

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50535447 (CHEMBL4453318) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem | PDB Article PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas Medical Branch Curated by ChEMBL | Assay Description Displacement of [3H]-SCH23390 from wild type human D1R expressed in HEK293 cell membranes incubated for 90 mins by scintillation counting based compe... | ACS Med Chem Lett 10: 792-799 (2019) Article DOI: 10.1021/acsmedchemlett.9b00050 BindingDB Entry DOI: 10.7270/Q26W9FKV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM86282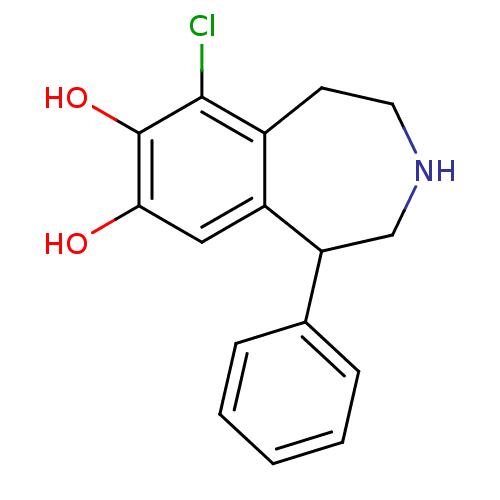 (6-Chloro-1-phenyl-2,3,4,5-tetrahydro-1H-benzo[d]az...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas Medical Branch Curated by ChEMBL | Assay Description Displacement of [3H]-SCH23390 from wild type human D1R expressed in HEK293 cell membranes incubated for 90 mins by scintillation counting based compe... | ACS Med Chem Lett 10: 792-799 (2019) Article DOI: 10.1021/acsmedchemlett.9b00050 BindingDB Entry DOI: 10.7270/Q26W9FKV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM21278 (5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human recombinant CB1 receptor expressed in HEK293 cells | J Med Chem 50: 5951-66 (2007) Article DOI: 10.1021/jm061490u BindingDB Entry DOI: 10.7270/Q2Q81CTK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM160875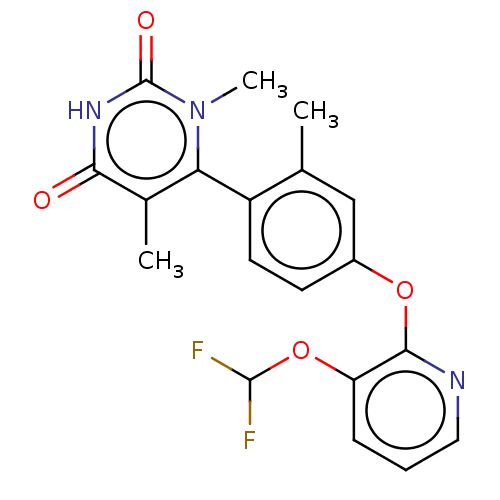 (US10093655, Example 45 | US11014909, Example 45 | ...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas Medical Branch Curated by ChEMBL | Assay Description Displacement of [3H]-SCH23390 from wild type human D1R expressed in HEK293 cell membranes incubated for 90 mins by scintillation counting based compe... | ACS Med Chem Lett 10: 792-799 (2019) Article DOI: 10.1021/acsmedchemlett.9b00050 BindingDB Entry DOI: 10.7270/Q26W9FKV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50535448 (CHEMBL4469983) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas Medical Branch Curated by ChEMBL | Assay Description Displacement of [3H]-SCH23390 from wild type human D1R expressed in HEK293 cell membranes incubated for 90 mins by scintillation counting based compe... | ACS Med Chem Lett 10: 792-799 (2019) Article DOI: 10.1021/acsmedchemlett.9b00050 BindingDB Entry DOI: 10.7270/Q26W9FKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50226179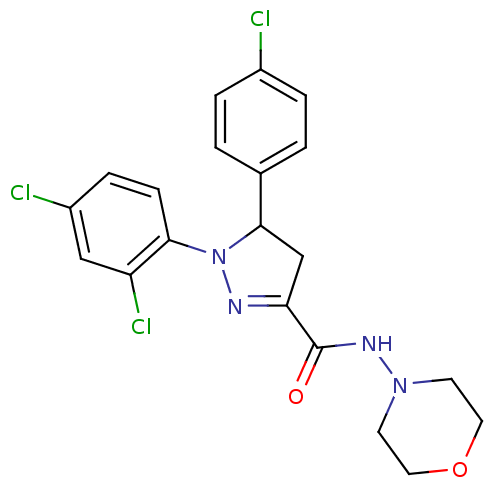 ((+)-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4,5-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human recombinant CB1 receptor expressed in HEK293 cells | J Med Chem 50: 5951-66 (2007) Article DOI: 10.1021/jm061490u BindingDB Entry DOI: 10.7270/Q2Q81CTK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50226179 ((+)-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4,5-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human recombinant CB1 receptor expressed in HEK293 cells | J Med Chem 50: 5951-66 (2007) Article DOI: 10.1021/jm061490u BindingDB Entry DOI: 10.7270/Q2Q81CTK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50226179 ((+)-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4,5-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 475 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human recombinant CB1 receptor expressed in HEK293 cells | J Med Chem 50: 5951-66 (2007) Article DOI: 10.1021/jm061490u BindingDB Entry DOI: 10.7270/Q2Q81CTK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM21278 (5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.99E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre Curated by ChEMBL | Assay Description Displacement of [3H]WIN552122 from human recombinant CB2 receptor expressed in CHOK1 cells | J Med Chem 50: 5951-66 (2007) Article DOI: 10.1021/jm061490u BindingDB Entry DOI: 10.7270/Q2Q81CTK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50226179 ((+)-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4,5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 2.45E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre Curated by ChEMBL | Assay Description Displacement of [3H]WIN552122 from human recombinant CB2 receptor expressed in CHOK1 cells | J Med Chem 50: 5951-66 (2007) Article DOI: 10.1021/jm061490u BindingDB Entry DOI: 10.7270/Q2Q81CTK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50226179 ((+)-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4,5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 2.45E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre Curated by ChEMBL | Assay Description Displacement of [3H]WIN552122 from human recombinant CB2 receptor expressed in CHOK1 cells | J Med Chem 50: 5951-66 (2007) Article DOI: 10.1021/jm061490u BindingDB Entry DOI: 10.7270/Q2Q81CTK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50226179 ((+)-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4,5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 2.64E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre Curated by ChEMBL | Assay Description Displacement of [3H]WIN552122 from human recombinant CB2 receptor expressed in CHOK1 cells | J Med Chem 50: 5951-66 (2007) Article DOI: 10.1021/jm061490u BindingDB Entry DOI: 10.7270/Q2Q81CTK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor alpha (Homo sapiens (Human)) | BDBM18869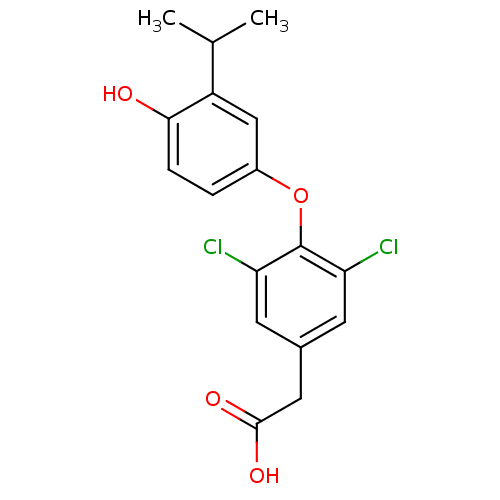 (2-{3,5-dichloro-4-[4-hydroxy-3-(propan-2-yl)phenox...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a |
Zydus Research Centre Curated by ChEMBL | Assay Description Agonist activity at human thyroid hormone receptor alpha expressed in CV1 cells by TRE-luciferase assay | Bioorg Med Chem Lett 18: 3919-24 (2008) Article DOI: 10.1016/j.bmcl.2008.06.038 BindingDB Entry DOI: 10.7270/Q2CZ36ZV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50523450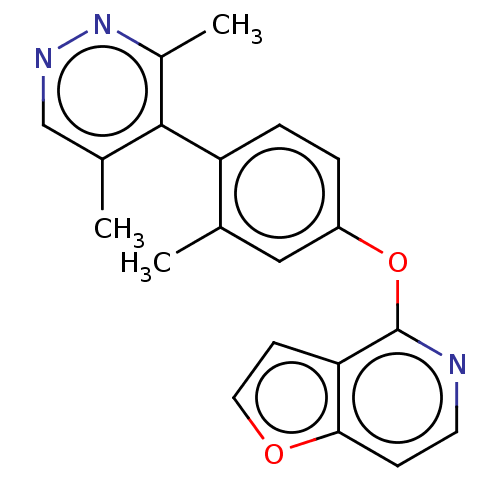 (CHEMBL4437012) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a |
