 Found 283 hits with Last Name = 'riggs' and Initial = 'jr'
Found 283 hits with Last Name = 'riggs' and Initial = 'jr' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Coagulation factor VII
(Homo sapiens (Human)) | BDBM50180400
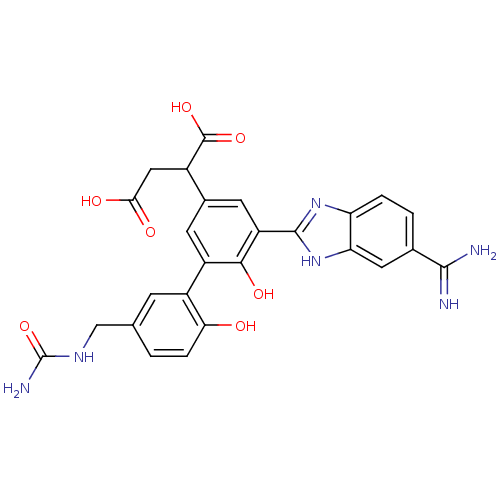
(2-[5-(5-carbamimidoyl-1H-benzoimidazol-2-yl)-6,2'-...)Show SMILES NC(=O)NCc1ccc(O)c(c1)-c1cc(cc(-c2nc3ccc(cc3[nH]2)C(N)=N)c1O)C(CC(O)=O)C(O)=O Show InChI InChI=1S/C26H24N6O7/c27-23(28)12-2-3-18-19(8-12)32-24(31-18)17-7-13(14(25(37)38)9-21(34)35)6-16(22(17)36)15-5-11(1-4-20(15)33)10-30-26(29)39/h1-8,14,33,36H,9-10H2,(H3,27,28)(H,31,32)(H,34,35)(H,37,38)(H3,29,30,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Binding affinity to f7a |
Bioorg Med Chem Lett 16: 3829-32 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.018
BindingDB Entry DOI: 10.7270/Q2DN44NK |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor VII/Tissue factor
(Homo sapiens (Human)) | BDBM50189939
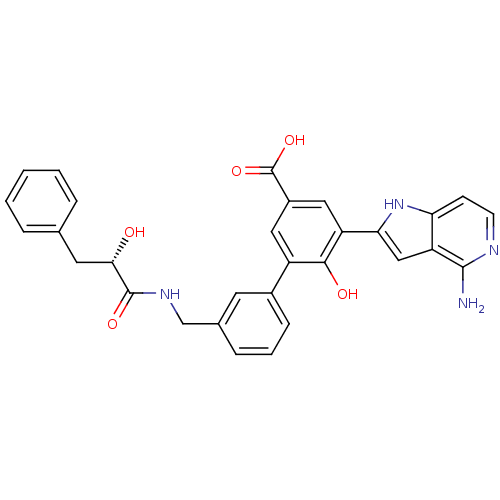
(5-(4-amino-1H-pyrrolo[3,2-c]pyridin-2-yl)-6-hydrox...)Show SMILES Nc1nccc2[nH]c(cc12)-c1cc(cc(-c2cccc(CNC(=O)[C@@H](O)Cc3ccccc3)c2)c1O)C(O)=O Show InChI InChI=1S/C30H26N4O5/c31-28-23-15-25(34-24(23)9-10-32-28)22-14-20(30(38)39)13-21(27(22)36)19-8-4-7-18(11-19)16-33-29(37)26(35)12-17-5-2-1-3-6-17/h1-11,13-15,26,34-36H,12,16H2,(H2,31,32)(H,33,37)(H,38,39)/t26-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics
Curated by ChEMBL
| Assay Description
Binding affinity to f7a/TF complex |
Bioorg Med Chem Lett 16: 4567-70 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.016
BindingDB Entry DOI: 10.7270/Q2NG4Q67 |
More data for this
Ligand-Target Pair | |
Coagulation factor VII/Tissue factor
(Homo sapiens (Human)) | BDBM50189938
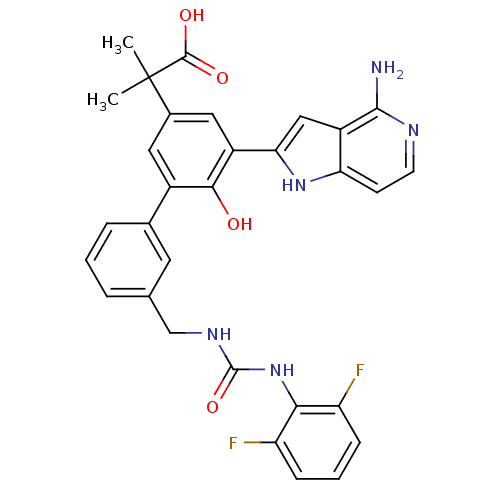
(2-{5-(4-amino-1H-pyrrolo[3,2-c]pyridin-2-yl)-3'-[3...)Show SMILES CC(C)(C(O)=O)c1cc(-c2cc3c(N)nccc3[nH]2)c(O)c(c1)-c1cccc(CNC(=O)Nc2c(F)cccc2F)c1 Show InChI InChI=1S/C31H27F2N5O4/c1-31(2,29(40)41)18-12-19(27(39)20(13-18)25-14-21-24(37-25)9-10-35-28(21)34)17-6-3-5-16(11-17)15-36-30(42)38-26-22(32)7-4-8-23(26)33/h3-14,37,39H,15H2,1-2H3,(H2,34,35)(H,40,41)(H2,36,38,42) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics
Curated by ChEMBL
| Assay Description
Binding affinity to f7a/TF complex |
Bioorg Med Chem Lett 16: 4567-70 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.016
BindingDB Entry DOI: 10.7270/Q2NG4Q67 |
More data for this
Ligand-Target Pair | |
Coagulation factor VII
(Homo sapiens (Human)) | BDBM50186211

(CHEMBL211482 | N-[3'-(5-carbamimidoyl-1H-indol-2-y...)Show SMILES NC(=N)c1ccc2[nH]c(cc2c1)-c1cccc(c1O)-c1cc(CNC(=O)CO)ccc1O Show InChI InChI=1S/C24H22N4O4/c25-24(26)14-5-6-19-15(9-14)10-20(28-19)17-3-1-2-16(23(17)32)18-8-13(4-7-21(18)30)11-27-22(31)12-29/h1-10,28-30,32H,11-12H2,(H3,25,26)(H,27,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Binding affinity to f7a |
Bioorg Med Chem Lett 16: 3829-32 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.018
BindingDB Entry DOI: 10.7270/Q2DN44NK |
More data for this
Ligand-Target Pair | |
Coagulation factor VII
(Homo sapiens (Human)) | BDBM50186210
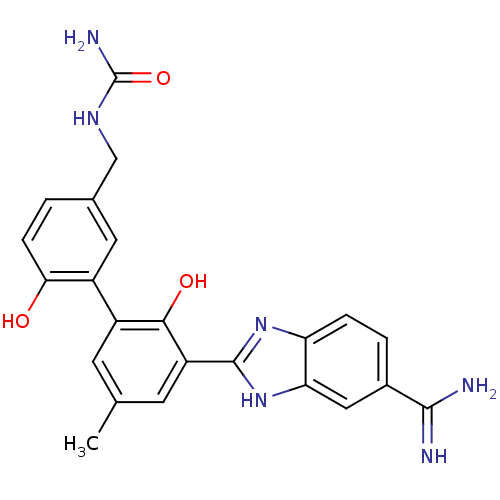
(2-(2,2'-dihydroxy-5-methyl-5'-ureidomethyl-bipheny...)Show SMILES Cc1cc(-c2nc3ccc(cc3[nH]2)C(N)=N)c(O)c(c1)-c1cc(CNC(N)=O)ccc1O Show InChI InChI=1S/C23H22N6O3/c1-11-6-15(14-8-12(2-5-19(14)30)10-27-23(26)32)20(31)16(7-11)22-28-17-4-3-13(21(24)25)9-18(17)29-22/h2-9,30-31H,10H2,1H3,(H3,24,25)(H,28,29)(H3,26,27,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Binding affinity to f7a |
Bioorg Med Chem Lett 16: 3829-32 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.018
BindingDB Entry DOI: 10.7270/Q2DN44NK |
More data for this
Ligand-Target Pair | |
Coagulation factor VII
(Homo sapiens (Human)) | BDBM50186216

