 Found 186 hits with Last Name = 'qiao' and Initial = 'w'
Found 186 hits with Last Name = 'qiao' and Initial = 'w' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50293451

(1-Methyl-1-[2-(7-oxo-7H-1-aza-benzo[de]anthracen-9...)Show SMILES C[N+]1(CCC(=O)Nc2ccc-3c(c2)C(=O)c2cccc4ccnc-3c24)CCCCC1 Show InChI InChI=1S/C25H25N3O2/c1-28(13-3-2-4-14-28)15-11-22(29)27-18-8-9-19-21(16-18)25(30)20-7-5-6-17-10-12-26-24(19)23(17)20/h5-10,12,16H,2-4,11,13-15H2,1H3/p+1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE by LB plot |
Eur J Med Chem 44: 2523-32 (2009)
Article DOI: 10.1016/j.ejmech.2009.01.021
BindingDB Entry DOI: 10.7270/Q2Z89CFV |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Escherichia coli) | BDBM210929
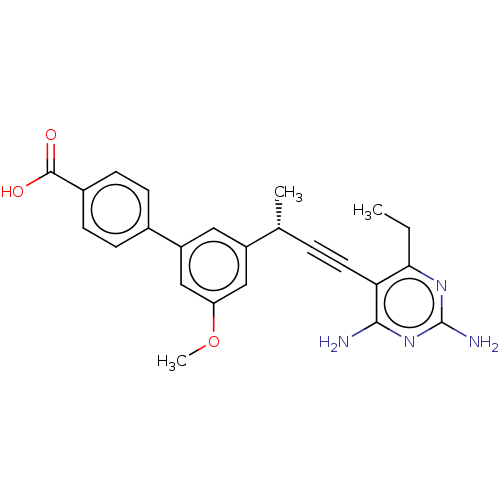
(UCP1172)Show SMILES CCc1nc(N)nc(N)c1C#C[C@@H](C)c1cc(OC)cc(c1)-c1ccc(cc1)C(O)=O |r| Show InChI InChI=1S/C24H24N4O3/c1-4-21-20(22(25)28-24(26)27-21)10-5-14(2)17-11-18(13-19(12-17)31-3)15-6-8-16(9-7-15)23(29)30/h6-9,11-14H,4H2,1-3H3,(H,29,30)(H4,25,26,27,28)/t14-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50230555

(CHEMBL5267078)Show SMILES [H][C@]12CCCC[C@@]1([H])c1cc(Cl)cc(C(=O)N[C@@]3([H])CN4CCC3CC4)c1O2 |wU:6.7,1.0,wD:17.18,(7.76,-5.43,;7.44,-3.92,;8.95,-4.25,;9.98,-3.08,;9.49,-1.63,;7.97,-1.31,;6.95,-2.47,;6.65,-.96,;5.43,-2.47,;4.41,-1.31,;2.9,-1.64,;1.87,-.49,;2.41,-3.08,;3.43,-4.24,;2.94,-5.69,;3.97,-6.85,;1.43,-5.99,;-.11,-5.92,;-.11,-7.46,;-1.02,-7.16,;-2.55,-7,;-3.16,-5.57,;-2.25,-4.34,;-.71,-4.52,;-.88,-6.04,;-2.4,-5.85,;4.95,-3.94,;6.2,-4.84,)| Show InChI InChI=1S/C20H25ClN2O2/c21-13-9-15-14-3-1-2-4-18(14)25-19(15)16(10-13)20(24)22-17-11-23-7-5-12(17)6-8-23/h9-10,12,14,17-18H,1-8,11H2,(H,22,24)/t14-,17-,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against beta-1 adrenergic receptor determined as pA2 in guinea pig atria |
J Med Chem 20: 430-9 (1977)
Article DOI: 10.1021/jm00213a022
BindingDB Entry DOI: 10.7270/Q2ZC85WV |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Staphylococcus aureus) | BDBM50608616
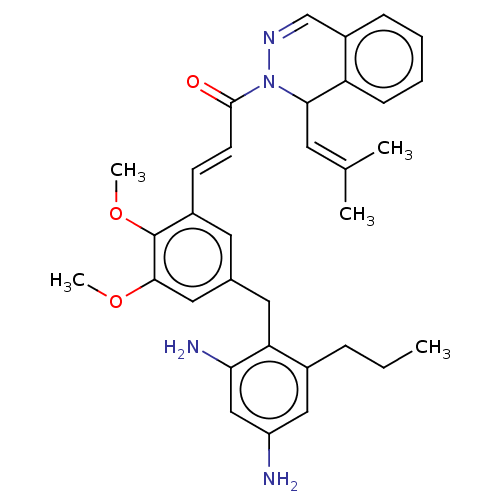
(CHEMBL5287382)Show SMILES [#6]-[#6]-[#6]-c1cc(-[#7])cc(-[#7])c1-[#6]-c1cc(-[#8]-[#6])c(-[#8]-[#6])c(\[#6]=[#6]\[#6](=O)-[#7]-2-[#7]=[#6]-c3ccccc3-[#6]-2\[#6]=[#6](\[#6])-[#6])c1 |c:27| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| UniChem
| | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Escherichia coli) | BDBM50608614
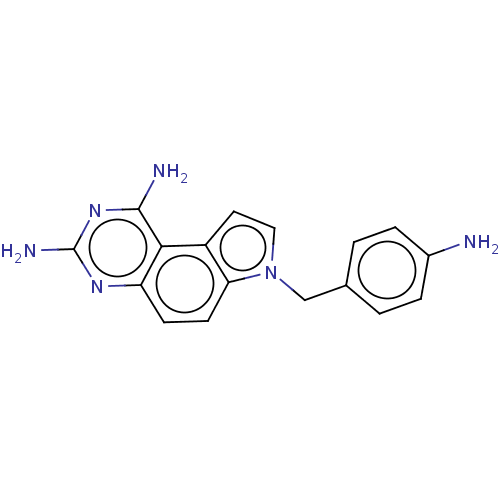
(CHEMBL1724189) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| UniChem
| | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50230554
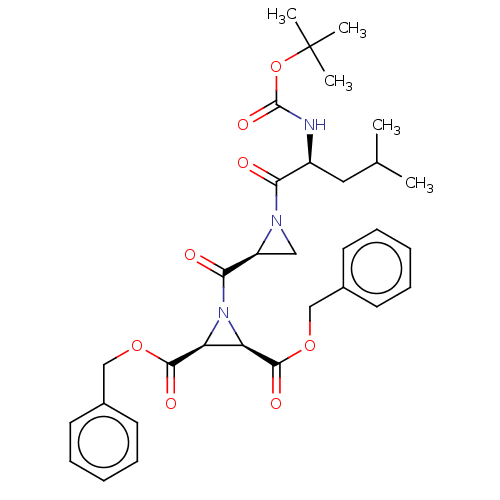
(CHEMBL5283523)Show InChI InChI=1S/C20H23ClN2O2/c1-23-10-4-5-14(23)8-9-22-20(24)17-12-13(21)11-16-15-6-2-3-7-18(15)25-19(16)17/h4-5,10-12,15,18H,2-3,6-9H2,1H3,(H,22,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 9.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against beta-1 adrenergic receptor determined as pA2 in guinea pig atria |
J Med Chem 20: 430-9 (1977)
Article DOI: 10.1021/jm00213a022
BindingDB Entry DOI: 10.7270/Q2ZC85WV |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50230554