University of Texas Medical Branch Curated by ChEMBL | Assay Description Agonist activity at wild type human D1R expressed in HEK293 cells assessed as effect on beta-arrestin2 recruitment by PRESTO-Tango beta-arrestin2 rec... | ACS Med Chem Lett 10: 792-799 (2019) Article DOI: 10.1021/acsmedchemlett.9b00050 BindingDB Entry DOI: 10.7270/Q26W9FKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor alpha (Homo sapiens (Human)) | BDBM50251497 (3-(4-(3-tert-butyl-4-hydroxyphenoxy)-3,5-dichlorop...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.06E+3 | n/a | n/a | n/a | n/a |
Zydus Research Centre Curated by ChEMBL | Assay Description Agonist activity at human thyroid hormone receptor alpha expressed in CV1 cells by TRE-luciferase assay | Bioorg Med Chem Lett 18: 3919-24 (2008) Article DOI: 10.1016/j.bmcl.2008.06.038 BindingDB Entry DOI: 10.7270/Q2CZ36ZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor alpha (Homo sapiens (Human)) | BDBM50251496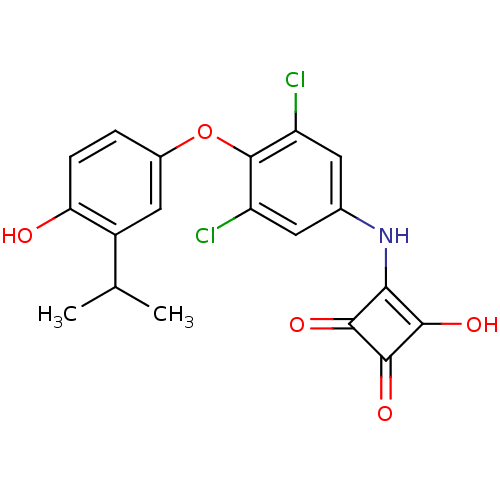 (3-(3,5-dichloro-4-(4-hydroxy-3-isopropylphenoxy)ph...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 222 | n/a | n/a | n/a | n/a |
Zydus Research Centre Curated by ChEMBL | Assay Description Agonist activity at human thyroid hormone receptor alpha expressed in CV1 cells by TRE-luciferase assay | Bioorg Med Chem Lett 18: 3919-24 (2008) Article DOI: 10.1016/j.bmcl.2008.06.038 BindingDB Entry DOI: 10.7270/Q2CZ36ZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor beta (Homo sapiens (Human)) | BDBM50251497 (3-(4-(3-tert-butyl-4-hydroxyphenoxy)-3,5-dichlorop...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 317 | n/a | n/a | n/a | n/a |
Zydus Research Centre Curated by ChEMBL | Assay Description Agonist activity at human thyroid hormone receptor beta expressed in CV1 cells by TRE-luciferase assay | Bioorg Med Chem Lett 18: 3919-24 (2008) Article DOI: 10.1016/j.bmcl.2008.06.038 BindingDB Entry DOI: 10.7270/Q2CZ36ZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor beta (Homo sapiens (Human)) | BDBM50251496 (3-(3,5-dichloro-4-(4-hydroxy-3-isopropylphenoxy)ph...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 70 | n/a | n/a | n/a | n/a |
Zydus Research Centre Curated by ChEMBL | Assay Description Agonist activity at human thyroid hormone receptor beta expressed in CV1 cells by TRE-luciferase assay | Bioorg Med Chem Lett 18: 3919-24 (2008) Article DOI: 10.1016/j.bmcl.2008.06.038 BindingDB Entry DOI: 10.7270/Q2CZ36ZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50349807 (CHEMBL1813003) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.00150 | n/a | n/a | n/a | n/a |
Zydus Research Centre Curated by ChEMBL | Assay Description Transactivation of human PPARgamma expressed in human HepG2 cells cotransfected with PPRE3-TK-Luc by luciferase reporter gene assay | Bioorg Med Chem Lett 21: 3103-9 (2011) Article DOI: 10.1016/j.bmcl.2011.03.020 BindingDB Entry DOI: 10.7270/Q2ZC837H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50349808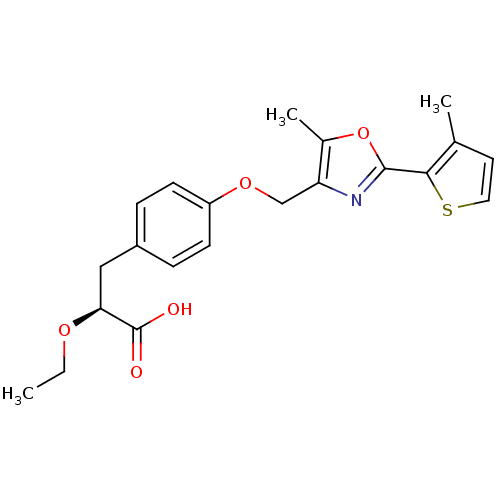 (CHEMBL1813005) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.000300 | n/a | n/a | n/a | n/a |
