

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50039635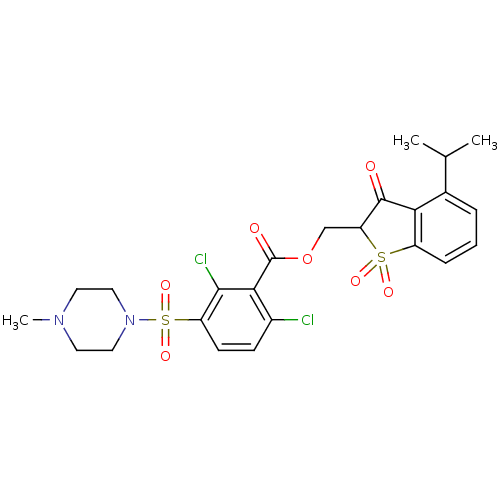 (2,6-Dichloro-3-(4-methyl-piperazine-1-sulfonyl)-be...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.00700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceutical Research Division Curated by ChEMBL | Assay Description In vitro inhibition constant of human leukocyte elastase. | J Med Chem 37: 2623-6 (1994) BindingDB Entry DOI: 10.7270/Q2ST7QGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50039646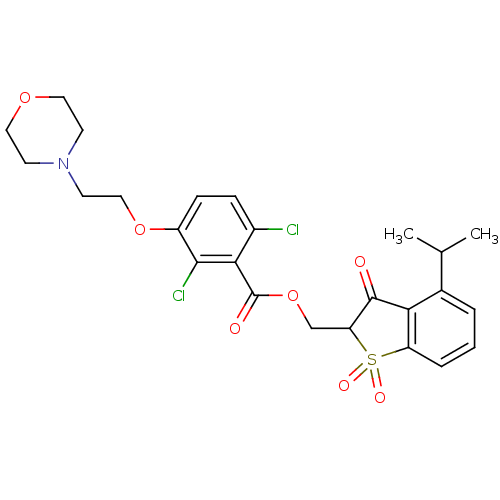 (2,6-Dichloro-3-(2-morpholin-4-yl-ethoxy)-benzoic a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceutical Research Division Curated by ChEMBL | Assay Description In vitro inhibition constant of human leukocyte elastase. | J Med Chem 37: 2623-6 (1994) BindingDB Entry DOI: 10.7270/Q2ST7QGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50039637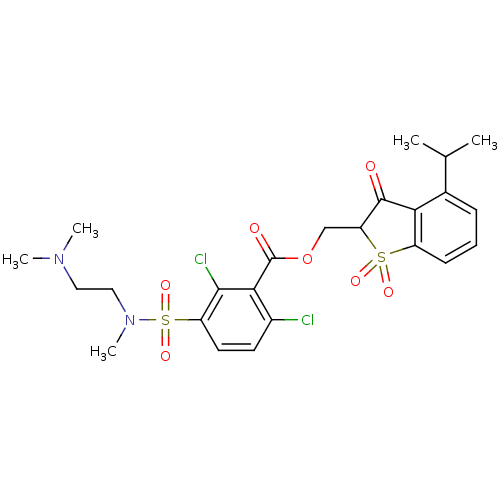 (2,6-Dichloro-3-[(2-dimethylamino-ethyl)-methyl-sul...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceutical Research Division Curated by ChEMBL | Assay Description In vitro inhibition constant of human leukocyte elastase. | J Med Chem 37: 2623-6 (1994) BindingDB Entry DOI: 10.7270/Q2ST7QGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50039631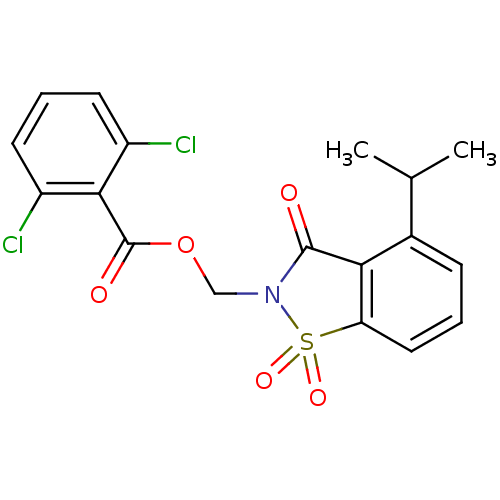 (2,6-Dichloro-benzoic acid 4-isopropyl-1,1,3-trioxo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceutical Research Division Curated by ChEMBL | Assay Description In vitro inhibition constant of human leukocyte elastase. | J Med Chem 37: 2623-6 (1994) BindingDB Entry DOI: 10.7270/Q2ST7QGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50039641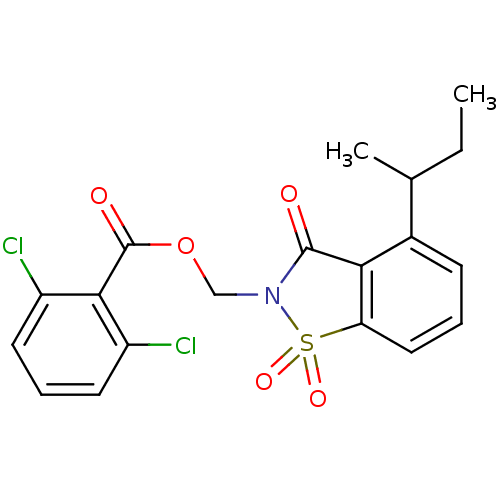 (2,6-Dichloro-benzoic acid 4-sec-butyl-1,1,3-trioxo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceutical Research Division Curated by ChEMBL | Assay Description In vitro inhibition constant of human leukocyte elastase. | J Med Chem 37: 2623-6 (1994) BindingDB Entry DOI: 10.7270/Q2ST7QGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50039642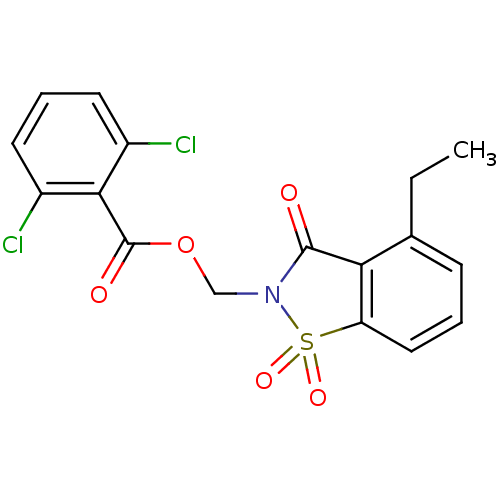 (2,6-Dichloro-benzoic acid 4-ethyl-1,1,3-trioxo-1,3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceutical Research Division Curated by ChEMBL | Assay Description In vitro inhibition constant of human leukocyte elastase. | J Med Chem 37: 2623-6 (1994) BindingDB Entry DOI: 10.7270/Q2ST7QGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50039634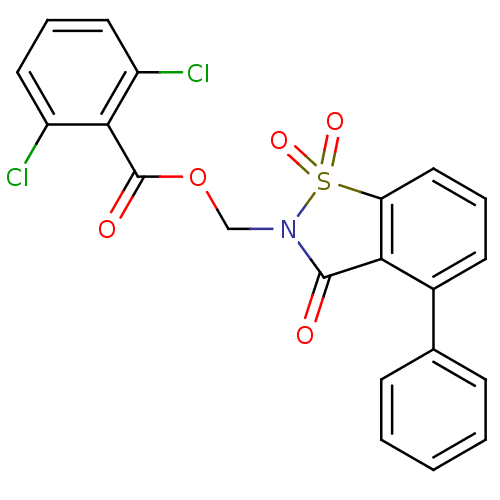 (2,6-Dichloro-benzoic acid 1,1,3-trioxo-4-phenyl-1,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceutical Research Division Curated by ChEMBL | Assay Description In vitro inhibition constant of human leukocyte elastase. | J Med Chem 37: 2623-6 (1994) BindingDB Entry DOI: 10.7270/Q2ST7QGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50039644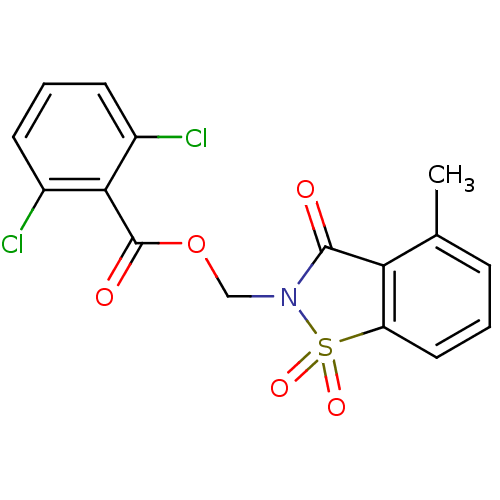 (2,6-Dichloro-benzoic acid 4-methyl-1,1,3-trioxo-1,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceutical Research Division Curated by ChEMBL | Assay Description In vitro inhibition constant of human leukocyte elastase. | J Med Chem 37: 2623-6 (1994) BindingDB Entry DOI: 10.7270/Q2ST7QGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50039636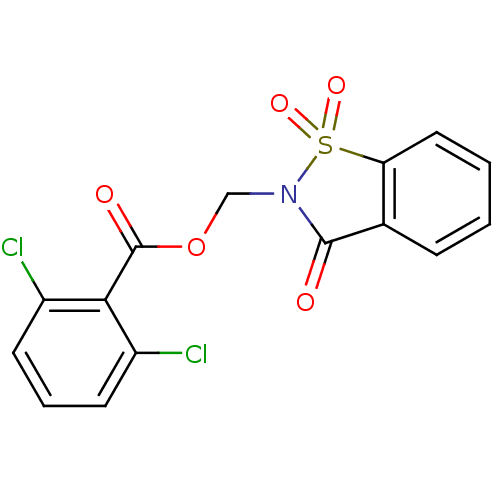 (2,6-Dichloro-benzoic acid 1,1,3-trioxo-1,3-dihydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceutical Research Division Curated by ChEMBL | Assay Description In vitro inhibition constant of human leukocyte elastase. | J Med Chem 37: 2623-6 (1994) BindingDB Entry DOI: 10.7270/Q2ST7QGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50039640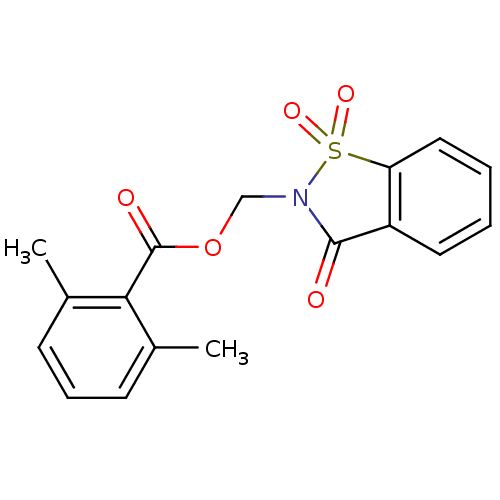 (2,6-Dimethyl-benzoic acid 1,1,3-trioxo-1,3-dihydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceutical Research Division Curated by ChEMBL | Assay Description In vitro inhibition constant of human leukocyte elastase. | J Med Chem 37: 2623-6 (1994) BindingDB Entry DOI: 10.7270/Q2ST7QGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50039638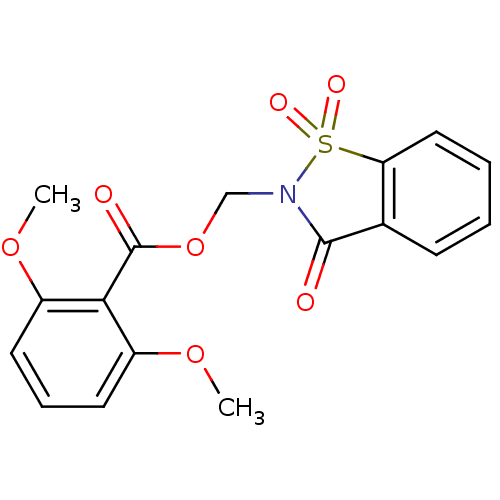 (2,6-Dimethoxy-benzoic acid 1,1,3-trioxo-1,3-dihydr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceutical Research Division Curated by ChEMBL | Assay Description In vitro inhibition constant of human leukocyte elastase. | J Med Chem 37: 2623-6 (1994) BindingDB Entry DOI: 10.7270/Q2ST7QGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50039639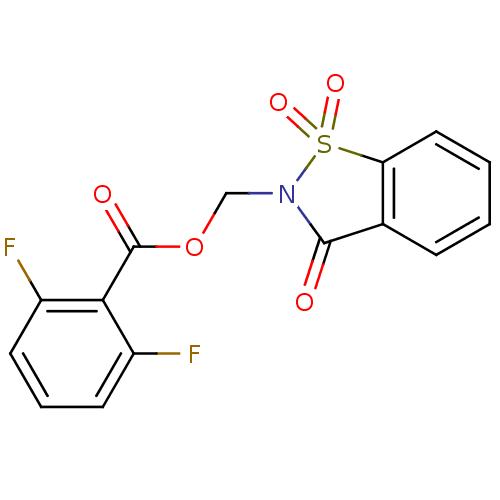 (2,6-Difluoro-benzoic acid 1,1,3-trioxo-1,3-dihydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceutical Research Division Curated by ChEMBL | Assay Description In vitro inhibition constant of human leukocyte elastase. | J Med Chem 37: 2623-6 (1994) BindingDB Entry DOI: 10.7270/Q2ST7QGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50039632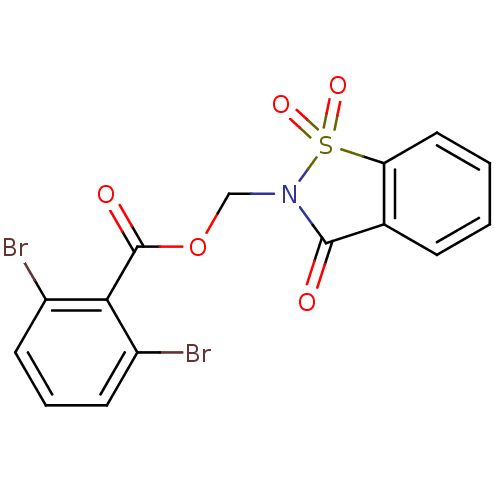 (2,6-Dibromo-benzoic acid 1,1,3-trioxo-1,3-dihydro-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceutical Research Division Curated by ChEMBL | Assay Description In vitro inhibition constant of human leukocyte elastase. | J Med Chem 37: 2623-6 (1994) BindingDB Entry DOI: 10.7270/Q2ST7QGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50039633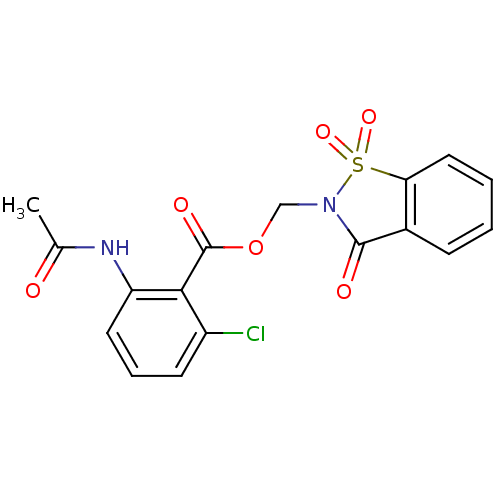 (2-Acetylamino-6-chloro-benzoic acid 1,1,3-trioxo-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceutical Research Division Curated by ChEMBL | Assay Description In vitro inhibition constant of human leukocyte elastase. | J Med Chem 37: 2623-6 (1994) BindingDB Entry DOI: 10.7270/Q2ST7QGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50039647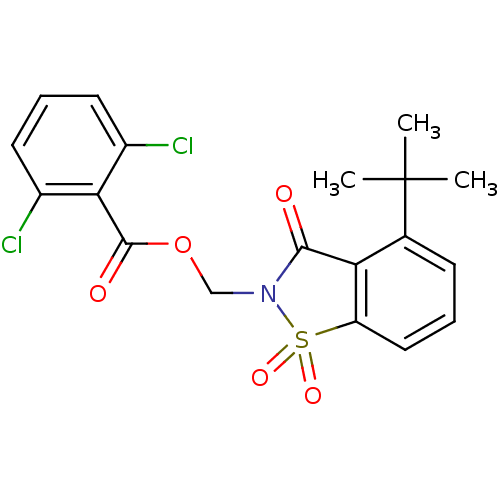 (2,6-Dichloro-benzoic acid 4-tert-butyl-1,1,3-triox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceutical Research Division Curated by ChEMBL | Assay Description In vitro inhibition constant of human leukocyte elastase. | J Med Chem 37: 2623-6 (1994) BindingDB Entry DOI: 10.7270/Q2ST7QGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50039643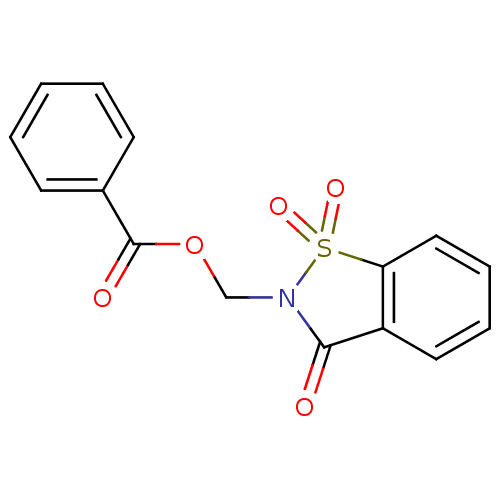 (Benzoic acid 1,1,3-trioxo-1,3-dihydro-1lambda*6*-b...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceutical Research Division Curated by ChEMBL | Assay Description In vitro inhibition constant of human leukocyte elastase. | J Med Chem 37: 2623-6 (1994) BindingDB Entry DOI: 10.7270/Q2ST7QGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50039645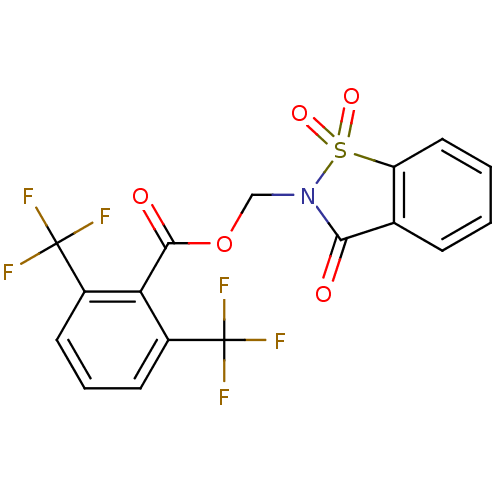 (2,6-Bis-trifluoromethyl-benzoic acid 1,1,3-trioxo-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceutical Research Division Curated by ChEMBL | Assay Description In vitro inhibition constant of human leukocyte elastase. | J Med Chem 37: 2623-6 (1994) BindingDB Entry DOI: 10.7270/Q2ST7QGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50039648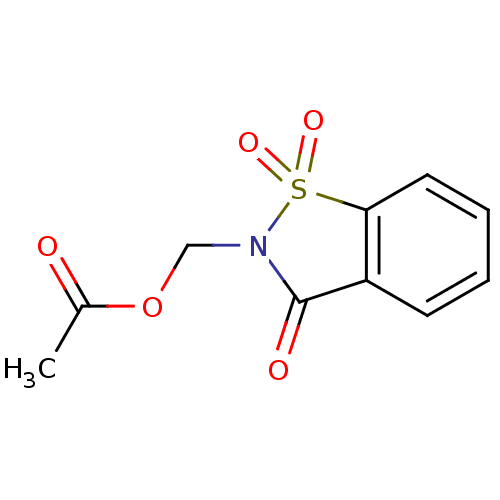 (Acetic acid 1,1,3-trioxo-1,3-dihydro-1lambda*6*-be...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 102 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceutical Research Division Curated by ChEMBL | Assay Description In vitro inhibition constant of human leukocyte elastase. | J Med Chem 37: 2623-6 (1994) BindingDB Entry DOI: 10.7270/Q2ST7QGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50443545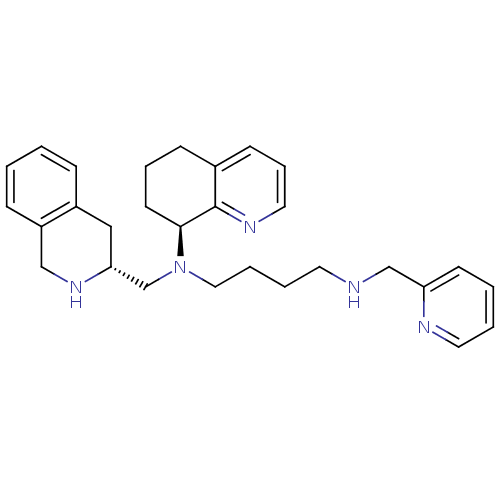 (CHEMBL3091683) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Antagonist activity at CXCR4 in human Chem-1 cells assessed as inhibition of SDF-1alpha-mediated calcium