 Found 1367 hits with Last Name = 'schröder' and Initial = 'j'
Found 1367 hits with Last Name = 'schröder' and Initial = 'j' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cathepsin K
(Homo sapiens (Human)) | BDBM50098577
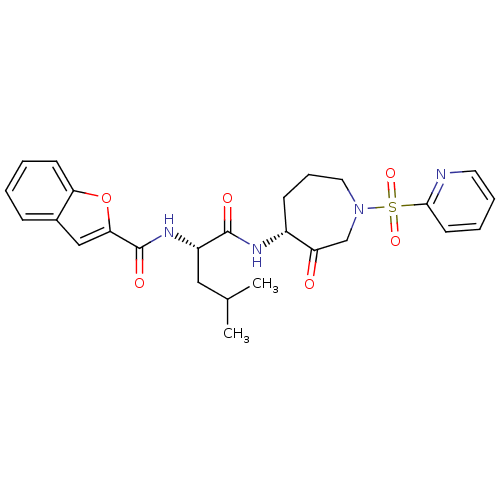
(Benzofuran-2-carboxylic acid {3-methyl-1-[3-oxo-1-...)Show SMILES CC(C)C[C@H](NC(=O)c1cc2ccccc2o1)C(=O)N[C@@H]1CCCN(CC1=O)S(=O)(=O)c1ccccn1 Show InChI InChI=1S/C26H30N4O6S/c1-17(2)14-20(29-26(33)23-15-18-8-3-4-10-22(18)36-23)25(32)28-19-9-7-13-30(16-21(19)31)37(34,35)24-11-5-6-12-27-24/h3-6,8,10-12,15,17,19-20H,7,9,13-14,16H2,1-2H3,(H,28,32)(H,29,33)/t19-,20+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MSD Animal Health Innovation GmbH
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin K |
J Med Chem 56: 1478-90 (2013)
Article DOI: 10.1021/jm3013932
BindingDB Entry DOI: 10.7270/Q2W09774 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Cathepsin K
(Homo sapiens (Human)) | BDBM50408519
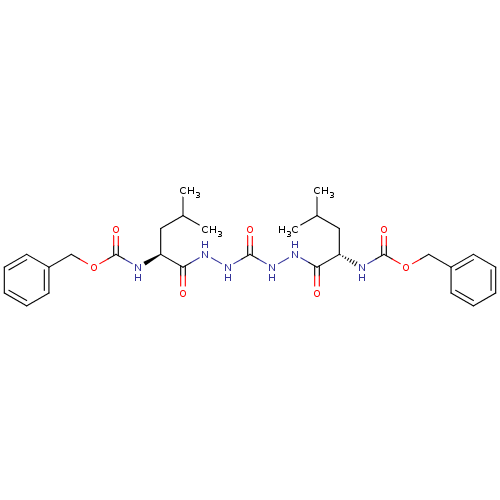
(CHEMBL115357)Show SMILES CC(C)C[C@H](NC(=O)OCc1ccccc1)C(=O)NNC(=O)NNC(=O)[C@H](CC(C)C)NC(=O)OCc1ccccc1 |r| Show InChI InChI=1S/C29H40N6O7/c1-19(2)15-23(30-28(39)41-17-21-11-7-5-8-12-21)25(36)32-34-27(38)35-33-26(37)24(16-20(3)4)31-29(40)42-18-22-13-9-6-10-14-22/h5-14,19-20,23-24H,15-18H2,1-4H3,(H,30,39)(H,31,40)(H,32,36)(H,33,37)(H2,34,35,38)/t23-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MSD Animal Health Innovation GmbH
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin K |
J Med Chem 56: 1478-90 (2013)
Article DOI: 10.1021/jm3013932
BindingDB Entry DOI: 10.7270/Q2W09774 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM12042

((2R,3R)-3-{[(1S)-1-[(4-carbamimidamidobutyl)carbam...)Show SMILES [#6]-[#6](-[#6])-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@@H]-1-[#8]-[#6@H]-1-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6]-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7] |r| Show InChI InChI=1S/C15H27N5O5/c1-8(2)7-9(20-13(22)10-11(25-10)14(23)24)12(21)18-5-3-4-6-19-15(16)17/h8-11H,3-7H2,1-2H3,(H,18,21)(H,20,22)(H,23,24)(H4,16,17,19)/t9-,10+,11+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MSD Animal Health Innovation GmbH
