

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glucose-dependent insulinotropic receptor (Homo sapiens (Human)) | BDBM50341309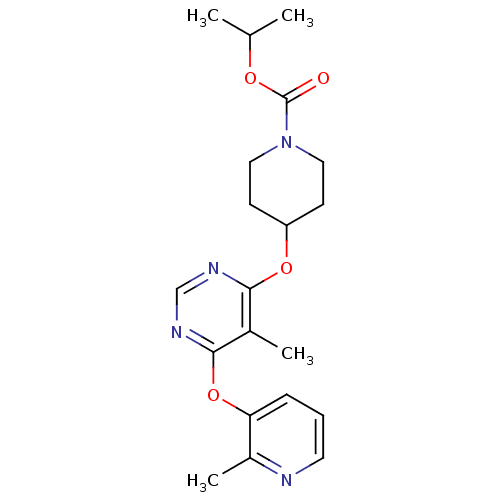 (CHEMBL1766081 | isopropyl 4-(5-methyl-6-(2-methylp...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]isopropyl 4-(1-(4-(methylsulfonyl)phenyl)-1H-pyrazolo[3,4-d]pyrimidin-4-yloxy)piperidine-1-carboxylate/tert-butyl 4-(1-(4-(methyl... | J Med Chem 54: 1948-52 (2011) Article DOI: 10.1021/jm200003p BindingDB Entry DOI: 10.7270/Q2CZ37G0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-dependent insulinotropic receptor (Homo sapiens (Human)) | BDBM50341310 (CHEMBL1766082 | Isopropyl 9-anti-({5-Methyl-6-[(2-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]isopropyl 4-(1-(4-(methylsulfonyl)phenyl)-1H-pyrazolo[3,4-d]pyrimidin-4-yloxy)piperidine-1-carboxylate/tert-butyl 4-(1-(4-(methyl... | J Med Chem 54: 1948-52 (2011) Article DOI: 10.1021/jm200003p BindingDB Entry DOI: 10.7270/Q2CZ37G0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-dependent insulinotropic receptor (Homo sapiens (Human)) | BDBM50341310 (CHEMBL1766082 | Isopropyl 9-anti-({5-Methyl-6-[(2-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]isopropyl 4-(1-(4-(methylsulfonyl)phenyl)-1H-pyrazolo[3,4-d]pyrimidin-4-yloxy)piperidine-1-carboxylate/tert-butyl 4-(1-(4-(methyl... | J Med Chem 54: 1948-52 (2011) Article DOI: 10.1021/jm200003p BindingDB Entry DOI: 10.7270/Q2CZ37G0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-dependent insulinotropic receptor (Rattus norvegicus) | BDBM50341310 (CHEMBL1766082 | Isopropyl 9-anti-({5-Methyl-6-[(2-...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 125 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]isopropyl 4-(1-(4-(methylsulfonyl)phenyl)-1H-pyrazolo[3,4-d]pyrimidin-4-yloxy)piperidine-1-carboxylate/tert-butyl 4-(1-(4-(methyl... | J Med Chem 54: 1948-52 (2011) Article DOI: 10.1021/jm200003p BindingDB Entry DOI: 10.7270/Q2CZ37G0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-dependent insulinotropic receptor (Rattus norvegicus) | BDBM50341310 (CHEMBL1766082 | Isopropyl 9-anti-({5-Methyl-6-[(2-...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 125 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]isopropyl 4-(1-(4-(methylsulfonyl)phenyl)-1H-pyrazolo[3,4-d]pyrimidin-4-yloxy)piperidine-1-carboxylate/tert-butyl 4-(1-(4-(methyl... | J Med Chem 54: 1948-52 (2011) Article DOI: 10.1021/jm200003p BindingDB Entry DOI: 10.7270/Q2CZ37G0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-dependent insulinotropic receptor (Rattus norvegicus) | BDBM50341309 (CHEMBL1766081 | isopropyl 4-(5-methyl-6-(2-methylp...