 Found 289 hits with Last Name = 'quartieri' and Initial = 'f'
Found 289 hits with Last Name = 'quartieri' and Initial = 'f' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM31541

(pyrazolo[4,3-h]quinazoline-3-carboxamide, 24)Show SMILES CN1CCN(CC1)c1cccc(Nc2ncc3CCc4c(nn(C)c4-c3n2)C(N)=O)c1 Show InChI InChI=1S/C22H26N8O/c1-28-8-10-30(11-9-28)16-5-3-4-15(12-16)25-22-24-13-14-6-7-17-19(21(23)31)27-29(2)20(17)18(14)26-22/h3-5,12-13H,6-11H2,1-2H3,(H2,23,31)(H,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.4 | 23 |
Nerviano Medical Sciences Srl
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzymes and substrates, followed by quantitation of the phosphorylat... |
J Med Chem 52: 5152-63 (2009)
Article DOI: 10.1021/jm9006559
BindingDB Entry DOI: 10.7270/Q2JH3JJP |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM31532
(pyrazolo[4,3-h]quinazoline-3-carboxamide, 1)Show InChI InChI=1S/C17H16N6O/c1-23-15-12(14(22-23)16(18)24)8-7-10-9-19-17(21-13(10)15)20-11-5-3-2-4-6-11/h2-6,9H,7-8H2,1H3,(H2,18,24)(H,19,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.4 | 23 |
Nerviano Medical Sciences Srl
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzymes and substrates, followed by quantitation of the phosphorylat... |
J Med Chem 52: 5152-63 (2009)
Article DOI: 10.1021/jm9006559
BindingDB Entry DOI: 10.7270/Q2JH3JJP |
More data for this
Ligand-Target Pair | |
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM31539
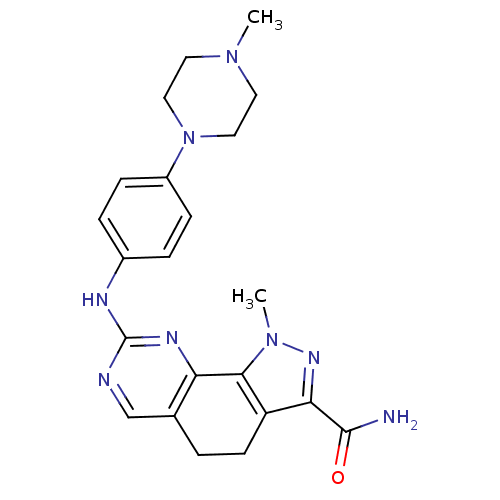
(pyrazolo[4,3-h]quinazoline-3-carboxamide, 22)Show SMILES CN1CCN(CC1)c1ccc(Nc2ncc3CCc4c(nn(C)c4-c3n2)C(N)=O)cc1 Show InChI InChI=1S/C22H26N8O/c1-28-9-11-30(12-10-28)16-6-4-15(5-7-16)25-22-24-13-14-3-8-17-19(21(23)31)27-29(2)20(17)18(14)26-22/h4-7,13H,3,8-12H2,1-2H3,(H2,23,31)(H,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.4 | 23 |
Nerviano Medical Sciences Srl
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzymes and substrates, followed by quantitation of the phosphorylat... |
J Med Chem 52: 5152-63 (2009)
Article DOI: 10.1021/jm9006559
BindingDB Entry DOI: 10.7270/Q2JH3JJP |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM31532

(pyrazolo[4,3-h]quinazoline-3-carboxamide, 1)Show InChI InChI=1S/C17H16N6O/c1-23-15-12(14(22-23)16(18)24)8-7-10-9-19-17(21-13(10)15)20-11-5-3-2-4-6-11/h2-6,9H,7-8H2,1H3,(H2,18,24)(H,19,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences srl
Curated by ChEMBL
| Assay Description
Inhibition of CDK2/Cyclin A assessed as [33P]-gamma-ATP incorporation into substrate by gamma counting |
Bioorg Med Chem Lett 21: 4507-11 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.122
BindingDB Entry DOI: 10.7270/Q2R211RS |
More data for this
Ligand-Target Pair | |
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50318085
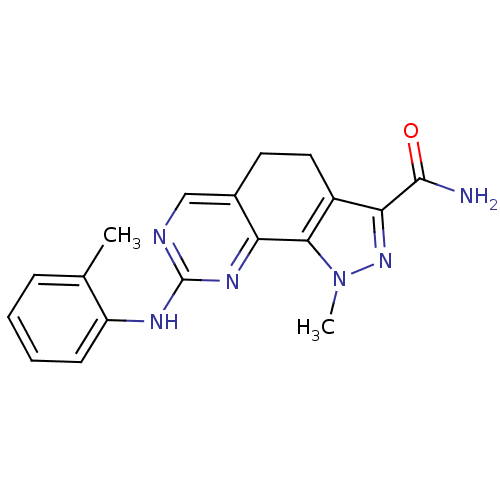
(1-Methyl-8-[(2-methylphenyl)amino]-4,5-dihydro-1H-...)Show InChI InChI=1S/C18H18N6O/c1-10-5-3-4-6-13(10)21-18-20-9-11-7-8-12-15(17(19)25)23-24(2)16(12)14(11)22-18/h3-6,9H,7-8H2,1-2H3,(H2,19,25)(H,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences srl
Curated by ChEMBL
| Assay Description
Inhibition of CDK2/Cyclin A assessed as [33P]-gamma-ATP incorporation into substrate by gamma counting |
Bioorg Med Chem Lett 21: 4507-11 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.122
BindingDB Entry DOI: 10.7270/Q2R211RS |
More data for this
Ligand-Target Pair | |
Choline kinase alpha
(Homo sapiens (Human)) | BDBM50579781

(CHEMBL5094847)Show SMILES CC1(C)Cc2nc(NC(=O)[C@H]3CC[C@H](CC3)n3cnc4c(N)nc(Cl)nc34)sc2C(C)(C)N1 |r,wU:13.16,10.9,(21.59,-25.94,;22.93,-25.18,;21.6,-24.4,;23.25,-26.69,;24.71,-27.16,;25.32,-28.56,;26.86,-28.42,;27.89,-29.57,;29.4,-29.25,;30.42,-30.4,;29.88,-27.79,;28.86,-26.64,;29.34,-25.18,;30.84,-24.87,;31.88,-26.01,;31.39,-27.48,;31.32,-23.41,;30.42,-22.16,;31.32,-20.92,;32.79,-21.4,;34.12,-20.62,;34.11,-19.08,;35.45,-21.39,;35.46,-22.94,;36.79,-23.7,;34.12,-23.71,;32.78,-22.94,;27.19,-26.9,;25.85,-26.13,;25.53,-24.64,;25.93,-23.14,;27.02,-24.23,;24.08,-24.16,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of ChoKalpha (unknown origin) by HTS assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128310
BindingDB Entry DOI: 10.7270/Q27H1PFF |
More data for this
Ligand-Target Pair | |
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM31542

(pyrazolo[4,3-h]quinazoline-3-carboxamide, 25)Show SMILES Cn1nc(C(N)=O)c2CCc3cnc(Nc4ccc(cc4)N4CCOCC4)nc3-c12 Show InChI InChI=1S/C21H23N7O2/c1-27-19-16(18(26-27)20(22)29)7-2-13-12-23-21(25-17(13)19)24-14-3-5-15(6-4-14)28-8-10-30-11-9-28/h3-6,12H,2,7-11H2,1H3,(H2,22,29)(H,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | 7.4 | 23 |
Nerviano Medical Sciences Srl
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzymes and substrates, followed by quantitation of the phosphorylat... |
J Med Chem 52: 5152-63 (2009)
Article DOI: 10.1021/jm9006559
BindingDB Entry DOI: 10.7270/Q2JH3JJP |
More data for this
Ligand-Target Pair | |
Choline kinase alpha
(Homo sapiens (Human)) | BDBM50579779
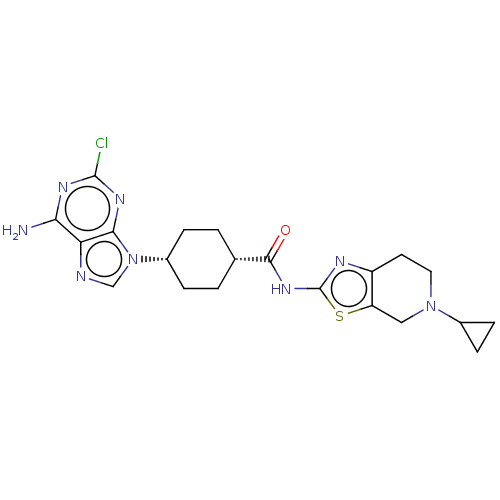
(CHEMBL5091519)Show SMILES Cl.Nc1nc(Cl)nc2n(cnc12)[C@@H]1CC[C@@H](CC1)C(=O)Nc1nc2CCN(Cc2s1)C1CC1 |r,wU:12.12,15.19,(65.78,-4.32,;78.94,-3.56,;78.95,-5.1,;80.28,-5.86,;80.29,-7.41,;81.63,-8.18,;78.95,-8.19,;77.62,-7.41,;76.15,-7.89,;75.25,-6.64,;76.15,-5.4,;77.62,-5.87,;75.67,-9.35,;74.17,-9.66,;73.69,-11.12,;74.71,-12.27,;76.22,-11.96,;76.71,-10.49,;74.23,-13.74,;75.25,-14.89,;72.72,-14.05,;71.69,-12.9,;70.15,-13.04,;69.54,-11.64,;68.08,-11.17,;67.76,-9.66,;68.91,-8.64,;70.37,-9.12,;70.68,-10.61,;72.01,-11.38,;68.59,-7.13,;69.08,-5.67,;67.57,-5.98,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of ChoKalpha (unknown origin) by HTS assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128310
BindingDB Entry DOI: 10.7270/Q27H1PFF |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM50318085

