 Found 383 hits with Last Name = 'qiao' and Initial = 'h'
Found 383 hits with Last Name = 'qiao' and Initial = 'h' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50064786
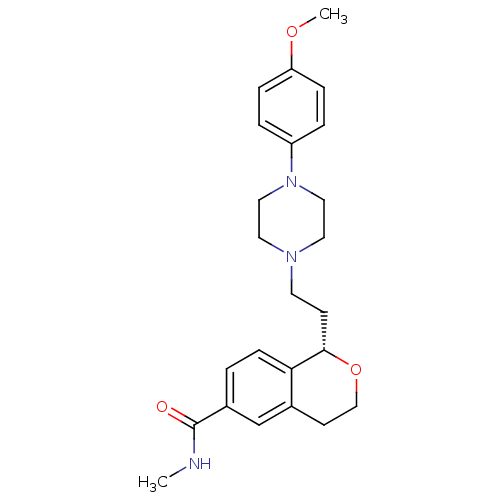
((S)-1-(2-(4-(4-methoxyphenyl)piperazin-1-yl)ethyl)...)Show SMILES CNC(=O)c1ccc2[C@H](CCN3CCN(CC3)c3ccc(OC)cc3)OCCc2c1 |r| Show InChI InChI=1S/C24H31N3O3/c1-25-24(28)19-3-8-22-18(17-19)10-16-30-23(22)9-11-26-12-14-27(15-13-26)20-4-6-21(29-2)7-5-20/h3-8,17,23H,9-16H2,1-2H3,(H,25,28)/t23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.113073
BindingDB Entry DOI: 10.7270/Q2FT8R1H |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Cavia porcellus (Guinea pig)) | BDBM50108659
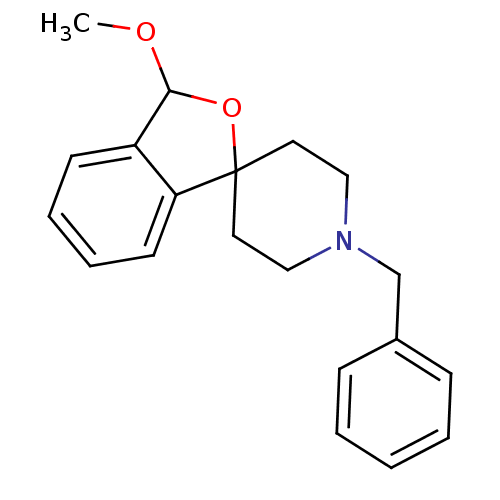
(1'-benzyl-3-methoxy-3H-spiro[2-benzofuran-1,4'-pip...)Show InChI InChI=1S/C20H23NO2/c1-22-19-17-9-5-6-10-18(17)20(23-19)11-13-21(14-12-20)15-16-7-3-2-4-8-16/h2-10,19H,11-15H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.113073
BindingDB Entry DOI: 10.7270/Q2FT8R1H |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Cavia porcellus (Guinea pig)) | BDBM50108653
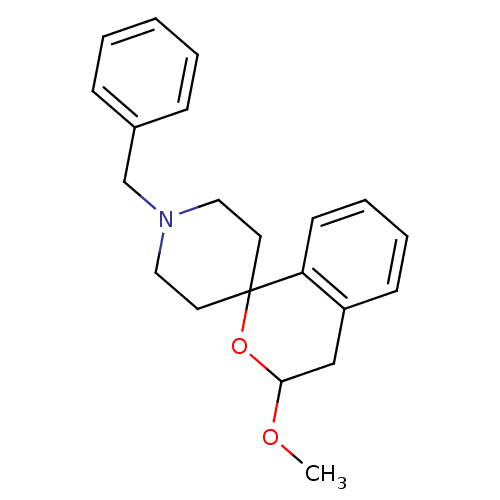
(1'-benzyl-3-methoxyspiro[3,4-dihydro-1H-isochromen...)Show InChI InChI=1S/C21H25NO2/c1-23-20-15-18-9-5-6-10-19(18)21(24-20)11-13-22(14-12-21)16-17-7-3-2-4-8-17/h2-10,20H,11-16H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.113073
BindingDB Entry DOI: 10.7270/Q2FT8R1H |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Cavia porcellus (Guinea pig)) | BDBM50120472
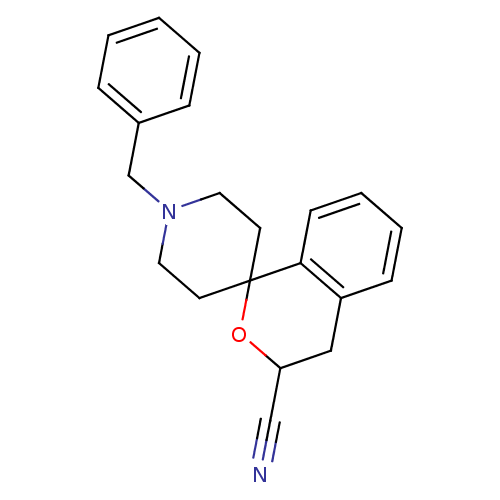
(1'-benzylspiro[3,4-dihydro-1H-isochromene-1,4'-(he...)Show InChI InChI=1S/C21H22N2O/c22-15-19-14-18-8-4-5-9-20(18)21(24-19)10-12-23(13-11-21)16-17-6-2-1-3-7-17/h1-9,19H,10-14,16H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.113073
BindingDB Entry DOI: 10.7270/Q2FT8R1H |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Cavia porcellus (Guinea pig)) | BDBM50108669
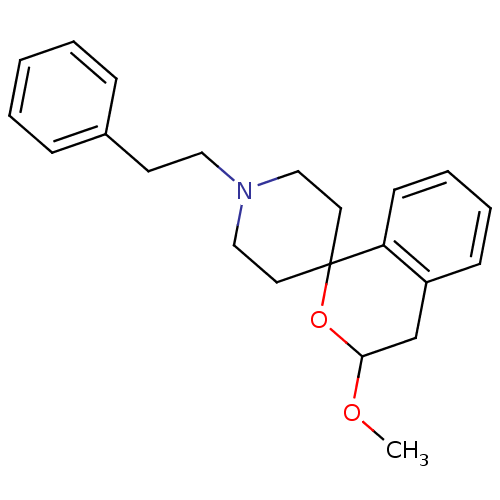
(1-[3-methoxyspiro[3,4-dihydro-1H-isochromene-1,4'-...)Show InChI InChI=1S/C22H27NO2/c1-24-21-17-19-9-5-6-10-20(19)22(25-21)12-15-23(16-13-22)14-11-18-7-3-2-4-8-18/h2-10,21H,11-17H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.113073
BindingDB Entry DOI: 10.7270/Q2FT8R1H |
More data for this
Ligand-Target Pair | |
DCN1-like protein 5
(Homo sapiens) | BDBM50525313