flux preincubated for 10 mins by FLIPR assay | ACS Med Chem Lett 4: 1025-30 (2013) Article DOI: 10.1021/ml400183q BindingDB Entry DOI: 10.7270/Q2ZS2Z0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50443541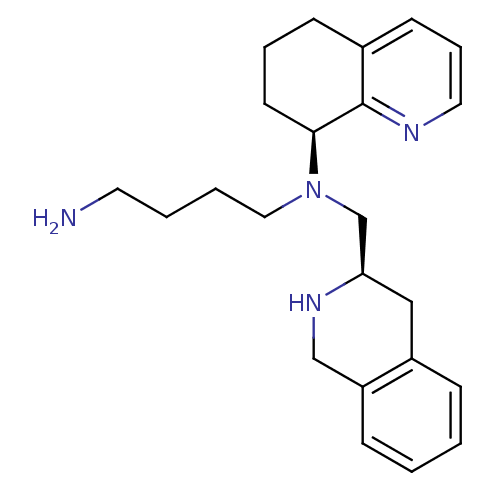 (CHEMBL3091687) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Antagonist activity at CXCR4 in human Chem-1 cells assessed as inhibition of SDF-1alpha-mediated calcium flux preincubated for 10 mins by FLIPR assay | ACS Med Chem Lett 4: 1025-30 (2013) Article DOI: 10.1021/ml400183q BindingDB Entry DOI: 10.7270/Q2ZS2Z0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50443543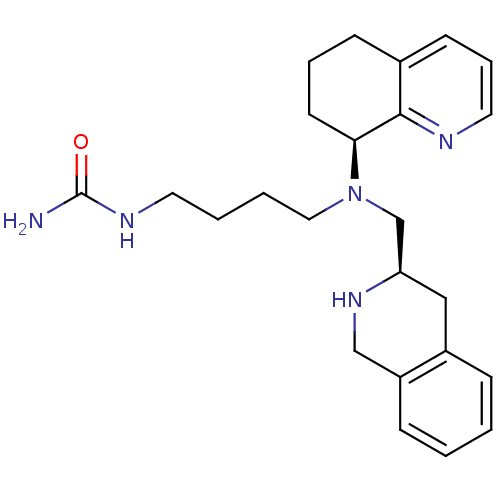 (CHEMBL3091685) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Antagonist activity at CXCR4 in human Chem-1 cells assessed as inhibition of SDF-1alpha-mediated calcium flux preincubated for 10 mins by FLIPR assay | ACS Med Chem Lett 4: 1025-30 (2013) Article DOI: 10.1021/ml400183q BindingDB Entry DOI: 10.7270/Q2ZS2Z0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50270038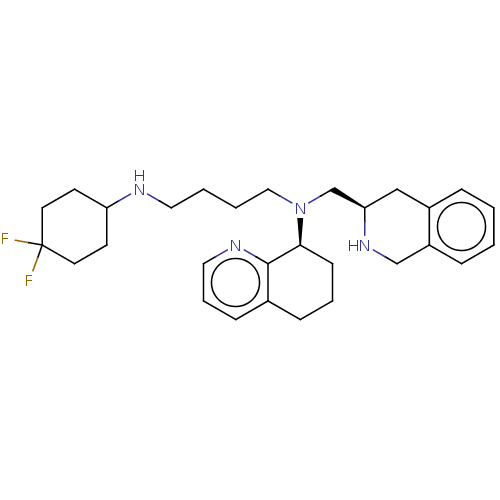 (CHEMBL4062223) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Antagonist activity at human CXCR4 in CCRF-CEM cells assessed as decrease in SDF-1alpha stimulated Ca2+ flux preincubated for 25 mins followed by SDF... | J Med Chem 61: 946-979 (2018) Article DOI: 10.1021/acs.jmedchem.7b01420 BindingDB Entry DOI: 10.7270/Q2KH0QTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50443544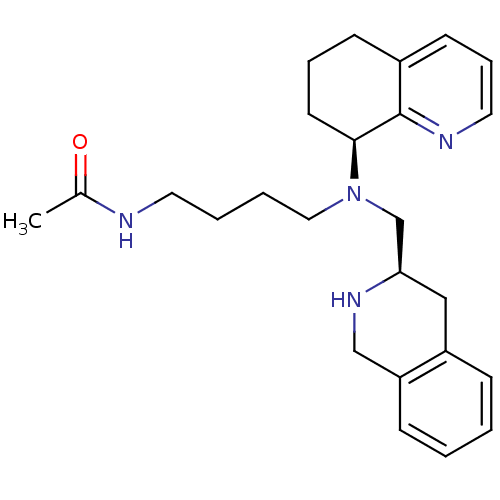 (CHEMBL3091684) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Antagonist activity at CXCR4 in human Chem-1 cells assessed as inhibition of SDF-1alpha-mediated calcium flux preincubated for 10 mins by FLIPR assay | ACS Med Chem Lett 4: 1025-30 (2013) Article DOI: 10.1021/ml400183q BindingDB Entry DOI: 10.7270/Q2ZS2Z0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50443541 (CHEMBL3091687) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Antagonist activity at CXCR4 in human MAGI-CCR5 cells assessed as inhibition of HIV-1 3B entry after 2 to 6 days by beta-galactosidase reporter gene ... | ACS Med Chem Lett 4: 1025-30 (2013) Article DOI: 10.1021/ml400183q BindingDB Entry DOI: 10.7270/Q2ZS2Z0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Mus musculus) | BDBM50270016 (CHEMBL4070320) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Antagonist activity at mouse CXCR4 | J Med Chem 61: 946-979 (2018) Article DOI: 10.1021/acs.jmedchem.7b01420 BindingDB