(CHEMBL5283523)Show InChI InChI=1S/C20H23ClN2O2/c1-23-10-4-5-14(23)8-9-22-20(24)17-12-13(21)11-16-15-6-2-3-7-18(15)25-19(16)17/h4-5,10-12,15,18H,2-3,6-9H2,1H3,(H,22,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against beta-1 adrenergic receptor determined as pA2 in guinea pig atria |
J Med Chem 20: 430-9 (1977)
Article DOI: 10.1021/jm00213a022
BindingDB Entry DOI: 10.7270/Q2ZC85WV |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50293452
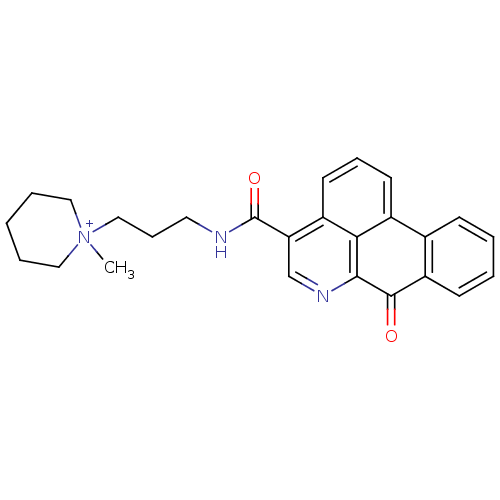
(1-methyl-1-(3-(7-oxo-7H-dibenzo[de,g]quinoline-4-c...)Show SMILES C[N+]1(CCCNC(=O)c2cnc3C(=O)c4ccccc4-c4cccc2c34)CCCCC1 Show InChI InChI=1S/C26H27N3O2/c1-29(14-5-2-6-15-29)16-8-13-27-26(31)22-17-28-24-23-19(11-7-12-20(22)23)18-9-3-4-10-21(18)25(24)30/h3-4,7,9-12,17H,2,5-6,8,13-16H2,1H3/p+1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE by LB plot |
Eur J Med Chem 44: 2523-32 (2009)
Article DOI: 10.1016/j.ejmech.2009.01.021
BindingDB Entry DOI: 10.7270/Q2Z89CFV |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50211247
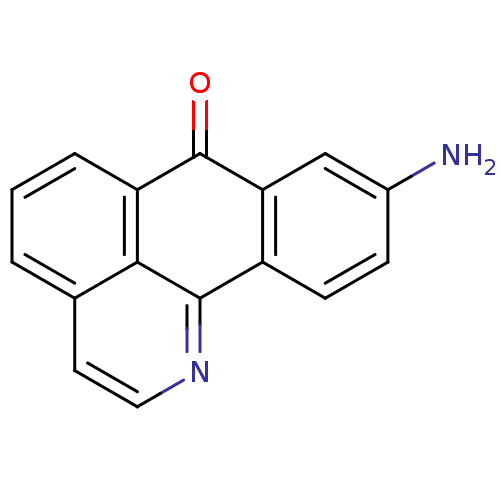
(9-Amino-1-azabenzanthrone | 9-amino-1-aza-benzo[de...)Show InChI InChI=1S/C16H10N2O/c17-10-4-5-11-13(8-10)16(19)12-3-1-2-9-6-7-18-15(11)14(9)12/h1-8H,17H2 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.86E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE by LB plot |
Eur J Med Chem 44: 2523-32 (2009)
Article DOI: 10.1016/j.ejmech.2009.01.021
BindingDB Entry DOI: 10.7270/Q2Z89CFV |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50293443
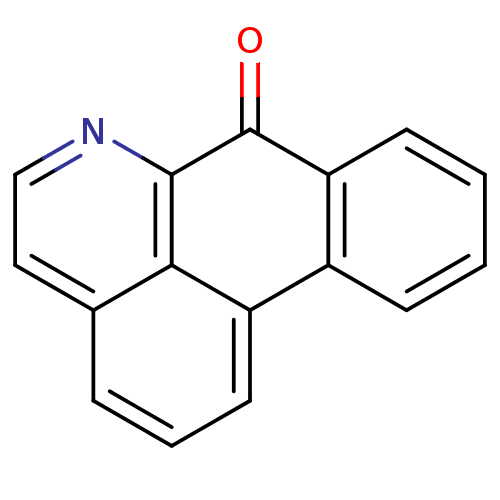
(7-Oxo-7H-dibenzo[de,g]quinoline | CHEMBL559502)Show InChI InChI=1S/C16H9NO/c18-16-13-6-2-1-5-11(13)12-7-3-4-10-8-9-17-15(16)14(10)12/h1-9H | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.46E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE by LB plot |
Eur J Med Chem 44: 2523-32 (2009)
Article DOI: 10.1016/j.ejmech.2009.01.021
BindingDB Entry DOI: 10.7270/Q2Z89CFV |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Escherichia coli) | BDBM50108046
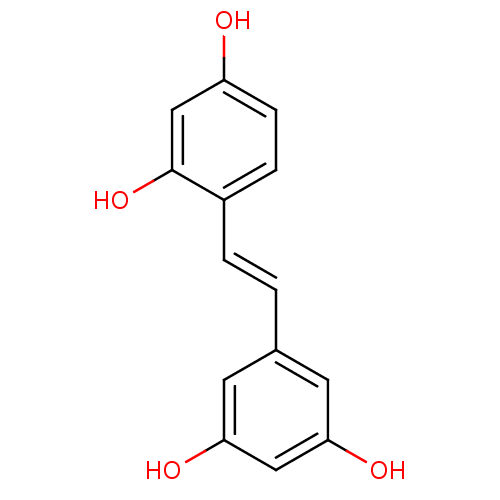
((oxyresveratrol)4-[(E)-2-(3,5-dihydroxyphenyl)viny...)Show InChI InChI=1S/C14H12O4/c15-11-4-3-10(14(18)8-11)2-1-9-5-12(16)7-13(17)6-9/h1-8,15-18H/b2-1+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| | 3.15E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Escherichia coli) | BDBM50608615
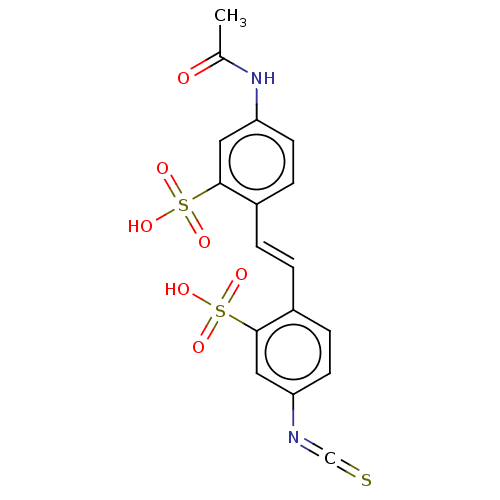
(CHEBI:34383 | CHEMBL1162149)Show SMILES CC(=O)Nc1ccc(\C=C\c2ccc(cc2S(O)(=O)=O)N=C=S)c(c1)S(O)(=O)=O | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| UniChem
| | 3.59E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50014323