(2-(5-fluoro-2,2'-dihydroxy-5'-ureidomethyl-bipheny...)Show SMILES NC(=O)NCc1ccc(O)c(c1)-c1cc(F)cc(-c2nc3ccc(cc3[nH]2)C(N)=N)c1O Show InChI InChI=1S/C22H19FN6O3/c23-12-7-14(13-5-10(1-4-18(13)30)9-27-22(26)32)19(31)15(8-12)21-28-16-3-2-11(20(24)25)6-17(16)29-21/h1-8,30-31H,9H2,(H3,24,25)(H,28,29)(H3,26,27,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Binding affinity to f7a |
Bioorg Med Chem Lett 16: 3829-32 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.018
BindingDB Entry DOI: 10.7270/Q2DN44NK |
More data for this
Ligand-Target Pair | |
Coagulation factor VII
(Homo sapiens (Human)) | BDBM14863
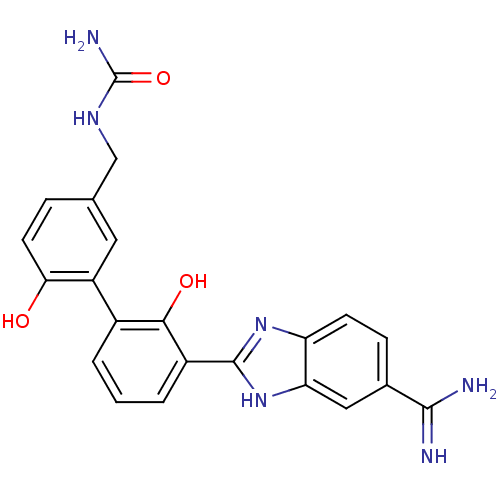
(({3-[3-(5-carbamimidoyl-1H-1,3-benzodiazol-2-yl)-2...)Show SMILES NC(=O)NCc1ccc(O)c(c1)-c1cccc(-c2nc3ccc(cc3[nH]2)C(N)=N)c1O Show InChI InChI=1S/C22H20N6O3/c23-20(24)12-5-6-16-17(9-12)28-21(27-16)14-3-1-2-13(19(14)30)15-8-11(4-7-18(15)29)10-26-22(25)31/h1-9,29-30H,10H2,(H3,23,24)(H,27,28)(H3,25,26,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 13 | -44.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Celera
| Assay Description
The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... |
Bioorg Med Chem Lett 16: 3197-200 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.049
BindingDB Entry DOI: 10.7270/Q20P0X9Q |
More data for this
Ligand-Target Pair | |
Coagulation factor VII/Tissue factor
(Homo sapiens (Human)) | BDBM14863

(({3-[3-(5-carbamimidoyl-1H-1,3-benzodiazol-2-yl)-2...)Show SMILES NC(=O)NCc1ccc(O)c(c1)-c1cccc(-c2nc3ccc(cc3[nH]2)C(N)=N)c1O Show InChI InChI=1S/C22H20N6O3/c23-20(24)12-5-6-16-17(9-12)28-21(27-16)14-3-1-2-13(19(14)30)15-8-11(4-7-18(15)29)10-26-22(25)31/h1-9,29-30H,10H2,(H3,23,24)(H,27,28)(H3,25,26,31) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics
Curated by ChEMBL
| Assay Description
Binding affinity to f7a/TF complex |
Bioorg Med Chem Lett 16: 4567-70 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.016
BindingDB Entry DOI: 10.7270/Q2NG4Q67 |
More data for this
Ligand-Target Pair | |
Coagulation factor VII
(Homo sapiens (Human)) | BDBM50186214
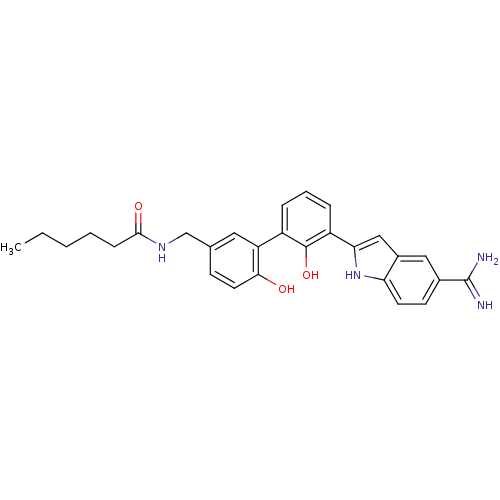
(CHEMBL380098 | hexanoic acid [3'-(5-carbamimidoyl-...)Show SMILES CCCCCC(=O)NCc1ccc(O)c(c1)-c1cccc(-c2cc3cc(ccc3[nH]2)C(N)=N)c1O Show InChI InChI=1S/C28H30N4O3/c1-2-3-4-8-26(34)31-16-17-9-12-25(33)22(13-17)20-6-5-7-21(27(20)35)24-15-19-14-18(28(29)30)10-11-23(19)32-24/h5-7,9-15,32-33,35H,2-4,8,16H2,1H3,(H3,29,30)(H,31,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Binding affinity to f7a |
Bioorg Med Chem Lett 16: 3829-32 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.018
BindingDB Entry DOI: 10.7270/Q2DN44NK |
More data for this
Ligand-Target Pair | |
Coagulation factor VII
(Homo sapiens (Human)) | BDBM14863

(({3-[3-(5-carbamimidoyl-1H-1,3-benzodiazol-2-yl)-2...)Show SMILES NC(=O)NCc1ccc(O)c(c1)-c1cccc(-c2nc3ccc(cc3[nH]2)C(N)=N)c1O Show InChI InChI=1S/C22H20N6O3/c23-20(24)12-5-6-16-17(9-12)28-21(27-16)14-3-1-2-13(19(14)30)15-8-11(4-7-18(15)29)10-26-22(25)31/h1-9,29-30H,10H2,(H3,23,24)(H,27,28)(H3,25,26,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Binding affinity to f7a |
Bioorg Med Chem Lett 16: 3829-32 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.018
BindingDB Entry DOI: 10.7270/Q2DN44NK |
More data for this
Ligand-Target Pair | |
Coagulation factor VII
(Homo sapiens (Human)) | BDBM50186217
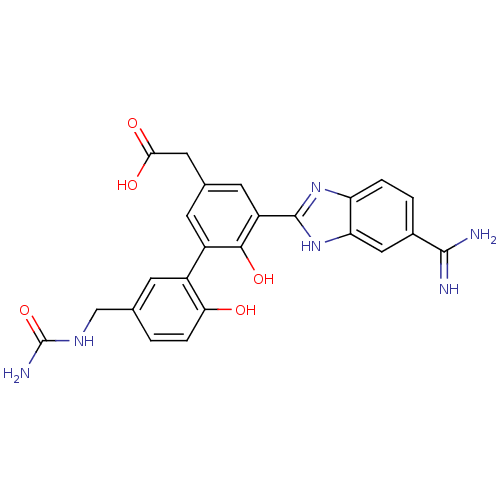
(CHEMBL377990 | [5-(5-carbamimidoyl-1H-benzoimidazo...)Show SMILES NC(=O)NCc1ccc(O)c(c1)-c1cc(CC(O)=O)cc(-c2nc3ccc(cc3[nH]2)C(N)=N)c1O Show InChI InChI=1S/C24H22N6O5/c25-22(26)13-2-3-17-18(9-13)30-23(29-17)16-7-12(8-20(32)33)6-15(21(16)34)14-5-11(1-4-19(14)31)10-28-24(27)35/h1-7,9,31,34H,8,10H2,(H3,25,26)(H,29,30)(H,32,33)(H3,27,28,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Binding affinity to f7a |
Bioorg Med Chem Lett 16: 3829-32 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.018
BindingDB Entry DOI: 10.7270/Q2DN44NK |
More data for this
Ligand-Target Pair | |
Coagulation factor VII/Tissue factor
(Homo sapiens (Human)) | BDBM50189941
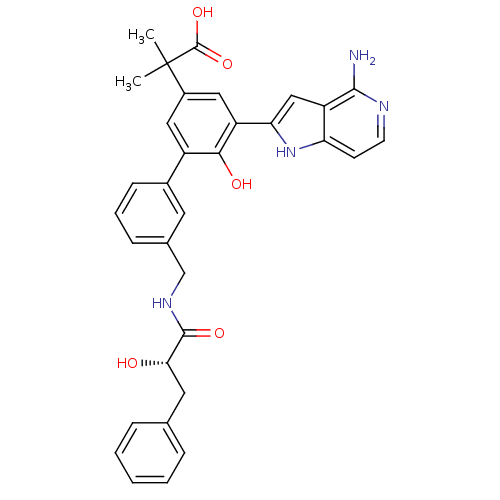
(2-{5-(4-amino-1H-pyrrolo[3,2-c]pyridin-2-yl)-6-hyd...)Show SMILES CC(C)(C(O)=O)c1cc(-c2cc3c(N)nccc3[nH]2)c(O)c(c1)-c1cccc(CNC(=O)[C@@H](O)Cc2ccccc2)c1 Show InChI InChI=1S/C33H32N4O5/c1-33(2,32(41)42)22-15-23(29(39)24(16-22)27-17-25-26(37-27)11-12-35-30(25)34)21-10-6-9-20(13-21)18-36-31(40)28(38)14-19-7-4-3-5-8-19/h3-13,15-17,28,37-39H,14,18H2,1-2H3,(H2,34,35)(H,36,40)(H,41,42)/t28-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics
Curated by ChEMBL
| Assay Description
Binding affinity to f7a/TF complex |
Bioorg Med Chem Lett 16: 4567-70 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.016
BindingDB Entry DOI: 10.7270/Q2NG4Q67 |
More data for this
Ligand-Target Pair | |
Coagulation factor VII
(Homo sapiens (Human)) | BDBM50186212
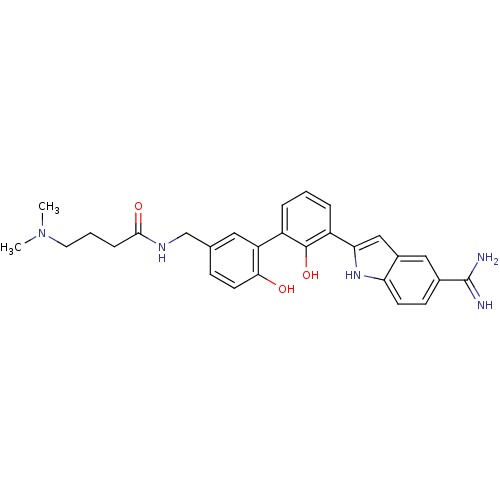
(CHEMBL214351 | N-[3'-(5-carbamimidoyl-1H-indol-2-y...)Show SMILES CN(C)CCCC(=O)NCc1ccc(O)c(c1)-c1cccc(-c2cc3cc(ccc3[nH]2)C(N)=N)c1O Show InChI InChI=1S/C28H31N5O3/c1-33(2)12-4-7-26(35)31-16-17-8-11-25(34)22(13-17)20-5-3-6-21(27(20)36)24-15-19-14-18(28(29)30)9-10-23(19)32-24/h3,5-6,8-11,13-15,32,34,36H,4,7,12,16H2,1-2H3,(H3,29,30)(H,31,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Binding affinity to f7a |
Bioorg Med Chem Lett 16: 3829-32 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.018
BindingDB Entry DOI: 10.7270/Q2DN44NK |
More data for this
Ligand-Target Pair | |
Coagulation factor VII
(Homo sapiens (Human)) | BDBM50186215

