 Found 488 hits with Last Name = 'ruan' and Initial = 'q'
Found 488 hits with Last Name = 'ruan' and Initial = 'q' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM164638

(BDBM166759 | US10604504, Example 223 | US11623921,...)Show SMILES CC#CC(=O)N[C@H]1CCCN(C1)c1c(F)cc(C(N)=O)c2[nH]c(C)c(C)c12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 116 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged BTK (unknown origin) after 1.5 hrs by HTRF analysis |
J Med Chem 62: 3228-3250 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00167
BindingDB Entry DOI: 10.7270/Q2ZK5M66 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50357312

(IBRUTINIB | PCI-32765 | US10124003, Ref. Ex. Compo...)Show SMILES Nc1ncnc2n(nc(-c3ccc(Oc4ccccc4)cc3)c12)[C@@H]1CCCN(C1)C(=O)C=C Show InChI InChI=1S/C25H24N6O2/c1-2-21(32)30-14-6-7-18(15-30)31-25-22(24(26)27-16-28-25)23(29-31)17-10-12-20(13-11-17)33-19-8-4-3-5-9-19/h2-5,8-13,16,18H,1,6-7,14-15H2,(H2,26,27,28)/t18-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 425 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged BTK (unknown origin) after 1.5 hrs by HTRF analysis |
J Med Chem 62: 3228-3250 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00167
BindingDB Entry DOI: 10.7270/Q2ZK5M66 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM166831
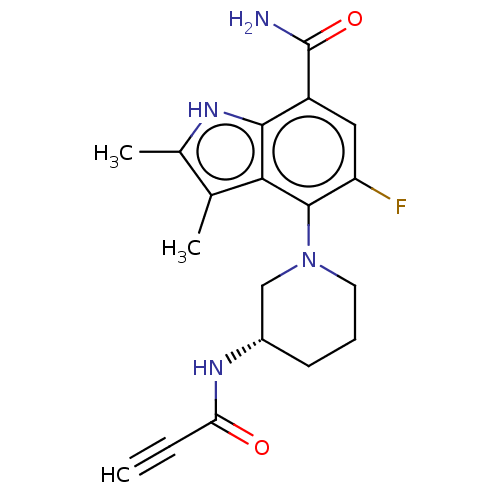
(US10604504, Example 242 | US11623921, Example 242 ...)Show SMILES Cc1[nH]c2c(cc(F)c(N3CCC[C@@H](C3)NC(=O)C#C)c2c1C)C(N)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human BTK using fluoresceinated peptide as substrate after 60 mins fluorescence assay |
J Med Chem 62: 3228-3250 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00167
BindingDB Entry DOI: 10.7270/Q2ZK5M66 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50357312

(IBRUTINIB | PCI-32765 | US10124003, Ref. Ex. Compo...)Show SMILES Nc1ncnc2n(nc(-c3ccc(Oc4ccccc4)cc3)c12)[C@@H]1CCCN(C1)C(=O)C=C Show InChI InChI=1S/C25H24N6O2/c1-2-21(32)30-14-6-7-18(15-30)31-25-22(24(26)27-16-28-25)23(29-31)17-10-12-20(13-11-17)33-19-8-4-3-5-9-19/h2-5,8-13,16,18H,1,6-7,14-15H2,(H2,26,27,28)/t18-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human BTK using fluoresceinated peptide as substrate after 60 mins fluorescence assay |
J Med Chem 62: 3228-3250 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00167
BindingDB Entry DOI: 10.7270/Q2ZK5M66 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM164638

(BDBM166759 | US10604504, Example 223 | US11623921,...)Show SMILES CC#CC(=O)N[C@H]1CCCN(C1)c1c(F)cc(C(N)=O)c2[nH]c(C)c(C)c12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human BTK using fluoresceinated peptide as substrate after 60 mins fluorescence assay |
J Med Chem 62: 3228-3250 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00167
BindingDB Entry DOI: 10.7270/Q2ZK5M66 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50239718
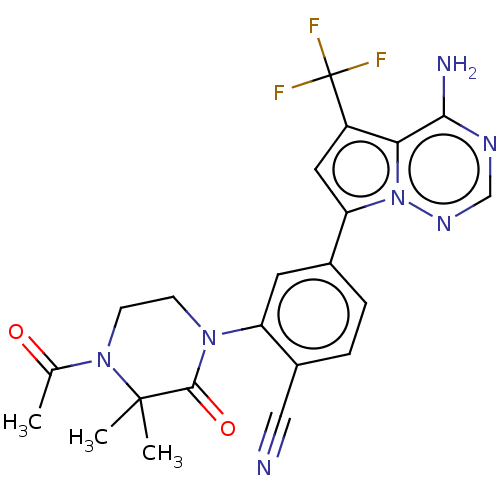
(CHEMBL4064666 | US10214537, Example 639)Show SMILES CC(=O)N1CCN(C(=O)C1(C)C)c1cc(ccc1C#N)-c1cc(c2c(N)ncnn12)C(F)(F)F Show InChI InChI=1S/C22H20F3N7O2/c1-12(33)31-7-6-30(20(34)21(31,2)3)16-8-13(4-5-14(16)10-26)17-9-15(22(23,24)25)18-19(27)28-11-29-32(17)18/h4-5,8-9,11H,6-7H2,1-3H3,(H2,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Antisecretory activity evaluated by the inhibition of 14C -AP uptake in isolated rabbit parietal cells stimulated by exogenous histamine |
J Med Chem 60: 5193-5208 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00618
BindingDB Entry DOI: 10.7270/Q2WW7KVJ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM165319
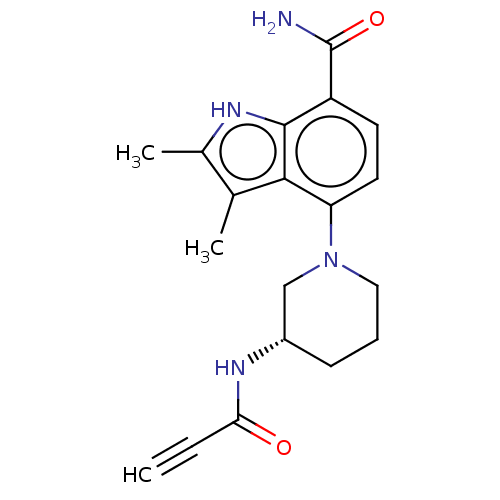
