 Found 1805 hits with Last Name = 'remiszewski' and Initial = 's'
Found 1805 hits with Last Name = 'remiszewski' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Dimer of Protein farnesyltransferase subunit beta
(Homo sapiens (Human)) | BDBM50079090

(CHEMBL90435 | [(4-{2-[4-((R)-3,10-Dibromo-8-chloro...)Show SMILES OC(=O)CNC(=O)N1CCC(CC(=O)N2CCC(CC2)[C@H]2c3ncc(Br)cc3CCc3cc(Cl)cc(Br)c23)CC1 Show InChI InChI=1S/C29H33Br2ClN4O4/c30-21-12-20-2-1-19-13-22(32)14-23(31)26(19)27(28(20)33-15-21)18-5-9-35(10-6-18)24(37)11-17-3-7-36(8-4-17)29(40)34-16-25(38)39/h12-15,17-18,27H,1-11,16H2,(H,34,40)(H,38,39)/t27-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity against Hras Farnesyltransferase (FPT). |
J Med Chem 42: 2651-61 (1999)
Article DOI: 10.1021/jm990059k
BindingDB Entry DOI: 10.7270/Q2ST7P2V |
More data for this
Ligand-Target Pair | |
Dimer of Protein farnesyltransferase subunit beta
(Homo sapiens (Human)) | BDBM50079085
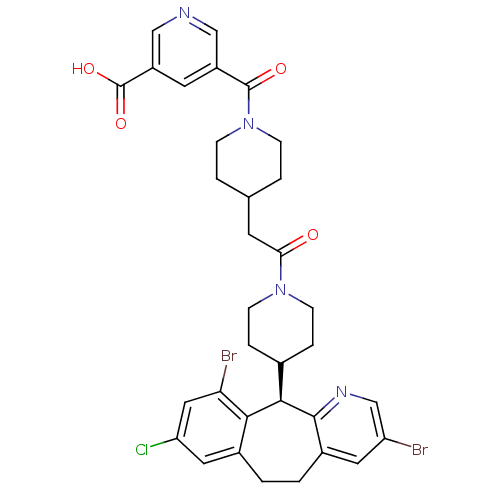
(5-(4-{2-[4-((R)-3,10-Dibromo-8-chloro-6,11-dihydro...)Show SMILES OC(=O)c1cncc(c1)C(=O)N1CCC(CC(=O)N2CCC(CC2)[C@H]2c3ncc(Br)cc3CCc3cc(Cl)cc(Br)c23)CC1 Show InChI InChI=1S/C33H33Br2ClN4O4/c34-25-13-22-2-1-21-14-26(36)15-27(35)29(21)30(31(22)38-18-25)20-5-9-39(10-6-20)28(41)11-19-3-7-40(8-4-19)32(42)23-12-24(33(43)44)17-37-16-23/h12-20,30H,1-11H2,(H,43,44)/t30-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity against Hras Farnesyltransferase (FPT). |
J Med Chem 42: 2651-61 (1999)
Article DOI: 10.1021/jm990059k
BindingDB Entry DOI: 10.7270/Q2ST7P2V |
More data for this
Ligand-Target Pair | |
Dimer of Protein farnesyltransferase subunit beta
(Homo sapiens (Human)) | BDBM50079075
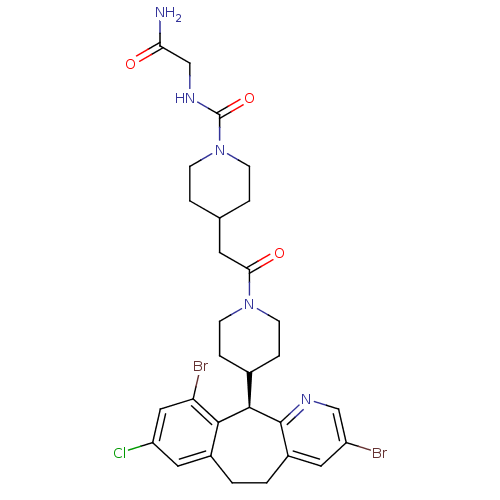
(4-{2-[4-((R)-3,10-Dibromo-8-chloro-6,11-dihydro-5H...)Show SMILES NC(=O)CNC(=O)N1CCC(CC(=O)N2CCC(CC2)[C@H]2c3ncc(Br)cc3CCc3cc(Cl)cc(Br)c23)CC1 Show InChI InChI=1S/C29H34Br2ClN5O3/c30-21-12-20-2-1-19-13-22(32)14-23(31)26(19)27(28(20)34-15-21)18-5-9-36(10-6-18)25(39)11-17-3-7-37(8-4-17)29(40)35-16-24(33)38/h12-15,17-18,27H,1-11,16H2,(H2,33,38)(H,35,40)/t27-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-FPP incorporation into His6-H-Ras-CVLS by farnesyl transferase |
J Med Chem 42: 2651-61 (1999)
Article DOI: 10.1021/jm990059k
BindingDB Entry DOI: 10.7270/Q2ST7P2V |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase XIAP [124-240]
(Homo sapiens (Human)) | BDBM243099