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]isopropyl 4-(1-(4-(methylsulfonyl)phenyl)-1H-pyrazolo[3,4-d]pyrimidin-4-yloxy)piperidine-1-carboxylate/tert-butyl 4-(1-(4-(methyl... | J Med Chem 54: 1948-52 (2011) Article DOI: 10.1021/jm200003p BindingDB Entry DOI: 10.7270/Q2CZ37G0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-dependent insulinotropic receptor (Homo sapiens (Human)) | BDBM50341312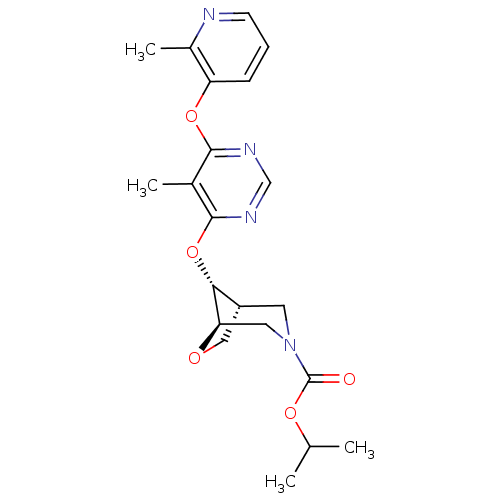 ((1R,5R,8R)-Isopropyl 8-(5-Methyl-6-(2-methylpyridi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.18E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]isopropyl 4-(1-(4-(methylsulfonyl)phenyl)-1H-pyrazolo[3,4-d]pyrimidin-4-yloxy)piperidine-1-carboxylate/tert-butyl 4-(1-(4-(methyl... | J Med Chem 54: 1948-52 (2011) Article DOI: 10.1021/jm200003p BindingDB Entry DOI: 10.7270/Q2CZ37G0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-dependent insulinotropic receptor (Homo sapiens (Human)) | BDBM50341311 ((+/-)-isopropyl 8-(5-methyl-6-(2-methylpyridin-3-y...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]isopropyl 4-(1-(4-(methylsulfonyl)phenyl)-1H-pyrazolo[3,4-d]pyrimidin-4-yloxy)piperidine-1-carboxylate/tert-butyl 4-(1-(4-(methyl... | J Med Chem 54: 1948-52 (2011) Article DOI: 10.1021/jm200003p BindingDB Entry DOI: 10.7270/Q2CZ37G0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-dependent insulinotropic receptor (Homo sapiens (Human)) | BDBM50341313 ((1S,5S,8S)-Isopropyl 8-(5-Methyl-6-(2-methylpyridi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.27E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]isopropyl 4-(1-(4-(methylsulfonyl)phenyl)-1H-pyrazolo[3,4-d]pyrimidin-4-yloxy)piperidine-1-carboxylate/tert-butyl 4-(1-(4-(methyl... | J Med Chem 54: 1948-52 (2011) Article DOI: 10.1021/jm200003p BindingDB Entry DOI: 10.7270/Q2CZ37G0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM50200182 ((1S,3R,5Z,7E)-9,10-secocholesta-5,7,10(19)-triene-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 0.0953 | n/a | n/a | n/a | n/a | n/a | n/a |
Marquette University Curated by ChEMBL | Assay Description Agonist activity at GAL4 DNA-binding domain fused VDR (unknown origin) ligand binding domain expressed in UAS-bla HEK 293T cells assessed as beta-lac... | Eur J Med Chem 157: 791-804 (2018) Article DOI: 10.1016/j.ejmech.2018.08.006 BindingDB Entry DOI: 10.7270/Q2K64MS7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor beta (Homo sapiens (Human)) | BDBM398047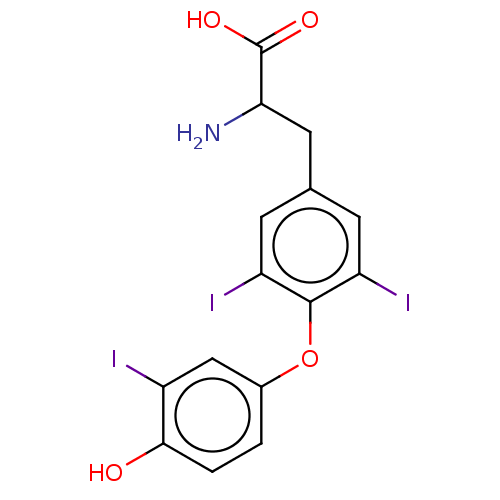 (US10322118, Entry 5) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.103 | n/a | n/a | n/a | n/a | n/a | n/a |
Marquette University Curated by ChEMBL | Assay Description Agonist activity at GAL4 DNA-binding domain fused TRbeta receptor (unknown origin) ligand binding domain expressed in UAS-bla HEK 293T cells assessed... | Eur J Med Chem 157: 791-804 (2018) Article DOI: 10.1016/j.ejmech.2018.08.006 BindingDB Entry DOI: 10.7270/Q2K64MS7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM17292 ((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.107 | n/a | n/a | n/a | n/a | n/a | n/a |
