 Found 271 hits with Last Name = 'rasmussen' and Initial = 'ba'
Found 271 hits with Last Name = 'rasmussen' and Initial = 'ba' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Beta-lactamase TEM
(Escherichia coli) | BDBM50076678
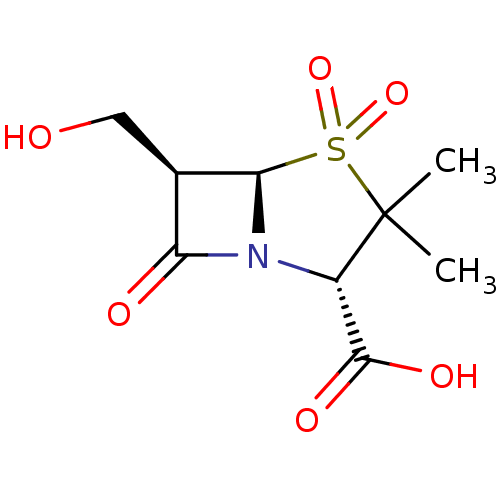
((2S,5R,6R)-6-Hydroxymethyl-3,3-dimethyl-4,4,7-trio...)Show SMILES CC1(C)[C@@H](N2[C@@H]([C@H](CO)C2=O)S1(=O)=O)C(O)=O Show InChI InChI=1S/C9H13NO6S/c1-9(2)5(8(13)14)10-6(12)4(3-11)7(10)17(9,15)16/h4-5,7,11H,3H2,1-2H3,(H,13,14)/t4-,5+,7-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against TEM-1 (class A) beta-lactamase |
Bioorg Med Chem Lett 9: 991-6 (1999)
BindingDB Entry DOI: 10.7270/Q2TB163Q |
More data for this
Ligand-Target Pair | |
Beta-lactamase TEM
(Escherichia coli) | BDBM50403733
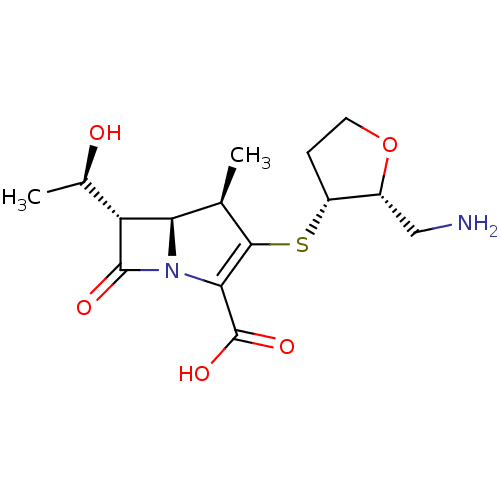
(CHEMBL1206880 | CL-191121)Show SMILES C[C@@H](O)[C@@H]1[C@H]2[C@@H](C)C(S[C@@H]3CCO[C@@H]3CN)=C(N2C1=O)C(O)=O |c:16| Show InChI InChI=1S/C15H22N2O5S/c1-6-11-10(7(2)18)14(19)17(11)12(15(20)21)13(6)23-9-3-4-22-8(9)5-16/h6-11,18H,3-5,16H2,1-2H3,(H,20,21)/t6-,7-,8-,9-,10-,11-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against TEM-1 (class A) beta-lactamase |
Bioorg Med Chem Lett 9: 991-6 (1999)
BindingDB Entry DOI: 10.7270/Q2TB163Q |
More data for this
Ligand-Target Pair | |
Beta-lactamase TEM
(Escherichia coli) | BDBM50157692
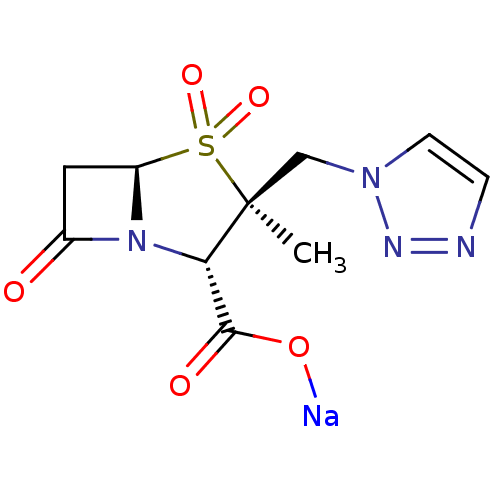
(CHEMBL1439 | CL-307579 | Sodium; (2S,3S,5R)-3-meth...)Show SMILES C[C@]1(Cn2ccnn2)[C@@H](N2[C@@H](CC2=O)S1(=O)=O)C(=O)O[Na] |r| Show InChI InChI=1S/C10H12N4O5S.Na/c1-10(5-13-3-2-11-12-13)8(9(16)17)14-6(15)4-7(14)20(10,18)19;/h2-3,7-8H,4-5H2,1H3,(H,16,17);/q;+1/p-1/t7-,8+,10+;/m1./s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against TEM-1 (class A) beta-lactamase |
Bioorg Med Chem Lett 9: 991-6 (1999)
BindingDB Entry DOI: 10.7270/Q2TB163Q |
More data for this
Ligand-Target Pair | |
Beta-lactamase
(Escherichia coli) | BDBM50076671
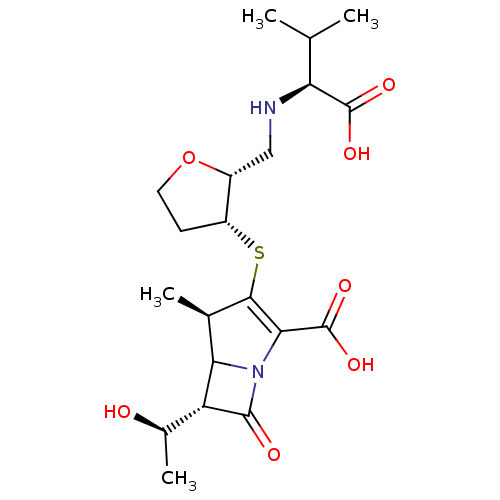
((4R,6S)-3-{(2R,3R)-2-[((S)-1-Carboxy-2-methyl-prop...)Show SMILES CC(C)[C@H](NC[C@H]1OCC[C@H]1SC1=C(N2C([C@@H]([C@@H](C)O)C2=O)[C@H]1C)C(O)=O)C(O)=O |t:13| Show InChI InChI=1S/C20H30N2O7S/c1-8(2)14(19(25)26)21-7-11-12(5-6-29-11)30-17-9(3)15-13(10(4)23)18(24)22(15)16(17)20(27)28/h8-15,21,23H,5-7H2,1-4H3,(H,25,26)(H,27,28)/t9-,10-,11-,12-,13-,14+,15?/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against AmpC (class C) beta-lactamase |
Bioorg Med Chem Lett 9: 991-6 (1999)
BindingDB Entry DOI: 10.7270/Q2TB163Q |
More data for this
Ligand-Target Pair | |
Beta-lactamase TEM
(Escherichia coli) | BDBM50076671

