 Found 1315 hits with Last Name = 'roberts' and Initial = 'm'
Found 1315 hits with Last Name = 'roberts' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Bromodomain-containing protein 3 [306-416]
(Homo sapiens (Human)) | BDBM205431

((6S+2S)-PEG1 (7))Show SMILES COC(=O)C[C@@H]1N=C(c2c(C)c(sc2-n2c(C)nnc12)C(=O)NCCOCCNC(=O)C[C@@H]1N=C(c2c(C)c(C)sc2-n2c(C)nnc12)c1ccc(Cl)cc1)c1ccc(Cl)cc1 |r,c:6,36| Show InChI InChI=1S/C43H42Cl2N10O5S2/c1-21-23(3)61-42-34(21)36(26-7-11-28(44)12-8-26)48-30(39-52-50-24(4)54(39)42)19-32(56)46-15-17-60-18-16-47-41(58)38-22(2)35-37(27-9-13-29(45)14-10-27)49-31(20-33(57)59-6)40-53-51-25(5)55(40)43(35)62-38/h7-14,30-31H,15-20H2,1-6H3,(H,46,56)(H,47,58)/t30-,31-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.00270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute
| Assay Description
Refer to DiscoverX |
Nat Chem Biol 12: 1089-1096 (2016)
Article DOI: 10.1038/nchembio.2209
BindingDB Entry DOI: 10.7270/Q2VQ31HW |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 4 [333-460]
(Homo sapiens (Human)) | BDBM205431

((6S+2S)-PEG1 (7))Show SMILES COC(=O)C[C@@H]1N=C(c2c(C)c(sc2-n2c(C)nnc12)C(=O)NCCOCCNC(=O)C[C@@H]1N=C(c2c(C)c(C)sc2-n2c(C)nnc12)c1ccc(Cl)cc1)c1ccc(Cl)cc1 |r,c:6,36| Show InChI InChI=1S/C43H42Cl2N10O5S2/c1-21-23(3)61-42-34(21)36(26-7-11-28(44)12-8-26)48-30(39-52-50-24(4)54(39)42)19-32(56)46-15-17-60-18-16-47-41(58)38-22(2)35-37(27-9-13-29(45)14-10-27)49-31(20-33(57)59-6)40-53-51-25(5)55(40)43(35)62-38/h7-14,30-31H,15-20H2,1-6H3,(H,46,56)(H,47,58)/t30-,31-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.00430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute
| Assay Description
Refer to DiscoverX |
Nat Chem Biol 12: 1089-1096 (2016)
Article DOI: 10.1038/nchembio.2209
BindingDB Entry DOI: 10.7270/Q2VQ31HW |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 2 [348-455]
(Homo sapiens (Human)) | BDBM205431

((6S+2S)-PEG1 (7))Show SMILES COC(=O)C[C@@H]1N=C(c2c(C)c(sc2-n2c(C)nnc12)C(=O)NCCOCCNC(=O)C[C@@H]1N=C(c2c(C)c(C)sc2-n2c(C)nnc12)c1ccc(Cl)cc1)c1ccc(Cl)cc1 |r,c:6,36| Show InChI InChI=1S/C43H42Cl2N10O5S2/c1-21-23(3)61-42-34(21)36(26-7-11-28(44)12-8-26)48-30(39-52-50-24(4)54(39)42)19-32(56)46-15-17-60-18-16-47-41(58)38-22(2)35-37(27-9-13-29(45)14-10-27)49-31(20-33(57)59-6)40-53-51-25(5)55(40)43(35)62-38/h7-14,30-31H,15-20H2,1-6H3,(H,46,56)(H,47,58)/t30-,31-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.00690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute
| Assay Description
Refer to DiscoverX |
Nat Chem Biol 12: 1089-1096 (2016)
Article DOI: 10.1038/nchembio.2209
BindingDB Entry DOI: 10.7270/Q2VQ31HW |
More data for this
Ligand-Target Pair | |
Isoform C of Bromodomain-containing protein 4 (Short)
(Homo sapiens (Human)) | BDBM205431

((6S+2S)-PEG1 (7))Show SMILES COC(=O)C[C@@H]1N=C(c2c(C)c(sc2-n2c(C)nnc12)C(=O)NCCOCCNC(=O)C[C@@H]1N=C(c2c(C)c(C)sc2-n2c(C)nnc12)c1ccc(Cl)cc1)c1ccc(Cl)cc1 |r,c:6,36| Show InChI InChI=1S/C43H42Cl2N10O5S2/c1-21-23(3)61-42-34(21)36(26-7-11-28(44)12-8-26)48-30(39-52-50-24(4)54(39)42)19-32(56)46-15-17-60-18-16-47-41(58)38-22(2)35-37(27-9-13-29(45)14-10-27)49-31(20-33(57)59-6)40-53-51-25(5)55(40)43(35)62-38/h7-14,30-31H,15-20H2,1-6H3,(H,46,56)(H,47,58)/t30-,31-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.00780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute
| Assay Description
Refer to DiscoverX |
Nat Chem Biol 12: 1089-1096 (2016)
Article DOI: 10.1038/nchembio.2209
BindingDB Entry DOI: 10.7270/Q2VQ31HW |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 2 [71-194,348-455]
(Homo sapiens (Human)) | BDBM205431

((6S+2S)-PEG1 (7))Show SMILES COC(=O)C[C@@H]1N=C(c2c(C)c(sc2-n2c(C)nnc12)C(=O)NCCOCCNC(=O)C[C@@H]1N=C(c2c(C)c(C)sc2-n2c(C)nnc12)c1ccc(Cl)cc1)c1ccc(Cl)cc1 |r,c:6,36| Show InChI InChI=1S/C43H42Cl2N10O5S2/c1-21-23(3)61-42-34(21)36(26-7-11-28(44)12-8-26)48-30(39-52-50-24(4)54(39)42)19-32(56)46-15-17-60-18-16-47-41(58)38-22(2)35-37(27-9-13-29(45)14-10-27)49-31(20-33(57)59-6)40-53-51-25(5)55(40)43(35)62-38/h7-14,30-31H,15-20H2,1-6H3,(H,46,56)(H,47,58)/t30-,31-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute
| Assay Description
Refer to DiscoverX |
Nat Chem Biol 12: 1089-1096 (2016)
Article DOI: 10.1038/nchembio.2209
BindingDB Entry DOI: 10.7270/Q2VQ31HW |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 3 [24-144,306-416]
(Homo sapiens (Human)) | BDBM205453

(MTI (35))Show SMILES Cc1sc-2c(c1C)C(=NC(CC(=O)NCCOCCOCCOCCOCCOCCOCCOCCNC(=O)C[C@@H]1N=C(c3c(C)c(C)sc3-n3c(C)nnc13)c1ccc(Cl)cc1)c1nnc(C)n-21)c1ccc(Cl)cc1 |r,c:8,43| Show InChI InChI=1S/C54H66Cl2N10O9S2/c1-33-35(3)76-53-47(33)49(39-7-11-41(55)12-8-39)59-43(51-63-61-37(5)65(51)53)31-45(67)57-15-17-69-19-21-71-23-25-73-27-29-75-30-28-74-26-24-72-22-20-70-18-16-58-46(68)32-44-52-64-62-38(6)66(52)54-48(34(2)36(4)77-54)50(60-44)40-9-13-42(56)14-10-40/h7-14,43-44H,15-32H2,1-6H3,(H,57,67)(H,58,68)/t43-,44?/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute
| Assay Description
Refer to DiscoverX |
Nat Chem Biol 12: 1089-1096 (2016)
Article DOI: 10.1038/nchembio.2209
BindingDB Entry DOI: 10.7270/Q2VQ31HW |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 4 [44-168,333-460]
(Homo sapiens (Human)) | BDBM205431

((6S+2S)-PEG1 (7))Show SMILES COC(=O)C[C@@H]1N=C(c2c(C)c(sc2-n2c(C)nnc12)C(=O)NCCOCCNC(=O)C[C@@H]1N=C(c2c(C)c(C)sc2-n2c(C)nnc12)c1ccc(Cl)cc1)c1ccc(Cl)cc1 |r,c:6,36| Show InChI InChI=1S/C43H42Cl2N10O5S2/c1-21-23(3)61-42-34(21)36(26-7-11-28(44)12-8-26)48-30(39-52-50-24(4)54(39)42)19-32(56)46-15-17-60-18-16-47-41(58)38-22(2)35-37(27-9-13-29(45)14-10-27)49-31(20-33(57)59-6)40-53-51-25(5)55(40)43(35)62-38/h7-14,30-31H,15-20H2,1-6H3,(H,46,56)(H,47,58)/t30-,31-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute
| Assay Description
Refer to DiscoverX |
Nat Chem Biol 12: 1089-1096 (2016)
Article DOI: 10.1038/nchembio.2209
BindingDB Entry DOI: 10.7270/Q2VQ31HW |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 2 [71-194]
(Homo sapiens (Human)) | BDBM205431