Marquette University Curated by ChEMBL | Assay Description Agonist activity at GAL4 DNA-binding domain fused ERalpha (unknown origin) ligand binding domain expressed in UAS-bla GripTite 293 cells assessed as ... | Eur J Med Chem 157: 791-804 (2018) Article DOI: 10.1016/j.ejmech.2018.08.006 BindingDB Entry DOI: 10.7270/Q2K64MS7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM18660 ((10S,11S,14S,15S)-14,15-dimethyl-14-propanoyltetra...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.236 | n/a | n/a | n/a | n/a | n/a | n/a |
Marquette University Curated by ChEMBL | Assay Description Agonist activity at GAL4 DNA-binding domain fused PR (unknown origin) ligand binding domain expressed in UAS-bla HEK 293T cells assessed as beta-lact... | Eur J Med Chem 157: 791-804 (2018) Article DOI: 10.1016/j.ejmech.2018.08.006 BindingDB Entry DOI: 10.7270/Q2K64MS7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50367916 (METHYLTRIENOLONE | Metribolone | R-1881) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank KEGG MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | n/a | n/a | 0.302 | n/a | n/a | n/a | n/a | n/a | n/a |
Marquette University Curated by ChEMBL | Assay Description Agonist activity at GAL4 DNA-binding domain fused androgen receptor (unknown origin) ligand binding domain expressed in UAS-bla GripTite 293 cells as... | Eur J Med Chem 157: 791-804 (2018) Article DOI: 10.1016/j.ejmech.2018.08.006 BindingDB Entry DOI: 10.7270/Q2K64MS7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mineralocorticoid receptor (Homo sapiens (Human)) | BDBM19214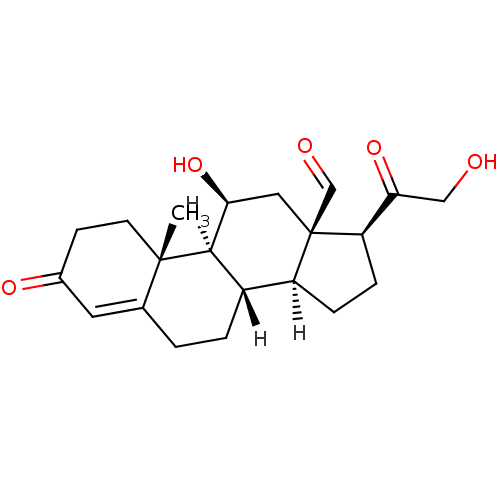 ((1S,2R,10S,11S,14S,15R,17S)-17-hydroxy-14-(2-hydro...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 0.305 | n/a | n/a | n/a | n/a | n/a | n/a |
Marquette University Curated by ChEMBL | Assay Description Agonist activity at GAL4 DNA-binding domain fused MR (unknown origin) ligand binding domain expressed in UAS-bla H cells assessed as beta-lactamase t... | Eur J Med Chem 157: 791-804 (2018) Article DOI: 10.1016/j.ejmech.2018.08.006 BindingDB Entry DOI: 10.7270/Q2K64MS7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM17292 ((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.579 | n/a | n/a | n/a | n/a | n/a | n/a |
Marquette University Curated by ChEMBL | Assay Description Agonist activity at GAL4 DNA-binding domain fused ERbeta (unknown origin) ligand binding domain expressed in UAS-bla GripTite 293 cells assessed as b... | Eur J Med Chem 157: 791-804 (2018) Article DOI: 10.1016/j.ejmech.2018.08.006 BindingDB Entry DOI: 10.7270/Q2K64MS7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM18207 ((1R,2S,10S,11S,13R,14R,15S,17S)-1-fluoro-14,17-dih...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Marquette University Curated by ChEMBL | Assay Description Agonist activity at GAL4 DNA-binding domain fused GR (unknown origin) ligand binding domain expressed in UAS-bla HEK 293T cells assessed as beta-lact... | Eur J Med Chem 157: 791-804 (2018) Article DOI: 10.1016/j.ejmech.2018.08.006 BindingDB Entry DOI: 10.7270/Q2K64MS7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM50085041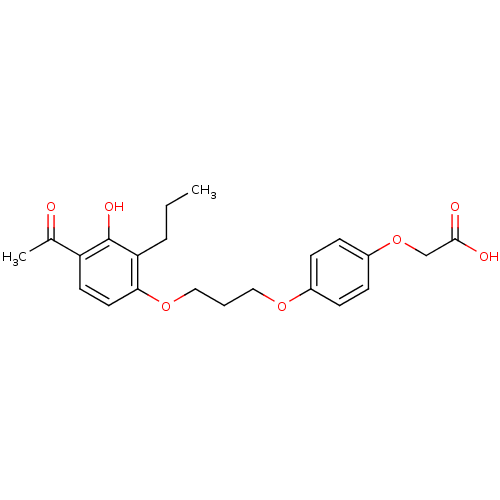 (2-(4-(3-(4-acetyl-3-hydroxy-2-propylphenoxy)propox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Marquette