((4R,6S)-3-{(2R,3R)-2-[((S)-1-Carboxy-2-methyl-prop...)Show SMILES CC(C)[C@H](NC[C@H]1OCC[C@H]1SC1=C(N2C([C@@H]([C@@H](C)O)C2=O)[C@H]1C)C(O)=O)C(O)=O |t:13| Show InChI InChI=1S/C20H30N2O7S/c1-8(2)14(19(25)26)21-7-11-12(5-6-29-11)30-17-9(3)15-13(10(4)23)18(24)22(15)16(17)20(27)28/h8-15,21,23H,5-7H2,1-4H3,(H,25,26)(H,27,28)/t9-,10-,11-,12-,13-,14+,15?/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 91 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against TEM-1 (class A) beta-lactamase |
Bioorg Med Chem Lett 9: 991-6 (1999)
BindingDB Entry DOI: 10.7270/Q2TB163Q |
More data for this
Ligand-Target Pair | |
Beta-lactamase
(Escherichia coli) | BDBM50076673

(CHEMBL175189 | Sodium; (2S,5R,6R)-6-((S)-1-hydroxy...)Show SMILES C[C@H](O)[C@H]1[C@@H]2N([C@@H](C([O-])=O)C(C)(C)S2(=O)=O)C1=O Show InChI InChI=1S/C10H15NO6S/c1-4(12)5-7(13)11-6(9(14)15)10(2,3)18(16,17)8(5)11/h4-6,8,12H,1-3H3,(H,14,15)/p-1/t4-,5+,6-,8+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against AmpC (class C) beta-lactamase |
Bioorg Med Chem Lett 9: 991-6 (1999)
BindingDB Entry DOI: 10.7270/Q2TB163Q |
More data for this
Ligand-Target Pair | |
Beta-lactamase
(Escherichia coli) | BDBM50403733

(CHEMBL1206880 | CL-191121)Show SMILES C[C@@H](O)[C@@H]1[C@H]2[C@@H](C)C(S[C@@H]3CCO[C@@H]3CN)=C(N2C1=O)C(O)=O |c:16| Show InChI InChI=1S/C15H22N2O5S/c1-6-11-10(7(2)18)14(19)17(11)12(15(20)21)13(6)23-9-3-4-22-8(9)5-16/h6-11,18H,3-5,16H2,1-2H3,(H,20,21)/t6-,7-,8-,9-,10-,11-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against AmpC (class C) beta-lactamase |
Bioorg Med Chem Lett 9: 991-6 (1999)
BindingDB Entry DOI: 10.7270/Q2TB163Q |
More data for this
Ligand-Target Pair | |
Beta-lactamase
(Escherichia coli) | BDBM50076678

((2S,5R,6R)-6-Hydroxymethyl-3,3-dimethyl-4,4,7-trio...)Show SMILES CC1(C)[C@@H](N2[C@@H]([C@H](CO)C2=O)S1(=O)=O)C(O)=O Show InChI InChI=1S/C9H13NO6S/c1-9(2)5(8(13)14)10-6(12)4(3-11)7(10)17(9,15)16/h4-5,7,11H,3H2,1-2H3,(H,13,14)/t4-,5+,7-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against AmpC (class C) beta-lactamase |
Bioorg Med Chem Lett 9: 991-6 (1999)
BindingDB Entry DOI: 10.7270/Q2TB163Q |
More data for this
Ligand-Target Pair | |
Beta-lactamase TEM
(Escherichia coli) | BDBM50021954
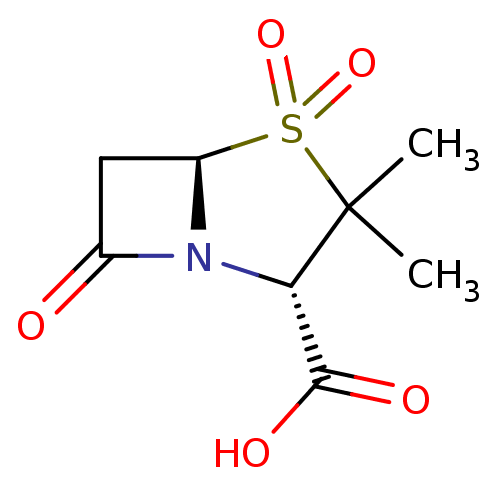
((2S,5R)-3,3-Dimethyl-4,4,7-trioxo-4lambda*6*-thia-...)Show SMILES CC1(C)[C@@H](N2[C@@H](CC2=O)S1(=O)=O)C(O)=O |r| Show InChI InChI=1S/C8H11NO5S/c1-8(2)6(7(11)12)9-4(10)3-5(9)15(8,13)14/h5-6H,3H2,1-2H3,(H,11,12)/t5-,6+/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against TEM-1 (class A) beta-lactamase |
Bioorg Med Chem Lett 9: 991-6 (1999)
BindingDB Entry DOI: 10.7270/Q2TB163Q |
More data for this
Ligand-Target Pair | |
Beta-lactamase
(Escherichia coli) | BDBM50076674

(CHEMBL177772 | Sodium; (2S,5R,6S)-6-((S)-1-hydroxy...)Show SMILES C[C@H](O)[C@@H]1[C@@H]2N([C@@H](C([O-])=O)C(C)(C)S2(=O)=O)C1=O Show InChI InChI=1S/C10H15NO6S/c1-4(12)5-7(13)11-6(9(14)15)10(2,3)18(16,17)8(5)11/h4-6,8,12H,1-3H3,(H,14,15)/p-1/t4-,5-,6-,8+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against AmpC (class C) beta-lactamase |
Bioorg Med Chem Lett 9: 991-6 (1999)
BindingDB Entry DOI: 10.7270/Q2TB163Q |
More data for this
Ligand-Target Pair | |
UDP-N-acetylglucosamine 1-carboxyvinyltransferase
(Escherichia coli K-12 (Enterobacteria)) | BDBM50475586