((S)—N—((S)-5-(4-Acetylbenzoyl)-1-((6-bro...)Show SMILES CCC(NC)C(=O)N[C@H]1CN(C(=O)c2ccc(cc2)C(C)=O)c2ccccc2N(Cc2c(OC)ccc3cc(Br)ccc23)C1=O |r| Show InChI InChI=1S/C35H35BrN4O5/c1-5-28(37-3)33(42)38-29-20-40(34(43)23-12-10-22(11-13-23)21(2)41)31-9-7-6-8-30(31)39(35(29)44)19-27-26-16-15-25(36)18-24(26)14-17-32(27)45-4/h6-18,28-29,37H,5,19-20H2,1-4H3,(H,38,42)/t28?,29-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Hoffmann-La Roche Inc.
US Patent
| Assay Description
Ten nanomolar of 6× Histidine-tagged BIR2 domain, corresponding to amino acids 124-240 of XIAP, or BIR3 domain, corresponding to amino acids 241-356 ... |
US Patent US10053431 (2018)
BindingDB Entry DOI: 10.7270/Q2W097X3 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50350832
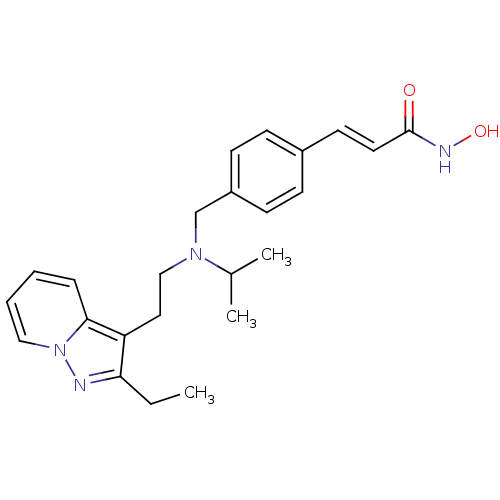
(CHEMBL1819274)Show SMILES CCc1nn2ccccc2c1CCN(Cc1ccc(\C=C\C(=O)NO)cc1)C(C)C Show InChI InChI=1S/C24H30N4O2/c1-4-22-21(23-7-5-6-15-28(23)25-22)14-16-27(18(2)3)17-20-10-8-19(9-11-20)12-13-24(29)26-30/h5-13,15,18,30H,4,14,16-17H2,1-3H3,(H,26,29)/b13-12+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 |
J Med Chem 54: 4752-72 (2011)
Article DOI: 10.1021/jm200388e
BindingDB Entry DOI: 10.7270/Q23N24DW |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50350831
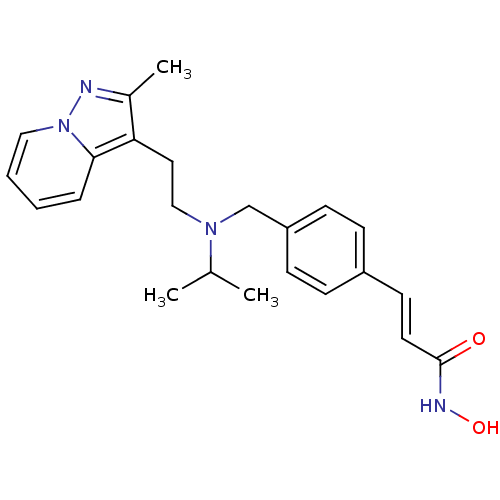
(CHEMBL1819273)Show SMILES CC(C)N(CCc1c(C)nn2ccccc12)Cc1ccc(\C=C\C(=O)NO)cc1 Show InChI InChI=1S/C23H28N4O2/c1-17(2)26(15-13-21-18(3)24-27-14-5-4-6-22(21)27)16-20-9-7-19(8-10-20)11-12-23(28)25-29/h4-12,14,17,29H,13,15-16H2,1-3H3,(H,25,28)/b12-11+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 |
J Med Chem 54: 4752-72 (2011)
Article DOI: 10.1021/jm200388e
BindingDB Entry DOI: 10.7270/Q23N24DW |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM14457
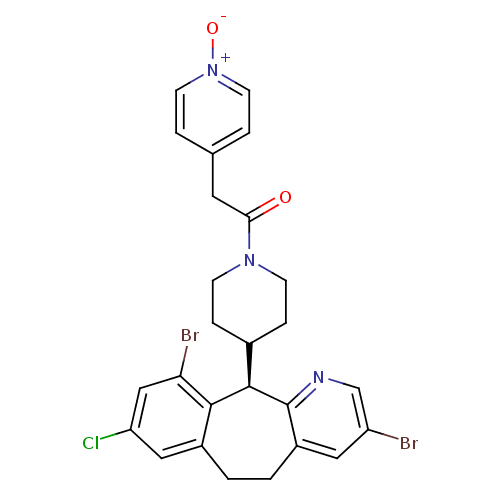
((+)-4-(8-Chloro-3,10-dibromo-6,11-dihydro-5H-benzo...)Show SMILES [O-][n+]1ccc(CC(=O)N2CCC(CC2)[C@H]2c3ncc(Br)cc3CCc3cc(Cl)cc(Br)c23)cc1 |r| Show InChI InChI=1S/C26H24Br2ClN3O2/c27-20-12-19-2-1-18-13-21(29)14-22(28)24(18)25(26(19)30-15-20)17-5-7-31(8-6-17)23(33)11-16-3-9-32(34)10-4-16/h3-4,9-10,12-15,17,25H,1-2,5-8,11H2/t25-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Schering-Plough Research Institute
| Assay Description
FPT activity was determined by measuring transfer of [3H] farnesyl from [3H]farnesyl pyrophosphate to the substrate His6-Ha-Ras-CVLS. The incorporate... |
J Med Chem 41: 4890-902 (1998)
Article DOI: 10.1021/jm980462b
BindingDB Entry DOI: 10.7270/Q2ZS2TRS |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50350818
(CHEMBL1819257)Show SMILES COc1ccncc1-c1[nH]c2ccccc2c1CCNCc1ccc(\C=C\C(=O)NO)cc1 Show InChI InChI=1S/C26H26N4O3/c1-33-24-13-15-28-17-22(24)26-21(20-4-2-3-5-23(20)29-26)12-14-27-16-19-8-6-18(7-9-19)10-11-25(31)30-32/h2-11,13,15,17,27,29,32H,12,14,16H2,1H3,(H,30,31)/b11-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 |
J Med Chem 54: 4752-72 (2011)
Article DOI: 10.1021/jm200388e
BindingDB Entry DOI: 10.7270/Q23N24DW |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50350827
(CHEMBL1819267)Show SMILES CC(C)(C)c1[nH]c2ncccc2c1CCNCc1ccc(C=CC(=O)NO)cc1 |w:21.22| Show InChI InChI=1S/C23H28N4O2/c1-23(2,3)21-18(19-5-4-13-25-22(19)26-21)12-14-24-15-17-8-6-16(7-9-17)10-11-20(28)27-29/h4-11,13,24,29H,12,14-15H2,1-3H3,(H,25,26)(H,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 |
J Med Chem 54: 4752-72 (2011)
Article DOI: 10.1021/jm200388e
BindingDB Entry DOI: 10.7270/Q23N24DW |
More data for this
Ligand-Target Pair | |
Dimer of Protein farnesyltransferase subunit beta
(Homo sapiens (Human)) | BDBM50079079
(1-(4-{2-[4-((R)-3,10-Dibromo-8-chloro-6,11-dihydro...)Show SMILES Clc1cc(Br)c2[C@@H](C3CCN(CC3)C(=O)CC3CCN(CC3)C(=O)Cn3cncn3)c3ncc(Br)cc3CCc2c1 Show InChI InChI=1S/C30H33Br2ClN6O2/c31-23-12-22-2-1-21-13-24(33)14-25(32)28(21)29(30(22)35-15-23)20-5-9-37(10-6-20)26(40)11-19-3-7-38(8-4-19)27(41)16-39-18-34-17-36-39/h12-15,17-20,29H,1-11,16H2/t29-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity against Hras Farnesyltransferase (FPT). |
J Med Chem 42: 2651-61 (1999)
Article DOI: 10.1021/jm990059k
BindingDB Entry DOI: 10.7270/Q2ST7P2V |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM14461
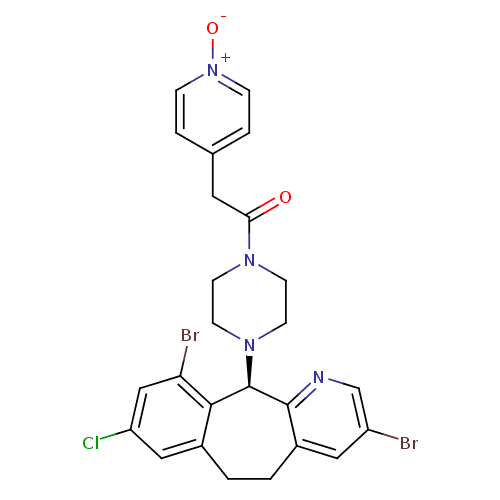
((+)-4-(3,10-Dibromo-8-chloro-6,11-dihydro-5H-benzo...)Show SMILES [O-][n+]1ccc(CC(=O)N2CCN(CC2)[C@H]2c3ncc(Br)cc3CCc3cc(Cl)cc(Br)c23)cc1 |r| Show InChI InChI=1S/C25H23Br2ClN4O2/c26-19-12-18-2-1-17-13-20(28)14-21(27)23(17)25(24(18)29-15-19)31-9-7-30(8-10-31)22(33)11-16-3-5-32(34)6-4-16/h3-6,12-15,25H,1-2,7-11H2/t25-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Schering-Plough Research Institute
| Assay Description
FPT activity was determined by measuring transfer of [3H] farnesyl from [3H]farnesyl pyrophosphate to the substrate His6-Ha-Ras-CVLS. The incorporate... |
J Med Chem 41: 4890-902 (1998)
Article DOI: 10.1021/jm980462b
BindingDB Entry DOI: 10.7270/Q2ZS2TRS |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM14459
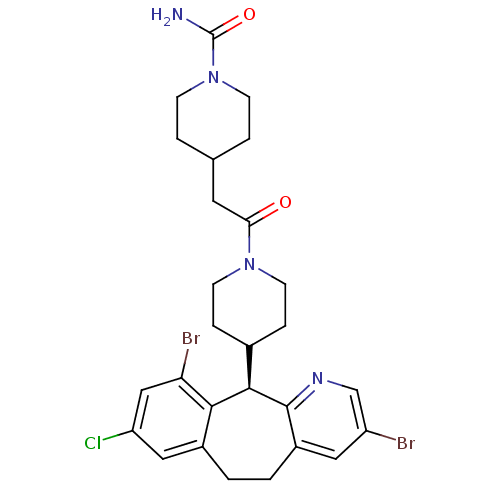
((+)-4-[2-[4-(8-Chloro-3,10-dibromo-6,11-dihydro-5H...)Show SMILES NC(=O)N1CCC(CC(=O)N2CCC(CC2)[C@H]2c3ncc(Br)cc3CCc3cc(Cl)cc(Br)c23)CC1 |r| Show InChI InChI=1S/C27H31Br2ClN4O2/c28-20-12-19-2-1-18-13-21(30)14-22(29)24(18)25(26(19)32-15-20)17-5-9-33(10-6-17)23(35)11-16-3-7-34(8-4-16)27(31)36/h12-17,25H,1-11H2,(H2,31,36)/t25-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Schering-Plough Research Institute
| Assay Description
FPT activity was determined by measuring transfer of [3H] farnesyl from [3H]farnesyl pyrophosphate to the substrate His6-Ha-Ras-CVLS. The incorporate... |
J Med Chem 41: 4890-902 (1998)
Article DOI: 10.1021/jm980462b
BindingDB Entry DOI: 10.7270/Q2ZS2TRS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dimer of Protein farnesyltransferase subunit beta
(Homo sapiens (Human)) | BDBM14459

((+)-4-[2-[4-(8-Chloro-3,10-dibromo-6,11-dihydro-5H...)Show SMILES NC(=O)N1CCC(CC(=O)N2CCC(CC2)[C@H]2c3ncc(Br)cc3CCc3cc(Cl)cc(Br)c23)CC1 |r| Show InChI InChI=1S/C27H31Br2ClN4O2/c28-20-12-19-2-1-18-13-21(30)14-22(29)24(18)25(26(19)32-15-20)17-5-9-33(10-6-17)23(35)11-16-3-7-34(8-4-16)27(31)36/h12-17,25H,1-11H2,(H2,31,36)/t25-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Farnesyl protein transferase |
J Med Chem 42: 2125-35 (1999)
Article DOI: 10.1021/jm990030g
BindingDB Entry DOI: 10.7270/Q2ZS2VP9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dimer of Protein farnesyltransferase subunit beta
(Homo sapiens (Human)) | BDBM14459