University Curated by ChEMBL | Assay Description Agonist activity at GAL4 DNA-binding domain fused PPARdelta receptor (unknown origin) ligand binding domain expressed in UAS-bla HEK 293T cells asses... | Eur J Med Chem 157: 791-804 (2018) Article DOI: 10.1016/j.ejmech.2018.08.006 BindingDB Entry DOI: 10.7270/Q2K64MS7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM50134329 (CHEMBL122708 | Sulfamic acid (11R,12S,15S,16S)-13-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Kingston University Curated by ChEMBL | Assay Description In vitro inhibition of estrone sulfatase. | Bioorg Med Chem Lett 12: 1279-82 (2002) BindingDB Entry DOI: 10.7270/Q2MC90K1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50466088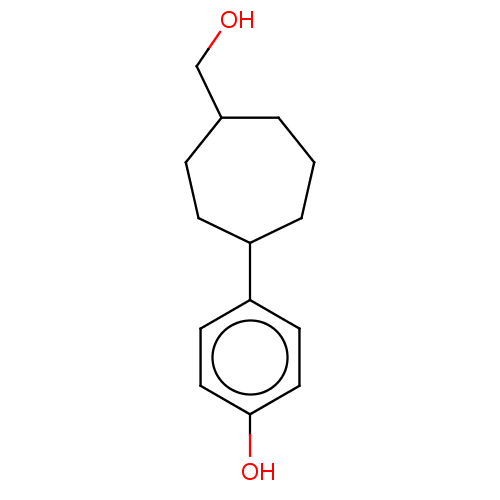 (CHEMBL4293810) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Marquette University Curated by ChEMBL | Assay Description Inhibition of CYP2C9 (unknown origin) using luciferin-H as substrate preincubated for 10 mins followed by NADPH regeneration system addition and meas... | Eur J Med Chem 157: 791-804 (2018) Article DOI: 10.1016/j.ejmech.2018.08.006 BindingDB Entry DOI: 10.7270/Q2K64MS7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM50051829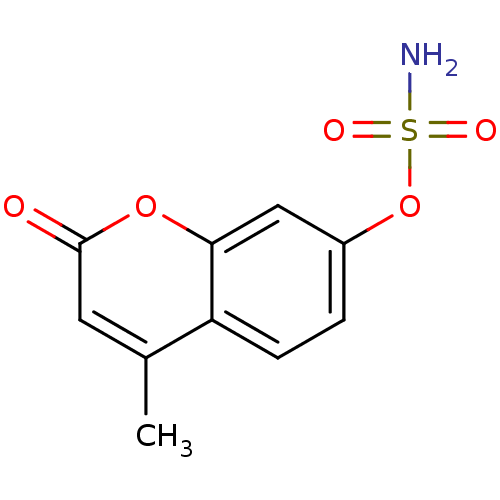 (4-methyl-2-oxo-2H-chromen-7-yl sulfamate | CHEMBL1...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kingston University Curated by ChEMBL | Assay Description In vitro inhibition of estrone sulfatase. | Bioorg Med Chem Lett 12: 1279-82 (2002) BindingDB Entry DOI: 10.7270/Q2MC90K1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50466088 (CHEMBL4293810) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Marquette University Curated by ChEMBL | Assay Description Inhibition of CYP3A4 (unknown origin) using luciferin-IPA as substrate preincubated for 10 mins followed by NADPH regeneration system addition and me... | Eur J Med Chem 157: 791-804 (2018) Article DOI: 10.1016/j.ejmech.2018.08.006 BindingDB Entry DOI: 10.7270/Q2K64MS7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50466088 (CHEMBL4293810) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Marquette University Curated by ChEMBL | Assay Description Inhibition of CYP2D6 (unknown origin) using luciferin-ME EGE as substrate preincubated for 10 mins followed by NADPH regeneration system addition and... | Eur J Med Chem 157: 791-804 (2018) Article DOI: 10.1016/j.ejmech.2018.08.006 BindingDB Entry DOI: 10.7270/Q2K64MS7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM50098109 (3-nitrophenyl sulfamate | CHEMBL283560 | Sulfamic ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 1.20E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Kingston University Curated by ChEMBL | Assay Description In vitro inhibition of estrone sulfatase. | Bioorg Med Chem Lett 12: 1279-82 (2002) BindingDB Entry DOI: 10.7270/Q2MC90K1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM50112406 (CHEMBL24063 | Sulfamic acid 