(US10604504, Example 115 | US11623921, Example 115 ...)Show SMILES Cc1[nH]c2c(ccc(N3CCC[C@@H](C3)NC(=O)C#C)c2c1C)C(N)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human BTK using fluoresceinated peptide as substrate after 60 mins fluorescence assay |
J Med Chem 62: 3228-3250 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00167
BindingDB Entry DOI: 10.7270/Q2ZK5M66 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50245571
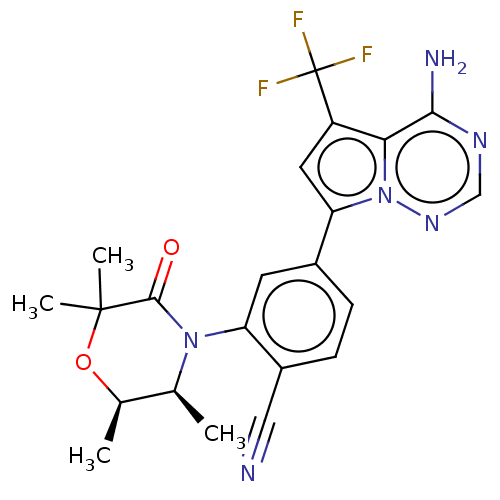
(CHEMBL4091875)Show SMILES C[C@H]1OC(C)(C)C(=O)N([C@H]1C)c1cc(ccc1C#N)-c1cc(c2c(N)ncnn12)C(F)(F)F |r| Show InChI InChI=1S/C22H21F3N6O2/c1-11-12(2)33-21(3,4)20(32)30(11)16-7-13(5-6-14(16)9-26)17-8-15(22(23,24)25)18-19(27)28-10-29-31(17)18/h5-8,10-12H,1-4H3,(H2,27,28,29)/t11-,12+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) after 60 mins using fluorescein-labeled kinase tracer by HTRF assay |
Bioorg Med Chem Lett 27: 2849-2853 (2017)
Article DOI: 10.1016/j.bmcl.2017.01.077
BindingDB Entry DOI: 10.7270/Q25M684R |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50245561
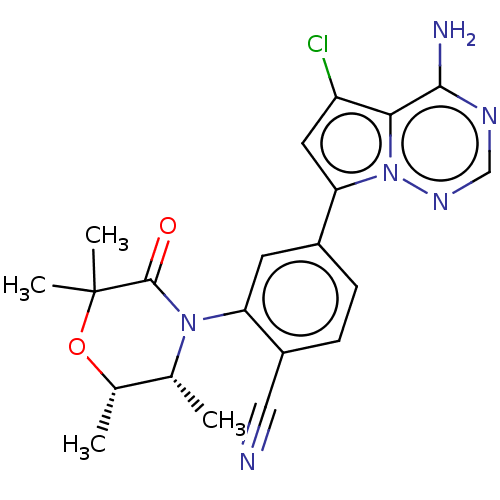
(CHEMBL4069213)Show SMILES C[C@@H]1OC(C)(C)C(=O)N([C@@H]1C)c1cc(ccc1C#N)-c1cc(Cl)c2c(N)ncnn12 |r| Show InChI InChI=1S/C21H21ClN6O2/c1-11-12(2)30-21(3,4)20(29)27(11)16-7-13(5-6-14(16)9-23)17-8-15(22)18-19(24)25-10-26-28(17)18/h5-8,10-12H,1-4H3,(H2,24,25,26)/t11-,12+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) after 60 mins using fluorescein-labeled kinase tracer by HTRF assay |
Bioorg Med Chem Lett 27: 2849-2853 (2017)
Article DOI: 10.1016/j.bmcl.2017.01.077
BindingDB Entry DOI: 10.7270/Q25M684R |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50245582
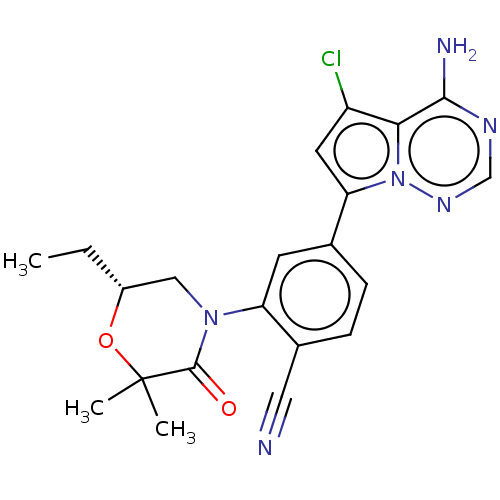
(CHEMBL4080090)Show SMILES CC[C@@H]1CN(C(=O)C(C)(C)O1)c1cc(ccc1C#N)-c1cc(Cl)c2c(N)ncnn12 |r| Show InChI InChI=1S/C21H21ClN6O2/c1-4-14-10-27(20(29)21(2,3)30-14)16-7-12(5-6-13(16)9-23)17-8-15(22)18-19(24)25-11-26-28(17)18/h5-8,11,14H,4,10H2,1-3H3,(H2,24,25,26)/t14-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) after 60 mins using fluorescein-labeled kinase tracer by HTRF assay |
Bioorg Med Chem Lett 27: 2849-2853 (2017)
Article DOI: 10.1016/j.bmcl.2017.01.077
BindingDB Entry DOI: 10.7270/Q25M684R |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50245550
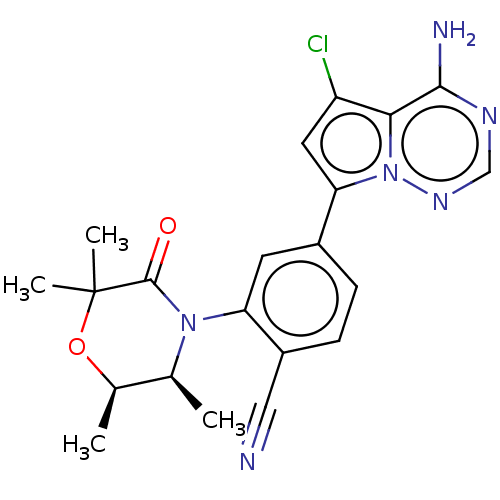
(CHEMBL4096456)Show SMILES C[C@H]1OC(C)(C)C(=O)N([C@H]1C)c1cc(ccc1C#N)-c1cc(Cl)c2c(N)ncnn12 |r| Show InChI InChI=1S/C21H21ClN6O2/c1-11-12(2)30-21(3,4)20(29)27(11)16-7-13(5-6-14(16)9-23)17-8-15(22)18-19(24)25-10-26-28(17)18/h5-8,10-12H,1-4H3,(H2,24,25,26)/t11-,12+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) after 60 mins using fluorescein-labeled kinase tracer by HTRF assay |
Bioorg Med Chem Lett 27: 2849-2853 (2017)
Article DOI: 10.1016/j.bmcl.2017.01.077
BindingDB Entry DOI: 10.7270/Q25M684R |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM358638
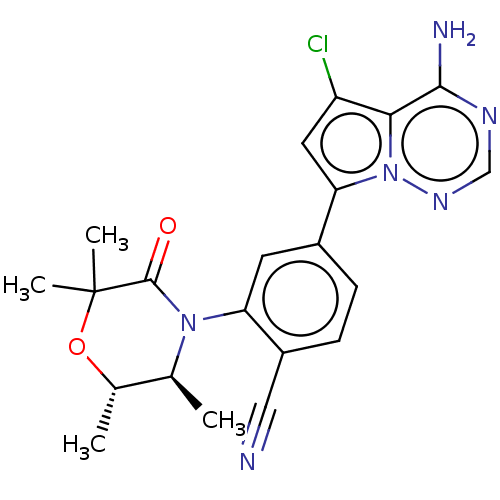
(4-(4-amino-5-chloropyrrolo[2,1-f] [1,2,4]triazin-7...)Show SMILES C[C@@H]1OC(C)(C)C(=O)N([C@H]1C)c1cc(ccc1C#N)-c1cc(Cl)c2c(N)ncnn12 |r| Show InChI InChI=1S/C21H21ClN6O2/c1-11-12(2)30-21(3,4)20(29)27(11)16-7-13(5-6-14(16)9-23)17-8-15(22)18-19(24)25-10-26-28(17)18/h5-8,10-12H,1-4H3,(H2,24,25,26)/t11-,12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) after 60 mins using fluorescein-labeled kinase tracer by HTRF assay |
Bioorg Med Chem Lett 27: 2849-2853 (2017)
Article DOI: 10.1016/j.bmcl.2017.01.077
BindingDB Entry DOI: 10.7270/Q25M684R |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM164638

(BDBM166759 | US10604504, Example 223 | US11623921,...)Show SMILES CC#CC(=O)N[C@H]1CCCN(C1)c1c(F)cc(C(N)=O)c2[nH]c(C)c(C)c12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of BTK in human PBMC assessed as reduction in TNFalpha expression |
J Med Chem 62: 3228-3250 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00167
