

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rho-associated protein kinase 2 [1-543,T431N] (Homo sapiens (Human)) | BDBM25472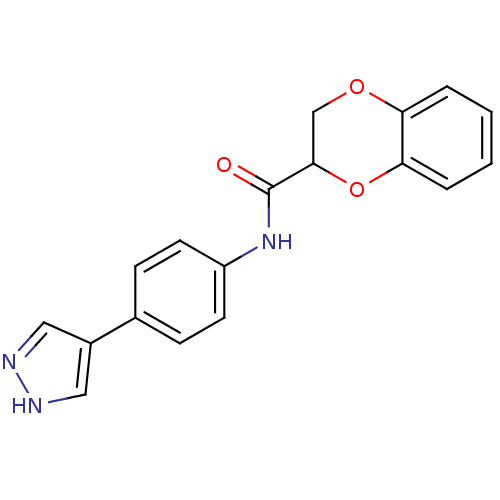 (CHEMBL519123 | N-[4-(1H-pyrazol-4-yl)phenyl]-2,3-d...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | 7.0 | 22 |
The Scripps Research Institute | Assay Description Assays were performed using the STK2 kinase system from Cisbio. Reaction mixture containing STK2 substrate, ATP and test compound was added to the we... | J Med Chem 51: 6642-5 (2008) Article DOI: 10.1021/jm800986w BindingDB Entry DOI: 10.7270/Q2JS9NRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 [1-543,T431N] (Homo sapiens (Human)) | BDBM25474 (JMC516642 Compound 5 | N-{2-[2-(dimethylamino)etho...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | 7.0 | 22 |
The Scripps Research Institute | Assay Description Assays were performed using the STK2 kinase system from Cisbio. Reaction mixture containing STK2 substrate, ATP and test compound was added to the we... | J Med Chem 51: 6642-5 (2008) Article DOI: 10.1021/jm800986w BindingDB Entry DOI: 10.7270/Q2JS9NRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 [1-543,T431N] (Homo sapiens (Human)) | BDBM25473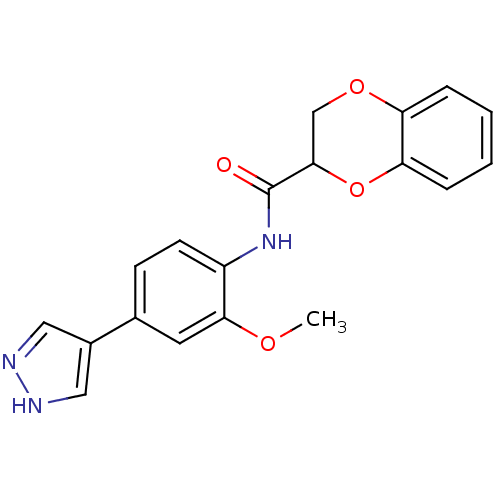 (N-[2-methoxy-4-(1H-pyrazol-4-yl)phenyl]-2,3-dihydr...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | 7.0 | 22 |
The Scripps Research Institute | Assay Description Assays were performed using the STK2 kinase system from Cisbio. Reaction mixture containing STK2 substrate, ATP and test compound was added to the we... | J Med Chem 51: 6642-5 (2008) Article DOI: 10.1021/jm800986w BindingDB Entry DOI: 10.7270/Q2JS9NRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 [1-543,T431N] (Homo sapiens (Human)) | BDBM25470 (N-[4-(pyridin-4-yl)-1,3-thiazol-2-yl]-2,3-dihydro-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.20 | n/a | n/a | n/a | n/a | 7.0 | 22 |
The Scripps Research Institute | Assay Description Assays were performed using the STK2 kinase system from Cisbio. Reaction mixture containing STK2 substrate, ATP and test compound was added to the we... | J Med Chem 51: 6642-5 (2008) Article DOI: 10.1021/jm800986w BindingDB Entry DOI: 10.7270/Q2JS9NRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 [1-543,T431N] (Homo sapiens (Human)) | BDBM25471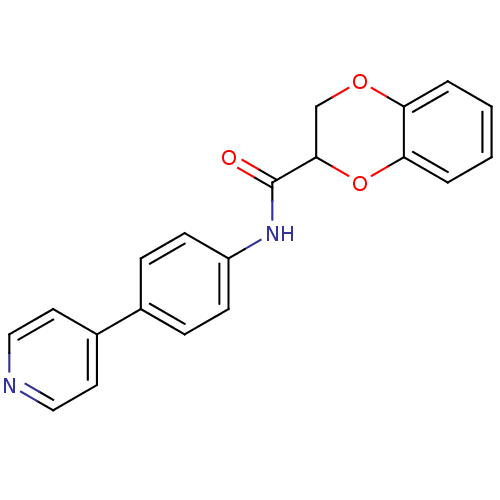 (N-[4-(pyridin-4-yl)phenyl]-2,3-dihydro-1,4-benzodi...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 46 | n/a | n/a | n/a | n/a | 7.0 | 22 |
The Scripps Research Institute | Assay Description Assays were performed using the STK2 kinase system from Cisbio. Reaction mixture containing STK2 substrate, ATP and test compound was added to the we... | J Med Chem 51: 6642-5 (2008) Article DOI: 10.1021/jm800986w BindingDB Entry DOI: 10.7270/Q2JS9NRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM22369 (4-(4-methanesulfonylphenyl)-3-phenyl-2,5-dihydrofu...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