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin K by gelatin zymographic analysis |
J Med Chem 56: 1478-90 (2013)
Article DOI: 10.1021/jm3013932
BindingDB Entry DOI: 10.7270/Q2W09774 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM19807
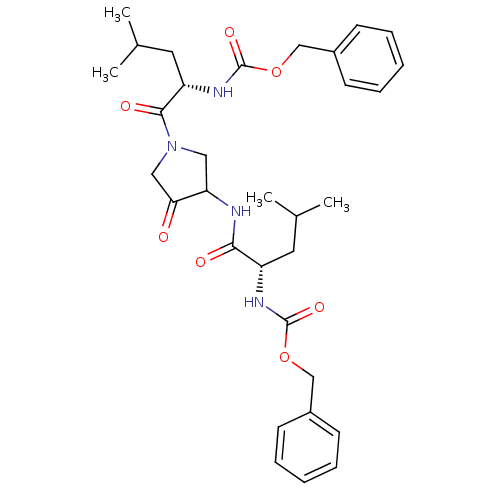
(CHEMBL100563 | benzyl N-[(1S)-1-({1-[(2S)-2-{[(ben...)Show SMILES CC(C)C[C@H](NC(=O)OCc1ccccc1)C(=O)NC1CN(CC1=O)C(=O)[C@H](CC(C)C)NC(=O)OCc1ccccc1 |r| Show InChI InChI=1S/C32H42N4O7/c1-21(2)15-25(34-31(40)42-19-23-11-7-5-8-12-23)29(38)33-27-17-36(18-28(27)37)30(39)26(16-22(3)4)35-32(41)43-20-24-13-9-6-10-14-24/h5-14,21-22,25-27H,15-20H2,1-4H3,(H,33,38)(H,34,40)(H,35,41)/t25-,26-,27?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MSD Animal Health Innovation GmbH
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin K (unknown origin) |
J Med Chem 56: 1478-90 (2013)
Article DOI: 10.1021/jm3013932
BindingDB Entry DOI: 10.7270/Q2W09774 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Cathepsin K
(Homo sapiens (Human)) | BDBM50426169
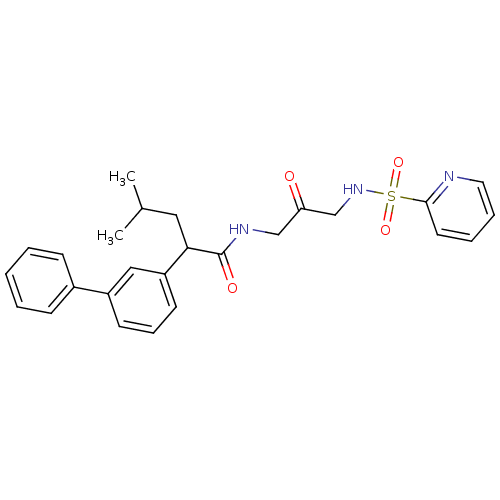
(CHEMBL2316589)Show SMILES CC(C)CC(C(=O)NCC(=O)CNS(=O)(=O)c1ccccn1)c1cccc(c1)-c1ccccc1 Show InChI InChI=1S/C26H29N3O4S/c1-19(2)15-24(22-12-8-11-21(16-22)20-9-4-3-5-10-20)26(31)28-17-23(30)18-29-34(32,33)25-13-6-7-14-27-25/h3-14,16,19,24,29H,15,17-18H2,1-2H3,(H,28,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MSD Animal Health Innovation GmbH
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin K (unknown origin) |
J Med Chem 56: 1478-90 (2013)
Article DOI: 10.1021/jm3013932
BindingDB Entry DOI: 10.7270/Q2W09774 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50066648
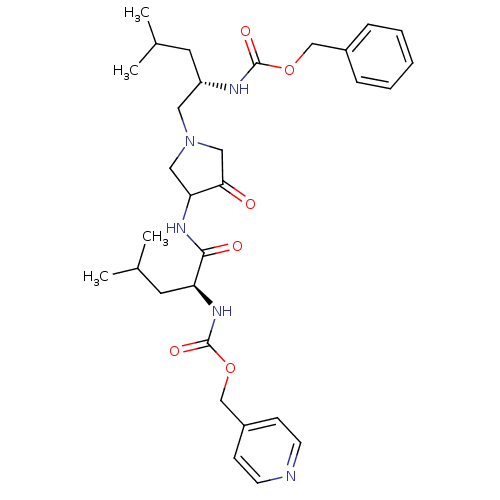
(((S)-3-Methyl-1-{3-[(S)-4-methyl-2-(pyridin-4-ylme...)Show SMILES CC(C)C[C@@H](CN1CC(NC(=O)[C@H](CC(C)C)NC(=O)OCc2ccncc2)C(=O)C1)NC(=O)OCc1ccccc1 Show InChI InChI=1S/C31H43N5O6/c1-21(2)14-25(33-30(39)41-19-23-8-6-5-7-9-23)16-36-17-27(28(37)18-36)34-29(38)26(15-22(3)4)35-31(40)42-20-24-10-12-32-13-11-24/h5-13,21-22,25-27H,14-20H2,1-4H3,(H,33,39)(H,34,38)(H,35,40)/t25-,26-,27?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MSD Animal Health Innovation GmbH