(CHEMBL214352 | morpholine-4-carboxylic acid [3'-(5...)Show SMILES NC(=N)c1ccc2[nH]c(cc2c1)-c1cccc(c1O)-c1cc(CNC(=O)N2CCOCC2)ccc1O Show InChI InChI=1S/C27H27N5O4/c28-26(29)17-5-6-22-18(13-17)14-23(31-22)20-3-1-2-19(25(20)34)21-12-16(4-7-24(21)33)15-30-27(35)32-8-10-36-11-9-32/h1-7,12-14,31,33-34H,8-11,15H2,(H3,28,29)(H,30,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Binding affinity to f7a |
Bioorg Med Chem Lett 16: 3829-32 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.018
BindingDB Entry DOI: 10.7270/Q2DN44NK |
More data for this
Ligand-Target Pair | |
Coagulation factor VII/Tissue factor
(Homo sapiens (Human)) | BDBM50189942
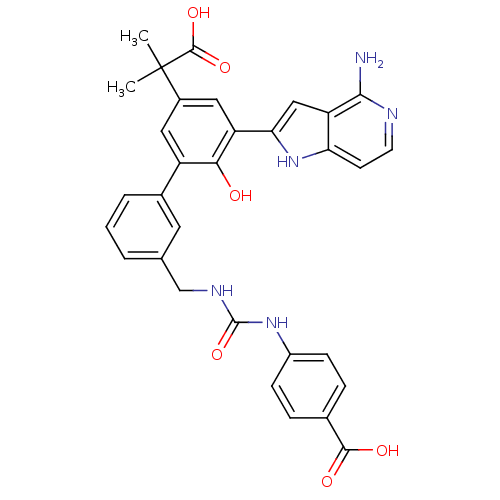
(4-{3-[3'-(4-amino-1H-pyrrolo[3,2-c]pyridin-2-yl)-5...)Show SMILES CC(C)(C(O)=O)c1cc(-c2cc3c(N)nccc3[nH]2)c(O)c(c1)-c1cccc(CNC(=O)Nc2ccc(cc2)C(O)=O)c1 Show InChI InChI=1S/C32H29N5O6/c1-32(2,30(41)42)20-13-22(27(38)23(14-20)26-15-24-25(37-26)10-11-34-28(24)33)19-5-3-4-17(12-19)16-35-31(43)36-21-8-6-18(7-9-21)29(39)40/h3-15,37-38H,16H2,1-2H3,(H2,33,34)(H,39,40)(H,41,42)(H2,35,36,43) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics
Curated by ChEMBL
| Assay Description
Binding affinity to f7a/TF complex |
Bioorg Med Chem Lett 16: 4567-70 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.016
BindingDB Entry DOI: 10.7270/Q2NG4Q67 |
More data for this
Ligand-Target Pair | |
Coagulation factor VII
(Homo sapiens (Human)) | BDBM14880
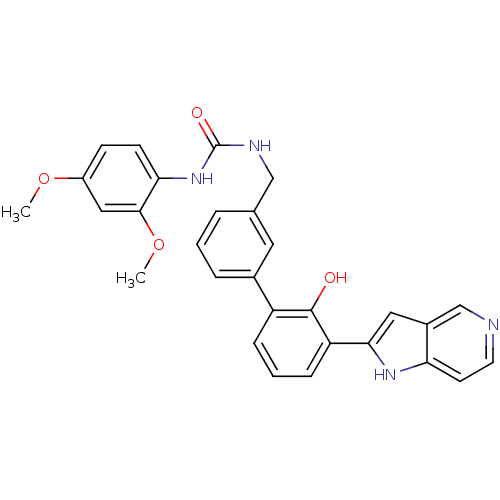
(1-(2,4-dimethoxyphenyl)-3-{[3-(2-hydroxy-3-{1H-pyr...)Show SMILES COc1ccc(NC(=O)NCc2cccc(c2)-c2cccc(-c3cc4cnccc4[nH]3)c2O)c(OC)c1 Show InChI InChI=1S/C29H26N4O4/c1-36-21-9-10-25(27(15-21)37-2)33-29(35)31-16-18-5-3-6-19(13-18)22-7-4-8-23(28(22)34)26-14-20-17-30-12-11-24(20)32-26/h3-15,17,32,34H,16H2,1-2H3,(H2,31,33,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
| Assay Description
The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... |
Bioorg Med Chem Lett 16: 3197-200 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.049
BindingDB Entry DOI: 10.7270/Q20P0X9Q |
More data for this
Ligand-Target Pair | |
Coagulation factor VII
(Homo sapiens (Human)) | BDBM14872
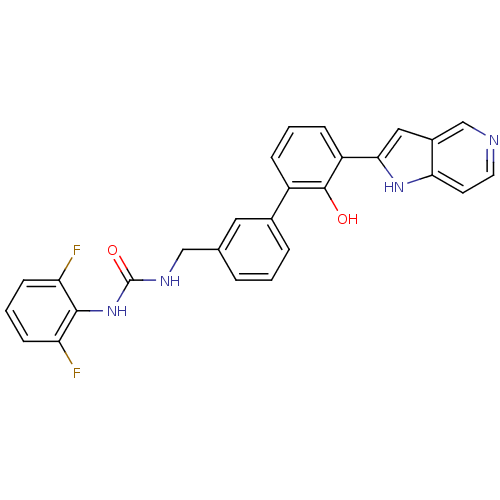
(1-(2,6-difluorophenyl)-3-{[3-(2-hydroxy-3-{1H-pyrr...)Show SMILES Oc1c(cccc1-c1cccc(CNC(=O)Nc2c(F)cccc2F)c1)-c1cc2cnccc2[nH]1 Show InChI InChI=1S/C27H20F2N4O2/c28-21-8-3-9-22(29)25(21)33-27(35)31-14-16-4-1-5-17(12-16)19-6-2-7-20(26(19)34)24-13-18-15-30-11-10-23(18)32-24/h1-13,15,32,34H,14H2,(H2,31,33,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 33 | -42.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Celera
| Assay Description
The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... |
Bioorg Med Chem Lett 16: 3197-200 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.049
BindingDB Entry DOI: 10.7270/Q20P0X9Q |
More data for this
Ligand-Target Pair | |
Coagulation factor VII
(Homo sapiens (Human)) | BDBM14877