Entry DOI: 10.7270/Q2KH0QTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50443541 (CHEMBL3091687) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Antagonist activity at human CXCR4 in CCRF-CEM cells assessed as decrease in SDF-1alpha stimulated Ca2+ flux preincubated for 25 mins followed by SDF... | J Med Chem 61: 946-979 (2018) Article DOI: 10.1021/acs.jmedchem.7b01420 BindingDB Entry DOI: 10.7270/Q2KH0QTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50443546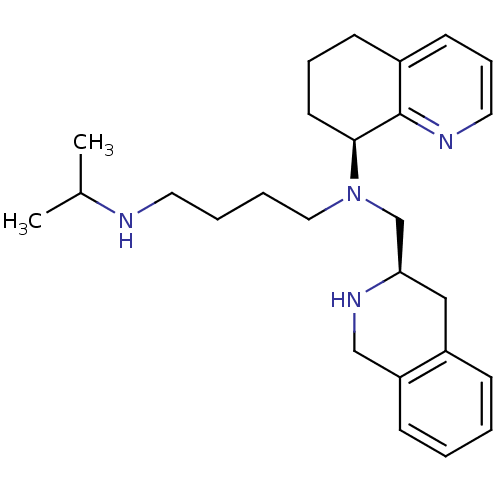 (CHEMBL3091682) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Antagonist activity at CXCR4 in human Chem-1 cells assessed as inhibition of SDF-1alpha-mediated calcium flux preincubated for 10 mins by FLIPR assay | ACS Med Chem Lett 4: 1025-30 (2013) Article DOI: 10.1021/ml400183q BindingDB Entry DOI: 10.7270/Q2ZS2Z0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50443547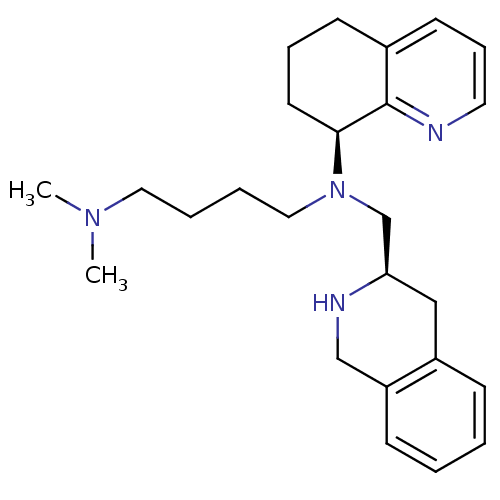 (CHEMBL3091681) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Antagonist activity at CXCR4 in human Chem-1 cells assessed as inhibition of SDF-1alpha-mediated calcium flux preincubated for 10 mins by FLIPR assay | ACS Med Chem Lett 4: 1025-30 (2013) Article DOI: 10.1021/ml400183q BindingDB Entry DOI: 10.7270/Q2ZS2Z0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Mus musculus) | BDBM50270018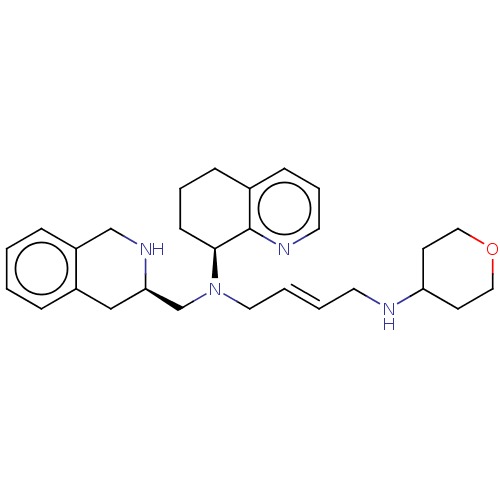 (CHEMBL4075205) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Antagonist activity at mouse CXCR4 | J Med Chem 61: 946-979 (2018) Article DOI: 10.1021/acs.jmedchem.7b01420 BindingDB Entry DOI: 10.7270/Q2KH0QTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50270049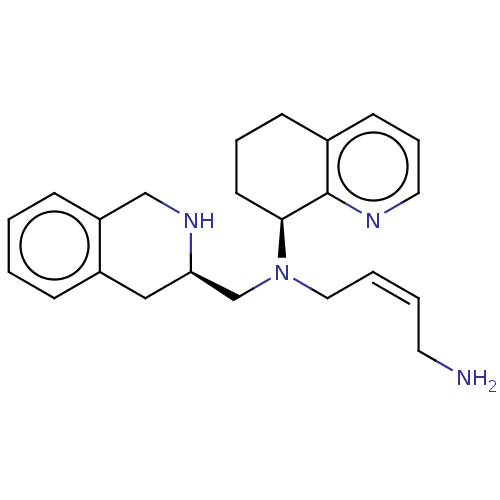 (CHEMBL4096305) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Antagonist activity at human CXCR4 in CCRF-CEM cells assessed as decrease in SDF-1alpha stimulated Ca2+ flux preincubated for 25 mins followed by SDF... | J Med Chem 61: 946-979 (2018) Article DOI: 10.1021/acs.jmedchem.7b01420 BindingDB Entry DOI: 10.7270/Q2KH0QTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50443541 (CHEMBL3091687) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Antagonist activity at CXCR4 (unknown origin) assessed as inhibition of SDF-1-induced beta-arrestin recruitment incubated for 30 mins prior to SDF-1 ... | ACS Med Chem Lett 4: 1025-30 (2013) Article DOI: 10.1021/ml400183q BindingDB Entry DOI: 10.7270/Q2ZS2Z0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50270028 (CHEMBL4071612) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Antagonist activity at human CXCR4 in CCRF-CEM cells assessed as decrease in SDF-1alpha stimulated Ca2+ flux preincubated for 25 mins followed by SDF... | J Med Chem 61: 946-979 (2018) Article DOI: 10.1021/acs.jmedchem.7b01420 BindingDB Entry DOI: 10.7270/Q2KH0QTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50270018 (CHEMBL4075205) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Antagonist activity at human CXCR4 in CCRF-CEM cells assessed as decrease in SDF-1alpha stimulated Ca2+ flux preincubated for 25 mins followed by SDF... | J Med Chem 61: 946-979 (2018) Article DOI: 10.1021/acs.jmedchem.7b01420 BindingDB Entry DOI: 10.7270/Q2KH0QTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50270037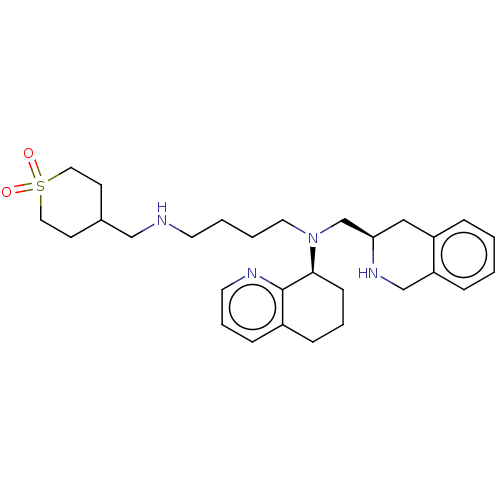 (CHEMBL4070843) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Antagonist activity at human CXCR4 in CCRF-CEM cells assessed as decrease in SDF-1alpha stimulated Ca2+ flux preincubated for 25 mins followed by SDF... | J Med Chem 61: 946-979 (2018) Article DOI: 10.1021/acs.jmedchem.7b01420 BindingDB Entry DOI: 10.7270/Q2KH0QTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50443541 (CHEMBL3091687) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Antagonist activity at CXCR4 (unknown origin) expressed in CHO-K1 cells assessed as inhibition of SDF-1alpha/forskolin-induced cAMP production | ACS Med Chem Lett 4: 1025-30 (2013) Article DOI: 10.1021/ml400183q BindingDB Entry DOI: 10.7270/Q2ZS2Z0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Mus musculus) | BDBM50270019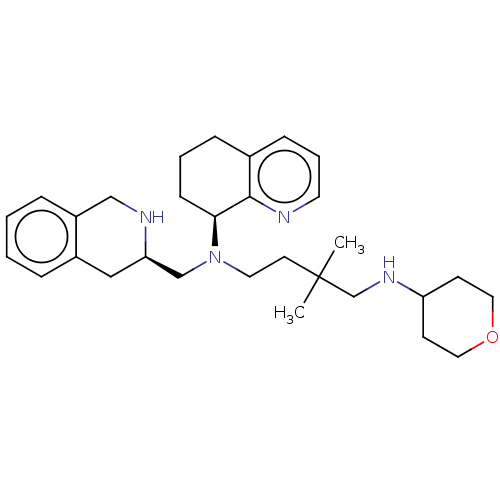 (CHEMBL4065224) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Antagonist activity at mouse CXCR4 | J Med Chem 61: 946-979 (2018) Article DOI: 10.1021/acs.jmedchem.7b01420 BindingDB Entry DOI: 10.7270/Q2KH0QTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50443549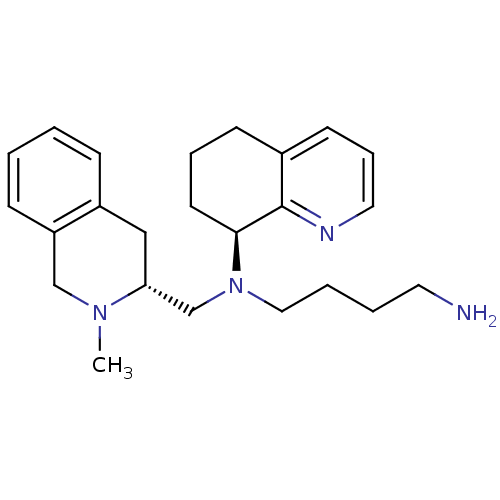 (CHEMBL3091693) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Antagonist activity at CXCR4 in human MAGI-CCR5 cells assessed as inhibition of HIV-1 3B entry after 2 to 6 days by beta-galactosidase reporter gene ... | ACS Med Chem Lett 4: 1025-30 (2013) Article DOI: 10.1021/ml400183q BindingDB Entry DOI: 10.7270/Q2ZS2Z0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50443548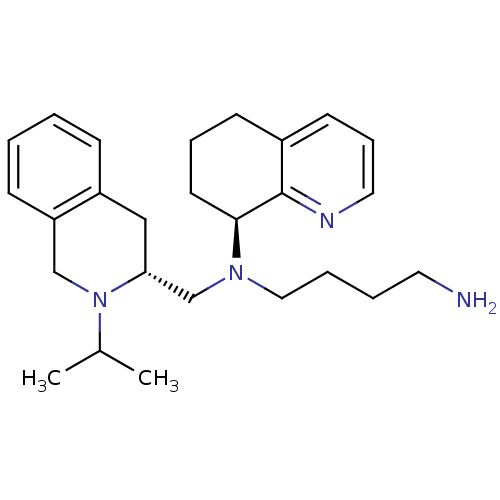 (CHEMBL3091694) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Antagonist activity at CXCR4 in human MAGI-CCR5 cells assessed