((6S+2S)-PEG1 (7))Show SMILES COC(=O)C[C@@H]1N=C(c2c(C)c(sc2-n2c(C)nnc12)C(=O)NCCOCCNC(=O)C[C@@H]1N=C(c2c(C)c(C)sc2-n2c(C)nnc12)c1ccc(Cl)cc1)c1ccc(Cl)cc1 |r,c:6,36| Show InChI InChI=1S/C43H42Cl2N10O5S2/c1-21-23(3)61-42-34(21)36(26-7-11-28(44)12-8-26)48-30(39-52-50-24(4)54(39)42)19-32(56)46-15-17-60-18-16-47-41(58)38-22(2)35-37(27-9-13-29(45)14-10-27)49-31(20-33(57)59-6)40-53-51-25(5)55(40)43(35)62-38/h7-14,30-31H,15-20H2,1-6H3,(H,46,56)(H,47,58)/t30-,31-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute
| Assay Description
Refer to DiscoverX |
Nat Chem Biol 12: 1089-1096 (2016)
Article DOI: 10.1038/nchembio.2209
BindingDB Entry DOI: 10.7270/Q2VQ31HW |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 2 [71-194,348-455]
(Homo sapiens (Human)) | BDBM205453

(MTI (35))Show SMILES Cc1sc-2c(c1C)C(=NC(CC(=O)NCCOCCOCCOCCOCCOCCOCCOCCNC(=O)C[C@@H]1N=C(c3c(C)c(C)sc3-n3c(C)nnc13)c1ccc(Cl)cc1)c1nnc(C)n-21)c1ccc(Cl)cc1 |r,c:8,43| Show InChI InChI=1S/C54H66Cl2N10O9S2/c1-33-35(3)76-53-47(33)49(39-7-11-41(55)12-8-39)59-43(51-63-61-37(5)65(51)53)31-45(67)57-15-17-69-19-21-71-23-25-73-27-29-75-30-28-74-26-24-72-22-20-70-18-16-58-46(68)32-44-52-64-62-38(6)66(52)54-48(34(2)36(4)77-54)50(60-44)40-9-13-42(56)14-10-40/h7-14,43-44H,15-32H2,1-6H3,(H,57,67)(H,58,68)/t43-,44?/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute
| Assay Description
Refer to DiscoverX |
Nat Chem Biol 12: 1089-1096 (2016)
Article DOI: 10.1038/nchembio.2209
BindingDB Entry DOI: 10.7270/Q2VQ31HW |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 2 [348-455]
(Homo sapiens (Human)) | BDBM205453

(MTI (35))Show SMILES Cc1sc-2c(c1C)C(=NC(CC(=O)NCCOCCOCCOCCOCCOCCOCCOCCNC(=O)C[C@@H]1N=C(c3c(C)c(C)sc3-n3c(C)nnc13)c1ccc(Cl)cc1)c1nnc(C)n-21)c1ccc(Cl)cc1 |r,c:8,43| Show InChI InChI=1S/C54H66Cl2N10O9S2/c1-33-35(3)76-53-47(33)49(39-7-11-41(55)12-8-39)59-43(51-63-61-37(5)65(51)53)31-45(67)57-15-17-69-19-21-71-23-25-73-27-29-75-30-28-74-26-24-72-22-20-70-18-16-58-46(68)32-44-52-64-62-38(6)66(52)54-48(34(2)36(4)77-54)50(60-44)40-9-13-42(56)14-10-40/h7-14,43-44H,15-32H2,1-6H3,(H,57,67)(H,58,68)/t43-,44?/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute
| Assay Description
Refer to DiscoverX |
Nat Chem Biol 12: 1089-1096 (2016)
Article DOI: 10.1038/nchembio.2209
BindingDB Entry DOI: 10.7270/Q2VQ31HW |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 4 [333-460]
(Homo sapiens (Human)) | BDBM205453

(MTI (35))Show SMILES Cc1sc-2c(c1C)C(=NC(CC(=O)NCCOCCOCCOCCOCCOCCOCCOCCNC(=O)C[C@@H]1N=C(c3c(C)c(C)sc3-n3c(C)nnc13)c1ccc(Cl)cc1)c1nnc(C)n-21)c1ccc(Cl)cc1 |r,c:8,43| Show InChI InChI=1S/C54H66Cl2N10O9S2/c1-33-35(3)76-53-47(33)49(39-7-11-41(55)12-8-39)59-43(51-63-61-37(5)65(51)53)31-45(67)57-15-17-69-19-21-71-23-25-73-27-29-75-30-28-74-26-24-72-22-20-70-18-16-58-46(68)32-44-52-64-62-38(6)66(52)54-48(34(2)36(4)77-54)50(60-44)40-9-13-42(56)14-10-40/h7-14,43-44H,15-32H2,1-6H3,(H,57,67)(H,58,68)/t43-,44?/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute
| Assay Description
Refer to DiscoverX |
Nat Chem Biol 12: 1089-1096 (2016)
Article DOI: 10.1038/nchembio.2209
BindingDB Entry DOI: 10.7270/Q2VQ31HW |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 3 [24-144]
(Homo sapiens (Human)) | BDBM205453

(MTI (35))Show SMILES Cc1sc-2c(c1C)C(=NC(CC(=O)NCCOCCOCCOCCOCCOCCOCCOCCNC(=O)C[C@@H]1N=C(c3c(C)c(C)sc3-n3c(C)nnc13)c1ccc(Cl)cc1)c1nnc(C)n-21)c1ccc(Cl)cc1 |r,c:8,43| Show InChI InChI=1S/C54H66Cl2N10O9S2/c1-33-35(3)76-53-47(33)49(39-7-11-41(55)12-8-39)59-43(51-63-61-37(5)65(51)53)31-45(67)57-15-17-69-19-21-71-23-25-73-27-29-75-30-28-74-26-24-72-22-20-70-18-16-58-46(68)32-44-52-64-62-38(6)66(52)54-48(34(2)36(4)77-54)50(60-44)40-9-13-42(56)14-10-40/h7-14,43-44H,15-32H2,1-6H3,(H,57,67)(H,58,68)/t43-,44?/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute
| Assay Description
Refer to DiscoverX |
Nat Chem Biol 12: 1089-1096 (2016)
Article DOI: 10.1038/nchembio.2209
BindingDB Entry DOI: 10.7270/Q2VQ31HW |
More data for this
Ligand-Target Pair | |
Isoform C of Bromodomain-containing protein 4 (Short)
(Homo sapiens (Human)) | BDBM205453

(MTI (35))Show SMILES Cc1sc-2c(c1C)C(=NC(CC(=O)NCCOCCOCCOCCOCCOCCOCCOCCNC(=O)C[C@@H]1N=C(c3c(C)c(C)sc3-n3c(C)nnc13)c1ccc(Cl)cc1)c1nnc(C)n-21)c1ccc(Cl)cc1 |r,c:8,43| Show InChI InChI=1S/C54H66Cl2N10O9S2/c1-33-35(3)76-53-47(33)49(39-7-11-41(55)12-8-39)59-43(51-63-61-37(5)65(51)53)31-45(67)57-15-17-69-19-21-71-23-25-73-27-29-75-30-28-74-26-24-72-22-20-70-18-16-58-46(68)32-44-52-64-62-38(6)66(52)54-48(34(2)36(4)77-54)50(60-44)40-9-13-42(56)14-10-40/h7-14,43-44H,15-32H2,1-6H3,(H,57,67)(H,58,68)/t43-,44?/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute
| Assay Description
Refer to DiscoverX |
Nat Chem Biol 12: 1089-1096 (2016)
Article DOI: 10.1038/nchembio.2209
BindingDB Entry DOI: 10.7270/Q2VQ31HW |
More data for this
Ligand-Target Pair | |
Bromodomain testis-specific protein [251-382]
(Homo sapiens (Human)) | BDBM205431

((6S+2S)-PEG1 (7))Show SMILES COC(=O)C[C@@H]1N=C(c2c(C)c(sc2-n2c(C)nnc12)C(=O)NCCOCCNC(=O)C[C@@H]1N=C(c2c(C)c(C)sc2-n2c(C)nnc12)c1ccc(Cl)cc1)c1ccc(Cl)cc1 |r,c:6,36| Show InChI InChI=1S/C43H42Cl2N10O5S2/c1-21-23(3)61-42-34(21)36(26-7-11-28(44)12-8-26)48-30(39-52-50-24(4)54(39)42)19-32(56)46-15-17-60-18-16-47-41(58)38-22(2)35-37(27-9-13-29(45)14-10-27)49-31(20-33(57)59-6)40-53-51-25(5)55(40)43(35)62-38/h7-14,30-31H,15-20H2,1-6H3,(H,46,56)(H,47,58)/t30-,31-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute
| Assay Description
Refer to DiscoverX |
Nat Chem Biol 12: 1089-1096 (2016)
Article DOI: 10.1038/nchembio.2209
BindingDB Entry DOI: 10.7270/Q2VQ31HW |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 3 [306-416]
(Homo sapiens (Human)) | BDBM205453