Zydus Research Centre Curated by ChEMBL | Assay Description Transactivation of human PPARgamma expressed in human HepG2 cells cotransfected with PPRE3-TK-Luc by luciferase reporter gene assay | Bioorg Med Chem Lett 21: 3103-9 (2011) Article DOI: 10.1016/j.bmcl.2011.03.020 BindingDB Entry DOI: 10.7270/Q2ZC837H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50349809 (CHEMBL1813006) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.000200 | n/a | n/a | n/a | n/a |
Zydus Research Centre Curated by ChEMBL | Assay Description Transactivation of human PPARgamma expressed in human HepG2 cells cotransfected with PPRE3-TK-Luc by luciferase reporter gene assay | Bioorg Med Chem Lett 21: 3103-9 (2011) Article DOI: 10.1016/j.bmcl.2011.03.020 BindingDB Entry DOI: 10.7270/Q2ZC837H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50349810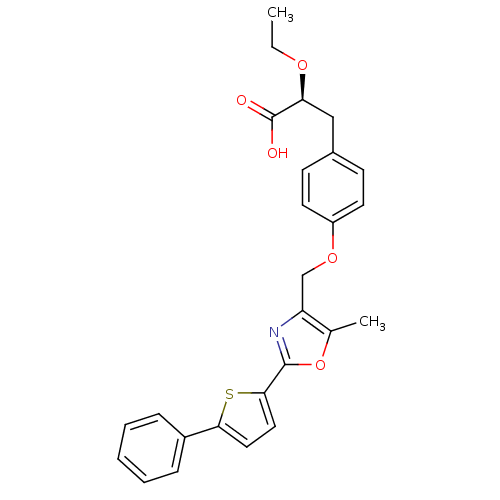 (CHEMBL1813007) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 3 | n/a | n/a | n/a | n/a |
Zydus Research Centre Curated by ChEMBL | Assay Description Transactivation of human PPARgamma expressed in human HepG2 cells cotransfected with PPRE3-TK-Luc by luciferase reporter gene assay | Bioorg Med Chem Lett 21: 3103-9 (2011) Article DOI: 10.1016/j.bmcl.2011.03.020 BindingDB Entry DOI: 10.7270/Q2ZC837H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50349811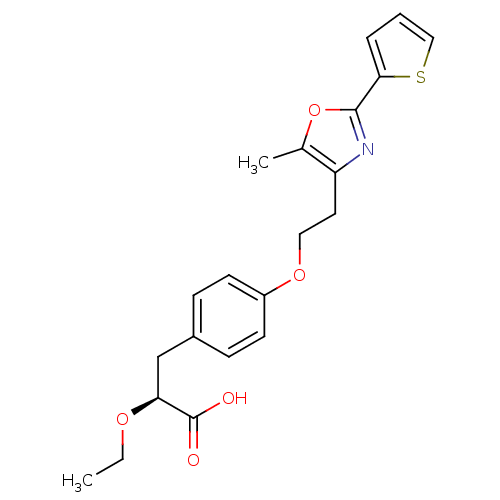 (CHEMBL1813008) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a |
Zydus Research Centre Curated by ChEMBL | Assay Description Transactivation of human PPARgamma expressed in human HepG2 cells cotransfected with PPRE3-TK-Luc by luciferase reporter gene assay | Bioorg Med Chem Lett 21: 3103-9 (2011) Article DOI: 10.1016/j.bmcl.2011.03.020 BindingDB Entry DOI: 10.7270/Q2ZC837H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50349812 (CHEMBL1813010) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.000100 | n/a | n/a | n/a | n/a |
Zydus Research Centre Curated by ChEMBL | Assay Description Transactivation of human PPARgamma expressed in human HepG2 cells cotransfected with PPRE3-TK-Luc by luciferase reporter gene assay | Bioorg Med Chem Lett 21: 3103-9 (2011) Article DOI: 10.1016/j.bmcl.2011.03.020 BindingDB Entry DOI: 10.7270/Q2ZC837H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50349813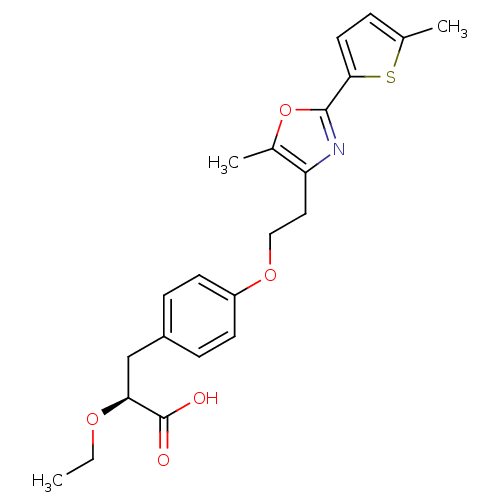 (CHEMBL1813011) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.0180 | n/a | n/a | n/a | n/a |
Zydus Research Centre Curated by ChEMBL | Assay Description Transactivation of human PPARgamma expressed in human HepG2 cells cotransfected with PPRE3-TK-Luc by luciferase reporter gene assay | Bioorg Med Chem Lett 21: 3103-9 (2011) Article DOI: 10.1016/j.bmcl.2011.03.020 BindingDB Entry DOI: 10.7270/Q2ZC837H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50349814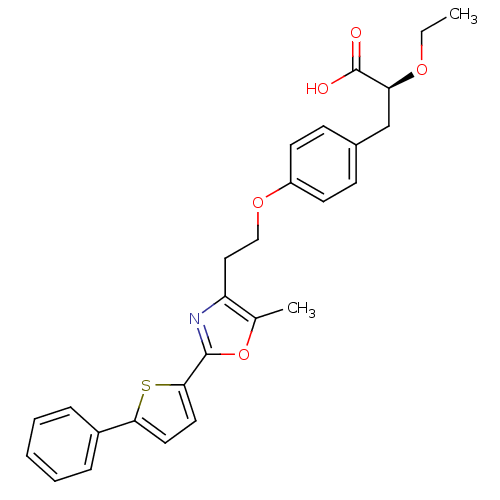 (CHEMBL1813012) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a |
