

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50000788 ((morphine)4-methyl-(1S,5R,13R,14S,17R)-12-oxa-4-az...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aix-Marseille Universit£ Curated by ChEMBL | Assay Description Binding affinity to mu opioid receptor | Bioorg Med Chem Lett 19: 6736-9 (2009) Article DOI: 10.1016/j.bmcl.2009.09.112 BindingDB Entry DOI: 10.7270/Q2VT1S67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM21015 ((2S)-2-{2-[(2R)-2-[(2S)-2-amino-3-(4-hydroxyphenyl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aix-Marseille Universit£ Curated by ChEMBL | Assay Description Binding affinity to mu opioid receptor | Bioorg Med Chem Lett 19: 6736-9 (2009) Article DOI: 10.1016/j.bmcl.2009.09.112 BindingDB Entry DOI: 10.7270/Q2VT1S67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM21173 (1,3-dipropyl-8-cyclopentylxanthine | 8-cyclopentyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 12.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aix-Marseille Universit£ Curated by ChEMBL | Assay Description Binding affinity to adenosine receptor A1 | Bioorg Med Chem Lett 19: 6736-9 (2009) Article DOI: 10.1016/j.bmcl.2009.09.112 BindingDB Entry DOI: 10.7270/Q2VT1S67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Farnesyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM25298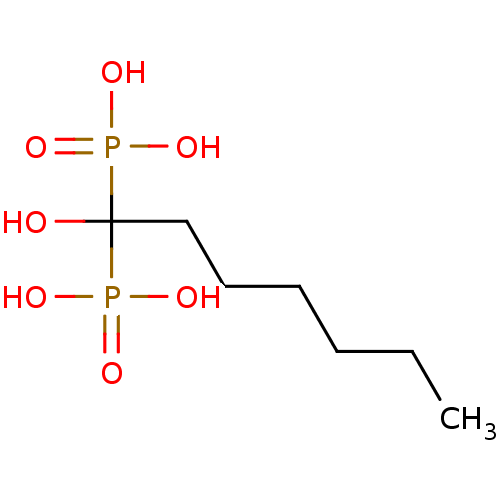 ((1-hydroxy-1-phosphonoheptyl)phosphonic acid | CHE...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Buenos Aires Curated by ChEMBL | Assay Description Inhibitory concentration of the compound against Trypanosoma brucei farnesyl pyrophosphate synthase activity | Bioorg Med Chem Lett 13: 3231-5 (2003) BindingDB Entry DOI: 10.7270/Q2ZK5G2R | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Farnesyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM50132606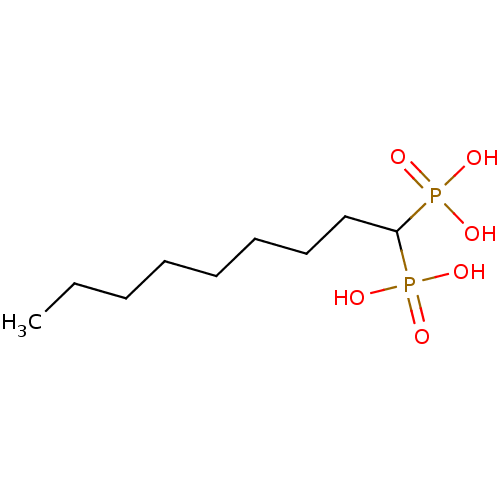 ((1-Phosphono-nonyl)-phosphonic acid | CHEMBL111695) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Buenos Aires Curated by ChEMBL | Assay Description Binding affinity of the compound towards T. cruzi farnesyl pyrophosphate synthase (TcFPPS) | Bioorg Med Chem Lett 13: 3231-5 (2003) BindingDB Entry DOI: 10.7270/Q2ZK5G2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Farnesyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM50097889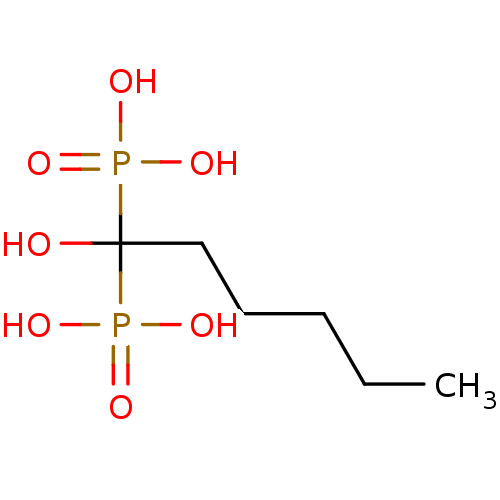 ((1-Hydroxy-1-phosphono-hexyl)-phosphonic acid | 1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Buenos Aires Curated by ChEMBL | Assay Description Inhibition of Trypanosoma cruzi farnesyl pyrophosphate synthase | Bioorg Med Chem Lett 13: 3231-5 (2003) BindingDB Entry DOI: 10.7270/Q2ZK5G2R | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Farnesyl diphosphate synthase (Trypanosoma cruzi) | BDBM25298 ((1-hydroxy-1-phosphonoheptyl)phosphonic acid | CHE...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Buenos Aires Curated by ChEMBL | Assay Description Inhibition of Trypanosoma cruzi FPPS | Bioorg Med Chem 16: 3283-90 (2008) Article DOI: 10.1016/j.bmc.2007.12.010 BindingDB Entry DOI: 10.7270/Q2JD4XPC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Farnesyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM25298 ((1-hydroxy-1-phosphonoheptyl)phosphonic acid | CHE...