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin K (unknown origin) |
J Med Chem 56: 1478-90 (2013)
Article DOI: 10.1021/jm3013932
BindingDB Entry DOI: 10.7270/Q2W09774 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Cathepsin K
(Homo sapiens (Human)) | BDBM50079596
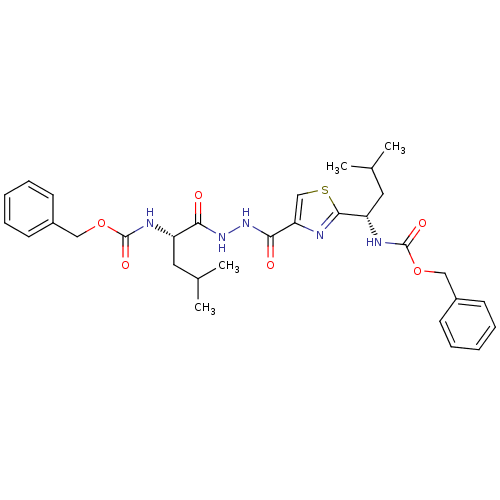
(((S)-1-{N'-[2-((S)-1-Benzyloxycarbonylamino-3-meth...)Show SMILES CC(C)C[C@H](NC(=O)OCc1ccccc1)C(=O)NNC(=O)c1csc(n1)[C@H](CC(C)C)NC(=O)OCc1ccccc1 Show InChI InChI=1S/C31H39N5O6S/c1-20(2)15-24(33-30(39)41-17-22-11-7-5-8-12-22)27(37)35-36-28(38)26-19-43-29(32-26)25(16-21(3)4)34-31(40)42-18-23-13-9-6-10-14-23/h5-14,19-21,24-25H,15-18H2,1-4H3,(H,33,39)(H,34,40)(H,35,37)(H,36,38)/t24-,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MSD Animal Health Innovation GmbH
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin K |
J Med Chem 56: 1478-90 (2013)
Article DOI: 10.1021/jm3013932
BindingDB Entry DOI: 10.7270/Q2W09774 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Cathepsin K
(Homo sapiens (Human)) | BDBM50426167
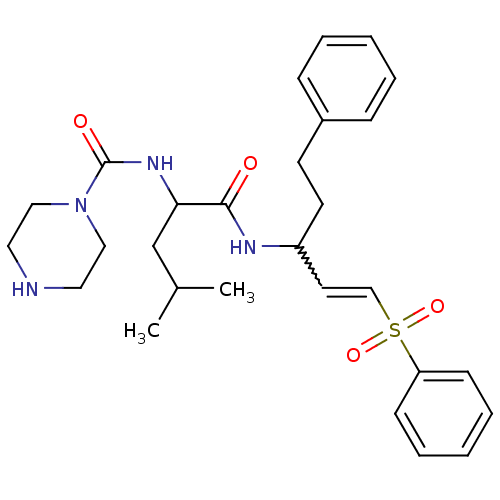
(CHEMBL2316590)Show SMILES CC(C)CC(NC(=O)N1CCNCC1)C(=O)NC(CCc1ccccc1)C=CS(=O)(=O)c1ccccc1 |w:26.27| Show InChI InChI=1S/C28H38N4O4S/c1-22(2)21-26(31-28(34)32-18-16-29-17-19-32)27(33)30-24(14-13-23-9-5-3-6-10-23)15-20-37(35,36)25-11-7-4-8-12-25/h3-12,15,20,22,24,26,29H,13-14,16-19,21H2,1-2H3,(H,30,33)(H,31,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MSD Animal Health Innovation GmbH
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin K |
J Med Chem 56: 1478-90 (2013)
Article DOI: 10.1021/jm3013932
BindingDB Entry DOI: 10.7270/Q2W09774 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50426170
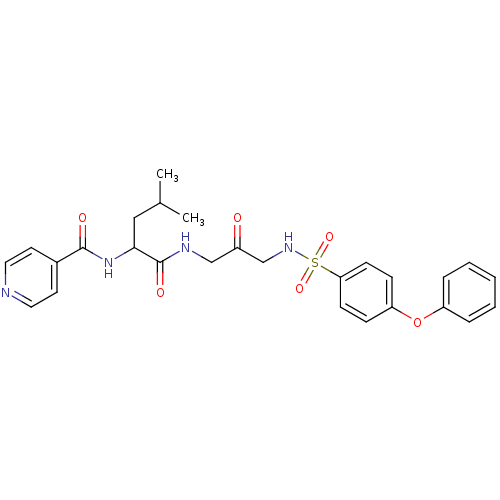
(CHEMBL2316313)Show SMILES CC(C)CC(NC(=O)c1ccncc1)C(=O)NCC(=O)CNS(=O)(=O)c1ccc(Oc2ccccc2)cc1 Show InChI InChI=1S/C27H30N4O6S/c1-19(2)16-25(31-26(33)20-12-14-28-15-13-20)27(34)29-17-21(32)18-30-38(35,36)24-10-8-23(9-11-24)37-22-6-4-3-5-7-22/h3-15,19,25,30H,16-18H2,1-2H3,(H,29,34)(H,31,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MSD Animal Health Innovation GmbH