Vittal Mallya Scientific Research Foundation Curated by ChEMBL | Assay Description Inhibitory activity against Prostaglandin G/H synthase 2 in human whole blood assay as LPS induced PGE-2 generation. | Bioorg Med Chem Lett 13: 657-60 (2003) BindingDB Entry DOI: 10.7270/Q2QF8S8R | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| cAMP-dependent protein kinase catalytic subunit alpha (Homo sapiens (Human)) | BDBM25472 (CHEMBL519123 | N-[4-(1H-pyrazol-4-yl)phenyl]-2,3-d...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 186 | n/a | n/a | n/a | n/a | 7.3 | 22 |
The Scripps Research Institute | Assay Description Reaction mixture of kemptide, ATP, and test compound was added to the wells using a BioRAPTR FRD Workstation (Aurora Discovery). Reaction was started... | J Med Chem 51: 6642-5 (2008) Article DOI: 10.1021/jm800986w BindingDB Entry DOI: 10.7270/Q2JS9NRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase MRCK alpha (Homo sapiens (Human)) | BDBM25473 (N-[2-methoxy-4-(1H-pyrazol-4-yl)phenyl]-2,3-dihydr...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 367 | n/a | n/a | n/a | n/a | 7.3 | 22 |
The Scripps Research Institute | Assay Description The mixture of a S6-peptide, ATP, and test compound was added to the wells using a BioRAPTR FRD Workstation (Aurora Discovery). Reaction was started ... | J Med Chem 51: 6642-5 (2008) Article DOI: 10.1021/jm800986w BindingDB Entry DOI: 10.7270/Q2JS9NRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-dependent protein kinase catalytic subunit alpha (Homo sapiens (Human)) | BDBM25470 (N-[4-(pyridin-4-yl)-1,3-thiazol-2-yl]-2,3-dihydro-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 637 | n/a | n/a | n/a | n/a | 7.3 | 22 |
The Scripps Research Institute | Assay Description Reaction mixture of kemptide, ATP, and test compound was added to the wells using a BioRAPTR FRD Workstation (Aurora Discovery). Reaction was started... | J Med Chem 51: 6642-5 (2008) Article DOI: 10.1021/jm800986w BindingDB Entry DOI: 10.7270/Q2JS9NRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-dependent protein kinase catalytic subunit alpha (Homo sapiens (Human)) | BDBM25471 (N-[4-(pyridin-4-yl)phenyl]-2,3-dihydro-1,4-benzodi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 692 | n/a | n/a | n/a | n/a | 7.3 | 22 |
The Scripps Research Institute | Assay Description Reaction mixture of kemptide, ATP, and test compound was added to the wells using a BioRAPTR FRD Workstation (Aurora Discovery). Reaction was started... | J Med Chem 51: 6642-5 (2008) Article DOI: 10.1021/jm800986w BindingDB Entry DOI: 10.7270/Q2JS9NRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 [1-543,T431N] (Homo sapiens (Human)) | BDBM25476 (N-{2-[2-(dimethylamino)ethoxy]-4-(1H-pyrazol-4-yl)...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 760 | n/a | n/a | n/a | n/a | 7.0 | 22 |