(MTI (35))Show SMILES Cc1sc-2c(c1C)C(=NC(CC(=O)NCCOCCOCCOCCOCCOCCOCCOCCNC(=O)C[C@@H]1N=C(c3c(C)c(C)sc3-n3c(C)nnc13)c1ccc(Cl)cc1)c1nnc(C)n-21)c1ccc(Cl)cc1 |r,c:8,43| Show InChI InChI=1S/C54H66Cl2N10O9S2/c1-33-35(3)76-53-47(33)49(39-7-11-41(55)12-8-39)59-43(51-63-61-37(5)65(51)53)31-45(67)57-15-17-69-19-21-71-23-25-73-27-29-75-30-28-74-26-24-72-22-20-70-18-16-58-46(68)32-44-52-64-62-38(6)66(52)54-48(34(2)36(4)77-54)50(60-44)40-9-13-42(56)14-10-40/h7-14,43-44H,15-32H2,1-6H3,(H,57,67)(H,58,68)/t43-,44?/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute
| Assay Description
Refer to DiscoverX |
Nat Chem Biol 12: 1089-1096 (2016)
Article DOI: 10.1038/nchembio.2209
BindingDB Entry DOI: 10.7270/Q2VQ31HW |
More data for this
Ligand-Target Pair | |
Bromodomain testis-specific protein [21-137,251-382]
(Homo sapiens (Human)) | BDBM205453

(MTI (35))Show SMILES Cc1sc-2c(c1C)C(=NC(CC(=O)NCCOCCOCCOCCOCCOCCOCCOCCNC(=O)C[C@@H]1N=C(c3c(C)c(C)sc3-n3c(C)nnc13)c1ccc(Cl)cc1)c1nnc(C)n-21)c1ccc(Cl)cc1 |r,c:8,43| Show InChI InChI=1S/C54H66Cl2N10O9S2/c1-33-35(3)76-53-47(33)49(39-7-11-41(55)12-8-39)59-43(51-63-61-37(5)65(51)53)31-45(67)57-15-17-69-19-21-71-23-25-73-27-29-75-30-28-74-26-24-72-22-20-70-18-16-58-46(68)32-44-52-64-62-38(6)66(52)54-48(34(2)36(4)77-54)50(60-44)40-9-13-42(56)14-10-40/h7-14,43-44H,15-32H2,1-6H3,(H,57,67)(H,58,68)/t43-,44?/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0760 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute
| Assay Description
Refer to DiscoverX |
Nat Chem Biol 12: 1089-1096 (2016)
Article DOI: 10.1038/nchembio.2209
BindingDB Entry DOI: 10.7270/Q2VQ31HW |
More data for this
Ligand-Target Pair | |
Bromodomain testis-specific protein [251-382]
(Homo sapiens (Human)) | BDBM205453

(MTI (35))Show SMILES Cc1sc-2c(c1C)C(=NC(CC(=O)NCCOCCOCCOCCOCCOCCOCCOCCNC(=O)C[C@@H]1N=C(c3c(C)c(C)sc3-n3c(C)nnc13)c1ccc(Cl)cc1)c1nnc(C)n-21)c1ccc(Cl)cc1 |r,c:8,43| Show InChI InChI=1S/C54H66Cl2N10O9S2/c1-33-35(3)76-53-47(33)49(39-7-11-41(55)12-8-39)59-43(51-63-61-37(5)65(51)53)31-45(67)57-15-17-69-19-21-71-23-25-73-27-29-75-30-28-74-26-24-72-22-20-70-18-16-58-46(68)32-44-52-64-62-38(6)66(52)54-48(34(2)36(4)77-54)50(60-44)40-9-13-42(56)14-10-40/h7-14,43-44H,15-32H2,1-6H3,(H,57,67)(H,58,68)/t43-,44?/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0860 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute
| Assay Description
Refer to DiscoverX |
Nat Chem Biol 12: 1089-1096 (2016)
Article DOI: 10.1038/nchembio.2209
BindingDB Entry DOI: 10.7270/Q2VQ31HW |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 4 [44-168,333-460]
(Homo sapiens (Human)) | BDBM205453

(MTI (35))Show SMILES Cc1sc-2c(c1C)C(=NC(CC(=O)NCCOCCOCCOCCOCCOCCOCCOCCNC(=O)C[C@@H]1N=C(c3c(C)c(C)sc3-n3c(C)nnc13)c1ccc(Cl)cc1)c1nnc(C)n-21)c1ccc(Cl)cc1 |r,c:8,43| Show InChI InChI=1S/C54H66Cl2N10O9S2/c1-33-35(3)76-53-47(33)49(39-7-11-41(55)12-8-39)59-43(51-63-61-37(5)65(51)53)31-45(67)57-15-17-69-19-21-71-23-25-73-27-29-75-30-28-74-26-24-72-22-20-70-18-16-58-46(68)32-44-52-64-62-38(6)66(52)54-48(34(2)36(4)77-54)50(60-44)40-9-13-42(56)14-10-40/h7-14,43-44H,15-32H2,1-6H3,(H,57,67)(H,58,68)/t43-,44?/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0870 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute
| Assay Description
Refer to DiscoverX |
Nat Chem Biol 12: 1089-1096 (2016)
Article DOI: 10.1038/nchembio.2209
BindingDB Entry DOI: 10.7270/Q2VQ31HW |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 4 [44-168]
(Homo sapiens (Human)) | BDBM205453

(MTI (35))Show SMILES Cc1sc-2c(c1C)C(=NC(CC(=O)NCCOCCOCCOCCOCCOCCOCCOCCNC(=O)C[C@@H]1N=C(c3c(C)c(C)sc3-n3c(C)nnc13)c1ccc(Cl)cc1)c1nnc(C)n-21)c1ccc(Cl)cc1 |r,c:8,43| Show InChI InChI=1S/C54H66Cl2N10O9S2/c1-33-35(3)76-53-47(33)49(39-7-11-41(55)12-8-39)59-43(51-63-61-37(5)65(51)53)31-45(67)57-15-17-69-19-21-71-23-25-73-27-29-75-30-28-74-26-24-72-22-20-70-18-16-58-46(68)32-44-52-64-62-38(6)66(52)54-48(34(2)36(4)77-54)50(60-44)40-9-13-42(56)14-10-40/h7-14,43-44H,15-32H2,1-6H3,(H,57,67)(H,58,68)/t43-,44?/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0990 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute
| Assay Description
Refer to DiscoverX |
Nat Chem Biol 12: 1089-1096 (2016)
Article DOI: 10.1038/nchembio.2209
BindingDB Entry DOI: 10.7270/Q2VQ31HW |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 3 [24-144,306-416]
(Homo sapiens (Human)) | BDBM205431

((6S+2S)-PEG1 (7))Show SMILES COC(=O)C[C@@H]1N=C(c2c(C)c(sc2-n2c(C)nnc12)C(=O)NCCOCCNC(=O)C[C@@H]1N=C(c2c(C)c(C)sc2-n2c(C)nnc12)c1ccc(Cl)cc1)c1ccc(Cl)cc1 |r,c:6,36| Show InChI InChI=1S/C43H42Cl2N10O5S2/c1-21-23(3)61-42-34(21)36(26-7-11-28(44)12-8-26)48-30(39-52-50-24(4)54(39)42)19-32(56)46-15-17-60-18-16-47-41(58)38-22(2)35-37(27-9-13-29(45)14-10-27)49-31(20-33(57)59-6)40-53-51-25(5)55(40)43(35)62-38/h7-14,30-31H,15-20H2,1-6H3,(H,46,56)(H,47,58)/t30-,31-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute
| Assay Description
Refer to DiscoverX |
Nat Chem Biol 12: 1089-1096 (2016)
Article DOI: 10.1038/nchembio.2209
BindingDB Entry DOI: 10.7270/Q2VQ31HW |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 3 [24-144]
(Homo sapiens (Human)) | BDBM205431

((6S+2S)-PEG1 (7))Show SMILES COC(=O)C[C@@H]1N=C(c2c(C)c(sc2-n2c(C)nnc12)C(=O)NCCOCCNC(=O)C[C@@H]1N=C(c2c(C)c(C)sc2-n2c(C)nnc12)c1ccc(Cl)cc1)c1ccc(Cl)cc1 |r,c:6,36| Show InChI InChI=1S/C43H42Cl2N10O5S2/c1-21-23(3)61-42-34(21)36(26-7-11-28(44)12-8-26)48-30(39-52-50-24(4)54(39)42)19-32(56)46-15-17-60-18-16-47-41(58)38-22(2)35-37(27-9-13-29(45)14-10-27)49-31(20-33(57)59-6)40-53-51-25(5)55(40)43(35)62-38/h7-14,30-31H,15-20H2,1-6H3,(H,46,56)(H,47,58)/t30-,31-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute
| Assay Description
Refer to DiscoverX |
Nat Chem Biol 12: 1089-1096 (2016)
Article DOI: 10.1038/nchembio.2209
BindingDB Entry DOI: 10.7270/Q2VQ31HW |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 2 [71-194]
(Homo sapiens (Human)) | BDBM205453