BindingDB Entry DOI: 10.7270/Q2ZK5M66 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM358628
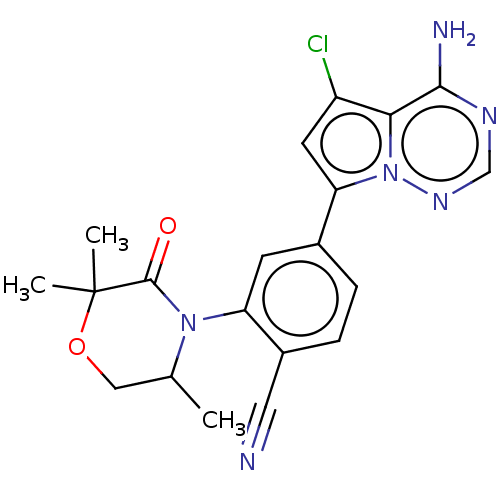
(4-(4-amino-5-chloropyrrolo[2,1-f] [1,2,4]triazin-7...)Show SMILES CC1COC(C)(C)C(=O)N1c1cc(ccc1C#N)-c1cc(Cl)c2c(N)ncnn12 Show InChI InChI=1S/C20H19ClN6O2/c1-11-9-29-20(2,3)19(28)26(11)15-6-12(4-5-13(15)8-22)16-7-14(21)17-18(23)24-10-25-27(16)17/h4-7,10-11H,9H2,1-3H3,(H2,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) after 60 mins using fluorescein-labeled kinase tracer by HTRF assay |
Bioorg Med Chem Lett 27: 2849-2853 (2017)
Article DOI: 10.1016/j.bmcl.2017.01.077
BindingDB Entry DOI: 10.7270/Q25M684R |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM164638

(BDBM166759 | US10604504, Example 223 | US11623921,...)Show SMILES CC#CC(=O)N[C@H]1CCCN(C1)c1c(F)cc(C(N)=O)c2[nH]c(C)c(C)c12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of BTK in human peripheral B cells assessed as reduction in CD86 surface expression |
J Med Chem 62: 3228-3250 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00167
BindingDB Entry DOI: 10.7270/Q2ZK5M66 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM358653
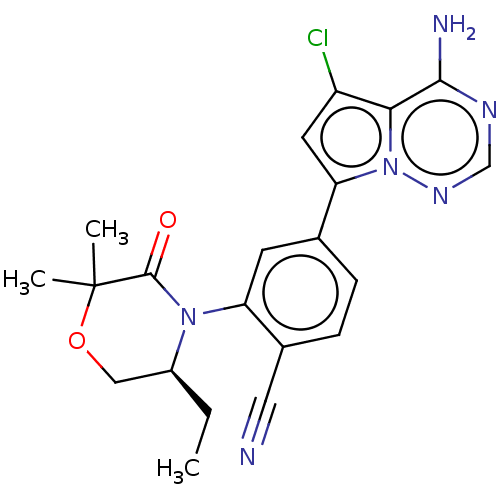
((S)-4-(4-amino-5-chloropyrrolo[2,1-f] [1,2,4]triaz...)Show SMILES CC[C@H]1COC(C)(C)C(=O)N1c1cc(ccc1C#N)-c1cc(Cl)c2c(N)ncnn12 |r| Show InChI InChI=1S/C21H21ClN6O2/c1-4-14-10-30-21(2,3)20(29)27(14)16-7-12(5-6-13(16)9-23)17-8-15(22)18-19(24)25-11-26-28(17)18/h5-8,11,14H,4,10H2,1-3H3,(H2,24,25,26)/t14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) after 60 mins using fluorescein-labeled kinase tracer by HTRF assay |
Bioorg Med Chem Lett 27: 2849-2853 (2017)
Article DOI: 10.1016/j.bmcl.2017.01.077
BindingDB Entry DOI: 10.7270/Q25M684R |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50245562

(CHEMBL4097187)Show SMILES [2H]C1([2H])OC(C)(C)C(=O)N(c2cc(ccc2C#N)-c2cc(Cl)c3c(N)ncnn23)C1([2H])[2H] Show InChI InChI=1S/C19H17ClN6O2/c1-19(2)18(27)25(5-6-28-19)14-7-11(3-4-12(14)9-21)15-8-13(20)16-17(22)23-10-24-26(15)16/h3-4,7-8,10H,5-6H2,1-2H3,(H2,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) after 60 mins using fluorescein-labeled kinase tracer by HTRF assay |
Bioorg Med Chem Lett 27: 2849-2853 (2017)
Article DOI: 10.1016/j.bmcl.2017.01.077
BindingDB Entry DOI: 10.7270/Q25M684R |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM358657
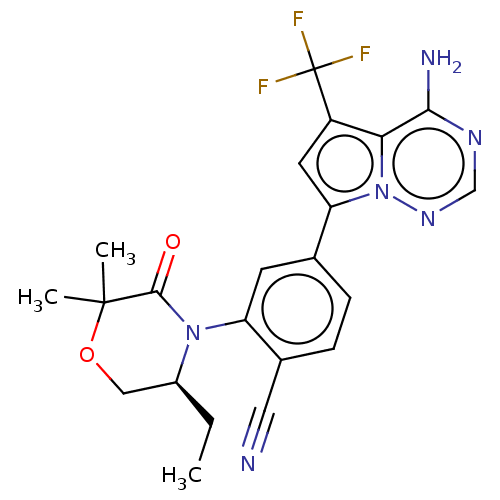
((S)-4-(4-amino-5-(trifluoromethyl) pyrrolo[2,1-f][...)Show SMILES CC[C@H]1COC(C)(C)C(=O)N1c1cc(ccc1C#N)-c1cc(c2c(N)ncnn12)C(F)(F)F |r| Show InChI InChI=1S/C22H21F3N6O2/c1-4-14-10-33-21(2,3)20(32)30(14)16-7-12(5-6-13(16)9-26)17-8-15(22(23,24)25)18-19(27)28-11-29-31(17)18/h5-8,11,14H,4,10H2,1-3H3,(H2,27,28,29)/t14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) after 60 mins using fluorescein-labeled kinase tracer by HTRF assay |
Bioorg Med Chem Lett 27: 2849-2853 (2017)
Article DOI: 10.1016/j.bmcl.2017.01.077
BindingDB Entry DOI: 10.7270/Q25M684R |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50232433
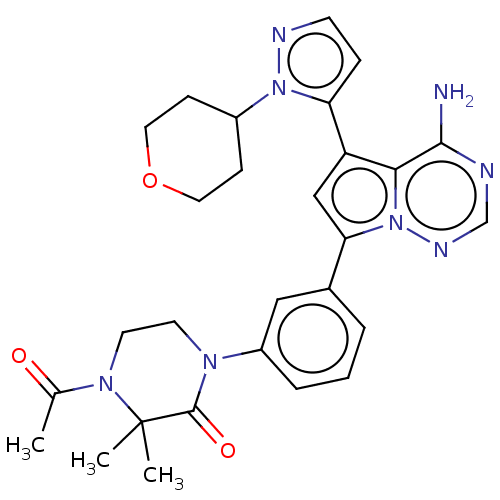
(CHEMBL4068514 | US10214537, Example 619)Show SMILES CC(=O)N1CCN(C(=O)C1(C)C)c1cccc(c1)-c1cc(-c2ccnn2C2CCOCC2)c2c(N)ncnn12 Show InChI InChI=1S/C28H32N8O3/c1-18(37)34-12-11-33(27(38)28(34,2)3)21-6-4-5-19(15-21)24-16-22(25-26(29)30-17-32-36(24)25)23-7-10-31-35(23)20-8-13-39-14-9-20/h4-7,10,15-17,20H,8-9,11-14H2,1-3H3,(H2,29,30,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Antisecretory activity evaluated by the inhibition of 14C -AP uptake in isolated rabbit parietal cells stimulated by exogenous histamine |
J Med Chem 60: 5193-5208 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00618