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Buenos Aires Curated by ChEMBL | Assay Description Inhibition of trypanosoma cruzi farnesyl pyrophosphate synthase (TcFPPS) | Bioorg Med Chem Lett 15: 4685-90 (2005) Article DOI: 10.1016/j.bmcl.2005.07.060 BindingDB Entry DOI: 10.7270/Q21Z43ZW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Farnesyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM50132608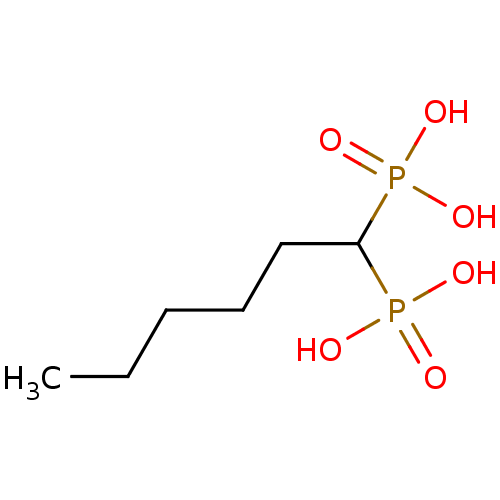 ((1-Phosphono-hexyl)-phosphonic acid | CHEMBL280463) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Buenos Aires Curated by ChEMBL | Assay Description Binding affinity of the compound towards T. cruzi farnesyl pyrophosphate synthase (TcFPPS) | Bioorg Med Chem Lett 13: 3231-5 (2003) BindingDB Entry DOI: 10.7270/Q2ZK5G2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Farnesyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM50132600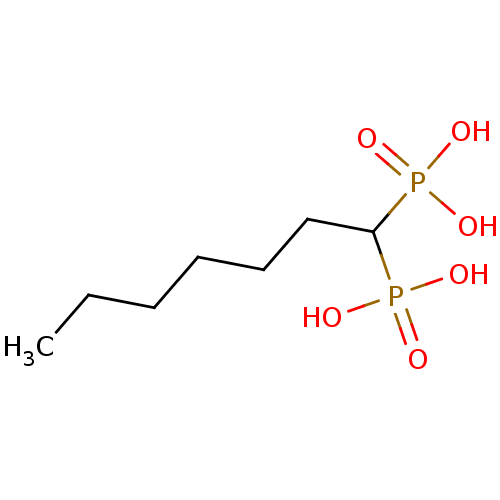 ((1-Phosphono-heptyl)-phosphonic acid | CHEMBL32208...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Buenos Aires Curated by ChEMBL | Assay Description Binding affinity of the compound towards T. cruzi farnesyl pyrophosphate synthase (TcFPPS) | Bioorg Med Chem Lett 13: 3231-5 (2003) BindingDB Entry DOI: 10.7270/Q2ZK5G2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Farnesyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM25265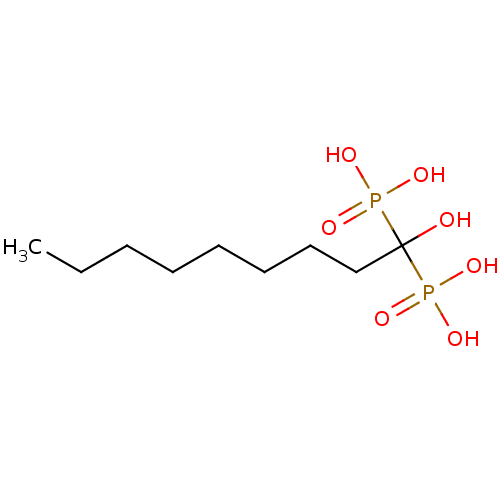 ((1-hydroxy-1-phosphonononyl)phosphonic acid | CHEM...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Buenos Aires Curated by ChEMBL | Assay Description Binding affinity of the compound towards T. cruzi farnesyl pyrophosphate synthase (TcFPPS) | Bioorg Med Chem Lett 13: 3231-5 (2003) BindingDB Entry DOI: 10.7270/Q2ZK5G2R | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50299696 ((2S,3S,4R,5S)-5-(6-(cyclopentylamino)-9H-purin-8-y...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 728 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aix-Marseille Universit£ Curated by ChEMBL | Assay Description Binding affinity to mu opioid receptor | Bioorg Med Chem Lett 19: 6736-9 (2009) Article DOI: 10.1016/j.bmcl.2009.09.112 BindingDB Entry DOI: 10.7270/Q2VT1S67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM31373 (CHEMBL28137 | terephthalamide scaffold, 9) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 780 | -34.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Yale University | Assay Description Fluorescence polarization experiments were conducted on a Photon Technology International instrument using a 0.3 cm path length cuvette. Spectra were... | J Am Chem Soc 127: 5463-8 (2005) Article DOI: 10.1021/ja0446404 BindingDB Entry DOI: 10.7270/Q29Z937V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Farnesyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM50132602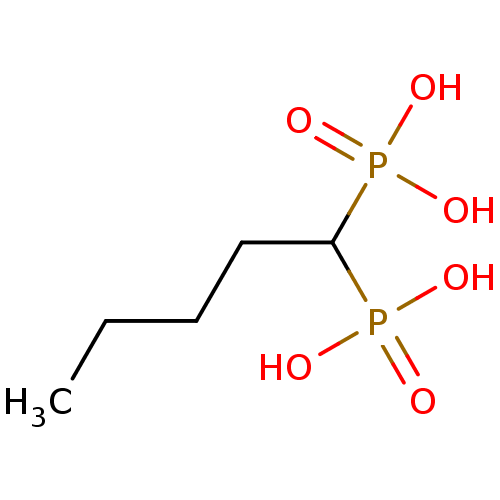 ((1-Phosphono-pentyl)-phosphonic acid | CHEMBL32255...