(CHEMBL198030)Show SMILES OC1=C(C(=O)O\C1=C/c1ccc(cc1)-c1cc(Cl)cc(Cl)c1)c1cc(Cl)cc(Cl)c1 |t:1| Show InChI InChI=1S/C23H12Cl4O3/c24-16-6-14(7-17(25)10-16)13-3-1-12(2-4-13)5-20-22(28)21(23(29)30-20)15-8-18(26)11-19(27)9-15/h1-11,28H/b20-5- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.09E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against MurA in Escherichia coli |
Bioorg Med Chem Lett 16: 176-80 (2006)
Article DOI: 10.1016/j.bmcl.2005.09.021
BindingDB Entry DOI: 10.7270/Q25M68G7 |
More data for this
Ligand-Target Pair | |
UDP-N-acetylglucosamine 1-carboxyvinyltransferase
(Escherichia coli K-12 (Enterobacteria)) | BDBM50475607
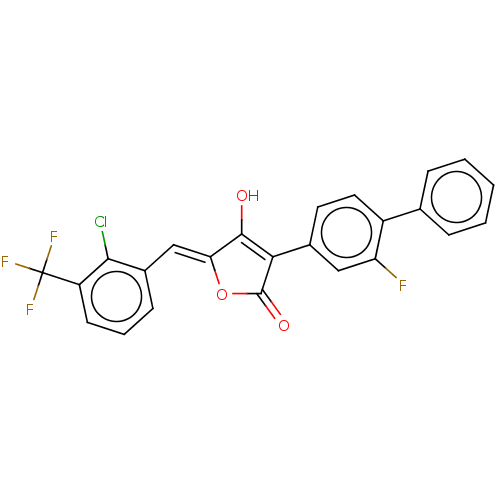
(CHEMBL199629)Show SMILES OC1=C(C(=O)O\C1=C/c1cccc(c1Cl)C(F)(F)F)c1ccc(c(F)c1)-c1ccccc1 |t:1| Show InChI InChI=1S/C24H13ClF4O3/c25-21-15(7-4-8-17(21)24(27,28)29)12-19-22(30)20(23(31)32-19)14-9-10-16(18(26)11-14)13-5-2-1-3-6-13/h1-12,30H/b19-12- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.17E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against MurA in Escherichia coli |
Bioorg Med Chem Lett 16: 176-80 (2006)
Article DOI: 10.1016/j.bmcl.2005.09.021
BindingDB Entry DOI: 10.7270/Q25M68G7 |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Escherichia coli K-12 (Enterobacteria)) | BDBM50475607

(CHEMBL199629)Show SMILES OC1=C(C(=O)O\C1=C/c1cccc(c1Cl)C(F)(F)F)c1ccc(c(F)c1)-c1ccccc1 |t:1| Show InChI InChI=1S/C24H13ClF4O3/c25-21-15(7-4-8-17(21)24(27,28)29)12-19-22(30)20(23(31)32-19)14-9-10-16(18(26)11-14)13-5-2-1-3-6-13/h1-12,30H/b19-12- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.17E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against MurB in Escherichia coli |
Bioorg Med Chem Lett 16: 176-80 (2006)
Article DOI: 10.1016/j.bmcl.2005.09.021
BindingDB Entry DOI: 10.7270/Q25M68G7 |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Escherichia coli K-12 (Enterobacteria)) | BDBM50475601

(CHEMBL199812)Show SMILES OC1=C(C(=O)O\C1=C/c1cc(ccc1Cl)C(F)(F)F)c1ccc(c(F)c1)-c1ccccc1 |t:1| Show InChI InChI=1S/C24H13ClF4O3/c25-18-9-7-16(24(27,28)29)10-15(18)12-20-22(30)21(23(31)32-20)14-6-8-17(19(26)11-14)13-4-2-1-3-5-13/h1-12,30H/b20-12- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.17E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against MurB in Escherichia coli |
Bioorg Med Chem Lett 16: 176-80 (2006)
Article DOI: 10.1016/j.bmcl.2005.09.021
BindingDB Entry DOI: 10.7270/Q25M68G7 |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Escherichia coli K-12 (Enterobacteria)) | BDBM50475590

(CHEMBL370421)Show SMILES OC1=C(C(=O)O\C1=C/c1cc(ccc1F)C(F)(F)F)c1ccc(c(F)c1)-c1ccccc1 |t:1| Show InChI InChI=1S/C24H13F5O3/c25-18-9-7-16(24(27,28)29)10-15(18)12-20-22(30)21(23(31)32-20)14-6-8-17(19(26)11-14)13-4-2-1-3-5-13/h1-12,30H/b20-12- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against MurB in Escherichia coli |
Bioorg Med Chem Lett 16: 176-80 (2006)
Article DOI: 10.1016/j.bmcl.2005.09.021
BindingDB Entry DOI: 10.7270/Q25M68G7 |
More data for this
Ligand-Target Pair | |
UDP-N-acetylglucosamine 1-carboxyvinyltransferase
(Escherichia coli K-12 (Enterobacteria)) | BDBM50475590

(CHEMBL370421)Show SMILES OC1=C(C(=O)O\C1=C/c1cc(ccc1F)C(F)(F)F)c1ccc(c(F)c1)-c1ccccc1 |t:1| Show InChI InChI=1S/C24H13F5O3/c25-18-9-7-16(24(27,28)29)10-15(18)12-20-22(30)21(23(31)32-20)14-6-8-17(19(26)11-14)13-4-2-1-3-5-13/h1-12,30H/b20-12- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against MurA in Escherichia coli |
Bioorg Med Chem Lett 16: 176-80 (2006)
Article DOI: 10.1016/j.bmcl.2005.09.021
BindingDB Entry DOI: 10.7270/Q25M68G7 |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Escherichia coli K-12 (Enterobacteria)) | BDBM50475599