(1-Methyl-8-[(2-methylphenyl)amino]-4,5-dihydro-1H-...)Show InChI InChI=1S/C18H18N6O/c1-10-5-3-4-6-13(10)21-18-20-9-11-7-8-12-15(17(19)25)23-24(2)16(12)14(11)22-18/h3-6,9H,7-8H2,1-2H3,(H2,19,25)(H,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences srl
Curated by ChEMBL
| Assay Description
Inhibition of PLK1 assessed as [33P]-gamma-ATP incorporation into substrate by gamma counting |
Bioorg Med Chem Lett 21: 4507-11 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.122
BindingDB Entry DOI: 10.7270/Q2R211RS |
More data for this
Ligand-Target Pair | |
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM31544

(pyrazolo[4,3-h]quinazoline-3-carboxamide, 27)Show SMILES CN1CCN(CC1)c1ccc(Nc2ncc3CC(C)(C)c4c(nn(C)c4-c3n2)C(N)=O)cc1 Show InChI InChI=1S/C24H30N8O/c1-24(2)13-15-14-26-23(28-19(15)21-18(24)20(22(25)33)29-31(21)4)27-16-5-7-17(8-6-16)32-11-9-30(3)10-12-32/h5-8,14H,9-13H2,1-4H3,(H2,25,33)(H,26,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | 7.4 | 23 |
Nerviano Medical Sciences Srl
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzymes and substrates, followed by quantitation of the phosphorylat... |
J Med Chem 52: 5152-63 (2009)
Article DOI: 10.1021/jm9006559
BindingDB Entry DOI: 10.7270/Q2JH3JJP |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM31533

(pyrazolo[4,3-h]quinazoline-3-carboxamide, 16)Show InChI InChI=1S/C18H18N6O/c1-19-17(25)15-13-9-8-11-10-20-18(21-12-6-4-3-5-7-12)22-14(11)16(13)24(2)23-15/h3-7,10H,8-9H2,1-2H3,(H,19,25)(H,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | 7.4 | 23 |
Nerviano Medical Sciences Srl
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzymes and substrates, followed by quantitation of the phosphorylat... |
J Med Chem 52: 5152-63 (2009)
Article DOI: 10.1021/jm9006559
BindingDB Entry DOI: 10.7270/Q2JH3JJP |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM31533

(pyrazolo[4,3-h]quinazoline-3-carboxamide, 16)Show InChI InChI=1S/C18H18N6O/c1-19-17(25)15-13-9-8-11-10-20-18(21-12-6-4-3-5-7-12)22-14(11)16(13)24(2)23-15/h3-7,10H,8-9H2,1-2H3,(H,19,25)(H,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences srl
Curated by ChEMBL
| Assay Description
Inhibition of CDK2/Cyclin A assessed as [33P]-gamma-ATP incorporation into substrate by gamma counting |
Bioorg Med Chem Lett 21: 4507-11 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.122
BindingDB Entry DOI: 10.7270/Q2R211RS |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Dual specificity protein kinase TTK
(Homo sapiens (Human)) | BDBM50349092
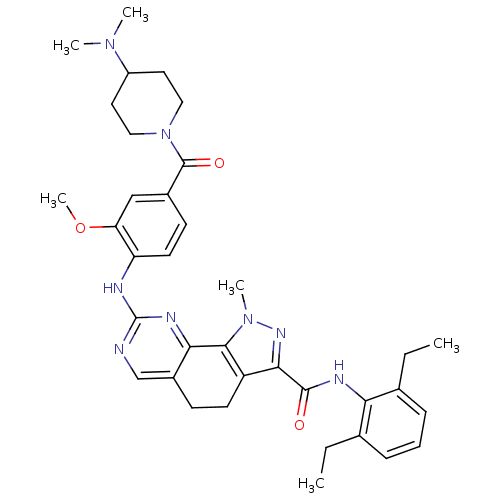
(CHEMBL1807303)Show SMILES CCc1cccc(CC)c1NC(=O)c1nn(C)c-2c1CCc1cnc(Nc3ccc(cc3OC)C(=O)N3CCC(CC3)N(C)C)nc-21 Show InChI InChI=1S/C36H44N8O3/c1-7-22-10-9-11-23(8-2)30(22)39-34(45)32-27-14-12-25-21-37-36(40-31(25)33(27)43(5)41-32)38-28-15-13-24(20-29(28)47-6)35(46)44-18-16-26(17-19-44)42(3)4/h9-11,13,15,20-21,26H,7-8,12,14,16-19H2,1-6H3,(H,39,45)(H,37,38,40) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences srl
Curated by ChEMBL
| Assay Description
Inhibition of MPS1 assessed as [33P]-gamma-ATP incorporation into substrate P38-betatide by gamma counting |
Bioorg Med Chem Lett 21: 4507-11 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.122
BindingDB Entry DOI: 10.7270/Q2R211RS |
More data for this
Ligand-Target Pair | |
Choline kinase alpha
(Homo sapiens (Human)) | BDBM50579782
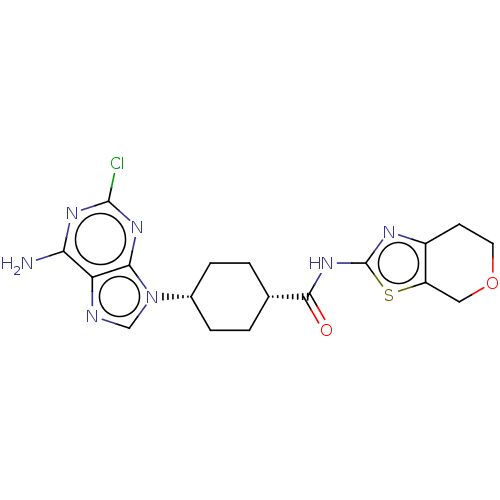
(CHEMBL5078600)Show SMILES Nc1nc(Cl)nc2n(cnc12)[C@@H]1CC[C@@H](CC1)C(=O)Nc1nc2CCOCc2s1 |r,wU:11.12,14.19,(53.68,-20.14,;53.69,-21.69,;55.03,-22.45,;55.03,-24,;56.37,-24.77,;53.69,-24.77,;52.36,-24,;50.89,-24.48,;49.99,-23.23,;50.9,-21.98,;52.36,-22.46,;50.41,-25.94,;48.91,-26.25,;48.43,-27.71,;49.45,-28.86,;50.96,-28.54,;51.45,-27.08,;48.97,-30.32,;49.99,-31.47,;47.46,-30.64,;46.43,-29.48,;44.89,-29.63,;44.28,-28.23,;42.82,-27.76,;42.51,-26.25,;43.65,-25.22,;45.11,-25.71,;45.42,-27.2,;46.76,-27.97,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of ChoKalpha (unknown origin) by HTS assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128310
BindingDB Entry DOI: 10.7270/Q27H1PFF |
More data for this
Ligand-Target Pair | |
Choline kinase alpha
(Homo sapiens (Human)) | BDBM50579773
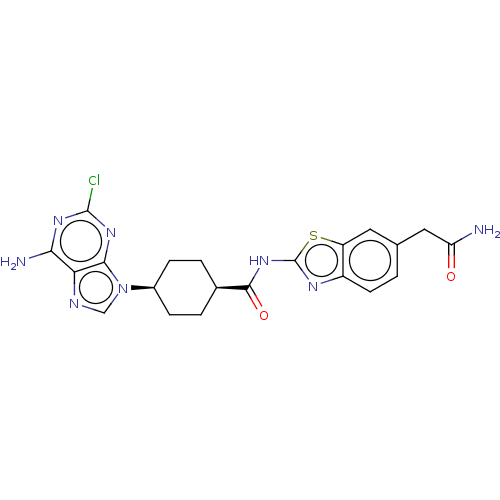
(CHEMBL5079663)Show SMILES NC(=O)Cc1ccc2nc(NC(=O)[C@H]3CC[C@H](CC3)n3cnc4c(N)nc(Cl)nc34)sc2c1 |r,wU:16.19,13.12,(25.2,-43.33,;25.51,-44.84,;26.98,-45.33,;24.36,-45.87,;24.68,-47.38,;23.53,-48.41,;23.85,-49.9,;25.3,-50.38,;25.92,-51.79,;27.46,-51.64,;28.48,-52.79,;29.99,-52.48,;31.01,-53.63,;30.47,-51.02,;29.45,-49.87,;29.93,-48.41,;31.44,-48.1,;32.47,-49.24,;31.98,-50.7,;31.91,-46.64,;31.01,-45.39,;31.92,-44.14,;33.38,-44.62,;34.71,-43.85,;34.7,-42.3,;36.05,-44.61,;36.05,-46.16,;37.39,-46.93,;34.72,-46.93,;33.38,-46.16,;27.78,-50.13,;26.44,-49.36,;26.13,-47.86,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of ChoKalpha (unknown origin) by HTS assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128310
BindingDB Entry DOI: 10.7270/Q27H1PFF |
More data for this
Ligand-Target Pair | |
Choline kinase alpha
(Homo sapiens (Human)) | BDBM50579777
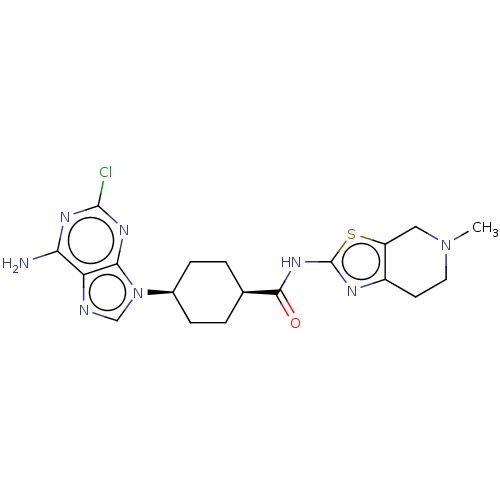
(CHEMBL5090932)Show SMILES CN1CCc2nc(NC(=O)[C@H]3CC[C@H](CC3)n3cnc4c(N)nc(Cl)nc34)sc2C1 |r,wU:13.16,10.9,(24.36,-7.31,;24.67,-8.82,;23.53,-9.84,;23.84,-11.35,;25.3,-11.82,;25.92,-13.22,;27.46,-13.08,;28.48,-14.23,;29.99,-13.91,;31.02,-15.06,;30.47,-12.45,;29.45,-11.3,;29.93,-9.84,;31.44,-9.53,;32.47,-10.67,;31.98,-12.14,;31.92,-8.07,;31.01,-6.82,;31.92,-5.58,;33.38,-6.05,;34.71,-5.28,;34.71,-3.74,;36.05,-6.04,;36.05,-7.59,;37.39,-8.36,;34.72,-8.36,;33.38,-7.59,;27.78,-11.56,;26.44,-10.79,;26.13,-9.3,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of ChoKalpha (unknown origin) by HTS assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128310
BindingDB Entry DOI: 10.7270/Q27H1PFF |
More data for this
Ligand-Target Pair | |
Dual specificity protein kinase TTK
(Homo sapiens (Human)) | BDBM50349094