(3-{[3-(2-hydroxy-3-{1H-pyrrolo[3,2-c]pyridin-2-yl}...)Show SMILES COc1ccccc1NC(=O)NCc1cccc(c1)-c1cccc(-c2cc3cnccc3[nH]2)c1O Show InChI InChI=1S/C28H24N4O3/c1-35-26-11-3-2-10-24(26)32-28(34)30-16-18-6-4-7-19(14-18)21-8-5-9-22(27(21)33)25-15-20-17-29-13-12-23(20)31-25/h2-15,17,31,33H,16H2,1H3,(H2,30,32,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 47 | -41.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Celera
| Assay Description
The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... |
Bioorg Med Chem Lett 16: 3197-200 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.049
BindingDB Entry DOI: 10.7270/Q20P0X9Q |
More data for this
Ligand-Target Pair | |
Coagulation factor VII
(Homo sapiens (Human)) | BDBM14876
(1-(2-chlorophenyl)-3-{[3-(2-hydroxy-3-{1H-pyrrolo[...)Show SMILES Oc1c(cccc1-c1cccc(CNC(=O)Nc2ccccc2Cl)c1)-c1cc2cnccc2[nH]1 Show InChI InChI=1S/C27H21ClN4O2/c28-22-9-1-2-10-24(22)32-27(34)30-15-17-5-3-6-18(13-17)20-7-4-8-21(26(20)33)25-14-19-16-29-12-11-23(19)31-25/h1-14,16,31,33H,15H2,(H2,30,32,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 57 | -40.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Celera
| Assay Description
The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... |
Bioorg Med Chem Lett 16: 3197-200 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.049
BindingDB Entry DOI: 10.7270/Q20P0X9Q |
More data for this
Ligand-Target Pair | |
Coagulation factor VII
(Homo sapiens (Human)) | BDBM14869

(1-(2-fluorophenyl)-3-{[3-(2-hydroxy-3-{1H-pyrrolo[...)Show SMILES Oc1c(cccc1-c1cccc(CNC(=O)Nc2ccccc2F)c1)-c1cc2cnccc2[nH]1 Show InChI InChI=1S/C27H21FN4O2/c28-22-9-1-2-10-24(22)32-27(34)30-15-17-5-3-6-18(13-17)20-7-4-8-21(26(20)33)25-14-19-16-29-12-11-23(19)31-25/h1-14,16,31,33H,15H2,(H2,30,32,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 57 | -40.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Celera
| Assay Description
The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... |
Bioorg Med Chem Lett 16: 3197-200 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.049
BindingDB Entry DOI: 10.7270/Q20P0X9Q |
More data for this
Ligand-Target Pair | |
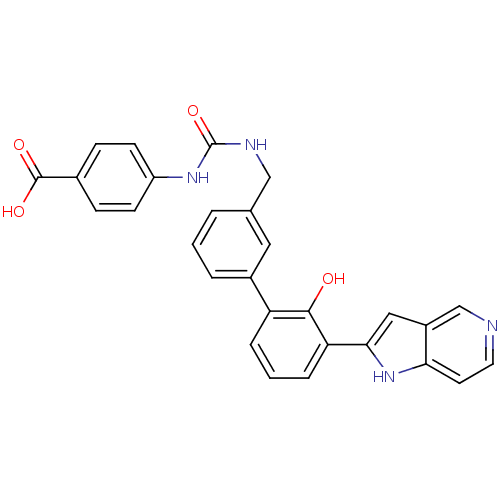
Coagulation factor VII
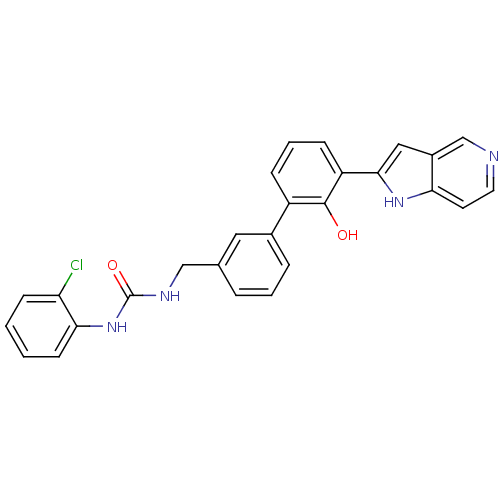
(Homo sapiens (Human)) | BDBM14883
(4-[({[3-(2-hydroxy-3-{1H-pyrrolo[3,2-c]pyridin-2-y...)Show SMILES OC(=O)c1ccc(NC(=O)NCc2cccc(c2)-c2cccc(-c3cc4cnccc4[nH]3)c2O)cc1 Show InChI InChI=1S/C28H22N4O4/c33-26-22(5-2-6-23(26)25-14-20-16-29-12-11-24(20)32-25)19-4-1-3-17(13-19)15-30-28(36)31-21-9-7-18(8-10-21)27(34)35/h1-14,16,32-33H,15H2,(H,34,35)(H2,30,31,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
| Assay Description
The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... |
Bioorg Med Chem Lett 16: 3197-200 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.049
BindingDB Entry DOI: 10.7270/Q20P0X9Q |
More data for this
Ligand-Target Pair | |
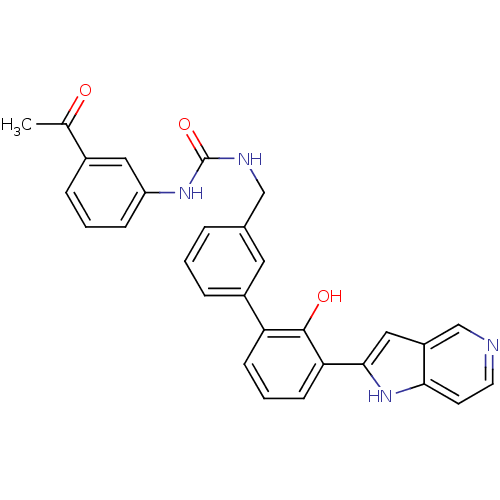
Coagulation factor VII
(Homo sapiens (Human)) | BDBM14881
(1-(3-acetylphenyl)-3-{[3-(2-hydroxy-3-{1H-pyrrolo[...)Show SMILES CC(=O)c1cccc(NC(=O)NCc2cccc(c2)-c2cccc(-c3cc4cnccc4[nH]3)c2O)c1 Show InChI InChI=1S/C29H24N4O3/c1-18(34)20-6-3-8-23(14-20)32-29(36)31-16-19-5-2-7-21(13-19)24-9-4-10-25(28(24)35)27-15-22-17-30-12-11-26(22)33-27/h2-15,17,33,35H,16H2,1H3,(H2,31,32,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
| Assay Description
The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... |
Bioorg Med Chem Lett 16: 3197-200 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.049
BindingDB Entry DOI: 10.7270/Q20P0X9Q |
More data for this
Ligand-Target Pair | |
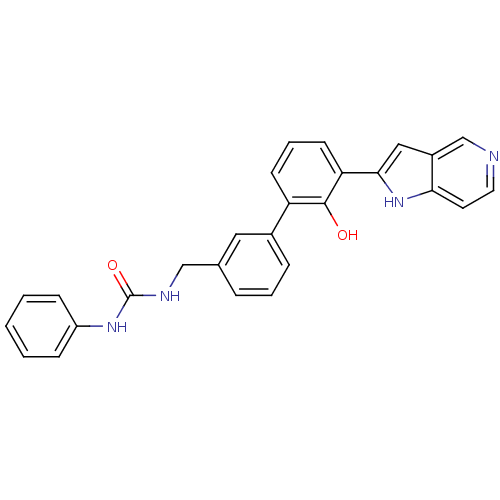
Coagulation factor VII
(Homo sapiens (Human)) | BDBM14868
(3-{[3-(2-hydroxy-3-{1H-pyrrolo[3,2-c]pyridin-2-yl}...)Show SMILES Oc1c(cccc1-c1cccc(CNC(=O)Nc2ccccc2)c1)-c1cc2cnccc2[nH]1 Show InChI InChI=1S/C27H22N4O2/c32-26-22(10-5-11-23(26)25-15-20-17-28-13-12-24(20)31-25)19-7-4-6-18(14-19)16-29-27(33)30-21-8-2-1-3-9-21/h1-15,17,31-32H,16H2,(H2,29,30,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 61.5 | -40.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Celera
| Assay Description
The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... |
Bioorg Med Chem Lett 16: 3197-200 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.049
BindingDB Entry DOI: 10.7270/Q20P0X9Q |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor VII
(Homo sapiens (Human)) | BDBM14896
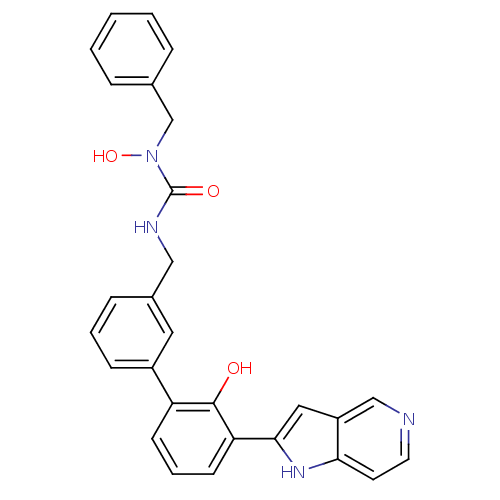
(1-benzyl-1-hydroxy-3-{[3-(2-hydroxy-3-{1H-pyrrolo[...)Show SMILES ON(Cc1ccccc1)C(=O)NCc1cccc(c1)-c1cccc(-c2cc3cnccc3[nH]2)c1O Show InChI InChI=1S/C28H24N4O3/c33-27-23(10-5-11-24(27)26-15-22-17-29-13-12-25(22)31-26)21-9-4-8-20(14-21)16-30-28(34)32(35)18-19-6-2-1-3-7-19/h1-15,17,31,33,35H,16,18H2,(H,30,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 72 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
| Assay Description
The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... |
Bioorg Med Chem Lett 16: 3197-200 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.049
BindingDB Entry DOI: 10.7270/Q20P0X9Q |
More data for this
Ligand-Target Pair | |
Coagulation factor VII/Tissue factor
(Homo sapiens (Human)) | BDBM50189937
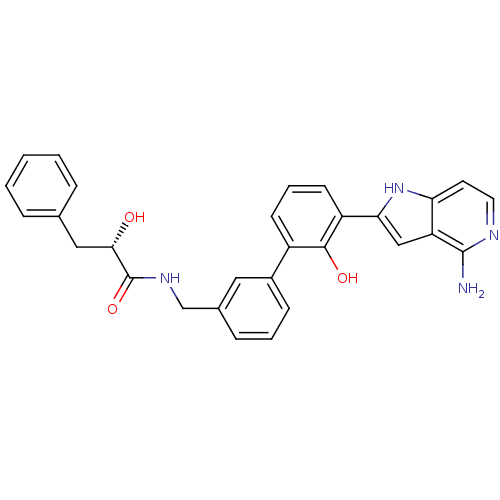
((S)-N-[3'-(4-amino-1H-pyrrolo[3,2-c]pyridin-2-yl)-...)Show SMILES Nc1nccc2[nH]c(cc12)-c1cccc(-c2cccc(CNC(=O)[C@@H](O)Cc3ccccc3)c2)c1O Show InChI InChI=1S/C29H26N4O3/c30-28-23-16-25(33-24(23)12-13-31-28)22-11-5-10-21(27(22)35)20-9-4-8-19(14-20)17-32-29(36)26(34)15-18-6-2-1-3-7-18/h1-14,16,26,33-35H,15,17H2,(H2,30,31)(H,32,36)/t26-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 81 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics
Curated by ChEMBL
| Assay Description
Binding affinity to f7a/TF complex |
Bioorg Med Chem Lett 16: 4567-70 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.016
BindingDB Entry DOI: 10.7270/Q2NG4Q67 |
More data for this
Ligand-Target Pair | |
Coagulation factor VII
(Homo sapiens (Human)) | BDBM14879