((+)-4-[2-[4-(8-Chloro-3,10-dibromo-6,11-dihydro-5H...)Show SMILES NC(=O)N1CCC(CC(=O)N2CCC(CC2)[C@H]2c3ncc(Br)cc3CCc3cc(Cl)cc(Br)c23)CC1 |r| Show InChI InChI=1S/C27H31Br2ClN4O2/c28-20-12-19-2-1-18-13-21(30)14-22(29)24(18)25(26(19)32-15-20)17-5-9-33(10-6-17)23(35)11-16-3-7-34(8-4-16)27(31)36/h12-17,25H,1-11H2,(H2,31,36)/t25-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity against Hras Farnesyltransferase (FPT). |
J Med Chem 42: 2651-61 (1999)
Article DOI: 10.1021/jm990059k
BindingDB Entry DOI: 10.7270/Q2ST7P2V |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dimer of Protein farnesyltransferase subunit beta
(Homo sapiens (Human)) | BDBM50079074

(4-{2-[4-((R)-3,10-Dibromo-8-chloro-6,11-dihydro-5H...)Show SMILES CNC(=O)N1CCC(CC(=O)N2CCC(CC2)[C@H]2c3ncc(Br)cc3CCc3cc(Cl)cc(Br)c23)CC1 Show InChI InChI=1S/C28H33Br2ClN4O2/c1-32-28(37)35-8-4-17(5-9-35)12-24(36)34-10-6-18(7-11-34)26-25-19(14-22(31)15-23(25)30)2-3-20-13-21(29)16-33-27(20)26/h13-18,26H,2-12H2,1H3,(H,32,37)/t26-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity against Hras Farnesyltransferase (FPT). |
J Med Chem 42: 2651-61 (1999)
Article DOI: 10.1021/jm990059k
BindingDB Entry DOI: 10.7270/Q2ST7P2V |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50350820
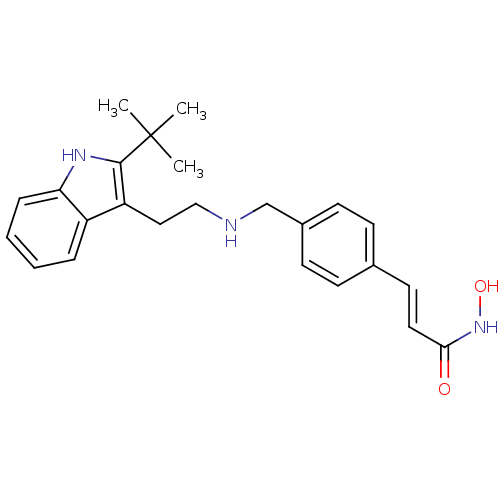
(CHEMBL1819260)Show SMILES CC(C)(C)c1[nH]c2ccccc2c1CCNCc1ccc(\C=C\C(=O)NO)cc1 Show InChI InChI=1S/C24H29N3O2/c1-24(2,3)23-20(19-6-4-5-7-21(19)26-23)14-15-25-16-18-10-8-17(9-11-18)12-13-22(28)27-29/h4-13,25-26,29H,14-16H2,1-3H3,(H,27,28)/b13-12+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 |
J Med Chem 54: 4752-72 (2011)
Article DOI: 10.1021/jm200388e
BindingDB Entry DOI: 10.7270/Q23N24DW |
More data for this
Ligand-Target Pair | |
Dimer of Protein farnesyltransferase subunit beta
(Homo sapiens (Human)) | BDBM50079084

(CHEMBL432573 | [(4-{2-[4-((R)-3,10-Dibromo-8-chlor...)Show SMILES CCOC(=O)CNC(=O)N1CCC(CC(=O)N2CCC(CC2)[C@H]2c3ncc(Br)cc3CCc3cc(Cl)cc(Br)c23)CC1 Show InChI InChI=1S/C31H37Br2ClN4O4/c1-2-42-27(40)18-36-31(41)38-9-5-19(6-10-38)13-26(39)37-11-7-20(8-12-37)29-28-21(15-24(34)16-25(28)33)3-4-22-14-23(32)17-35-30(22)29/h14-17,19-20,29H,2-13,18H2,1H3,(H,36,41)/t29-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity against Hras Farnesyltransferase (FPT). |
J Med Chem 42: 2651-61 (1999)
Article DOI: 10.1021/jm990059k
BindingDB Entry DOI: 10.7270/Q2ST7P2V |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50350835
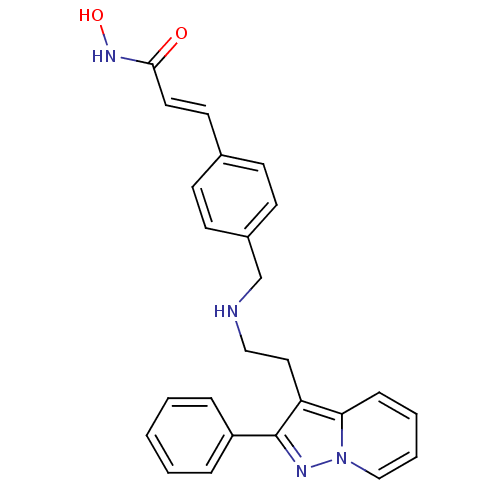
(CHEMBL1819272)Show SMILES ONC(=O)\C=C\c1ccc(CNCCc2c(nn3ccccc23)-c2ccccc2)cc1 Show InChI InChI=1S/C25H24N4O2/c30-24(28-31)14-13-19-9-11-20(12-10-19)18-26-16-15-22-23-8-4-5-17-29(23)27-25(22)21-6-2-1-3-7-21/h1-14,17,26,31H,15-16,18H2,(H,28,30)/b14-13+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 |
J Med Chem 54: 4752-72 (2011)
Article DOI: 10.1021/jm200388e
BindingDB Entry DOI: 10.7270/Q23N24DW |
More data for this
Ligand-Target Pair | |
Dimer of Protein farnesyltransferase subunit beta
(Homo sapiens (Human)) | BDBM50079089

(1-[4-((R)-3,10-Dibromo-8-chloro-6,11-dihydro-5H-be...)Show SMILES CS(=O)(=O)N1CCC(CC(=O)N2CCC(CC2)[C@H]2c3ncc(Br)cc3CCc3cc(Cl)cc(Br)c23)CC1 Show InChI InChI=1S/C27H32Br2ClN3O3S/c1-37(35,36)33-10-4-17(5-11-33)12-24(34)32-8-6-18(7-9-32)26-25-19(14-22(30)15-23(25)29)2-3-20-13-21(28)16-31-27(20)26/h13-18,26H,2-12H2,1H3/t26-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity against Hras Farnesyltransferase (FPT). |
J Med Chem 42: 2651-61 (1999)
Article DOI: 10.1021/jm990059k
BindingDB Entry DOI: 10.7270/Q2ST7P2V |
More data for this
Ligand-Target Pair | |
Dimer of Protein farnesyltransferase subunit beta
(Homo sapiens (Human)) | BDBM50079083

(1-[4-((R)-3,10-Dibromo-8-chloro-6,11-dihydro-5H-be...)Show SMILES [O-][n+]1cccc(c1)C(=O)N1CCC(CC(=O)N2CCC(CC2)[C@H]2c3ncc(Br)cc3CCc3cc(Cl)cc(Br)c23)CC1 Show InChI InChI=1S/C32H33Br2ClN4O3/c33-25-15-23-4-3-22-16-26(35)17-27(34)29(22)30(31(23)36-18-25)21-7-12-37(13-8-21)28(40)14-20-5-10-38(11-6-20)32(41)24-2-1-9-39(42)19-24/h1-2,9,15-21,30H,3-8,10-14H2/t30-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Hras Farnesyl protein transferase(FPT) |
J Med Chem 42: 2651-61 (1999)
Article DOI: 10.1021/jm990059k
BindingDB Entry DOI: 10.7270/Q2ST7P2V |
More data for this
Ligand-Target Pair | |
Dimer of Protein farnesyltransferase subunit beta
(Homo sapiens (Human)) | BDBM50079091