(CHEMBL200097)Show SMILES Cc1ccccc1-c1ccc(\C=C2/OC(O)=C(C2=O)c2cc(Cl)cc(Cl)c2)cc1C |c:16| Show InChI InChI=1S/C25H18Cl2O3/c1-14-5-3-4-6-20(14)21-8-7-16(9-15(21)2)10-22-24(28)23(25(29)30-22)17-11-18(26)13-19(27)12-17/h3-13,29H,1-2H3/b22-10- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.28E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against MurB in Escherichia coli |
Bioorg Med Chem Lett 16: 176-80 (2006)
Article DOI: 10.1016/j.bmcl.2005.09.021
BindingDB Entry DOI: 10.7270/Q25M68G7 |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Escherichia coli K-12 (Enterobacteria)) | BDBM50475582
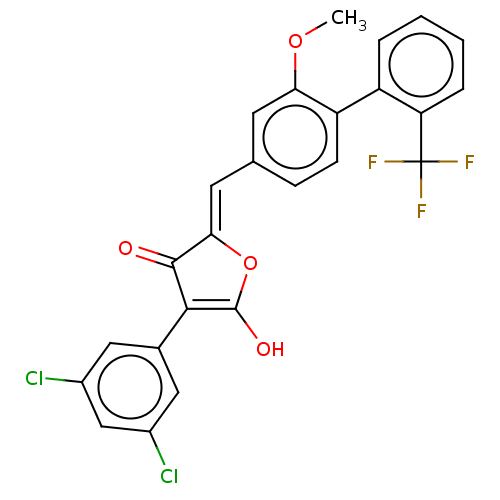
(CHEMBL199511)Show SMILES COc1cc(\C=C2/OC(O)=C(C2=O)c2cc(Cl)cc(Cl)c2)ccc1-c1ccccc1C(F)(F)F |c:9| Show InChI InChI=1S/C25H15Cl2F3O4/c1-33-20-8-13(6-7-18(20)17-4-2-3-5-19(17)25(28,29)30)9-21-23(31)22(24(32)34-21)14-10-15(26)12-16(27)11-14/h2-12,32H,1H3/b21-9- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.94E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against MurB in Escherichia coli |
Bioorg Med Chem Lett 16: 176-80 (2006)
Article DOI: 10.1016/j.bmcl.2005.09.021
BindingDB Entry DOI: 10.7270/Q25M68G7 |
More data for this
Ligand-Target Pair | |
UDP-N-acetylglucosamine 1-carboxyvinyltransferase 1
(Staphylococcus aureus subsp. aureus N315) | BDBM50475596
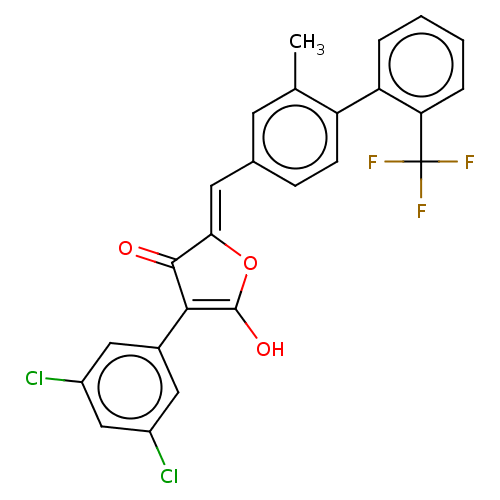
(CHEMBL200015)Show SMILES Cc1cc(\C=C2/OC(O)=C(C2=O)c2cc(Cl)cc(Cl)c2)ccc1-c1ccccc1C(F)(F)F |c:8| Show InChI InChI=1S/C25H15Cl2F3O3/c1-13-8-14(6-7-18(13)19-4-2-3-5-20(19)25(28,29)30)9-21-23(31)22(24(32)33-21)15-10-16(26)12-17(27)11-15/h2-12,32H,1H3/b21-9- | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against MurA in Staphylococcus aureus |
Bioorg Med Chem Lett 16: 176-80 (2006)
Article DOI: 10.1016/j.bmcl.2005.09.021
BindingDB Entry DOI: 10.7270/Q25M68G7 |
More data for this
Ligand-Target Pair | |
UDP-N-acetylglucosamine 1-carboxyvinyltransferase
(Escherichia coli K-12 (Enterobacteria)) | BDBM50475591

(CHEMBL382328)Show SMILES OC1=C(C(=O)O\C1=C/c1cccc(c1)-c1cc(Cl)cc(Cl)c1)c1cc(Cl)cc(Cl)c1 |t:1| Show InChI InChI=1S/C23H12Cl4O3/c24-16-6-14(7-17(25)10-16)13-3-1-2-12(4-13)5-20-22(28)21(23(29)30-20)15-8-18(26)11-19(27)9-15/h1-11,28H/b20-5- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.18E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against MurA in Escherichia coli |
Bioorg Med Chem Lett 16: 176-80 (2006)
Article DOI: 10.1016/j.bmcl.2005.09.021
BindingDB Entry DOI: 10.7270/Q25M68G7 |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Escherichia coli K-12 (Enterobacteria)) | BDBM50475587

(CHEMBL426651)Show SMILES OC1=C(C(=O)O\C1=C/c1ccccc1-c1ccc(Cl)c(Cl)c1)c1ccc(Cl)c(Cl)c1 |t:1| Show InChI InChI=1S/C23H12Cl4O3/c24-16-7-5-13(9-18(16)26)15-4-2-1-3-12(15)11-20-22(28)21(23(29)30-20)14-6-8-17(25)19(27)10-14/h1-11,28H/b20-11- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.18E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against MurB in Escherichia coli |
Bioorg Med Chem Lett 16: 176-80 (2006)
Article DOI: 10.1016/j.bmcl.2005.09.021
BindingDB Entry DOI: 10.7270/Q25M68G7 |
More data for this
Ligand-Target Pair | |
UDP-N-acetylmuramate--L-alanine ligase
(Escherichia coli) | BDBM50475590

(CHEMBL370421)Show SMILES OC1=C(C(=O)O\C1=C/c1cc(ccc1F)C(F)(F)F)c1ccc(c(F)c1)-c1ccccc1 |t:1| Show InChI InChI=1S/C24H13F5O3/c25-18-9-7-16(24(27,28)29)10-15(18)12-20-22(30)21(23(31)32-20)14-6-8-17(19(26)11-14)13-4-2-1-3-5-13/h1-12,30H/b20-12- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against MurC in Escherichia coli |
Bioorg Med Chem Lett 16: 176-80 (2006)
Article DOI: 10.1016/j.bmcl.2005.09.021
BindingDB Entry DOI: 10.7270/Q25M68G7 |
More data for this
Ligand-Target Pair | |
UDP-N-acetylglucosamine 1-carboxyvinyltransferase
(Escherichia coli K-12 (Enterobacteria)) | BDBM50475599

(CHEMBL200097)Show SMILES Cc1ccccc1-c1ccc(\C=C2/OC(O)=C(C2=O)c2cc(Cl)cc(Cl)c2)cc1C |c:16| Show InChI InChI=1S/C25H18Cl2O3/c1-14-5-3-4-6-20(14)21-8-7-16(9-15(21)2)10-22-24(28)23(25(29)30-22)17-11-18(26)13-19(27)12-17/h3-13,29H,1-2H3/b22-10- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.57E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against MurA in Escherichia coli |
Bioorg Med Chem Lett 16: 176-80 (2006)
Article DOI: 10.1016/j.bmcl.2005.09.021
BindingDB Entry DOI: 10.7270/Q25M68G7 |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Staphylococcus aureus (Firmicutes)) | BDBM50166493