(2-PHENYL-4H-BENZO[H]CHROMEN-4-ONE | 2-Phenyl-benzo...)Show InChI InChI=1S/C19H12O2/c20-17-12-18(14-7-2-1-3-8-14)21-19-15-9-5-4-6-13(15)10-11-16(17)19/h1-12H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02071
BindingDB Entry DOI: 10.7270/Q2833X3S |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50230550

(CHEMBL5268799)Show SMILES [H][C@@]12CCC[C@@]([H])(C1)c1cc(Cl)cc(C(=O)N[C@]3([H])CN4CCC3CC4)c1O2 |wU:17.18,wD:5.5,1.0,(7.89,-6.13,;7.49,-4.65,;8.97,-4.24,;9.49,-2.71,;8.41,-1.62,;6.93,-2.03,;6.53,-.54,;7.97,-3.18,;5.43,-2.35,;4.41,-1.2,;2.9,-1.52,;1.87,-.37,;2.41,-2.97,;3.43,-4.13,;2.94,-5.58,;3.97,-6.74,;1.43,-5.88,;-.11,-5.81,;-.11,-7.35,;-1.03,-7.05,;-2.56,-6.88,;-2.4,-5.74,;-.88,-5.93,;-.71,-4.41,;-2.25,-4.23,;-3.16,-5.46,;4.95,-3.82,;5.97,-4.96,)| Show InChI InChI=1S/C20H25ClN2O2/c21-14-9-16-13-2-1-3-15(8-13)25-19(16)17(10-14)20(24)22-18-11-23-6-4-12(18)5-7-23/h9-10,12-13,15,18H,1-8,11H2,(H,22,24)/t13-,15+,18+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against Dihydrofolate reductase of rat liver |
J Med Chem 20: 430-9 (1977)
Article DOI: 10.1021/jm00213a022
BindingDB Entry DOI: 10.7270/Q2ZC85WV |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50293451

(1-Methyl-1-[2-(7-oxo-7H-1-aza-benzo[de]anthracen-9...)Show SMILES C[N+]1(CCC(=O)Nc2ccc-3c(c2)C(=O)c2cccc4ccnc-3c24)CCCCC1 Show InChI InChI=1S/C25H25N3O2/c1-28(13-3-2-4-14-28)15-11-22(29)27-18-8-9-19-21(16-18)25(30)20-7-5-6-17-10-12-26-24(19)23(17)20/h5-10,12,16H,2-4,11,13-15H2,1H3/p+1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE by Ellman's method |
Eur J Med Chem 44: 2523-32 (2009)
Article DOI: 10.1016/j.ejmech.2009.01.021
BindingDB Entry DOI: 10.7270/Q2Z89CFV |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50230550

(CHEMBL5268799)Show SMILES [H][C@@]12CCC[C@@]([H])(C1)c1cc(Cl)cc(C(=O)N[C@]3([H])CN4CCC3CC4)c1O2 |wU:17.18,wD:5.5,1.0,(7.89,-6.13,;7.49,-4.65,;8.97,-4.24,;9.49,-2.71,;8.41,-1.62,;6.93,-2.03,;6.53,-.54,;7.97,-3.18,;5.43,-2.35,;4.41,-1.2,;2.9,-1.52,;1.87,-.37,;2.41,-2.97,;3.43,-4.13,;2.94,-5.58,;3.97,-6.74,;1.43,-5.88,;-.11,-5.81,;-.11,-7.35,;-1.03,-7.05,;-2.56,-6.88,;-2.4,-5.74,;-.88,-5.93,;-.71,-4.41,;-2.25,-4.23,;-3.16,-5.46,;4.95,-3.82,;5.97,-4.96,)| Show InChI InChI=1S/C20H25ClN2O2/c21-14-9-16-13-2-1-3-15(8-13)25-19(16)17(10-14)20(24)22-18-11-23-6-4-12(18)5-7-23/h9-10,12-13,15,18H,1-8,11H2,(H,22,24)/t13-,15+,18+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.670 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonistic activity against beta-1 adrenergic receptor of isolated guinea pig atria |
J Med Chem 20: 430-9 (1977)
Article DOI: 10.1021/jm00213a022
BindingDB Entry DOI: 10.7270/Q2ZC85WV |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Mycobacterium tuberculosis) | BDBM50608610
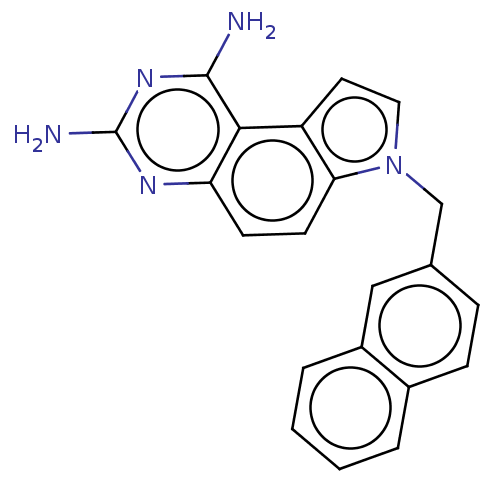
(CHEMBL5279457) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 0.960 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50166256