(CHEMBL4592844)Show InChI InChI=1S/C18H12N4S3/c1-3-9-23-17-21-15(13-6-4-12(2)5-7-13)14(11-19)16(22-17)25-18-20-8-10-24-18/h1,4-8,10H,9H2,2H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhengzhou University
Curated by ChEMBL
| Assay Description
Binding affinity to N-terminal GST tagged recombinant DCN5 (unknown origin) (47 to 237 residues) expressed in Escherichia coli BL21(DE3) assessed as ... |
J Med Chem 62: 5382-5403 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00003
BindingDB Entry DOI: 10.7270/Q2GT5RM9 |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Homo sapiens (Human)) | BDBM22165
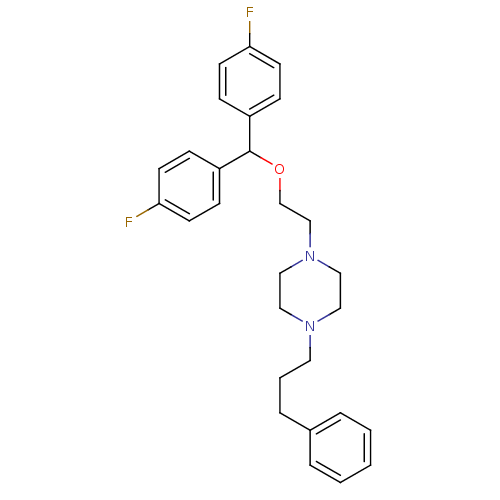
(1-{2-[bis(4-fluorophenyl)methoxy]ethyl}-4-(3-pheny...)Show SMILES Fc1ccc(cc1)C(OCCN1CCN(CCCc2ccccc2)CC1)c1ccc(F)cc1 Show InChI InChI=1S/C28H32F2N2O/c29-26-12-8-24(9-13-26)28(25-10-14-27(30)15-11-25)33-22-21-32-19-17-31(18-20-32)16-4-7-23-5-2-1-3-6-23/h1-3,5-6,8-15,28H,4,7,16-22H2 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Normal University
Curated by ChEMBL
| Assay Description
Displacement of [125I]IPT from DAT overexpressed in LLC-PK1 cell membrane by competitive binding assay |
Bioorg Med Chem Lett 22: 4303-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.030
BindingDB Entry DOI: 10.7270/Q2XG9S50 |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Homo sapiens (Human)) | BDBM50386906

(CHEMBL2048520)Show SMILES CN(CCCF)C[C@@H]1[C@H]2CC[C@@H](C[C@@H]1c1ccc(Cl)cc1)N2C |r,THB:22:21:13.7.12:10.9| Show InChI InChI=1S/C19H28ClFN2/c1-22(11-3-10-21)13-18-17(14-4-6-15(20)7-5-14)12-16-8-9-19(18)23(16)2/h4-7,16-19H,3,8-13H2,1-2H3/t16-,17+,18-,19+/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.64 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Normal University
Curated by ChEMBL
| Assay Description
Displacement of [125I]IPT from DAT overexpressed in LLC-PK1 cell membrane by competitive binding assay |
Bioorg Med Chem Lett 22: 4303-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.030
BindingDB Entry DOI: 10.7270/Q2XG9S50 |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Homo sapiens (Human)) | BDBM50386905
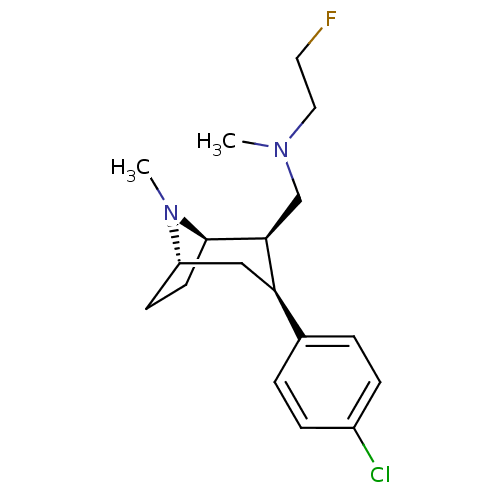
(CHEMBL2048519)Show SMILES CN(CCF)C[C@@H]1[C@H]2CC[C@@H](C[C@@H]1c1ccc(Cl)cc1)N2C |r,THB:21:20:12.6.11:9.8| Show InChI InChI=1S/C18H26ClFN2/c1-21(10-9-20)12-17-16(13-3-5-14(19)6-4-13)11-15-7-8-18(17)22(15)2/h3-6,15-18H,7-12H2,1-2H3/t15-,16+,17-,18+/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Normal University
Curated by ChEMBL
| Assay Description
Displacement of [125I]IPT from DAT overexpressed in LLC-PK1 cell membrane by competitive binding assay |
Bioorg Med Chem Lett 22: 4303-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.030
BindingDB Entry DOI: 10.7270/Q2XG9S50 |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Sus scrofa) | BDBM50033116
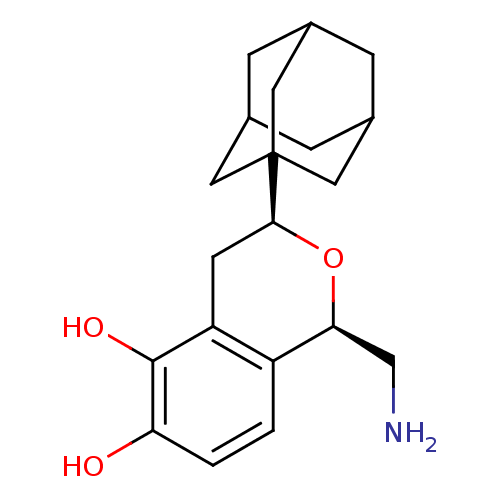
((1R,3S)-3-Adamantan-1-yl-1-aminomethyl-isochroman-...)Show SMILES NC[C@@H]1O[C@@H](Cc2c(O)c(O)ccc12)C12CC3CC(CC(C3)C1)C2 |TLB:21:16:23:22.20.19,21:20:23:15.16.17,THB:19:18:15:22.20.21,19:20:15:23.18.17| Show InChI InChI=1S/C20H27NO3/c21-10-17-14-1-2-16(22)19(23)15(14)6-18(24-17)20-7-11-3-12(8-20)5-13(4-11)9-20/h1-2,11-13,17-18,22-23H,3-10,21H2/t11?,12?,13?,17-,18-,20?/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.113073
BindingDB Entry DOI: 10.7270/Q2FT8R1H |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Sodium-dependent dopamine transporter
(Homo sapiens (Human)) | BDBM50386907
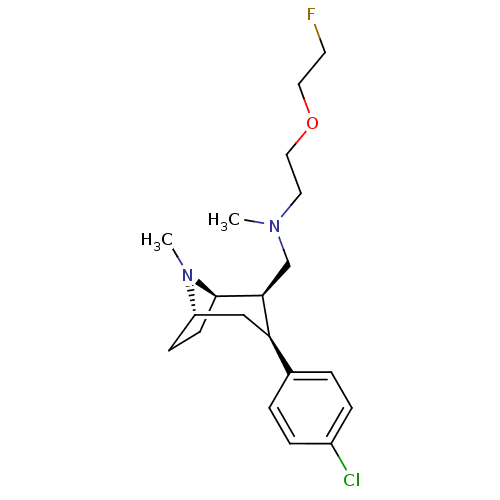
(CHEMBL2048521)Show SMILES CN(CCOCCF)C[C@@H]1[C@H]2CC[C@@H](C[C@@H]1c1ccc(Cl)cc1)N2C |r,THB:24:23:15.9.14:12.11| Show InChI InChI=1S/C20H30ClFN2O/c1-23(10-12-25-11-9-22)14-19-18(15-3-5-16(21)6-4-15)13-17-7-8-20(19)24(17)2/h3-6,17-20H,7-14H2,1-2H3/t17-,18+,19-,20+/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 4.63 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Normal University
Curated by ChEMBL
| Assay Description
Displacement of [125I]IPT from DAT overexpressed in LLC-PK1 cell membrane by competitive binding assay |
Bioorg Med Chem Lett 22: 4303-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.030
BindingDB Entry DOI: 10.7270/Q2XG9S50 |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Homo sapiens (Human)) | BDBM50386903
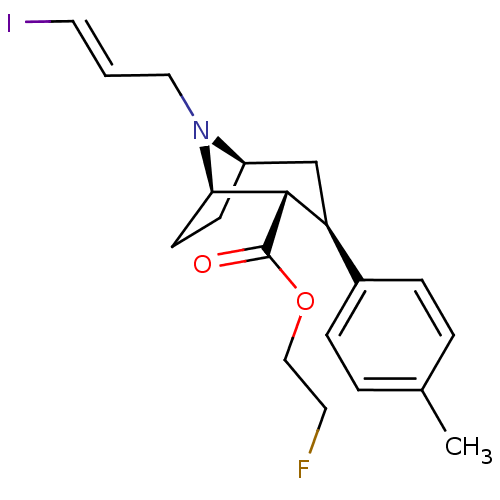
(CHEMBL559741)Show SMILES [H][C@@]12CC[C@]([H])([C@H]([C@@H](C1)c1ccc(C)cc1)C(=O)OCCF)N2C\C=C\I |r| Show InChI InChI=1S/C20H25FINO2/c1-14-3-5-15(6-4-14)17-13-16-7-8-18(23(16)11-2-10-22)19(17)20(24)25-12-9-21/h2-6,10,16-19H,7-9,11-13H2,1H3/b10-2+/t16-,17+,18-,19+/m1/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Normal University
Curated by ChEMBL
| Assay Description
Binding affinity to DAT |
Bioorg Med Chem Lett 22: 4303-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.030
BindingDB Entry DOI: 10.7270/Q2XG9S50 |
More data for this
Ligand-Target Pair | |
DCN1-like protein 1
(Homo sapiens) | BDBM50525313