(CHEMBL1807305)Show SMILES CCc1cccc(CC)c1NC(=O)c1nn(C)c-2c1CCc1cnc(Nc3ccc(cc3OC)C(=O)N3CCCN(C)CC3)nc-21 Show InChI InChI=1S/C35H42N8O3/c1-6-22-10-8-11-23(7-2)29(22)38-33(44)31-26-14-12-25-21-36-35(39-30(25)32(26)42(4)40-31)37-27-15-13-24(20-28(27)46-5)34(45)43-17-9-16-41(3)18-19-43/h8,10-11,13,15,20-21H,6-7,9,12,14,16-19H2,1-5H3,(H,38,44)(H,36,37,39) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences srl
Curated by ChEMBL
| Assay Description
Inhibition of MPS1 assessed as [33P]-gamma-ATP incorporation into substrate P38-betatide by gamma counting |
Bioorg Med Chem Lett 21: 4507-11 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.122
BindingDB Entry DOI: 10.7270/Q2R211RS |
More data for this
Ligand-Target Pair | |
Choline kinase alpha
(Homo sapiens (Human)) | BDBM50579771
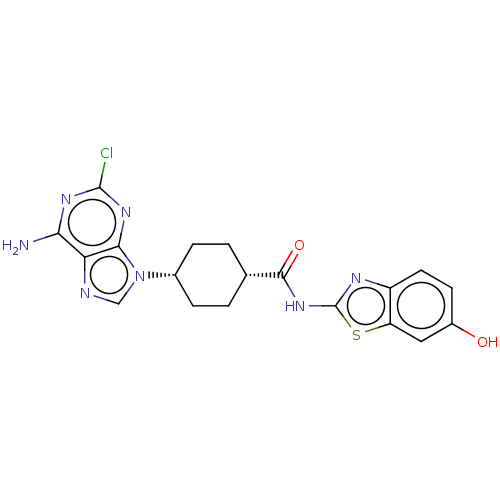
(CHEMBL5077194)Show SMILES Nc1nc(Cl)nc2n(cnc12)[C@@H]1CC[C@@H](CC1)C(=O)Nc1nc2ccc(O)cc2s1 |r,wU:11.12,14.19,(76.62,-21.9,;76.63,-23.44,;77.96,-24.2,;77.97,-25.75,;79.3,-26.52,;76.63,-26.53,;75.29,-25.76,;73.83,-26.23,;72.92,-24.98,;73.83,-23.74,;75.3,-24.22,;73.35,-27.69,;71.84,-28,;71.36,-29.46,;72.39,-30.61,;73.9,-30.3,;74.38,-28.83,;71.9,-32.08,;72.93,-33.23,;70.39,-32.39,;69.37,-31.24,;67.83,-31.38,;67.21,-29.97,;65.76,-29.49,;65.44,-28,;66.59,-26.97,;66.28,-25.46,;68.05,-27.45,;68.36,-28.95,;69.69,-29.72,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of ChoKalpha (unknown origin) by HTS assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128310
BindingDB Entry DOI: 10.7270/Q27H1PFF |
More data for this
Ligand-Target Pair | |
Choline kinase alpha
(Homo sapiens (Human)) | BDBM50579769

(CHEMBL5075042)Show SMILES COc1ccc2nc(NC(=O)[C@H]3CC[C@H](CC3)n3cnc4c(N)nc(Cl)nc34)sc2c1 |r,wU:14.17,11.10,(24.57,-24.01,;23.42,-25.03,;23.74,-26.54,;22.59,-27.57,;22.91,-29.07,;24.36,-29.54,;24.98,-30.95,;26.52,-30.81,;27.54,-31.96,;29.05,-31.65,;30.08,-32.8,;29.53,-30.18,;28.51,-29.03,;28.99,-27.57,;30.5,-27.27,;31.53,-28.4,;31.04,-29.87,;30.97,-25.8,;30.07,-24.55,;30.98,-23.31,;32.44,-23.79,;33.77,-23.01,;33.77,-21.47,;35.11,-23.77,;35.11,-25.33,;36.45,-26.09,;33.78,-26.1,;32.44,-25.33,;26.84,-29.3,;25.5,-28.53,;25.19,-27.03,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of ChoKalpha (unknown origin) by HTS assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128310
BindingDB Entry DOI: 10.7270/Q27H1PFF |
More data for this
Ligand-Target Pair | |
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM31543

(pyrazolo[4,3-h]quinazoline-3-carboxamide, 26)Show SMILES Cn1nc(C(N)=O)c2c1-c1nc(Nc3ccccc3)ncc1CC2(C)C Show InChI InChI=1S/C19H20N6O/c1-19(2)9-11-10-21-18(22-12-7-5-4-6-8-12)23-14(11)16-13(19)15(17(20)26)24-25(16)3/h4-8,10H,9H2,1-3H3,(H2,20,26)(H,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | 7.4 | 23 |
Nerviano Medical Sciences Srl
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzymes and substrates, followed by quantitation of the phosphorylat... |
J Med Chem 52: 5152-63 (2009)
Article DOI: 10.1021/jm9006559
BindingDB Entry DOI: 10.7270/Q2JH3JJP |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Choline kinase alpha
(Homo sapiens (Human)) | BDBM50579780