The Scripps Research Institute | Assay Description Assays were performed using the STK2 kinase system from Cisbio. Reaction mixture containing STK2 substrate, ATP and test compound was added to the we... | J Med Chem 51: 6642-5 (2008) Article DOI: 10.1021/jm800986w BindingDB Entry DOI: 10.7270/Q2JS9NRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 [1-543,T431N] (Homo sapiens (Human)) | BDBM25475 (2-[2-(dimethylamino)ethoxy]-4-(1H-pyrazol-4-yl)ani...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 920 | n/a | n/a | n/a | n/a | 7.0 | 22 |
The Scripps Research Institute | Assay Description Assays were performed using the STK2 kinase system from Cisbio. Reaction mixture containing STK2 substrate, ATP and test compound was added to the we... | J Med Chem 51: 6642-5 (2008) Article DOI: 10.1021/jm800986w BindingDB Entry DOI: 10.7270/Q2JS9NRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-dependent protein kinase catalytic subunit alpha (Homo sapiens (Human)) | BDBM25473 (N-[2-methoxy-4-(1H-pyrazol-4-yl)phenyl]-2,3-dihydr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 940 | n/a | n/a | n/a | n/a | 7.3 | 22 |
The Scripps Research Institute | Assay Description Reaction mixture of kemptide, ATP, and test compound was added to the wells using a BioRAPTR FRD Workstation (Aurora Discovery). Reaction was started... | J Med Chem 51: 6642-5 (2008) Article DOI: 10.1021/jm800986w BindingDB Entry DOI: 10.7270/Q2JS9NRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50124787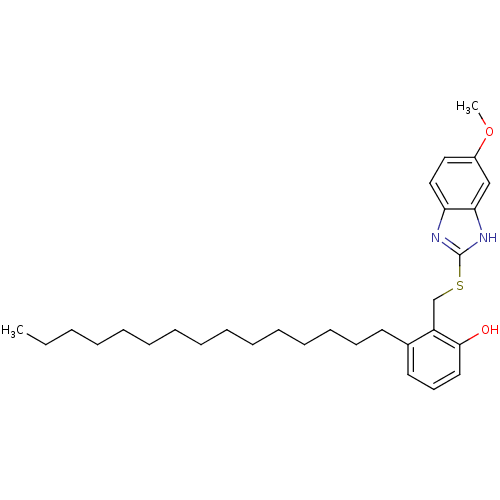 (2-(5-Methoxy-1H-benzoimidazol-2-ylsulfanylmethyl)-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Vittal Mallya Scientific Research Foundation Curated by ChEMBL | Assay Description Inhibitory activity against Prostaglandin G/H synthase 2 in human whole blood assay as LPS induced PGE-2 generation. | Bioorg Med Chem Lett 13: 657-60 (2003) BindingDB Entry DOI: 10.7270/Q2QF8S8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50124792 (2-(2-Methoxy-6-pentadecyl-benzylsulfanyl)-benzothi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.06E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Vittal Mallya Scientific Research Foundation Curated by ChEMBL | Assay Description Inhibitory activity against Prostaglandin G/H synthase 2 in human whole blood assay as LPS induced PGE-2 generation. | Bioorg Med Chem Lett 13: 657-60 (2003) BindingDB Entry DOI: 10.7270/Q2QF8S8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase MRCK alpha (Homo sapiens (Human)) | BDBM25474 (JMC516642 Compound 5 | N-{2-[2-(dimethylamino)etho...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.19E+3 | n/a | n/a | n/a | n/a | 7.3 | 22 |
The Scripps Research Institute | Assay Description The mixture of a S6-peptide, ATP, and test compound was added to the wells using a BioRAPTR FRD Workstation (Aurora Discovery). Reaction was started ... | J Med Chem 51: 6642-5 (2008) Article DOI: 10.1021/jm800986w BindingDB Entry DOI: 10.7270/Q2JS9NRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase MRCK alpha (Homo sapiens (Human)) | BDBM25472 (CHEMBL519123 | N-[4-(1H-pyrazol-4-yl)phenyl]-2,3-d...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.19E+3 | n/a | n/a | n/a | n/a | 7.3 | 22 |