(5-[4-(4-Bromo-phenoxy)-phenyl]-5-(4-isopropyl-pipe...)Show SMILES CC(C)N1CCN(CC1)C1(C(=O)NC(=O)NC1=O)c1ccc(Oc2ccc(Br)cc2)cc1 Show InChI InChI=1S/C23H25BrN4O4/c1-15(2)27-11-13-28(14-12-27)23(20(29)25-22(31)26-21(23)30)16-3-7-18(8-4-16)32-19-9-5-17(24)6-10-19/h3-10,15H,11-14H2,1-2H3,(H2,25,26,29,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against beta-1 adrenergic receptor determined as pA2 in guinea pig atria |
J Med Chem 20: 430-9 (1977)
Article DOI: 10.1021/jm00213a022
BindingDB Entry DOI: 10.7270/Q2ZC85WV |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50069985
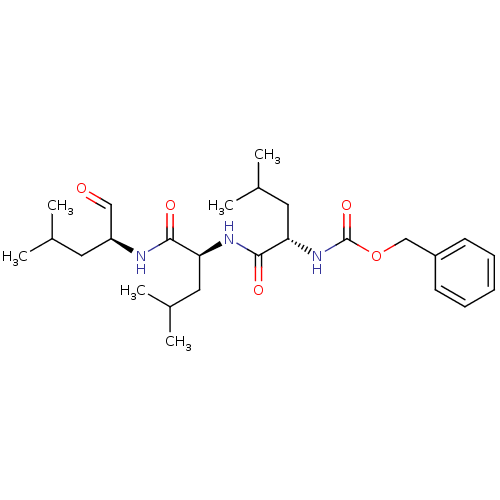
((S)-4-methyl-2-(3-phenyl-propionylamino)-pentanoic...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)NC(=O)OCc1ccccc1)C=O |r| Show InChI InChI=1S/C26H41N3O5/c1-17(2)12-21(15-30)27-24(31)22(13-18(3)4)28-25(32)23(14-19(5)6)29-26(33)34-16-20-10-8-7-9-11-20/h7-11,15,17-19,21-23H,12-14,16H2,1-6H3,(H,27,31)(H,28,32)(H,29,33)/t21-,22-,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human platelet Thromboxane synthetase |
J Med Chem 20: 430-9 (1977)
Article DOI: 10.1021/jm00213a022
BindingDB Entry DOI: 10.7270/Q2ZC85WV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50329819
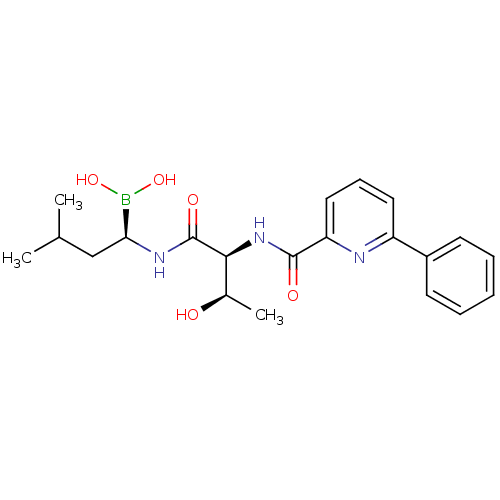
((R)-1-((2S,3R)-3-hydroxy-2-(6-phenylpicolinamido)b...)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](NC(=O)c1cccc(n1)-c1ccccc1)[C@@H](C)O)B(O)O Show InChI InChI=1S/C21H28BN3O5/c1-13(2)12-18(22(29)30)24-21(28)19(14(3)26)25-20(27)17-11-7-10-16(23-17)15-8-5-4-6-9-15/h4-11,13-14,18-19,26,29-30H,12H2,1-3H3,(H,24,28)(H,25,27)/t14-,18+,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonistic activity against beta-1 adrenergic receptor of isolated guinea pig atria |
J Med Chem 20: 430-9 (1977)
Article DOI: 10.1021/jm00213a022
BindingDB Entry DOI: 10.7270/Q2ZC85WV |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50069989

((R)-3-methyl-1-((S)-3-phenyl-2-(pyrazine-2-carboxa...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)c1cnccn1)B(O)O |r| Show InChI InChI=1S/C19H25BN4O4/c1-13(2)10-17(20(27)28)24-18(25)15(11-14-6-4-3-5-7-14)23-19(26)16-12-21-8-9-22-16/h3-9,12-13,15,17,27-28H,10-11H2,1-2H3,(H,23,26)(H,24,25)/t15-,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of dihydropteroate synthase from Escherichia coli. |
J Med Chem 20: 430-9 (1977)
Article DOI: 10.1021/jm00213a022
BindingDB Entry DOI: 10.7270/Q2ZC85WV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50230543
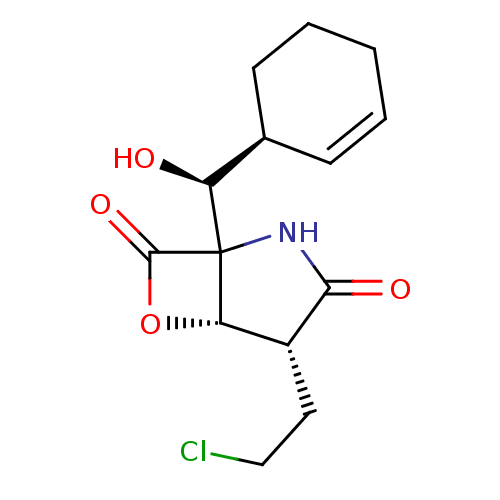
(CHEMBL5283576)Show InChI InChI=1S/C19H27ClN2O2/c1-3-22(4-2)10-9-21-19(23)16-12-13(20)11-15-14-7-5-6-8-17(14)24-18(15)16/h11-12,14,17H,3-10H2,1-2H3,(H,21,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonistic activity against beta-1 adrenergic receptor of isolated guinea pig atria |
J Med Chem 20: 430-9 (1977)
Article DOI: 10.1021/jm00213a022
BindingDB Entry DOI: 10.7270/Q2ZC85WV |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50293446