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Buenos Aires Curated by ChEMBL | Assay Description Inhibition of Trypanosoma cruzi farnesyl pyrophosphate synthase | Bioorg Med Chem Lett 13: 3231-5 (2003) BindingDB Entry DOI: 10.7270/Q2ZK5G2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50299696 ((2S,3S,4R,5S)-5-(6-(cyclopentylamino)-9H-purin-8-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aix-Marseille Universit£ Curated by ChEMBL | Assay Description Binding affinity to adenosine receptor A1 | Bioorg Med Chem Lett 19: 6736-9 (2009) Article DOI: 10.1016/j.bmcl.2009.09.112 BindingDB Entry DOI: 10.7270/Q2VT1S67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein phosphatase 3 (Homo sapiens (Human)) | BDBM50303176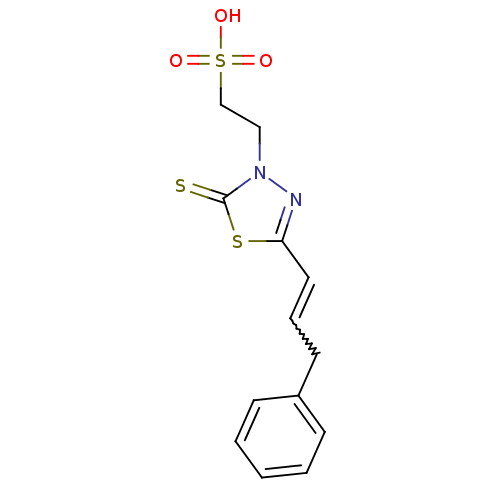 (2-((Z)-4-oxo-5-((E)-3-phenylallylidene)-2-thioxoth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | Article PubMed | 809 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Medical Research Curated by ChEMBL | Assay Description Inhibition of recombinant vaccina H1-related phosphatase expressed in Escherichia coli by Michaelis-Menton kinetic studies | J Med Chem 52: 6716-23 (2009) Article DOI: 10.1021/jm901016k BindingDB Entry DOI: 10.7270/Q2VX0GK3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM31374 (CHEMBL286715 | terephthalamide scaffold, 10) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 840 | -34.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Yale University | Assay Description Fluorescence polarization experiments were conducted on a Photon Technology International instrument using a 0.3 cm path length cuvette. Spectra were... | J Am Chem Soc 127: 5463-8 (2005) Article DOI: 10.1021/ja0446404 BindingDB Entry DOI: 10.7270/Q29Z937V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Farnesyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM50097886 ((1-Hydroxy-1-phosphono-octyl)-phosphonic acid | 1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 980 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Buenos Aires Curated by ChEMBL | Assay Description Binding affinity of the compound towards T. cruzi farnesyl pyrophosphate synthase (TcFPPS) | Bioorg Med Chem Lett 13: 3231-5 (2003) BindingDB Entry DOI: 10.7270/Q2ZK5G2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM31381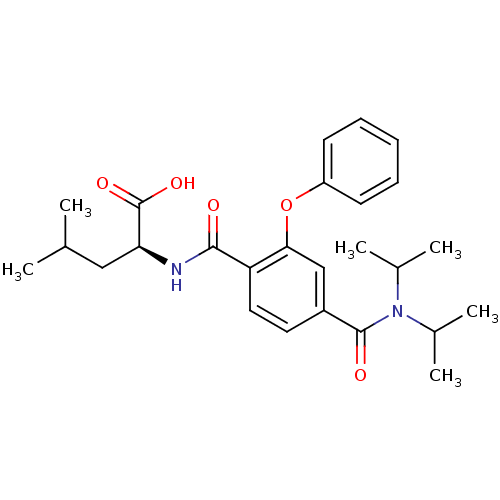 (CHEMBL282624 | terephthalamide scaffold, 29) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.01E+3 | -34.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Yale University | Assay Description Fluorescence polarization experiments were conducted on a Photon Technology International instrument using a 0.3 cm path length cuvette. Spectra were... | J Am Chem Soc 127: 5463-8 (2005) Article DOI: 10.1021/ja0446404 BindingDB Entry DOI: 10.7270/Q29Z937V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein phosphatase 3 (Homo sapiens (Human)) | BDBM50303177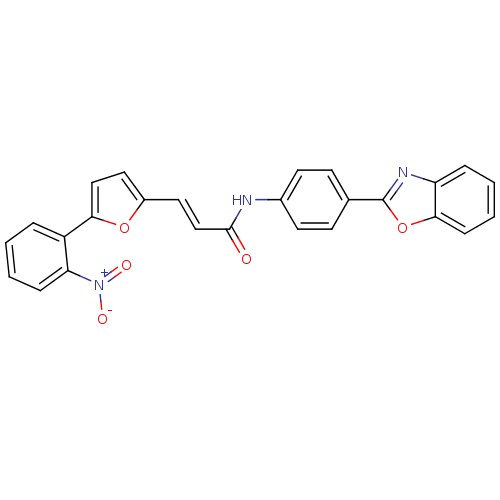 (CHEMBL565369 | N-(4-(benzo[d]oxazol-2-yl)phenyl)-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.76E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Medical Research Curated by ChEMBL | Assay Description Inhibition of recombinant vaccina H1-related phosphatase expressed in Escherichia coli by Michaelis-Menton kinetic studies | J Med Chem 52: 6716-23 (2009) Article DOI: 10.1021/jm901016k BindingDB Entry DOI: 10.7270/Q2VX0GK3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM31379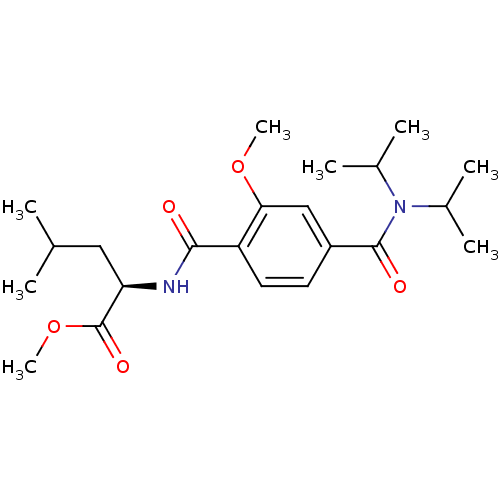 (CHEMBL28872 | terephthalamide scaffold, 27) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.79E+3 | -32.