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin K (unknown origin) |
J Med Chem 56: 1478-90 (2013)
Article DOI: 10.1021/jm3013932
BindingDB Entry DOI: 10.7270/Q2W09774 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50066650
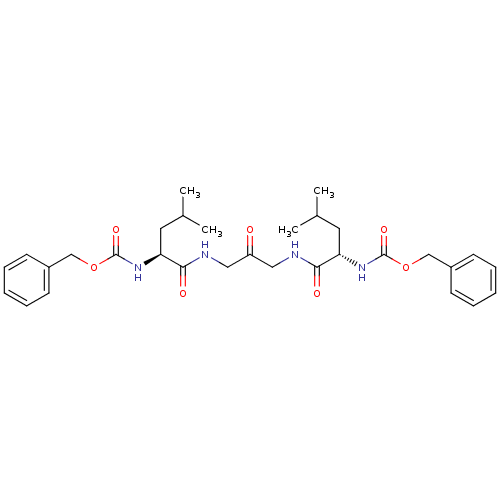
(1,3-BIS[[N-[(PHENYLMETHOXY)CARBONYL]-L-LEUCYL]AMIN...)Show SMILES CC(C)C[C@H](NC(=O)OCc1ccccc1)C(=O)NCC(=O)CNC(=O)[C@H](CC(C)C)NC(=O)OCc1ccccc1 Show InChI InChI=1S/C31H42N4O7/c1-21(2)15-26(34-30(39)41-19-23-11-7-5-8-12-23)28(37)32-17-25(36)18-33-29(38)27(16-22(3)4)35-31(40)42-20-24-13-9-6-10-14-24/h5-14,21-22,26-27H,15-20H2,1-4H3,(H,32,37)(H,33,38)(H,34,39)(H,35,40)/t26-,27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MSD Animal Health Innovation GmbH
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin K (unknown origin) |
J Med Chem 56: 1478-90 (2013)
Article DOI: 10.1021/jm3013932
BindingDB Entry DOI: 10.7270/Q2W09774 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50104706
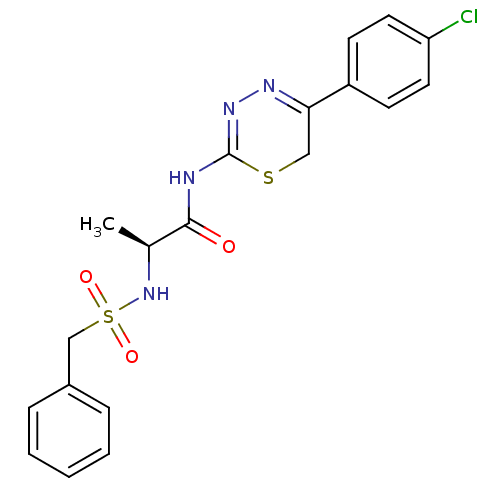
(CHEMBL111179 | N-[5-(4-Chloro-phenyl)-6H-[1,3,4]th...)Show SMILES C[C@H](NS(=O)(=O)Cc1ccccc1)C(=O)NC1=NN=C(CS1)c1ccc(Cl)cc1 |c:19,t:17| Show InChI InChI=1S/C19H19ClN4O3S2/c1-13(24-29(26,27)12-14-5-3-2-4-6-14)18(25)21-19-23-22-17(11-28-19)15-7-9-16(20)10-8-15/h2-10,13,24H,11-12H2,1H3,(H,21,23,25)/t13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universität Bielefeld
Curated by ChEMBL
| Assay Description
In vitro inhibition of human matrix metalloprotease-9 |
J Med Chem 44: 3231-43 (2001)
BindingDB Entry DOI: 10.7270/Q2RN3758 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50104718

((R)-N-(5-(4-bromophenyl)-6H-1,3,4-thiadiazin-2-yl)...)Show SMILES C[C@@H](NS(=O)(=O)c1ccccc1)C(=O)NC1=NN=C(CS1)c1ccc(Br)cc1 |c:18,t:16| Show InChI InChI=1S/C18H17BrN4O3S2/c1-12(23-28(25,26)15-5-3-2-4-6-15)17(24)20-18-22-21-16(11-27-18)13-7-9-14(19)10-8-13/h2-10,12,23H,11H2,1H3,(H,20,22,24)/t12-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universität Bielefeld
Curated by ChEMBL
| Assay Description