(CHEMBL556249 | Diethyl-methyl-[2-(7-oxo-7H-1-aza-b...)Show SMILES CC[N+](C)(CC)CCC(=O)Nc1ccc-2c(c1)C(=O)c1cccc3ccnc-2c13 Show InChI InChI=1S/C24H25N3O2/c1-4-27(3,5-2)14-12-21(28)26-17-9-10-18-20(15-17)24(29)19-8-6-7-16-11-13-25-23(18)22(16)19/h6-11,13,15H,4-5,12,14H2,1-3H3/p+1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.62 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE by Ellman's method |
Eur J Med Chem 44: 2523-32 (2009)
Article DOI: 10.1016/j.ejmech.2009.01.021
BindingDB Entry DOI: 10.7270/Q2Z89CFV |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Staphylococcus aureus) | BDBM50335530
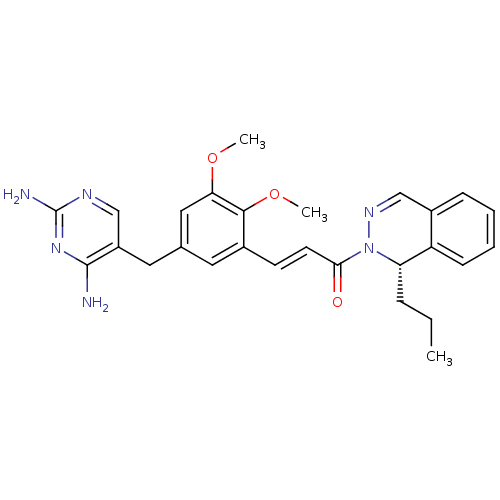
((S)-3-(5-((2,4-diaminopyrimidin-5-yl)methyl)-2,3-d...)Show SMILES CCC[C@@H]1N(N=Cc2ccccc12)C(=O)\C=C\c1cc(Cc2cnc(N)nc2N)cc(OC)c1OC |r,c:5| Show InChI InChI=1S/C27H30N6O3/c1-4-7-22-21-9-6-5-8-19(21)16-31-33(22)24(34)11-10-18-12-17(14-23(35-2)25(18)36-3)13-20-15-30-27(29)32-26(20)28/h5-6,8-12,14-16,22H,4,7,13H2,1-3H3,(H4,28,29,30,32)/b11-10+/t22-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50398607
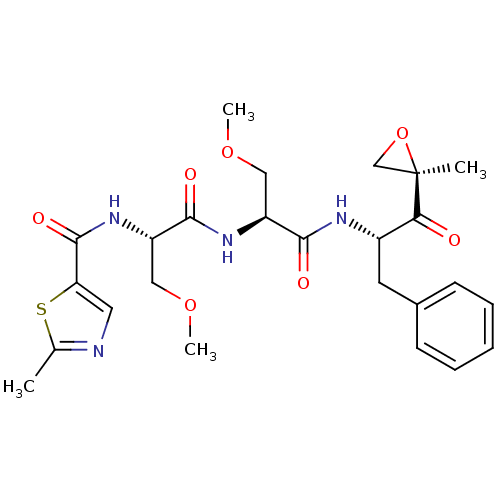
(ONX 0912 | OPROZOMIB | US10640533, Identification ...)Show SMILES COC[C@H](NC(=O)[C@H](COC)NC(=O)c1cnc(C)s1)C(=O)N[C@@H](Cc1ccccc1)C(=O)[C@@]1(C)CO1 |r| Show InChI InChI=1S/C25H32N4O7S/c1-15-26-11-20(37-15)24(33)29-19(13-35-4)23(32)28-18(12-34-3)22(31)27-17(21(30)25(2)14-36-25)10-16-8-6-5-7-9-16/h5-9,11,17-19H,10,12-14H2,1-4H3,(H,27,31)(H,28,32)(H,29,33)/t17-,18-,19-,25+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonistic activity against beta-1 adrenergic receptor of isolated guinea pig atria |
J Med Chem 20: 430-9 (1977)
Article DOI: 10.1021/jm00213a022
BindingDB Entry DOI: 10.7270/Q2ZC85WV |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50093572
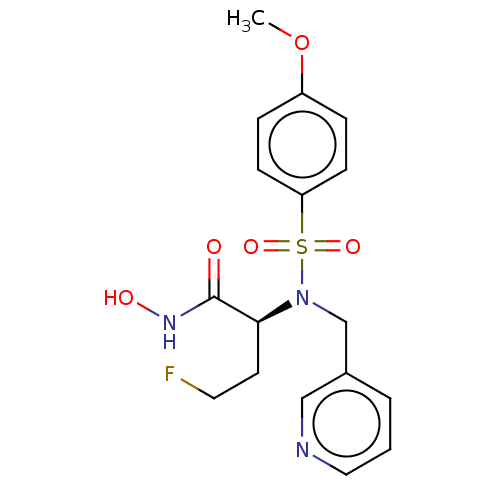
(CHEMBL3585766)Show SMILES COc1ccc(cc1)S(=O)(=O)N(Cc1cccnc1)[C@@H](CCF)C(=O)NO |r| Show InChI InChI=1S/C17H20FN3O5S/c1-26-14-4-6-15(7-5-14)27(24,25)21(12-13-3-2-10-19-11-13)16(8-9-18)17(22)20-23/h2-7,10-11,16,23H,8-9,12H2,1H3,(H,20,22)/t16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against Dihydrofolate reductase of rat liver |
J Med Chem 20: 430-9 (1977)
Article DOI: 10.1021/jm00213a022
BindingDB Entry DOI: 10.7270/Q2ZC85WV |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50398609
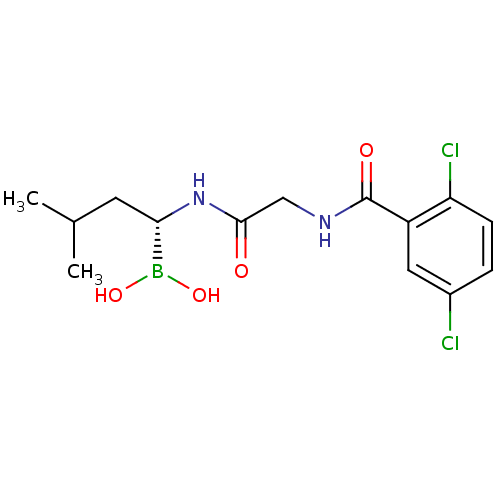
(CHEMBL2141296 | IXAZOMIB CITRATE | Ixazomib | MLN2...)Show SMILES CC(C)C[C@H](NC(=O)CNC(=O)c1cc(Cl)ccc1Cl)B(O)O Show InChI InChI=1S/C14H19BCl2N2O4/c1-8(2)5-12(15(22)23)19-13(20)7-18-14(21)10-6-9(16)3-4-11(10)17/h3-4,6,8,12,22-23H,5,7H2,1-2H3,(H,18,21)(H,19,20)/t12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonistic activity against beta-1 adrenergic receptor of isolated guinea pig atria |
J Med Chem 20: 430-9 (1977)
Article DOI: 10.1021/jm00213a022
BindingDB Entry DOI: 10.7270/Q2ZC85WV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50230552
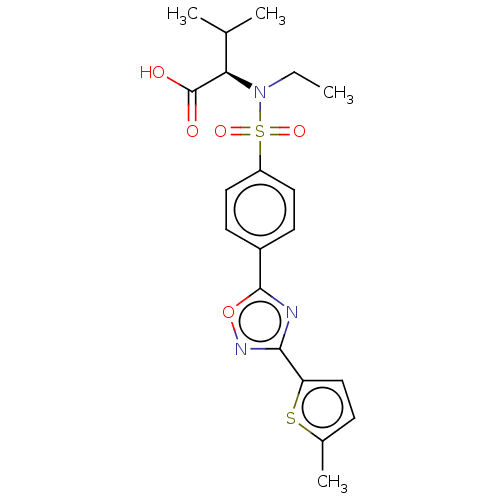
(CHEMBL5267731)Show InChI InChI=1S/C20H27ClN2O2/c1-23-10-4-5-14(23)8-9-22-20(24)17-12-13(21)11-16-15-6-2-3-7-18(15)25-19(16)17/h11-12,14-15,18H,2-10H2,1H3,(H,22,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against Dihydrofolate reductase of rat liver |
J Med Chem 20: 430-9 (1977)
Article DOI: 10.1021/jm00213a022
BindingDB Entry DOI: 10.7270/Q2ZC85WV |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50100089
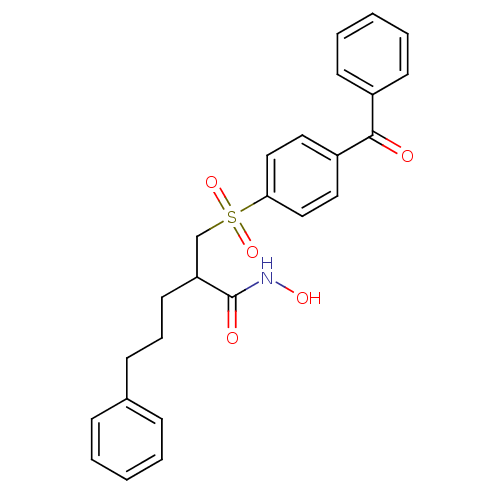
(2-(4-Benzoyl-benzenesulfonylmethyl)-5-phenyl-penta...)Show SMILES ONC(=O)C(CCCc1ccccc1)CS(=O)(=O)c1ccc(cc1)C(=O)c1ccccc1 Show InChI InChI=1S/C25H25NO5S/c27-24(20-11-5-2-6-12-20)21-14-16-23(17-15-21)32(30,31)18-22(25(28)26-29)13-7-10-19-8-3-1-4-9-19/h1-6,8-9,11-12,14-17,22,29H,7,10,13,18H2,(H,26,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against Dihydrofolate reductase of rat liver |
J Med Chem 20: 430-9 (1977)
Article DOI: 10.1021/jm00213a022
BindingDB Entry DOI: 10.7270/Q2ZC85WV |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50230551