BindingDB Entry DOI: 10.7270/Q2WW7KVJ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM358627
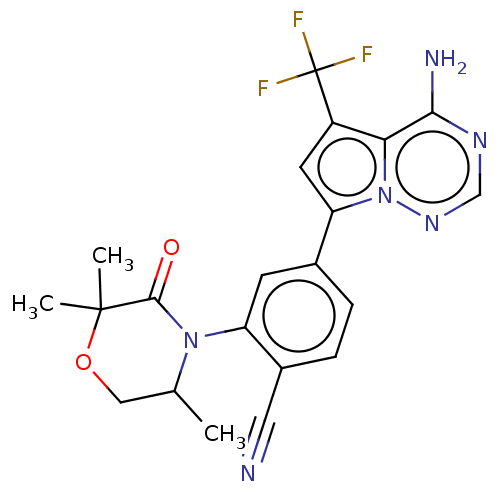
(4-(4-amino-5-(trifluoromethyl)pyrrolo [2,1-f][1,2,...)Show SMILES CC1COC(C)(C)C(=O)N1c1cc(ccc1C#N)-c1cc(c2c(N)ncnn12)C(F)(F)F Show InChI InChI=1S/C21H19F3N6O2/c1-11-9-32-20(2,3)19(31)29(11)15-6-12(4-5-13(15)8-25)16-7-14(21(22,23)24)17-18(26)27-10-28-30(16)17/h4-7,10-11H,9H2,1-3H3,(H2,26,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) after 60 mins using fluorescein-labeled kinase tracer by HTRF assay |
Bioorg Med Chem Lett 27: 2849-2853 (2017)
Article DOI: 10.1016/j.bmcl.2017.01.077
BindingDB Entry DOI: 10.7270/Q25M684R |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM165320
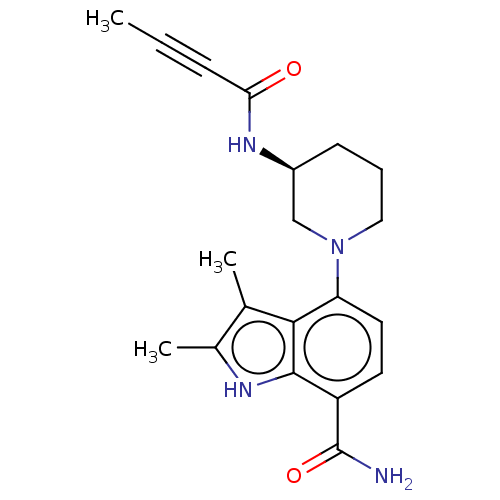
(US10604504, Example 116 | US11623921, Example 116 ...)Show SMILES CC#CC(=O)N[C@H]1CCCN(C1)c1ccc(C(N)=O)c2[nH]c(C)c(C)c12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human BTK using fluoresceinated peptide as substrate after 60 mins fluorescence assay |
J Med Chem 62: 3228-3250 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00167
BindingDB Entry DOI: 10.7270/Q2ZK5M66 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM165463
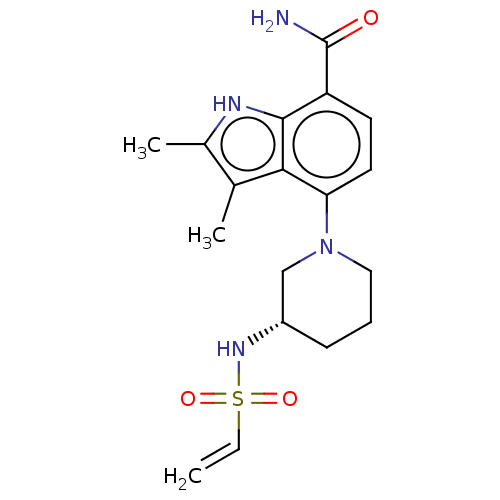
(US10604504, Example 141 | US11623921, Example 141 ...)Show SMILES Cc1[nH]c2c(ccc(N3CCC[C@@H](C3)NS(=O)(=O)C=C)c2c1C)C(N)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human BTK using fluoresceinated peptide as substrate after 60 mins fluorescence assay |
J Med Chem 62: 3228-3250 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00167
BindingDB Entry DOI: 10.7270/Q2ZK5M66 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM358577
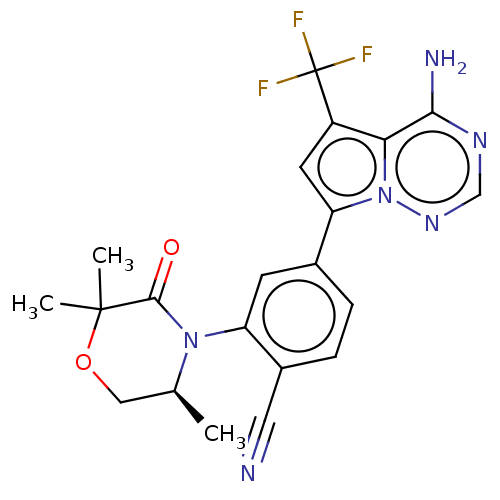
((S)-4-(4-amino-5-(trifluoromethyl) pyrrolo[2,1-f][...)Show SMILES C[C@H]1COC(C)(C)C(=O)N1c1cc(ccc1C#N)-c1cc(c2c(N)ncnn12)C(F)(F)F |r| Show InChI InChI=1S/C21H19F3N6O2/c1-11-9-32-20(2,3)19(31)29(11)15-6-12(4-5-13(15)8-25)16-7-14(21(22,23)24)17-18(26)27-10-28-30(16)17/h4-7,10-11H,9H2,1-3H3,(H2,26,27,28)/t11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) after 60 mins using fluorescein-labeled kinase tracer by HTRF assay |
Bioorg Med Chem Lett 27: 2849-2853 (2017)
Article DOI: 10.1016/j.bmcl.2017.01.077
BindingDB Entry DOI: 10.7270/Q25M684R |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM358632
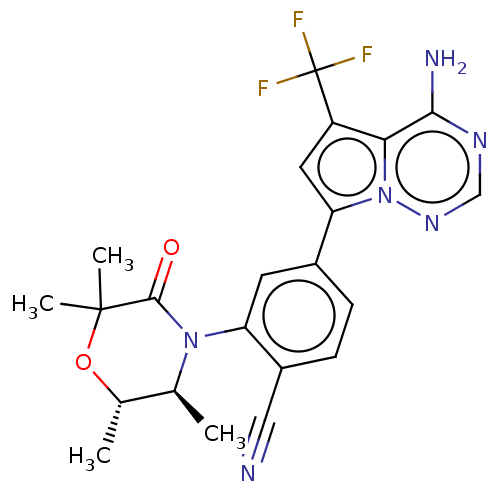
(4-(4-amino-5-(trifluoromethyl)pyrrolo [2,1-f][1,2,...)Show SMILES C[C@@H]1OC(C)(C)C(=O)N([C@H]1C)c1cc(ccc1C#N)-c1cc(c2c(N)ncnn12)C(F)(F)F |r| Show InChI InChI=1S/C22H21F3N6O2/c1-11-12(2)33-21(3,4)20(32)30(11)16-7-13(5-6-14(16)9-26)17-8-15(22(23,24)25)18-19(27)28-10-29-31(17)18/h5-8,10-12H,1-4H3,(H2,27,28,29)/t11-,12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) after 60 mins using fluorescein-labeled kinase tracer by HTRF assay |
Bioorg Med Chem Lett 27: 2849-2853 (2017)
Article DOI: 10.1016/j.bmcl.2017.01.077
BindingDB Entry DOI: 10.7270/Q25M684R |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50245552
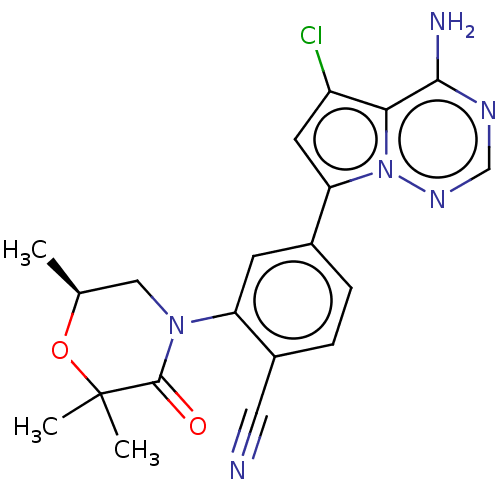
(CHEMBL4105636)Show SMILES C[C@H]1CN(C(=O)C(C)(C)O1)c1cc(ccc1C#N)-c1cc(Cl)c2c(N)ncnn12 |r| Show InChI InChI=1S/C20H19ClN6O2/c1-11-9-26(19(28)20(2,3)29-11)15-6-12(4-5-13(15)8-22)16-7-14(21)17-18(23)24-10-25-27(16)17/h4-7,10-11H,9H2,1-3H3,(H2,23,24,25)/t11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) after 60 mins using fluorescein-labeled kinase tracer by HTRF assay |
Bioorg Med Chem Lett 27: 2849-2853 (2017)
Article DOI: 10.1016/j.bmcl.2017.01.077
BindingDB Entry DOI: 10.7270/Q25M684R |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50245521
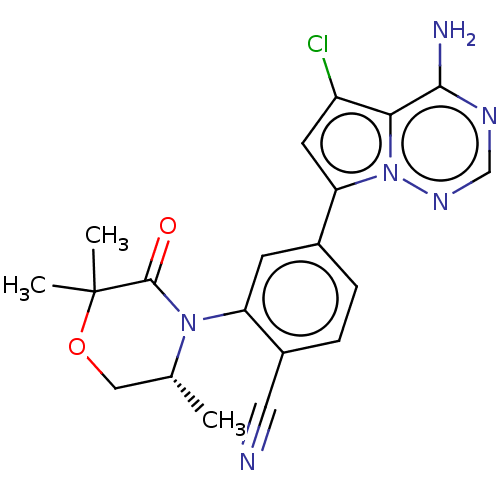