(CHEMBL5089356)Show SMILES Nc1nc(Cl)nc2n(cnc12)[C@@H]1CC[C@@H](CC1)C(=O)Nc1nc2CCNCc2s1 |r,wU:11.12,14.19,(14.03,-18.46,;14.04,-20,;15.37,-20.77,;15.38,-22.32,;16.72,-23.09,;14.04,-23.09,;12.71,-22.32,;11.24,-22.79,;10.34,-21.55,;11.24,-20.3,;12.71,-20.78,;10.76,-24.26,;9.26,-24.57,;8.78,-26.03,;9.8,-27.18,;11.31,-26.86,;11.8,-25.4,;9.32,-28.64,;10.34,-29.79,;7.81,-28.95,;6.78,-27.8,;5.24,-27.95,;4.63,-26.54,;3.17,-26.08,;2.85,-24.57,;4,-23.54,;5.46,-24.02,;5.77,-25.52,;7.1,-26.29,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of ChoKalpha (unknown origin) by HTS assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128310
BindingDB Entry DOI: 10.7270/Q27H1PFF |
More data for this
Ligand-Target Pair | |
Choline kinase alpha
(Homo sapiens (Human)) | BDBM50579775
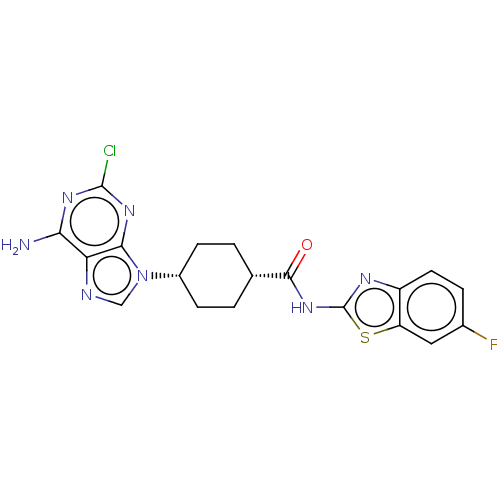
(CHEMBL5085262)Show SMILES Nc1nc(Cl)nc2n(cnc12)[C@@H]1CC[C@@H](CC1)C(=O)Nc1nc2ccc(F)cc2s1 |r,wU:11.12,14.19,(75.4,-43.26,;75.4,-44.81,;76.74,-45.57,;76.75,-47.12,;78.08,-47.89,;75.41,-47.89,;74.07,-47.12,;72.61,-47.6,;71.7,-46.35,;72.61,-45.1,;74.07,-45.58,;72.13,-49.06,;70.62,-49.37,;70.14,-50.83,;71.16,-51.98,;72.68,-51.67,;73.16,-50.2,;70.68,-53.44,;71.71,-54.59,;69.17,-53.76,;68.15,-52.61,;66.61,-52.75,;65.99,-51.34,;64.54,-50.86,;64.22,-49.37,;65.37,-48.34,;65.06,-46.83,;66.83,-48.82,;67.13,-50.32,;68.47,-51.09,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of ChoKalpha (unknown origin) by HTS assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128310
BindingDB Entry DOI: 10.7270/Q27H1PFF |
More data for this
Ligand-Target Pair | |
Choline kinase alpha
(Homo sapiens (Human)) | BDBM50579757
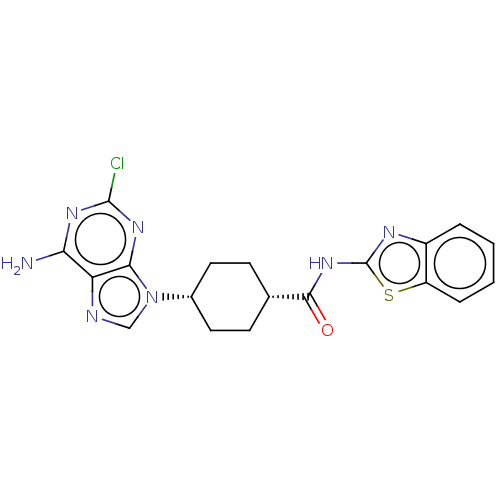
(CHEMBL5084844)Show SMILES Nc1nc(Cl)nc2n(cnc12)[C@@H]1CC[C@@H](CC1)C(=O)Nc1nc2ccccc2s1 |r,wU:11.12,14.19,(17.43,-18.47,;17.43,-20.01,;18.77,-20.78,;18.77,-22.33,;20.11,-23.1,;17.44,-23.1,;16.1,-22.33,;14.64,-22.8,;13.73,-21.56,;14.64,-20.31,;16.1,-20.79,;14.16,-24.27,;12.65,-24.58,;12.17,-26.03,;13.19,-27.19,;14.71,-26.87,;15.19,-25.4,;12.71,-28.65,;13.74,-29.8,;11.2,-28.96,;10.18,-27.81,;8.64,-27.96,;8.02,-26.55,;6.57,-26.07,;6.25,-24.57,;7.4,-23.55,;8.86,-24.03,;9.16,-25.53,;10.5,-26.3,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of ChoKalpha (unknown origin) by HTS assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128310
BindingDB Entry DOI: 10.7270/Q27H1PFF |
More data for this
Ligand-Target Pair | |
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM31549

(pyrazolo[4,3-h]quinazoline-3-carboxamide, 32)Show SMILES CNC(=O)c1nn(C)c-2c1C(C)(C)Cc1cnc(Nc3ccc(OC4CCN(C)CC4)cc3)nc-21 Show InChI InChI=1S/C26H33N7O2/c1-26(2)14-16-15-28-25(30-21(16)23-20(26)22(24(34)27-3)31-33(23)5)29-17-6-8-18(9-7-17)35-19-10-12-32(4)13-11-19/h6-9,15,19H,10-14H2,1-5H3,(H,27,34)(H,28,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | 7.4 | 23 |
Nerviano Medical Sciences Srl
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzymes and substrates, followed by quantitation of the phosphorylat... |
J Med Chem 52: 5152-63 (2009)
Article DOI: 10.1021/jm9006559
BindingDB Entry DOI: 10.7270/Q2JH3JJP |
More data for this
Ligand-Target Pair | |
Choline kinase alpha
(Homo sapiens (Human)) | BDBM50579770
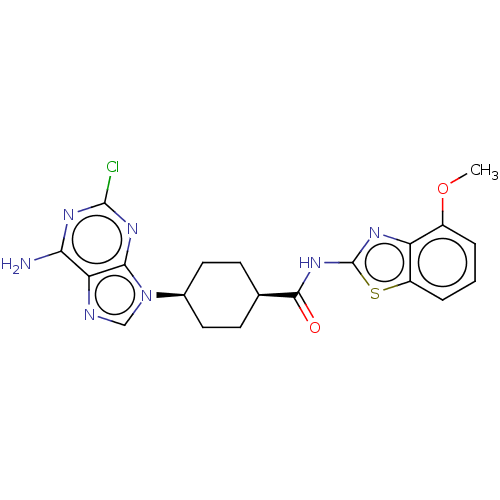
(CHEMBL5090132)Show SMILES COc1cccc2sc(NC(=O)[C@H]3CC[C@H](CC3)n3cnc4c(N)nc(Cl)nc34)nc12 |r,wU:15.18,12.11,(43.36,-30.19,;44.82,-30.67,;45.97,-29.64,;45.66,-28.15,;46.8,-27.12,;48.26,-27.6,;48.57,-29.1,;49.9,-29.87,;49.58,-31.39,;50.61,-32.54,;52.12,-32.22,;53.14,-33.37,;52.6,-30.76,;51.58,-29.61,;52.05,-28.15,;53.56,-27.84,;54.6,-28.98,;54.11,-30.45,;54.04,-26.38,;53.14,-25.13,;54.04,-23.88,;55.51,-24.36,;56.84,-23.59,;56.83,-22.04,;58.17,-24.35,;58.18,-25.9,;59.52,-26.67,;56.84,-26.67,;55.51,-25.9,;48.04,-31.53,;47.42,-30.12,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of ChoKalpha (unknown origin) by HTS assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128310
BindingDB Entry DOI: 10.7270/Q27H1PFF |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM31541

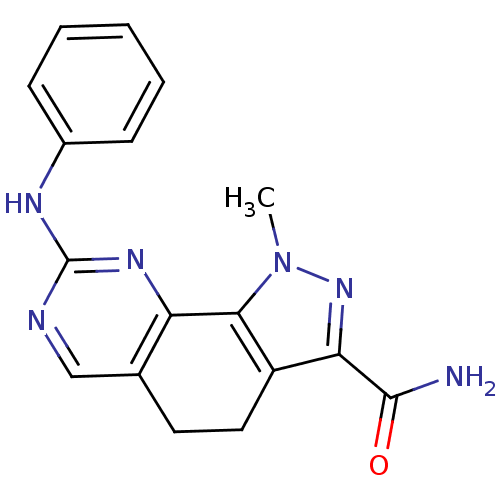
(pyrazolo[4,3-h]quinazoline-3-carboxamide, 24)Show SMILES CN1CCN(CC1)c1cccc(Nc2ncc3CCc4c(nn(C)c4-c3n2)C(N)=O)c1 Show InChI InChI=1S/C22H26N8O/c1-28-8-10-30(11-9-28)16-5-3-4-15(12-16)25-22-24-13-14-6-7-17-19(21(23)31)27-29(2)20(17)18(14)26-22/h3-5,12-13H,6-11H2,1-2H3,(H2,23,31)(H,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | 25 |
Nerviano Medical Sciences Srl
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzymes and substrates, followed by quantitation of the phosphorylat... |
J Med Chem 52: 5152-63 (2009)
Article DOI: 10.1021/jm9006559
BindingDB Entry DOI: 10.7270/Q2JH3JJP |
More data for this
Ligand-Target Pair | |
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM31546