3-iodo-phenyl ester) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.20E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Kingston University Curated by ChEMBL | Assay Description In vitro inhibition of estrone sulfatase. | Bioorg Med Chem Lett 12: 1279-82 (2002) BindingDB Entry DOI: 10.7270/Q2MC90K1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50466088 (CHEMBL4293810) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.30E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Marquette University Curated by ChEMBL | Assay Description Inhibition of CYP1A2 (unknown origin) using luciferin-ME as substrate preincubated for 10 mins followed by NADPH regeneration system addition and mea... | Eur J Med Chem 157: 791-804 (2018) Article DOI: 10.1016/j.ejmech.2018.08.006 BindingDB Entry DOI: 10.7270/Q2K64MS7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM50098099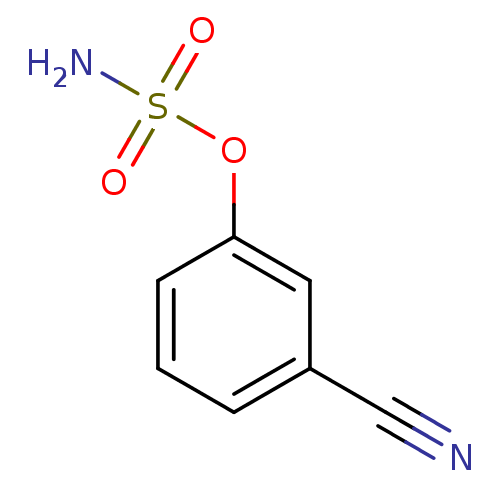 (CHEMBL23306 | Sulfamic acid 3-cyano-phenyl ester) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.91E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Kingston University Curated by ChEMBL | Assay Description In vitro inhibition of estrone sulfatase. | Bioorg Med Chem Lett 12: 1279-82 (2002) BindingDB Entry DOI: 10.7270/Q2MC90K1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM50098104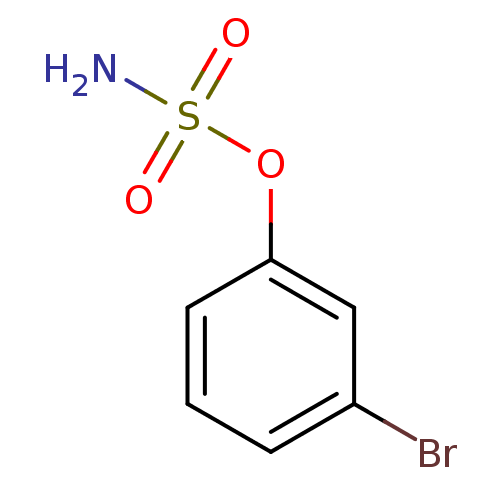 (CHEMBL23305 | Sulfamic acid 3-bromo-phenyl ester) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 2.57E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Kingston University Curated by ChEMBL | Assay Description In vitro inhibition of estrone sulfatase. | Bioorg Med Chem Lett 12: 1279-82 (2002) BindingDB Entry DOI: 10.7270/Q2MC90K1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM50098113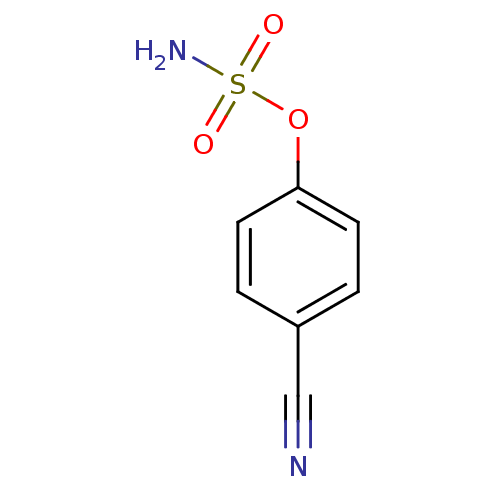 (CHEMBL24119 | Sulfamic acid 4-cyano-phenyl ester) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Kingston University Curated by ChEMBL | Assay Description In vitro inhibition of estrone sulfatase. | Bioorg Med Chem Lett 12: 1279-82 (2002) BindingDB Entry DOI: 10.7270/Q2MC90K1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM50098105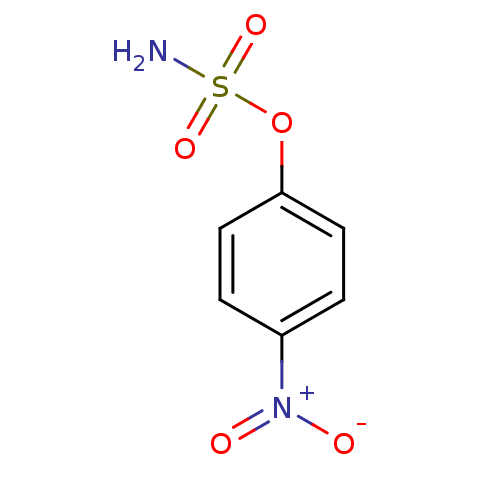 (CHEMBL23258 | Sulfamic acid 4-nitro-phenyl ester) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 3.30E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Kingston University Curated by ChEMBL | Assay Description In vitro inhibition of estrone sulfatase. | Bioorg Med Chem Lett 12: 1279-82 (2002) BindingDB Entry DOI: 10.7270/Q2MC90K1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM50098103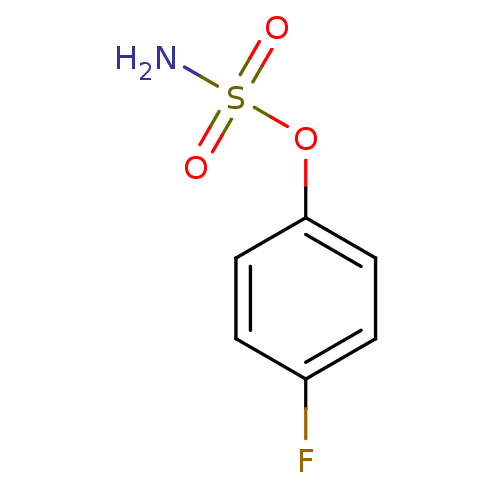 (4-fluorophenyl sulfamate | CHEMBL23865 | Sulfamic ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 5.37E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Kingston University Curated by ChEMBL | Assay Description In vitro inhibition of estrone sulfatase. | Bioorg Med Chem Lett 12: 1279-82 (2002) BindingDB Entry DOI: 