(CHEMBL5281153)Show InChI InChI=1S/C19H25ClN2O3/c20-13-11-15-14-3-1-2-4-17(14)25-18(15)16(12-13)19(23)21-5-6-22-7-9-24-10-8-22/h11-12,14,17H,1-10H2,(H,21,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against beta-2 adrenergic receptor determined as pA2 in guinea pig trachea |
J Med Chem 20: 430-9 (1977)
Article DOI: 10.1021/jm00213a022
BindingDB Entry DOI: 10.7270/Q2ZC85WV |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50230551

(CHEMBL5281153)Show InChI InChI=1S/C19H25ClN2O3/c20-13-11-15-14-3-1-2-4-17(14)25-18(15)16(12-13)19(23)21-5-6-22-7-9-24-10-8-22/h11-12,14,17H,1-10H2,(H,21,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against Dihydrofolate reductase of rat liver |
J Med Chem 20: 430-9 (1977)
Article DOI: 10.1021/jm00213a022
BindingDB Entry DOI: 10.7270/Q2ZC85WV |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50211244

(CHEMBL395926 | Trimethyl-[2-(7-oxo-7H-1-aza-benzo[...)Show SMILES C[N+](C)(C)CCC(=O)Nc1ccc-2c(c1)C(=O)c1cccc3ccnc-2c13 Show InChI InChI=1S/C22H21N3O2/c1-25(2,3)12-10-19(26)24-15-7-8-16-18(13-15)22(27)17-6-4-5-14-9-11-23-21(16)20(14)17/h4-9,11,13H,10,12H2,1-3H3/p+1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.81 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE by Ellman's method |
Eur J Med Chem 44: 2523-32 (2009)
Article DOI: 10.1016/j.ejmech.2009.01.021
BindingDB Entry DOI: 10.7270/Q2Z89CFV |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50095487

(CHEMBL8796 | Cyclohexanecarboxylic acid (1-cyano-a...)Show InChI InChI=1S/C12H19N3O/c13-9-15-7-10(8-15)6-14-12(16)11-4-2-1-3-5-11/h10-11H,1-8H2,(H,14,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against beta-1 adrenergic receptor determined as pA2 in guinea pig atria |
J Med Chem 20: 430-9 (1977)
Article DOI: 10.1021/jm00213a022
BindingDB Entry DOI: 10.7270/Q2ZC85WV |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50100089

