 Found 301 hits with Last Name = 'pullar' and Initial = 'ia'
Found 301 hits with Last Name = 'pullar' and Initial = 'ia' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Glutamate receptor ionotropic, kainate 2
(Rattus norvegicus) | BDBM50053067
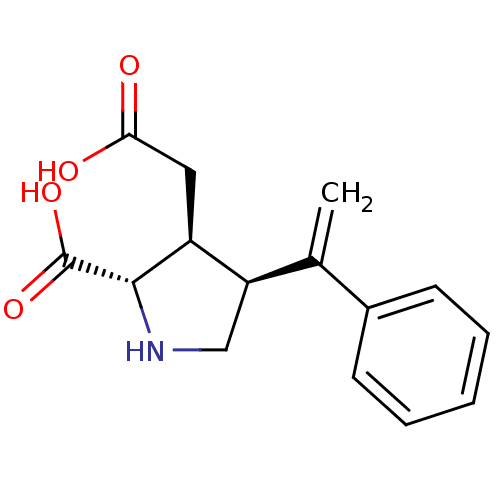
((2S,3S,4S)-3-Carboxymethyl-4-(1-phenyl-vinyl)-pyrr...)Show SMILES OC(=O)C[C@H]1[C@H](CN[C@@H]1C(O)=O)C(=C)c1ccccc1 Show InChI InChI=1S/C15H17NO4/c1-9(10-5-3-2-4-6-10)12-8-16-14(15(19)20)11(12)7-13(17)18/h2-6,11-12,14,16H,1,7-8H2,(H,17,18)(H,19,20)/t11-,12+,14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-kainate binding at rat forebrain ionotropic glutamate receptor kainate 2 |
J Med Chem 39: 3617-24 (1996)
Article DOI: 10.1021/jm960155a
BindingDB Entry DOI: 10.7270/Q2WW7GRZ |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Glutamate receptor ionotropic, kainate 2
(Rattus norvegicus) | BDBM50053071
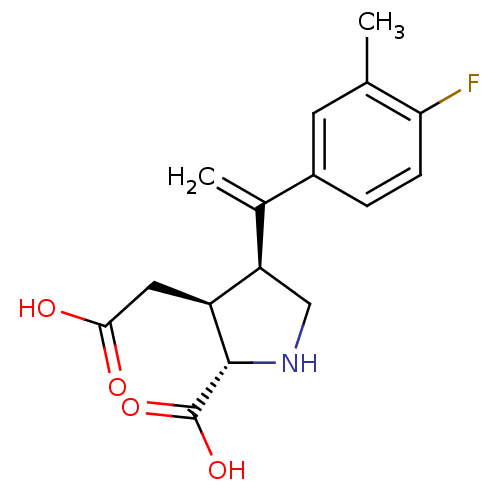
((2S,3S,4S)-3-Carboxymethyl-4-[1-(4-fluoro-3-methyl...)Show SMILES Cc1cc(ccc1F)C(=C)[C@H]1CN[C@@H]([C@H]1CC(O)=O)C(O)=O Show InChI InChI=1S/C16H18FNO4/c1-8-5-10(3-4-13(8)17)9(2)12-7-18-15(16(21)22)11(12)6-14(19)20/h3-5,11-12,15,18H,2,6-7H2,1H3,(H,19,20)(H,21,22)/t11-,12+,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-kainate binding at rat forebrain ionotropic glutamate receptor kainate 2 |
J Med Chem 39: 3617-24 (1996)
Article DOI: 10.1021/jm960155a
BindingDB Entry DOI: 10.7270/Q2WW7GRZ |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, kainate 2
(Rattus norvegicus) | BDBM50053075
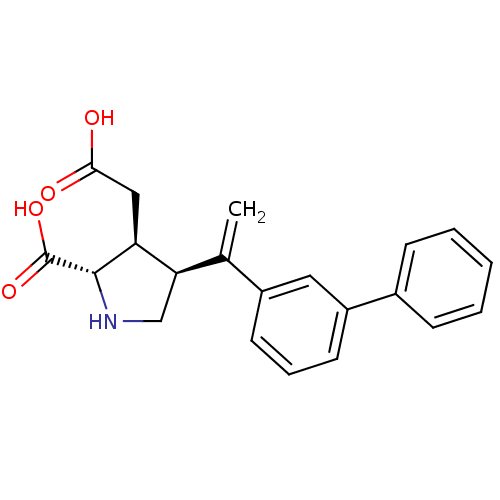
((2S,3S,4S)-4-(1-Biphenyl-3-yl-vinyl)-3-carboxymeth...)Show SMILES OC(=O)C[C@H]1[C@H](CN[C@@H]1C(O)=O)C(=C)c1cccc(c1)-c1ccccc1 Show InChI InChI=1S/C21H21NO4/c1-13(18-12-22-20(21(25)26)17(18)11-19(23)24)15-8-5-9-16(10-15)14-6-3-2-4-7-14/h2-10,17-18,20,22H,1,11-12H2,(H,23,24)(H,25,26)/t17-,18+,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-kainate binding at rat forebrain ionotropic glutamate receptor kainate 2 |
J Med Chem 39: 3617-24 (1996)
Article DOI: 10.1021/jm960155a
BindingDB Entry DOI: 10.7270/Q2WW7GRZ |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, kainate 2
(Rattus norvegicus) | BDBM50053083
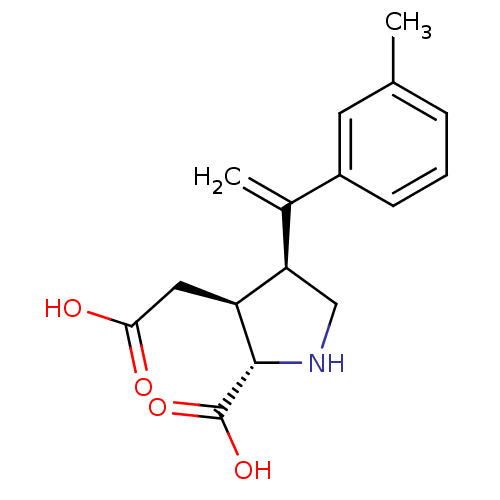
((2S,3S,4S)-3-Carboxymethyl-4-(1-m-tolyl-vinyl)-pyr...)Show SMILES Cc1cccc(c1)C(=C)[C@H]1CN[C@@H]([C@H]1CC(O)=O)C(O)=O Show InChI InChI=1S/C16H19NO4/c1-9-4-3-5-11(6-9)10(2)13-8-17-15(16(20)21)12(13)7-14(18)19/h3-6,12-13,15,17H,2,7-8H2,1H3,(H,18,19)(H,20,21)/t12-,13+,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-kainate binding at rat forebrain ionotropic glutamate receptor kainate 2 |
J Med Chem 39: 3617-24 (1996)
Article DOI: 10.1021/jm960155a
BindingDB Entry DOI: 10.7270/Q2WW7GRZ |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Glutamate receptor ionotropic, kainate 2
(Rattus norvegicus) | BDBM50053080
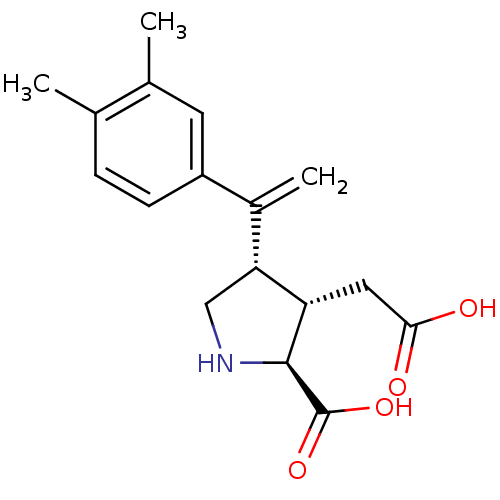
((2S,3S,4S)-3-Carboxymethyl-4-[1-(3,4-dimethyl-phen...)Show SMILES Cc1ccc(cc1C)C(=C)[C@H]1CN[C@@H]([C@H]1CC(O)=O)C(O)=O Show InChI InChI=1S/C17H21NO4/c1-9-4-5-12(6-10(9)2)11(3)14-8-18-16(17(21)22)13(14)7-15(19)20/h4-6,13-14,16,18H,3,7-8H2,1-2H3,(H,19,20)(H,21,22)/t13-,14+,16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-kainate binding at rat forebrain ionotropic glutamate receptor kainate 2 |
J Med Chem 39: 3617-24 (1996)
Article DOI: 10.1021/jm960155a
BindingDB Entry DOI: 10.7270/Q2WW7GRZ |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50132686
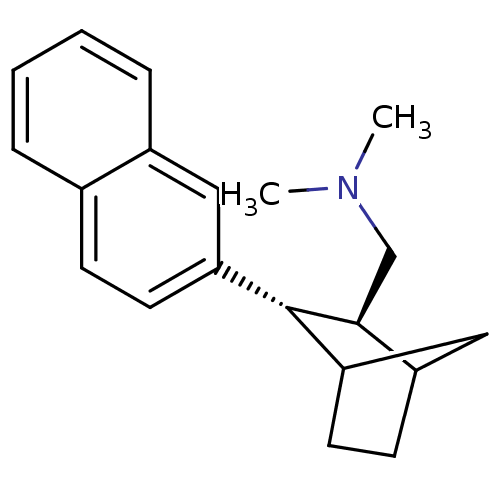
(CHEMBL326466 | Dimethyl-((2S,3S)-3-naphthalen-2-yl...)Show InChI InChI=1S/C20H25N/c1-21(2)13-19-16-8-10-18(12-16)20(19)17-9-7-14-5-3-4-6-15(14)11-17/h3-7,9,11,16,18-20H,8,10,12-13H2,1-2H3/t16?,18?,19-,20-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Centre
Curated by ChEMBL
| Assay Description
Displacement of [3H]-citalopram from Serotonin transporter |
Bioorg Med Chem Lett 13: 3277-80 (2003)
BindingDB Entry DOI: 10.7270/Q29W0DXF |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50132687
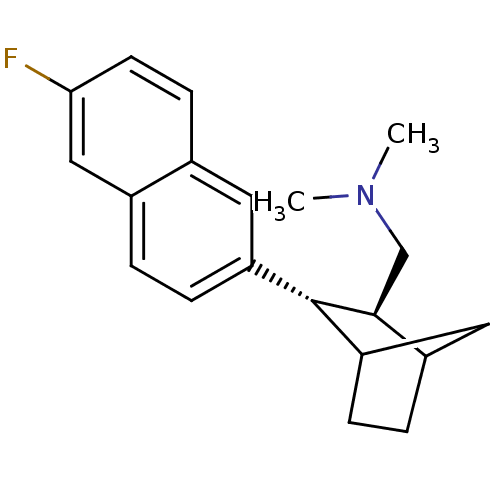
(CHEMBL324269 | [(2S,3S)-3-(6-Fluoro-naphthalen-2-y...)Show SMILES CN(C)C[C@H]1C2CCC(C2)[C@@H]1c1ccc2cc(F)ccc2c1 Show InChI InChI=1S/C20H24FN/c1-22(2)12-19-15-4-6-17(10-15)20(19)16-5-3-14-11-18(21)8-7-13(14)9-16/h3,5,7-9,11,15,17,19-20H,4,6,10,12H2,1-2H3/t15?,17?,19-,20-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Centre
Curated by ChEMBL
| Assay Description
Displacement of [3H]-citalopram from Serotonin transporter |
Bioorg Med Chem Lett 13: 3277-80 (2003)
BindingDB Entry DOI: 10.7270/Q29W0DXF |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, kainate 2
(Rattus norvegicus) | BDBM50053088
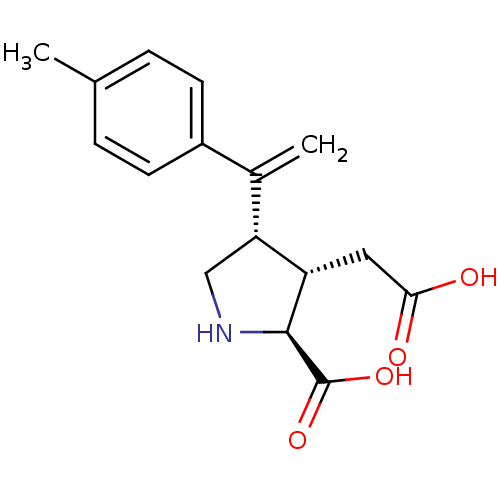
((2S,3S,4S)-3-Carboxymethyl-4-(1-p-tolyl-vinyl)-pyr...)Show SMILES Cc1ccc(cc1)C(=C)[C@H]1CN[C@@H]([C@H]1CC(O)=O)C(O)=O Show InChI InChI=1S/C16H19NO4/c1-9-3-5-11(6-4-9)10(2)13-8-17-15(16(20)21)12(13)7-14(18)19/h3-6,12-13,15,17H,2,7-8H2,1H3,(H,18,19)(H,20,21)/t12-,13+,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-kainate binding at rat forebrain ionotropic glutamate receptor kainate 2 |
J Med Chem 39: 3617-24 (1996)
Article DOI: 10.1021/jm960155a
BindingDB Entry DOI: 10.7270/Q2WW7GRZ |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Glutamate receptor ionotropic, kainate 2
(Rattus norvegicus) | BDBM50053087
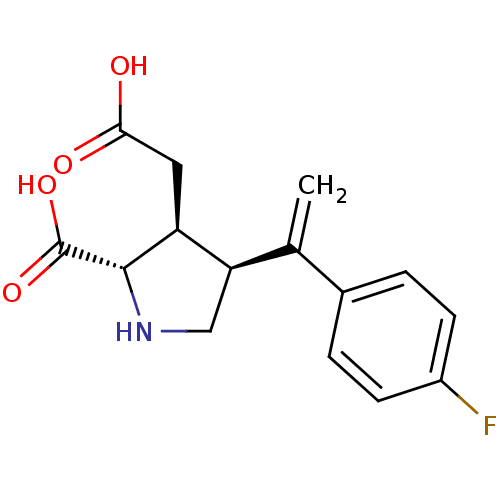
((2S,3S,4S)-3-Carboxymethyl-4-[1-(4-fluoro-phenyl)-...)Show SMILES OC(=O)C[C@H]1[C@H](CN[C@@H]1C(O)=O)C(=C)c1ccc(F)cc1 Show InChI InChI=1S/C15H16FNO4/c1-8(9-2-4-10(16)5-3-9)12-7-17-14(15(20)21)11(12)6-13(18)19/h2-5,11-12,14,17H,1,6-7H2,(H,18,19)(H,20,21)/t11-,12+,14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-kainate binding at rat forebrain ionotropic glutamate receptor kainate 2 |
J Med Chem 39: 3617-24 (1996)
Article DOI: 10.1021/jm960155a
BindingDB Entry DOI: 10.7270/Q2WW7GRZ |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50132685