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Yale University | Assay Description Fluorescence polarization experiments were conducted on a Photon Technology International instrument using a 0.3 cm path length cuvette. Spectra were... | J Am Chem Soc 127: 5463-8 (2005) Article DOI: 10.1021/ja0446404 BindingDB Entry DOI: 10.7270/Q29Z937V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM31378 (CHEMBL284686 | terephthalamide scaffold, 26) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.85E+3 | -32.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Yale University | Assay Description Fluorescence polarization experiments were conducted on a Photon Technology International instrument using a 0.3 cm path length cuvette. Spectra were... | J Am Chem Soc 127: 5463-8 (2005) Article DOI: 10.1021/ja0446404 BindingDB Entry DOI: 10.7270/Q29Z937V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein phosphatase 3 (Homo sapiens (Human)) | BDBM50303178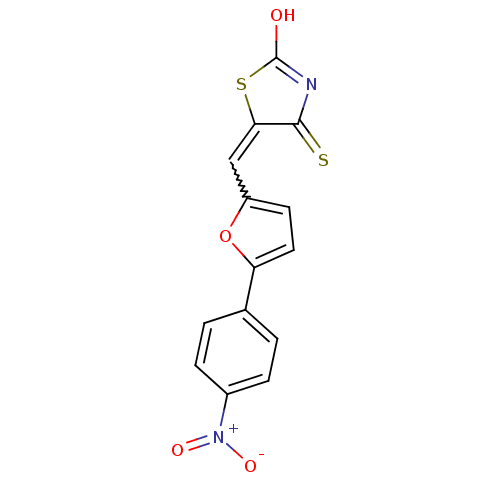 (5-((5-(4-nitrophenyl)furan-2-yl)methylene)-4-thiox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.86E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Medical Research Curated by ChEMBL | Assay Description Inhibition of recombinant vaccina H1-related phosphatase expressed in Escherichia coli by Michaelis-Menton kinetic studies | J Med Chem 52: 6716-23 (2009) Article DOI: 10.1021/jm901016k BindingDB Entry DOI: 10.7270/Q2VX0GK3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Farnesyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM50132605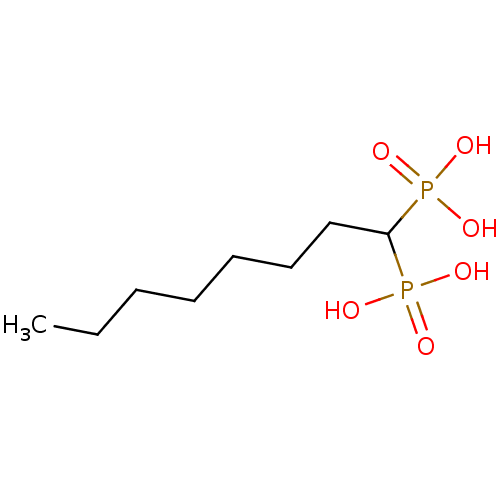 ((1-Phosphono-octyl)-phosphonic acid | CHEMBL324003) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.88E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Buenos Aires Curated by ChEMBL | Assay Description Binding affinity of the compound towards T. cruzi farnesyl pyrophosphate synthase (TcFPPS) | Bioorg Med Chem Lett 13: 3231-5 (2003) BindingDB Entry DOI: 10.7270/Q2ZK5G2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein phosphatase 3 (Homo sapiens (Human)) | BDBM50303179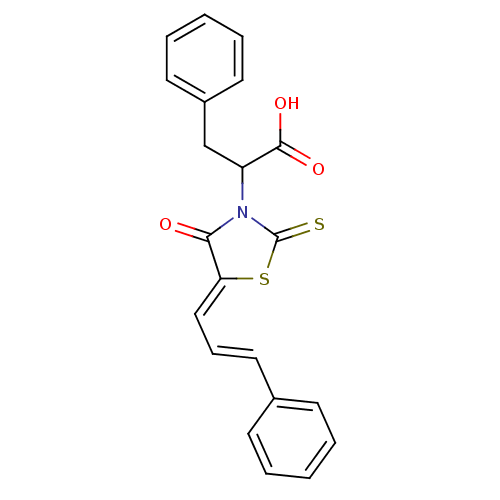 (2-(4-oxo-5-(3-phenylallylidene)-2-thioxothiazolidi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Medical Research Curated by ChEMBL | Assay Description Inhibition of recombinant vaccina H1-related phosphatase expressed in Escherichia coli by Michaelis-Menton kinetic studies | J Med Chem 52: 6716-23 (2009) Article DOI: 10.1021/jm901016k BindingDB Entry DOI: 10.7270/Q2VX0GK3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein phosphatase 3 (Homo sapiens (Human)) | BDBM11994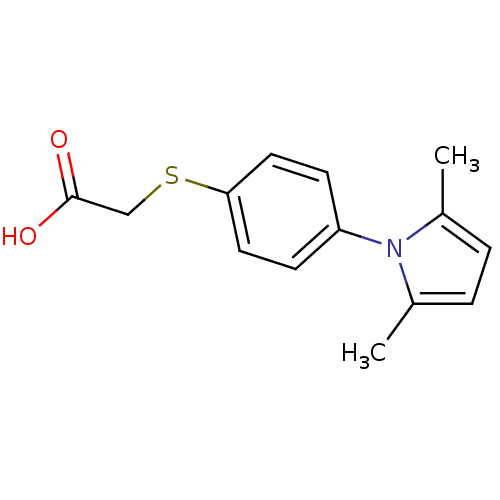 (2-{[4-(2,5-dimethyl-1H-pyrrol-1-yl)phenyl]sulfanyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents | Article PubMed | 2.09E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Medical Research Curated by ChEMBL | Assay Description Inhibition of recombinant vaccina H1-related phosphatase expressed in Escherichia coli by Michaelis-Menton kinetic studies | J Med Chem 52: 6716-23 (2009) Article DOI: 10.1021/jm901016k BindingDB Entry DOI: 10.7270/Q2VX0GK3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM31380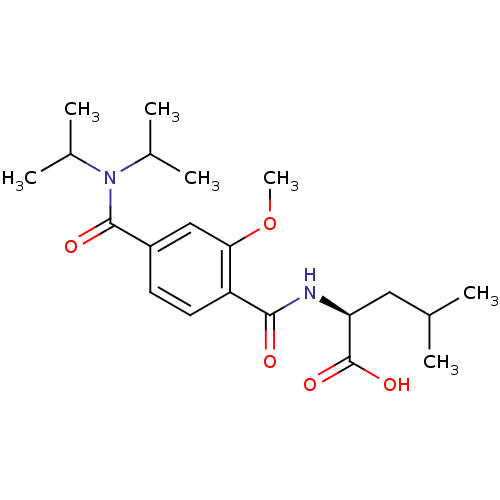 (terephthalamide scaffold, 28) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.31E+3 | -32.