(pyrazolo[4,3-h]quinazoline-3-carboxamide, 29)Show SMILES CNC(=O)c1nn(C)c-2c1C(C)(C)Cc1cnc(Nc3cccc(c3)N3CCN(C)CC3)nc-21 Show InChI InChI=1S/C25H32N8O/c1-25(2)14-16-15-27-24(29-20(16)22-19(25)21(23(34)26-3)30-32(22)5)28-17-7-6-8-18(13-17)33-11-9-31(4)10-12-33/h6-8,13,15H,9-12,14H2,1-5H3,(H,26,34)(H,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | 7.4 | 23 |
Nerviano Medical Sciences Srl
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzymes and substrates, followed by quantitation of the phosphorylat... |
J Med Chem 52: 5152-63 (2009)
Article DOI: 10.1021/jm9006559
BindingDB Entry DOI: 10.7270/Q2JH3JJP |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Choline kinase alpha
(Homo sapiens (Human)) | BDBM50579786
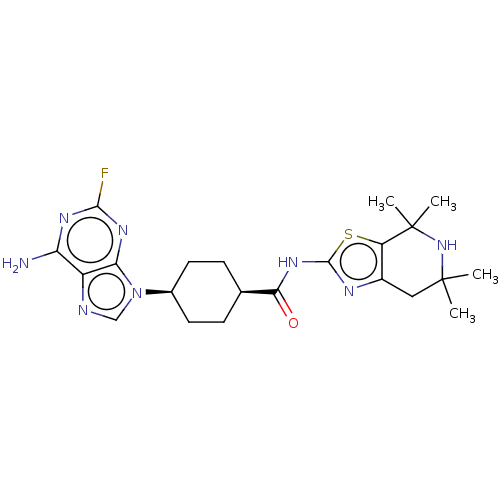
(CHEMBL5093152)Show SMILES CC1(C)Cc2nc(NC(=O)[C@H]3CC[C@H](CC3)n3cnc4c(N)nc(F)nc34)sc2C(C)(C)N1 |r,wU:13.16,10.9,(42.91,-44.68,;44.24,-43.92,;42.91,-43.14,;44.56,-45.43,;46.02,-45.89,;46.64,-47.3,;48.17,-47.15,;49.2,-48.3,;50.71,-47.99,;51.73,-49.14,;51.19,-46.53,;50.17,-45.38,;50.65,-43.92,;52.15,-43.61,;53.19,-44.75,;52.7,-46.21,;52.63,-42.15,;51.73,-40.9,;52.64,-39.66,;54.1,-40.13,;55.43,-39.36,;55.42,-37.82,;56.76,-40.12,;56.77,-41.67,;58.1,-42.44,;55.43,-42.44,;54.1,-41.67,;48.5,-45.64,;47.16,-44.87,;46.84,-43.37,;47.24,-41.88,;48.33,-42.97,;45.39,-42.89,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of ChoKalpha (unknown origin) by HTS assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128310
BindingDB Entry DOI: 10.7270/Q27H1PFF |
More data for this
Ligand-Target Pair | |
Choline kinase alpha
(Homo sapiens (Human)) | BDBM50579785
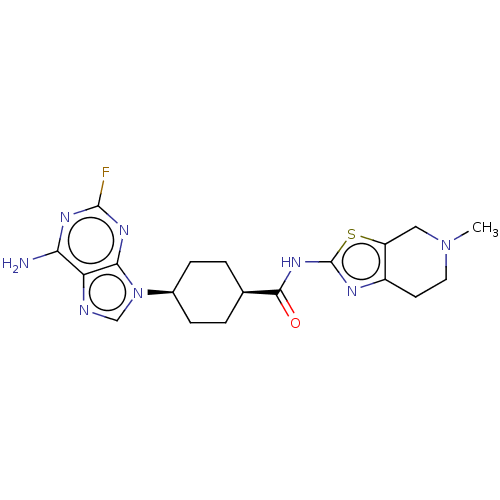
(CHEMBL5075110)Show SMILES CN1CCc2nc(NC(=O)[C@H]3CC[C@H](CC3)n3cnc4c(N)nc(F)nc34)sc2C1 |r,wU:13.16,10.9,(23.79,-42.21,;24.11,-43.72,;22.96,-44.75,;23.28,-46.25,;24.73,-46.72,;25.35,-48.13,;26.89,-47.98,;27.91,-49.13,;29.42,-48.82,;30.45,-49.97,;29.91,-47.36,;28.88,-46.2,;29.36,-44.75,;30.87,-44.44,;31.9,-45.57,;31.42,-47.04,;31.35,-42.97,;30.45,-41.73,;31.35,-40.48,;32.82,-40.96,;34.15,-40.18,;34.14,-38.64,;35.48,-40.95,;35.49,-42.5,;36.82,-43.27,;34.15,-43.27,;32.81,-42.5,;27.21,-46.47,;25.88,-45.7,;25.56,-44.2,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of ChoKalpha (unknown origin) by HTS assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128310
BindingDB Entry DOI: 10.7270/Q27H1PFF |
More data for this
Ligand-Target Pair | |
Choline kinase alpha
(Homo sapiens (Human)) | BDBM50579774