as inhibition of HIV-1 3B entry after 2 to 6 days by beta-galactosidase reporter gene ... | ACS Med Chem Lett 4: 1025-30 (2013) Article DOI: 10.1021/ml400183q BindingDB Entry DOI: 10.7270/Q2ZS2Z0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50270288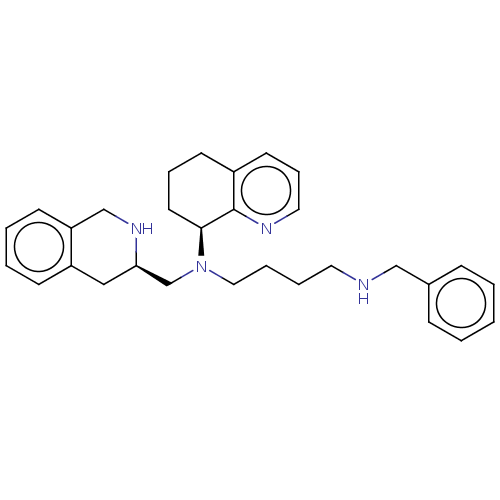 (CHEMBL4062981) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Antagonist activity at human CXCR4 in CCRF-CEM cells assessed as decrease in SDF-1alpha stimulated Ca2+ flux preincubated for 25 mins followed by SDF... | J Med Chem 61: 946-979 (2018) Article DOI: 10.1021/acs.jmedchem.7b01420 BindingDB Entry DOI: 10.7270/Q2KH0QTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50270032 (CHEMBL4092405) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Antagonist activity at human CXCR4 in CCRF-CEM cells assessed as decrease in SDF-1alpha stimulated Ca2+ flux preincubated for 25 mins followed by SDF... | J Med Chem 61: 946-979 (2018) Article DOI: 10.1021/acs.jmedchem.7b01420 BindingDB Entry DOI: 10.7270/Q2KH0QTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50270016 (CHEMBL4070320) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Antagonist activity at human CXCR4 in CCRF-CEM cells assessed as decrease in SDF-1alpha stimulated Ca2+ flux preincubated for 25 mins followed by SDF... | J Med Chem 61: 946-979 (2018) Article DOI: 10.1021/acs.jmedchem.7b01420 BindingDB Entry DOI: 10.7270/Q2KH0QTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50270052 (CHEMBL4080718) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Antagonist activity at human CXCR4 in CCRF-CEM cells assessed as decrease in SDF-1alpha stimulated Ca2+ flux preincubated for 25 mins followed by SDF... | J Med Chem 61: 946-979 (2018) Article DOI: 10.1021/acs.jmedchem.7b01420 BindingDB Entry DOI: 10.7270/Q2KH0QTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50270043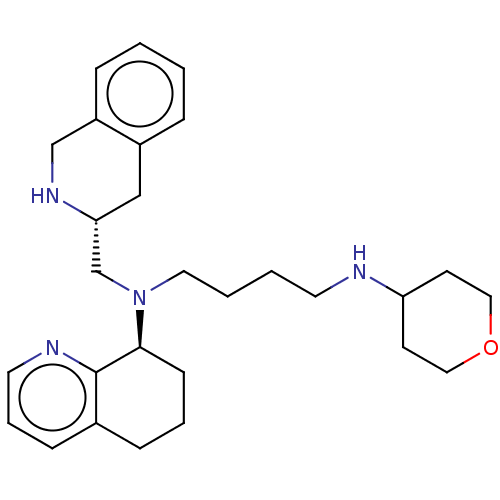 (CHEMBL4090235) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Antagonist activity at human CXCR4 in CCRF-CEM cells assessed as decrease in SDF-1alpha stimulated Ca2+ flux preincubated for 25 mins followed by SDF... | J Med Chem 61: 946-979 (2018) Article DOI: 10.1021/acs.jmedchem.7b01420 BindingDB Entry DOI: 10.7270/Q2KH0QTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50035696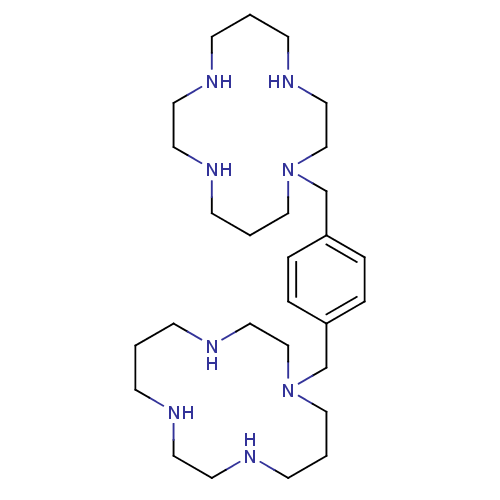 (1,1''-{1,4-phenylenebis(methylene)}-bis{1,4,8,11-t...