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Yale University | Assay Description Fluorescence polarization experiments were conducted on a Photon Technology International instrument using a 0.3 cm path length cuvette. Spectra were... | J Am Chem Soc 127: 5463-8 (2005) Article DOI: 10.1021/ja0446404 BindingDB Entry DOI: 10.7270/Q29Z937V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM31390 (terephthalamide scaffold, 37) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.44E+3 | -32.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Yale University | Assay Description Fluorescence polarization experiments were conducted on a Photon Technology International instrument using a 0.3 cm path length cuvette. Spectra were... | J Am Chem Soc 127: 5463-8 (2005) Article DOI: 10.1021/ja0446404 BindingDB Entry DOI: 10.7270/Q29Z937V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathionylspermidine synthase (Crithidia fasciculata) | BDBM50117542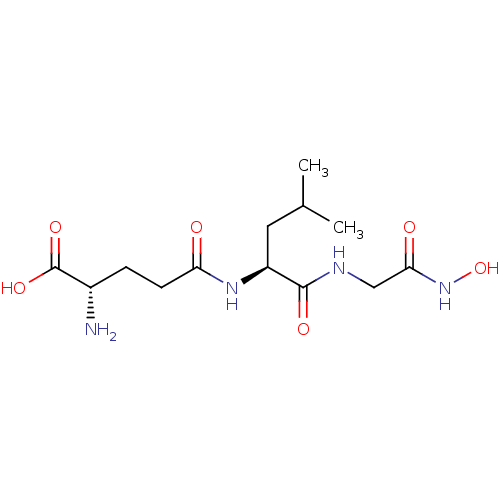 (2-Amino-4-[1-(hydroxycarbamoylmethyl-carbamoyl)-3-...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp (UIA) Curated by ChEMBL | Assay Description Inhibitory constant against amidase free Glutathionylspermidine Synthetase mutant (C79A) | Bioorg Med Chem Lett 12: 2553-6 (2002) BindingDB Entry DOI: 10.7270/Q2057F8Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein phosphatase 3 (Homo sapiens (Human)) | BDBM50303180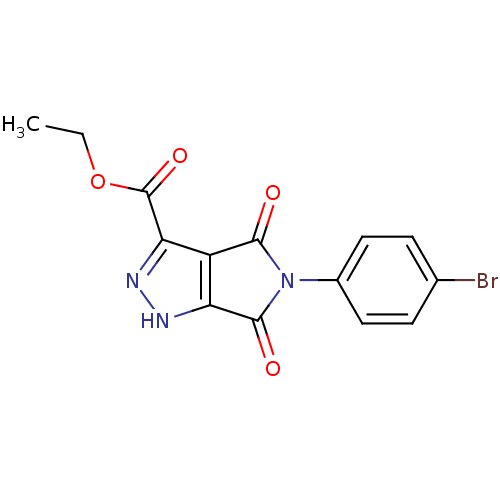 (CHEMBL577311 | ethyl 5-(4-bromophenyl)-4,6-dioxo-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.64E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Medical Research Curated by ChEMBL | Assay Description Inhibition of recombinant vaccina H1-related phosphatase expressed in Escherichia coli by Michaelis-Menton kinetic studies | J Med Chem 52: 6716-23 (2009) Article DOI: 10.1021/jm901016k BindingDB Entry DOI: 10.7270/Q2VX0GK3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein phosphatase 3 (Homo sapiens (Human)) | BDBM50303181 (1-(dibenzo[b,d]furan-3-yl)-3-(naphthalen-1-yl)thio...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | 2.94E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Medical Research Curated by ChEMBL | Assay Description Inhibition of recombinant vaccina H1-related phosphatase expressed in Escherichia coli by Michaelis-Menton kinetic studies | J Med Chem 52: 6716-23 (2009) Article DOI: 10.1021/jm901016k BindingDB Entry DOI: 10.7270/Q2VX0GK3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein phosphatase 3 (Homo sapiens (Human)) | BDBM50303182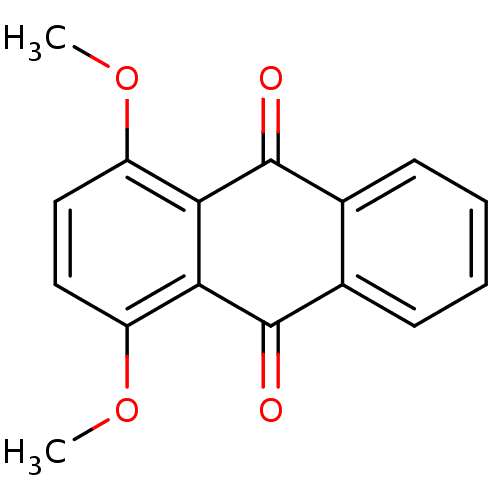 (1,4-dimethoxyanthracene-9,10-dione | CHEMBL570408 ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Medical Research Curated by ChEMBL | Assay Description Inhibition of recombinant vaccina H1-related phosphatase expressed in Escherichia coli by Michaelis-Menton kinetic studies | J Med Chem 52: 6716-23 (2009) Article DOI: 10.1021/jm901016k BindingDB Entry DOI: 10.7270/Q2VX0GK3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein phosphatase 3 (Homo sapiens (Human)) | BDBM28851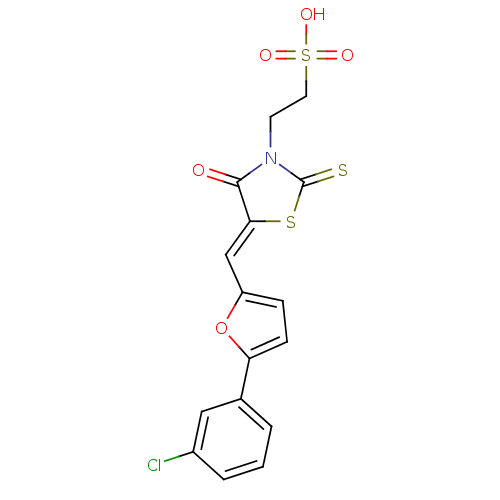 (2-[(5Z)-5-{[5-(3-chlorophenyl)furan-2-yl]methylide...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem Similars | Article PubMed | 3.