(3-{[3-(2-hydroxy-3-{1H-pyrrolo[3,2-c]pyridin-2-yl}...)Show SMILES COc1ccc(NC(=O)NCc2cccc(c2)-c2cccc(-c3cc4cnccc4[nH]3)c2O)cc1 Show InChI InChI=1S/C28H24N4O3/c1-35-22-10-8-21(9-11-22)31-28(34)30-16-18-4-2-5-19(14-18)23-6-3-7-24(27(23)33)26-15-20-17-29-13-12-25(20)32-26/h2-15,17,32-33H,16H2,1H3,(H2,30,31,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 88 | -39.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Celera
| Assay Description
The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... |
Bioorg Med Chem Lett 16: 3197-200 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.049
BindingDB Entry DOI: 10.7270/Q20P0X9Q |
More data for this
Ligand-Target Pair | |
Coagulation factor VII
(Homo sapiens (Human)) | BDBM14878

(3-{[3-(2-hydroxy-3-{1H-pyrrolo[3,2-c]pyridin-2-yl}...)Show SMILES COc1cccc(NC(=O)NCc2cccc(c2)-c2cccc(-c3cc4cnccc4[nH]3)c2O)c1 Show InChI InChI=1S/C28H24N4O3/c1-35-22-8-3-7-21(15-22)31-28(34)30-16-18-5-2-6-19(13-18)23-9-4-10-24(27(23)33)26-14-20-17-29-12-11-25(20)32-26/h2-15,17,32-33H,16H2,1H3,(H2,30,31,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 100 | -39.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Celera
| Assay Description
The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... |
Bioorg Med Chem Lett 16: 3197-200 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.049
BindingDB Entry DOI: 10.7270/Q20P0X9Q |
More data for this
Ligand-Target Pair | |
Coagulation factor VII
(Homo sapiens (Human)) | BDBM14873

(1-(2,4-difluorophenyl)-3-{[3-(2-hydroxy-3-{1H-pyrr...)Show SMILES Oc1c(cccc1-c1cccc(CNC(=O)Nc2ccc(F)cc2F)c1)-c1cc2cnccc2[nH]1 Show InChI InChI=1S/C27H20F2N4O2/c28-19-7-8-24(22(29)13-19)33-27(35)31-14-16-3-1-4-17(11-16)20-5-2-6-21(26(20)34)25-12-18-15-30-10-9-23(18)32-25/h1-13,15,32,34H,14H2,(H2,31,33,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 100 | -39.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Celera
| Assay Description
The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... |
Bioorg Med Chem Lett 16: 3197-200 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.049
BindingDB Entry DOI: 10.7270/Q20P0X9Q |
More data for this
Ligand-Target Pair | |
Coagulation factor VII
(Homo sapiens (Human)) | BDBM14871
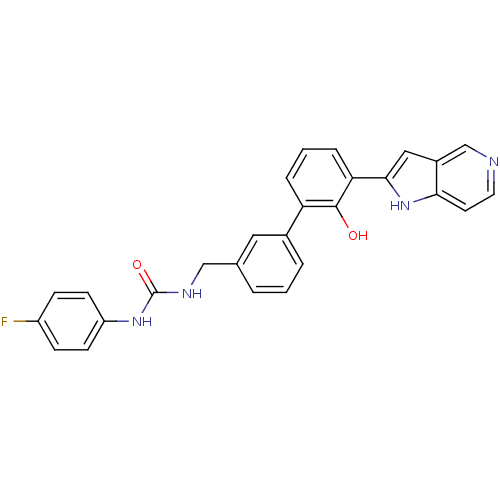
(1-(4-fluorophenyl)-3-{[3-(2-hydroxy-3-{1H-pyrrolo[...)Show SMILES Oc1c(cccc1-c1cccc(CNC(=O)Nc2ccc(F)cc2)c1)-c1cc2cnccc2[nH]1 Show InChI InChI=1S/C27H21FN4O2/c28-20-7-9-21(10-8-20)31-27(34)30-15-17-3-1-4-18(13-17)22-5-2-6-23(26(22)33)25-14-19-16-29-12-11-24(19)32-25/h1-14,16,32-33H,15H2,(H2,30,31,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 110 | -39.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Celera
| Assay Description
The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... |
Bioorg Med Chem Lett 16: 3197-200 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.049
BindingDB Entry DOI: 10.7270/Q20P0X9Q |
More data for this
Ligand-Target Pair | |
Coagulation factor VII
(Homo sapiens (Human)) | BDBM50186213
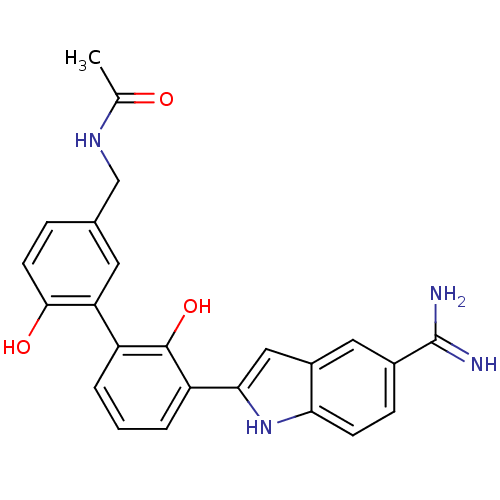
(CHEMBL211227 | N-[3'-(5-carbamimidoyl-1H-indol-2-y...)Show SMILES CC(=O)NCc1ccc(O)c(c1)-c1cccc(-c2cc3cc(ccc3[nH]2)C(N)=N)c1O Show InChI InChI=1S/C24H22N4O3/c1-13(29)27-12-14-5-8-22(30)19(9-14)17-3-2-4-18(23(17)31)21-11-16-10-15(24(25)26)6-7-20(16)28-21/h2-11,28,30-31H,12H2,1H3,(H3,25,26)(H,27,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Binding affinity to f7a |
Bioorg Med Chem Lett 16: 3829-32 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.018
BindingDB Entry DOI: 10.7270/Q2DN44NK |
More data for this
Ligand-Target Pair | |
Coagulation factor VII
(Homo sapiens (Human)) | BDBM14885