(4-(2-Benzylsulfanyl-ethyl)-1,2-bis-(4-chloro-pheny...)Show SMILES Oc1c(CCSCc2ccccc2)c(=O)n(-c2ccc(Cl)cc2)n1-c1ccc(Cl)cc1 Show InChI InChI=1S/C24H20Cl2N2O2S/c25-18-6-10-20(11-7-18)27-23(29)22(14-15-31-16-17-4-2-1-3-5-17)24(30)28(27)21-12-8-19(26)9-13-21/h1-13,29H,14-16H2 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MurB enzyme in Staphylococcus aureus |
Bioorg Med Chem Lett 15: 2527-31 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.058
BindingDB Entry DOI: 10.7270/Q2BG2NHW |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Escherichia coli K-12 (Enterobacteria)) | BDBM50475602
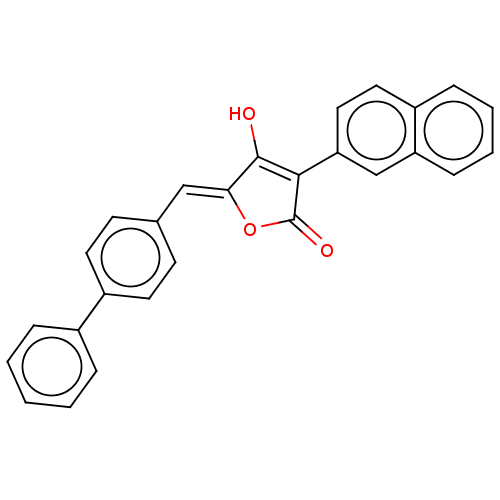
(CHEMBL199929)Show SMILES OC1=C(C(=O)O\C1=C/c1ccc(cc1)-c1ccccc1)c1ccc2ccccc2c1 |t:1| Show InChI InChI=1S/C27H18O3/c28-26-24(16-18-10-12-21(13-11-18)19-6-2-1-3-7-19)30-27(29)25(26)23-15-14-20-8-4-5-9-22(20)17-23/h1-17,28H/b24-16- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.12E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against MurB in Escherichia coli |
Bioorg Med Chem Lett 16: 176-80 (2006)
Article DOI: 10.1016/j.bmcl.2005.09.021
BindingDB Entry DOI: 10.7270/Q25M68G7 |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Staphylococcus aureus (Firmicutes)) | BDBM50166480
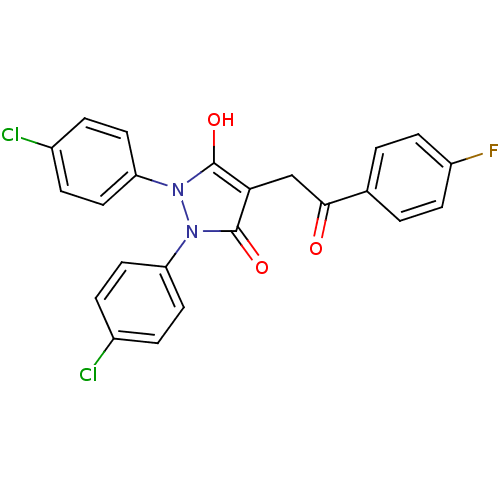
(1,2-Bis-(4-chloro-phenyl)-4-[2-(4-fluoro-phenyl)-2...)Show SMILES Oc1c(CC(=O)c2ccc(F)cc2)c(=O)n(-c2ccc(Cl)cc2)n1-c1ccc(Cl)cc1 Show InChI InChI=1S/C23H15Cl2FN2O3/c24-15-3-9-18(10-4-15)27-22(30)20(13-21(29)14-1-7-17(26)8-2-14)23(31)28(27)19-11-5-16(25)6-12-19/h1-12,30H,13H2 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MurB enzyme in Staphylococcus aureus |
Bioorg Med Chem Lett 15: 2527-31 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.058
BindingDB Entry DOI: 10.7270/Q2BG2NHW |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Staphylococcus aureus (Firmicutes)) | BDBM50166485
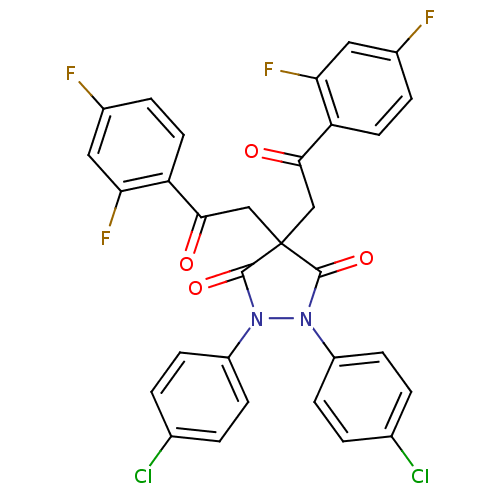
(1,2-Bis-(4-chloro-phenyl)-4,4-bis-[2-(2,4-difluoro...)Show SMILES Fc1ccc(C(=O)CC2(CC(=O)c3ccc(F)cc3F)C(=O)N(N(C2=O)c2ccc(Cl)cc2)c2ccc(Cl)cc2)c(F)c1 Show InChI InChI=1S/C31H18Cl2F4N2O4/c32-17-1-7-21(8-2-17)38-29(42)31(15-27(40)23-11-5-19(34)13-25(23)36,16-28(41)24-12-6-20(35)14-26(24)37)30(43)39(38)22-9-3-18(33)4-10-22/h1-14H,15-16H2 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MurB enzyme in Staphylococcus aureus |
Bioorg Med Chem Lett 15: 2527-31 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.058
BindingDB Entry DOI: 10.7270/Q2BG2NHW |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Escherichia coli K-12 (Enterobacteria)) | BDBM50166493

(4-(2-Benzylsulfanyl-ethyl)-1,2-bis-(4-chloro-pheny...)Show SMILES Oc1c(CCSCc2ccccc2)c(=O)n(-c2ccc(Cl)cc2)n1-c1ccc(Cl)cc1 Show InChI InChI=1S/C24H20Cl2N2O2S/c25-18-6-10-20(11-7-18)27-23(29)22(14-15-31-16-17-4-2-1-3-5-17)24(30)28(27)21-12-8-19(26)9-13-21/h1-13,29H,14-16H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MurB enzyme in Escherichia coli |
Bioorg Med Chem Lett 15: 2527-31 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.058
BindingDB Entry DOI: 10.7270/Q2BG2NHW |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Escherichia coli K-12 (Enterobacteria)) | BDBM50166480