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Medical Research Curated by ChEMBL | Assay Description Inhibition of recombinant vaccina H1-related phosphatase expressed in Escherichia coli by Michaelis-Menton kinetic studies | J Med Chem 52: 6716-23 (2009) Article DOI: 10.1021/jm901016k BindingDB Entry DOI: 10.7270/Q2VX0GK3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM31387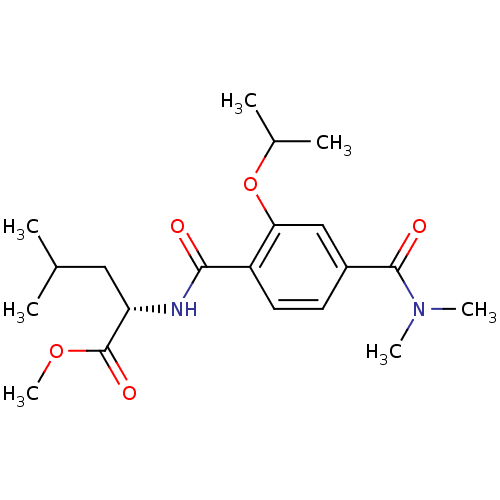 (terephthalamide scaffold, 34) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.14E+3 | -31.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Yale University | Assay Description Fluorescence polarization experiments were conducted on a Photon Technology International instrument using a 0.3 cm path length cuvette. Spectra were... | J Am Chem Soc 127: 5463-8 (2005) Article DOI: 10.1021/ja0446404 BindingDB Entry DOI: 10.7270/Q29Z937V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein phosphatase 3 (Homo sapiens (Human)) | BDBM50303184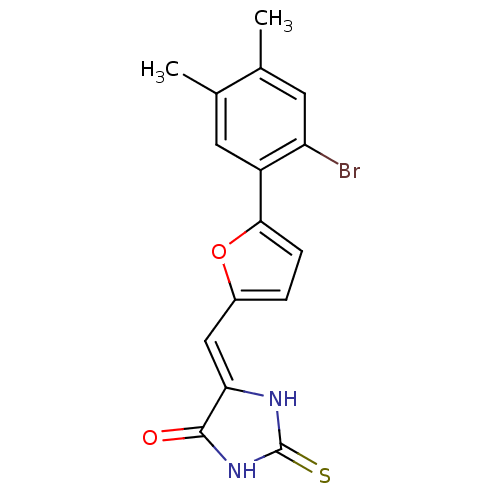 (5-((5-(2-bromo-4,5-dimethylphenyl)furan-2-yl)methy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.19E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Medical Research Curated by ChEMBL | Assay Description Inhibition of recombinant vaccina H1-related phosphatase expressed in Escherichia coli by Michaelis-Menton kinetic studies | J Med Chem 52: 6716-23 (2009) Article DOI: 10.1021/jm901016k BindingDB Entry DOI: 10.7270/Q2VX0GK3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM31385 (CHEMBL281240 | terephthalamide scaffold, 17) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 3.31E+3 | -31.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Yale University | Assay Description Fluorescence polarization experiments were conducted on a Photon Technology International instrument using a 0.3 cm path length cuvette. Spectra were... | J Am Chem Soc 127: 5463-8 (2005) Article DOI: 10.1021/ja0446404 BindingDB Entry DOI: 10.7270/Q29Z937V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein phosphatase 3 (Homo sapiens (Human)) | BDBM50303185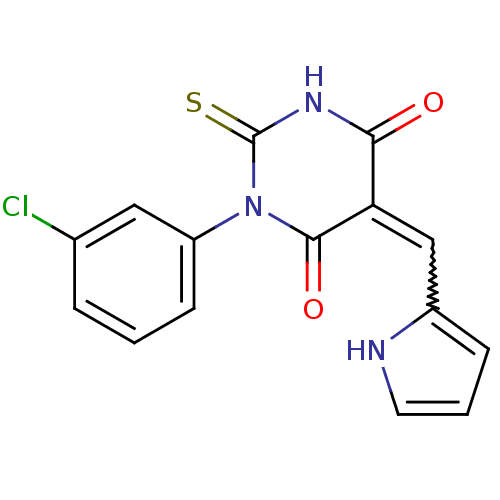 ((E/Z)-5-((1H-pyrrol-2-yl)methylene)-1-(3-chlorophe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | 3.49E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Medical Research Curated by ChEMBL | Assay Description Inhibition of recombinant vaccina H1-related phosphatase expressed in Escherichia coli by Michaelis-Menton kinetic studies | J Med Chem 52: 6716-23 (2009) Article DOI: 10.1021/jm901016k BindingDB Entry DOI: 10.7270/Q2VX0GK3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein phosphatase 3 (Homo sapiens (Human)) | BDBM15186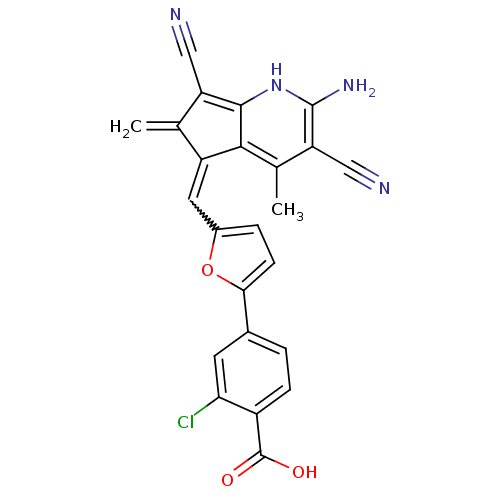 (4-(5-{[(5E)-2-amino-3,7-dicyano-4,6-dimethyl-5H-cy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 3.87E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Medical Research Curated by ChEMBL | Assay Description Inhibition of recombinant vaccina H1-related phosphatase expressed in Escherichia coli by Michaelis-Menton kinetic studies | J Med Chem 52: 6716-23 (2009) Article DOI: 10.1021/jm901016k BindingDB Entry DOI: 10.7270/Q2VX0GK3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein phosphatase 3 (Homo sapiens (Human)) | BDBM50303186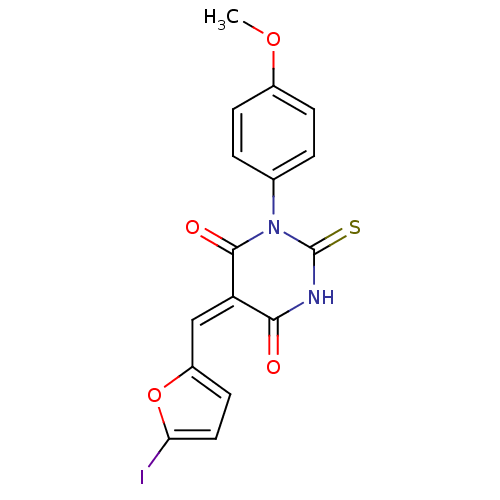 ((E/Z)-5-((5-iodofuran-2-yl)methylene)-1-(4-methoxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.44E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Medical Research Curated by ChEMBL | Assay Description Inhibition of recombinant vaccina H1-related phosphatase expressed in Escherichia coli by Michaelis-Menton kinetic studies | J Med Chem 52: 6716-23 (2009) Article DOI: 10.1021/jm901016k BindingDB Entry DOI: 10.7270/Q2VX0GK3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Farnesyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM50097884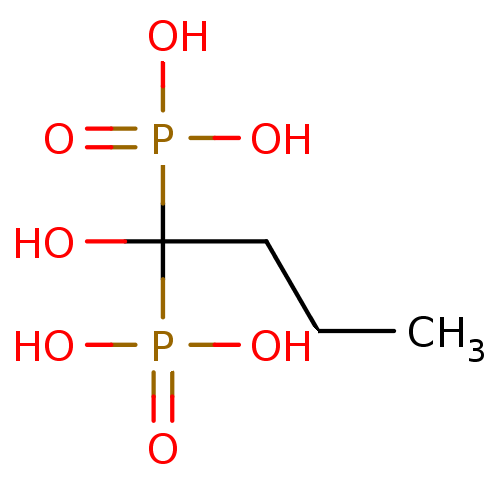 ((1-hydroxy-1-phosphono-butyl)-phosphonic acid | 1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 5.04E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Buenos Aires Curated by ChEMBL | Assay Description Binding affinity of the compound towards T. cruzi farnesyl pyrophosphate synthase (TcFPPS) | Bioorg Med Chem Lett 13: 3231-5 (2003) BindingDB Entry DOI: 10.7270/Q2ZK5G2R | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM31376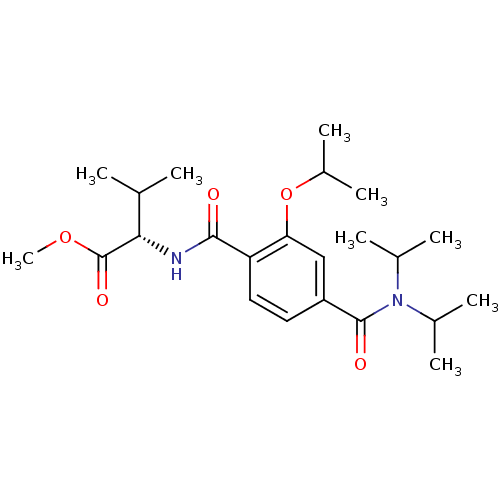 (CHEMBL433422 | terephthalamide scaffold, 24) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.85E+3 | -29.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Yale University | Assay Description Fluorescence polarization experiments were conducted on a Photon Technology International instrument using a 0.3 cm path length cuvette. Spectra were... | J Am Chem Soc 127: 5463-8 (2005) Article DOI: 10.1021/ja0446404 BindingDB Entry DOI: 10.7270/Q29Z937V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM31383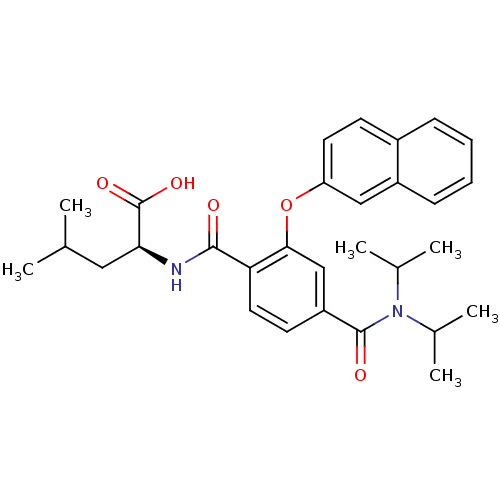 (terephthalamide scaffold, 31) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 7.63E+3 | -29.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Yale University | Assay Description Fluorescence polarization experiments were conducted on a Photon Technology International instrument using a 0.3 cm path length cuvette. Spectra were... | J Am Chem Soc 127: 5463-8 (2005) Article DOI: 10.1021/ja0446404 BindingDB Entry DOI: 10.7270/Q29Z937V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM31386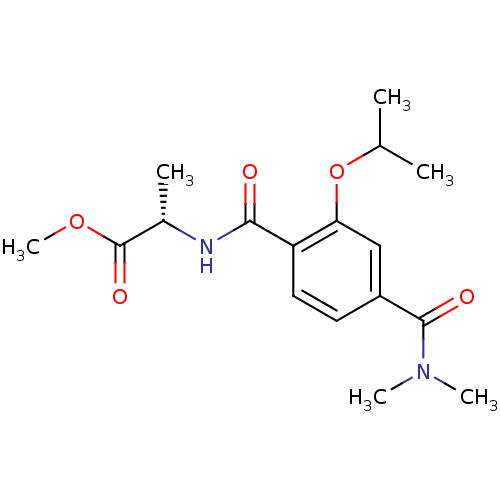 (terephthalamide scaffold, 33) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 8.34E+3 | -29.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Yale University | Assay Description Fluorescence polarization experiments were conducted on a Photon Technology International instrument using a 0.3 cm path length cuvette. Spectra were... | J Am Chem Soc 127: 5463-8 (2005) Article DOI: 10.1021/ja0446404 BindingDB Entry DOI: 10.7270/Q29Z937V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM31393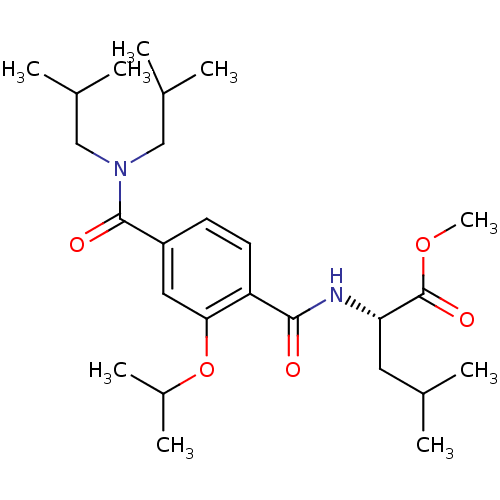 (terephthalamide scaffold, 40) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 8.36E+3 | -29.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Yale University | Assay Description Fluorescence polarization experiments were conducted on a Photon Technology International instrument using a 0.3 cm path length cuvette. Spectra were... | J Am Chem Soc 127: 5463-8 (2005) Article DOI: 10.1021/ja0446404 BindingDB Entry DOI: 10.7270/Q29Z937V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM31392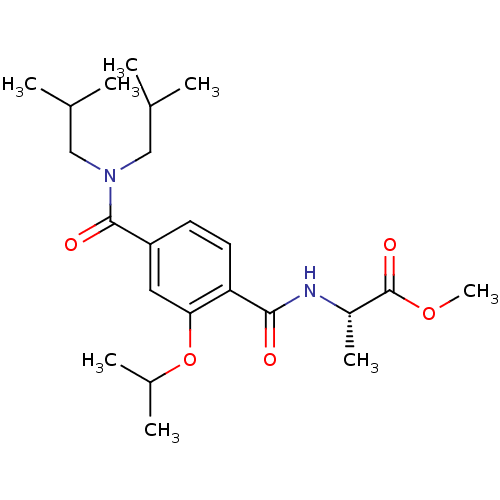 (terephthalamide scaffold, 39) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 8.76E+3 | -28.