(1-{[3-(2-hydroxy-3-{1H-pyrrolo[3,2-c]pyridin-2-yl}...)Show SMILES Oc1c(cccc1-c1cccc(CNC(=O)Nc2ccsc2)c1)-c1cc2cnccc2[nH]1 Show InChI InChI=1S/C25H20N4O2S/c30-24-20(5-2-6-21(24)23-12-18-14-26-9-7-22(18)29-23)17-4-1-3-16(11-17)13-27-25(31)28-19-8-10-32-15-19/h1-12,14-15,29-30H,13H2,(H2,27,28,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
| Assay Description
The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... |
Bioorg Med Chem Lett 16: 3197-200 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.049
BindingDB Entry DOI: 10.7270/Q20P0X9Q |
More data for this
Ligand-Target Pair | |
Coagulation factor VII
(Homo sapiens (Human)) | BDBM14870
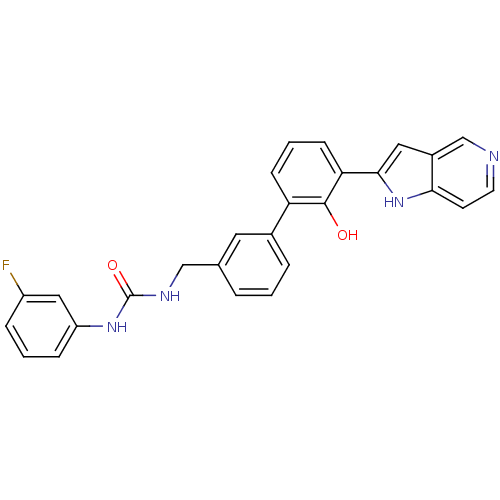
(1-(3-fluorophenyl)-3-{[3-(2-hydroxy-3-{1H-pyrrolo[...)Show SMILES Oc1c(cccc1-c1cccc(CNC(=O)Nc2cccc(F)c2)c1)-c1cc2cnccc2[nH]1 Show InChI InChI=1S/C27H21FN4O2/c28-20-6-2-7-21(14-20)31-27(34)30-15-17-4-1-5-18(12-17)22-8-3-9-23(26(22)33)25-13-19-16-29-11-10-24(19)32-25/h1-14,16,32-33H,15H2,(H2,30,31,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 130 | -38.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Celera
| Assay Description
The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... |
Bioorg Med Chem Lett 16: 3197-200 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.049
BindingDB Entry DOI: 10.7270/Q20P0X9Q |
More data for this
Ligand-Target Pair | |
Coagulation factor VII
(Homo sapiens (Human)) | BDBM14887
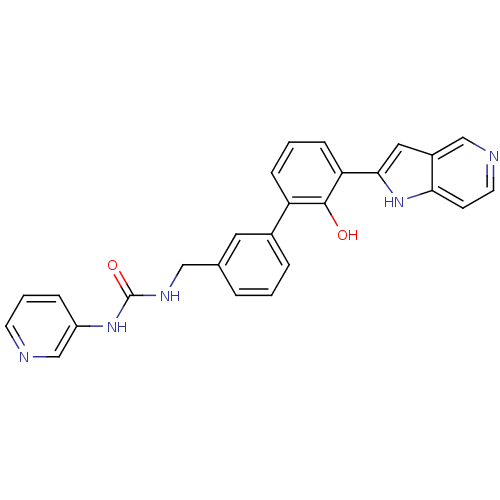
(3-{[3-(2-hydroxy-3-{1H-pyrrolo[3,2-c]pyridin-2-yl}...)Show SMILES Oc1c(cccc1-c1cccc(CNC(=O)Nc2cccnc2)c1)-c1cc2cnccc2[nH]1 Show InChI InChI=1S/C26H21N5O2/c32-25-21(7-2-8-22(25)24-13-19-15-28-11-9-23(19)31-24)18-5-1-4-17(12-18)14-29-26(33)30-20-6-3-10-27-16-20/h1-13,15-16,31-32H,14H2,(H2,29,30,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 155 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
| Assay Description
The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... |
Bioorg Med Chem Lett 16: 3197-200 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.049
BindingDB Entry DOI: 10.7270/Q20P0X9Q |
More data for this
Ligand-Target Pair | |
Coagulation factor VII
(Homo sapiens (Human)) | BDBM14884
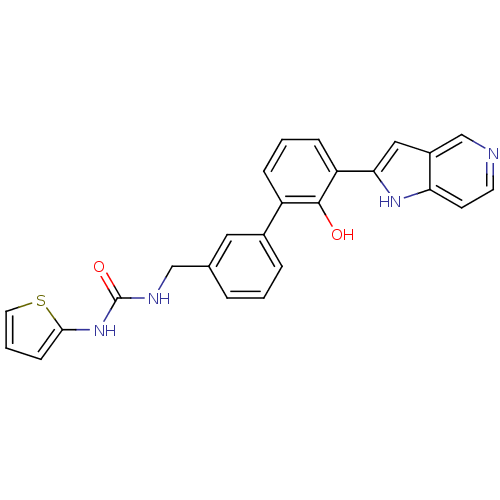
(1-{[3-(2-hydroxy-3-{1H-pyrrolo[3,2-c]pyridin-2-yl}...)Show SMILES Oc1c(cccc1-c1cccc(CNC(=O)Nc2cccs2)c1)-c1cc2cnccc2[nH]1 Show InChI InChI=1S/C25H20N4O2S/c30-24-19(6-2-7-20(24)22-13-18-15-26-10-9-21(18)28-22)17-5-1-4-16(12-17)14-27-25(31)29-23-8-3-11-32-23/h1-13,15,28,30H,14H2,(H2,27,29,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
| Assay Description
The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... |
Bioorg Med Chem Lett 16: 3197-200 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.049
BindingDB Entry DOI: 10.7270/Q20P0X9Q |
More data for this
Ligand-Target Pair | |
Coagulation factor VII
(Homo sapiens (Human)) | BDBM14882
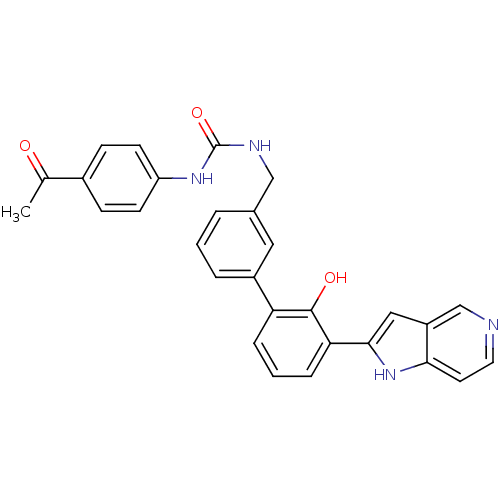
(1-(4-acetylphenyl)-3-{[3-(2-hydroxy-3-{1H-pyrrolo[...)Show SMILES CC(=O)c1ccc(NC(=O)NCc2cccc(c2)-c2cccc(-c3cc4cnccc4[nH]3)c2O)cc1 Show InChI InChI=1S/C29H24N4O3/c1-18(34)20-8-10-23(11-9-20)32-29(36)31-16-19-4-2-5-21(14-19)24-6-3-7-25(28(24)35)27-15-22-17-30-13-12-26(22)33-27/h2-15,17,33,35H,16H2,1H3,(H2,31,32,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
| Assay Description
The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... |
Bioorg Med Chem Lett 16: 3197-200 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.049
BindingDB Entry DOI: 10.7270/Q20P0X9Q |
More data for this
Ligand-Target Pair | |
Coagulation factor VII
(Homo sapiens (Human)) | BDBM14892
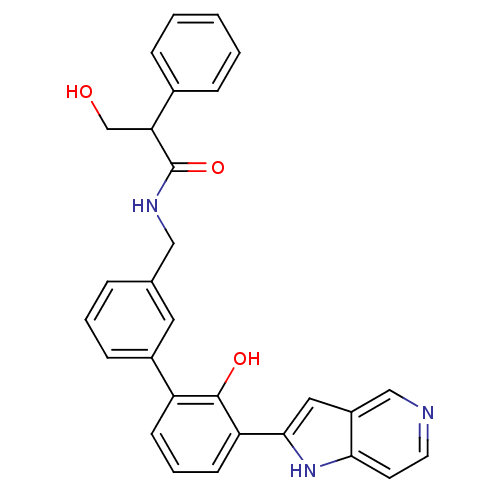
(3-hydroxy-N-{[3-(2-hydroxy-3-{1H-pyrrolo[3,2-c]pyr...)Show SMILES OCC(C(=O)NCc1cccc(c1)-c1cccc(-c2cc3cnccc3[nH]2)c1O)c1ccccc1 Show InChI InChI=1S/C29H25N3O3/c33-18-25(20-7-2-1-3-8-20)29(35)31-16-19-6-4-9-21(14-19)23-10-5-11-24(28(23)34)27-15-22-17-30-13-12-26(22)32-27/h1-15,17,25,32-34H,16,18H2,(H,31,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
| Assay Description
The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... |
Bioorg Med Chem Lett 16: 3197-200 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.049
BindingDB Entry DOI: 10.7270/Q20P0X9Q |
More data for this
Ligand-Target Pair | |
Coagulation factor VII/Tissue factor
(Homo sapiens (Human)) | BDBM50189940
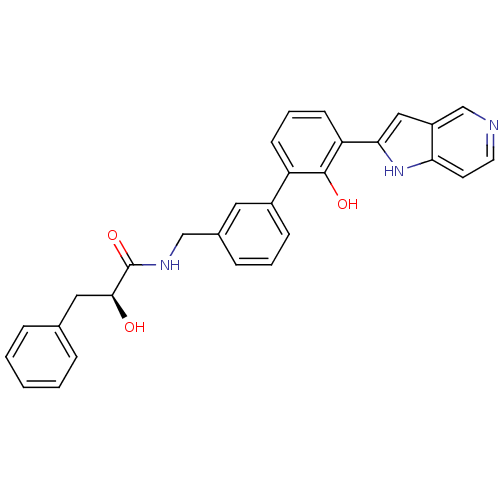
((S)-2-hydroxy-N-[2'-hydroxy-3'-(1H-pyrrolo[3,2-c]p...)Show SMILES O[C@@H](Cc1ccccc1)C(=O)NCc1cccc(c1)-c1cccc(-c2cc3cnccc3[nH]2)c1O Show InChI InChI=1S/C29H25N3O3/c33-27(15-19-6-2-1-3-7-19)29(35)31-17-20-8-4-9-21(14-20)23-10-5-11-24(28(23)34)26-16-22-18-30-13-12-25(22)32-26/h1-14,16,18,27,32-34H,15,17H2,(H,31,35)/t27-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics
Curated by ChEMBL
| Assay Description
Binding affinity to f7a/TF complex |
Bioorg Med Chem Lett 16: 4567-70 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.016
BindingDB Entry DOI: 10.7270/Q2NG4Q67 |
More data for this
Ligand-Target Pair | |
Coagulation factor VII
(Homo sapiens (Human)) | BDBM14866
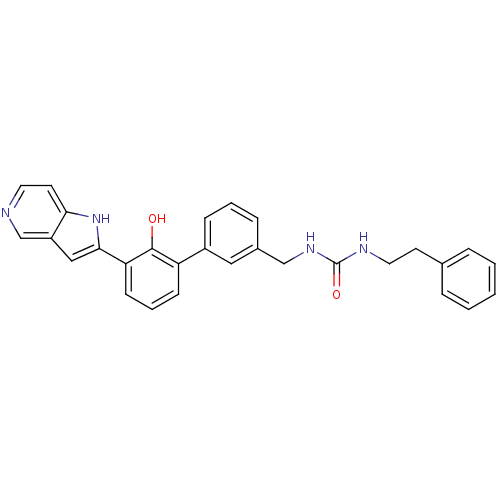
(3-{[3-(2-hydroxy-3-{1H-pyrrolo[3,2-c]pyridin-2-yl}...)Show SMILES Oc1c(cccc1-c1cccc(CNC(=O)NCCc2ccccc2)c1)-c1cc2cnccc2[nH]1 Show InChI InChI=1S/C29H26N4O2/c34-28-24(10-5-11-25(28)27-17-23-19-30-14-13-26(23)33-27)22-9-4-8-21(16-22)18-32-29(35)31-15-12-20-6-2-1-3-7-20/h1-11,13-14,16-17,19,33-34H,12,15,18H2,(H2,31,32,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 270 | -37.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Celera
| Assay Description
The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... |
Bioorg Med Chem Lett 16: 3197-200 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.049
BindingDB Entry DOI: 10.7270/Q20P0X9Q |
More data for this
Ligand-Target Pair | |
Coagulation factor VII
(Homo sapiens (Human)) | BDBM14874