In vitro inhibition of human matrix metalloprotease-9 |
J Med Chem 44: 3231-43 (2001)
BindingDB Entry DOI: 10.7270/Q2RN3758 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50104717
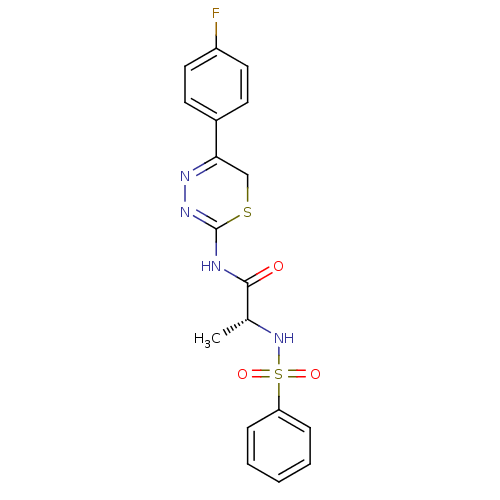
((R)-N-(5-(4-fluorophenyl)-6H-1,3,4-thiadiazin-2-yl...)Show SMILES C[C@@H](NS(=O)(=O)c1ccccc1)C(=O)NC1=NN=C(CS1)c1ccc(F)cc1 |c:18,t:16| Show InChI InChI=1S/C18H17FN4O3S2/c1-12(23-28(25,26)15-5-3-2-4-6-15)17(24)20-18-22-21-16(11-27-18)13-7-9-14(19)10-8-13/h2-10,12,23H,11H2,1H3,(H,20,22,24)/t12-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universität Bielefeld
Curated by ChEMBL
| Assay Description
In vitro inhibition of human matrix metalloprotease-9 |
J Med Chem 44: 3231-43 (2001)
BindingDB Entry DOI: 10.7270/Q2RN3758 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-14
(Homo sapiens (Human)) | BDBM50104710
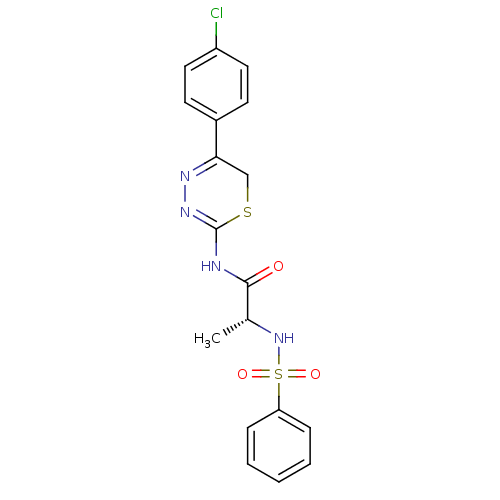
((R)-N-(5-(4-chlorophenyl)-6H-1,3,4-thiadiazin-2-yl...)Show SMILES C[C@@H](NS(=O)(=O)c1ccccc1)C(=O)NC1=NN=C(CS1)c1ccc(Cl)cc1 |c:18,t:16| Show InChI InChI=1S/C18H17ClN4O3S2/c1-12(23-28(25,26)15-5-3-2-4-6-15)17(24)20-18-22-21-16(11-27-18)13-7-9-14(19)10-8-13/h2-10,12,23H,11H2,1H3,(H,20,22,24)/t12-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universität Bielefeld
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloproteinase-14 |
J Med Chem 44: 3231-43 (2001)
BindingDB Entry DOI: 10.7270/Q2RN3758 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50104721
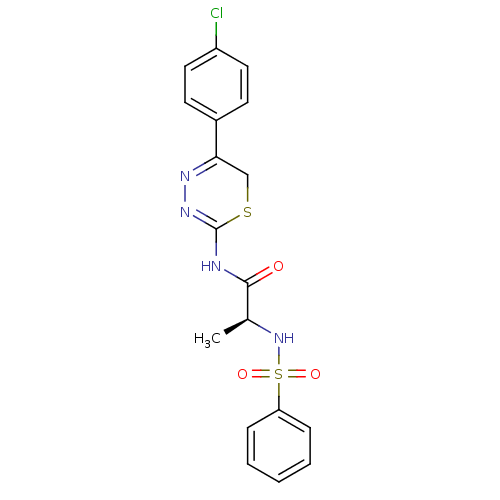
((S)-N-(5-(4-chlorophenyl)-6H-1,3,4-thiadiazin-2-yl...)Show SMILES C[C@H](NS(=O)(=O)c1ccccc1)C(=O)NC1=NN=C(CS1)c1ccc(Cl)cc1 |c:18,t:16| Show InChI InChI=1S/C18H17ClN4O3S2/c1-12(23-28(25,26)15-5-3-2-4-6-15)17(24)20-18-22-21-16(11-27-18)13-7-9-14(19)10-8-13/h2-10,12,23H,11H2,1H3,(H,20,22,24)/t12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universität Bielefeld
Curated by ChEMBL
| Assay Description
In vitro inhibition of human matrix metalloprotease-9 |
J Med Chem 44: 3231-43 (2001)
BindingDB Entry DOI: 10.7270/Q2RN3758 |
More data for this
Ligand-Target Pair | |
Neutrophil collagenase
(Homo sapiens (Human)) | BDBM50104714