(2-(4-{2-[4-((R)-3,10-Dibromo-8-chloro-6,11-dihydro...)Show SMILES NC(=O)CN1CCC(CC(=O)N2CCC(CC2)[C@H]2c3ncc(Br)cc3CCc3cc(Cl)cc(Br)c23)CC1 Show InChI InChI=1S/C28H33Br2ClN4O2/c29-21-12-20-2-1-19-13-22(31)14-23(30)26(19)27(28(20)33-15-21)18-5-9-35(10-6-18)25(37)11-17-3-7-34(8-4-17)16-24(32)36/h12-15,17-18,27H,1-11,16H2,(H2,32,36)/t27-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-FPP incorporation into His6-H-Ras-CVLS by farnesyl transferase |
J Med Chem 42: 2651-61 (1999)
Article DOI: 10.1021/jm990059k
BindingDB Entry DOI: 10.7270/Q2ST7P2V |
More data for this
Ligand-Target Pair | |
Dimer of Protein farnesyltransferase subunit beta
(Homo sapiens (Human)) | BDBM50079073
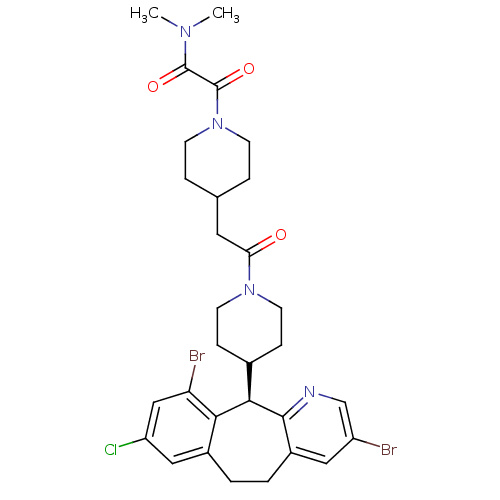
(2-(4-{2-[4-((R)-3,10-Dibromo-8-chloro-6,11-dihydro...)Show SMILES CN(C)C(=O)C(=O)N1CCC(CC(=O)N2CCC(CC2)[C@H]2c3ncc(Br)cc3CCc3cc(Cl)cc(Br)c23)CC1 Show InChI InChI=1S/C30H35Br2ClN4O3/c1-35(2)29(39)30(40)37-9-5-18(6-10-37)13-25(38)36-11-7-19(8-12-36)27-26-20(15-23(33)16-24(26)32)3-4-21-14-22(31)17-34-28(21)27/h14-19,27H,3-13H2,1-2H3/t27-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity against Hras Farnesyltransferase (FPT). |
J Med Chem 42: 2651-61 (1999)
Article DOI: 10.1021/jm990059k
BindingDB Entry DOI: 10.7270/Q2ST7P2V |
More data for this
Ligand-Target Pair | |
Dimer of Protein farnesyltransferase subunit beta
(Homo sapiens (Human)) | BDBM50079076
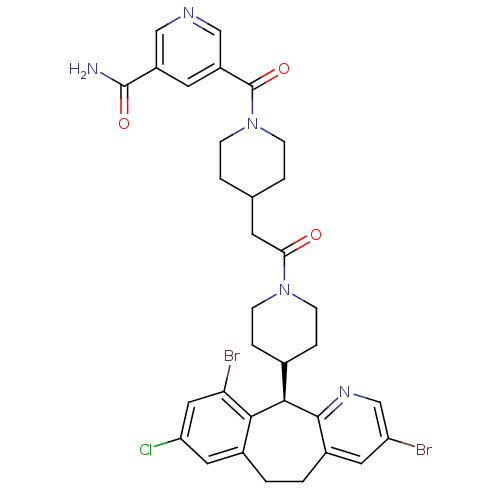
(5-(4-{2-[4-((R)-3,10-Dibromo-8-chloro-6,11-dihydro...)Show SMILES NC(=O)c1cncc(c1)C(=O)N1CCC(CC(=O)N2CCC(CC2)[C@H]2c3ncc(Br)cc3CCc3cc(Cl)cc(Br)c23)CC1 Show InChI InChI=1S/C33H34Br2ClN5O3/c34-25-13-22-2-1-21-14-26(36)15-27(35)29(21)30(31(22)39-18-25)20-5-9-40(10-6-20)28(42)11-19-3-7-41(8-4-19)33(44)24-12-23(32(37)43)16-38-17-24/h12-20,30H,1-11H2,(H2,37,43)/t30-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity against Hras Farnesyltransferase (FPT). |
J Med Chem 42: 2651-61 (1999)
Article DOI: 10.1021/jm990059k
BindingDB Entry DOI: 10.7270/Q2ST7P2V |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM14473
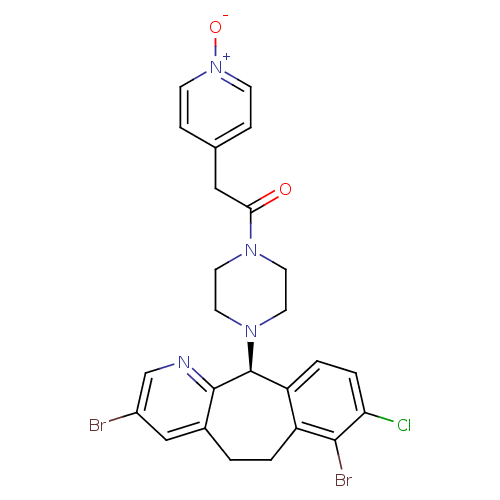
((-)-1-(8-Chloro-3,7-dibromo-6,11-dihydro-5H-benzo-...)Show SMILES [O-][n+]1ccc(CC(=O)N2CCN(CC2)[C@H]2c3ccc(Cl)c(Br)c3CCc3cc(Br)cnc23)cc1 |r| Show InChI InChI=1S/C25H23Br2ClN4O2/c26-18-14-17-1-2-19-20(3-4-21(28)23(19)27)25(24(17)29-15-18)31-11-9-30(10-12-31)22(33)13-16-5-7-32(34)8-6-16/h3-8,14-15,25H,1-2,9-13H2/t25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Schering-Plough Research Institute
| Assay Description
FPT activity was determined by measuring transfer of [3H] farnesyl from [3H]farnesyl pyrophosphate to the substrate His6-Ha-Ras-CVLS. The incorporate... |
J Med Chem 41: 4890-902 (1998)
Article DOI: 10.1021/jm980462b
BindingDB Entry DOI: 10.7270/Q2ZS2TRS |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM14467
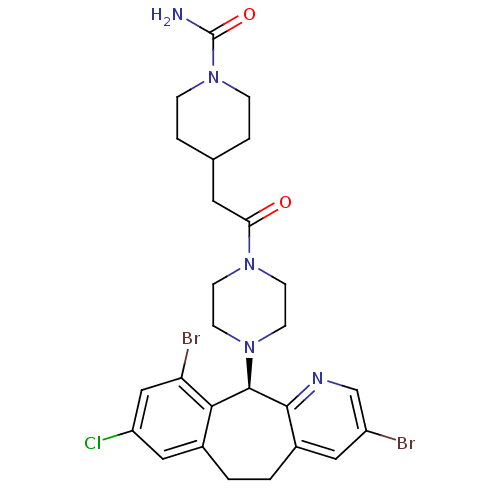
((+)-4-[2-[4-(8-Chloro-3,10-dibromo-6,11-dihydro-5H...)Show SMILES NC(=O)N1CCC(CC(=O)N2CCN(CC2)[C@H]2c3ncc(Br)cc3CCc3cc(Cl)cc(Br)c23)CC1 |r| Show InChI InChI=1S/C26H30Br2ClN5O2/c27-19-12-18-2-1-17-13-20(29)14-21(28)23(17)25(24(18)31-15-19)33-9-7-32(8-10-33)22(35)11-16-3-5-34(6-4-16)26(30)36/h12-16,25H,1-11H2,(H2,30,36)/t25-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Schering-Plough Research Institute
| Assay Description
FPT activity was determined by measuring transfer of [3H] farnesyl from [3H]farnesyl pyrophosphate to the substrate His6-Ha-Ras-CVLS. The incorporate... |
J Med Chem 41: 4890-902 (1998)
Article DOI: 10.1021/jm980462b
BindingDB Entry DOI: 10.7270/Q2ZS2TRS |
More data for this
Ligand-Target Pair | |
Dimer of Protein farnesyltransferase subunit beta
(Homo sapiens (Human)) | BDBM50079070
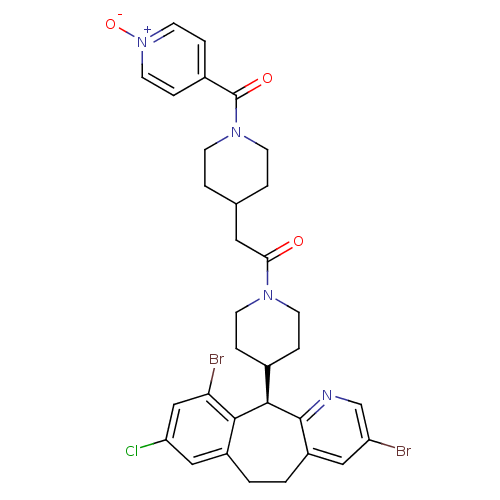
(1-[4-((R)-3,10-Dibromo-8-chloro-6,11-dihydro-5H-be...)Show SMILES [O-][n+]1ccc(cc1)C(=O)N1CCC(CC(=O)N2CCC(CC2)[C@H]2c3ncc(Br)cc3CCc3cc(Cl)cc(Br)c23)CC1 Show InChI InChI=1S/C32H33Br2ClN4O3/c33-25-16-24-2-1-23-17-26(35)18-27(34)29(23)30(31(24)36-19-25)21-5-11-37(12-6-21)28(40)15-20-3-9-38(10-4-20)32(41)22-7-13-39(42)14-8-22/h7-8,13-14,16-21,30H,1-6,9-12,15H2/t30-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Hras Farnesyl protein transferase(FPT) |
J Med Chem 42: 2651-61 (1999)
Article DOI: 10.1021/jm990059k
BindingDB Entry DOI: 10.7270/Q2ST7P2V |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM14469
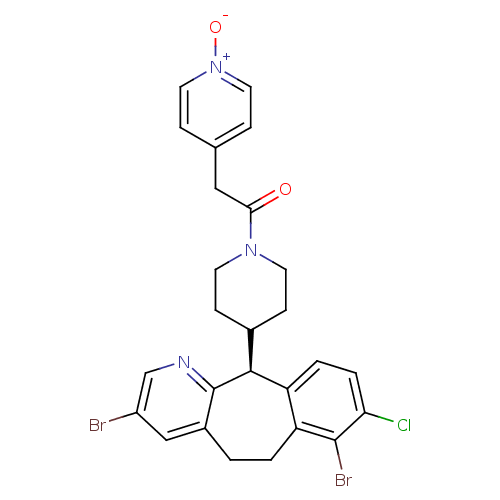
((-)-4-(8-Chloro-3,7-dibromo-6,11-dihydro-5H-benzo-...)Show SMILES [O-][n+]1ccc(CC(=O)N2CCC(CC2)[C@H]2c3ccc(Cl)c(Br)c3CCc3cc(Br)cnc23)cc1 |r| Show InChI InChI=1S/C26H24Br2ClN3O2/c27-19-14-18-1-2-21-20(3-4-22(29)25(21)28)24(26(18)30-15-19)17-7-9-31(10-8-17)23(33)13-16-5-11-32(34)12-6-16/h3-6,11-12,14-15,17,24H,1-2,7-10,13H2/t24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Schering-Plough Research Institute
| Assay Description
FPT activity was determined by measuring transfer of [3H] farnesyl from [3H]farnesyl pyrophosphate to the substrate His6-Ha-Ras-CVLS. The incorporate... |
J Med Chem 41: 4890-902 (1998)
Article DOI: 10.1021/jm980462b
BindingDB Entry DOI: 10.7270/Q2ZS2TRS |
More data for this
Ligand-Target Pair | |
Dimer of Protein farnesyltransferase subunit beta
(Homo sapiens (Human)) | BDBM50079086