(MTI (35))Show SMILES Cc1sc-2c(c1C)C(=NC(CC(=O)NCCOCCOCCOCCOCCOCCOCCOCCNC(=O)C[C@@H]1N=C(c3c(C)c(C)sc3-n3c(C)nnc13)c1ccc(Cl)cc1)c1nnc(C)n-21)c1ccc(Cl)cc1 |r,c:8,43| Show InChI InChI=1S/C54H66Cl2N10O9S2/c1-33-35(3)76-53-47(33)49(39-7-11-41(55)12-8-39)59-43(51-63-61-37(5)65(51)53)31-45(67)57-15-17-69-19-21-71-23-25-73-27-29-75-30-28-74-26-24-72-22-20-70-18-16-58-46(68)32-44-52-64-62-38(6)66(52)54-48(34(2)36(4)77-54)50(60-44)40-9-13-42(56)14-10-40/h7-14,43-44H,15-32H2,1-6H3,(H,57,67)(H,58,68)/t43-,44?/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute
| Assay Description
Refer to DiscoverX |
Nat Chem Biol 12: 1089-1096 (2016)
Article DOI: 10.1038/nchembio.2209
BindingDB Entry DOI: 10.7270/Q2VQ31HW |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 4 [44-168]
(Homo sapiens (Human)) | BDBM205431

((6S+2S)-PEG1 (7))Show SMILES COC(=O)C[C@@H]1N=C(c2c(C)c(sc2-n2c(C)nnc12)C(=O)NCCOCCNC(=O)C[C@@H]1N=C(c2c(C)c(C)sc2-n2c(C)nnc12)c1ccc(Cl)cc1)c1ccc(Cl)cc1 |r,c:6,36| Show InChI InChI=1S/C43H42Cl2N10O5S2/c1-21-23(3)61-42-34(21)36(26-7-11-28(44)12-8-26)48-30(39-52-50-24(4)54(39)42)19-32(56)46-15-17-60-18-16-47-41(58)38-22(2)35-37(27-9-13-29(45)14-10-27)49-31(20-33(57)59-6)40-53-51-25(5)55(40)43(35)62-38/h7-14,30-31H,15-20H2,1-6H3,(H,46,56)(H,47,58)/t30-,31-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute
| Assay Description
Refer to DiscoverX |
Nat Chem Biol 12: 1089-1096 (2016)
Article DOI: 10.1038/nchembio.2209
BindingDB Entry DOI: 10.7270/Q2VQ31HW |
More data for this
Ligand-Target Pair | |
Bromodomain testis-specific protein [21-137]
(Homo sapiens (Human)) | BDBM205431

((6S+2S)-PEG1 (7))Show SMILES COC(=O)C[C@@H]1N=C(c2c(C)c(sc2-n2c(C)nnc12)C(=O)NCCOCCNC(=O)C[C@@H]1N=C(c2c(C)c(C)sc2-n2c(C)nnc12)c1ccc(Cl)cc1)c1ccc(Cl)cc1 |r,c:6,36| Show InChI InChI=1S/C43H42Cl2N10O5S2/c1-21-23(3)61-42-34(21)36(26-7-11-28(44)12-8-26)48-30(39-52-50-24(4)54(39)42)19-32(56)46-15-17-60-18-16-47-41(58)38-22(2)35-37(27-9-13-29(45)14-10-27)49-31(20-33(57)59-6)40-53-51-25(5)55(40)43(35)62-38/h7-14,30-31H,15-20H2,1-6H3,(H,46,56)(H,47,58)/t30-,31-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute
| Assay Description
Refer to DiscoverX |
Nat Chem Biol 12: 1089-1096 (2016)
Article DOI: 10.1038/nchembio.2209
BindingDB Entry DOI: 10.7270/Q2VQ31HW |
More data for this
Ligand-Target Pair | |
Bromodomain testis-specific protein [21-137,251-382]
(Homo sapiens (Human)) | BDBM205431

((6S+2S)-PEG1 (7))Show SMILES COC(=O)C[C@@H]1N=C(c2c(C)c(sc2-n2c(C)nnc12)C(=O)NCCOCCNC(=O)C[C@@H]1N=C(c2c(C)c(C)sc2-n2c(C)nnc12)c1ccc(Cl)cc1)c1ccc(Cl)cc1 |r,c:6,36| Show InChI InChI=1S/C43H42Cl2N10O5S2/c1-21-23(3)61-42-34(21)36(26-7-11-28(44)12-8-26)48-30(39-52-50-24(4)54(39)42)19-32(56)46-15-17-60-18-16-47-41(58)38-22(2)35-37(27-9-13-29(45)14-10-27)49-31(20-33(57)59-6)40-53-51-25(5)55(40)43(35)62-38/h7-14,30-31H,15-20H2,1-6H3,(H,46,56)(H,47,58)/t30-,31-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute
| Assay Description
Refer to DiscoverX |
Nat Chem Biol 12: 1089-1096 (2016)
Article DOI: 10.1038/nchembio.2209
BindingDB Entry DOI: 10.7270/Q2VQ31HW |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50347782
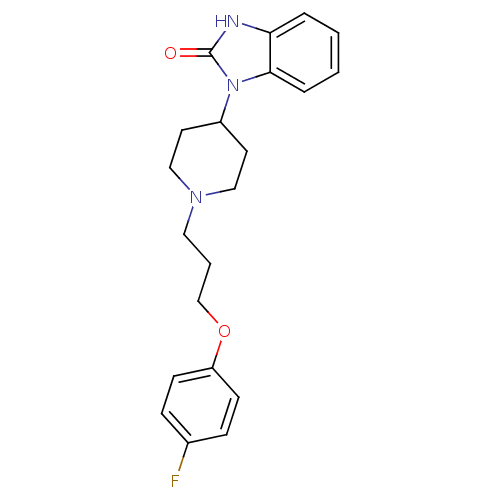
(CHEMBL1802360)Show SMILES Fc1ccc(OCCCN2CCC(CC2)n2c3ccccc3[nH]c2=O)cc1 Show InChI InChI=1S/C21H24FN3O2/c22-16-6-8-18(9-7-16)27-15-3-12-24-13-10-17(11-14-24)25-20-5-2-1-4-19(20)23-21(25)26/h1-2,4-9,17H,3,10-15H2,(H,23,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to dopamine D2 receptor |
ACS Med Chem Lett 1: 244-248 (2010)
Article DOI: 10.1021/ml100105x
BindingDB Entry DOI: 10.7270/Q20R9QGK |
More data for this
Ligand-Target Pair | |
Bromodomain testis-specific protein [21-137]
(Homo sapiens (Human)) | BDBM205453