(CHEMBL109571 | Dimethyl-(3-naphthalen-2-yl-bicyclo...)Show InChI InChI=1S/C20H25N/c1-21(2)13-19-16-8-10-18(12-16)20(19)17-9-7-14-5-3-4-6-15(14)11-17/h3-7,9,11,16,18-20H,8,10,12-13H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Centre
Curated by ChEMBL
| Assay Description
Displacement of [3H]-citalopram from Serotonin transporter |
Bioorg Med Chem Lett 13: 3277-80 (2003)
BindingDB Entry DOI: 10.7270/Q29W0DXF |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, kainate 2
(Rattus norvegicus) | BDBM50053064
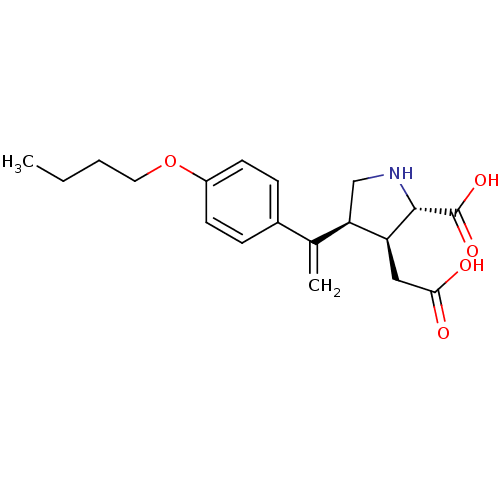
((2S,3S,4S)-4-[1-(4-Butoxy-phenyl)-vinyl]-3-carboxy...)Show SMILES CCCCOc1ccc(cc1)C(=C)[C@H]1CN[C@@H]([C@H]1CC(O)=O)C(O)=O Show InChI InChI=1S/C19H25NO5/c1-3-4-9-25-14-7-5-13(6-8-14)12(2)16-11-20-18(19(23)24)15(16)10-17(21)22/h5-8,15-16,18,20H,2-4,9-11H2,1H3,(H,21,22)(H,23,24)/t15-,16+,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-kainate binding at rat forebrain ionotropic glutamate receptor kainate 2 |
J Med Chem 39: 3617-24 (1996)
Article DOI: 10.1021/jm960155a
BindingDB Entry DOI: 10.7270/Q2WW7GRZ |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, kainate 2
(Rattus norvegicus) | BDBM50053063