Zydus Research Centre Curated by ChEMBL | Assay Description Transactivation of human PPARgamma expressed in human HepG2 cells cotransfected with PPRE3-TK-Luc by luciferase reporter gene assay | Bioorg Med Chem Lett 21: 3103-9 (2011) Article DOI: 10.1016/j.bmcl.2011.03.020 BindingDB Entry DOI: 10.7270/Q2ZC837H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM28681 (5-[(4-{2-[methyl(pyridin-2-yl)amino]ethoxy}phenyl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 50 | n/a | n/a | n/a | n/a |
Zydus Research Centre Curated by ChEMBL | Assay Description Transactivation of human PPARgamma expressed in human HepG2 cells cotransfected with PPRE3-TK-Luc by luciferase reporter gene assay | Bioorg Med Chem Lett 21: 3103-9 (2011) Article DOI: 10.1016/j.bmcl.2011.03.020 BindingDB Entry DOI: 10.7270/Q2ZC837H | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50349807 (CHEMBL1813003) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.0260 | n/a | n/a | n/a | n/a |
Zydus Research Centre Curated by ChEMBL | Assay Description Transactivation of human PPARalpha expressed in human HepG2 cells cotransfected with PPRE3-TK-Luc by luciferase reporter gene assay | Bioorg Med Chem Lett 21: 3103-9 (2011) Article DOI: 10.1016/j.bmcl.2011.03.020 BindingDB Entry DOI: 10.7270/Q2ZC837H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50349815 (CHEMBL1813004) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 3 | n/a | n/a | n/a | n/a |
Zydus Research Centre Curated by ChEMBL | Assay Description Transactivation of human PPARalpha expressed in human HepG2 cells cotransfected with PPRE3-TK-Luc by luciferase reporter gene assay | Bioorg Med Chem Lett 21: 3103-9 (2011) Article DOI: 10.1016/j.bmcl.2011.03.020 BindingDB Entry DOI: 10.7270/Q2ZC837H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50349808 (CHEMBL1813005) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.0000600 | n/a | n/a | n/a | n/a |
Zydus Research Centre Curated by ChEMBL | Assay Description Transactivation of human PPARalpha expressed in human HepG2 cells cotransfected with PPRE3-TK-Luc by luciferase reporter gene assay | Bioorg Med Chem Lett 21: 3103-9 (2011) Article DOI: 10.1016/j.bmcl.2011.03.020 BindingDB Entry DOI: 10.7270/Q2ZC837H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50349809 (CHEMBL1813006) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.0000500 | n/a | n/a | n/a | n/a |
Zydus Research Centre Curated by ChEMBL | Assay Description Transactivation of human PPARalpha expressed in human HepG2 cells cotransfected with PPRE3-TK-Luc by luciferase reporter gene assay | Bioorg Med Chem Lett 21: 3103-9 (2011) Article DOI: 10.1016/j.bmcl.2011.03.020 BindingDB Entry DOI: 10.7270/Q2ZC837H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50349810 (CHEMBL1813007) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 20 | n/a | n/a | n/a | n/a |
Zydus Research Centre Curated by ChEMBL | Assay Description Transactivation of human PPARalpha expressed in human HepG2 cells cotransfected with PPRE3-TK-Luc by luciferase reporter gene assay | Bioorg Med Chem Lett 21: 3103-9 (2011) Article DOI: 10.1016/j.bmcl.2011.03.020 BindingDB Entry DOI: 10.7270/Q2ZC837H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50349811 (CHEMBL1813008) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a |