(CHEMBL4592844)Show InChI InChI=1S/C18H12N4S3/c1-3-9-23-17-21-15(13-6-4-12(2)5-7-13)14(11-19)16(22-17)25-18-20-8-10-24-18/h1,4-8,10H,9H2,2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhengzhou University
Curated by ChEMBL
| Assay Description
Binding affinity to N-terminal GST tagged recombinant human DCN1 (58 to 259 residues) expressed in Escherichia coli BL21(DE3) assessed as reduction i... |
J Med Chem 62: 5382-5403 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00003
BindingDB Entry DOI: 10.7270/Q2GT5RM9 |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Homo sapiens (Human)) | BDBM50386910
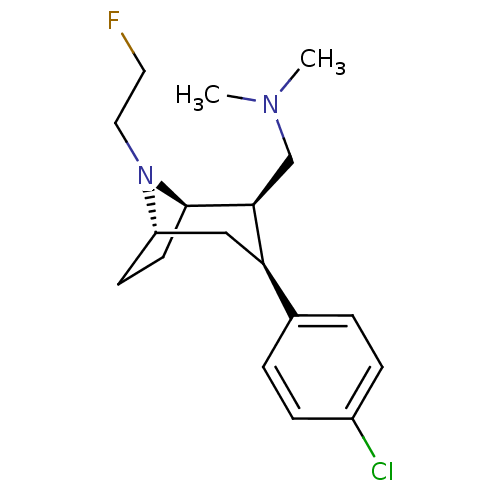
(CHEMBL2048524)Show SMILES CN(C)C[C@@H]1[C@H]2CC[C@@H](C[C@@H]1c1ccc(Cl)cc1)N2CCF |r,TLB:19:18:10.4.9:7.6| Show InChI InChI=1S/C18H26ClFN2/c1-21(2)12-17-16(13-3-5-14(19)6-4-13)11-15-7-8-18(17)22(15)10-9-20/h3-6,15-18H,7-12H2,1-2H3/t15-,16+,17-,18+/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 18.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Normal University
Curated by ChEMBL
| Assay Description
Displacement of [125I]IPT from DAT overexpressed in LLC-PK1 cell membrane by competitive binding assay |
Bioorg Med Chem Lett 22: 4303-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.030
BindingDB Entry DOI: 10.7270/Q2XG9S50 |
More data for this
Ligand-Target Pair | |
DCN1-like protein 1
(Homo sapiens) | BDBM50525313

(CHEMBL4592844)Show InChI InChI=1S/C18H12N4S3/c1-3-9-23-17-21-15(13-6-4-12(2)5-7-13)14(11-19)16(22-17)25-18-20-8-10-24-18/h1,4-8,10H,9H2,2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhengzhou University
Curated by ChEMBL
| Assay Description
Binding affinity to N-terminal His6-tagged recombinant human DCN1 expressed in Escherichia coli BL21-AI assessed as reduction in DCN1-FAM-labelled N-... |
J Med Chem 62: 5382-5403 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00003
BindingDB Entry DOI: 10.7270/Q2GT5RM9 |
More data for this
Ligand-Target Pair | |
DCN1-like protein 1
(Homo sapiens) | BDBM50569336