((2S,3S,4S)-3-Carboxymethyl-4-[1-(3-methoxy-phenyl)...)Show SMILES COc1cccc(c1)C(=C)[C@H]1CN[C@@H]([C@H]1CC(O)=O)C(O)=O Show InChI InChI=1S/C16H19NO5/c1-9(10-4-3-5-11(6-10)22-2)13-8-17-15(16(20)21)12(13)7-14(18)19/h3-6,12-13,15,17H,1,7-8H2,2H3,(H,18,19)(H,20,21)/t12-,13+,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-kainate binding at rat forebrain ionotropic glutamate receptor kainate 2 |
J Med Chem 39: 3617-24 (1996)
Article DOI: 10.1021/jm960155a
BindingDB Entry DOI: 10.7270/Q2WW7GRZ |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, kainate 2
(Rattus norvegicus) | BDBM50053059

((2S,3S,4S)-3-Carboxymethyl-4-[1-(4-propyl-phenyl)-...)Show SMILES CCCc1ccc(cc1)C(=C)[C@H]1CN[C@@H]([C@H]1CC(O)=O)C(O)=O Show InChI InChI=1S/C18H23NO4/c1-3-4-12-5-7-13(8-6-12)11(2)15-10-19-17(18(22)23)14(15)9-16(20)21/h5-8,14-15,17,19H,2-4,9-10H2,1H3,(H,20,21)(H,22,23)/t14-,15+,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-kainate binding at rat forebrain ionotropic glutamate receptor kainate 2 |
J Med Chem 39: 3617-24 (1996)
Article DOI: 10.1021/jm960155a
BindingDB Entry DOI: 10.7270/Q2WW7GRZ |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50132679
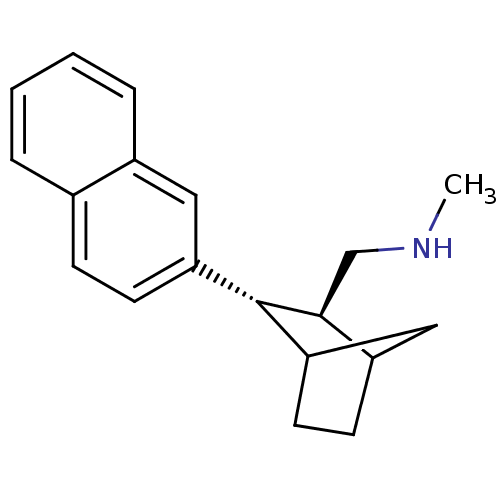
(CHEMBL111128 | Methyl-((2S,3S)-3-naphthalen-2-yl-b...)Show InChI InChI=1S/C19H23N/c1-20-12-18-15-7-9-17(11-15)19(18)16-8-6-13-4-2-3-5-14(13)10-16/h2-6,8,10,15,17-20H,7,9,11-12H2,1H3/t15?,17?,18-,19-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Centre
Curated by ChEMBL
| Assay Description
Displacement of [3H]-citalopram from Serotonin transporter |
Bioorg Med Chem Lett 13: 3277-80 (2003)
BindingDB Entry DOI: 10.7270/Q29W0DXF |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, kainate 2
(Rattus norvegicus) | BDBM50053068
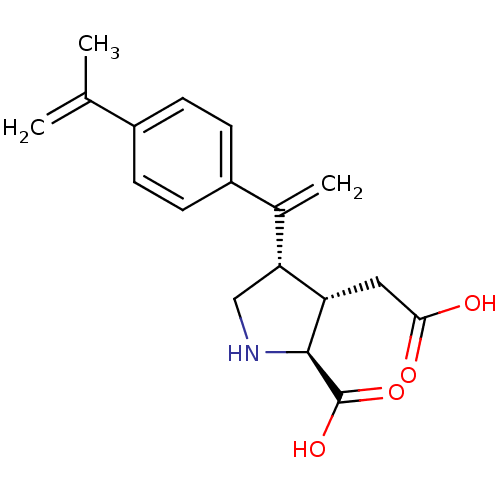
((2S,3S,4S)-3-Carboxymethyl-4-[1-(4-isopropenyl-phe...)Show SMILES CC(=C)c1ccc(cc1)C(=C)[C@H]1CN[C@@H]([C@H]1CC(O)=O)C(O)=O Show InChI InChI=1S/C18H21NO4/c1-10(2)12-4-6-13(7-5-12)11(3)15-9-19-17(18(22)23)14(15)8-16(20)21/h4-7,14-15,17,19H,1,3,8-9H2,2H3,(H,20,21)(H,22,23)/t14-,15+,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-kainate binding at rat forebrain ionotropic glutamate receptor kainate 2 |
J Med Chem 39: 3617-24 (1996)
Article DOI: 10.1021/jm960155a
BindingDB Entry DOI: 10.7270/Q2WW7GRZ |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Glutamate receptor ionotropic, kainate 2
(Rattus norvegicus) | BDBM50002369
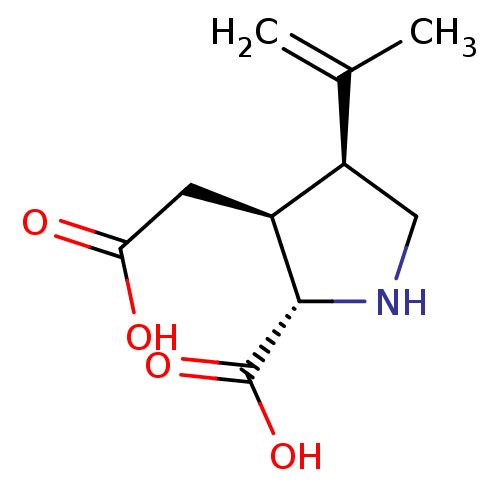
((2S-(2alpha,3beta,4beta))-2-carboxy-4-(1-methyleth...)Show SMILES CC(=C)[C@H]1CN[C@@H]([C@H]1CC(O)=O)C(O)=O |r| Show InChI InChI=1S/C10H15NO4/c1-5(2)7-4-11-9(10(14)15)6(7)3-8(12)13/h6-7,9,11H,1,3-4H2,2H3,(H,12,13)(H,14,15)/t6-,7+,9-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-kainate binding at rat forebrain ionotropic glutamate receptor kainate 2 |
J Med Chem 39: 3617-24 (1996)
Article DOI: 10.1021/jm960155a
BindingDB Entry DOI: 10.7270/Q2WW7GRZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glutamate receptor ionotropic, kainate 2
(Rattus norvegicus) | BDBM50053073