The Scripps Research Institute | Assay Description The mixture of a S6-peptide, ATP, and test compound was added to the wells using a BioRAPTR FRD Workstation (Aurora Discovery). Reaction was started ... | J Med Chem 51: 6642-5 (2008) Article DOI: 10.1021/jm800986w BindingDB Entry DOI: 10.7270/Q2JS9NRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM25472 (CHEMBL519123 | N-[4-(1H-pyrazol-4-yl)phenyl]-2,3-d...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.41E+3 | n/a | n/a | n/a | n/a | 7.3 | 22 |
The Scripps Research Institute | Assay Description The mixture of a S6-peptide, ATP, and test compound was added to the wells using a BioRAPTR FRD Workstation (Aurora Discovery). Reaction was started ... | J Med Chem 51: 6642-5 (2008) Article DOI: 10.1021/jm800986w BindingDB Entry DOI: 10.7270/Q2JS9NRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50124790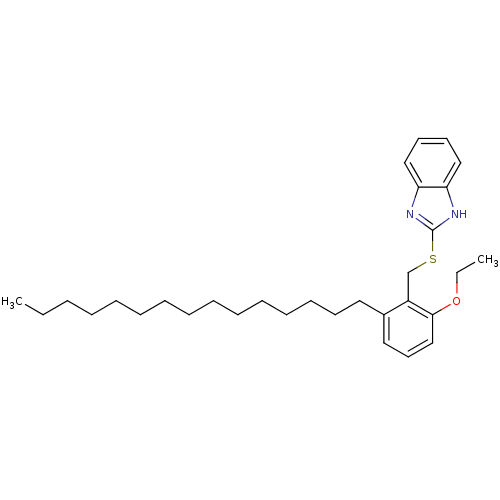 (2-(2-Ethoxy-6-pentadecyl-benzylsulfanyl)-1H-benzoi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.47E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Vittal Mallya Scientific Research Foundation Curated by ChEMBL | Assay Description Inhibitory activity against Prostaglandin G/H synthase 2 in human whole blood assay as LPS induced PGE-2 generation. | Bioorg Med Chem Lett 13: 657-60 (2003) BindingDB Entry DOI: 10.7270/Q2QF8S8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50124793 (2-(2-Methoxy-6-pentadecyl-benzylsulfanyl)-5-nitro-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.27E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Vittal Mallya Scientific Research Foundation Curated by ChEMBL | Assay Description Inhibitory activity against Prostaglandin G/H synthase 2 in human whole blood assay as LPS induced PGE-2 generation. | Bioorg Med Chem Lett 13: 657-60 (2003) BindingDB Entry DOI: 10.7270/Q2QF8S8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50124788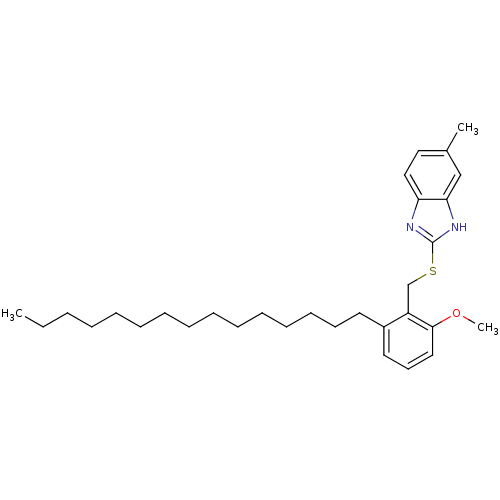 (2-(2-Methoxy-6-pentadecyl-benzylsulfanyl)-5-methyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.63E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Vittal Mallya Scientific Research Foundation Curated by ChEMBL | Assay Description Inhibitory activity against Prostaglandin G/H synthase 2 in human whole blood assay as LPS induced PGE-2 generation. | Bioorg Med Chem Lett 13: 657-60 (2003) BindingDB Entry DOI: 10.7270/Q2QF8S8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50124782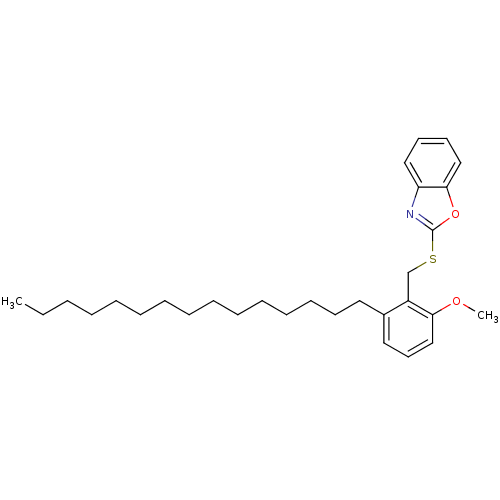 (2-(2-Methoxy-6-pentadecyl-benzylsulfanyl)-benzooxa...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.77E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Vittal Mallya Scientific Research Foundation Curated by ChEMBL | Assay Description Inhibitory activity against Prostaglandin G/H synthase 2 in human whole blood assay as LPS induced PGE-2 generation. | Bioorg Med Chem Lett 13: 657-60 (2003) BindingDB Entry DOI: 10.7270/Q2QF8S8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-dependent protein kinase catalytic subunit alpha (Homo sapiens (Human)) | BDBM25474 (JMC516642 Compound 5 | N-{2-[2-(dimethylamino)etho...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.97E+3 | n/a | n/a | n/a | n/a | 7.3 | 22 |