(CHEMBL4865016)Show SMILES S(Sc1nnc([nH]1)-c1ccccc1-c1ccccc1)c1nnc([nH]1)-c1ccccc1-c1ccccc1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human His-tagged DCN1 (58 to 259 residues) expressed in Escherichia coli BL21 (DE3) using MIKLFSLKQQKKEEESAGGTK-biotin as s... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113326
BindingDB Entry DOI: 10.7270/Q25H7M1W |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Homo sapiens (Human)) | BDBM50386911
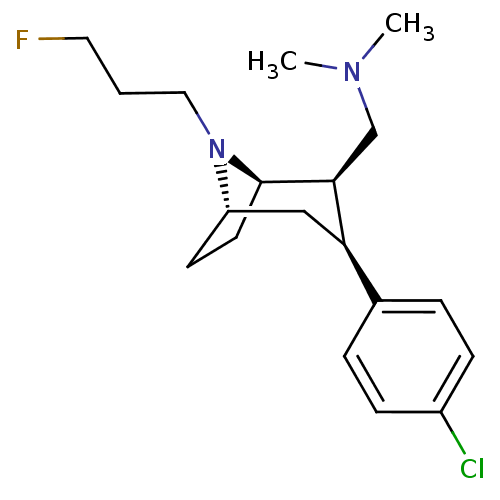
(CHEMBL2048525)Show SMILES CN(C)C[C@@H]1[C@H]2CC[C@@H](C[C@@H]1c1ccc(Cl)cc1)N2CCCF |r,TLB:19:18:10.4.9:7.6| Show InChI InChI=1S/C19H28ClFN2/c1-22(2)13-18-17(14-4-6-15(20)7-5-14)12-16-8-9-19(18)23(16)11-3-10-21/h4-7,16-19H,3,8-13H2,1-2H3/t16-,17+,18-,19+/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 26.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Normal University
Curated by ChEMBL
| Assay Description
Displacement of [125I]IPT from DAT overexpressed in LLC-PK1 cell membrane by competitive binding assay |
Bioorg Med Chem Lett 22: 4303-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.030
BindingDB Entry DOI: 10.7270/Q2XG9S50 |
More data for this
Ligand-Target Pair | |
DCN1-like protein 1
(Homo sapiens) | BDBM50569336

(CHEMBL4865016)Show SMILES S(Sc1nnc([nH]1)-c1ccccc1-c1ccccc1)c1nnc([nH]1)-c1ccccc1-c1ccccc1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human His-tagged DCN1 (58 to 259 residues) expressed in Escherichia coli BL21 (DE3) assessed as reduction in DCN1/UBC12 int... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113326
BindingDB Entry DOI: 10.7270/Q25H7M1W |
More data for this
Ligand-Target Pair | |
DCN1-like protein 2
(Homo sapiens) | BDBM50525313

(CHEMBL4592844)Show InChI InChI=1S/C18H12N4S3/c1-3-9-23-17-21-15(13-6-4-12(2)5-7-13)14(11-19)16(22-17)25-18-20-8-10-24-18/h1,4-8,10H,9H2,2H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 66 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhengzhou University
Curated by ChEMBL
| Assay Description
Binding affinity to N-terminal GST tagged recombinant DCN2 (unknown origin) (62 to 259 residues) expressed in Escherichia coli BL21(DE3) assessed as ... |
J Med Chem 62: 5382-5403 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00003
BindingDB Entry DOI: 10.7270/Q2GT5RM9 |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Homo sapiens (Human)) | BDBM50386909
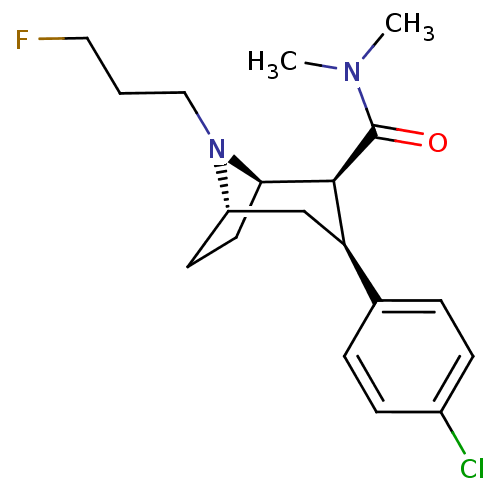
(CHEMBL2048523)Show SMILES CN(C)C(=O)[C@@H]1[C@H]2CC[C@@H](C[C@@H]1c1ccc(Cl)cc1)N2CCCF |r,TLB:20:19:11.5.10:8.7| Show InChI InChI=1S/C19H26ClFN2O/c1-22(2)19(24)18-16(13-4-6-14(20)7-5-13)12-15-8-9-17(18)23(15)11-3-10-21/h4-7,15-18H,3,8-12H2,1-2H3/t15-,16+,17+,18-/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 135 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Normal University
Curated by ChEMBL
| Assay Description
Displacement of [125I]IPT from DAT overexpressed in LLC-PK1 cell membrane by competitive binding assay |
Bioorg Med Chem Lett 22: 4303-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.030
BindingDB Entry DOI: 10.7270/Q2XG9S50 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50064786

((S)-1-(2-(4-(4-methoxyphenyl)piperazin-1-yl)ethyl)...)Show SMILES CNC(=O)c1ccc2[C@H](CCN3CCN(CC3)c3ccc(OC)cc3)OCCc2c1 |r| Show InChI InChI=1S/C24H31N3O3/c1-25-24(28)19-3-8-22-18(17-19)10-16-30-23(22)9-11-26-12-14-27(15-13-26)20-4-6-21(29-2)7-5-20/h3-8,17,23H,9-16H2,1-2H3,(H,25,28)/t23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 168 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.113073
BindingDB Entry DOI: 10.7270/Q2FT8R1H |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50064786

((S)-1-(2-(4-(4-methoxyphenyl)piperazin-1-yl)ethyl)...)Show SMILES CNC(=O)c1ccc2[C@H](CCN3CCN(CC3)c3ccc(OC)cc3)OCCc2c1 |r| Show InChI InChI=1S/C24H31N3O3/c1-25-24(28)19-3-8-22-18(17-19)10-16-30-23(22)9-11-26-12-14-27(15-13-26)20-4-6-21(29-2)7-5-20/h3-8,17,23H,9-16H2,1-2H3,(H,25,28)/t23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 241 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.113073
BindingDB Entry DOI: 10.7270/Q2FT8R1H |
More data for this
Ligand-Target Pair | |
DCN1-like protein 2
(Homo sapiens) | BDBM50569336

(CHEMBL4865016)Show SMILES S(Sc1nnc([nH]1)-c1ccccc1-c1ccccc1)c1nnc([nH]1)-c1ccccc1-c1ccccc1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 313 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human GST-tagged DCN2 (62 to 259 residues) expressed in Escherichia coli BL21 (DE3) using MIKLFSLKQQKKEEESAGGTK-biotin as s... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113326
BindingDB Entry DOI: 10.7270/Q25H7M1W |
More data for this
Ligand-Target Pair | |
DCN1-like protein 3
(Homo sapiens) | BDBM50525313

(CHEMBL4592844)Show InChI InChI=1S/C18H12N4S3/c1-3-9-23-17-21-15(13-6-4-12(2)5-7-13)14(11-19)16(22-17)25-18-20-8-10-24-18/h1,4-8,10H,9H2,2H3 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 592 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhengzhou University
Curated by ChEMBL
| Assay Description
Binding affinity to N-terminal GST tagged recombinant DCN3 (unknown origin) (86 to 304 residues) expressed in Escherichia coli BL21(DE3) assessed as ... |
J Med Chem 62: 5382-5403 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00003
BindingDB Entry DOI: 10.7270/Q2GT5RM9 |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Homo sapiens (Human)) | BDBM50386908