(CHEMBL5081222)Show SMILES NC(=O)c1ccc2nc(NC(=O)[C@H]3CC[C@H](CC3)n3cnc4c(N)nc(Cl)nc34)sc2c1 |r,wU:15.18,12.11,(43.29,-45.93,;44.75,-46.41,;45.9,-45.38,;45.07,-47.92,;43.92,-48.94,;44.23,-50.44,;45.68,-50.92,;46.3,-52.33,;47.84,-52.18,;48.87,-53.33,;50.38,-53.02,;51.4,-54.17,;50.86,-51.56,;49.84,-50.4,;50.32,-48.95,;51.82,-48.64,;52.86,-49.77,;52.37,-51.24,;52.3,-47.17,;51.4,-45.93,;52.31,-44.68,;53.77,-45.16,;55.1,-44.38,;55.09,-42.84,;56.44,-45.15,;56.44,-46.7,;57.78,-47.47,;55.1,-47.47,;53.77,-46.7,;48.16,-50.67,;46.83,-49.9,;46.52,-48.4,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of ChoKalpha (unknown origin) by HTS assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128310
BindingDB Entry DOI: 10.7270/Q27H1PFF |
More data for this
Ligand-Target Pair | |
Dual specificity protein kinase TTK
(Homo sapiens (Human)) | BDBM50349103
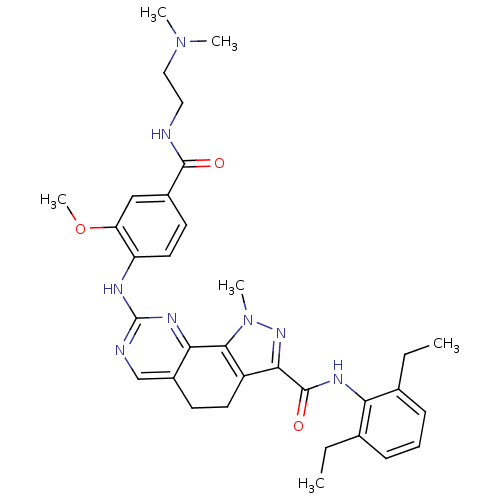
(CHEMBL1808341)Show SMILES CCc1cccc(CC)c1NC(=O)c1nn(C)c-2c1CCc1cnc(Nc3ccc(cc3OC)C(=O)NCCN(C)C)nc-21 Show InChI InChI=1S/C33H40N8O3/c1-7-20-10-9-11-21(8-2)27(20)37-32(43)29-24-14-12-23-19-35-33(38-28(23)30(24)41(5)39-29)36-25-15-13-22(18-26(25)44-6)31(42)34-16-17-40(3)4/h9-11,13,15,18-19H,7-8,12,14,16-17H2,1-6H3,(H,34,42)(H,37,43)(H,35,36,38) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences srl
Curated by ChEMBL
| Assay Description
Inhibition of MPS1 assessed as [33P]-gamma-ATP incorporation into substrate P38-betatide by gamma counting |
Bioorg Med Chem Lett 21: 4507-11 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.122
BindingDB Entry DOI: 10.7270/Q2R211RS |
More data for this
Ligand-Target Pair | |
Choline kinase alpha
(Homo sapiens (Human)) | BDBM50579772
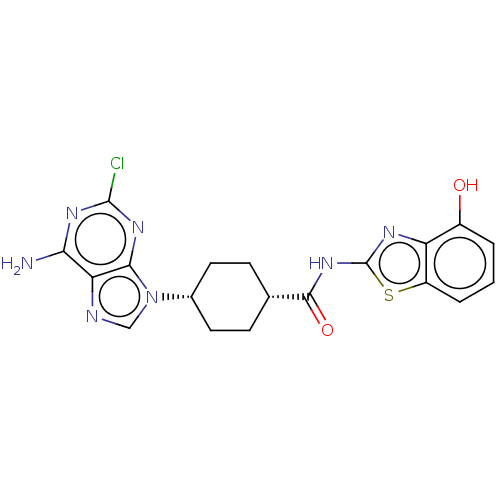
(CHEMBL5088776)Show SMILES Nc1nc(Cl)nc2n(cnc12)[C@@H]1CC[C@@H](CC1)C(=O)Nc1nc2c(O)cccc2s1 |r,wU:11.12,14.19,(14.05,-42.03,;14.05,-43.58,;15.39,-44.34,;15.4,-45.89,;16.73,-46.66,;14.06,-46.66,;12.72,-45.89,;11.26,-46.37,;10.35,-45.12,;11.26,-43.87,;12.72,-44.35,;10.78,-47.83,;9.27,-48.14,;8.79,-49.6,;9.82,-50.75,;11.33,-50.44,;11.81,-48.97,;9.33,-52.21,;10.36,-53.36,;7.82,-52.53,;6.8,-51.38,;5.26,-51.52,;4.64,-50.11,;3.19,-49.63,;2.04,-50.66,;2.87,-48.14,;4.02,-47.11,;5.48,-47.59,;5.79,-49.09,;7.12,-49.86,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of ChoKalpha (unknown origin) by HTS assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128310
BindingDB Entry DOI: 10.7270/Q27H1PFF |
More data for this
Ligand-Target Pair | |
Choline kinase alpha
(Homo sapiens (Human)) | BDBM50579784
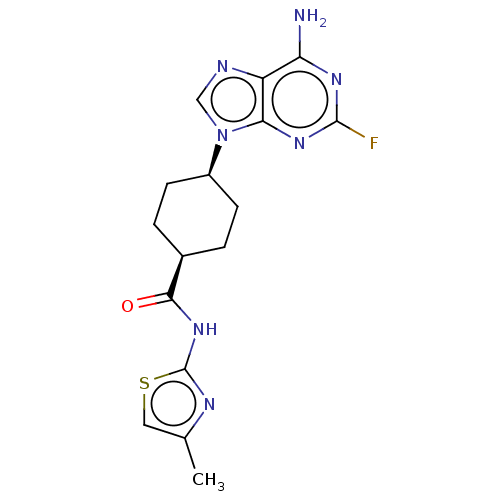
(CHEMBL5091201)Show SMILES Cc1csc(NC(=O)[C@H]2CC[C@H](CC2)n2cnc3c(N)nc(F)nc23)n1 |r,wU:11.14,8.7,(4.23,-45.63,;5.68,-46.1,;6.83,-45.08,;8.16,-45.85,;7.84,-47.36,;8.87,-48.51,;10.37,-48.2,;11.4,-49.35,;10.86,-46.74,;9.83,-45.58,;10.31,-44.13,;11.82,-43.82,;12.86,-44.95,;12.37,-46.42,;12.3,-42.35,;11.4,-41.11,;12.3,-39.86,;13.77,-40.34,;15.1,-39.56,;15.09,-38.02,;16.43,-40.33,;16.44,-41.88,;17.77,-42.65,;15.1,-42.65,;13.76,-41.88,;6.3,-47.51,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of ChoKalpha (unknown origin) by HTS assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128310
BindingDB Entry DOI: 10.7270/Q27H1PFF |
More data for this
Ligand-Target Pair | |
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM31557