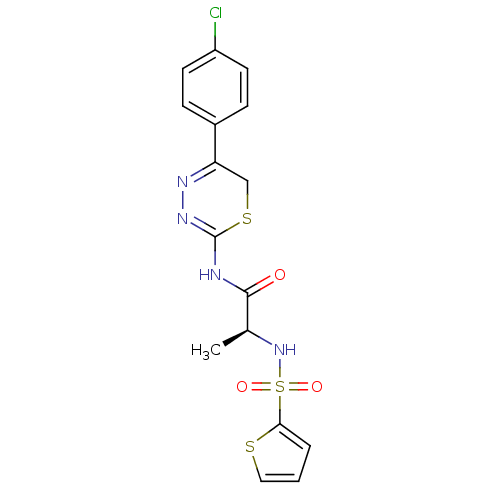
(CHEMBL109861 | N-[5-(4-Chloro-phenyl)-6H-[1,3,4]th...)Show SMILES C[C@H](NS(=O)(=O)c1cccs1)C(=O)NC1=NN=C(CS1)c1ccc(Cl)cc1 |c:17,t:15| Show InChI InChI=1S/C16H15ClN4O3S3/c1-10(21-27(23,24)14-3-2-8-25-14)15(22)18-16-20-19-13(9-26-16)11-4-6-12(17)7-5-11/h2-8,10,21H,9H2,1H3,(H,18,20,22)/t10-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universität Bielefeld
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloprotease-8 |
J Med Chem 44: 3231-43 (2001)
BindingDB Entry DOI: 10.7270/Q2RN3758 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50104710

((R)-N-(5-(4-chlorophenyl)-6H-1,3,4-thiadiazin-2-yl...)Show SMILES C[C@@H](NS(=O)(=O)c1ccccc1)C(=O)NC1=NN=C(CS1)c1ccc(Cl)cc1 |c:18,t:16| Show InChI InChI=1S/C18H17ClN4O3S2/c1-12(23-28(25,26)15-5-3-2-4-6-15)17(24)20-18-22-21-16(11-27-18)13-7-9-14(19)10-8-13/h2-10,12,23H,11H2,1H3,(H,20,22,24)/t12-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universität Bielefeld
Curated by ChEMBL
| Assay Description
In vitro inhibition of human matrix metalloprotease-9 |
J Med Chem 44: 3231-43 (2001)
BindingDB Entry DOI: 10.7270/Q2RN3758 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50104709
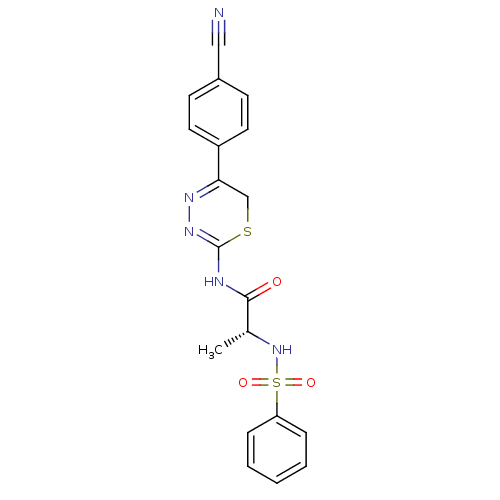
((R)-N-(5-(4-cyanophenyl)-6H-1,3,4-thiadiazin-2-yl)...)Show SMILES C[C@@H](NS(=O)(=O)c1ccccc1)C(=O)NC1=NN=C(CS1)c1ccc(cc1)C#N |c:18,t:16| Show InChI InChI=1S/C19H17N5O3S2/c1-13(24-29(26,27)16-5-3-2-4-6-16)18(25)21-19-23-22-17(12-28-19)15-9-7-14(11-20)8-10-15/h2-10,13,24H,12H2,1H3,(H,21,23,25)/t13-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universität Bielefeld
Curated by ChEMBL
| Assay Description
In vitro inhibition of human matrix metalloprotease-9 |
J Med Chem 44: 3231-43 (2001)
BindingDB Entry DOI: 10.7270/Q2RN3758 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50104723
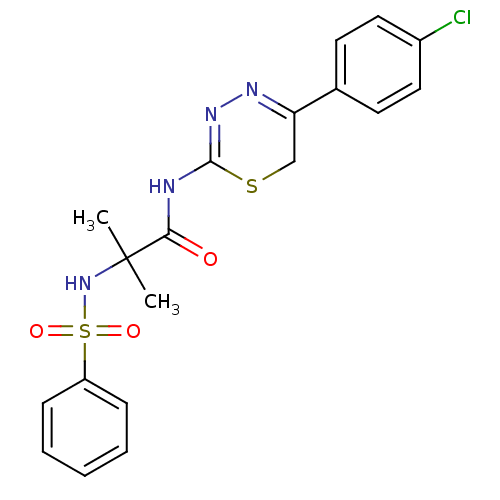
(2-Benzenesulfonylamino-N-[5-(4-chloro-phenyl)-6H-[...)Show SMILES CC(C)(NS(=O)(=O)c1ccccc1)C(=O)NC1=NN=C(CS1)c1ccc(Cl)cc1 |c:19,t:17| Show InChI InChI=1S/C19H19ClN4O3S2/c1-19(2,24-29(26,27)15-6-4-3-5-7-15)17(25)21-18-23-22-16(12-28-18)13-8-10-14(20)11-9-13/h3-11,24H,12H2,1-2H3,(H,21,23,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universität Bielefeld