(CHEMBL2048522)Show SMILES CN(C)C(=O)[C@@H]1[C@H]2CC[C@@H](C[C@@H]1c1ccc(Cl)cc1)N2CCF |r,TLB:20:19:11.5.10:8.7| Show InChI InChI=1S/C18H24ClFN2O/c1-21(2)18(23)17-15(12-3-5-13(19)6-4-12)11-14-7-8-16(17)22(14)10-9-20/h3-6,14-17H,7-11H2,1-2H3/t14-,15+,16+,17-/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 699 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Normal University
Curated by ChEMBL
| Assay Description
Displacement of [125I]IPT from DAT overexpressed in LLC-PK1 cell membrane by competitive binding assay |
Bioorg Med Chem Lett 22: 4303-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.030
BindingDB Entry DOI: 10.7270/Q2XG9S50 |
More data for this
Ligand-Target Pair | |
DCN1-like protein 4
(Homo sapiens) | BDBM50525313

(CHEMBL4592844)Show InChI InChI=1S/C18H12N4S3/c1-3-9-23-17-21-15(13-6-4-12(2)5-7-13)14(11-19)16(22-17)25-18-20-8-10-24-18/h1,4-8,10H,9H2,2H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 807 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhengzhou University
Curated by ChEMBL
| Assay Description
Binding affinity to N-terminal GST tagged recombinant DCN4 (unknown origin) (102 to 292 residues) expressed in Escherichia coli BL21(DE3) assessed as... |
J Med Chem 62: 5382-5403 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00003
BindingDB Entry DOI: 10.7270/Q2GT5RM9 |
More data for this
Ligand-Target Pair | |
DCN1-like protein 3
(Homo sapiens) | BDBM50569336

(CHEMBL4865016)Show SMILES S(Sc1nnc([nH]1)-c1ccccc1-c1ccccc1)c1nnc([nH]1)-c1ccccc1-c1ccccc1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human GST-tagged DCN3 (86 to 304 residues) expressed in Escherichia coli BL21 (DE3) using MIKLFSLKQQKKEEESAGGTK-biotin as s... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113326
BindingDB Entry DOI: 10.7270/Q25H7M1W |
More data for this
Ligand-Target Pair | |
DCN1-like protein 4
(Homo sapiens) | BDBM50569336

(CHEMBL4865016)Show SMILES S(Sc1nnc([nH]1)-c1ccccc1-c1ccccc1)c1nnc([nH]1)-c1ccccc1-c1ccccc1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human GST-tagged DCN4 (102 to 292 residues) expressed in Escherichia coli BL21 (DE3) using MIKLFSLKQQKKEEESAGGTK-biotin as ... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113326
BindingDB Entry DOI: 10.7270/Q25H7M1W |
More data for this
Ligand-Target Pair | |
DCN1-like protein 5
(Homo sapiens) | BDBM50569336

(CHEMBL4865016)Show SMILES S(Sc1nnc([nH]1)-c1ccccc1-c1ccccc1)c1nnc([nH]1)-c1ccccc1-c1ccccc1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human GST-tagged DCN5 (47 to 237 residues) expressed in Escherichia coli BL21 (DE3) using MIKLFSLKQQKKEEESAGGTK-biotin as s... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113326
BindingDB Entry DOI: 10.7270/Q25H7M1W |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50064786

((S)-1-(2-(4-(4-methoxyphenyl)piperazin-1-yl)ethyl)...)Show SMILES CNC(=O)c1ccc2[C@H](CCN3CCN(CC3)c3ccc(OC)cc3)OCCc2c1 |r| Show InChI InChI=1S/C24H31N3O3/c1-25-24(28)19-3-8-22-18(17-19)10-16-30-23(22)9-11-26-12-14-27(15-13-26)20-4-6-21(29-2)7-5-20/h3-8,17,23H,9-16H2,1-2H3,(H,25,28)/t23-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.09E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.113073
BindingDB Entry DOI: 10.7270/Q2FT8R1H |
More data for this
Ligand-Target Pair | |
Sigma intracellular receptor 2
(Rattus norvegicus (Rat)) | BDBM50120472

(1'-benzylspiro[3,4-dihydro-1H-isochromene-1,4'-(he...)Show InChI InChI=1S/C21H22N2O/c22-15-19-14-18-8-4-5-9-20(18)21(24-19)10-12-23(13-11-21)16-17-6-2-1-3-7-17/h1-9,19H,10-14,16H2 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.59E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.113073
BindingDB Entry DOI: 10.7270/Q2FT8R1H |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50064786

((S)-1-(2-(4-(4-methoxyphenyl)piperazin-1-yl)ethyl)...)Show SMILES CNC(=O)c1ccc2[C@H](CCN3CCN(CC3)c3ccc(OC)cc3)OCCc2c1 |r| Show InChI InChI=1S/C24H31N3O3/c1-25-24(28)19-3-8-22-18(17-19)10-16-30-23(22)9-11-26-12-14-27(15-13-26)20-4-6-21(29-2)7-5-20/h3-8,17,23H,9-16H2,1-2H3,(H,25,28)/t23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.113073
BindingDB Entry DOI: 10.7270/Q2FT8R1H |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
(Homo sapiens (Human)) | BDBM50064786

((S)-1-(2-(4-(4-methoxyphenyl)piperazin-1-yl)ethyl)...)Show SMILES CNC(=O)c1ccc2[C@H](CCN3CCN(CC3)c3ccc(OC)cc3)OCCc2c1 |r| Show InChI InChI=1S/C24H31N3O3/c1-25-24(28)19-3-8-22-18(17-19)10-16-30-23(22)9-11-26-12-14-27(15-13-26)20-4-6-21(29-2)7-5-20/h3-8,17,23H,9-16H2,1-2H3,(H,25,28)/t23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5.78E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.113073
BindingDB Entry DOI: 10.7270/Q2FT8R1H |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50591658
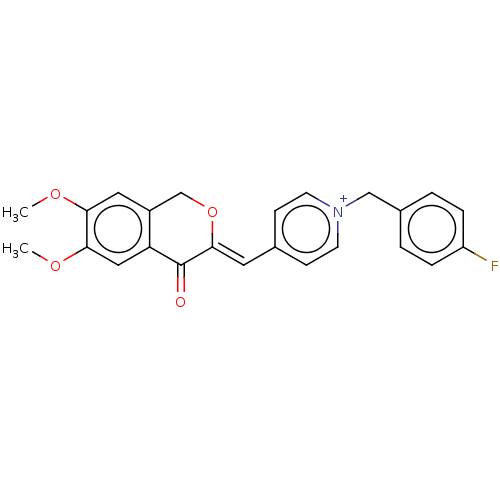
(CHEMBL5187881)Show SMILES [Br-].COc1cc2CO\C(=C/c3cc[n+](Cc4ccc(F)cc4)cc3)C(=O)c2cc1OC | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.113073
BindingDB Entry DOI: 10.7270/Q2FT8R1H |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50322823