(1,2-Bis-(4-chloro-phenyl)-4-[2-(4-fluoro-phenyl)-2...)Show SMILES Oc1c(CC(=O)c2ccc(F)cc2)c(=O)n(-c2ccc(Cl)cc2)n1-c1ccc(Cl)cc1 Show InChI InChI=1S/C23H15Cl2FN2O3/c24-15-3-9-18(10-4-15)27-22(30)20(13-21(29)14-1-7-17(26)8-2-14)23(31)28(27)19-11-5-16(25)6-12-19/h1-12,30H,13H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MurB enzyme in Escherichia coli |
Bioorg Med Chem Lett 15: 2527-31 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.058
BindingDB Entry DOI: 10.7270/Q2BG2NHW |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Escherichia coli K-12 (Enterobacteria)) | BDBM50166500
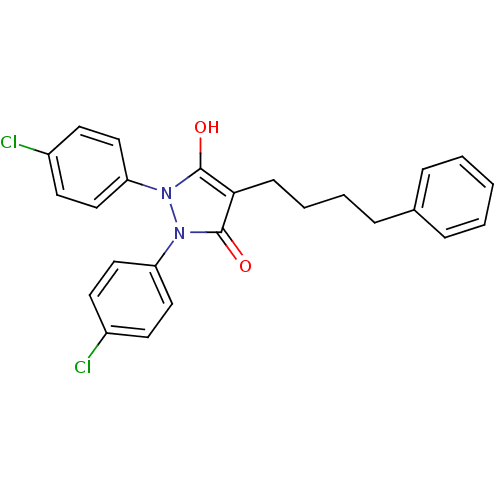
(1,2-Bis-(4-chloro-phenyl)-4-(4-phenyl-butyl)-pyraz...)Show SMILES Oc1c(CCCCc2ccccc2)c(=O)n(-c2ccc(Cl)cc2)n1-c1ccc(Cl)cc1 Show InChI InChI=1S/C25H22Cl2N2O2/c26-19-10-14-21(15-11-19)28-24(30)23(9-5-4-8-18-6-2-1-3-7-18)25(31)29(28)22-16-12-20(27)13-17-22/h1-3,6-7,10-17,30H,4-5,8-9H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MurB enzyme in Escherichia coli |
Bioorg Med Chem Lett 15: 2527-31 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.058
BindingDB Entry DOI: 10.7270/Q2BG2NHW |
More data for this
Ligand-Target Pair | |
Beta-lactamase
(Escherichia coli) | BDBM50076672
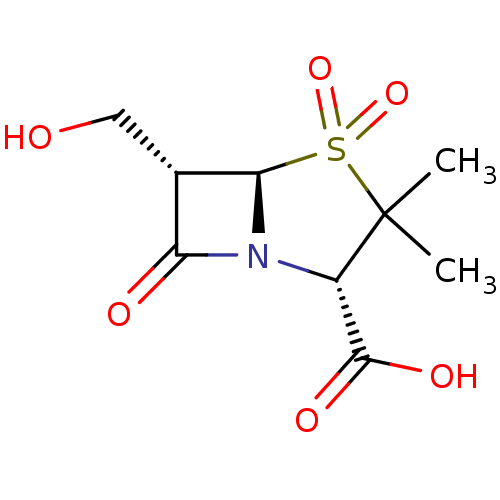
((2S,5R,6S)-6-Hydroxymethyl-3,3-dimethyl-4,4,7-trio...)Show SMILES CC1(C)[C@@H](N2[C@@H]([C@@H](CO)C2=O)S1(=O)=O)C(O)=O Show InChI InChI=1S/C9H13NO6S/c1-9(2)5(8(13)14)10-6(12)4(3-11)7(10)17(9,15)16/h4-5,7,11H,3H2,1-2H3,(H,13,14)/t4-,5-,7+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against AmpC (class C) beta-lactamase |
Bioorg Med Chem Lett 9: 991-6 (1999)
BindingDB Entry DOI: 10.7270/Q2TB163Q |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Escherichia coli K-12 (Enterobacteria)) | BDBM50166485

(1,2-Bis-(4-chloro-phenyl)-4,4-bis-[2-(2,4-difluoro...)Show SMILES Fc1ccc(C(=O)CC2(CC(=O)c3ccc(F)cc3F)C(=O)N(N(C2=O)c2ccc(Cl)cc2)c2ccc(Cl)cc2)c(F)c1 Show InChI InChI=1S/C31H18Cl2F4N2O4/c32-17-1-7-21(8-2-17)38-29(42)31(15-27(40)23-11-5-19(34)13-25(23)36,16-28(41)24-12-6-20(35)14-26(24)37)30(43)39(38)22-9-3-18(33)4-10-22/h1-14H,15-16H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MurB enzyme in Escherichia coli |
Bioorg Med Chem Lett 15: 2527-31 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.058
BindingDB Entry DOI: 10.7270/Q2BG2NHW |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Escherichia coli K-12 (Enterobacteria)) | BDBM50475596

(CHEMBL200015)Show SMILES Cc1cc(\C=C2/OC(O)=C(C2=O)c2cc(Cl)cc(Cl)c2)ccc1-c1ccccc1C(F)(F)F |c:8| Show InChI InChI=1S/C25H15Cl2F3O3/c1-13-8-14(6-7-18(13)19-4-2-3-5-20(19)25(28,29)30)9-21-23(31)22(24(32)33-21)15-10-16(26)12-17(27)11-15/h2-12,32H,1H3/b21-9- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against MurB in Escherichia coli |
Bioorg Med Chem Lett 16: 176-80 (2006)
Article DOI: 10.1016/j.bmcl.2005.09.021
BindingDB Entry DOI: 10.7270/Q25M68G7 |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Escherichia coli K-12 (Enterobacteria)) | BDBM50475591

(CHEMBL382328)Show SMILES OC1=C(C(=O)O\C1=C/c1cccc(c1)-c1cc(Cl)cc(Cl)c1)c1cc(Cl)cc(Cl)c1 |t:1| Show InChI InChI=1S/C23H12Cl4O3/c24-16-6-14(7-17(25)10-16)13-3-1-2-12(4-13)5-20-22(28)21(23(29)30-20)15-8-18(26)11-19(27)9-15/h1-11,28H/b20-5- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.27E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against MurB in Escherichia coli |
Bioorg Med Chem Lett 16: 176-80 (2006)
Article DOI: 10.1016/j.bmcl.2005.09.021
BindingDB Entry DOI: 10.7270/Q25M68G7 |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Escherichia coli K-12 (Enterobacteria)) | BDBM50475586

(CHEMBL198030)Show SMILES OC1=C(C(=O)O\C1=C/c1ccc(cc1)-c1cc(Cl)cc(Cl)c1)c1cc(Cl)cc(Cl)c1 |t:1| Show InChI InChI=1S/C23H12Cl4O3/c24-16-6-14(7-17(25)10-16)13-3-1-12(2-4-13)5-20-22(28)21(23(29)30-20)15-8-18(26)11-19(27)9-15/h1-11,28H/b20-5- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.27E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against MurB in Escherichia coli |
Bioorg Med Chem Lett 16: 176-80 (2006)
Article DOI: 10.1016/j.bmcl.2005.09.021
BindingDB Entry DOI: 10.7270/Q25M68G7 |
More data for this
Ligand-Target Pair | |
UDP-N-acetylglucosamine 1-carboxyvinyltransferase
(Escherichia coli K-12 (Enterobacteria)) | BDBM50475587

(CHEMBL426651)Show SMILES OC1=C(C(=O)O\C1=C/c1ccccc1-c1ccc(Cl)c(Cl)c1)c1ccc(Cl)c(Cl)c1 |t:1| Show InChI InChI=1S/C23H12Cl4O3/c24-16-7-5-13(9-18(16)26)15-4-2-1-3-12(15)11-20-22(28)21(23(29)30-20)14-6-8-17(25)19(27)10-14/h1-11,28H/b20-11- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.27E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against MurA in Escherichia coli |
Bioorg Med Chem Lett 16: 176-80 (2006)
Article DOI: 10.1016/j.bmcl.2005.09.021
BindingDB Entry DOI: 10.7270/Q25M68G7 |
More data for this
Ligand-Target Pair | |
UDP-N-acetylglucosamine 1-carboxyvinyltransferase
(Escherichia coli K-12 (Enterobacteria)) | BDBM50475601