(CHEMBL4065999)Show SMILES C[C@@H]1COC(C)(C)C(=O)N1c1cc(ccc1C#N)-c1cc(Cl)c2c(N)ncnn12 |r| Show InChI InChI=1S/C20H19ClN6O2/c1-11-9-29-20(2,3)19(28)26(11)15-6-12(4-5-13(15)8-22)16-7-14(21)17-18(23)24-10-25-27(16)17/h4-7,10-11H,9H2,1-3H3,(H2,23,24,25)/t11-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) after 60 mins using fluorescein-labeled kinase tracer by HTRF assay |
Bioorg Med Chem Lett 27: 2849-2853 (2017)
Article DOI: 10.1016/j.bmcl.2017.01.077
BindingDB Entry DOI: 10.7270/Q25M684R |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50168472
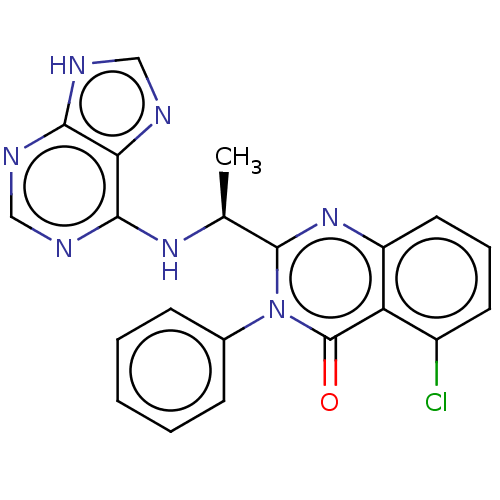
(CHEMBL3805348 | US9765060, Compound X)Show SMILES C[C@H](Nc1ncnc2[nH]cnc12)c1nc2cccc(Cl)c2c(=O)n1-c1ccccc1 |r| Show InChI InChI=1S/C21H16ClN7O/c1-12(27-19-17-18(24-10-23-17)25-11-26-19)20-28-15-9-5-8-14(22)16(15)21(30)29(20)13-6-3-2-4-7-13/h2-12H,1H3,(H2,23,24,25,26,27)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Antisecretory activity evaluated by the inhibition of 14C -AP uptake in isolated rabbit parietal cells stimulated by exogenous histamine |
J Med Chem 60: 5193-5208 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00618
BindingDB Entry DOI: 10.7270/Q2WW7KVJ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50168472

(CHEMBL3805348 | US9765060, Compound X)Show SMILES C[C@H](Nc1ncnc2[nH]cnc12)c1nc2cccc(Cl)c2c(=O)n1-c1ccccc1 |r| Show InChI InChI=1S/C21H16ClN7O/c1-12(27-19-17-18(24-10-23-17)25-11-26-19)20-28-15-9-5-8-14(22)16(15)21(30)29(20)13-6-3-2-4-7-13/h2-12H,1H3,(H2,23,24,25,26,27)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) after 60 mins using fluorescein-labeled kinase tracer by HTRF assay |
Bioorg Med Chem Lett 27: 2849-2853 (2017)
Article DOI: 10.1016/j.bmcl.2017.01.077
BindingDB Entry DOI: 10.7270/Q25M684R |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50245551

(CHEMBL4081613)Show SMILES C[C@@H]1OC(C)(C)C(=O)N([C@@H]1C)c1cc(ccc1C#N)-c1cc(c2c(N)ncnn12)C(F)(F)F |r| Show InChI InChI=1S/C22H21F3N6O2/c1-11-12(2)33-21(3,4)20(32)30(11)16-7-13(5-6-14(16)9-26)17-8-15(22(23,24)25)18-19(27)28-10-29-31(17)18/h5-8,10-12H,1-4H3,(H2,27,28,29)/t11-,12+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) after 60 mins using fluorescein-labeled kinase tracer by HTRF assay |
Bioorg Med Chem Lett 27: 2849-2853 (2017)
Article DOI: 10.1016/j.bmcl.2017.01.077
BindingDB Entry DOI: 10.7270/Q25M684R |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM165318

(US10604504, Example 114 | US11623921, Example 114 ...)Show SMILES Cc1[nH]c2c(ccc(NC3CCN(CC3)C(=O)C#C)c2c1C)C(N)=O Show InChI InChI=1S/C19H22N4O2/c1-4-16(24)23-9-7-13(8-10-23)22-15-6-5-14(19(20)25)18-17(15)11(2)12(3)21-18/h1,5-6,13,21-22H,7-10H2,2-3H3,(H2,20,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.850 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human BTK using fluoresceinated peptide as substrate after 60 mins fluorescence assay |
J Med Chem 62: 3228-3250 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00167
BindingDB Entry DOI: 10.7270/Q2ZK5M66 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Tec
(Homo sapiens (Human)) | BDBM164638

(BDBM166759 | US10604504, Example 223 | US11623921,...)Show SMILES CC#CC(=O)N[C@H]1CCCN(C1)c1c(F)cc(C(N)=O)c2[nH]c(C)c(C)c12 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of TEC (unknown origin) |
J Med Chem 62: 3228-3250 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00167
BindingDB Entry DOI: 10.7270/Q2ZK5M66 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50245542
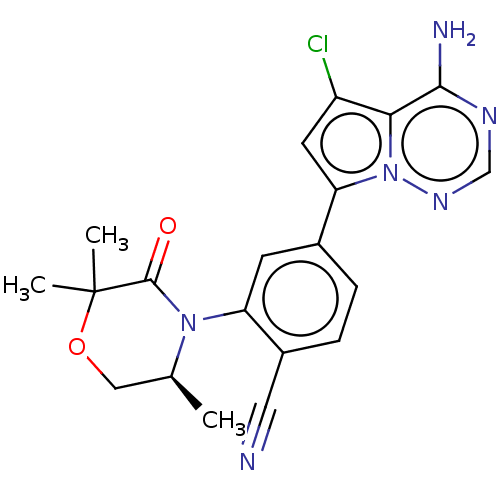
(CHEMBL4077544)Show SMILES C[C@H]1COC(C)(C)C(=O)N1c1cc(ccc1C#N)-c1cc(Cl)c2c(N)ncnn12 |r| Show InChI InChI=1S/C20H19ClN6O2/c1-11-9-29-20(2,3)19(28)26(11)15-6-12(4-5-13(15)8-22)16-7-14(21)17-18(23)24-10-25-27(16)17/h4-7,10-11H,9H2,1-3H3,(H2,23,24,25)/t11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) after 60 mins using fluorescein-labeled kinase tracer by HTRF assay |