((S)-N-(4-(3-chloro-4-fluorophenylamino)-7-(tetrahy...)Show SMILES CN(C)C\C=C\C(=O)Nc1cc2c(Nc3ccc(F)c(Cl)c3)ncnc2cc1O[C@H]1CCOC1 |r| Show InChI InChI=1S/C24H25ClFN5O3/c1-31(2)8-3-4-23(32)30-21-11-17-20(12-22(21)34-16-7-9-33-13-16)27-14-28-24(17)29-15-5-6-19(26)18(25)10-15/h3-6,10-12,14,16H,7-9,13H2,1-2H3,(H,30,32)(H,27,28,29)/b4-3+/t16-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of N-terminal GST-fused human wild type EGFR cytoplasmic domain (669 to 1210 residues) expressed in baculovirus expression system using 5-... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112388
BindingDB Entry DOI: 10.7270/Q24X5CJS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50029668

(AZD-9291 | Osimertinib | US10085983, Compound AZD-...)Show SMILES COc1cc(N(C)CCN(C)C)c(NC(=O)C=C)cc1Nc1nccc(n1)-c1cn(C)c2ccccc12 Show InChI InChI=1S/C28H33N7O2/c1-7-27(36)30-22-16-23(26(37-6)17-25(22)34(4)15-14-33(2)3)32-28-29-13-12-21(31-28)20-18-35(5)24-11-9-8-10-19(20)24/h7-13,16-18H,1,14-15H2,2-6H3,(H,30,36)(H,29,31,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of N-terminal GST-fused human EGFR L858R/T790M double mutant cytoplasmic domain (669 to 1210 residues) expressed in baculovirus expression... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112388
BindingDB Entry DOI: 10.7270/Q24X5CJS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Ephrin type-A receptor 2 [596-900]
(Homo sapiens (Human)) | BDBM13216

(BMS-354825 | CHEMBL1421 | DASATINIB | N-(2-Chloro-...)Show SMILES Cc1nc(Nc2ncc(s2)C(=O)Nc2c(C)cccc2Cl)cc(n1)N1CCN(CCO)CC1 Show InChI InChI=1S/C22H26ClN7O2S/c1-14-4-3-5-16(23)20(14)28-21(32)17-13-24-22(33-17)27-18-12-19(26-15(2)25-18)30-8-6-29(7-9-30)10-11-31/h3-5,12-13,31H,6-11H2,1-2H3,(H,28,32)(H,24,25,26,27) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Technical University of Munich
| Assay Description
Refer to Reaction Biology Corps. |
ACS Chem Biol 11: 3400-3411 (2016)
Article DOI: 10.1021/acschembio.6b00709
BindingDB Entry DOI: 10.7270/Q2TD9W5M |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50160871

(BI 1482694 | HM61713 | Olmutinib)Show SMILES CN1CCN(CC1)c1ccc(Nc2nc(Oc3cccc(NC(=O)C=C)c3)c3sccc3n2)cc1 Show InChI InChI=1S/C26H26N6O2S/c1-3-23(33)27-19-5-4-6-21(17-19)34-25-24-22(11-16-35-24)29-26(30-25)28-18-7-9-20(10-8-18)32-14-12-31(2)13-15-32/h3-11,16-17H,1,12-15H2,2H3,(H,27,33)(H,28,29,30) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of N-terminal GST-fused human EGFR L858R/T790M double mutant cytoplasmic domain (669 to 1210 residues) expressed in baculovirus expression... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112388
BindingDB Entry DOI: 10.7270/Q24X5CJS |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 2 [596-900]
(Homo sapiens (Human)) | BDBM50299218

(8-(2-Methoxyphenyl)-1-methyl-7-(2'-methyl-5'-hydro...)Show SMILES COc1ccccc1-n1c(cn2c1nc1n(C)c(=O)[nH]c(=O)c21)-c1cc(O)ccc1C |(16.89,-42.99,;16.89,-41.45,;18.23,-40.68,;19.55,-41.45,;20.88,-40.68,;20.88,-39.14,;19.55,-38.38,;18.23,-39.15,;16.9,-38.38,;16.91,-36.84,;15.44,-36.35,;14.53,-37.6,;15.43,-38.85,;14.53,-40.09,;13.06,-39.61,;11.74,-40.37,;11.73,-41.91,;10.41,-39.61,;9.07,-40.38,;10.41,-38.07,;11.74,-37.29,;11.74,-35.75,;13.06,-38.07,;18.24,-36.06,;19.58,-36.83,;20.9,-36.05,;22.24,-36.81,;20.9,-34.51,;19.55,-33.75,;18.23,-34.53,;16.89,-33.77,)| Show InChI InChI=1S/C22H19N5O4/c1-12-8-9-13(28)10-14(12)16-11-26-18-19(25(2)22(30)24-20(18)29)23-21(26)27(16)15-6-4-5-7-17(15)31-3/h4-11,28H,1-3H3,(H,24,29,30) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Technical University of Munich
| Assay Description
Refer to Reaction Biology Corps. |
ACS Chem Biol 11: 3400-3411 (2016)
Article DOI: 10.1021/acschembio.6b00709
BindingDB Entry DOI: 10.7270/Q2TD9W5M |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Ephrin type-A receptor 2 [596-900]
(Homo sapiens (Human)) | BDBM50322535

(3-(imidazo[1,2-b]pyridazin-3-ylethynyl)-4-methyl-N...)Show SMILES CN1CCN(Cc2ccc(NC(=O)c3ccc(C)c(c3)C#Cc3cnc4cccnn34)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C29H27F3N6O/c1-20-5-6-22(16-21(20)8-10-25-18-33-27-4-3-11-34-38(25)27)28(39)35-24-9-7-23(26(17-24)29(30,31)32)19-37-14-12-36(2)13-15-37/h3-7,9,11,16-18H,12-15,19H2,1-2H3,(H,35,39) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Technical University of Munich
| Assay Description
Refer to Reaction Biology Corps. |
ACS Chem Biol 11: 3400-3411 (2016)
Article DOI: 10.1021/acschembio.6b00709
BindingDB Entry DOI: 10.7270/Q2TD9W5M |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 2 [596-900]
(Homo sapiens (Human)) | BDBM50399540

(FORETINIB | US10464902, Foretinib | US10882853, Co...)Show SMILES COc1cc2c(Oc3ccc(NC(=O)C4(CC4)C(=O)Nc4ccc(F)cc4)cc3F)ccnc2cc1OCCCN1CCOCC1 Show InChI InChI=1S/C34H34F2N4O6/c1-43-30-20-25-27(21-31(30)45-16-2-13-40-14-17-44-18-15-40)37-12-9-28(25)46-29-8-7-24(19-26(29)36)39-33(42)34(10-11-34)32(41)38-23-5-3-22(35)4-6-23/h3-9,12,19-21H,2,10-11,13-18H2,1H3,(H,38,41)(H,39,42) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Technical University of Munich
| Assay Description
Refer to Reaction Biology Corps. |
ACS Chem Biol 11: 3400-3411 (2016)
Article DOI: 10.1021/acschembio.6b00709
BindingDB Entry DOI: 10.7270/Q2TD9W5M |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Ephrin type-A receptor 2 [596-900]
(Homo sapiens (Human)) | BDBM4552

