32/h4-12,14H,13H2,1-3H3,(H,25,26,27,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]ZM241385 from human adenosine A2A receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 1269-73 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.036
BindingDB Entry DOI: 10.7270/Q2K35VGF |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50377543
(CHEMBL260146)Show InChI InChI=1S/C17H14N6O2/c18-16-21-14(13-7-4-8-25-13)12-10-20-23(15(12)22-16)17(24)19-9-11-5-2-1-3-6-11/h1-8,10H,9H2,(H,19,24)(H2,18,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd
Curated by ChEMBL
| Assay Description
Binding affinity at human adenosine A2A receptor |
Bioorg Med Chem Lett 18: 2924-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.072
BindingDB Entry DOI: 10.7270/Q23779KZ |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50377538

(CHEMBL257757)Show InChI InChI=1S/C16H12ClN5O/c17-11-4-1-3-10(7-11)9-22-15-12(8-19-22)14(20-16(18)21-15)13-5-2-6-23-13/h1-8H,9H2,(H2,18,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd
Curated by ChEMBL
| Assay Description
Binding affinity at human adenosine A2A receptor |
Bioorg Med Chem Lett 18: 2924-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.072
BindingDB Entry DOI: 10.7270/Q23779KZ |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50237088
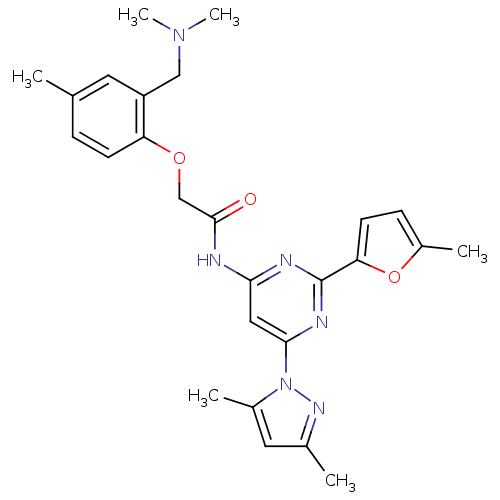
(CHEMBL256548 | N-(6-(3,5-dimethyl-1H-pyrazol-1-yl)...)Show SMILES CN(C)Cc1cc(C)ccc1OCC(=O)Nc1cc(nc(n1)-c1ccc(C)o1)-n1nc(C)cc1C Show InChI InChI=1S/C26H30N6O3/c1-16-7-9-21(20(11-16)14-31(5)6)34-15-25(33)27-23-13-24(32-18(3)12-17(2)30-32)29-26(28-23)22-10-8-19(4)35-22/h7-13H,14-15H2,1-6H3,(H,27,28,29,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]-ZM241385 from human adenosine A2A receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 1778-83 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.032
BindingDB Entry DOI: 10.7270/Q2VD6Z62 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50239036
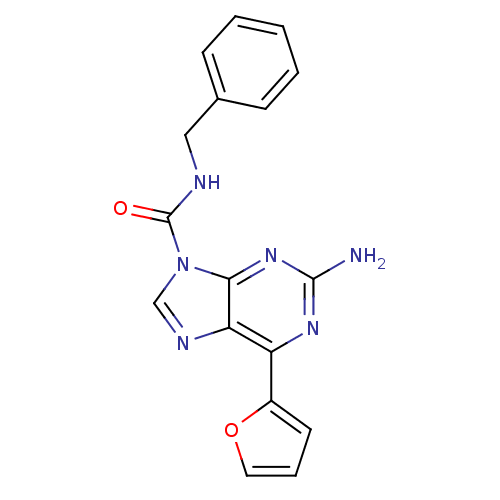
(2-amino-N-benzyl-6-(furan-2-yl)-9H-purine-9-carbox...)Show InChI InChI=1S/C17H14N6O2/c18-16-21-13(12-7-4-8-25-12)14-15(22-16)23(10-20-14)17(24)19-9-11-5-2-1-3-6-11/h1-8,10H,9H2,(H,19,24)(H2,18,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd
Curated by ChEMBL
| Assay Description
Binding affinity at human adenosine A2A receptor |
Bioorg Med Chem Lett 18: 2924-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.072
BindingDB Entry DOI: 10.7270/Q23779KZ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data