(3-(4-{2-[4-((R)-3,10-Dibromo-8-chloro-6,11-dihydro...)Show SMILES Clc1cc(Br)c2[C@@H](C3CCN(CC3)C(=O)CC3CCN(CC3)C(=O)CC#N)c3ncc(Br)cc3CCc2c1 Show InChI InChI=1S/C29H31Br2ClN4O2/c30-22-14-21-2-1-20-15-23(32)16-24(31)27(20)28(29(21)34-17-22)19-6-11-36(12-7-19)26(38)13-18-4-9-35(10-5-18)25(37)3-8-33/h14-19,28H,1-7,9-13H2/t28-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Hras Farnesyl protein transferase(FPT) |
J Med Chem 42: 2651-61 (1999)
Article DOI: 10.1021/jm990059k
BindingDB Entry DOI: 10.7270/Q2ST7P2V |
More data for this
Ligand-Target Pair | |
Dimer of Protein farnesyltransferase subunit beta
(Homo sapiens (Human)) | BDBM50079077
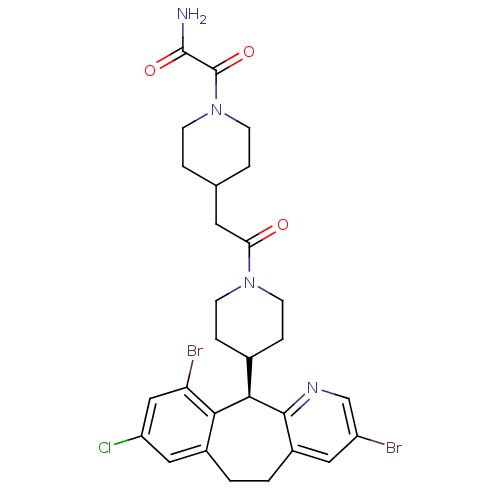
(2-(4-{2-[4-((R)-3,10-Dibromo-8-chloro-6,11-dihydro...)Show SMILES NC(=O)C(=O)N1CCC(CC(=O)N2CCC(CC2)[C@H]2c3ncc(Br)cc3CCc3cc(Cl)cc(Br)c23)CC1 Show InChI InChI=1S/C28H31Br2ClN4O3/c29-20-12-19-2-1-18-13-21(31)14-22(30)24(18)25(26(19)33-15-20)17-5-9-34(10-6-17)23(36)11-16-3-7-35(8-4-16)28(38)27(32)37/h12-17,25H,1-11H2,(H2,32,37)/t25-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity against Hras Farnesyltransferase (FPT). |
J Med Chem 42: 2651-61 (1999)
Article DOI: 10.1021/jm990059k
BindingDB Entry DOI: 10.7270/Q2ST7P2V |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50350830
(CHEMBL1819270)Show InChI InChI=1S/C20H22N4O2/c1-15-18(19-4-2-3-13-24(19)22-15)11-12-21-14-17-7-5-16(6-8-17)9-10-20(25)23-26/h2-10,13,21,26H,11-12,14H2,1H3,(H,23,25)/b10-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 |
J Med Chem 54: 4752-72 (2011)
Article DOI: 10.1021/jm200388e
BindingDB Entry DOI: 10.7270/Q23N24DW |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50350833
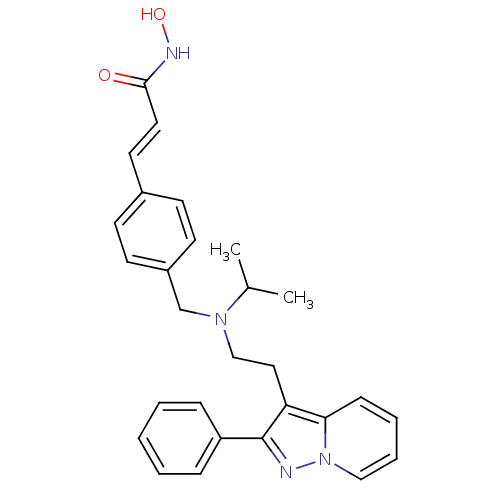
(CHEMBL1819275)Show SMILES CC(C)N(CCc1c(nn2ccccc12)-c1ccccc1)Cc1ccc(\C=C\C(=O)NO)cc1 Show InChI InChI=1S/C28H30N4O2/c1-21(2)31(20-23-13-11-22(12-14-23)15-16-27(33)30-34)19-17-25-26-10-6-7-18-32(26)29-28(25)24-8-4-3-5-9-24/h3-16,18,21,34H,17,19-20H2,1-2H3,(H,30,33)/b16-15+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 |
J Med Chem 54: 4752-72 (2011)
Article DOI: 10.1021/jm200388e
BindingDB Entry DOI: 10.7270/Q23N24DW |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase XIAP [124-214,C202A,C213G]
(Artificial Organism) | BDBM242783