(MTI (35))Show SMILES Cc1sc-2c(c1C)C(=NC(CC(=O)NCCOCCOCCOCCOCCOCCOCCOCCNC(=O)C[C@@H]1N=C(c3c(C)c(C)sc3-n3c(C)nnc13)c1ccc(Cl)cc1)c1nnc(C)n-21)c1ccc(Cl)cc1 |r,c:8,43| Show InChI InChI=1S/C54H66Cl2N10O9S2/c1-33-35(3)76-53-47(33)49(39-7-11-41(55)12-8-39)59-43(51-63-61-37(5)65(51)53)31-45(67)57-15-17-69-19-21-71-23-25-73-27-29-75-30-28-74-26-24-72-22-20-70-18-16-58-46(68)32-44-52-64-62-38(6)66(52)54-48(34(2)36(4)77-54)50(60-44)40-9-13-42(56)14-10-40/h7-14,43-44H,15-32H2,1-6H3,(H,57,67)(H,58,68)/t43-,44?/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute
| Assay Description
Refer to DiscoverX |
Nat Chem Biol 12: 1089-1096 (2016)
Article DOI: 10.1038/nchembio.2209
BindingDB Entry DOI: 10.7270/Q2VQ31HW |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM19622
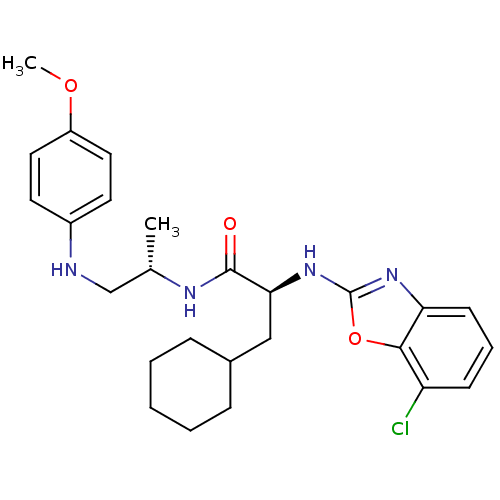
((2S)-2-[(7-chloro-1,3-benzoxazol-2-yl)amino]-3-cyc...)Show SMILES COc1ccc(NC[C@H](C)NC(=O)[C@H](CC2CCCCC2)Nc2nc3cccc(Cl)c3o2)cc1 |r| Show InChI InChI=1S/C26H33ClN4O3/c1-17(16-28-19-11-13-20(33-2)14-12-19)29-25(32)23(15-18-7-4-3-5-8-18)31-26-30-22-10-6-9-21(27)24(22)34-26/h6,9-14,17-18,23,28H,3-5,7-8,15-16H2,1-2H3,(H,29,32)(H,30,31)/t17-,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | -53.4 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
GNF
| Assay Description
The recombinant human cathepsin enzyme was preincubated with inhibitor for 20 minutes prior to addition of substrate. The substrate hydrolysis was mo... |
Bioorg Med Chem Lett 16: 1975-80 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.095
BindingDB Entry DOI: 10.7270/Q2PZ573M |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM19564
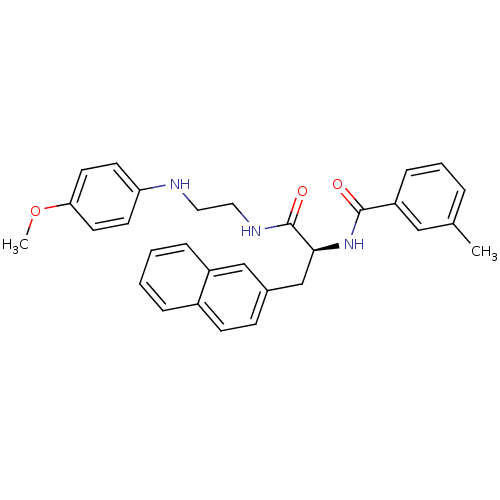
((2S)-N-{2-[(4-methoxyphenyl)amino]ethyl}-2-[(3-met...)Show SMILES COc1ccc(NCCNC(=O)[C@H](Cc2ccc3ccccc3c2)NC(=O)c2cccc(C)c2)cc1 |r| Show InChI InChI=1S/C30H31N3O3/c1-21-6-5-9-25(18-21)29(34)33-28(20-22-10-11-23-7-3-4-8-24(23)19-22)30(35)32-17-16-31-26-12-14-27(36-2)15-13-26/h3-15,18-19,28,31H,16-17,20H2,1-2H3,(H,32,35)(H,33,34)/t28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GNF
| Assay Description
The recombinant human cathepsin enzyme was preincubated with inhibitor for 20 minutes prior to addition of substrate. The substrate hydrolysis was mo... |
Bioorg Med Chem Lett 15: 4979-84 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.017
BindingDB Entry DOI: 10.7270/Q2ZG6QJ8 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM21221

((2S,3S,4R,5R)-5-(2-chloro-6-{[(3-iodophenyl)methyl...)Show SMILES CNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NCc3cccc(I)c3)nc(Cl)nc12 Show InChI InChI=1S/C18H18ClIN6O4/c1-21-16(29)13-11(27)12(28)17(30-13)26-7-23-10-14(24-18(19)25-15(10)26)22-6-8-3-2-4-9(20)5-8/h2-5,7,11-13,17,27-28H,6H2,1H3,(H,21,29)(H,22,24,25)/t11-,12+,13-,17+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Binding affinity for adenosine A3 receptor as inhibition of [125I]-AB-MECA binding to human receptor expressed in HEK 293 cells |
Bioorg Med Chem Lett 8: 1767-70 (1999)
BindingDB Entry DOI: 10.7270/Q2PR7V3R |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM19566
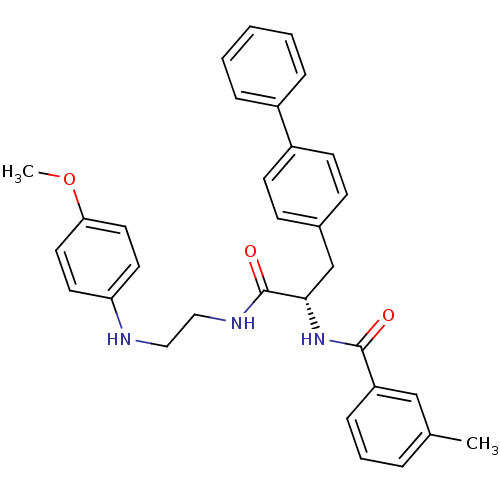
((2S)-N-{2-[(4-methoxyphenyl)amino]ethyl}-2-[(3-met...)Show SMILES COc1ccc(NCCNC(=O)[C@H](Cc2ccc(cc2)-c2ccccc2)NC(=O)c2cccc(C)c2)cc1 |r| Show InChI InChI=1S/C32H33N3O3/c1-23-7-6-10-27(21-23)31(36)35-30(22-24-11-13-26(14-12-24)25-8-4-3-5-9-25)32(37)34-20-19-33-28-15-17-29(38-2)18-16-28/h3-18,21,30,33H,19-20,22H2,1-2H3,(H,34,37)(H,35,36)/t30-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GNF
| Assay Description
The recombinant human cathepsin enzyme was preincubated with inhibitor for 20 minutes prior to addition of substrate. The substrate hydrolysis was mo... |
Bioorg Med Chem Lett 15: 4979-84 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.017
BindingDB Entry DOI: 10.7270/Q2ZG6QJ8 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50347782