Zydus Research Centre Curated by ChEMBL | Assay Description Transactivation of human PPARalpha expressed in human HepG2 cells cotransfected with PPRE3-TK-Luc by luciferase reporter gene assay | Bioorg Med Chem Lett 21: 3103-9 (2011) Article DOI: 10.1016/j.bmcl.2011.03.020 BindingDB Entry DOI: 10.7270/Q2ZC837H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50349816 (CHEMBL1813009) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a |
Zydus Research Centre Curated by ChEMBL | Assay Description Transactivation of human PPARalpha expressed in human HepG2 cells cotransfected with PPRE3-TK-Luc by luciferase reporter gene assay | Bioorg Med Chem Lett 21: 3103-9 (2011) Article DOI: 10.1016/j.bmcl.2011.03.020 BindingDB Entry DOI: 10.7270/Q2ZC837H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50349812 (CHEMBL1813010) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.0000700 | n/a | n/a | n/a | n/a |
Zydus Research Centre Curated by ChEMBL | Assay Description Transactivation of human PPARalpha expressed in human HepG2 cells cotransfected with PPRE3-TK-Luc by luciferase reporter gene assay | Bioorg Med Chem Lett 21: 3103-9 (2011) Article DOI: 10.1016/j.bmcl.2011.03.020 BindingDB Entry DOI: 10.7270/Q2ZC837H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50349813 (CHEMBL1813011) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.000310 | n/a | n/a | n/a | n/a |
Zydus Research Centre Curated by ChEMBL | Assay Description Transactivation of human PPARalpha expressed in human HepG2 cells cotransfected with PPRE3-TK-Luc by luciferase reporter gene assay | Bioorg Med Chem Lett 21: 3103-9 (2011) Article DOI: 10.1016/j.bmcl.2011.03.020 BindingDB Entry DOI: 10.7270/Q2ZC837H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50349814 (CHEMBL1813012) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 22 | n/a | n/a | n/a | n/a |
Zydus Research Centre Curated by ChEMBL | Assay Description Transactivation of human PPARalpha expressed in human HepG2 cells cotransfected with PPRE3-TK-Luc by luciferase reporter gene assay | Bioorg Med Chem Lett 21: 3103-9 (2011) Article DOI: 10.1016/j.bmcl.2011.03.020 BindingDB Entry DOI: 10.7270/Q2ZC837H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM24566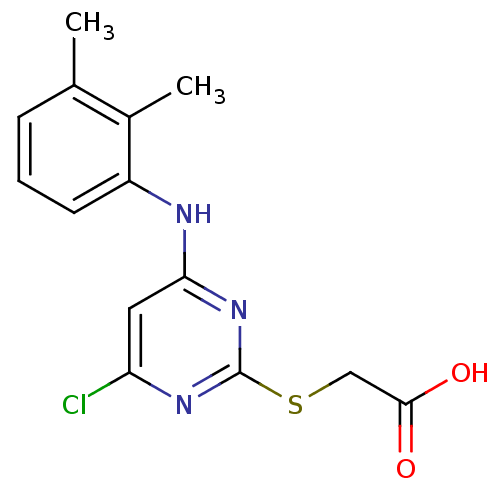 (2-({4-chloro-6-[(2,3-dimethylphenyl)amino]pyrimidi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 4.80E+3 | n/a | n/a | n/a | n/a |
Zydus Research Centre Curated by ChEMBL | Assay Description Transactivation of human PPARalpha expressed in human HepG2 cells cotransfected with PPRE3-TK-Luc by luciferase reporter gene assay | Bioorg Med Chem Lett 21: 3103-9 (2011) Article DOI: 10.1016/j.bmcl.2011.03.020 BindingDB Entry DOI: 10.7270/Q2ZC837H | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50349816 (CHEMBL1813009) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a |