(US9422331, 1)Show SMILES CN[C@@H](C)C(=O)N[C@H]1[C@H](C)N(C(=O)c2ccc(cc2)C(C)=O)c2ccccc2N(Cc2c(OC)ccc3c(Br)cccc23)C1=O |r| Show InChI InChI=1S/C35H35BrN4O5/c1-20(37-4)33(42)38-32-21(2)40(34(43)24-15-13-23(14-16-24)22(3)41)30-12-7-6-11-29(30)39(35(32)44)19-27-25-9-8-10-28(36)26(25)17-18-31(27)45-5/h6-18,20-21,32,37H,19H2,1-5H3,(H,38,42)/t20-,21-,32-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.5 | 37 |
HOFFMANN-LA ROCHE INC.
US Patent
| Assay Description
The peptide AVPIAQKSEK-(ε-biotin)-OH 1:2 TFA ("Peptide A") was identified as a substrate for the TR-FRET assay by screening the 6× Hist... |
US Patent US9422331 (2016)
BindingDB Entry DOI: 10.7270/Q2736PT4 |
More data for this
Ligand-Target Pair | |
Dimer of Protein farnesyltransferase subunit beta
(Homo sapiens (Human)) | BDBM50079072
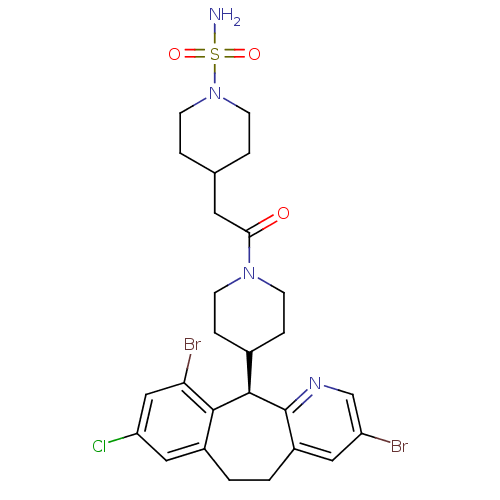
(4-{2-[4-((R)-3,10-Dibromo-8-chloro-6,11-dihydro-5H...)Show SMILES NS(=O)(=O)N1CCC(CC(=O)N2CCC(CC2)[C@H]2c3ncc(Br)cc3CCc3cc(Cl)cc(Br)c23)CC1 Show InChI InChI=1S/C26H31Br2ClN4O3S/c27-20-12-19-2-1-18-13-21(29)14-22(28)24(18)25(26(19)31-15-20)17-5-7-32(8-6-17)23(34)11-16-3-9-33(10-4-16)37(30,35)36/h12-17,25H,1-11H2,(H2,30,35,36)/t25-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity against Hras Farnesyltransferase (FPT). |
J Med Chem 42: 2651-61 (1999)
Article DOI: 10.1021/jm990059k
BindingDB Entry DOI: 10.7270/Q2ST7P2V |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM14477
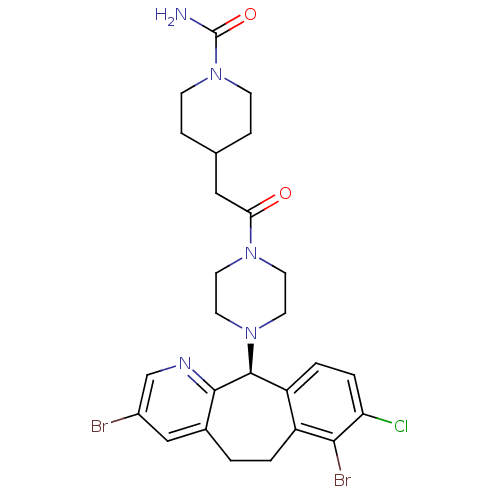
((-)-4-[2-[4-(3,7-Dibromo-8-chloro-6,11-dihydro-5Hb...)Show SMILES NC(=O)N1CCC(CC(=O)N2CCN(CC2)[C@H]2c3ccc(Cl)c(Br)c3CCc3cc(Br)cnc23)CC1 |r| Show InChI InChI=1S/C26H30Br2ClN5O2/c27-18-14-17-1-2-19-20(3-4-21(29)23(19)28)25(24(17)31-15-18)33-11-9-32(10-12-33)22(35)13-16-5-7-34(8-6-16)26(30)36/h3-4,14-16,25H,1-2,5-13H2,(H2,30,36)/t25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Schering-Plough Research Institute
| Assay Description
FPT activity was determined by measuring transfer of [3H] farnesyl from [3H]farnesyl pyrophosphate to the substrate His6-Ha-Ras-CVLS. The incorporate... |
J Med Chem 41: 4890-902 (1998)
Article DOI: 10.1021/jm980462b
BindingDB Entry DOI: 10.7270/Q2ZS2TRS |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM14463
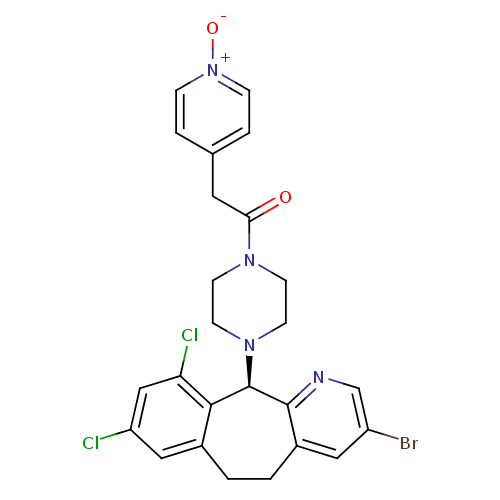
((+)-4-(3-Bromo-8,10-dichloro-6,11-dihydro-5H-benzo...)Show SMILES [O-][n+]1ccc(CC(=O)N2CCN(CC2)[C@H]2c3ncc(Br)cc3CCc3cc(Cl)cc(Cl)c23)cc1 |r| Show InChI InChI=1S/C25H23BrCl2N4O2/c26-19-12-18-2-1-17-13-20(27)14-21(28)23(17)25(24(18)29-15-19)31-9-7-30(8-10-31)22(33)11-16-3-5-32(34)6-4-16/h3-6,12-15,25H,1-2,7-11H2/t25-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <3.80 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Schering-Plough Research Institute
| Assay Description
FPT activity was determined by measuring transfer of [3H] farnesyl from [3H]farnesyl pyrophosphate to the substrate His6-Ha-Ras-CVLS. The incorporate... |
J Med Chem 41: 4890-902 (1998)
Article DOI: 10.1021/jm980462b
BindingDB Entry DOI: 10.7270/Q2ZS2TRS |
More data for this
Ligand-Target Pair | |
Dimer of Protein farnesyltransferase subunit beta
(Homo sapiens (Human)) | BDBM50077853

(1-[4-(3,10-Dibromo-8-chloro-5,6-dihydro-benzo[5,6]...)Show SMILES [#8-]-[n+]1ccc(-[#6]-[#6](=O)-[#7]-2-[#6]-[#6]\[#6](-[#6]-[#6]-2)=[#6]-2/c3ncc(Br)cc3-[#6]-[#6]-c3cc(Cl)cc(Br)c-23)cc1 Show InChI InChI=1S/C26H22Br2ClN3O2/c27-20-12-19-2-1-18-13-21(29)14-22(28)24(18)25(26(19)30-15-20)17-5-7-31(8-6-17)23(33)11-16-3-9-32(34)10-4-16/h3-4,9-10,12-15H,1-2,5-8,11H2 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Farnesyl protein transferase |
J Med Chem 42: 2125-35 (1999)
Article DOI: 10.1021/jm990030g
BindingDB Entry DOI: 10.7270/Q2ZS2VP9 |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Homo sapiens (Human)) | BDBM50422364
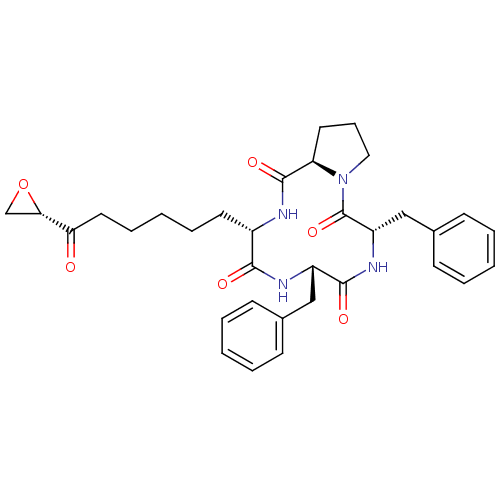
(TRAPOXIN B)Show SMILES O=C(CCCCC[C@@H]1NC(=O)[C@H]2CCCN2C(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccccc2)NC1=O)[C@@H]1CO1 Show InChI InChI=1S/C33H40N4O6/c38-28(29-21-43-29)17-9-3-8-15-24-30(39)35-25(19-22-11-4-1-5-12-22)31(40)36-26(20-23-13-6-2-7-14-23)33(42)37-18-10-16-27(37)32(41)34-24/h1-2,4-7,11-14,24-27,29H,3,8-10,15-21H2,(H,34,41)(H,35,39)(H,36,40)/t24-,25-,26-,27+,29-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Concentration required to inhibit the partially purified HDAC enzyme by 50% obtained from H1299 cell lysate |
J Med Chem 45: 753-7 (2002)
Article DOI: 10.1021/jm015568c
BindingDB Entry DOI: 10.7270/Q2T156D5 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50350821
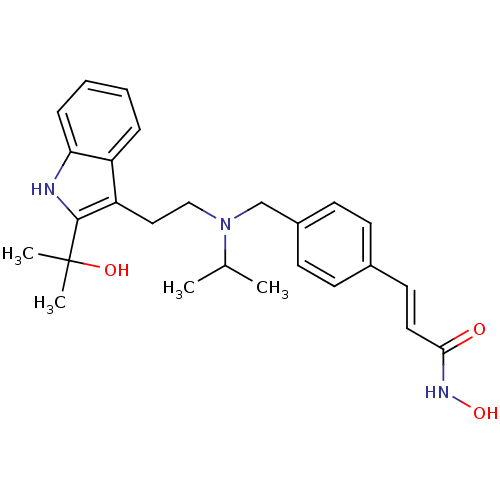
(CHEMBL1819261)Show SMILES CC(C)N(CCc1c([nH]c2ccccc12)C(C)(C)O)Cc1ccc(\C=C\C(=O)NO)cc1 Show InChI InChI=1S/C26H33N3O3/c1-18(2)29(17-20-11-9-19(10-12-20)13-14-24(30)28-32)16-15-22-21-7-5-6-8-23(21)27-25(22)26(3,4)31/h5-14,18,27,31-32H,15-17H2,1-4H3,(H,28,30)/b14-13+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 |
J Med Chem 54: 4752-72 (2011)
Article DOI: 10.1021/jm200388e
BindingDB Entry DOI: 10.7270/Q23N24DW |
More data for this
Ligand-Target Pair | |
Dimer of Protein farnesyltransferase subunit beta
(Homo sapiens (Human)) | BDBM50079088
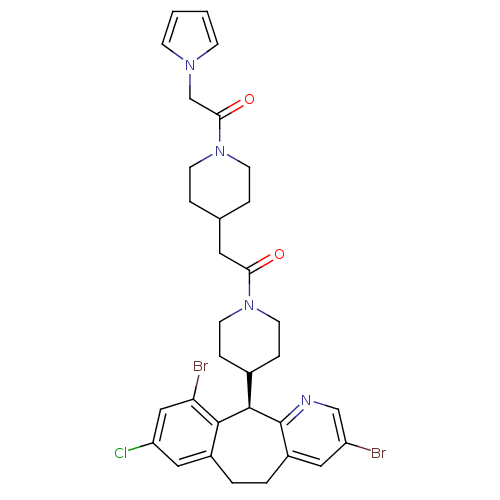
(1-(4-{2-[4-((R)-3,10-Dibromo-8-chloro-6,11-dihydro...)Show SMILES Clc1cc(Br)c2[C@@H](C3CCN(CC3)C(=O)CC3CCN(CC3)C(=O)Cn3cccc3)c3ncc(Br)cc3CCc2c1 Show InChI InChI=1S/C32H35Br2ClN4O2/c33-25-16-24-4-3-23-17-26(35)18-27(34)30(23)31(32(24)36-19-25)22-7-13-38(14-8-22)28(40)15-21-5-11-39(12-6-21)29(41)20-37-9-1-2-10-37/h1-2,9-10,16-19,21-22,31H,3-8,11-15,20H2/t31-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity against Hras Farnesyltransferase (FPT). |
J Med Chem 42: 2651-61 (1999)
Article DOI: 10.1021/jm990059k
BindingDB Entry DOI: 10.7270/Q2ST7P2V |
More data for this
Ligand-Target Pair | |
Dimer of Protein farnesyltransferase subunit beta
(Homo sapiens (Human)) | BDBM50079081
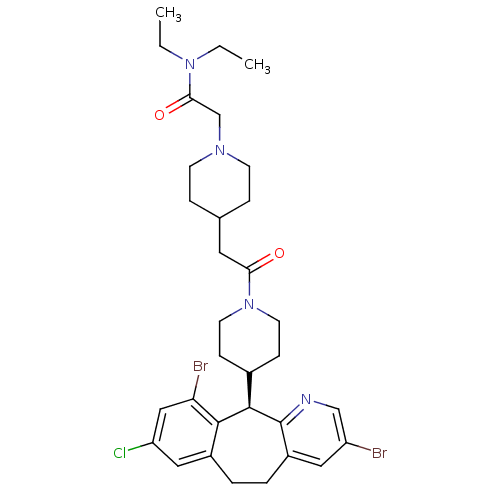
(2-(4-{2-[4-((R)-3,10-Dibromo-8-chloro-6,11-dihydro...)Show SMILES CCN(CC)C(=O)CN1CCC(CC(=O)N2CCC(CC2)[C@H]2c3ncc(Br)cc3CCc3cc(Cl)cc(Br)c23)CC1 Show InChI InChI=1S/C32H41Br2ClN4O2/c1-3-38(4-2)29(41)20-37-11-7-21(8-12-37)15-28(40)39-13-9-22(10-14-39)31-30-23(17-26(35)18-27(30)34)5-6-24-16-25(33)19-36-32(24)31/h16-19,21-22,31H,3-15,20H2,1-2H3/t31-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-FPP incorporation into His6-H-Ras-CVLS by farnesyl transferase |
J Med Chem 42: 2651-61 (1999)
Article DOI: 10.1021/jm990059k
BindingDB Entry DOI: 10.7270/Q2ST7P2V |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase XIAP [124-240]
(Homo sapiens (Human)) | BDBM242611
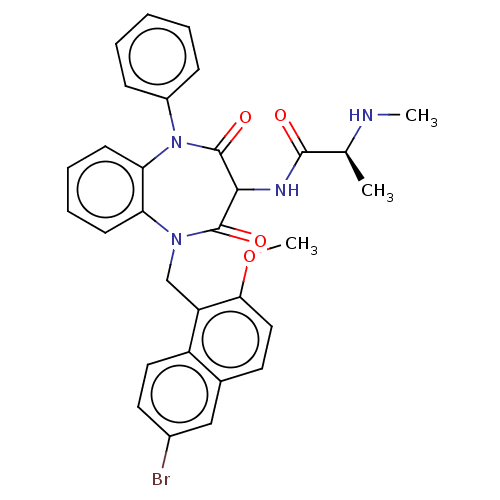
((2S)-N-(1-((6-bromo-2- methoxynaphthalen-1-yl)meth...)Show SMILES CN[C@@H](C)C(=O)NC1C(=O)N(Cc2c(OC)ccc3cc(Br)ccc23)c2ccccc2N(c2ccccc2)C1=O |r| Show InChI InChI=1S/C31H29BrN4O4/c1-19(33-2)29(37)34-28-30(38)35(18-24-23-15-14-21(32)17-20(23)13-16-27(24)40-3)25-11-7-8-12-26(25)36(31(28)39)22-9-5-4-6-10-22/h4-17,19,28,33H,18H2,1-3H3,(H,34,37)/t19-,28?/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 4.99 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Hoffmann-La Roche Inc.
US Patent
| Assay Description
Ten nanomolar of 6× Histidine-tagged BIR2 domain, corresponding to amino acids 124-240 of XIAP, or BIR3 domain, corresponding to amino acids 241-356 ... |
US Patent US10053431 (2018)
BindingDB Entry DOI: 10.7270/Q2W097X3 |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Homo sapiens (Human)) | BDBM50422364