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Antagonist activity at CXCR4 in human PBMC assessed as inhibition of HIV-1 3B infection | ACS Med Chem Lett 4: 1025-30 (2013) Article DOI: 10.1021/ml400183q BindingDB Entry DOI: 10.7270/Q2ZS2Z0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50315305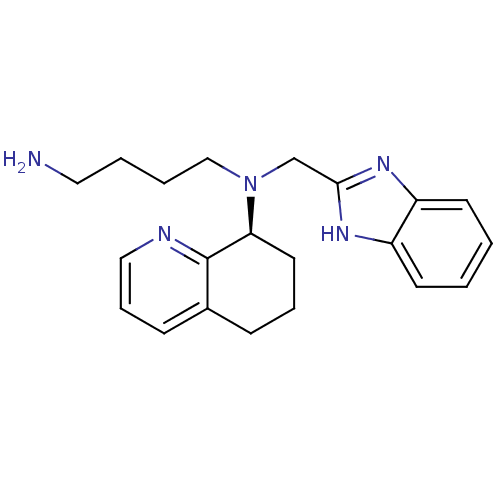 ((S)-N-((1H-benzo[d]imidazol-2-yl)methyl)-N-(4-amin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Antagonist activity at CXCR4 in human PBMC assessed as inhibition of HIV-1 3B infection | ACS Med Chem Lett 4: 1025-30 (2013) Article DOI: 10.1021/ml400183q BindingDB Entry DOI: 10.7270/Q2ZS2Z0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50443547 (CHEMBL3091681) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Antagonist activity at CXCR4 in human MAGI-CCR5 cells assessed as inhibition of HIV-1 3B entry after 2 to 6 days by beta-galactosidase reporter gene ... | ACS Med Chem Lett 4: 1025-30 (2013) Article DOI: 10.1021/ml400183q BindingDB Entry DOI: 10.7270/Q2ZS2Z0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50270047 (CHEMBL4075694) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Inhibition of recombinant human CYP2D6 expressed in microsomes of insect cells using AMMC as substrate preincubated for 30 mins followed by NADP addi... | J Med Chem 61: 946-979 (2018) Article DOI: 10.1021/acs.jmedchem.7b01420 BindingDB Entry DOI: 10.7270/Q2KH0QTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50443541 (CHEMBL3091687) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Antagonist activity at CXCR4 in human PBMC assessed as inhibition of HIV-1 3B infection | ACS Med Chem Lett 4: 1025-30 (2013) Article DOI: 10.1021/ml400183q BindingDB Entry DOI: 10.7270/Q2ZS2Z0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50270019 (CHEMBL4065224) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Antagonist activity at human CXCR4 in CCRF-CEM cells assessed as decrease in SDF-1alpha stimulated Ca2+ flux preincubated for 25 mins followed by SDF... | J Med Chem 61: 946-979 (2018) Article DOI: 10.1021/acs.jmedchem.7b01420 BindingDB Entry DOI: 10.7270/Q2KH0QTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50270035 (CHEMBL4076841) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Antagonist activity at human CXCR4 in CCRF-CEM cells assessed as decrease in SDF-1alpha stimulated Ca2+ flux preincubated for 25 mins followed by SDF... | J Med Chem 61: 946-979 (2018) Article DOI: 10.1021/acs.jmedchem.7b01420 BindingDB Entry DOI: 10.7270/Q2KH0QTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 379 total ) | Next | Last >> |