Zydus Research Centre Curated by ChEMBL | Assay Description Transactivation of human PPARgamma expressed in human HepG2 cells cotransfected with PPRE3-TK-Luc by luciferase reporter gene assay | Bioorg Med Chem Lett 21: 3103-9 (2011) Article DOI: 10.1016/j.bmcl.2011.03.020 BindingDB Entry DOI: 10.7270/Q2ZC837H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50349815 (CHEMBL1813004) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a |
Zydus Research Centre Curated by ChEMBL | Assay Description Transactivation of human PPARgamma expressed in human HepG2 cells cotransfected with PPRE3-TK-Luc by luciferase reporter gene assay | Bioorg Med Chem Lett 21: 3103-9 (2011) Article DOI: 10.1016/j.bmcl.2011.03.020 BindingDB Entry DOI: 10.7270/Q2ZC837H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM55121 (3-HYDROXYTYRAMINE HYDROCHLORIDE | 4-(2-aminoethyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank KEGG PC cid PC sid PDB UniChem Similars | DrugBank Article PubMed | n/a | n/a | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a |
University of Texas Medical Branch Curated by ChEMBL | Assay Description Agonist activity at D2R (unknown origin) expressed in HEK293 cells assessed as effect on cAMP accumulation incubated for 10 mins by Gi-cAMP Glosensor... | ACS Med Chem Lett 10: 792-799 (2019) Article DOI: 10.1021/acsmedchemlett.9b00050 BindingDB Entry DOI: 10.7270/Q26W9FKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM50018958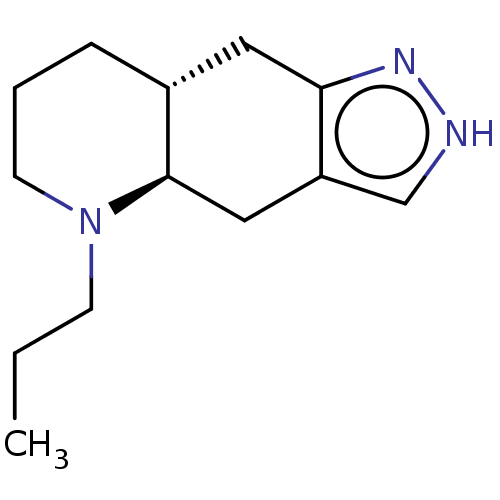 (CHEBI:75401 | QUINPIROLE) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a |
University of Texas Medical Branch Curated by ChEMBL | Assay Description Agonist activity at D4R (unknown origin) expressed in HEK293 cells assessed as effect on cAMP accumulation incubated for 10 mins by Gi-cAMP Glosensor... | ACS Med Chem Lett 10: 792-799 (2019) Article DOI: 10.1021/acsmedchemlett.9b00050 BindingDB Entry DOI: 10.7270/Q26W9FKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1B) dopamine receptor (Homo sapiens (Human)) | BDBM86282 (6-Chloro-1-phenyl-2,3,4,5-tetrahydro-1H-benzo[d]az...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a |
University of Texas Medical Branch Curated by ChEMBL | Assay Description Agonist activity at D5R (unknown origin) expressed in HEK293 cells assessed as effect on cAMP accumulation incubated for 10 mins by Gs-cAMP Glosensor... | ACS Med Chem Lett 10: 792-799 (2019) Article DOI: 10.1021/acsmedchemlett.9b00050 BindingDB Entry DOI: 10.7270/Q26W9FKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1B) dopamine receptor (Homo sapiens (Human)) | BDBM160875 (US10093655, Example 45 | US11014909, Example 45 | ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 12 | n/a | n/a | n/a | n/a |
University of Texas Medical Branch Curated by ChEMBL | Assay Description Agonist activity at D5R (unknown origin) expressed in HEK293 cells assessed as effect on cAMP accumulation incubated for 10 mins by Gs-cAMP Glosensor... | ACS Med Chem Lett 10: 792-799 (2019) Article DOI: 10.1021/acsmedchemlett.9b00050 BindingDB Entry DOI: 10.7270/Q26W9FKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50535455 (CHEMBL4538358) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 450 | n/a | n/a | n/a | n/a |