(2-(4-Benzoyl-benzenesulfonylmethyl)-5-phenyl-penta...)Show SMILES ONC(=O)C(CCCc1ccccc1)CS(=O)(=O)c1ccc(cc1)C(=O)c1ccccc1 Show InChI InChI=1S/C25H25NO5S/c27-24(20-11-5-2-6-12-20)21-14-16-23(17-15-21)32(30,31)18-22(25(28)26-29)13-7-10-19-8-3-1-4-9-19/h1-6,8-9,11-12,14-17,22,29H,7,10,13,18H2,(H,26,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Angiotensin I converting enzyme |
J Med Chem 20: 430-9 (1977)
Article DOI: 10.1021/jm00213a022
BindingDB Entry DOI: 10.7270/Q2ZC85WV |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50095487

(CHEMBL8796 | Cyclohexanecarboxylic acid (1-cyano-a...)Show InChI InChI=1S/C12H19N3O/c13-9-15-7-10(8-15)6-14-12(16)11-4-2-1-3-5-11/h10-11H,1-8H2,(H,14,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against beta-1 adrenergic receptor determined as pA2 in guinea pig atria |
J Med Chem 20: 430-9 (1977)
Article DOI: 10.1021/jm00213a022
BindingDB Entry DOI: 10.7270/Q2ZC85WV |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Mycobacterium tuberculosis) | BDBM50608612

(CHEMBL1904209)Show SMILES CC(O)=O.Nc1nc(N)c2c3ccn(Cc4cccc(c4)[N+]([O-])=O)c3ccc2n1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50277889
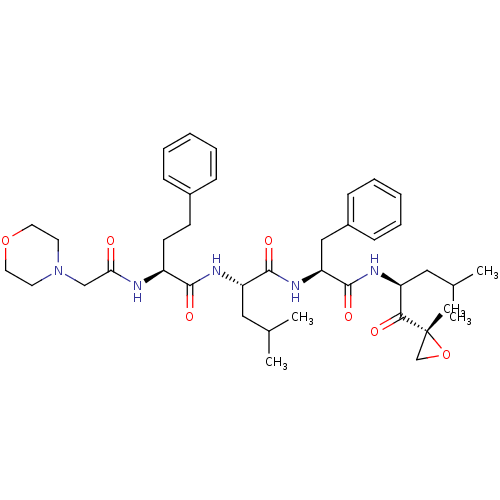
(CARFILZOMIB | CHEMBL451887)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCc1ccccc1)NC(=O)CN1CCOCC1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CC(C)C)C(=O)[C@@]1(C)CO1 |r| Show InChI InChI=1S/C40H57N5O7/c1-27(2)22-32(36(47)40(5)26-52-40)42-39(50)34(24-30-14-10-7-11-15-30)44-38(49)33(23-28(3)4)43-37(48)31(17-16-29-12-8-6-9-13-29)41-35(46)25-45-18-20-51-21-19-45/h6-15,27-28,31-34H,16-26H2,1-5H3,(H,41,46)(H,42,50)(H,43,48)(H,44,49)/t31-,32-,33-,34-,40+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human platelet Thromboxane synthetase |
J Med Chem 20: 430-9 (1977)
Article DOI: 10.1021/jm00213a022
BindingDB Entry DOI: 10.7270/Q2ZC85WV |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-2
(Homo sapiens (Human)) | BDBM50230543

(CHEMBL5283576)Show InChI InChI=1S/C19H27ClN2O2/c1-3-22(4-2)10-9-21-19(23)16-12-13(20)11-15-14-7-5-6-8-17(14)24-18(15)16/h11-12,14,17H,3-10H2,1-2H3,(H,21,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human platelet Thromboxane synthetase |
J Med Chem 20: 430-9 (1977)
Article DOI: 10.1021/jm00213a022
BindingDB Entry DOI: 10.7270/Q2ZC85WV |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM8610

(1-[4-(4-{[(2R,4S)-2-(2,4-dichlorophenyl)-2-(1H-imi...)Show SMILES [H][C@]1(COc2ccc(cc2)N2CCN(CC2)C(C)=O)CO[C@@](Cn2ccnc2)(O1)c1ccc(Cl)cc1Cl |r| Show InChI InChI=1S/C26H28Cl2N4O4/c1-19(33)31-10-12-32(13-11-31)21-3-5-22(6-4-21)34-15-23-16-35-26(36-23,17-30-9-8-29-18-30)24-7-2-20(27)14-25(24)28/h2-9,14,18,23H,10-13,15-17H2,1H3/t23-,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02071
BindingDB Entry DOI: 10.7270/Q2833X3S |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Staphylococcus aureus) | BDBM50536186

(CHEMBL4483572)Show SMILES CCc1nc(N)nc(N)c1C#C[C@H](C)c1cc(OC)cc(c1)-c1ccncc1 |r| Show InChI InChI=1S/C22H23N5O/c1-4-20-19(21(23)27-22(24)26-20)6-5-14(2)16-11-17(13-18(12-16)28-3)15-7-9-25-10-8-15/h7-14H,4H2,1-3H3,(H4,23,24,26,27)/t14-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Staphylococcus aureus) | BDBM50608617
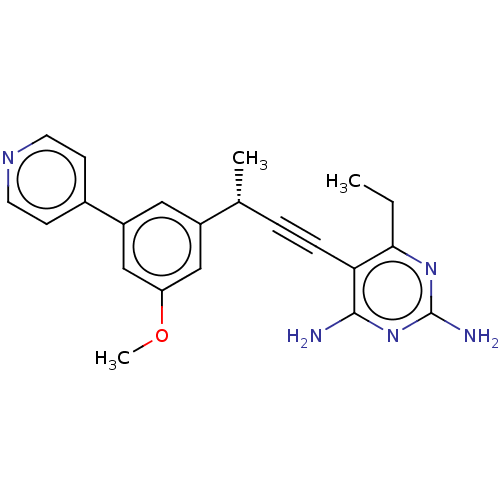
(CHEMBL5284400)Show SMILES CCc1nc(N)nc(N)c1C#C[C@@H](C)c1cc(OC)cc(c1)-c1ccncc1 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PDB
UniChem
| PDB
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50121975