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against the catalytic domain of the human matrix metalloprotease-2 |
J Med Chem 44: 3231-43 (2001)
BindingDB Entry DOI: 10.7270/Q2RN3758 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50104707
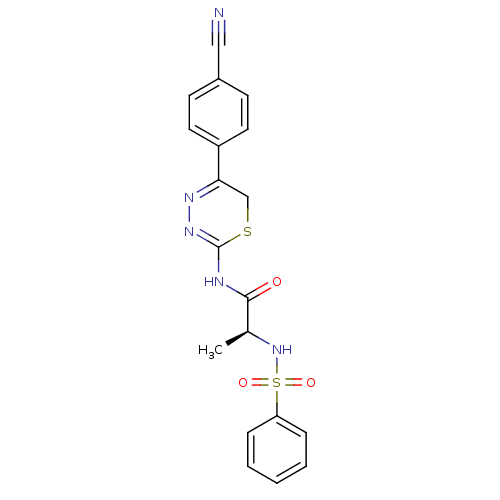
((S)-N-(5-(4-cyanophenyl)-6H-1,3,4-thiadiazin-2-yl)...)Show SMILES C[C@H](NS(=O)(=O)c1ccccc1)C(=O)NC1=NN=C(CS1)c1ccc(cc1)C#N |c:18,t:16| Show InChI InChI=1S/C19H17N5O3S2/c1-13(24-29(26,27)16-5-3-2-4-6-16)18(25)21-19-23-22-17(12-28-19)15-9-7-14(11-20)8-10-15/h2-10,13,24H,12H2,1H3,(H,21,23,25)/t13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universität Bielefeld
Curated by ChEMBL
| Assay Description
In vitro inhibition of human matrix metalloprotease-9 |
J Med Chem 44: 3231-43 (2001)
BindingDB Entry DOI: 10.7270/Q2RN3758 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50104706

(CHEMBL111179 | N-[5-(4-Chloro-phenyl)-6H-[1,3,4]th...)Show SMILES C[C@H](NS(=O)(=O)Cc1ccccc1)C(=O)NC1=NN=C(CS1)c1ccc(Cl)cc1 |c:19,t:17| Show InChI InChI=1S/C19H19ClN4O3S2/c1-13(24-29(26,27)12-14-5-3-2-4-6-14)18(25)21-19-23-22-17(11-28-19)15-7-9-16(20)10-8-15/h2-10,13,24H,11-12H2,1H3,(H,21,23,25)/t13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universität Bielefeld
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against the catalytic domain of the human matrix metalloprotease-2 |
J Med Chem 44: 3231-43 (2001)
BindingDB Entry DOI: 10.7270/Q2RN3758 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50104723

(2-Benzenesulfonylamino-N-[5-(4-chloro-phenyl)-6H-[...)Show SMILES CC(C)(NS(=O)(=O)c1ccccc1)C(=O)NC1=NN=C(CS1)c1ccc(Cl)cc1 |c:19,t:17| Show InChI InChI=1S/C19H19ClN4O3S2/c1-19(2,24-29(26,27)15-6-4-3-5-7-15)17(25)21-18-23-22-16(12-28-18)13-8-10-14(20)11-9-13/h3-11,24H,12H2,1-2H3,(H,21,23,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universität Bielefeld
Curated by ChEMBL
| Assay Description
In vitro inhibition of human matrix metalloprotease-9 |
J Med Chem 44: 3231-43 (2001)