University of Texas Medical Branch Curated by ChEMBL | Assay Description Agonist activity at wild type human D1R expressed in HEK293 cells assessed as effect on cAMP accumulation incubated for 10 mins by Gs-cAMP Glosensor ... | ACS Med Chem Lett 10: 792-799 (2019) Article DOI: 10.1021/acsmedchemlett.9b00050 BindingDB Entry DOI: 10.7270/Q26W9FKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50535447 (CHEMBL4453318) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | n/a | n/a | 35 | n/a | n/a | n/a | n/a |
University of Texas Medical Branch Curated by ChEMBL | Assay Description Agonist activity at wild type human D1R expressed in HEK293 cells assessed as effect on beta-arrestin2 recruitment by PRESTO-Tango beta-arrestin2 rec... | ACS Med Chem Lett 10: 792-799 (2019) Article DOI: 10.1021/acsmedchemlett.9b00050 BindingDB Entry DOI: 10.7270/Q26W9FKV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| D(1B) dopamine receptor (Homo sapiens (Human)) | BDBM55121 (3-HYDROXYTYRAMINE HYDROCHLORIDE | 4-(2-aminoethyl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2 | n/a | n/a | n/a | n/a |
University of Texas Medical Branch Curated by ChEMBL | Assay Description Agonist activity at D5R (unknown origin) expressed in HEK293 cells assessed as effect on cAMP accumulation incubated for 10 mins by Gs-cAMP Glosensor... | ACS Med Chem Lett 10: 792-799 (2019) Article DOI: 10.1021/acsmedchemlett.9b00050 BindingDB Entry DOI: 10.7270/Q26W9FKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50465935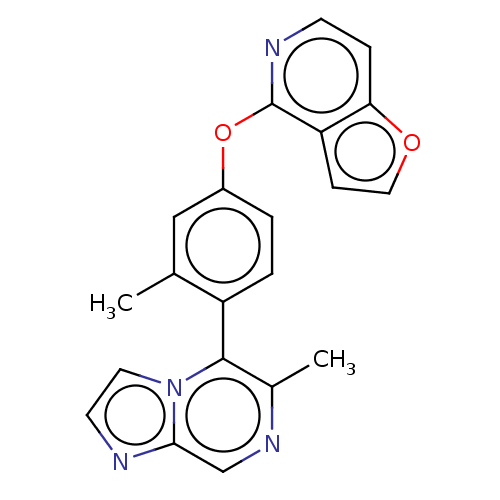 (CHEMBL4277264) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 11 | n/a | n/a | n/a | n/a |
University of Texas Medical Branch Curated by ChEMBL | Assay Description Agonist activity at wild type human D1R expressed in HEK293 cells assessed as effect on cAMP accumulation incubated for 10 mins by Gs-cAMP Glosensor ... | ACS Med Chem Lett 10: 792-799 (2019) Article DOI: 10.1021/acsmedchemlett.9b00050 BindingDB Entry DOI: 10.7270/Q26W9FKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM86282 (6-Chloro-1-phenyl-2,3,4,5-tetrahydro-1H-benzo[d]az...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a |
University of Texas Medical Branch Curated by ChEMBL | Assay Description Agonist activity at wild type human D1R expressed in HEK293 cells assessed as effect on cAMP accumulation incubated for 10 mins by Gs-cAMP Glosensor ... | ACS Med Chem Lett 10: 792-799 (2019) Article DOI: 10.1021/acsmedchemlett.9b00050 BindingDB Entry DOI: 10.7270/Q26W9FKV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50523450 (CHEMBL4437012) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 81 | n/a | n/a | n/a | n/a |
University of Texas Medical Branch Curated by ChEMBL | Assay Description Agonist activity at wild type human D1R expressed in HEK293 cells assessed as effect on cAMP accumulation incubated for 10 mins by Gs-cAMP Glosensor ... | ACS Med Chem Lett 10: 792-799 (2019) Article DOI: 10.1021/acsmedchemlett.9b00050 BindingDB Entry DOI: 10.7270/Q26W9FKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 71 total ) | Next | Last >> |