Bioorg Med Chem Lett 27: 2849-2853 (2017)
Article DOI: 10.1016/j.bmcl.2017.01.077
BindingDB Entry DOI: 10.7270/Q25M684R |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM164638

(BDBM166759 | US10604504, Example 223 | US11623921,...)Show SMILES CC#CC(=O)N[C@H]1CCCN(C1)c1c(F)cc(C(N)=O)c2[nH]c(C)c(C)c12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of BTK in human memory B cells assessed as reduction in CD86 surface expression |
J Med Chem 62: 3228-3250 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00167
BindingDB Entry DOI: 10.7270/Q2ZK5M66 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50544198
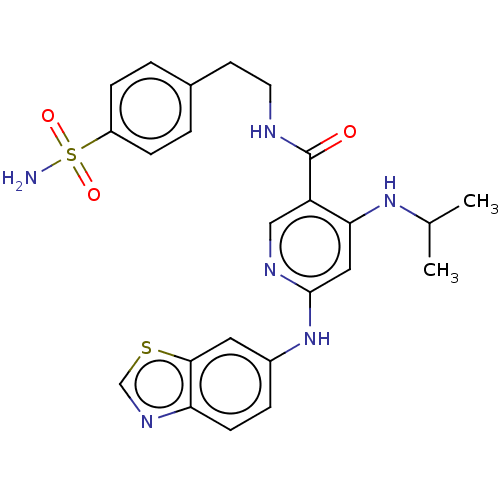
(CHEMBL4636136)Show SMILES CC(C)Nc1cc(Nc2ccc3ncsc3c2)ncc1C(=O)NCCc1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C24H26N6O3S2/c1-15(2)29-21-12-23(30-17-5-8-20-22(11-17)34-14-28-20)27-13-19(21)24(31)26-10-9-16-3-6-18(7-4-16)35(25,32)33/h3-8,11-15H,9-10H2,1-2H3,(H,26,31)(H2,25,32,33)(H2,27,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Biocon Bristol Myers Squibb Research Center
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
ACS Med Chem Lett 11: 1402-1409 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00082
BindingDB Entry DOI: 10.7270/Q2542S4M |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM358576
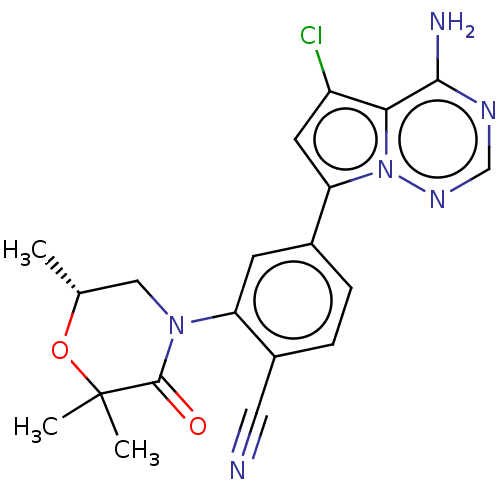
((R)-4-(4-amino-5-chloropyrrolo[2,1-f] [1,2,4]triaz...)Show SMILES C[C@@H]1CN(C(=O)C(C)(C)O1)c1cc(ccc1C#N)-c1cc(Cl)c2c(N)ncnn12 |r| Show InChI InChI=1S/C20H19ClN6O2/c1-11-9-26(19(28)20(2,3)29-11)15-6-12(4-5-13(15)8-22)16-7-14(21)17-18(23)24-10-25-27(16)17/h4-7,10-11H,9H2,1-3H3,(H2,23,24,25)/t11-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) after 60 mins using fluorescein-labeled kinase tracer by HTRF assay |
Bioorg Med Chem Lett 27: 2849-2853 (2017)
Article DOI: 10.1016/j.bmcl.2017.01.077
BindingDB Entry DOI: 10.7270/Q25M684R |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50245545
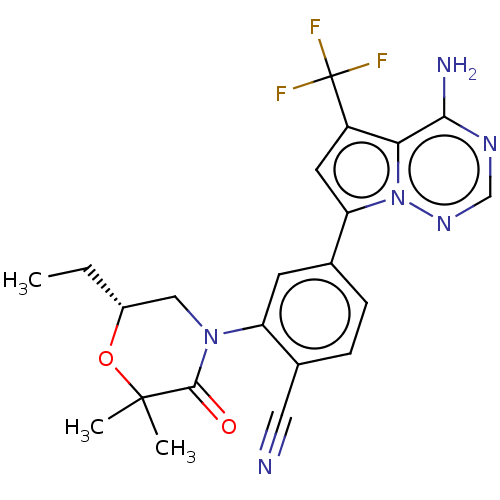
(CHEMBL4076745)Show SMILES CC[C@@H]1CN(C(=O)C(C)(C)O1)c1cc(ccc1C#N)-c1cc(c2c(N)ncnn12)C(F)(F)F |r| Show InChI InChI=1S/C22H21F3N6O2/c1-4-14-10-30(20(32)21(2,3)33-14)16-7-12(5-6-13(16)9-26)17-8-15(22(23,24)25)18-19(27)28-11-29-31(17)18/h5-8,11,14H,4,10H2,1-3H3,(H2,27,28,29)/t14-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) after 60 mins using fluorescein-labeled kinase tracer by HTRF assay |
Bioorg Med Chem Lett 27: 2849-2853 (2017)
Article DOI: 10.1016/j.bmcl.2017.01.077
BindingDB Entry DOI: 10.7270/Q25M684R |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50239736
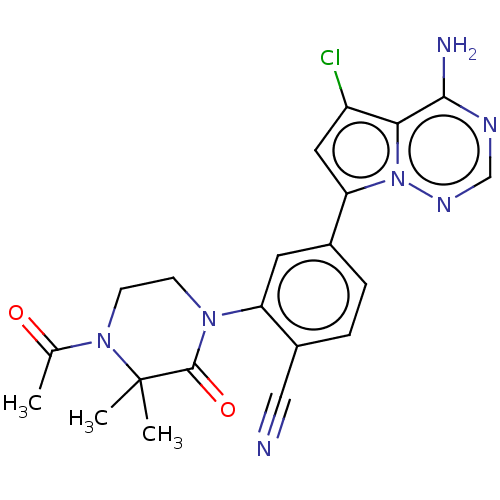
(CHEMBL4074315 | US10214537, Example 637)Show SMILES CC(=O)N1CCN(C(=O)C1(C)C)c1cc(ccc1C#N)-c1cc(Cl)c2c(N)ncnn12 Show InChI InChI=1S/C21H20ClN7O2/c1-12(30)28-7-6-27(20(31)21(28,2)3)16-8-13(4-5-14(16)10-23)17-9-15(22)18-19(24)25-11-26-29(17)18/h4-5,8-9,11H,6-7H2,1-3H3,(H2,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Antisecretory activity evaluated by the inhibition of 14C -AP uptake in isolated rabbit parietal cells stimulated by exogenous histamine |
J Med Chem 60: 5193-5208 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00618
BindingDB Entry DOI: 10.7270/Q2WW7KVJ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50245585
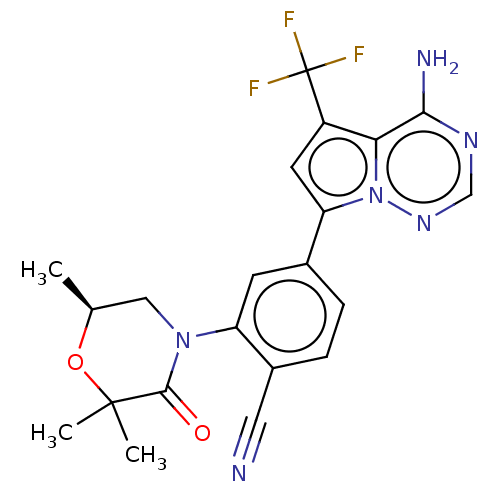
(CHEMBL4072098)Show SMILES C[C@H]1CN(C(=O)C(C)(C)O1)c1cc(ccc1C#N)-c1cc(c2c(N)ncnn12)C(F)(F)F |r| Show InChI InChI=1S/C21H19F3N6O2/c1-11-9-29(19(31)20(2,3)32-11)15-6-12(4-5-13(15)8-25)16-7-14(21(22,23)24)17-18(26)27-10-28-30(16)17/h4-7,10-11H,9H2,1-3H3,(H2,26,27,28)/t11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) after 60 mins using fluorescein-labeled kinase tracer by HTRF assay |