((2S,3S,4S)-3-Carboxymethyl-4-[1-(4-methoxy-phenyl)...)Show SMILES COc1ccc(cc1)C(=C)[C@H]1CN[C@@H]([C@H]1CC(O)=O)C(O)=O Show InChI InChI=1S/C16H19NO5/c1-9(10-3-5-11(22-2)6-4-10)13-8-17-15(16(20)21)12(13)7-14(18)19/h3-6,12-13,15,17H,1,7-8H2,2H3,(H,18,19)(H,20,21)/t12-,13+,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-kainate binding at rat forebrain ionotropic glutamate receptor kainate 2 |
J Med Chem 39: 3617-24 (1996)
Article DOI: 10.1021/jm960155a
BindingDB Entry DOI: 10.7270/Q2WW7GRZ |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, kainate 2
(Rattus norvegicus) | BDBM50053061
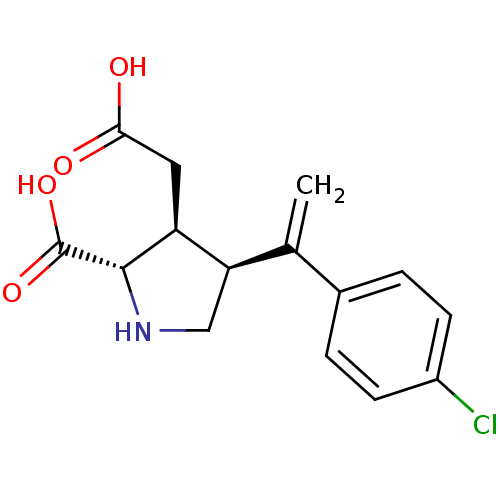
((2S,3S,4S)-3-Carboxymethyl-4-[1-(4-chloro-phenyl)-...)Show SMILES OC(=O)C[C@H]1[C@H](CN[C@@H]1C(O)=O)C(=C)c1ccc(Cl)cc1 Show InChI InChI=1S/C15H16ClNO4/c1-8(9-2-4-10(16)5-3-9)12-7-17-14(15(20)21)11(12)6-13(18)19/h2-5,11-12,14,17H,1,6-7H2,(H,18,19)(H,20,21)/t11-,12+,14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-kainate binding at rat forebrain ionotropic glutamate receptor kainate 2 |
J Med Chem 39: 3617-24 (1996)
Article DOI: 10.1021/jm960155a
BindingDB Entry DOI: 10.7270/Q2WW7GRZ |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50132678

(CHEMBL432022 | [(2S,3S)-3-(6-Fluoro-naphthalen-2-y...)Show InChI InChI=1S/C19H22FN/c1-21-11-18-14-3-5-16(9-14)19(18)15-4-2-13-10-17(20)7-6-12(13)8-15/h2,4,6-8,10,14,16,18-19,21H,3,5,9,11H2,1H3/t14?,16?,18-,19-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Centre
Curated by ChEMBL
| Assay Description
Displacement of [3H]-citalopram from Serotonin transporter |
Bioorg Med Chem Lett 13: 3277-80 (2003)
BindingDB Entry DOI: 10.7270/Q29W0DXF |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, kainate 2
(Rattus norvegicus) | BDBM50053082
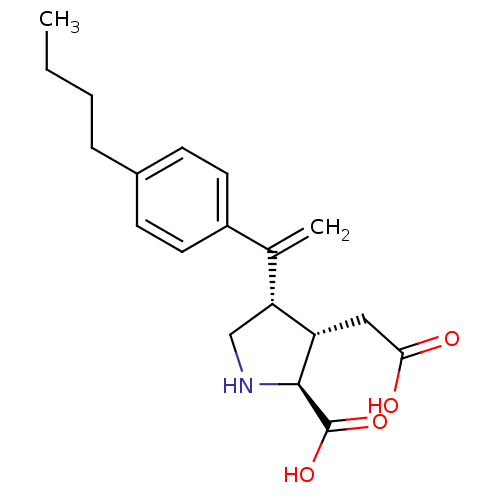
((2S,3S,4S)-4-[1-(4-Butyl-phenyl)-vinyl]-3-carboxym...)Show SMILES CCCCc1ccc(cc1)C(=C)[C@H]1CN[C@@H]([C@H]1CC(O)=O)C(O)=O Show InChI InChI=1S/C19H25NO4/c1-3-4-5-13-6-8-14(9-7-13)12(2)16-11-20-18(19(23)24)15(16)10-17(21)22/h6-9,15-16,18,20H,2-5,10-11H2,1H3,(H,21,22)(H,23,24)/t15-,16+,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-kainate binding at rat forebrain ionotropic glutamate receptor kainate 2 |
J Med Chem 39: 3617-24 (1996)
Article DOI: 10.1021/jm960155a
BindingDB Entry DOI: 10.7270/Q2WW7GRZ |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50132684

(CHEMBL331799 | Methyl-(3-naphthalen-2-yl-bicyclo[2...)Show InChI InChI=1S/C19H23N/c1-20-12-18-15-7-9-17(11-15)19(18)16-8-6-13-4-2-3-5-14(13)10-16/h2-6,8,10,15,17-20H,7,9,11-12H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Centre
Curated by ChEMBL
| Assay Description
Displacement of [3H]-citalopram from Serotonin transporter |
Bioorg Med Chem Lett 13: 3277-80 (2003)
BindingDB Entry DOI: 10.7270/Q29W0DXF |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50132677

(CHEMBL109517 | Dimethyl-((2R,3R)-3-naphthalen-2-yl...)Show InChI InChI=1S/C20H25N/c1-21(2)13-19-16-8-10-18(12-16)20(19)17-9-7-14-5-3-4-6-15(14)11-17/h3-7,9,11,16,18-20H,8,10,12-13H2,1-2H3/t16?,18?,19-,20-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 5.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Centre
Curated by ChEMBL
| Assay Description
Displacement of [3H]-citalopram from Serotonin transporter |
Bioorg Med Chem Lett 13: 3277-80 (2003)
BindingDB Entry DOI: 10.7270/Q29W0DXF |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Homo sapiens (Human)) | BDBM50132683