(4-[(2,4-Dichloro-5-methoxyphenyl)amino]-6-methoxy-...)Show SMILES COc1cc(Nc2c(cnc3cc(OCCCN4CCN(C)CC4)c(OC)cc23)C#N)c(Cl)cc1Cl Show InChI InChI=1S/C26H29Cl2N5O3/c1-32-6-8-33(9-7-32)5-4-10-36-25-13-21-18(11-24(25)35-3)26(17(15-29)16-30-21)31-22-14-23(34-2)20(28)12-19(22)27/h11-14,16H,4-10H2,1-3H3,(H,30,31) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Technical University of Munich
| Assay Description
Refer to Reaction Biology Corps. |
ACS Chem Biol 11: 3400-3411 (2016)
Article DOI: 10.1021/acschembio.6b00709
BindingDB Entry DOI: 10.7270/Q2TD9W5M |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Ephrin type-A receptor 2 [596-900]
(Homo sapiens (Human)) | BDBM209859
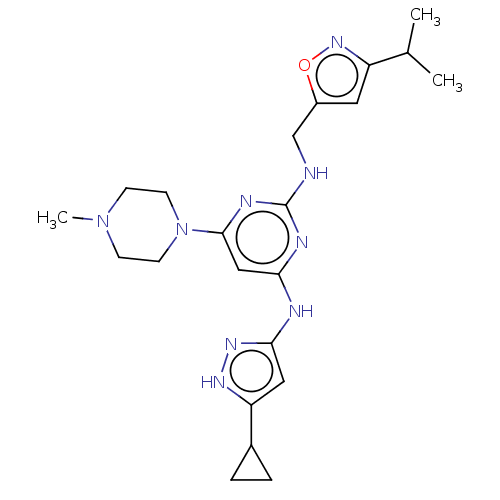
(4-N-(5-cyclopropyl-1H-pyrazol-3-yl)-6-(4-methylpip...)Show SMILES CC(C)c1cc(CNc2nc(Nc3cc([nH]n3)C3CC3)cc(n2)N2CCN(C)CC2)on1 Show InChI InChI=1S/C22H31N9O/c1-14(2)17-10-16(32-29-17)13-23-22-25-19(24-20-11-18(27-28-20)15-4-5-15)12-21(26-22)31-8-6-30(3)7-9-31/h10-12,14-15H,4-9,13H2,1-3H3,(H3,23,24,25,26,27,28) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Technical University of Munich
| Assay Description
Refer to Reaction Biology Corps. |
ACS Chem Biol 11: 3400-3411 (2016)
Article DOI: 10.1021/acschembio.6b00709
BindingDB Entry DOI: 10.7270/Q2TD9W5M |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 2 [596-900]
(Homo sapiens (Human)) | BDBM50311316
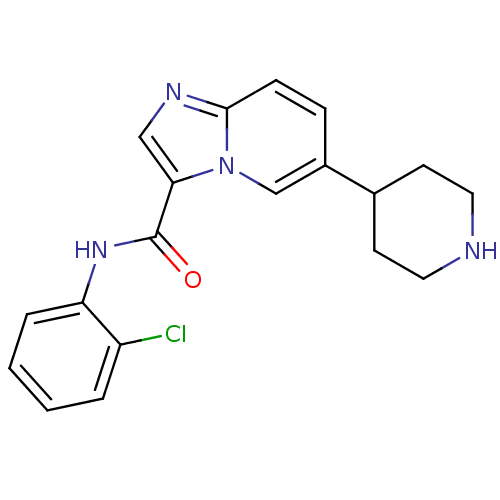
(CHEMBL1077739 | LDN-211904 | N-(2-chlorophenyl)-6-...)Show InChI InChI=1S/C19H19ClN4O/c20-15-3-1-2-4-16(15)23-19(25)17-11-22-18-6-5-14(12-24(17)18)13-7-9-21-10-8-13/h1-6,11-13,21H,7-10H2,(H,23,25) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Technical University of Munich
| Assay Description
Refer to Reaction Biology Corps. |
ACS Chem Biol 11: 3400-3411 (2016)
Article DOI: 10.1021/acschembio.6b00709
BindingDB Entry DOI: 10.7270/Q2TD9W5M |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Ephrin type-A receptor 2 [596-900]
(Homo sapiens (Human)) | BDBM209858
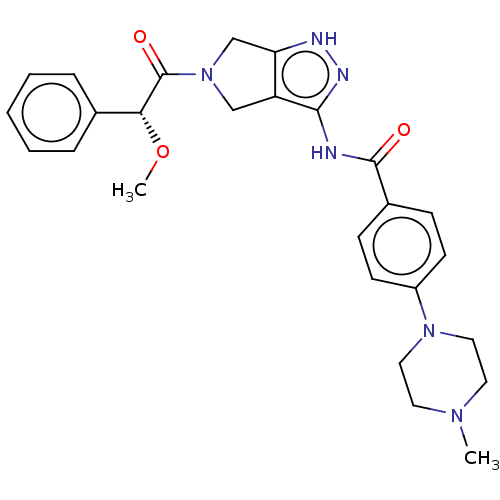
(Danusertib | N-[5-[(2R)-2-methoxy-2-phenylacetyl]-...)Show SMILES CO[C@@H](C(=O)N1Cc2[nH]nc(NC(=O)c3ccc(cc3)N3CCN(C)CC3)c2C1)c1ccccc1 Show InChI InChI=1S/C26H30N6O3/c1-30-12-14-31(15-13-30)20-10-8-19(9-11-20)25(33)27-24-21-16-32(17-22(21)28-29-24)26(34)23(35-2)18-6-4-3-5-7-18/h3-11,23H,12-17H2,1-2H3,(H2,27,28,29,33)/t23-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Technical University of Munich
| Assay Description
Refer to Reaction Biology Corps. |
ACS Chem Biol 11: 3400-3411 (2016)
Article DOI: 10.1021/acschembio.6b00709
BindingDB Entry DOI: 10.7270/Q2TD9W5M |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Ephrin type-A receptor 2 [596-900]
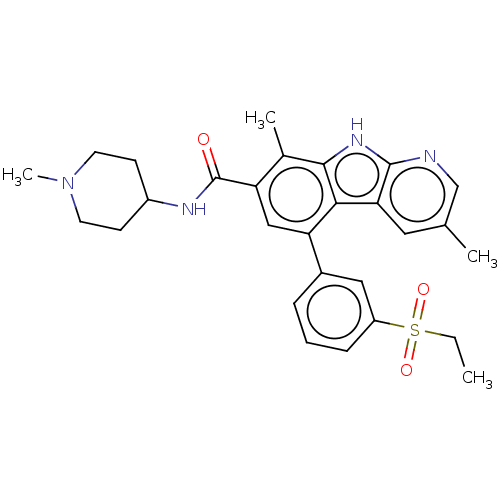
(Homo sapiens (Human)) | BDBM209861
(5-(3-ethylsulfonylphenyl)-3,8-dimethyl-N-(1-methyl...)Show SMILES CCS(=O)(=O)c1cccc(c1)-c1cc(C(=O)NC2CCN(C)CC2)c(C)c2[nH]c3ncc(C)cc3c12 Show InChI InChI=1S/C28H32N4O3S/c1-5-36(34,35)21-8-6-7-19(14-21)23-15-22(28(33)30-20-9-11-32(4)12-10-20)18(3)26-25(23)24-13-17(2)16-29-27(24)31-26/h6-8,13-16,20H,5,9-12H2,1-4H3,(H,29,31)(H,30,33) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Technical University of Munich
| Assay Description
Refer to Reaction Biology Corps. |
ACS Chem Biol 11: 3400-3411 (2016)
Article DOI: 10.1021/acschembio.6b00709
BindingDB Entry DOI: 10.7270/Q2TD9W5M |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 2 [596-900]
(Homo sapiens (Human)) | BDBM6568