(CHEMBL199812)Show SMILES OC1=C(C(=O)O\C1=C/c1cc(ccc1Cl)C(F)(F)F)c1ccc(c(F)c1)-c1ccccc1 |t:1| Show InChI InChI=1S/C24H13ClF4O3/c25-18-9-7-16(24(27,28)29)10-15(18)12-20-22(30)21(23(31)32-20)14-6-8-17(19(26)11-14)13-4-2-1-3-5-13/h1-12,30H/b20-12- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.51E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against MurA in Escherichia coli |
Bioorg Med Chem Lett 16: 176-80 (2006)
Article DOI: 10.1016/j.bmcl.2005.09.021
BindingDB Entry DOI: 10.7270/Q25M68G7 |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Escherichia coli K-12 (Enterobacteria)) | BDBM50475603
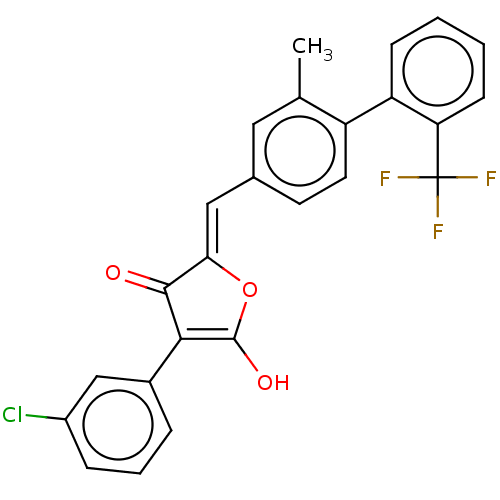
(CHEMBL199320)Show SMILES Cc1cc(\C=C2/OC(O)=C(C2=O)c2cccc(Cl)c2)ccc1-c1ccccc1C(F)(F)F |c:8| Show InChI InChI=1S/C25H16ClF3O3/c1-14-11-15(9-10-18(14)19-7-2-3-8-20(19)25(27,28)29)12-21-23(30)22(24(31)32-21)16-5-4-6-17(26)13-16/h2-13,31H,1H3/b21-12- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.56E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against MurB in Escherichia coli |
Bioorg Med Chem Lett 16: 176-80 (2006)
Article DOI: 10.1016/j.bmcl.2005.09.021
BindingDB Entry DOI: 10.7270/Q25M68G7 |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Staphylococcus aureus (Firmicutes)) | BDBM50166500

(1,2-Bis-(4-chloro-phenyl)-4-(4-phenyl-butyl)-pyraz...)Show SMILES Oc1c(CCCCc2ccccc2)c(=O)n(-c2ccc(Cl)cc2)n1-c1ccc(Cl)cc1 Show InChI InChI=1S/C25H22Cl2N2O2/c26-19-10-14-21(15-11-19)28-24(30)23(9-5-4-8-18-6-2-1-3-7-18)25(31)29(28)22-16-12-20(27)13-17-22/h1-3,6-7,10-17,30H,4-5,8-9H2 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MurB enzyme in Staphylococcus aureus |
Bioorg Med Chem Lett 15: 2527-31 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.058
BindingDB Entry DOI: 10.7270/Q2BG2NHW |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Escherichia coli K-12 (Enterobacteria)) | BDBM50166475

(1,2-Bis-(4-chloro-phenyl)-4,4-bis-(6-phenyl-hexyl)...)Show SMILES Clc1ccc(cc1)N1N(C(=O)C(CCCCCCc2ccccc2)(CCCCCCc2ccccc2)C1=O)c1ccc(Cl)cc1 Show InChI InChI=1S/C39H42Cl2N2O2/c40-33-21-25-35(26-22-33)42-37(44)39(38(45)43(42)36-27-23-34(41)24-28-36,29-13-3-1-7-15-31-17-9-5-10-18-31)30-14-4-2-8-16-32-19-11-6-12-20-32/h5-6,9-12,17-28H,1-4,7-8,13-16,29-30H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MurB enzyme in Escherichia coli |
Bioorg Med Chem Lett 15: 2527-31 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.058
BindingDB Entry DOI: 10.7270/Q2BG2NHW |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Escherichia coli K-12 (Enterobacteria)) | BDBM50166499

(4,4-Bis-(2-benzyloxy-ethyl)-1,2-bis-(4-chloro-phen...)Show SMILES Clc1ccc(cc1)N1N(C(=O)C(CCOCc2ccccc2)(CCOCc2ccccc2)C1=O)c1ccc(Cl)cc1 Show InChI InChI=1S/C33H30Cl2N2O4/c34-27-11-15-29(16-12-27)36-31(38)33(19-21-40-23-25-7-3-1-4-8-25,20-22-41-24-26-9-5-2-6-10-26)32(39)37(36)30-17-13-28(35)14-18-30/h1-18H,19-24H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MurB enzyme in Escherichia coli |
Bioorg Med Chem Lett 15: 2527-31 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.058
BindingDB Entry DOI: 10.7270/Q2BG2NHW |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Staphylococcus aureus (Firmicutes)) | BDBM50166499

(4,4-Bis-(2-benzyloxy-ethyl)-1,2-bis-(4-chloro-phen...)Show SMILES Clc1ccc(cc1)N1N(C(=O)C(CCOCc2ccccc2)(CCOCc2ccccc2)C1=O)c1ccc(Cl)cc1 Show InChI InChI=1S/C33H30Cl2N2O4/c34-27-11-15-29(16-12-27)36-31(38)33(19-21-40-23-25-7-3-1-4-8-25,20-22-41-24-26-9-5-2-6-10-26)32(39)37(36)30-17-13-28(35)14-18-30/h1-18H,19-24H2 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MurB enzyme in Staphylococcus aureus |
Bioorg Med Chem Lett 15: 2527-31 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.058
BindingDB Entry DOI: 10.7270/Q2BG2NHW |
More data for this
Ligand-Target Pair | |
UDP-N-acetylglucosamine 1-carboxyvinyltransferase
(Escherichia coli K-12 (Enterobacteria)) | BDBM50475582

(CHEMBL199511)Show SMILES COc1cc(\C=C2/OC(O)=C(C2=O)c2cc(Cl)cc(Cl)c2)ccc1-c1ccccc1C(F)(F)F |c:9| Show InChI InChI=1S/C25H15Cl2F3O4/c1-33-20-8-13(6-7-18(20)17-4-2-3-5-19(17)25(28,29)30)9-21-23(31)22(24(32)34-21)14-10-15(26)12-16(27)11-14/h2-12,32H,1H3/b21-9- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.88E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against MurA in Escherichia coli |
Bioorg Med Chem Lett 16: 176-80 (2006)
Article DOI: 10.1016/j.bmcl.2005.09.021
BindingDB Entry DOI: 10.7270/Q25M68G7 |
More data for this
Ligand-Target Pair | |
Beta-lactamase TEM
(Escherichia coli) | BDBM50076672