10.7270/Q2MC90K1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM50098100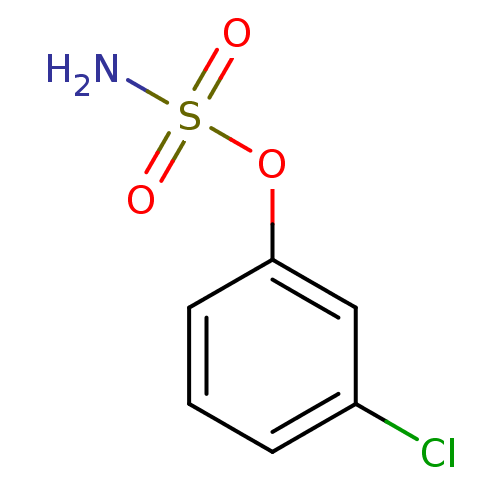 (CHEMBL282717 | Sulfamic acid 3-chloro-phenyl ester) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 5.37E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Kingston University Curated by ChEMBL | Assay Description In vitro inhibition of estrone sulfatase. | Bioorg Med Chem Lett 12: 1279-82 (2002) BindingDB Entry DOI: 10.7270/Q2MC90K1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM50112403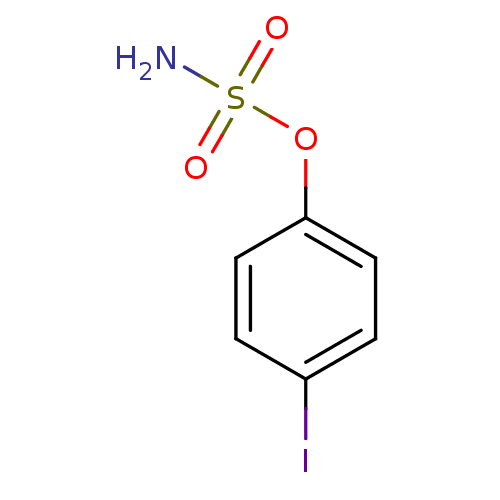 (CHEMBL24120 | Sulfamic acid 4-iodo-phenyl ester) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.60E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Kingston University Curated by ChEMBL | Assay Description In vitro inhibition of estrone sulfatase. | Bioorg Med Chem Lett 12: 1279-82 (2002) BindingDB Entry DOI: 10.7270/Q2MC90K1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM50112404 (CHEMBL23067 | Sulfamic acid 2,2,2-trichloro-ethyl ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | PubMed | n/a | n/a | 7.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Kingston University Curated by ChEMBL | Assay Description In vitro inhibition of estrone sulfatase. | Bioorg Med Chem Lett 12: 1279-82 (2002) BindingDB Entry DOI: 10.7270/Q2MC90K1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM50098108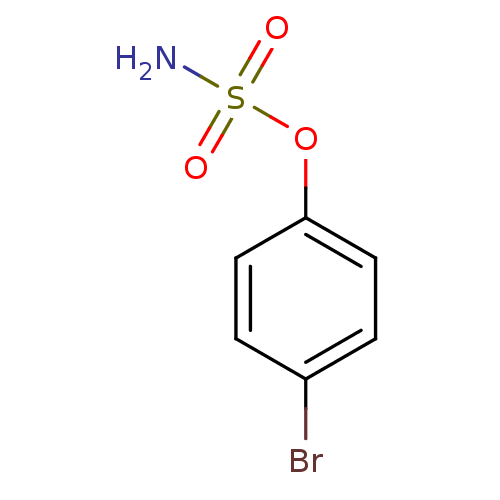 (CHEMBL283121 | Sulfamic acid 4-bromo-phenyl ester) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 9.12E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Kingston University Curated by ChEMBL | Assay Description In vitro inhibition of estrone sulfatase. | Bioorg Med Chem Lett 12: 1279-82 (2002) BindingDB Entry DOI: 10.7270/Q2MC90K1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM50098102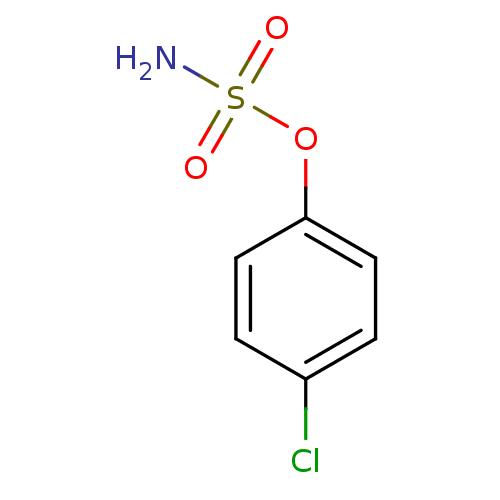 (CHEMBL23350 | Sulfamic acid 4-chloro-phenyl ester) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.58E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Kingston University Curated by ChEMBL | Assay Description In vitro inhibition of estrone sulfatase. | Bioorg Med Chem Lett 12: 1279-82 (2002) BindingDB Entry DOI: 10.7270/Q2MC90K1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM50098107 (CHEMBL287279 | Sulfamic acid m-tolyl ester) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.08E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Kingston University Curated by ChEMBL | Assay Description In vitro inhibition of estrone sulfatase. | Bioorg Med Chem Lett 12: 1279-82 (2002) BindingDB Entry DOI: 10.7270/Q2MC90K1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM50098112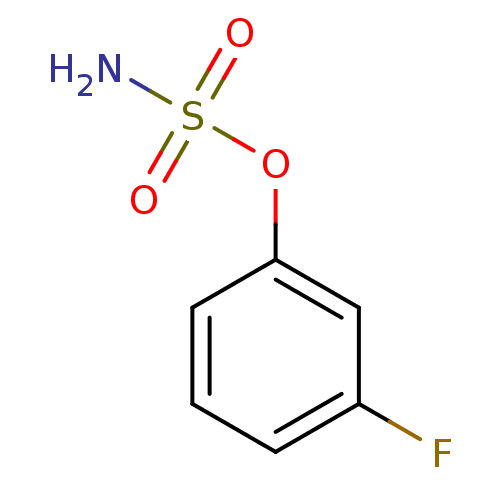 (CHEMBL23864 | Sulfamic acid 3-fluoro-phenyl ester) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 2.08E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Kingston University Curated by ChEMBL | Assay Description In vitro inhibition of estrone sulfatase. | Bioorg Med Chem Lett 12: 1279-82 (2002) BindingDB Entry DOI: 10.7270/Q2MC90K1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM50098106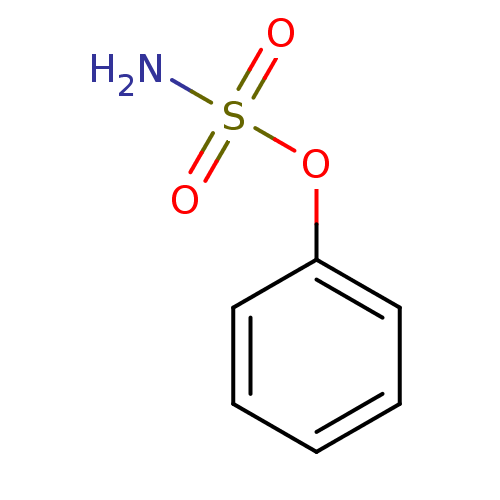 (CHEMBL24259 | PHENYLSULFAMATE | Sulfamic acid phen...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >1.00E+7 | n/a | n/a | n/a | n/a | n/a | n/a |