Bioorg Med Chem Lett 27: 2849-2853 (2017)
Article DOI: 10.1016/j.bmcl.2017.01.077
BindingDB Entry DOI: 10.7270/Q25M684R |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50245581
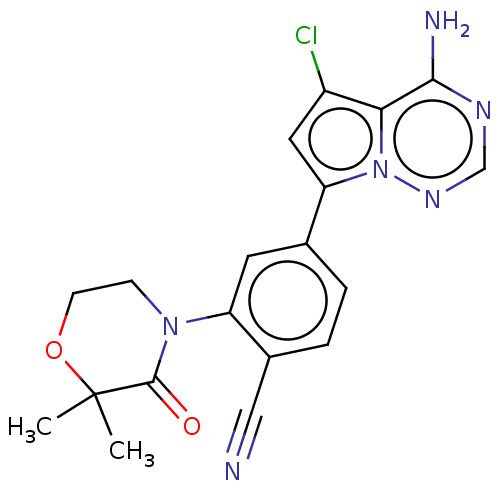
(CHEMBL4092654)Show SMILES CC1(C)OCCN(C1=O)c1cc(ccc1C#N)-c1cc(Cl)c2c(N)ncnn12 Show InChI InChI=1S/C19H17ClN6O2/c1-19(2)18(27)25(5-6-28-19)14-7-11(3-4-12(14)9-21)15-8-13(20)16-17(22)23-10-24-26(15)16/h3-4,7-8,10H,5-6H2,1-2H3,(H2,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) after 60 mins using fluorescein-labeled kinase tracer by HTRF assay |
Bioorg Med Chem Lett 27: 2849-2853 (2017)
Article DOI: 10.1016/j.bmcl.2017.01.077
BindingDB Entry DOI: 10.7270/Q25M684R |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50239744
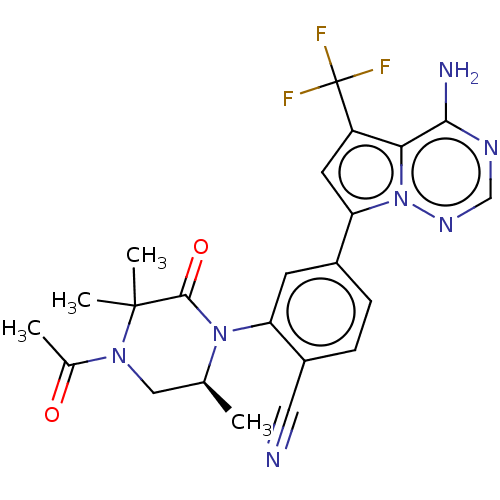
(CHEMBL4071965 | US10214537, Example 643)Show SMILES C[C@H]1CN(C(C)=O)C(C)(C)C(=O)N1c1cc(ccc1C#N)-c1cc(c2c(N)ncnn12)C(F)(F)F |r| Show InChI InChI=1S/C23H22F3N7O2/c1-12-10-31(13(2)34)22(3,4)21(35)32(12)17-7-14(5-6-15(17)9-27)18-8-16(23(24,25)26)19-20(28)29-11-30-33(18)19/h5-8,11-12H,10H2,1-4H3,(H2,28,29,30)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Antisecretory activity evaluated by the inhibition of 14C -AP uptake in isolated rabbit parietal cells stimulated by dibutyryl cyclic adenosine 3', 5... |
J Med Chem 60: 5193-5208 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00618
BindingDB Entry DOI: 10.7270/Q2WW7KVJ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50239752
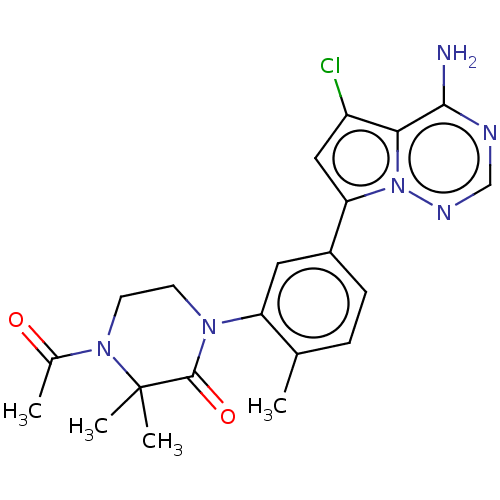
(CHEMBL4067315 | US10214537, Example 585)Show SMILES CC(=O)N1CCN(C(=O)C1(C)C)c1cc(ccc1C)-c1cc(Cl)c2c(N)ncnn12 Show InChI InChI=1S/C21H23ClN6O2/c1-12-5-6-14(17-10-15(22)18-19(23)24-11-25-28(17)18)9-16(12)26-7-8-27(13(2)29)21(3,4)20(26)30/h5-6,9-11H,7-8H2,1-4H3,(H2,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Antisecretory activity evaluated by the inhibition of 14C -AP uptake in isolated rabbit parietal cells stimulated by exogenous histamine |
J Med Chem 60: 5193-5208 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00618
BindingDB Entry DOI: 10.7270/Q2WW7KVJ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM165465
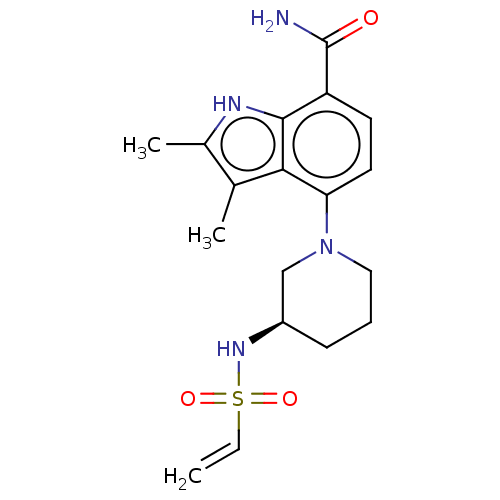
(US10604504, Example 143 | US11623921, Example 143 ...)Show SMILES Cc1[nH]c2c(ccc(N3CCC[C@H](C3)NS(=O)(=O)C=C)c2c1C)C(N)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human BTK using fluoresceinated peptide as substrate after 60 mins fluorescence assay |
J Med Chem 62: 3228-3250 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00167
BindingDB Entry DOI: 10.7270/Q2ZK5M66 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50239741
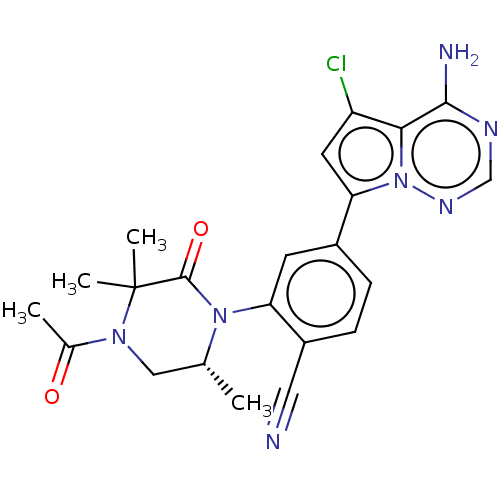
(CHEMBL4095752)Show SMILES C[C@@H]1CN(C(C)=O)C(C)(C)C(=O)N1c1cc(ccc1C#N)-c1cc(Cl)c2c(N)ncnn12 |r| Show InChI InChI=1S/C22H22ClN7O2/c1-12-10-28(13(2)31)22(3,4)21(32)29(12)17-7-14(5-6-15(17)9-24)18-8-16(23)19-20(25)26-11-27-30(18)19/h5-8,11-12H,10H2,1-4H3,(H2,25,26,27)/t12-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Antisecretory activity evaluated by the inhibition of 14C -AP uptake in isolated rabbit parietal cells stimulated by dibutyryl cyclic adenosine 3', 5... |
J Med Chem 60: 5193-5208 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00618
BindingDB Entry DOI: 10.7270/Q2WW7KVJ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50239723
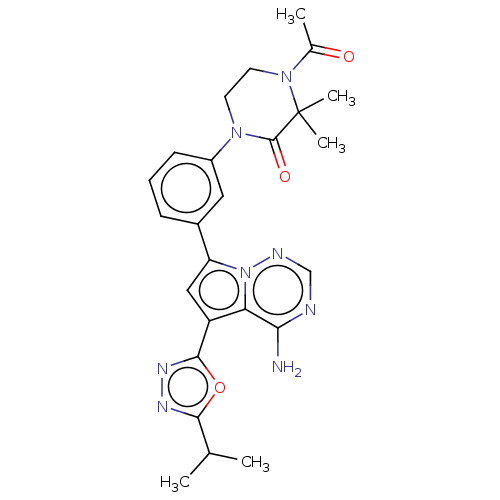
(CHEMBL4074193 | US10214537, Example 478)Show SMILES CC(C)c1nnc(o1)-c1cc(-c2cccc(c2)N2CCN(C(C)=O)C(C)(C)C2=O)n2ncnc(N)c12 Show InChI InChI=1S/C25H28N8O3/c1-14(2)22-29-30-23(36-22)18-12-19(33-20(18)21(26)27-13-28-33)16-7-6-8-17(11-16)31-9-10-32(15(3)34)25(4,5)24(31)35/h6-8,11-14H,9-10H2,1-5H3,(H2,26,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Antisecretory activity evaluated by the inhibition of 14C -AP uptake in isolated rabbit parietal cells stimulated by exogenous histamine |