(pyrazolo[4,3-h]quinazoline-3-carboxamide, 40)Show SMILES CNC(=O)c1nn(C)c-2c1C(C)(C)Cc1cnc(Nc3ccc(CN(C)C)cc3)nc-21 Show InChI InChI=1S/C23H29N7O/c1-23(2)11-15-12-25-22(26-16-9-7-14(8-10-16)13-29(4)5)27-18(15)20-17(23)19(21(31)24-3)28-30(20)6/h7-10,12H,11,13H2,1-6H3,(H,24,31)(H,25,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | 7.4 | 23 |
Nerviano Medical Sciences Srl
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzymes and substrates, followed by quantitation of the phosphorylat... |
J Med Chem 52: 5152-63 (2009)
Article DOI: 10.1021/jm9006559
BindingDB Entry DOI: 10.7270/Q2JH3JJP |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Dual specificity protein kinase TTK
(Homo sapiens (Human)) | BDBM50349099
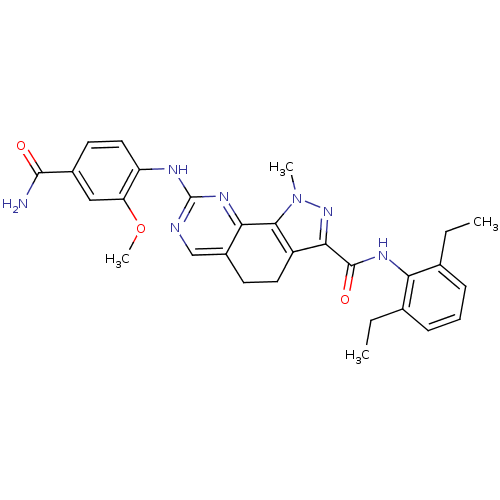
(CHEMBL1808338)Show SMILES CCc1cccc(CC)c1NC(=O)c1nn(C)c-2c1CCc1cnc(Nc3ccc(cc3OC)C(N)=O)nc-21 Show InChI InChI=1S/C29H31N7O3/c1-5-16-8-7-9-17(6-2)23(16)33-28(38)25-20-12-10-19-15-31-29(34-24(19)26(20)36(3)35-25)32-21-13-11-18(27(30)37)14-22(21)39-4/h7-9,11,13-15H,5-6,10,12H2,1-4H3,(H2,30,37)(H,33,38)(H,31,32,34) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences srl
Curated by ChEMBL
| Assay Description
Inhibition of MPS1 assessed as [33P]-gamma-ATP incorporation into substrate P38-betatide by gamma counting |
Bioorg Med Chem Lett 21: 4507-11 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.122
BindingDB Entry DOI: 10.7270/Q2R211RS |
More data for this
Ligand-Target Pair | |
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM31553
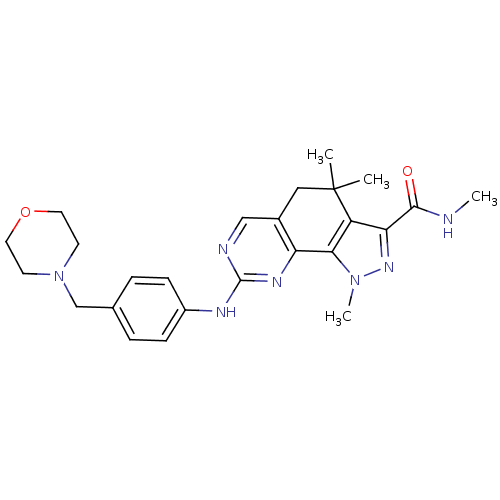
(pyrazolo[4,3-h]quinazoline-3-carboxamide, 36)Show SMILES CNC(=O)c1nn(C)c-2c1C(C)(C)Cc1cnc(Nc3ccc(CN4CCOCC4)cc3)nc-21 Show InChI InChI=1S/C25H31N7O2/c1-25(2)13-17-14-27-24(29-20(17)22-19(25)21(23(33)26-3)30-31(22)4)28-18-7-5-16(6-8-18)15-32-9-11-34-12-10-32/h5-8,14H,9-13,15H2,1-4H3,(H,26,33)(H,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | 7.4 | 23 |
Nerviano Medical Sciences Srl
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzymes and substrates, followed by quantitation of the phosphorylat... |
J Med Chem 52: 5152-63 (2009)
Article DOI: 10.1021/jm9006559
BindingDB Entry DOI: 10.7270/Q2JH3JJP |
More data for this
Ligand-Target Pair | |
Choline kinase alpha
(Homo sapiens (Human)) | BDBM50579764
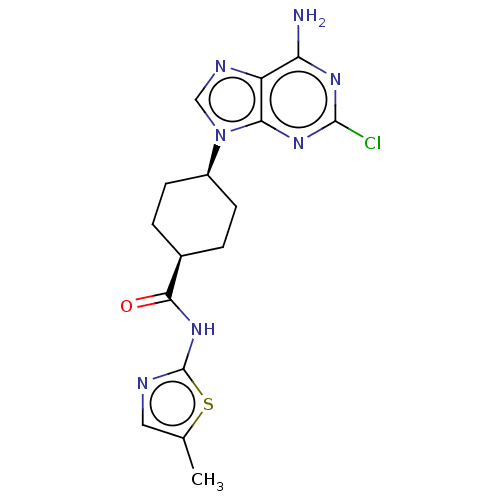
(CHEMBL5086885)Show SMILES Cc1cnc(NC(=O)[C@H]2CC[C@H](CC2)n2cnc3c(N)nc(Cl)nc23)s1 |r,wU:11.14,8.7,(4.59,-8.31,;4.75,-9.84,;3.6,-10.86,;4.22,-12.27,;5.76,-12.12,;6.78,-13.27,;8.29,-12.96,;9.31,-14.11,;8.77,-11.5,;7.75,-10.35,;8.23,-8.89,;9.74,-8.58,;10.77,-9.72,;10.28,-11.19,;10.21,-7.12,;9.31,-5.87,;10.22,-4.63,;11.68,-5.11,;13.01,-4.33,;13,-2.79,;14.34,-5.1,;14.35,-6.65,;15.68,-7.41,;13.01,-7.42,;11.68,-6.65,;6.08,-10.61,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of ChoKalpha (unknown origin) by HTS assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128310
BindingDB Entry DOI: 10.7270/Q27H1PFF |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM50318081
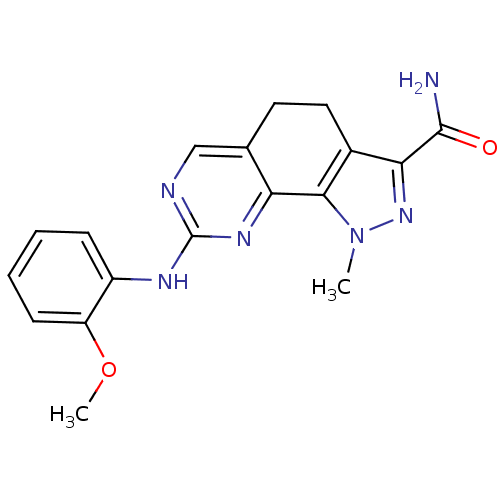
(8-[(2-Methoxyphenyl)amino]-1-methyl-4,5-dihydro-1H...)Show InChI InChI=1S/C18H18N6O2/c1-24-16-11(15(23-24)17(19)25)8-7-10-9-20-18(22-14(10)16)21-12-5-3-4-6-13(12)26-2/h3-6,9H,7-8H2,1-2H3,(H2,19,25)(H,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences srl
Curated by ChEMBL
| Assay Description
Inhibition of PLK1 assessed as [33P]-gamma-ATP incorporation into substrate by gamma counting |
Bioorg Med Chem Lett 21: 4507-11 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.122
BindingDB Entry DOI: 10.7270/Q2R211RS |
More data for this
Ligand-Target Pair | |
Choline kinase alpha
(Homo sapiens (Human)) | BDBM50579778
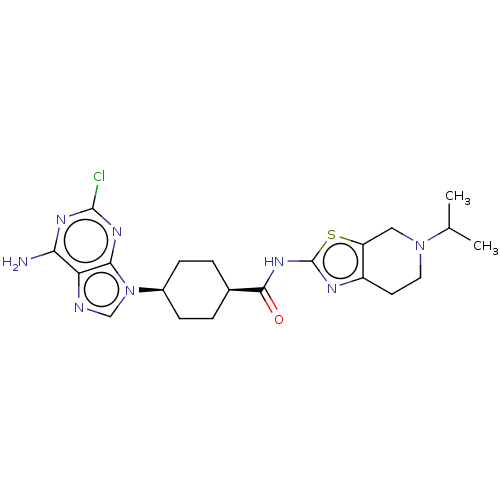
(CHEMBL5078057)Show SMILES CC(C)N1CCc2nc(NC(=O)[C@H]3CC[C@H](CC3)n3cnc4c(N)nc(Cl)nc34)sc2C1 |r,wU:15.18,12.11,(45.88,-7.03,;44.73,-8.05,;43.27,-7.57,;45.05,-9.56,;43.9,-10.59,;44.22,-12.09,;45.68,-12.56,;46.29,-13.97,;47.83,-13.82,;48.86,-14.97,;50.37,-14.66,;51.39,-15.81,;50.85,-13.2,;49.83,-12.04,;50.31,-10.59,;51.81,-10.28,;52.85,-11.41,;52.36,-12.88,;52.29,-8.81,;51.39,-7.57,;52.29,-6.32,;53.76,-6.8,;55.09,-6.02,;55.08,-4.48,;56.42,-6.79,;56.43,-8.34,;57.76,-9.11,;55.09,-9.11,;53.76,-8.34,;48.15,-12.31,;46.82,-11.54,;46.5,-10.04,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of ChoKalpha (unknown origin) by HTS assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128310
BindingDB Entry DOI: 10.7270/Q27H1PFF |
More data for this
Ligand-Target Pair | |
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM31545
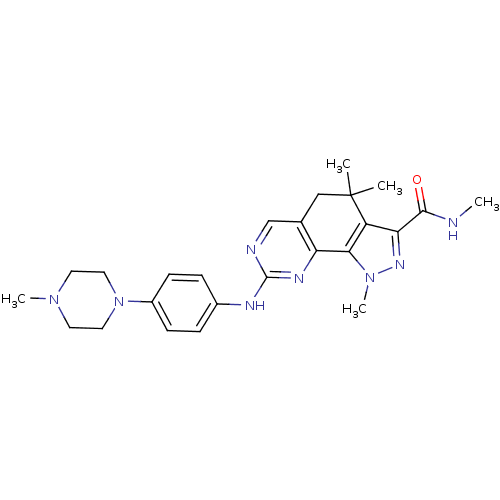
(Milciclib | pyrazolo[4,3-h]quinazoline-3-carboxami...)Show SMILES CNC(=O)c1nn(C)c-2c1C(C)(C)Cc1cnc(Nc3ccc(cc3)N3CCN(C)CC3)nc-21 Show InChI InChI=1S/C25H32N8O/c1-25(2)14-16-15-27-24(29-20(16)22-19(25)21(23(34)26-3)30-32(22)5)28-17-6-8-18(9-7-17)33-12-10-31(4)11-13-33/h6-9,15H,10-14H2,1-5H3,(H,26,34)(H,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | 7.4 | 23 |
Nerviano Medical Sciences Srl
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzymes and substrates, followed by quantitation of the phosphorylat... |
J Med Chem 52: 5152-63 (2009)
Article DOI: 10.1021/jm9006559
BindingDB Entry DOI: 10.7270/Q2JH3JJP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM31540
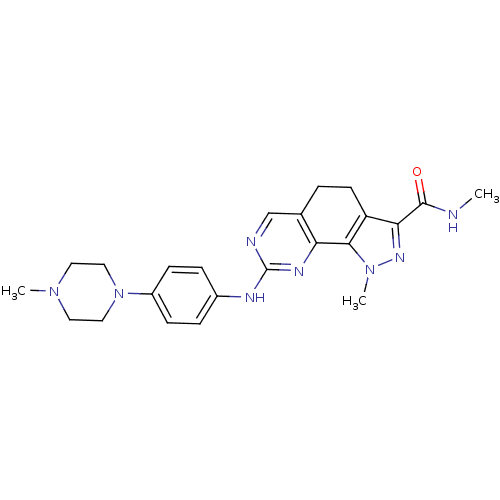
(pyrazolo[4,3-h]quinazoline-3-carboxamide, 23)Show SMILES CNC(=O)c1nn(C)c-2c1CCc1cnc(Nc3ccc(cc3)N3CCN(C)CC3)nc-21 Show InChI InChI=1S/C23H28N8O/c1-24-22(32)20-18-9-4-15-14-25-23(27-19(15)21(18)30(3)28-20)26-16-5-7-17(8-6-16)31-12-10-29(2)11-13-31/h5-8,14H,4,9-13H2,1-3H3,(H,24,32)(H,25,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | 7.4 | 23 |
Nerviano Medical Sciences Srl
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzymes and substrates, followed by quantitation of the phosphorylat... |
J Med Chem 52: 5152-63 (2009)
Article DOI: 10.1021/jm9006559
BindingDB Entry DOI: 10.7270/Q2JH3JJP |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM31550
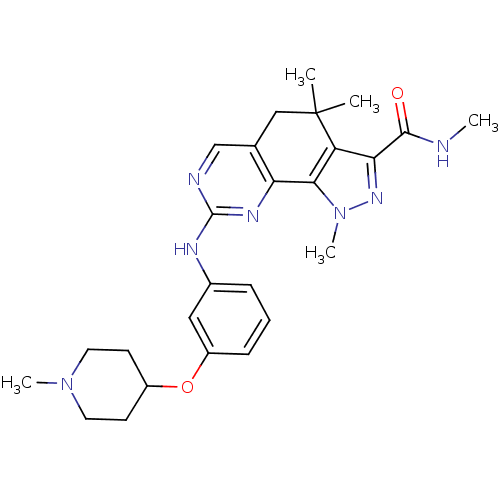
(pyrazolo[4,3-h]quinazoline-3-carboxamide, 33)Show SMILES CNC(=O)c1nn(C)c-2c1C(C)(C)Cc1cnc(Nc3cccc(OC4CCN(C)CC4)c3)nc-21 Show InChI InChI=1S/C26H33N7O2/c1-26(2)14-16-15-28-25(30-21(16)23-20(26)22(24(34)27-3)31-33(23)5)29-17-7-6-8-19(13-17)35-18-9-11-32(4)12-10-18/h6-8,13,15,18H,9-12,14H2,1-5H3,(H,27,34)(H,28,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 47 | n/a | n/a | n/a | n/a | 7.4 | 23 |
Nerviano Medical Sciences Srl
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzymes and substrates, followed by quantitation of the phosphorylat... |
J Med Chem 52: 5152-63 (2009)
Article DOI: 10.1021/jm9006559
BindingDB Entry DOI: 10.7270/Q2JH3JJP |
More data for this
Ligand-Target Pair | |
Choline kinase alpha
(Homo sapiens (Human)) | BDBM50579783