(6-((2S,3S)-3-Dimethylaminomethyl-bicyclo[2.2.1]hep...)Show SMILES CN(C)C[C@H]1C2CCC(C2)[C@@H]1c1ccc2cc(O)ccc2c1 Show InChI InChI=1S/C20H25NO/c1-21(2)12-19-15-4-6-17(10-15)20(19)16-5-3-14-11-18(22)8-7-13(14)9-16/h3,5,7-9,11,15,17,19-20,22H,4,6,10,12H2,1-2H3/t15?,17?,19-,20-/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Centre
Curated by ChEMBL
| Assay Description
Displacement of [3H]-WIN-35,428 from Dopamine transporter |
Bioorg Med Chem Lett 13: 3277-80 (2003)
BindingDB Entry DOI: 10.7270/Q29W0DXF |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, kainate 2
(Rattus norvegicus) | BDBM50053065
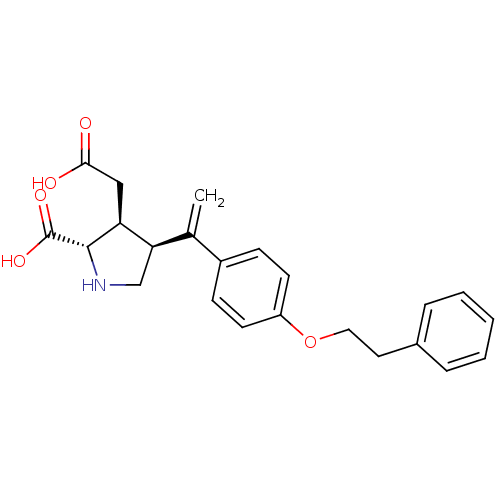
((2S,3S,4S)-3-Carboxymethyl-4-[1-(4-phenethyloxy-ph...)Show SMILES OC(=O)C[C@H]1[C@H](CN[C@@H]1C(O)=O)C(=C)c1ccc(OCCc2ccccc2)cc1 Show InChI InChI=1S/C23H25NO5/c1-15(20-14-24-22(23(27)28)19(20)13-21(25)26)17-7-9-18(10-8-17)29-12-11-16-5-3-2-4-6-16/h2-10,19-20,22,24H,1,11-14H2,(H,25,26)(H,27,28)/t19-,20+,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-kainate binding at rat forebrain ionotropic glutamate receptor kainate 2 |
J Med Chem 39: 3617-24 (1996)
Article DOI: 10.1021/jm960155a
BindingDB Entry DOI: 10.7270/Q2WW7GRZ |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Homo sapiens (Human)) | BDBM50026952

((cis) [3-(3,4-Dichloro-phenyl)-bicyclo[2.2.2]oct-2...)Show SMILES CN(C)C[C@@H]1C2CCC(CC2)[C@@H]1c1ccc(Cl)c(Cl)c1 |wU:11.13,4.3,TLB:12:11:6.7:10.9,THB:3:4:6.7:10.9,(4.42,-5.07,;3.76,-3.67,;2.22,-3.53,;4.65,-2.41,;4,-1.01,;5.68,-.26,;5.79,1.35,;5.19,2.75,;5.16,1.21,;6.61,.69,;7.15,-.75,;3.76,.53,;2.43,1.3,;1.1,.53,;-.23,1.3,;-.23,2.84,;-1.58,3.63,;1.1,3.61,;1.1,5.15,;2.43,2.84,)| Show InChI InChI=1S/C17H23Cl2N/c1-20(2)10-14-11-3-5-12(6-4-11)17(14)13-7-8-15(18)16(19)9-13/h7-9,11-12,14,17H,3-6,10H2,1-2H3/t11?,12?,14-,17-/m1/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 7.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Centre
Curated by ChEMBL
| Assay Description
In vitro inhibition of [3H]-NE re-uptake into synaptosome |
Bioorg Med Chem Lett 13: 3277-80 (2003)
BindingDB Entry DOI: 10.7270/Q29W0DXF |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, kainate 2
(Rattus norvegicus) | BDBM50053089
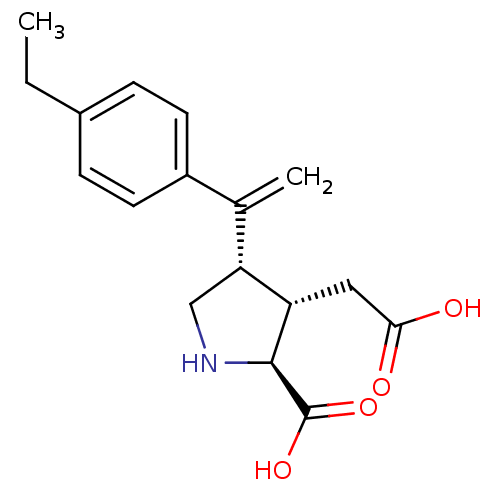
((2S,3S,4S)-3-Carboxymethyl-4-[1-(4-ethyl-phenyl)-v...)Show SMILES CCc1ccc(cc1)C(=C)[C@H]1CN[C@@H]([C@H]1CC(O)=O)C(O)=O Show InChI InChI=1S/C17H21NO4/c1-3-11-4-6-12(7-5-11)10(2)14-9-18-16(17(21)22)13(14)8-15(19)20/h4-7,13-14,16,18H,2-3,8-9H2,1H3,(H,19,20)(H,21,22)/t13-,14+,16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-kainate binding at rat forebrain ionotropic glutamate receptor kainate 2 |
J Med Chem 39: 3617-24 (1996)
Article DOI: 10.1021/jm960155a
BindingDB Entry DOI: 10.7270/Q2WW7GRZ |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Glutamate receptor ionotropic, kainate 2
(Rattus norvegicus) | BDBM50053070
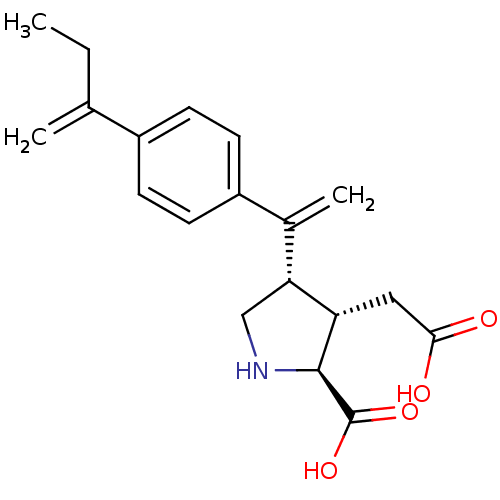
((2S,3S,4S)-3-Carboxymethyl-4-{1-[4-(1-methylene-pr...)Show SMILES CCC(=C)c1ccc(cc1)C(=C)[C@H]1CN[C@@H]([C@H]1CC(O)=O)C(O)=O Show InChI InChI=1S/C19H23NO4/c1-4-11(2)13-5-7-14(8-6-13)12(3)16-10-20-18(19(23)24)15(16)9-17(21)22/h5-8,15-16,18,20H,2-4,9-10H2,1H3,(H,21,22)(H,23,24)/t15-,16+,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-kainate binding at rat forebrain ionotropic glutamate receptor kainate 2 |
J Med Chem 39: 3617-24 (1996)
Article DOI: 10.1021/jm960155a
BindingDB Entry DOI: 10.7270/Q2WW7GRZ |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Glutamate receptor ionotropic, kainate 2
(Rattus norvegicus) | BDBM50053076
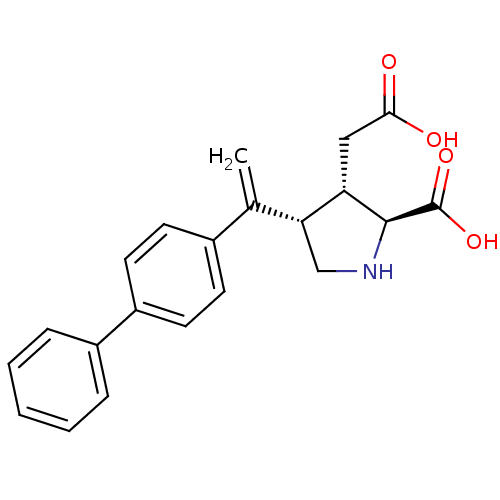
((2S,3S,4S)-4-(1-Biphenyl-4-yl-vinyl)-3-carboxymeth...)Show SMILES OC(=O)C[C@H]1[C@H](CN[C@@H]1C(O)=O)C(=C)c1ccc(cc1)-c1ccccc1 Show InChI InChI=1S/C21H21NO4/c1-13(18-12-22-20(21(25)26)17(18)11-19(23)24)14-7-9-16(10-8-14)15-5-3-2-4-6-15/h2-10,17-18,20,22H,1,11-12H2,(H,23,24)(H,25,26)/t17-,18+,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-kainate binding at rat forebrain ionotropic glutamate receptor kainate 2 |
J Med Chem 39: 3617-24 (1996)
Article DOI: 10.1021/jm960155a
BindingDB Entry DOI: 10.7270/Q2WW7GRZ |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, kainate 2
(Rattus norvegicus) | BDBM50053062