(TRAPOXIN B)Show SMILES O=C(CCCCC[C@@H]1NC(=O)[C@H]2CCCN2C(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccccc2)NC1=O)[C@@H]1CO1 Show InChI InChI=1S/C33H40N4O6/c38-28(29-21-43-29)17-9-3-8-15-24-30(39)35-25(19-22-11-4-1-5-12-22)31(40)36-26(20-23-13-6-2-7-14-23)33(42)37-18-10-16-27(37)32(41)34-24/h1-2,4-7,11-14,24-27,29H,3,8-10,15-21H2,(H,34,41)(H,35,39)(H,36,40)/t24-,25-,26-,27+,29-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against histone deacetylase (HDAC) enzyme obtained from H1299 human lung carcinoma cell lysates |
J Med Chem 46: 4609-24 (2003)
Article DOI: 10.1021/jm030235w
BindingDB Entry DOI: 10.7270/Q2736TP0 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase XIAP [124-214,C202A,C213G]
(Artificial Organism) | BDBM242790
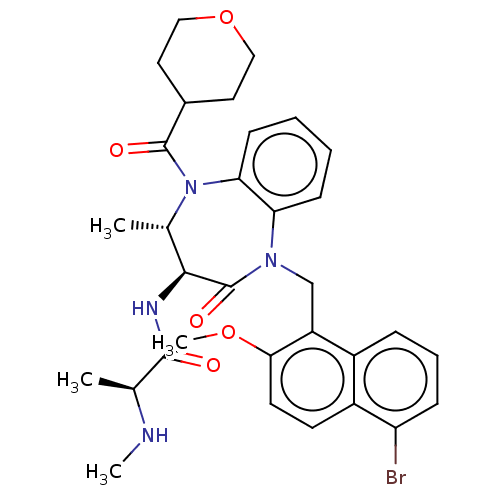
(US9422331, 8)Show SMILES CN[C@@H](C)C(=O)N[C@H]1[C@H](C)N(C(=O)C2CCOCC2)c2ccccc2N(Cc2c(OC)ccc3c(Br)cccc23)C1=O |r| Show InChI InChI=1S/C32H37BrN4O5/c1-19(34-3)30(38)35-29-20(2)37(31(39)21-14-16-42-17-15-21)27-11-6-5-10-26(27)36(32(29)40)18-24-22-8-7-9-25(33)23(22)12-13-28(24)41-4/h5-13,19-21,29,34H,14-18H2,1-4H3,(H,35,38)/t19-,20-,29-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.5 | 37 |
HOFFMANN-LA ROCHE INC.
US Patent
| Assay Description
The peptide AVPIAQKSEK-(ε-biotin)-OH 1:2 TFA ("Peptide A") was identified as a substrate for the TR-FRET assay by screening the 6× Hist... |
US Patent US9422331 (2016)
BindingDB Entry DOI: 10.7270/Q2736PT4 |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Homo sapiens (Human)) | BDBM50422364

(TRAPOXIN B)Show SMILES O=C(CCCCC[C@@H]1NC(=O)[C@H]2CCCN2C(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccccc2)NC1=O)[C@@H]1CO1 Show InChI InChI=1S/C33H40N4O6/c38-28(29-21-43-29)17-9-3-8-15-24-30(39)35-25(19-22-11-4-1-5-12-22)31(40)36-26(20-23-13-6-2-7-14-23)33(42)37-18-10-16-27(37)32(41)34-24/h1-2,4-7,11-14,24-27,29H,3,8-10,15-21H2,(H,34,41)(H,35,39)(H,36,40)/t24-,25-,26-,27+,29-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Concentration required to inhibit human Histone deacetylase (HDAC) enzyme by 50% |
J Med Chem 45: 753-7 (2002)
Article DOI: 10.1021/jm015568c
BindingDB Entry DOI: 10.7270/Q2T156D5 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase XIAP [124-240]
(Homo sapiens (Human)) | BDBM243181