(6-(2,6-dichlorophenyl)-8-methyl-2-{[3-(methylsulfa...)Show SMILES CSc1cccc(Nc2ncc3cc(-c4c(Cl)cccc4Cl)c(=O)n(C)c3n2)c1 |(-12.18,1.86,;-10.84,1.09,;-9.51,1.86,;-9.51,3.4,;-8.18,4.17,;-6.84,3.4,;-6.84,1.86,;-5.51,1.08,;-4.18,1.85,;-4.18,3.39,;-2.84,4.16,;-1.51,3.4,;-.18,4.17,;1.16,3.4,;2.49,4.17,;2.49,5.71,;1.16,6.48,;3.83,6.48,;5.16,5.71,;5.16,4.17,;3.83,3.39,;3.83,1.85,;1.16,1.86,;2.49,1.09,;-.18,1.09,;-.18,-.45,;-1.51,1.86,;-2.84,1.08,;-8.18,1.08,)| Show InChI InChI=1S/C21H16Cl2N4OS/c1-27-19-12(9-15(20(27)28)18-16(22)7-4-8-17(18)23)11-24-21(26-19)25-13-5-3-6-14(10-13)29-2/h3-11H,1-2H3,(H,24,25,26) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Technical University of Munich
| Assay Description
Refer to Reaction Biology Corps. |
ACS Chem Biol 11: 3400-3411 (2016)
Article DOI: 10.1021/acschembio.6b00709
BindingDB Entry DOI: 10.7270/Q2TD9W5M |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50498654
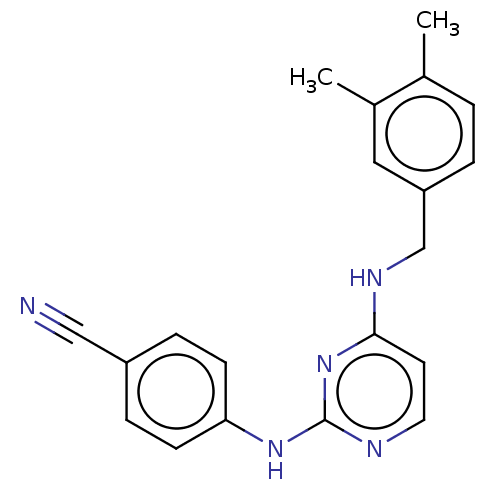
(CHEMBL3618319)Show InChI InChI=1S/C20H19N5/c1-14-3-4-17(11-15(14)2)13-23-19-9-10-22-20(25-19)24-18-7-5-16(12-21)6-8-18/h3-11H,13H2,1-2H3,(H2,22,23,24,25) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan Institute of Technology
Curated by ChEMBL
| Assay Description
Inhibition of recombinant HIV1 Reverse transcriptase p66/p51 using poly (rA)-oligo (dT) as template primer after 40 mins by spectrofluorometric analy... |
Bioorg Med Chem 23: 6587-93 (2015)
Article DOI: 10.1016/j.bmc.2015.09.020
BindingDB Entry DOI: 10.7270/Q2ZS30JJ |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50103642

(4-(6-amino-5-bromo-2-(4-cyanophenylamino)pyrimidin...)Show SMILES Cc1cc(cc(C)c1Oc1nc(Nc2ccc(cc2)C#N)nc(N)c1Br)C#N Show InChI InChI=1S/C20H15BrN6O/c1-11-7-14(10-23)8-12(2)17(11)28-19-16(21)18(24)26-20(27-19)25-15-5-3-13(9-22)4-6-15/h3-8H,1-2H3,(H3,24,25,26,27) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan Institute of Technology
Curated by ChEMBL
| Assay Description
Inhibition of recombinant HIV1 Reverse transcriptase p66/p51 using poly (rA)-oligo (dT) as template primer after 40 mins by spectrofluorometric analy... |
Bioorg Med Chem 23: 6587-93 (2015)
Article DOI: 10.1016/j.bmc.2015.09.020
BindingDB Entry DOI: 10.7270/Q2ZS30JJ |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 2 [596-900]
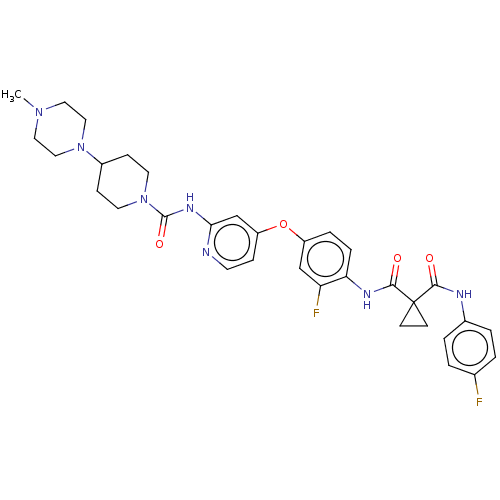
(Homo sapiens (Human)) | BDBM50100615
(E-7050 | E7050 | Golvatinib)Show SMILES CN1CCN(CC1)C1CCN(CC1)C(=O)Nc1cc(Oc2ccc(NC(=O)C3(CC3)C(=O)Nc3ccc(F)cc3)c(F)c2)ccn1 Show InChI InChI=1S/C33H37F2N7O4/c1-40-16-18-41(19-17-40)24-9-14-42(15-10-24)32(45)39-29-21-26(8-13-36-29)46-25-6-7-28(27(35)20-25)38-31(44)33(11-12-33)30(43)37-23-4-2-22(34)3-5-23/h2-8,13,20-21,24H,9-12,14-19H2,1H3,(H,37,43)(H,38,44)(H,36,39,45) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Technical University of Munich
| Assay Description
Refer to Reaction Biology Corps. |
ACS Chem Biol 11: 3400-3411 (2016)
Article DOI: 10.1021/acschembio.6b00709
BindingDB Entry DOI: 10.7270/Q2TD9W5M |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data