((2S,3S,4S)-3-Carboxymethyl-4-[1-(3,5-dimethyl-phen...)Show SMILES Cc1cc(C)cc(c1)C(=C)[C@H]1CN[C@@H]([C@H]1CC(O)=O)C(O)=O Show InChI InChI=1S/C17H21NO4/c1-9-4-10(2)6-12(5-9)11(3)14-8-18-16(17(21)22)13(14)7-15(19)20/h4-6,13-14,16,18H,3,7-8H2,1-2H3,(H,19,20)(H,21,22)/t13-,14+,16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-kainate binding at rat forebrain ionotropic glutamate receptor kainate 2 |
J Med Chem 39: 3617-24 (1996)
Article DOI: 10.1021/jm960155a
BindingDB Entry DOI: 10.7270/Q2WW7GRZ |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Glutamate receptor ionotropic, kainate 2
(Rattus norvegicus) | BDBM50053074
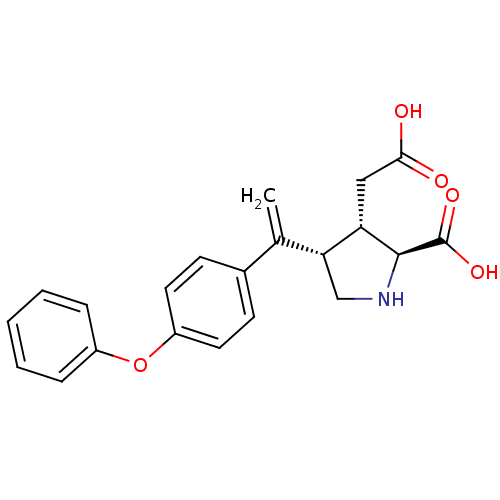
((2S,3S,4S)-3-Carboxymethyl-4-[1-(4-phenoxy-phenyl)...)Show SMILES OC(=O)C[C@H]1[C@H](CN[C@@H]1C(O)=O)C(=C)c1ccc(Oc2ccccc2)cc1 Show InChI InChI=1S/C21H21NO5/c1-13(18-12-22-20(21(25)26)17(18)11-19(23)24)14-7-9-16(10-8-14)27-15-5-3-2-4-6-15/h2-10,17-18,20,22H,1,11-12H2,(H,23,24)(H,25,26)/t17-,18+,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-kainate binding at rat forebrain ionotropic glutamate receptor kainate 2 |
J Med Chem 39: 3617-24 (1996)
Article DOI: 10.1021/jm960155a
BindingDB Entry DOI: 10.7270/Q2WW7GRZ |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50132680

(CHEMBL322348 | [(2S,3S)-3-(6-Methoxy-naphthalen-2-...)Show SMILES CNC[C@H]1C2CCC(C2)[C@@H]1c1ccc2cc(OC)ccc2c1 Show InChI InChI=1S/C20H25NO/c1-21-12-19-15-4-6-17(10-15)20(19)16-5-3-14-11-18(22-2)8-7-13(14)9-16/h3,5,7-9,11,15,17,19-21H,4,6,10,12H2,1-2H3/t15?,17?,19-,20-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Centre
Curated by ChEMBL
| Assay Description
Displacement of [3H]-citalopram from Serotonin transporter |
Bioorg Med Chem Lett 13: 3277-80 (2003)
BindingDB Entry DOI: 10.7270/Q29W0DXF |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, kainate 2
(Rattus norvegicus) | BDBM50053077
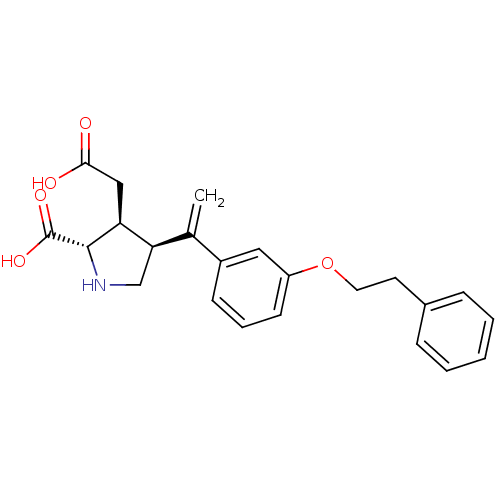
((2S,3S,4S)-3-Carboxymethyl-4-[1-(3-phenethyloxy-ph...)Show SMILES OC(=O)C[C@H]1[C@H](CN[C@@H]1C(O)=O)C(=C)c1cccc(OCCc2ccccc2)c1 Show InChI InChI=1S/C23H25NO5/c1-15(20-14-24-22(23(27)28)19(20)13-21(25)26)17-8-5-9-18(12-17)29-11-10-16-6-3-2-4-7-16/h2-9,12,19-20,22,24H,1,10-11,13-14H2,(H,25,26)(H,27,28)/t19-,20+,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-kainate binding at rat forebrain ionotropic glutamate receptor kainate 2 |
J Med Chem 39: 3617-24 (1996)
Article DOI: 10.1021/jm960155a
BindingDB Entry DOI: 10.7270/Q2WW7GRZ |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Homo sapiens (Human)) | BDBM50132685