((2S,5R,6S)-6-Hydroxymethyl-3,3-dimethyl-4,4,7-trio...)Show SMILES CC1(C)[C@@H](N2[C@@H]([C@@H](CO)C2=O)S1(=O)=O)C(O)=O Show InChI InChI=1S/C9H13NO6S/c1-9(2)5(8(13)14)10-6(12)4(3-11)7(10)17(9,15)16/h4-5,7,11H,3H2,1-2H3,(H,13,14)/t4-,5-,7+/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against TEM-1 (class A) beta-lactamase |
Bioorg Med Chem Lett 9: 991-6 (1999)
BindingDB Entry DOI: 10.7270/Q2TB163Q |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Staphylococcus aureus (Firmicutes)) | BDBM50166475

(1,2-Bis-(4-chloro-phenyl)-4,4-bis-(6-phenyl-hexyl)...)Show SMILES Clc1ccc(cc1)N1N(C(=O)C(CCCCCCc2ccccc2)(CCCCCCc2ccccc2)C1=O)c1ccc(Cl)cc1 Show InChI InChI=1S/C39H42Cl2N2O2/c40-33-21-25-35(26-22-33)42-37(44)39(38(45)43(42)36-27-23-34(41)24-28-36,29-13-3-1-7-15-31-17-9-5-10-18-31)30-14-4-2-8-16-32-19-11-6-12-20-32/h5-6,9-12,17-28H,1-4,7-8,13-16,29-30H2 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MurB enzyme in Staphylococcus aureus |
Bioorg Med Chem Lett 15: 2527-31 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.058
BindingDB Entry DOI: 10.7270/Q2BG2NHW |
More data for this
Ligand-Target Pair | |
UDP-N-acetylglucosamine 1-carboxyvinyltransferase
(Escherichia coli K-12 (Enterobacteria)) | BDBM50166480

(1,2-Bis-(4-chloro-phenyl)-4-[2-(4-fluoro-phenyl)-2...)Show SMILES Oc1c(CC(=O)c2ccc(F)cc2)c(=O)n(-c2ccc(Cl)cc2)n1-c1ccc(Cl)cc1 Show InChI InChI=1S/C23H15Cl2FN2O3/c24-15-3-9-18(10-4-15)27-22(30)20(13-21(29)14-1-7-17(26)8-2-14)23(31)28(27)19-11-5-16(25)6-12-19/h1-12,30H,13H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MurA enzyme in Escherichia coli |
Bioorg Med Chem Lett 15: 2527-31 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.058
BindingDB Entry DOI: 10.7270/Q2BG2NHW |
More data for this
Ligand-Target Pair | |
UDP-N-acetylglucosamine 1-carboxyvinyltransferase
(Escherichia coli K-12 (Enterobacteria)) | BDBM50475603

(CHEMBL199320)Show SMILES Cc1cc(\C=C2/OC(O)=C(C2=O)c2cccc(Cl)c2)ccc1-c1ccccc1C(F)(F)F |c:8| Show InChI InChI=1S/C25H16ClF3O3/c1-14-11-15(9-10-18(14)19-7-2-3-8-20(19)25(27,28)29)12-21-23(30)22(24(31)32-21)16-5-4-6-17(26)13-16/h2-13,31H,1H3/b21-12- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against MurA in Escherichia coli |
Bioorg Med Chem Lett 16: 176-80 (2006)
Article DOI: 10.1016/j.bmcl.2005.09.021
BindingDB Entry DOI: 10.7270/Q25M68G7 |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Escherichia coli K-12 (Enterobacteria)) | BDBM50166478

(1,2-Bis-(4-chloro-phenyl)-4-(6-phenyl-hexyl)-pyraz...)Show SMILES Oc1c(CCCCCCc2ccccc2)c(=O)n(-c2ccc(Cl)cc2)n1-c1ccc(Cl)cc1 Show InChI InChI=1S/C27H26Cl2N2O2/c28-21-12-16-23(17-13-21)30-26(32)25(27(33)31(30)24-18-14-22(29)15-19-24)11-7-2-1-4-8-20-9-5-3-6-10-20/h3,5-6,9-10,12-19,32H,1-2,4,7-8,11H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MurB enzyme in Escherichia coli |
Bioorg Med Chem Lett 15: 2527-31 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.058
BindingDB Entry DOI: 10.7270/Q2BG2NHW |
More data for this
Ligand-Target Pair | |
UDP-N-acetylglucosamine 1-carboxyvinyltransferase
(Escherichia coli K-12 (Enterobacteria)) | BDBM50166485

(1,2-Bis-(4-chloro-phenyl)-4,4-bis-[2-(2,4-difluoro...)Show SMILES Fc1ccc(C(=O)CC2(CC(=O)c3ccc(F)cc3F)C(=O)N(N(C2=O)c2ccc(Cl)cc2)c2ccc(Cl)cc2)c(F)c1 Show InChI InChI=1S/C31H18Cl2F4N2O4/c32-17-1-7-21(8-2-17)38-29(42)31(15-27(40)23-11-5-19(34)13-25(23)36,16-28(41)24-12-6-20(35)14-26(24)37)30(43)39(38)22-9-3-18(33)4-10-22/h1-14H,15-16H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MurA enzyme in Escherichia coli |
Bioorg Med Chem Lett 15: 2527-31 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.058
BindingDB Entry DOI: 10.7270/Q2BG2NHW |
More data for this
Ligand-Target Pair | |
UDP-N-acetylenolpyruvoylglucosamine reductase
(Escherichia coli K-12 (Enterobacteria)) | BDBM50475579
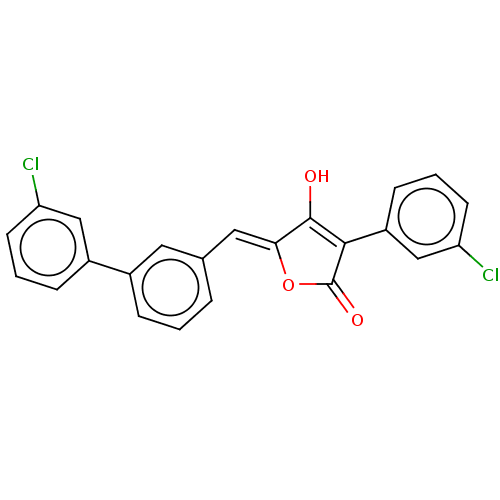
(CHEMBL382202)Show SMILES OC1=C(C(=O)O\C1=C/c1cccc(c1)-c1cccc(Cl)c1)c1cccc(Cl)c1 |t:1| Show InChI InChI=1S/C23H14Cl2O3/c24-18-8-2-6-16(12-18)15-5-1-4-14(10-15)11-20-22(26)21(23(27)28-20)17-7-3-9-19(25)13-17/h1-13,26H/b20-11- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.77E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against MurB in Escherichia coli |
Bioorg Med Chem Lett 16: 176-80 (2006)
Article DOI: 10.1016/j.bmcl.2005.09.021
BindingDB Entry DOI: 10.7270/Q25M68G7 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data