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Yale University | Assay Description Fluorescence polarization experiments were conducted on a Photon Technology International instrument using a 0.3 cm path length cuvette. Spectra were... | J Am Chem Soc 127: 5463-8 (2005) Article DOI: 10.1021/ja0446404 BindingDB Entry DOI: 10.7270/Q29Z937V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM31388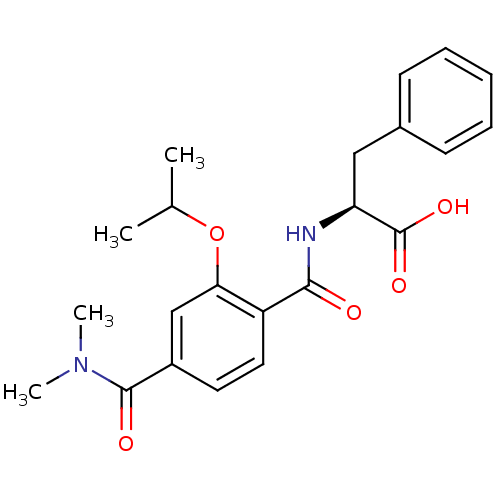 (terephthalamide scaffold, 35) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 8.92E+3 | -28.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Yale University | Assay Description Fluorescence polarization experiments were conducted on a Photon Technology International instrument using a 0.3 cm path length cuvette. Spectra were... | J Am Chem Soc 127: 5463-8 (2005) Article DOI: 10.1021/ja0446404 BindingDB Entry DOI: 10.7270/Q29Z937V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM31377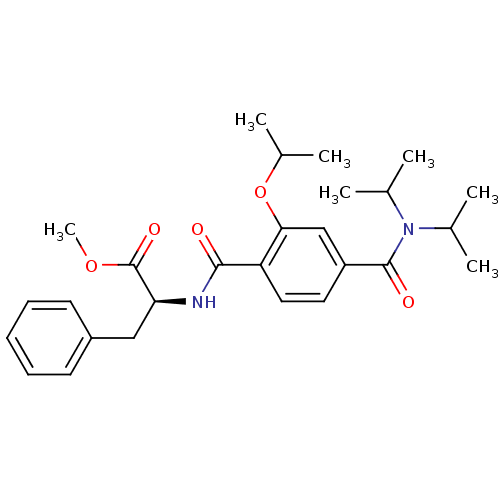 (terephthalamide scaffold, 25) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.09E+4 | -28.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Yale University | Assay Description Fluorescence polarization experiments were conducted on a Photon Technology International instrument using a 0.3 cm path length cuvette. Spectra were... | J Am Chem Soc 127: 5463-8 (2005) Article DOI: 10.1021/ja0446404 BindingDB Entry DOI: 10.7270/Q29Z937V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM31375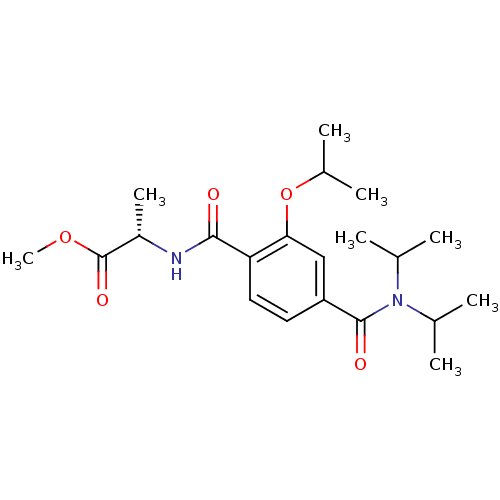 (CHEMBL28504 | terephthalamide scaffold, 23) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10E+4 | -28.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Yale University | Assay Description Fluorescence polarization experiments were conducted on a Photon Technology International instrument using a 0.3 cm path length cuvette. Spectra were... | J Am Chem Soc 127: 5463-8 (2005) Article DOI: 10.1021/ja0446404 BindingDB Entry DOI: 10.7270/Q29Z937V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM31391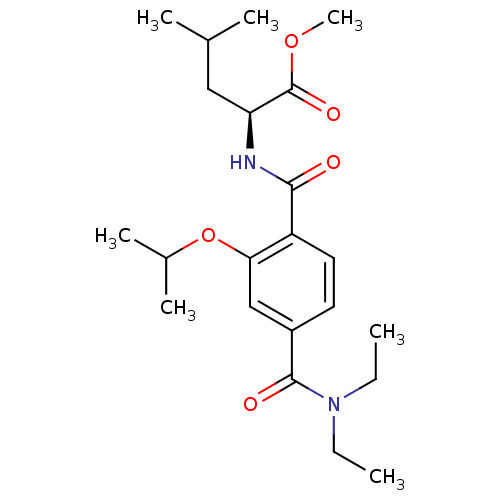 (terephthalamide scaffold, 38) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.14E+4 | -28.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Yale University | Assay Description Fluorescence polarization experiments were conducted on a Photon Technology International instrument using a 0.3 cm path length cuvette. Spectra were... | J Am Chem Soc 127: 5463-8 (2005) Article DOI: 10.1021/ja0446404 BindingDB Entry DOI: 10.7270/Q29Z937V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein phosphatase 3 (Homo sapiens (Human)) | BDBM50303187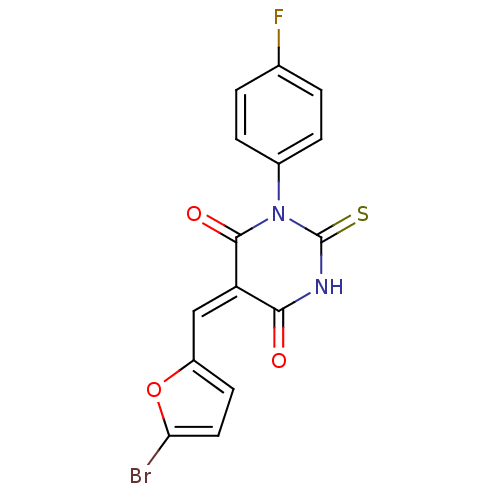 (5-((5-bromofuran-2-yl)methylene)-1-(4-fluorophenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.21E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Medical Research Curated by ChEMBL | Assay Description Inhibition of recombinant vaccina H1-related phosphatase expressed in Escherichia coli by Michaelis-Menton kinetic studies | J Med Chem 52: 6716-23 (2009) Article DOI: 10.1021/jm901016k BindingDB Entry DOI: 10.7270/Q2VX0GK3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 5606 total ) | Next | Last >> |