(CHEMBL109571 | Dimethyl-(3-naphthalen-2-yl-bicyclo...)Show InChI InChI=1S/C20H25N/c1-21(2)13-19-16-8-10-18(12-16)20(19)17-9-7-14-5-3-4-6-15(14)11-17/h3-7,9,11,16,18-20H,8,10,12-13H2,1-2H3 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Centre
Curated by ChEMBL
| Assay Description
Displacement of [3H]-WIN-35,428 from Dopamine transporter |
Bioorg Med Chem Lett 13: 3277-80 (2003)
BindingDB Entry DOI: 10.7270/Q29W0DXF |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, kainate 2
(Homo sapiens (Human)) | BDBM50053058
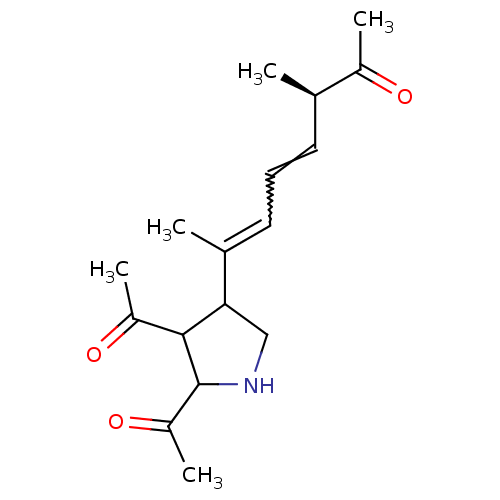
((4E,6E)-(R)-7-[4,5-Bis-(1-hydroxy-vinyl)-pyrrolidi...)Show SMILES C[C@H](C=CC=C(C)C1CNC(C1C(C)=O)C(C)=O)C(C)=O |w:4.3| Show InChI InChI=1S/C17H25NO3/c1-10(12(3)19)7-6-8-11(2)15-9-18-17(14(5)21)16(15)13(4)20/h6-8,10,15-18H,9H2,1-5H3/t10-,15?,16?,17?/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 18.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-kainate binding at ionotropic glutamate receptor kainate 2 |
J Med Chem 39: 3617-24 (1996)
Article DOI: 10.1021/jm960155a
BindingDB Entry DOI: 10.7270/Q2WW7GRZ |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, kainate 2
(Rattus norvegicus) | BDBM50053060
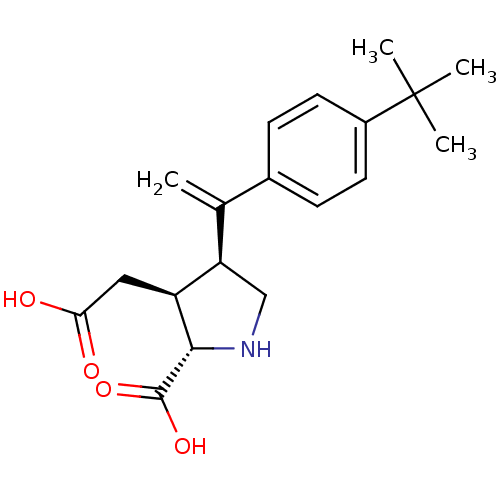
((2S,3S,4S)-4-[1-(4-tert-Butyl-phenyl)-vinyl]-3-car...)Show SMILES CC(C)(C)c1ccc(cc1)C(=C)[C@H]1CN[C@@H]([C@H]1CC(O)=O)C(O)=O Show InChI InChI=1S/C19H25NO4/c1-11(12-5-7-13(8-6-12)19(2,3)4)15-10-20-17(18(23)24)14(15)9-16(21)22/h5-8,14-15,17,20H,1,9-10H2,2-4H3,(H,21,22)(H,23,24)/t14-,15+,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 18.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-kainate binding at rat forebrain ionotropic glutamate receptor kainate 2 |
J Med Chem 39: 3617-24 (1996)
Article DOI: 10.1021/jm960155a
BindingDB Entry DOI: 10.7270/Q2WW7GRZ |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Glutamate receptor ionotropic, kainate 2
(Rattus norvegicus) | BDBM50053079
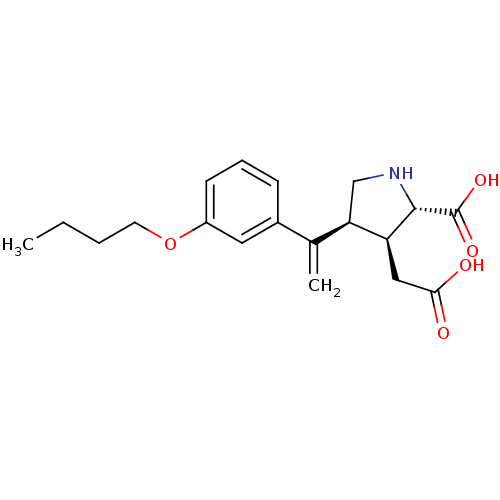
((2S,3S,4S)-4-[1-(3-Butoxy-phenyl)-vinyl]-3-carboxy...)Show SMILES CCCCOc1cccc(c1)C(=C)[C@H]1CN[C@@H]([C@H]1CC(O)=O)C(O)=O Show InChI InChI=1S/C19H25NO5/c1-3-4-8-25-14-7-5-6-13(9-14)12(2)16-11-20-18(19(23)24)15(16)10-17(21)22/h5-7,9,15-16,18,20H,2-4,8,10-11H2,1H3,(H,21,22)(H,23,24)/t15-,16+,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 23.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-kainate binding at rat forebrain ionotropic glutamate receptor kainate 2 |
J Med Chem 39: 3617-24 (1996)
Article DOI: 10.1021/jm960155a
BindingDB Entry DOI: 10.7270/Q2WW7GRZ |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Homo sapiens (Human)) | BDBM50132684

(CHEMBL331799 | Methyl-(3-naphthalen-2-yl-bicyclo[2...)Show InChI InChI=1S/C19H23N/c1-20-12-18-15-7-9-17(11-15)19(18)16-8-6-13-4-2-3-5-14(13)10-16/h2-6,8,10,15,17-20H,7,9,11-12H2,1H3 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Centre
Curated by ChEMBL
| Assay Description
In vitro inhibition of DA re-uptake into synaptosome |
Bioorg Med Chem Lett 13: 3277-80 (2003)
BindingDB Entry DOI: 10.7270/Q29W0DXF |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50132681

(CHEMBL323678 | [(2S,3S)-3-(6-Methoxy-naphthalen-2-...)Show SMILES COc1ccc2cc(ccc2c1)[C@H]1C2CCC(C2)[C@@H]1CN(C)C Show InChI InChI=1S/C21H27NO/c1-22(2)13-20-16-5-7-18(11-16)21(20)17-6-4-15-12-19(23-3)9-8-14(15)10-17/h4,6,8-10,12,16,18,20-21H,5,7,11,13H2,1-3H3/t16?,18?,20-,21-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Centre
Curated by ChEMBL
| Assay Description
Displacement of [3H]-citalopram from Serotonin transporter |
Bioorg Med Chem Lett 13: 3277-80 (2003)
BindingDB Entry DOI: 10.7270/Q29W0DXF |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Homo sapiens (Human)) | BDBM50132686

(CHEMBL326466 | Dimethyl-((2S,3S)-3-naphthalen-2-yl...)Show InChI InChI=1S/C20H25N/c1-21(2)13-19-16-8-10-18(12-16)20(19)17-9-7-14-5-3-4-6-15(14)11-17/h3-7,9,11,16,18-20H,8,10,12-13H2,1-2H3/t16?,18?,19-,20-/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Centre
Curated by ChEMBL
| Assay Description
Displacement of [3H]-WIN-35,428 from Dopamine transporter |
Bioorg Med Chem Lett 13: 3277-80 (2003)
BindingDB Entry DOI: 10.7270/Q29W0DXF |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50132682

(CHEMBL113136 | [(2S,3S)-3-(3-Chloro-phenyl)-bicycl...)Show SMILES CN(C)C[C@H]1C2CCC(CC2)[C@@H]1c1cccc(Cl)c1 |wU:11.13,wD:4.3,TLB:12:11:6.7:10.9,THB:3:4:6.7:10.9,(3.18,-2.78,;1.64,-2.8,;.89,-4.14,;.86,-1.47,;1.62,-.12,;3.16,.53,;3.41,2.42,;2.97,3.54,;2.9,1.88,;4.25,1.28,;4.53,-.1,;1.43,1.25,;.09,2.02,;-1.25,1.25,;-2.58,2.02,;-2.58,3.56,;-1.25,4.33,;-1.25,5.87,;.09,3.56,)| Show InChI InChI=1S/C17H24ClN/c1-19(2)11-16-12-6-8-13(9-7-12)17(16)14-4-3-5-15(18)10-14/h3-5,10,12-13,16-17H,6-9,11H2,1-2H3/t12?,13?,16-,17+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Centre
Curated by ChEMBL
| Assay Description
In vitro ability of compound to inhibit 5-HT re-uptake of radiolabelled [3H]-tritium trasmitter into synaptosome |
Bioorg Med Chem Lett 13: 3277-80 (2003)
BindingDB Entry DOI: 10.7270/Q29W0DXF |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, kainate 2
(Homo sapiens (Human)) | BDBM50053067

((2S,3S,4S)-3-Carboxymethyl-4-(1-phenyl-vinyl)-pyrr...)Show SMILES OC(=O)C[C@H]1[C@H](CN[C@@H]1C(O)=O)C(=C)c1ccccc1 Show InChI InChI=1S/C15H17NO4/c1-9(10-5-3-2-4-6-10)12-8-16-14(15(19)20)11(12)7-13(17)18/h2-6,11-12,14,16H,1,7-8H2,(H,17,18)(H,19,20)/t11-,12+,14-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 29.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-kainate binding at ionotropic glutamate receptor kainate 2 |
J Med Chem 39: 3617-24 (1996)
Article DOI: 10.1021/jm960155a
BindingDB Entry DOI: 10.7270/Q2WW7GRZ |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, kainate 2
(Homo sapiens (Human)) | BDBM50053061