The Scripps Research Institute | Assay Description Reaction mixture of kemptide, ATP, and test compound was added to the wells using a BioRAPTR FRD Workstation (Aurora Discovery). Reaction was started... | J Med Chem 51: 6642-5 (2008) Article DOI: 10.1021/jm800986w BindingDB Entry DOI: 10.7270/Q2JS9NRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase MRCK alpha (Homo sapiens (Human)) | BDBM25470 (N-[4-(pyridin-4-yl)-1,3-thiazol-2-yl]-2,3-dihydro-...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.85E+3 | n/a | n/a | n/a | n/a | 7.3 | 22 |
The Scripps Research Institute | Assay Description The mixture of a S6-peptide, ATP, and test compound was added to the wells using a BioRAPTR FRD Workstation (Aurora Discovery). Reaction was started ... | J Med Chem 51: 6642-5 (2008) Article DOI: 10.1021/jm800986w BindingDB Entry DOI: 10.7270/Q2JS9NRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50124786 (2-(2-Ethoxy-6-pentadecyl-benzylsulfanyl)-5-methoxy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Vittal Mallya Scientific Research Foundation Curated by ChEMBL | Assay Description Inhibitory activity against Prostaglandin G/H synthase 2 in human whole blood assay as LPS induced PGE-2 generation. | Bioorg Med Chem Lett 13: 657-60 (2003) BindingDB Entry DOI: 10.7270/Q2QF8S8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM25471 (N-[4-(pyridin-4-yl)phenyl]-2,3-dihydro-1,4-benzodi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.44E+3 | n/a | n/a | n/a | n/a | 7.3 | 22 |
The Scripps Research Institute | Assay Description The mixture of a S6-peptide, ATP, and test compound was added to the wells using a BioRAPTR FRD Workstation (Aurora Discovery). Reaction was started ... | J Med Chem 51: 6642-5 (2008) Article DOI: 10.1021/jm800986w BindingDB Entry DOI: 10.7270/Q2JS9NRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase MRCK alpha (Homo sapiens (Human)) | BDBM25471 (N-[4-(pyridin-4-yl)phenyl]-2,3-dihydro-1,4-benzodi...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.05E+3 | n/a | n/a | n/a | n/a | 7.3 | 22 |
The Scripps Research Institute | Assay Description The mixture of a S6-peptide, ATP, and test compound was added to the wells using a BioRAPTR FRD Workstation (Aurora Discovery). Reaction was started ... | J Med Chem 51: 6642-5 (2008) Article DOI: 10.1021/jm800986w BindingDB Entry DOI: 10.7270/Q2JS9NRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM25474 (JMC516642 Compound 5 | N-{2-[2-(dimethylamino)etho...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.49E+3 | n/a | n/a | n/a | n/a | 7.3 | 22 |