((6-Methoxy-quinolin-4-yl)-(5-vinyl-1-aza-bicyclo[2...)Show SMILES COc1ccc2nccc([C@H](O)[C@H]3C[C@@H]4CCN3C[C@@H]4C=C)c2c1 |r,THB:20:19:12.13:16.15,10:12:18.19:16.15| Show InChI InChI=1S/C20H24N2O2/c1-3-13-12-22-9-7-14(13)10-19(22)20(23)16-6-8-21-18-5-4-15(24-2)11-17(16)18/h3-6,8,11,13-14,19-20,23H,1,7,9-10,12H2,2H3/t13-,14-,19+,20-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02071
BindingDB Entry DOI: 10.7270/Q2833X3S |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dihydrofolate reductase
(Mycobacterium tuberculosis) | BDBM50608609
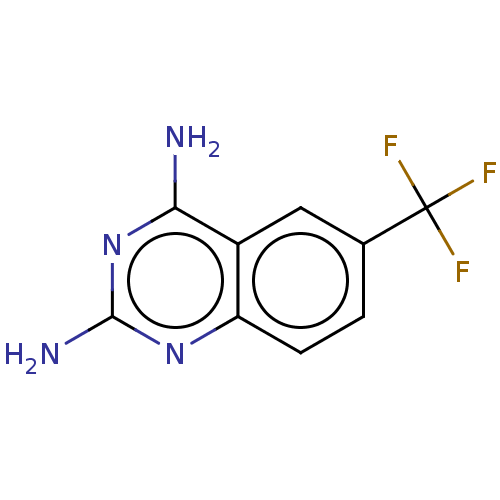
(CHEMBL586115 | TCMDC-124259) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-1
(Homo sapiens (Human)) | BDBM50069989

((R)-3-methyl-1-((S)-3-phenyl-2-(pyrazine-2-carboxa...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)c1cnccn1)B(O)O |r| Show InChI InChI=1S/C19H25BN4O4/c1-13(2)10-17(20(27)28)24-18(25)15(11-14-6-4-3-5-7-14)23-19(26)16-12-21-8-9-22-16/h3-9,12-13,15,17,27-28H,10-11H2,1-2H3,(H,23,26)(H,24,25)/t15-,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Evaluated for norepinephrine as agonist. |
J Med Chem 20: 430-9 (1977)
Article DOI: 10.1021/jm00213a022
BindingDB Entry DOI: 10.7270/Q2ZC85WV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50166256

(5-[4-(4-Bromo-phenoxy)-phenyl]-5-(4-isopropyl-pipe...)Show SMILES CC(C)N1CCN(CC1)C1(C(=O)NC(=O)NC1=O)c1ccc(Oc2ccc(Br)cc2)cc1 Show InChI InChI=1S/C23H25BrN4O4/c1-15(2)27-11-13-28(14-12-27)23(20(29)25-22(31)26-21(23)30)16-3-7-18(8-4-16)32-19-9-5-17(24)6-10-19/h3-10,15H,11-14H2,1-2H3,(H2,25,26,29,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against Dihydrofolate reductase of rat liver |
J Med Chem 20: 430-9 (1977)
Article DOI: 10.1021/jm00213a022
BindingDB Entry DOI: 10.7270/Q2ZC85WV |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50264809

(2-((4-phenoxyphenylsulfonyl)methyl)thiirane | CHEM...)Show InChI InChI=1S/C15H14O3S2/c16-20(17,11-14-10-19-14)15-8-6-13(7-9-15)18-12-4-2-1-3-5-12/h1-9,14H,10-11H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against Dihydrofolate reductase of chicken liver |
J Med Chem 20: 430-9 (1977)
Article DOI: 10.1021/jm00213a022
BindingDB Entry DOI: 10.7270/Q2ZC85WV |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-1
(Homo sapiens (Human)) | BDBM50398609

(CHEMBL2141296 | IXAZOMIB CITRATE | Ixazomib | MLN2...)Show SMILES CC(C)C[C@H](NC(=O)CNC(=O)c1cc(Cl)ccc1Cl)B(O)O Show InChI InChI=1S/C14H19BCl2N2O4/c1-8(2)5-12(15(22)23)19-13(20)7-18-14(21)10-6-9(16)3-4-11(10)17/h3-4,6,8,12,22-23H,5,7H2,1-2H3,(H,18,21)(H,19,20)/t12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro relative blocking action (pA2) of the post-synaptic Alpha-1 adrenergic receptor from rat and rabbit aorta |
J Med Chem 20: 430-9 (1977)
Article DOI: 10.1021/jm00213a022
BindingDB Entry DOI: 10.7270/Q2ZC85WV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Proteasome subunit beta type-1
(Homo sapiens (Human)) | BDBM50230543

(CHEMBL5283576)Show InChI InChI=1S/C19H27ClN2O2/c1-3-22(4-2)10-9-21-19(23)16-12-13(20)11-15-14-7-5-6-8-17(14)24-18(15)16/h11-12,14,17H,3-10H2,1-2H3,(H,21,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Evaluated for norepinephrine as agonist. |
J Med Chem 20: 430-9 (1977)
Article DOI: 10.1021/jm00213a022
BindingDB Entry DOI: 10.7270/Q2ZC85WV |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50093572

(CHEMBL3585766)Show SMILES COc1ccc(cc1)S(=O)(=O)N(Cc1cccnc1)[C@@H](CCF)C(=O)NO |r| Show InChI InChI=1S/C17H20FN3O5S/c1-26-14-4-6-15(7-5-14)27(24,25)21(12-13-3-2-10-19-11-13)16(8-9-18)17(22)20-23/h2-7,10-11,16,23H,8-9,12H2,1H3,(H,20,22)/t16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonistic activity against beta-1 adrenergic receptor of isolated guinea pig atria |
J Med Chem 20: 430-9 (1977)
Article DOI: 10.1021/jm00213a022
BindingDB Entry DOI: 10.7270/Q2ZC85WV |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50230552

(CHEMBL5267731)Show InChI InChI=1S/C20H27ClN2O2/c1-23-10-4-5-14(23)8-9-22-20(24)17-12-13(21)11-16-15-6-2-3-7-18(15)25-19(16)17/h11-12,14-15,18H,2-10H2,1H3,(H,22,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against beta-1 adrenergic receptor determined as pA2 in guinea pig atria |
J Med Chem 20: 430-9 (1977)
Article DOI: 10.1021/jm00213a022
BindingDB Entry DOI: 10.7270/Q2ZC85WV |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data