((2S,3S,4S)-3-Carboxymethyl-4-[1-(4-chloro-phenyl)-...)Show SMILES OC(=O)C[C@H]1[C@H](CN[C@@H]1C(O)=O)C(=C)c1ccc(Cl)cc1 Show InChI InChI=1S/C15H16ClNO4/c1-8(9-2-4-10(16)5-3-9)12-7-17-14(15(20)21)11(12)6-13(18)19/h2-5,11-12,14,17H,1,6-7H2,(H,18,19)(H,20,21)/t11-,12+,14-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 31.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-kainate binding at ionotropic glutamate receptor kainate 2 |
J Med Chem 39: 3617-24 (1996)
Article DOI: 10.1021/jm960155a
BindingDB Entry DOI: 10.7270/Q2WW7GRZ |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Homo sapiens (Human)) | BDBM50132677

(CHEMBL109517 | Dimethyl-((2R,3R)-3-naphthalen-2-yl...)Show InChI InChI=1S/C20H25N/c1-21(2)13-19-16-8-10-18(12-16)20(19)17-9-7-14-5-3-4-6-15(14)11-17/h3-7,9,11,16,18-20H,8,10,12-13H2,1-2H3/t16?,18?,19-,20-/m1/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Centre
Curated by ChEMBL
| Assay Description
Displacement of [3H]-WIN-35,428 from Dopamine transporter |
Bioorg Med Chem Lett 13: 3277-80 (2003)
BindingDB Entry DOI: 10.7270/Q29W0DXF |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, kainate 2
(Homo sapiens (Human)) | BDBM50053087

((2S,3S,4S)-3-Carboxymethyl-4-[1-(4-fluoro-phenyl)-...)Show SMILES OC(=O)C[C@H]1[C@H](CN[C@@H]1C(O)=O)C(=C)c1ccc(F)cc1 Show InChI InChI=1S/C15H16FNO4/c1-8(9-2-4-10(16)5-3-9)12-7-17-14(15(20)21)11(12)6-13(18)19/h2-5,11-12,14,17H,1,6-7H2,(H,18,19)(H,20,21)/t11-,12+,14-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 38.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-kainate binding at ionotropic glutamate receptor kainate 2 |
J Med Chem 39: 3617-24 (1996)
Article DOI: 10.1021/jm960155a
BindingDB Entry DOI: 10.7270/Q2WW7GRZ |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Homo sapiens (Human)) | BDBM50132679

(CHEMBL111128 | Methyl-((2S,3S)-3-naphthalen-2-yl-b...)Show InChI InChI=1S/C19H23N/c1-20-12-18-15-7-9-17(11-15)19(18)16-8-6-13-4-2-3-5-14(13)10-16/h2-6,8,10,15,17-20H,7,9,11-12H2,1H3/t15?,17?,18-,19-/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Centre
Curated by ChEMBL
| Assay Description
Displacement of [3H]-WIN-35,428 from Dopamine transporter |
Bioorg Med Chem Lett 13: 3277-80 (2003)
BindingDB Entry DOI: 10.7270/Q29W0DXF |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, kainate 2
(Homo sapiens (Human)) | BDBM50053088

((2S,3S,4S)-3-Carboxymethyl-4-(1-p-tolyl-vinyl)-pyr...)Show SMILES Cc1ccc(cc1)C(=C)[C@H]1CN[C@@H]([C@H]1CC(O)=O)C(O)=O Show InChI InChI=1S/C16H19NO4/c1-9-3-5-11(6-4-9)10(2)13-8-17-15(16(20)21)12(13)7-14(18)19/h3-6,12-13,15,17H,2,7-8H2,1H3,(H,18,19)(H,20,21)/t12-,13+,15-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 41.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-kainate binding at ionotropic glutamate receptor kainate 2 |
J Med Chem 39: 3617-24 (1996)
Article DOI: 10.1021/jm960155a
BindingDB Entry DOI: 10.7270/Q2WW7GRZ |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50026952

((cis) [3-(3,4-Dichloro-phenyl)-bicyclo[2.2.2]oct-2...)Show SMILES CN(C)C[C@@H]1C2CCC(CC2)[C@@H]1c1ccc(Cl)c(Cl)c1 |wU:11.13,4.3,TLB:12:11:6.7:10.9,THB:3:4:6.7:10.9,(4.42,-5.07,;3.76,-3.67,;2.22,-3.53,;4.65,-2.41,;4,-1.01,;5.68,-.26,;5.79,1.35,;5.19,2.75,;5.16,1.21,;6.61,.69,;7.15,-.75,;3.76,.53,;2.43,1.3,;1.1,.53,;-.23,1.3,;-.23,2.84,;-1.58,3.63,;1.1,3.61,;1.1,5.15,;2.43,2.84,)| Show InChI InChI=1S/C17H23Cl2N/c1-20(2)10-14-11-3-5-12(6-4-11)17(14)13-7-8-15(18)16(19)9-13/h7-9,11-12,14,17H,3-6,10H2,1-2H3/t11?,12?,14-,17-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Centre
Curated by ChEMBL
| Assay Description
Displacement of [3H]-WIN-35,428 from Dopamine transporter |
Bioorg Med Chem Lett 13: 3277-80 (2003)
BindingDB Entry DOI: 10.7270/Q29W0DXF |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50132683

(6-((2S,3S)-3-Dimethylaminomethyl-bicyclo[2.2.1]hep...)Show SMILES CN(C)C[C@H]1C2CCC(C2)[C@@H]1c1ccc2cc(O)ccc2c1 Show InChI InChI=1S/C20H25NO/c1-21(2)12-19-15-4-6-17(10-15)20(19)16-5-3-14-11-18(22)8-7-13(14)9-16/h3,5,7-9,11,15,17,19-20,22H,4,6,10,12H2,1-2H3/t15?,17?,19-,20-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Centre
Curated by ChEMBL
| Assay Description
Displacement of [3H]-citalopram from Serotonin transporter |
Bioorg Med Chem Lett 13: 3277-80 (2003)
BindingDB Entry DOI: 10.7270/Q29W0DXF |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Homo sapiens (Human)) | BDBM50132687

(CHEMBL324269 | [(2S,3S)-3-(6-Fluoro-naphthalen-2-y...)Show SMILES CN(C)C[C@H]1C2CCC(C2)[C@@H]1c1ccc2cc(F)ccc2c1 Show InChI InChI=1S/C20H24FN/c1-22(2)12-19-15-4-6-17(10-15)20(19)16-5-3-14-11-18(21)8-7-13(14)9-16/h3,5,7-9,11,15,17,19-20H,4,6,10,12H2,1-2H3/t15?,17?,19-,20-/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Centre
Curated by ChEMBL
| Assay Description
Displacement of [3H]-WIN-35,428 from Dopamine transporter |
Bioorg Med Chem Lett 13: 3277-80 (2003)
BindingDB Entry DOI: 10.7270/Q29W0DXF |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50132683

(6-((2S,3S)-3-Dimethylaminomethyl-bicyclo[2.2.1]hep...)Show SMILES CN(C)C[C@H]1C2CCC(C2)[C@@H]1c1ccc2cc(O)ccc2c1 Show InChI InChI=1S/C20H25NO/c1-21(2)12-19-15-4-6-17(10-15)20(19)16-5-3-14-11-18(22)8-7-13(14)9-16/h3,5,7-9,11,15,17,19-20,22H,4,6,10,12H2,1-2H3/t15?,17?,19-,20-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Centre
Curated by ChEMBL
| Assay Description
Displacement of [3H]-Nisoxetine from norepinephrin transpoter |
Bioorg Med Chem Lett 13: 3277-80 (2003)
BindingDB Entry DOI: 10.7270/Q29W0DXF |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data