(CHEMBL1802360)Show SMILES Fc1ccc(OCCCN2CCC(CC2)n2c3ccccc3[nH]c2=O)cc1 Show InChI InChI=1S/C21H24FN3O2/c22-16-6-8-18(9-7-16)27-15-3-12-24-13-10-17(11-14-24)25-20-5-2-1-4-19(20)23-21(25)26/h1-2,4-9,17H,3,10-15H2,(H,23,26) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to dopamine D3 receptor |
ACS Med Chem Lett 1: 244-248 (2010)
Article DOI: 10.1021/ml100105x
BindingDB Entry DOI: 10.7270/Q20R9QGK |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM19553
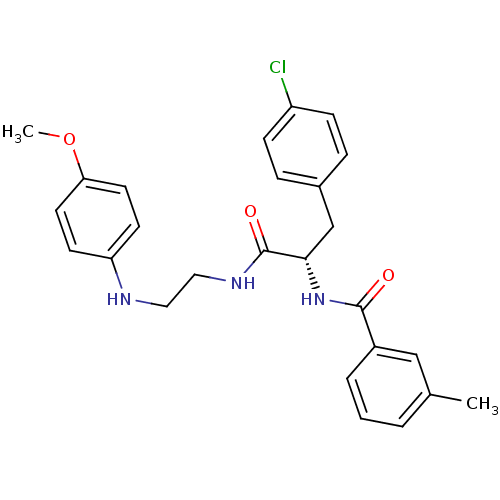
((2S)-3-(4-chlorophenyl)-N-{2-[(4-methoxyphenyl)ami...)Show SMILES COc1ccc(NCCNC(=O)[C@H](Cc2ccc(Cl)cc2)NC(=O)c2cccc(C)c2)cc1 |r| Show InChI InChI=1S/C26H28ClN3O3/c1-18-4-3-5-20(16-18)25(31)30-24(17-19-6-8-21(27)9-7-19)26(32)29-15-14-28-22-10-12-23(33-2)13-11-22/h3-13,16,24,28H,14-15,17H2,1-2H3,(H,29,32)(H,30,31)/t24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GNF
| Assay Description
The recombinant human cathepsin enzyme was preincubated with inhibitor for 20 minutes prior to addition of substrate. The substrate hydrolysis was mo... |
Bioorg Med Chem Lett 15: 4979-84 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.017
BindingDB Entry DOI: 10.7270/Q2ZG6QJ8 |
More data for this
Ligand-Target Pair | |
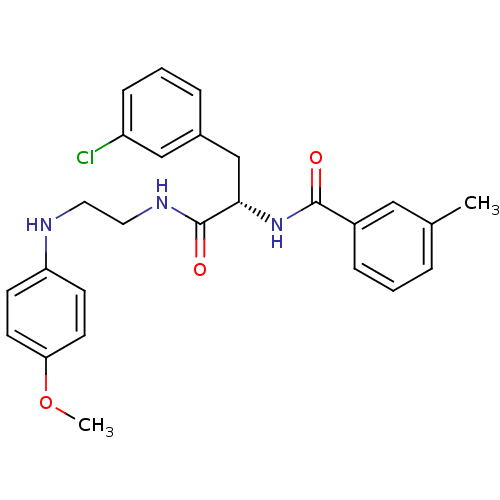
Procathepsin L
(Homo sapiens (Human)) | BDBM19554
((2S)-3-(3-chlorophenyl)-N-{2-[(4-methoxyphenyl)ami...)Show SMILES COc1ccc(NCCNC(=O)[C@H](Cc2cccc(Cl)c2)NC(=O)c2cccc(C)c2)cc1 |r| Show InChI InChI=1S/C26H28ClN3O3/c1-18-5-3-7-20(15-18)25(31)30-24(17-19-6-4-8-21(27)16-19)26(32)29-14-13-28-22-9-11-23(33-2)12-10-22/h3-12,15-16,24,28H,13-14,17H2,1-2H3,(H,29,32)(H,30,31)/t24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GNF
| Assay Description
The recombinant human cathepsin enzyme was preincubated with inhibitor for 20 minutes prior to addition of substrate. The substrate hydrolysis was mo... |
Bioorg Med Chem Lett 15: 4979-84 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.017
BindingDB Entry DOI: 10.7270/Q2ZG6QJ8 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM19577
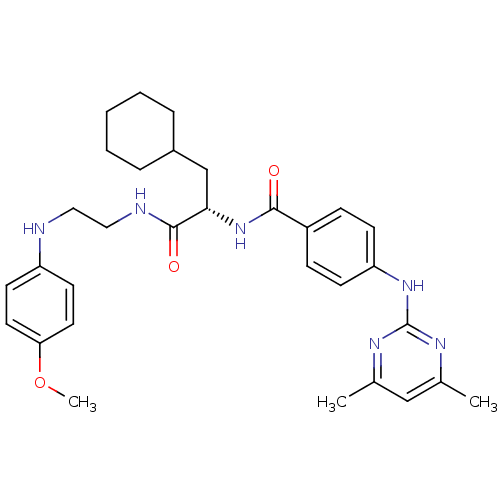
((2S)-3-cyclohexyl-2-({4-[(4,6-dimethylpyrimidin-2-...)Show SMILES COc1ccc(NCCNC(=O)[C@H](CC2CCCCC2)NC(=O)c2ccc(Nc3nc(C)cc(C)n3)cc2)cc1 |r| Show InChI InChI=1S/C31H40N6O3/c1-21-19-22(2)35-31(34-21)36-26-11-9-24(10-12-26)29(38)37-28(20-23-7-5-4-6-8-23)30(39)33-18-17-32-25-13-15-27(40-3)16-14-25/h9-16,19,23,28,32H,4-8,17-18,20H2,1-3H3,(H,33,39)(H,37,38)(H,34,35,36)/t28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GNF
| Assay Description
The recombinant human cathepsin enzyme was preincubated with inhibitor for 20 minutes prior to addition of substrate. The substrate hydrolysis was mo... |
Bioorg Med Chem Lett 15: 4979-84 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.017
BindingDB Entry DOI: 10.7270/Q2ZG6QJ8 |
More data for this
Ligand-Target Pair | |
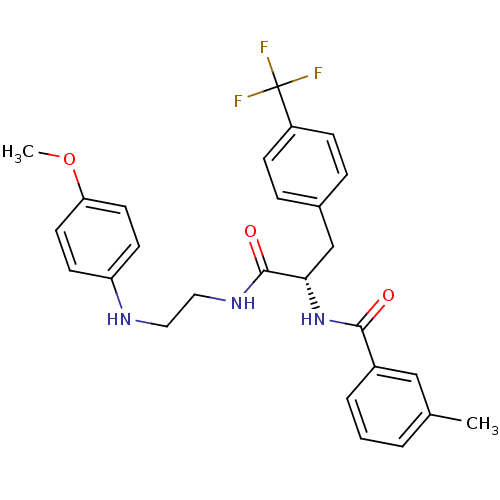
Procathepsin L
(Homo sapiens (Human)) | BDBM19557
((2S)-N-{2-[(4-methoxyphenyl)amino]ethyl}-2-[(3-met...)Show SMILES COc1ccc(NCCNC(=O)[C@H](Cc2ccc(cc2)C(F)(F)F)NC(=O)c2cccc(C)c2)cc1 |r| Show InChI InChI=1S/C27H28F3N3O3/c1-18-4-3-5-20(16-18)25(34)33-24(17-19-6-8-21(9-7-19)27(28,29)30)26(35)32-15-14-31-22-10-12-23(36-2)13-11-22/h3-13,16,24,31H,14-15,17H2,1-2H3,(H,32,35)(H,33,34)/t24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GNF
| Assay Description
The recombinant human cathepsin enzyme was preincubated with inhibitor for 20 minutes prior to addition of substrate. The substrate hydrolysis was mo... |
Bioorg Med Chem Lett 15: 4979-84 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.017
BindingDB Entry DOI: 10.7270/Q2ZG6QJ8 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM19570
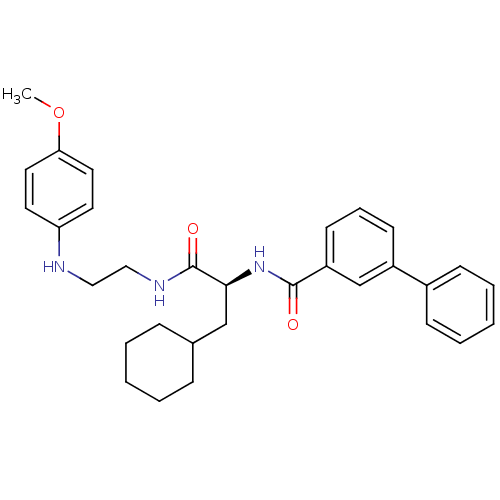
((2S)-3-cyclohexyl-N-{2-[(4-methoxyphenyl)amino]eth...)Show SMILES COc1ccc(NCCNC(=O)[C@H](CC2CCCCC2)NC(=O)c2cccc(c2)-c2ccccc2)cc1 |r| Show InChI InChI=1S/C31H37N3O3/c1-37-28-17-15-27(16-18-28)32-19-20-33-31(36)29(21-23-9-4-2-5-10-23)34-30(35)26-14-8-13-25(22-26)24-11-6-3-7-12-24/h3,6-8,11-18,22-23,29,32H,2,4-5,9-10,19-21H2,1H3,(H,33,36)(H,34,35)/t29-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GNF
| Assay Description
The recombinant human cathepsin enzyme was preincubated with inhibitor for 20 minutes prior to addition of substrate. The substrate hydrolysis was mo... |
Bioorg Med Chem Lett 15: 4979-84 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.017
BindingDB Entry DOI: 10.7270/Q2ZG6QJ8 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM19627
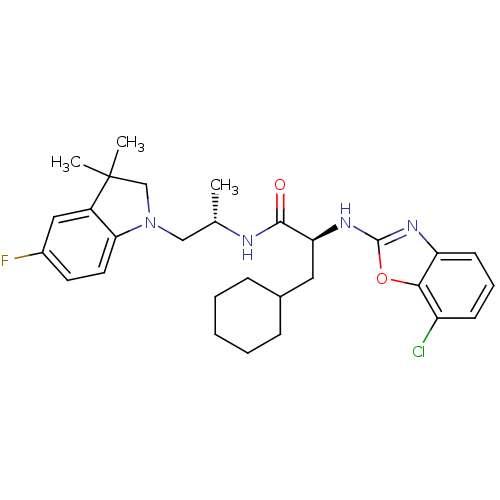
((2S)-2-[(7-chloro-1,3-benzoxazol-2-yl)amino]-3-cyc...)Show SMILES C[C@@H](CN1CC(C)(C)c2cc(F)ccc12)NC(=O)[C@H](CC1CCCCC1)Nc1nc2cccc(Cl)c2o1 |r| Show InChI InChI=1S/C29H36ClFN4O2/c1-18(16-35-17-29(2,3)21-15-20(31)12-13-25(21)35)32-27(36)24(14-19-8-5-4-6-9-19)34-28-33-23-11-7-10-22(30)26(23)37-28/h7,10-13,15,18-19,24H,4-6,8-9,14,16-17H2,1-3H3,(H,32,36)(H,33,34)/t18-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | -50.6 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
GNF
| Assay Description
The recombinant human cathepsin enzyme was preincubated with inhibitor for 20 minutes prior to addition of substrate. The substrate hydrolysis was mo... |
Bioorg Med Chem Lett 16: 1975-80 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.095
BindingDB Entry DOI: 10.7270/Q2PZ573M |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50347782