(1-(3,4-difluorophenyl)-3-{[3-(2-hydroxy-3-{1H-pyrr...)Show SMILES Oc1c(cccc1-c1cccc(CNC(=O)Nc2ccc(F)c(F)c2)c1)-c1cc2cnccc2[nH]1 Show InChI InChI=1S/C27H20F2N4O2/c28-22-8-7-19(13-23(22)29)32-27(35)31-14-16-3-1-4-17(11-16)20-5-2-6-21(26(20)34)25-12-18-15-30-10-9-24(18)33-25/h1-13,15,33-34H,14H2,(H2,31,32,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 330 | -36.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Celera
| Assay Description
The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... |
Bioorg Med Chem Lett 16: 3197-200 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.049
BindingDB Entry DOI: 10.7270/Q20P0X9Q |
More data for this
Ligand-Target Pair | |
Coagulation factor VII
(Homo sapiens (Human)) | BDBM14865

(1-{[3-(2-hydroxy-3-{1H-pyrrolo[3,2-c]pyridin-2-yl}...)Show SMILES CCCCCNC(=O)NCc1cccc(c1)-c1cccc(-c2cc3cnccc3[nH]2)c1O Show InChI InChI=1S/C26H28N4O2/c1-2-3-4-12-28-26(32)29-16-18-7-5-8-19(14-18)21-9-6-10-22(25(21)31)24-15-20-17-27-13-11-23(20)30-24/h5-11,13-15,17,30-31H,2-4,12,16H2,1H3,(H2,28,29,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 335 | -36.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Celera
| Assay Description
The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... |
Bioorg Med Chem Lett 16: 3197-200 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.049
BindingDB Entry DOI: 10.7270/Q20P0X9Q |
More data for this
Ligand-Target Pair | |
Coagulation factor VII
(Homo sapiens (Human)) | BDBM14867