J Med Chem 60: 5193-5208 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00618
BindingDB Entry DOI: 10.7270/Q2WW7KVJ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM165321
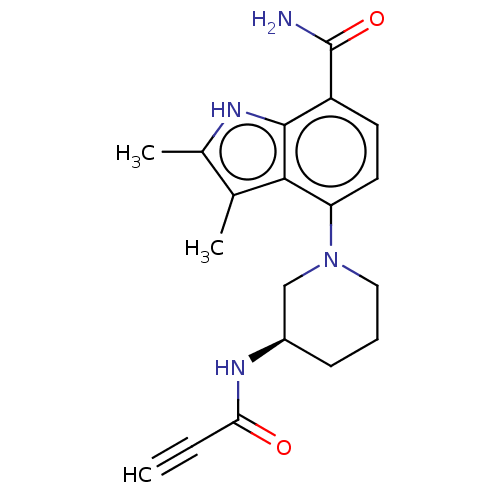
(US10604504, Example 117 | US11623921, Example 117 ...)Show SMILES Cc1[nH]c2c(ccc(N3CCC[C@H](C3)NC(=O)C#C)c2c1C)C(N)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human BTK using fluoresceinated peptide as substrate after 60 mins fluorescence assay |
J Med Chem 62: 3228-3250 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00167
BindingDB Entry DOI: 10.7270/Q2ZK5M66 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM165461
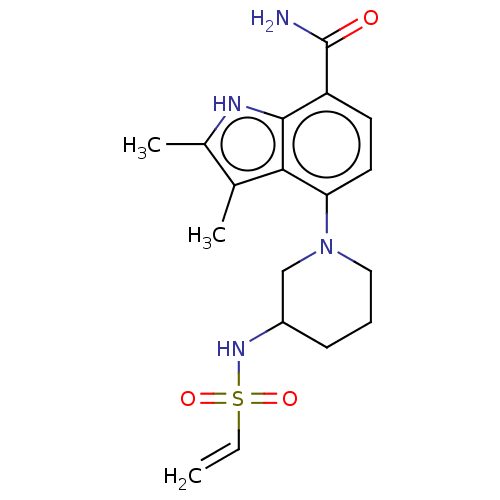
(US10604504, Example 139 | US11623921, Example 139 ...)Show SMILES Cc1[nH]c2c(ccc(N3CCCC(C3)NS(=O)(=O)C=C)c2c1C)C(N)=O | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human BTK using fluoresceinated peptide as substrate after 60 mins fluorescence assay |
J Med Chem 62: 3228-3250 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00167
BindingDB Entry DOI: 10.7270/Q2ZK5M66 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50245543
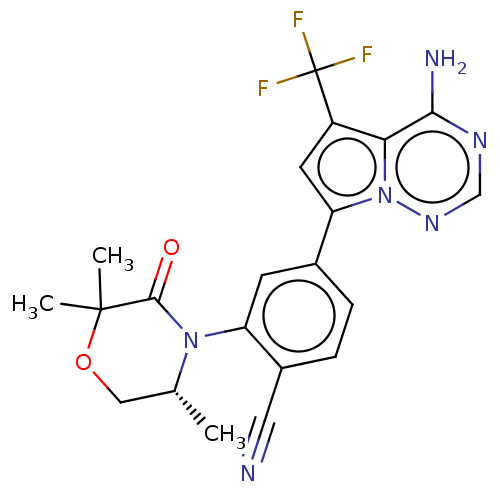
(CHEMBL4084394)Show SMILES C[C@@H]1COC(C)(C)C(=O)N1c1cc(ccc1C#N)-c1cc(c2c(N)ncnn12)C(F)(F)F |r| Show InChI InChI=1S/C21H19F3N6O2/c1-11-9-32-20(2,3)19(31)29(11)15-6-12(4-5-13(15)8-25)16-7-14(21(22,23)24)17-18(26)27-10-28-30(16)17/h4-7,10-11H,9H2,1-3H3,(H2,26,27,28)/t11-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) after 60 mins using fluorescein-labeled kinase tracer by HTRF assay |
Bioorg Med Chem Lett 27: 2849-2853 (2017)
Article DOI: 10.1016/j.bmcl.2017.01.077
BindingDB Entry DOI: 10.7270/Q25M684R |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50239746
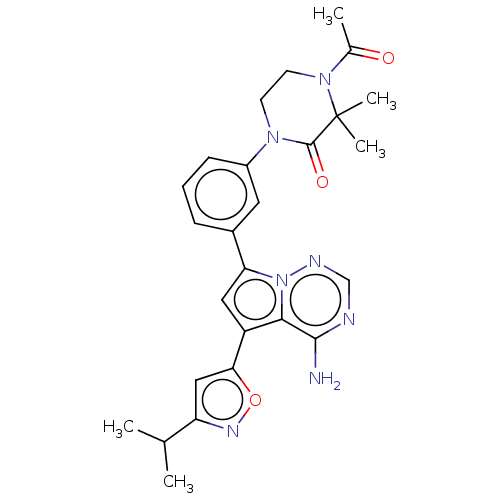
(CHEMBL4094693)Show SMILES CC(C)c1cc(on1)-c1cc(-c2cccc(c2)N2CCN(C(C)=O)C(C)(C)C2=O)n2ncnc(N)c12 Show InChI InChI=1S/C26H29N7O3/c1-15(2)20-13-22(36-30-20)19-12-21(33-23(19)24(27)28-14-29-33)17-7-6-8-18(11-17)31-9-10-32(16(3)34)26(4,5)25(31)35/h6-8,11-15H,9-10H2,1-5H3,(H2,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Antisecretory activity evaluated by the inhibition of 14C -AP uptake in isolated rabbit parietal cells stimulated by exogenous histamine |
J Med Chem 60: 5193-5208 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00618
BindingDB Entry DOI: 10.7270/Q2WW7KVJ |
More data for this
Ligand-Target Pair | |
Cytoplasmic tyrosine-protein kinase BMX
(Homo sapiens (Human)) | BDBM164638

(BDBM166759 | US10604504, Example 223 | US11623921,...)Show SMILES CC#CC(=O)N[C@H]1CCCN(C1)c1c(F)cc(C(N)=O)c2[nH]c(C)c(C)c12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of BMX (unknown origin) |
J Med Chem 62: 3228-3250 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00167
BindingDB Entry DOI: 10.7270/Q2ZK5M66 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50239734
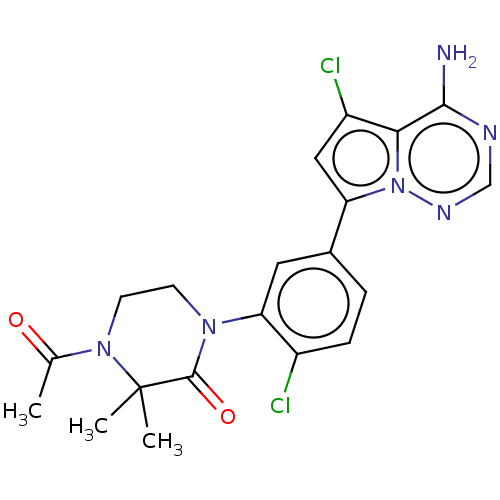
(CHEMBL4097222 | US10214537, Example 628)Show SMILES CC(=O)N1CCN(C(=O)C1(C)C)c1cc(ccc1Cl)-c1cc(Cl)c2c(N)ncnn12 Show InChI InChI=1S/C20H20Cl2N6O2/c1-11(29)27-7-6-26(19(30)20(27,2)3)16-8-12(4-5-13(16)21)15-9-14(22)17-18(23)24-10-25-28(15)17/h4-5,8-10H,6-7H2,1-3H3,(H2,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Antisecretory activity evaluated by the inhibition of 14C -AP uptake in isolated rabbit parietal cells stimulated by dibutyryl cyclic adenosine 3', 5... |
J Med Chem 60: 5193-5208 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00618
BindingDB Entry DOI: 10.7270/Q2WW7KVJ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data