The Scripps Research Institute | Assay Description The mixture of a S6-peptide, ATP, and test compound was added to the wells using a BioRAPTR FRD Workstation (Aurora Discovery). Reaction was started ... | J Med Chem 51: 6642-5 (2008) Article DOI: 10.1021/jm800986w BindingDB Entry DOI: 10.7270/Q2JS9NRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM25473 (N-[2-methoxy-4-(1H-pyrazol-4-yl)phenyl]-2,3-dihydr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8.82E+3 | n/a | n/a | n/a | n/a | 7.3 | 22 |
The Scripps Research Institute | Assay Description The mixture of a S6-peptide, ATP, and test compound was added to the wells using a BioRAPTR FRD Workstation (Aurora Discovery). Reaction was started ... | J Med Chem 51: 6642-5 (2008) Article DOI: 10.1021/jm800986w BindingDB Entry DOI: 10.7270/Q2JS9NRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50124796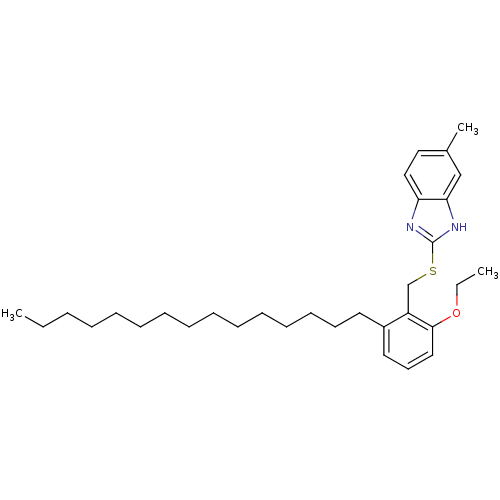 (2-(2-Ethoxy-6-pentadecyl-benzylsulfanyl)-5-methyl-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vittal Mallya Scientific Research Foundation Curated by ChEMBL | Assay Description Inhibitory activity against Prostaglandin G/H synthase 2 in human whole blood assay as LPS induced PGE-2 generation. | Bioorg Med Chem Lett 13: 657-60 (2003) BindingDB Entry DOI: 10.7270/Q2QF8S8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50124795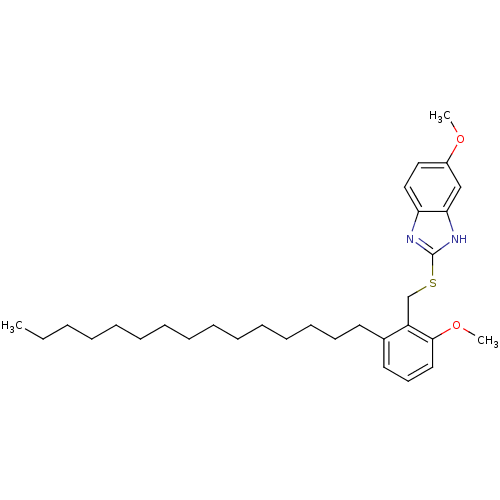 (5-Methoxy-2-(2-methoxy-6-pentadecyl-benzylsulfanyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vittal Mallya Scientific Research Foundation Curated by ChEMBL | Assay Description Inhibitory activity against Prostaglandin G/H synthase 2 in human whole blood assay as LPS induced PGE-2 generation. | Bioorg Med Chem Lett 13: 657-60 (2003) BindingDB Entry DOI: 10.7270/Q2QF8S8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50124794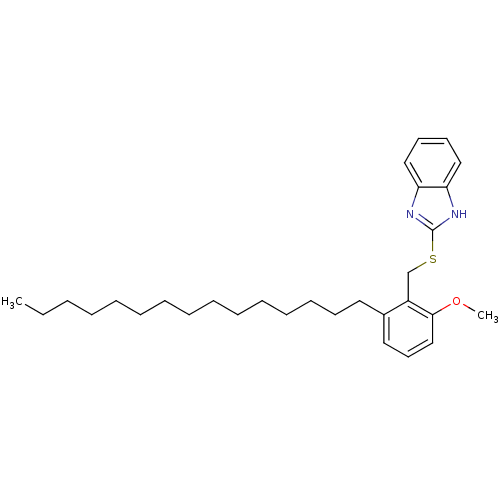 (2-(2-Methoxy-6-pentadecyl-benzylsulfanyl)-1H-benzo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vittal Mallya Scientific Research Foundation Curated by ChEMBL | Assay Description Inhibitory activity against Prostaglandin G/H synthase 2 in human whole blood assay as LPS induced PGE-2 generation. | Bioorg Med Chem Lett 13: 657-60 (2003) BindingDB Entry DOI: 10.7270/Q2QF8S8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50124791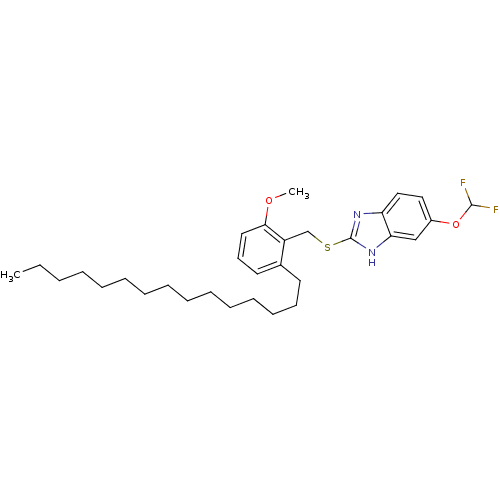 (5-Difluoromethoxy-2-(2-methoxy-6-pentadecyl-benzyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vittal Mallya Scientific Research Foundation Curated by ChEMBL | Assay Description Inhibitory activity against Prostaglandin G/H synthase 2 in human whole blood assay as LPS induced PGE-2 generation. | Bioorg Med Chem Lett 13: 657-60 (2003) BindingDB Entry DOI: 10.7270/Q2QF8S8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50124789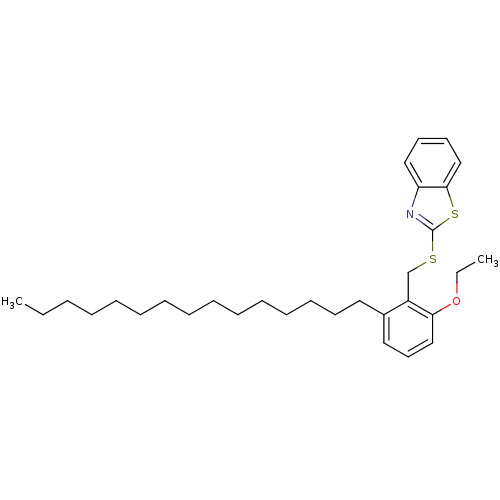 (2-(2-Ethoxy-6-pentadecyl-benzylsulfanyl)-benzothia...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vittal Mallya Scientific Research Foundation Curated by ChEMBL | Assay Description Inhibitory activity against Prostaglandin G/H synthase 2 in human whole blood assay as LPS induced PGE-2 generation. | Bioorg Med Chem Lett 13: 657-60 (2003) BindingDB Entry DOI: 10.7270/Q2QF8S8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50124784 (2-(2-Ethoxy-6-pentadecyl-benzylsulfanyl)-benzooxaz...