(CHEMBL1802360)Show SMILES Fc1ccc(OCCCN2CCC(CC2)n2c3ccccc3[nH]c2=O)cc1 Show InChI InChI=1S/C21H24FN3O2/c22-16-6-8-18(9-7-16)27-15-3-12-24-13-10-17(11-14-24)25-20-5-2-1-4-19(20)23-21(25)26/h1-2,4-9,17H,3,10-15H2,(H,23,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to 5HT2A receptor |
ACS Med Chem Lett 1: 244-248 (2010)
Article DOI: 10.1021/ml100105x
BindingDB Entry DOI: 10.7270/Q20R9QGK |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM19582
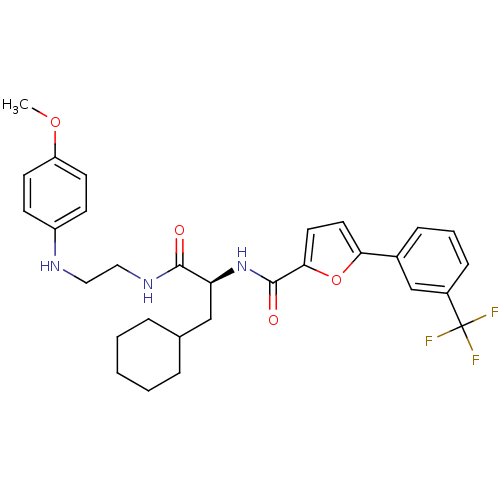
((2S)-3-cyclohexyl-N-{2-[(4-methoxyphenyl)amino]eth...)Show SMILES COc1ccc(NCCNC(=O)[C@H](CC2CCCCC2)NC(=O)c2ccc(o2)-c2cccc(c2)C(F)(F)F)cc1 |r| Show InChI InChI=1S/C30H34F3N3O4/c1-39-24-12-10-23(11-13-24)34-16-17-35-28(37)25(18-20-6-3-2-4-7-20)36-29(38)27-15-14-26(40-27)21-8-5-9-22(19-21)30(31,32)33/h5,8-15,19-20,25,34H,2-4,6-7,16-18H2,1H3,(H,35,37)(H,36,38)/t25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GNF
| Assay Description
The recombinant human cathepsin enzyme was preincubated with inhibitor for 20 minutes prior to addition of substrate. The substrate hydrolysis was mo... |
Bioorg Med Chem Lett 15: 4979-84 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.017
BindingDB Entry DOI: 10.7270/Q2ZG6QJ8 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM19556
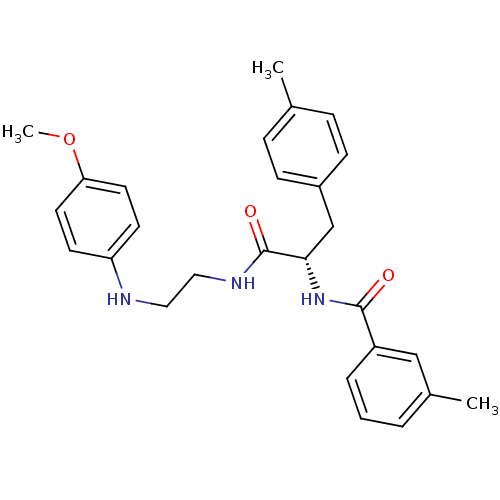
((2S)-N-{2-[(4-methoxyphenyl)amino]ethyl}-3-(4-meth...)Show SMILES COc1ccc(NCCNC(=O)[C@H](Cc2ccc(C)cc2)NC(=O)c2cccc(C)c2)cc1 |r| Show InChI InChI=1S/C27H31N3O3/c1-19-7-9-21(10-8-19)18-25(30-26(31)22-6-4-5-20(2)17-22)27(32)29-16-15-28-23-11-13-24(33-3)14-12-23/h4-14,17,25,28H,15-16,18H2,1-3H3,(H,29,32)(H,30,31)/t25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GNF
| Assay Description
The recombinant human cathepsin enzyme was preincubated with inhibitor for 20 minutes prior to addition of substrate. The substrate hydrolysis was mo... |
Bioorg Med Chem Lett 15: 4979-84 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.017
BindingDB Entry DOI: 10.7270/Q2ZG6QJ8 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM19552
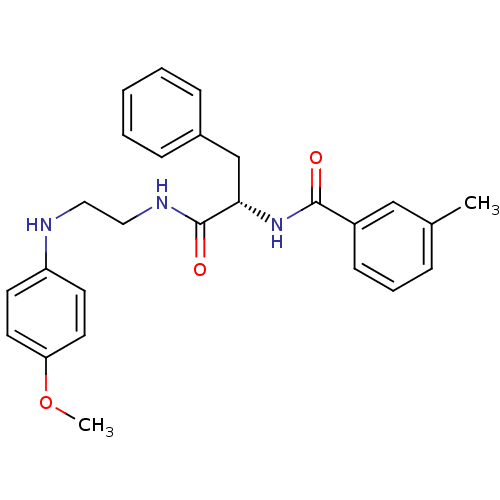
((2S)-N-{2-[(4-methoxyphenyl)amino]ethyl}-2-[(3-met...)Show SMILES COc1ccc(NCCNC(=O)[C@H](Cc2ccccc2)NC(=O)c2cccc(C)c2)cc1 |r| Show InChI InChI=1S/C26H29N3O3/c1-19-7-6-10-21(17-19)25(30)29-24(18-20-8-4-3-5-9-20)26(31)28-16-15-27-22-11-13-23(32-2)14-12-22/h3-14,17,24,27H,15-16,18H2,1-2H3,(H,28,31)(H,29,30)/t24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GNF
| Assay Description
The recombinant human cathepsin enzyme was preincubated with inhibitor for 20 minutes prior to addition of substrate. The substrate hydrolysis was mo... |
Bioorg Med Chem Lett 15: 4979-84 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.017
BindingDB Entry DOI: 10.7270/Q2ZG6QJ8 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM19716
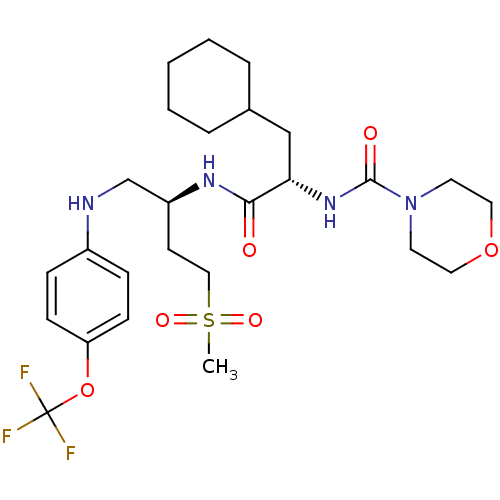
((2S)-3-cyclohexyl-N-[(2S)-4-methanesulfonyl-1-{[4-...)Show SMILES CS(=O)(=O)CC[C@@H](CNc1ccc(OC(F)(F)F)cc1)NC(=O)[C@H](CC1CCCCC1)NC(=O)N1CCOCC1 |r| Show InChI InChI=1S/C26H39F3N4O6S/c1-40(36,37)16-11-21(18-30-20-7-9-22(10-8-20)39-26(27,28)29)31-24(34)23(17-19-5-3-2-4-6-19)32-25(35)33-12-14-38-15-13-33/h7-10,19,21,23,30H,2-6,11-18H2,1H3,(H,31,34)(H,32,35)/t21-,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GNF
| Assay Description
The recombinant human cathepsin enzyme was preincubated with inhibitor for 20 minutes prior to addition of substrate. The substrate hydrolysis was mo... |
Bioorg Med Chem Lett 16: 5112-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.033
BindingDB Entry DOI: 10.7270/Q29P2ZXR |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM19575
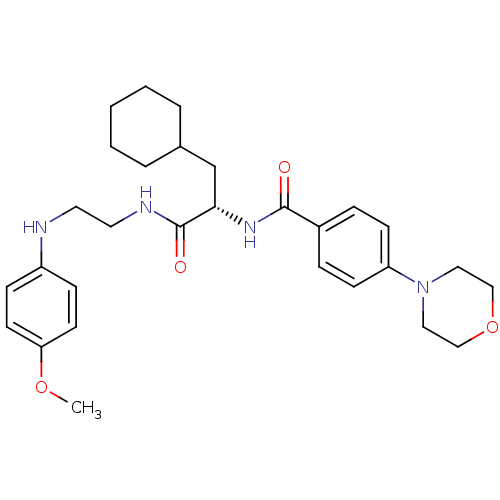
((2S)-3-cyclohexyl-N-{2-[(4-methoxyphenyl)amino]eth...)Show SMILES COc1ccc(NCCNC(=O)[C@H](CC2CCCCC2)NC(=O)c2ccc(cc2)N2CCOCC2)cc1 |r| Show InChI InChI=1S/C29H40N4O4/c1-36-26-13-9-24(10-14-26)30-15-16-31-29(35)27(21-22-5-3-2-4-6-22)32-28(34)23-7-11-25(12-8-23)33-17-19-37-20-18-33/h7-14,22,27,30H,2-6,15-21H2,1H3,(H,31,35)(H,32,34)/t27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GNF
| Assay Description
The recombinant human cathepsin enzyme was preincubated with inhibitor for 20 minutes prior to addition of substrate. The substrate hydrolysis was mo... |
Bioorg Med Chem Lett 15: 4979-84 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.017
BindingDB Entry DOI: 10.7270/Q2ZG6QJ8 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM19549
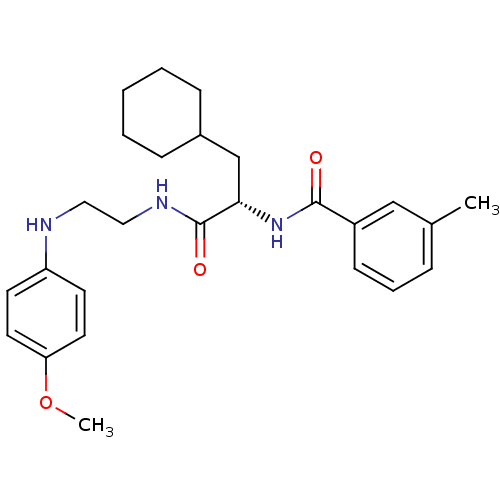
((2S)-3-cyclohexyl-N-{2-[(4-methoxyphenyl)amino]eth...)Show SMILES COc1ccc(NCCNC(=O)[C@H](CC2CCCCC2)NC(=O)c2cccc(C)c2)cc1 |r| Show InChI InChI=1S/C26H35N3O3/c1-19-7-6-10-21(17-19)25(30)29-24(18-20-8-4-3-5-9-20)26(31)28-16-15-27-22-11-13-23(32-2)14-12-22/h6-7,10-14,17,20,24,27H,3-5,8-9,15-16,18H2,1-2H3,(H,28,31)(H,29,30)/t24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GNF
| Assay Description
The recombinant human cathepsin enzyme was preincubated with inhibitor for 20 minutes prior to addition of substrate. The substrate hydrolysis was mo... |
Bioorg Med Chem Lett 15: 4979-84 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.017
BindingDB Entry DOI: 10.7270/Q2ZG6QJ8 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM19716