(US10053431, 76d)Show SMILES CN[C@@H](C)C(=O)N[C@H]1CN(C(=O)c2ccc(NC(C)=O)cc2)c2ccccc2N(Cc2c(OC)ccc3cc(Br)ccc23)C1=O |r| Show InChI InChI=1S/C34H34BrN5O5/c1-20(36-3)32(42)38-28-19-40(33(43)22-9-13-25(14-10-22)37-21(2)41)30-8-6-5-7-29(30)39(34(28)44)18-27-26-15-12-24(35)17-23(26)11-16-31(27)45-4/h5-17,20,28,36H,18-19H2,1-4H3,(H,37,41)(H,38,42)/t20-,28-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Hoffmann-La Roche Inc.
US Patent
| Assay Description
Ten nanomolar of 6× Histidine-tagged BIR2 domain, corresponding to amino acids 124-240 of XIAP, or BIR3 domain, corresponding to amino acids 241-356 ... |
US Patent US10053431 (2018)
BindingDB Entry DOI: 10.7270/Q2W097X3 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase XIAP [124-214,C202A,C213G]
(Artificial Organism) | BDBM242789
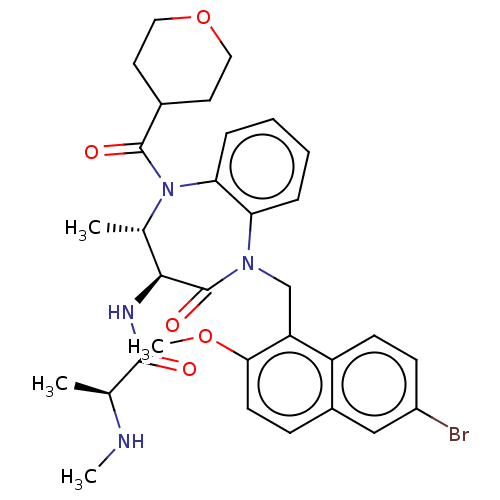
(US9422331, 7)Show SMILES CN[C@@H](C)C(=O)N[C@H]1[C@H](C)N(C(=O)C2CCOCC2)c2ccccc2N(Cc2c(OC)ccc3cc(Br)ccc23)C1=O |r| Show InChI InChI=1S/C32H37BrN4O5/c1-19(34-3)30(38)35-29-20(2)37(31(39)21-13-15-42-16-14-21)27-8-6-5-7-26(27)36(32(29)40)18-25-24-11-10-23(33)17-22(24)9-12-28(25)41-4/h5-12,17,19-21,29,34H,13-16,18H2,1-4H3,(H,35,38)/t19-,20-,29-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.5 | 37 |
HOFFMANN-LA ROCHE INC.
US Patent
| Assay Description
The peptide AVPIAQKSEK-(ε-biotin)-OH 1:2 TFA ("Peptide A") was identified as a substrate for the TR-FRET assay by screening the 6× Hist... |
US Patent US9422331 (2016)
BindingDB Entry DOI: 10.7270/Q2736PT4 |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM14471
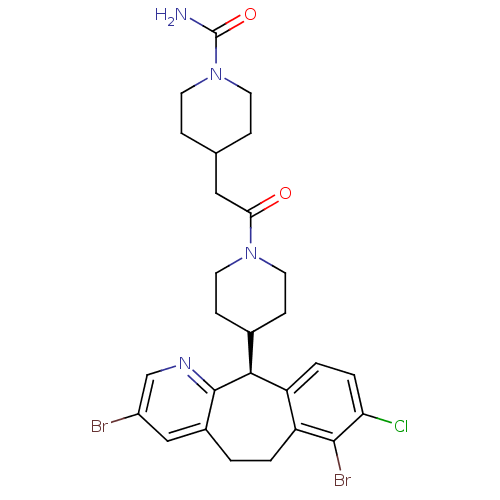
((-)-4-[2-[4-(3,7-Dibromo-8-chloro-6,11-dihydro-5H-...)Show SMILES NC(=O)N1CCC(CC(=O)N2CCC(CC2)[C@H]2c3ccc(Cl)c(Br)c3CCc3cc(Br)cnc23)CC1 |r| Show InChI InChI=1S/C27H31Br2ClN4O2/c28-19-14-18-1-2-21-20(3-4-22(30)25(21)29)24(26(18)32-15-19)17-7-11-33(12-8-17)23(35)13-16-5-9-34(10-6-16)27(31)36/h3-4,14-17,24H,1-2,5-13H2,(H2,31,36)/t24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Schering-Plough Research Institute
| Assay Description
FPT activity was determined by measuring transfer of [3H] farnesyl from [3H]farnesyl pyrophosphate to the substrate His6-Ha-Ras-CVLS. The incorporate... |
J Med Chem 41: 4890-902 (1998)
Article DOI: 10.1021/jm980462b
BindingDB Entry DOI: 10.7270/Q2ZS2TRS |
More data for this
Ligand-Target Pair | |
Dimer of Protein farnesyltransferase subunit beta
(Homo sapiens (Human)) | BDBM14471

((-)-4-[2-[4-(3,7-Dibromo-8-chloro-6,11-dihydro-5H-...)Show SMILES NC(=O)N1CCC(CC(=O)N2CCC(CC2)[C@H]2c3ccc(Cl)c(Br)c3CCc3cc(Br)cnc23)CC1 |r| Show InChI InChI=1S/C27H31Br2ClN4O2/c28-19-14-18-1-2-21-20(3-4-22(30)25(21)29)24(26(18)32-15-19)17-7-11-33(12-8-17)23(35)13-16-5-9-34(10-6-16)27(31)36/h3-4,14-17,24H,1-2,5-13H2,(H2,31,36)/t24-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Farnesyltransferase |
J Med Chem 42: 2125-35 (1999)
Article DOI: 10.1021/jm990030g
BindingDB Entry DOI: 10.7270/Q2ZS2VP9 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase XIAP [124-240]
(Artificial Sequence) | BDBM242836
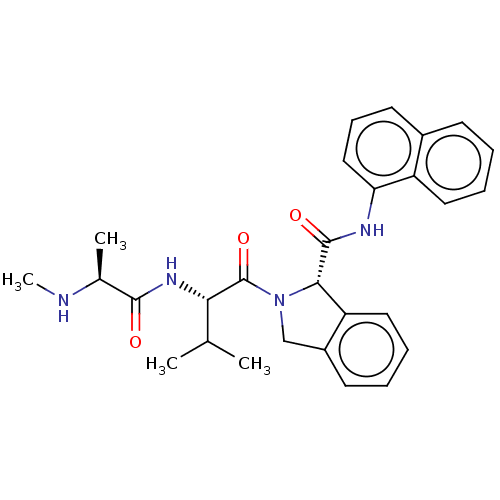
(US9422332, 21)Show SMILES CN[C@@H](C)C(=O)N[C@@H](C(C)C)C(=O)N1Cc2ccccc2[C@H]1C(=O)Nc1cccc2ccccc12 |r| Show InChI InChI=1S/C28H32N4O3/c1-17(2)24(31-26(33)18(3)29-4)28(35)32-16-20-11-6-8-14-22(20)25(32)27(34)30-23-15-9-12-19-10-5-7-13-21(19)23/h5-15,17-18,24-25,29H,16H2,1-4H3,(H,30,34)(H,31,33)/t18-,24-,25-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.5 | 37 |
HOFFMANN-LA ROCHE INC.
US Patent
| Assay Description
The peptide AVPIAQKSEK-(ε-biotin)-OH 1:2 TFA ("Peptide A") was identified as a substrate for the TR-FRET assay by screening the 6× Hist... |
US Patent US9422332 (2016)
BindingDB Entry DOI: 10.7270/Q23F4NJK |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase XIAP
(Homo sapiens (Human)) | BDBM50441819
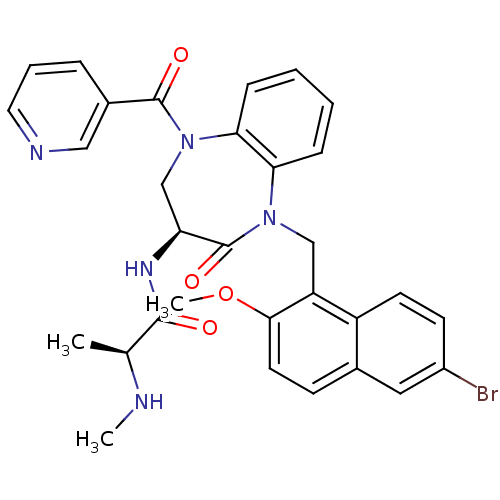
(CHEMBL2436209 | US10053431, 89b)Show SMILES CN[C@@H](C)C(=O)N[C@H]1CN(C(=O)c2cccnc2)c2ccccc2N(Cc2c(OC)ccc3cc(Br)ccc23)C1=O |r| Show InChI InChI=1S/C31H30BrN5O4/c1-19(33-2)29(38)35-25-18-37(30(39)21-7-6-14-34-16-21)27-9-5-4-8-26(27)36(31(25)40)17-24-23-12-11-22(32)15-20(23)10-13-28(24)41-3/h4-16,19,25,33H,17-18H2,1-3H3,(H,35,38)/t19-,25-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Displacement of AVPIAQKSEK (epsilon-biotin)-OH from 6xhistidine-thrombin-TEV-tagged XIAP BIR2 domain (124 to 240) (unknown origin) after 45 mins by T... |
J Med Chem 56: 7788-803 (2013)
Article DOI: 10.1021/jm400732v
BindingDB Entry DOI: 10.7270/Q2M32X6V |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data