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vittal Mallya Scientific Research Foundation Curated by ChEMBL | Assay Description Inhibitory activity against Prostaglandin G/H synthase 2 in human whole blood assay as LPS induced PGE-2 generation. | Bioorg Med Chem Lett 13: 657-60 (2003) BindingDB Entry DOI: 10.7270/Q2QF8S8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50124783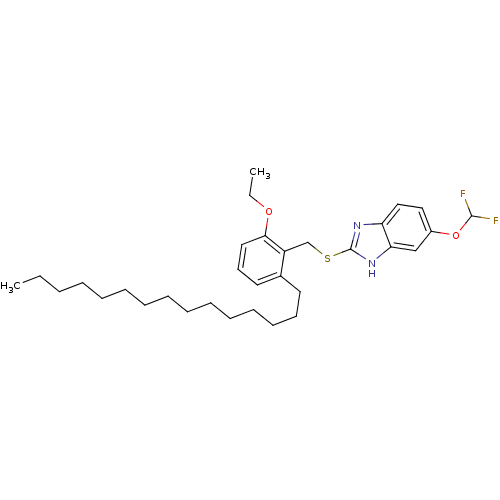 (5-Difluoromethoxy-2-(2-ethoxy-6-pentadecyl-benzyls...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vittal Mallya Scientific Research Foundation Curated by ChEMBL | Assay Description Inhibitory activity against Prostaglandin G/H synthase 2 in human whole blood assay as LPS induced PGE-2 generation. | Bioorg Med Chem Lett 13: 657-60 (2003) BindingDB Entry DOI: 10.7270/Q2QF8S8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM22369 (4-(4-methanesulfonylphenyl)-3-phenyl-2,5-dihydrofu...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 1.14E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vittal Mallya Scientific Research Foundation Curated by ChEMBL | Assay Description Inhibitory activity against Prostaglandin G/H synthase 1 in human whole blood assay as TXB2 generation | Bioorg Med Chem Lett 13: 657-60 (2003) BindingDB Entry DOI: 10.7270/Q2QF8S8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM25470 (N-[4-(pyridin-4-yl)-1,3-thiazol-2-yl]-2,3-dihydro-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | 7.3 | 22 |
The Scripps Research Institute | Assay Description The mixture of a S6-peptide, ATP, and test compound was added to the wells using a BioRAPTR FRD Workstation (Aurora Discovery). Reaction was started ... | J Med Chem 51: 6642-5 (2008) Article DOI: 10.1021/jm800986w BindingDB Entry DOI: 10.7270/Q2JS9NRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50124787 (2-(5-Methoxy-1H-benzoimidazol-2-ylsulfanylmethyl)-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.84E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Vittal Mallya Scientific Research Foundation Curated by ChEMBL | Assay Description Inhibitory activity against Prostaglandin G/H synthase 1 in human whole blood assay as TXB2 generation | Bioorg Med Chem Lett 13: 657-60 (2003) BindingDB Entry DOI: 10.7270/Q2QF8S8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50124785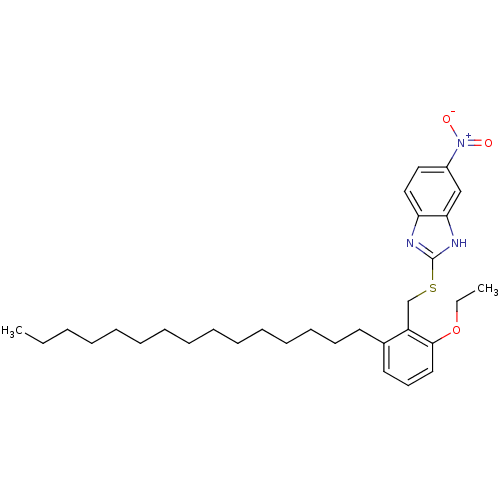 (2-(2-Ethoxy-6-pentadecyl-benzylsulfanyl)-5-nitro-1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.84E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Vittal Mallya Scientific Research Foundation Curated by ChEMBL | Assay Description Inhibitory activity against Prostaglandin G/H synthase 2 in human whole blood assay as LPS induced PGE-2 generation. | Bioorg Med Chem Lett 13: 657-60 (2003) BindingDB Entry DOI: 10.7270/Q2QF8S8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50124785 (2-(2-Ethoxy-6-pentadecyl-benzylsulfanyl)-5-nitro-1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Vittal Mallya Scientific Research Foundation Curated by ChEMBL | Assay Description Inhibitory activity against Prostaglandin G/H synthase 1 in human whole blood assay as TXB2 generation | Bioorg Med Chem Lett 13: 657-60 (2003) BindingDB Entry DOI: 10.7270/Q2QF8S8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50124782 (2-(2-Methoxy-6-pentadecyl-benzylsulfanyl)-benzooxa...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Vittal Mallya Scientific Research Foundation Curated by ChEMBL | Assay Description Inhibitory activity against Prostaglandin G/H synthase 1 in human whole blood assay as TXB2 generation | Bioorg Med Chem Lett 13: 657-60 (2003) BindingDB Entry DOI: 10.7270/Q2QF8S8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50124790 (2-(2-Ethoxy-6-pentadecyl-benzylsulfanyl)-1H-benzoi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Vittal Mallya Scientific Research Foundation Curated by ChEMBL | Assay Description Inhibitory activity against Prostaglandin G/H synthase 1 in human whole blood assay as TXB2 generation | Bioorg Med Chem Lett 13: 657-60 (2003) BindingDB Entry DOI: 10.7270/Q2QF8S8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50124792 (2-(2-Methoxy-6-pentadecyl-benzylsulfanyl)-benzothi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Vittal Mallya Scientific Research Foundation Curated by ChEMBL | Assay Description Inhibitory activity against Prostaglandin G/H synthase 1 in human whole blood assay as TXB2 generation | Bioorg Med Chem Lett 13: 657-60 (2003) BindingDB Entry DOI: 10.7270/Q2QF8S8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50124793 (2-(2-Methoxy-6-pentadecyl-benzylsulfanyl)-5-nitro-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Vittal Mallya Scientific Research Foundation Curated by ChEMBL | Assay Description Inhibitory activity against Prostaglandin G/H synthase 1 in human whole blood assay as TXB2 generation | Bioorg Med Chem Lett 13: 657-60 (2003) BindingDB Entry DOI: 10.7270/Q2QF8S8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50124786 (2-(2-Ethoxy-6-pentadecyl-benzylsulfanyl)-5-methoxy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Vittal Mallya Scientific Research Foundation Curated by ChEMBL | Assay Description Inhibitory activity against Prostaglandin G/H synthase 1 in human whole blood assay as TXB2 generation | Bioorg Med Chem Lett 13: 657-60 (2003) BindingDB Entry DOI: 10.7270/Q2QF8S8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50124788 (2-(2-Methoxy-6-pentadecyl-benzylsulfanyl)-5-methyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Vittal Mallya Scientific Research Foundation Curated by ChEMBL | Assay Description Inhibitory activity against Prostaglandin G/H synthase 1 in human whole blood assay as TXB2 generation | Bioorg Med Chem Lett 13: 657-60 (2003) BindingDB Entry DOI: 10.7270/Q2QF8S8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||