(CHEMBL5088850)Show SMILES Nc1nc(Cl)nc2n(cnc12)[C@@H]1CC[C@@H](CC1)C(=O)Nc1nc2CCS(=O)(=O)Cc2s1 |r,wU:11.12,14.19,(78.25,-20.67,;78.26,-22.21,;79.6,-22.97,;79.6,-24.52,;80.94,-25.29,;78.26,-25.3,;76.93,-24.52,;75.46,-25,;74.56,-23.75,;75.47,-22.51,;76.93,-22.98,;74.98,-26.46,;73.48,-26.77,;73,-28.23,;74.02,-29.38,;75.53,-29.07,;76.02,-27.6,;73.54,-30.85,;74.56,-32,;72.03,-31.16,;71,-30.01,;69.46,-30.15,;68.85,-28.75,;67.39,-28.28,;67.08,-26.77,;68.21,-25.74,;67.12,-24.65,;68.62,-24.24,;69.68,-26.23,;69.99,-27.72,;71.33,-28.49,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of ChoKalpha (unknown origin) by HTS assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128310
BindingDB Entry DOI: 10.7270/Q27H1PFF |
More data for this
Ligand-Target Pair | |
Choline kinase alpha
(Homo sapiens (Human)) | BDBM50579758
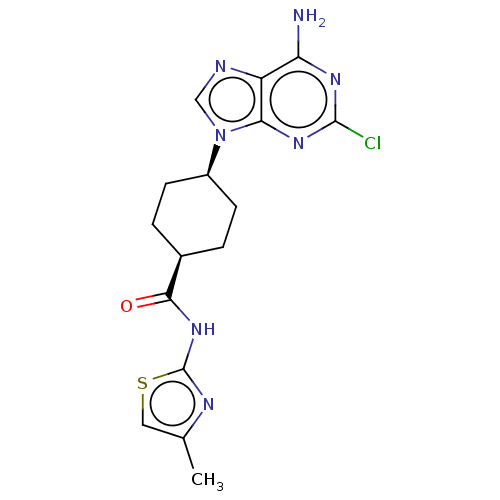
(CHEMBL5085127)Show SMILES Cc1csc(NC(=O)[C@H]2CC[C@H](CC2)n2cnc3c(N)nc(Cl)nc23)n1 |r,wU:11.14,8.7,(28.37,-27.42,;29.87,-27.75,;31.02,-26.72,;32.35,-27.5,;32.03,-29.01,;33.06,-30.16,;34.56,-29.85,;35.59,-31,;35.05,-28.39,;34.03,-27.23,;34.5,-25.78,;36.01,-25.47,;37.04,-26.61,;36.56,-28.07,;36.49,-24.01,;35.59,-22.76,;36.49,-21.52,;37.95,-21.99,;39.28,-21.22,;39.28,-19.68,;40.62,-21.98,;40.62,-23.53,;41.96,-24.3,;39.29,-24.3,;37.95,-23.53,;30.49,-29.16,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of ChoKalpha (unknown origin) by HTS assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128310
BindingDB Entry DOI: 10.7270/Q27H1PFF |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM31532

(pyrazolo[4,3-h]quinazoline-3-carboxamide, 1)Show InChI InChI=1S/C17H16N6O/c1-23-15-12(14(22-23)16(18)24)8-7-10-9-19-17(21-13(10)15)20-11-5-3-2-4-6-11/h2-6,9H,7-8H2,1H3,(H2,18,24)(H,19,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences srl
Curated by ChEMBL
| Assay Description
Inhibition of Aur-A assessed as [33P]-gamma-ATP incorporation into substrate by gamma counting |
Bioorg Med Chem Lett 21: 4507-11 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.122
BindingDB Entry DOI: 10.7270/Q2R211RS |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM31532

(pyrazolo[4,3-h]quinazoline-3-carboxamide, 1)Show InChI InChI=1S/C17H16N6O/c1-23-15-12(14(22-23)16(18)24)8-7-10-9-19-17(21-13(10)15)20-11-5-3-2-4-6-11/h2-6,9H,7-8H2,1H3,(H2,18,24)(H,19,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | 25 |
Nerviano Medical Sciences Srl
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzymes and substrates, followed by quantitation of the phosphorylat... |
J Med Chem 52: 5152-63 (2009)
Article DOI: 10.1021/jm9006559
BindingDB Entry DOI: 10.7270/Q2JH3JJP |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM31542

(pyrazolo[4,3-h]quinazoline-3-carboxamide, 25)Show SMILES Cn1nc(C(N)=O)c2CCc3cnc(Nc4ccc(cc4)N4CCOCC4)nc3-c12 Show InChI InChI=1S/C21H23N7O2/c1-27-19-16(18(26-27)20(22)29)7-2-13-12-23-21(25-17(13)19)24-14-3-5-15(6-4-14)28-8-10-30-11-9-28/h3-6,12H,2,7-11H2,1H3,(H2,22,29)(H,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | 25 |
Nerviano Medical Sciences Srl
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzymes and substrates, followed by quantitation of the phosphorylat... |
J Med Chem 52: 5152-63 (2009)
Article DOI: 10.1021/jm9006559
BindingDB Entry DOI: 10.7270/Q2JH3JJP |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM31539

(pyrazolo[4,3-h]quinazoline-3-carboxamide, 22)Show SMILES CN1CCN(CC1)c1ccc(Nc2ncc3CCc4c(nn(C)c4-c3n2)C(N)=O)cc1 Show InChI InChI=1S/C22H26N8O/c1-28-9-11-30(12-10-28)16-6-4-15(5-7-16)25-22-24-13-14-3-8-17-19(21(23)31)27-29(2)20(17)18(14)26-22/h4-7,13H,3,8-12H2,1-2H3,(H2,23,31)(H,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | 25 |
Nerviano Medical Sciences Srl
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzymes and substrates, followed by quantitation of the phosphorylat... |
J Med Chem 52: 5152-63 (2009)
Article DOI: 10.1021/jm9006559
BindingDB Entry DOI: 10.7270/Q2JH3JJP |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM31545

(Milciclib | pyrazolo[4,3-h]quinazoline-3-carboxami...)Show SMILES CNC(=O)c1nn(C)c-2c1C(C)(C)Cc1cnc(Nc3ccc(cc3)N3CCN(C)CC3)nc-21 Show InChI InChI=1S/C25H32N8O/c1-25(2)14-16-15-27-24(29-20(16)22-19(25)21(23(34)26-3)30-32(22)5)28-17-6-8-18(9-7-17)33-12-10-31(4)11-13-33/h6-9,15H,10-14H2,1-5H3,(H,26,34)(H,27,28,29) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 53 | n/a | n/a | n/a | n/a | 7.5 | 23 |
Nerviano Medical Sciences Srl
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzymes and substrates, followed by quantitation of the phosphorylat... |
J Med Chem 52: 5152-63 (2009)
Article DOI: 10.1021/jm9006559
BindingDB Entry DOI: 10.7270/Q2JH3JJP |
More data for this
Ligand-Target Pair | |
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM31551

(pyrazolo[4,3-h]quinazoline-3-carboxamide, 34)Show SMILES CNC(=O)c1nn(C)c-2c1C(C)(C)Cc1cnc(Nc3ccc(CN4CCN(C)CC4)cc3)nc-21 Show InChI InChI=1S/C26H34N8O/c1-26(2)14-18-15-28-25(30-21(18)23-20(26)22(24(35)27-3)31-33(23)5)29-19-8-6-17(7-9-19)16-34-12-10-32(4)11-13-34/h6-9,15H,10-14,16H2,1-5H3,(H,27,35)(H,28,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 55 | n/a | n/a | n/a | n/a | 7.4 | 23 |
Nerviano Medical Sciences Srl
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzymes and substrates, followed by quantitation of the phosphorylat... |
J Med Chem 52: 5152-63 (2009)
Article DOI: 10.1021/jm9006559
BindingDB Entry DOI: 10.7270/Q2JH3JJP |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data