((2S)-3-cyclohexyl-N-[(2S)-4-methanesulfonyl-1-{[4-...)Show SMILES CS(=O)(=O)CC[C@@H](CNc1ccc(OC(F)(F)F)cc1)NC(=O)[C@H](CC1CCCCC1)NC(=O)N1CCOCC1 |r| Show InChI InChI=1S/C26H39F3N4O6S/c1-40(36,37)16-11-21(18-30-20-7-9-22(10-8-20)39-26(27,28)29)31-24(34)23(17-19-5-3-2-4-6-19)32-25(35)33-12-14-38-15-13-33/h7-10,19,21,23,30H,2-6,11-18H2,1H3,(H,31,34)(H,32,35)/t21-,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5 | -49.3 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
GNF
| Assay Description
The recombinant human cathepsin enzyme was preincubated with inhibitor for 20 minutes prior to addition of substrate. The substrate hydrolysis was mo... |
Bioorg Med Chem Lett 16: 5112-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.033
BindingDB Entry DOI: 10.7270/Q29P2ZXR |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM19568
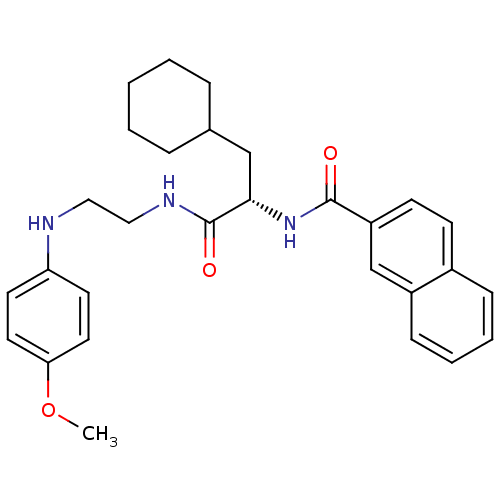
((2S)-3-cyclohexyl-N-{2-[(4-methoxyphenyl)amino]eth...)Show SMILES COc1ccc(NCCNC(=O)[C@H](CC2CCCCC2)NC(=O)c2ccc3ccccc3c2)cc1 |r| Show InChI InChI=1S/C29H35N3O3/c1-35-26-15-13-25(14-16-26)30-17-18-31-29(34)27(19-21-7-3-2-4-8-21)32-28(33)24-12-11-22-9-5-6-10-23(22)20-24/h5-6,9-16,20-21,27,30H,2-4,7-8,17-19H2,1H3,(H,31,34)(H,32,33)/t27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GNF
| Assay Description
The recombinant human cathepsin enzyme was preincubated with inhibitor for 20 minutes prior to addition of substrate. The substrate hydrolysis was mo... |
Bioorg Med Chem Lett 15: 4979-84 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.017
BindingDB Entry DOI: 10.7270/Q2ZG6QJ8 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM19649
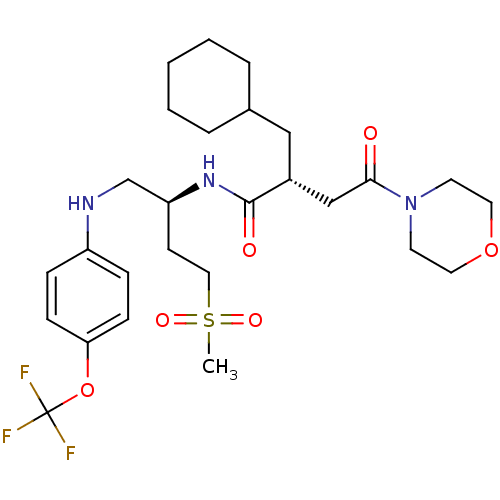
((2R)-2-(cyclohexylmethyl)-N-[(2S)-4-methanesulfony...)Show SMILES CS(=O)(=O)CC[C@@H](CNc1ccc(OC(F)(F)F)cc1)NC(=O)[C@H](CC1CCCCC1)CC(=O)N1CCOCC1 |r| Show InChI InChI=1S/C27H40F3N3O6S/c1-40(36,37)16-11-23(19-31-22-7-9-24(10-8-22)39-27(28,29)30)32-26(35)21(17-20-5-3-2-4-6-20)18-25(34)33-12-14-38-15-13-33/h7-10,20-21,23,31H,2-6,11-19H2,1H3,(H,32,35)/t21-,23+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5 | -49.3 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
GNF
| Assay Description
The recombinant human cathepsin enzyme was preincubated with inhibitor for 20 minutes prior to addition of substrate. The substrate hydrolysis was mo... |
Bioorg Med Chem Lett 17: 2899-903 (2007)
Article DOI: 10.1016/j.bmcl.2007.02.049
BindingDB Entry DOI: 10.7270/Q2K64GBR |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM19631

((2S)-2-(1,3-benzoxazol-2-ylamino)-N-[(2R)-1-(benzy...)Show SMILES Fc1ccc2N(C[C@H](COCc3ccccc3)NC(=O)[C@H](CC3CCCCC3)Nc3nc4ccccc4o3)CCc2c1 |r| Show InChI InChI=1S/C34H39FN4O3/c35-27-15-16-31-26(20-27)17-18-39(31)21-28(23-41-22-25-11-5-2-6-12-25)36-33(40)30(19-24-9-3-1-4-10-24)38-34-37-29-13-7-8-14-32(29)42-34/h2,5-8,11-16,20,24,28,30H,1,3-4,9-10,17-19,21-23H2,(H,36,40)(H,37,38)/t28-,30+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | -49.3 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
GNF
| Assay Description
The recombinant human cathepsin enzyme was preincubated with inhibitor for 20 minutes prior to addition of substrate. The substrate hydrolysis was mo... |
Bioorg Med Chem Lett 16: 1975-80 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.095
BindingDB Entry DOI: 10.7270/Q2PZ573M |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM19555
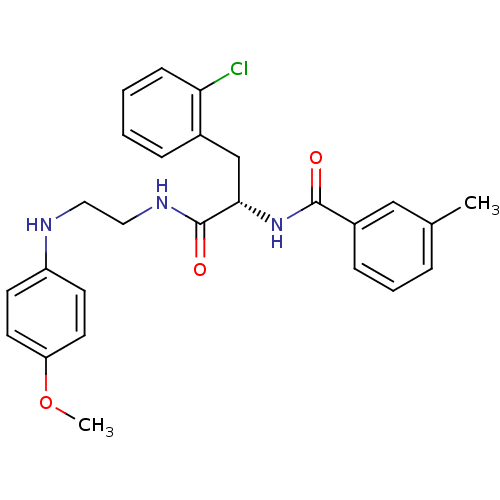
((2S)-3-(2-chlorophenyl)-N-{2-[(4-methoxyphenyl)ami...)Show SMILES COc1ccc(NCCNC(=O)[C@H](Cc2ccccc2Cl)NC(=O)c2cccc(C)c2)cc1 |r| Show InChI InChI=1S/C26H28ClN3O3/c1-18-6-5-8-20(16-18)25(31)30-24(17-19-7-3-4-9-23(19)27)26(32)29-15-14-28-21-10-12-22(33-2)13-11-21/h3-13,16,24,28H,14-15,17H2,1-2H3,(H,29,32)(H,30,31)/t24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GNF
| Assay Description
The recombinant human cathepsin enzyme was preincubated with inhibitor for 20 minutes prior to addition of substrate. The substrate hydrolysis was mo... |
Bioorg Med Chem Lett 15: 4979-84 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.017
BindingDB Entry DOI: 10.7270/Q2ZG6QJ8 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data