Kingston University Curated by ChEMBL | Assay Description In vitro inhibition of estrone sulfatase. | Bioorg Med Chem Lett 12: 1279-82 (2002) BindingDB Entry DOI: 10.7270/Q2MC90K1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM50098101 (CHEMBL24210 | Sulfamic acid p-tolyl ester) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >1.00E+7 | n/a | n/a | n/a | n/a | n/a | n/a |
Kingston University Curated by ChEMBL | Assay Description In vitro inhibition of estrone sulfatase. | Bioorg Med Chem Lett 12: 1279-82 (2002) BindingDB Entry DOI: 10.7270/Q2MC90K1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-dependent insulinotropic receptor (Rattus norvegicus) | BDBM50341309 (CHEMBL1766081 | isopropyl 4-(5-methyl-6-(2-methylp...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 84 | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Agonist activity at rat GPR119 expressed in human HEK293 cells assessed as increase in cAMP level after 16 hrs by beta-lactamase reporter gene assay | J Med Chem 54: 1948-52 (2011) Article DOI: 10.1021/jm200003p BindingDB Entry DOI: 10.7270/Q2CZ37G0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-dependent insulinotropic receptor (Rattus norvegicus) | BDBM50341310 (CHEMBL1766082 | Isopropyl 9-anti-({5-Methyl-6-[(2-...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 228 | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Agonist activity at rat GPR119 expressed in human HEK293 cells assessed as increase in cAMP level after 16 hrs by beta-lactamase reporter gene assay | J Med Chem 54: 1948-52 (2011) Article DOI: 10.1021/jm200003p BindingDB Entry DOI: 10.7270/Q2CZ37G0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-dependent insulinotropic receptor (Rattus norvegicus) | BDBM50341310 (CHEMBL1766082 | Isopropyl 9-anti-({5-Methyl-6-[(2-...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 227 | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Agonist activity at rat GPR119 expressed in human HEK293 cells assessed as increase in cAMP level after 16 hrs by beta-lactamase reporter gene assay | J Med Chem 54: 1948-52 (2011) Article DOI: 10.1021/jm200003p BindingDB Entry DOI: 10.7270/Q2CZ37G0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-dependent insulinotropic receptor (Homo sapiens (Human)) | BDBM50341311 ((+/-)-isopropyl 8-(5-methyl-6-(2-methylpyridin-3-y...