(1-{[3-(2-hydroxy-3-{1H-pyrrolo[3,2-c]pyridin-2-yl}...)Show SMILES Oc1c(cccc1-c1cccc(CNC(=O)NCCc2cccs2)c1)-c1cc2cnccc2[nH]1 Show InChI InChI=1S/C27H24N4O2S/c32-26-22(7-2-8-23(26)25-15-20-17-28-11-10-24(20)31-25)19-5-1-4-18(14-19)16-30-27(33)29-12-9-21-6-3-13-34-21/h1-8,10-11,13-15,17,31-32H,9,12,16H2,(H2,29,30,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 350 | -36.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Celera
| Assay Description
The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... |
Bioorg Med Chem Lett 16: 3197-200 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.049
BindingDB Entry DOI: 10.7270/Q20P0X9Q |
More data for this
Ligand-Target Pair | |
Coagulation factor VII
(Homo sapiens (Human)) | BDBM50182057
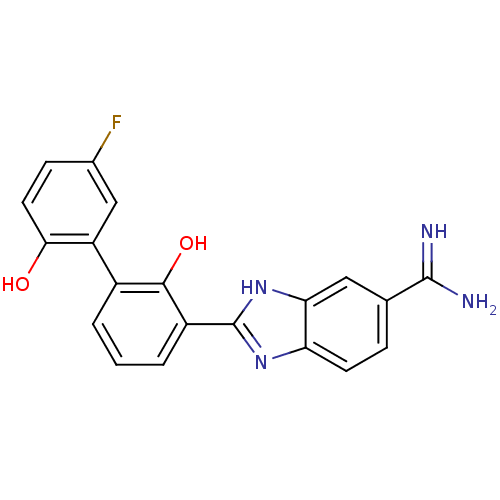
(2-(5'-fluoro-2,2'-dihydroxy-biphenyl-3-yl)-1H-benz...)Show SMILES NC(=N)c1ccc2nc([nH]c2c1)-c1cccc(c1O)-c1cc(F)ccc1O Show InChI InChI=1S/C20H15FN4O2/c21-11-5-7-17(26)14(9-11)12-2-1-3-13(18(12)27)20-24-15-6-4-10(19(22)23)8-16(15)25-20/h1-9,26-27H,(H3,22,23)(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics
Curated by ChEMBL
| Assay Description
Inhibition of factor7a in Sprague-Dawley rats |
Bioorg Med Chem Lett 16: 2224-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.039
BindingDB Entry DOI: 10.7270/Q2TM79Q2 |
More data for this
Ligand-Target Pair | |
Coagulation factor VII
(Homo sapiens (Human)) | BDBM14893
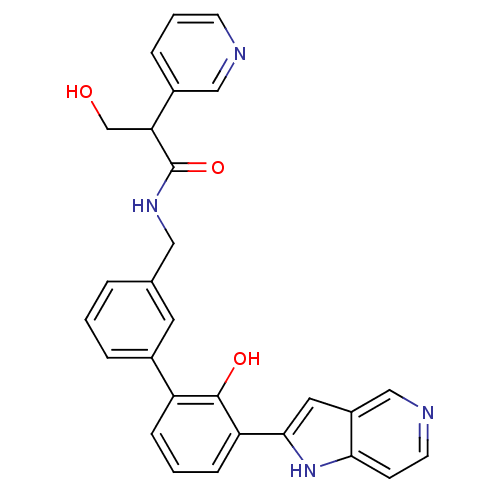
(3-hydroxy-N-{[3-(2-hydroxy-3-{1H-pyrrolo[3,2-c]pyr...)Show SMILES OCC(C(=O)NCc1cccc(c1)-c1cccc(-c2cc3cnccc3[nH]2)c1O)c1cccnc1 Show InChI InChI=1S/C28H24N4O3/c33-17-24(20-6-3-10-29-15-20)28(35)31-14-18-4-1-5-19(12-18)22-7-2-8-23(27(22)34)26-13-21-16-30-11-9-25(21)32-26/h1-13,15-16,24,32-34H,14,17H2,(H,31,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
| Assay Description
The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... |
Bioorg Med Chem Lett 16: 3197-200 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.049
BindingDB Entry DOI: 10.7270/Q20P0X9Q |
More data for this
Ligand-Target Pair | |
Coagulation factor VII
(Homo sapiens (Human)) | BDBM14864
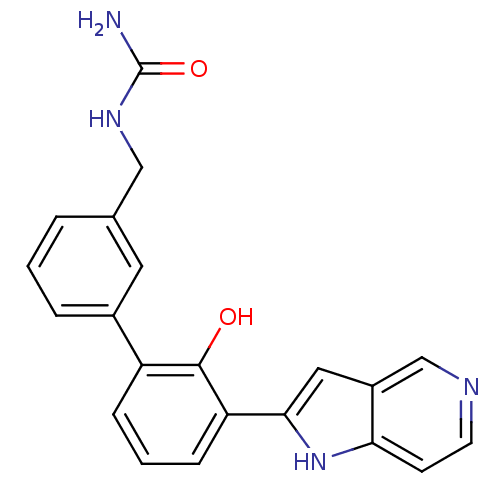
(5-azaindole analog 2 | {[3-(2-hydroxy-3-{1H-pyrrol...)Show SMILES NC(=O)NCc1cccc(c1)-c1cccc(-c2cc3cnccc3[nH]2)c1O Show InChI InChI=1S/C21H18N4O2/c22-21(27)24-11-13-3-1-4-14(9-13)16-5-2-6-17(20(16)26)19-10-15-12-23-8-7-18(15)25-19/h1-10,12,25-26H,11H2,(H3,22,24,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 800 | -34.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Celera
| Assay Description
The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... |
Bioorg Med Chem Lett 16: 3197-200 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.049
BindingDB Entry DOI: 10.7270/Q20P0X9Q |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor VII
(Homo sapiens (Human)) | BDBM14875
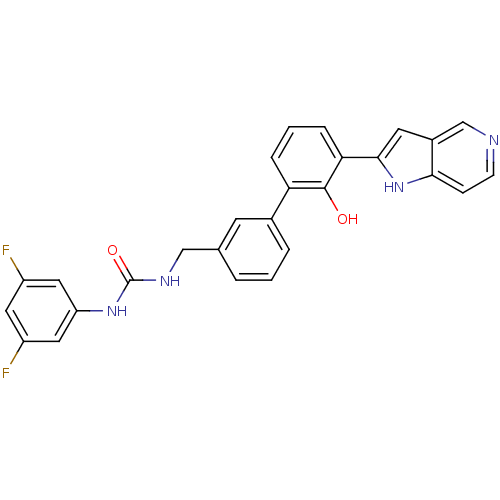
(1-(3,5-difluorophenyl)-3-{[3-(2-hydroxy-3-{1H-pyrr...)Show SMILES Oc1c(cccc1-c1cccc(CNC(=O)Nc2cc(F)cc(F)c2)c1)-c1cc2cnccc2[nH]1 Show InChI InChI=1S/C27H20F2N4O2/c28-19-11-20(29)13-21(12-19)32-27(35)31-14-16-3-1-4-17(9-16)22-5-2-6-23(26(22)34)25-10-18-15-30-8-7-24(18)33-25/h1-13,15,33-34H,14H2,(H2,31,32,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 830 | -34.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Celera
| Assay Description
The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... |
Bioorg Med Chem Lett 16: 3197-200 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.049
BindingDB Entry DOI: 10.7270/Q20P0X9Q |
More data for this
Ligand-Target Pair | |
Coagulation factor VII
(Homo sapiens (Human)) | BDBM14894

(2-hydroxy-N-{[3-(2-hydroxy-3-{1H-pyrrolo[3,2-c]pyr...)Show SMILES OC(C(=O)NCc1cccc(c1)-c1cccc(-c2cc3cnccc3[nH]2)c1O)c1ccccc1 Show InChI InChI=1S/C28H23N3O3/c32-26(19-7-2-1-3-8-19)28(34)30-16-18-6-4-9-20(14-18)22-10-5-11-23(27(22)33)25-15-21-17-29-13-12-24(21)31-25/h1-15,17,26,31-33H,16H2,(H,30,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
| Assay Description
The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... |
Bioorg Med Chem Lett 16: 3197-200 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.049
BindingDB Entry DOI: 10.7270/Q20P0X9Q |
More data for this
Ligand-Target Pair | |
Coagulation factor VII
(Homo sapiens (Human)) | BDBM14890
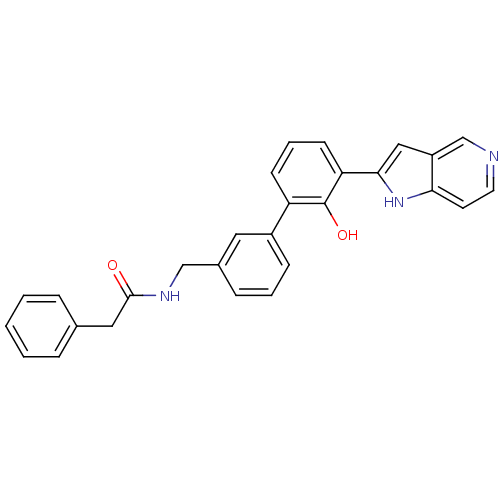
(5-azaindole analog 28 | N-{[3-(2-hydroxy-3-{1H-pyr...)Show SMILES Oc1c(cccc1-c1cccc(CNC(=O)Cc2ccccc2)c1)-c1cc2cnccc2[nH]1 Show InChI InChI=1S/C28H23N3O2/c32-27(15-19-6-2-1-3-7-19)30-17-20-8-4-9-21(14-20)23-10-5-11-24(28(23)33)26-16-22-18-29-13-12-25(22)31-26/h1-14,16,18,31,33H,15,17H2,(H,30,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
| Assay Description
The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... |
Bioorg Med Chem Lett 16: 3197-200 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.049
BindingDB Entry DOI: 10.7270/Q20P0X9Q |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM14863

(({3-[3-(5-carbamimidoyl-1H-1,3-benzodiazol-2-yl)-2...)Show SMILES NC(=O)NCc1ccc(O)c(c1)-c1cccc(-c2nc3ccc(cc3[nH]2)C(N)=N)c1O Show InChI InChI=1S/C22H20N6O3/c23-20(24)12-5-6-16-17(9-12)28-21(27-16)14-3-1-2-13(19(14)30)15-8-11(4-7-18(15)29)10-26-22(25)31/h1-9,29-30H,10H2,(H3,23,24)(H,27,28)(H3,25,26,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.70E+3 | -31.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Celera
| Assay Description
The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... |
Bioorg Med Chem Lett 16: 3197-200 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.049
BindingDB Entry DOI: 10.7270/Q20P0X9Q |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM14863

(({3-[3-(5-carbamimidoyl-1H-1,3-benzodiazol-2-yl)-2...)Show SMILES NC(=O)NCc1ccc(O)c(c1)-c1cccc(-c2nc3ccc(cc3[nH]2)C(N)=N)c1O Show InChI InChI=1S/C22H20N6O3/c23-20(24)12-5-6-16-17(9-12)28-21(27-16)14-3-1-2-13(19(14)30)15-8-11(4-7-18(15)29)10-26-22(25)31/h1-9,29-30H,10H2,(H3,23,24)(H,27,28)(H3,25,26,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics
Curated by ChEMBL
| Assay Description
Binding affinity to f10a |
Bioorg Med Chem Lett 16: 4567-70 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.016
BindingDB Entry DOI: 10.7270/Q2NG4Q67 |
More data for this
Ligand-Target Pair | |
Coagulation factor VII
(Homo sapiens (Human)) | BDBM14886

(3-{[3-(2-hydroxy-3-{1H-pyrrolo[3,2-c]pyridin-2-yl}...)Show SMILES Oc1c(cccc1-c1cccc(CNC(=O)Nc2ccccn2)c1)-c1cc2cnccc2[nH]1 Show InChI InChI=1S/C26H21N5O2/c32-25-20(7-4-8-21(25)23-14-19-16-27-12-10-22(19)30-23)18-6-3-5-17(13-18)15-29-26(33)31-24-9-1-2-11-28-24/h1-14,16,30,32H,15H2,(H2,28,29,31,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
| Assay Description
The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... |
Bioorg Med Chem Lett 16: 3197-200 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.049
BindingDB Entry DOI: 10.7270/Q20P0X9Q |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data