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.24E+3 | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Agonist activity at GPR119 in human HEK293 cells assessed as increase in cAMP level after 16 hrs by beta-lactamase reporter gene assay | J Med Chem 54: 1948-52 (2011) Article DOI: 10.1021/jm200003p BindingDB Entry DOI: 10.7270/Q2CZ37G0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-dependent insulinotropic receptor (Homo sapiens (Human)) | BDBM50341312 ((1R,5R,8R)-Isopropyl 8-(5-Methyl-6-(2-methylpyridi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 740 | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Agonist activity at GPR119 in human HEK293 cells assessed as increase in cAMP level after 16 hrs by beta-lactamase reporter gene assay | J Med Chem 54: 1948-52 (2011) Article DOI: 10.1021/jm200003p BindingDB Entry DOI: 10.7270/Q2CZ37G0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-dependent insulinotropic receptor (Homo sapiens (Human)) | BDBM50341313 ((1S,5S,8S)-Isopropyl 8-(5-Methyl-6-(2-methylpyridi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.07E+3 | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Agonist activity at GPR119 in human HEK293 cells assessed as increase in cAMP level after 16 hrs by beta-lactamase reporter gene assay | J Med Chem 54: 1948-52 (2011) Article DOI: 10.1021/jm200003p BindingDB Entry DOI: 10.7270/Q2CZ37G0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-dependent insulinotropic receptor (Homo sapiens (Human)) | BDBM50341309 (CHEMBL1766081 | isopropyl 4-(5-methyl-6-(2-methylp...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 14 | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Agonist activity at GPR119 in human HEK293 cells assessed as increase in cAMP level after 16 hrs by beta-lactamase reporter gene assay | J Med Chem 54: 1948-52 (2011) Article DOI: 10.1021/jm200003p BindingDB Entry DOI: 10.7270/Q2CZ37G0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-dependent insulinotropic receptor (Homo sapiens (Human)) | BDBM50341310 (CHEMBL1766082 | Isopropyl 9-anti-({5-Methyl-6-[(2-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Agonist activity at GPR119 in human HEK293 cells assessed as increase in cAMP level after 16 hrs by beta-lactamase reporter gene assay | J Med Chem 54: 1948-52 (2011) Article DOI: 10.1021/jm200003p BindingDB Entry DOI: 10.7270/Q2CZ37G0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-dependent insulinotropic receptor (Homo sapiens (Human)) | BDBM50341310 (CHEMBL1766082 | Isopropyl 9-anti-({5-Methyl-6-[(2-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 65 | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Agonist activity at GPR119 in human HEK293 cells assessed as increase in cAMP level after 16 hrs by beta-lactamase reporter gene assay | J Med Chem 54: 1948-52 (2011) Article DOI: 10.1021/jm200003p BindingDB Entry DOI: 10.7270/Q2CZ37G0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50466084 (CHEMBL3099433) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Marquette University Curated by ChEMBL | Assay Description Agonist activity at full length ERalpha (unknown origin) after 24 hrs by ERE-driven luciferase reporter gene assay | Eur J Med Chem 157: 791-804 (2018) Article DOI: 10.1016/j.ejmech.2018.08.006 BindingDB Entry DOI: 10.7270/Q2K64MS7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 102 total ) | Next | Last >> |