BindingDB Entry DOI: 10.7270/Q2RN3758 |
More data for this
Ligand-Target Pair | |
Macrophage metalloelastase
(Homo sapiens (Human)) | BDBM50104716
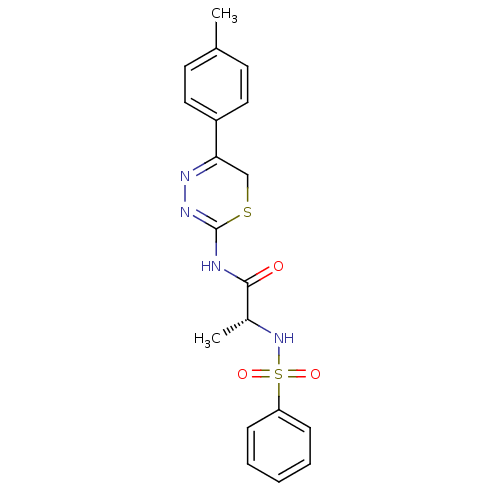
((R)-2-(phenylsulfonamido)-N-(5-p-tolyl-6H-1,3,4-th...)Show SMILES C[C@@H](NS(=O)(=O)c1ccccc1)C(=O)NC1=NN=C(CS1)c1ccc(C)cc1 |c:18,t:16| Show InChI InChI=1S/C19H20N4O3S2/c1-13-8-10-15(11-9-13)17-12-27-19(22-21-17)20-18(24)14(2)23-28(25,26)16-6-4-3-5-7-16/h3-11,14,23H,12H2,1-2H3,(H,20,22,24)/t14-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universität Bielefeld
Curated by ChEMBL
| Assay Description
In Vitro inhibitory activity against the catalytic domain of the matrix metalloprotease-12 |
J Med Chem 44: 3231-43 (2001)
BindingDB Entry DOI: 10.7270/Q2RN3758 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-14
(Homo sapiens (Human)) | BDBM50104721

((S)-N-(5-(4-chlorophenyl)-6H-1,3,4-thiadiazin-2-yl...)Show SMILES C[C@H](NS(=O)(=O)c1ccccc1)C(=O)NC1=NN=C(CS1)c1ccc(Cl)cc1 |c:18,t:16| Show InChI InChI=1S/C18H17ClN4O3S2/c1-12(23-28(25,26)15-5-3-2-4-6-15)17(24)20-18-22-21-16(11-27-18)13-7-9-14(19)10-8-13/h2-10,12,23H,11H2,1H3,(H,20,22,24)/t12-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universität Bielefeld
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against the catalytic domain of human matrix metalloprotease-14 |
J Med Chem 44: 3231-43 (2001)
BindingDB Entry DOI: 10.7270/Q2RN3758 |
More data for this
Ligand-Target Pair | |
Neutrophil collagenase
(Homo sapiens (Human)) | BDBM50104722
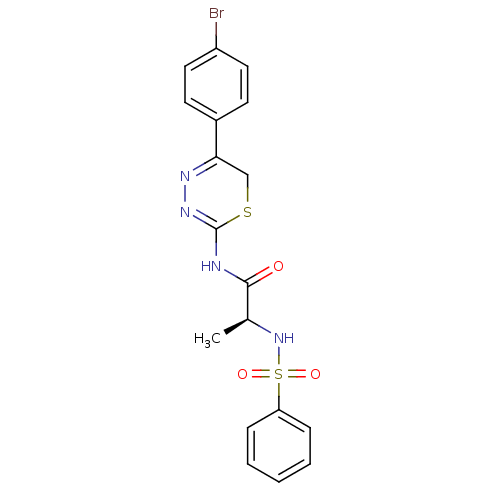
((S)-N-(5-(4-bromophenyl)-6H-1,3,4-thiadiazin-2-yl)...)Show SMILES C[C@H](NS(=O)(=O)c1ccccc1)C(=O)NC1=NN=C(CS1)c1ccc(Br)cc1 |c:18,t:16| Show InChI InChI=1S/C18H17BrN4O3S2/c1-12(23-28(25,26)15-5-3-2-4-6-15)17(24)20-18-22-21-16(11-27-18)13-7-9-14(19)10-8-13/h2-10,12,23H,11H2,1H3,(H,20,22,24)/t12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universität Bielefeld
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloprotease-8 |
J Med Chem 44: 3231-43 (2001)
BindingDB Entry DOI: 10.7270/Q2RN3758 |
More data for this
Ligand-Target Pair | |
Macrophage metalloelastase
(Homo sapiens (Human)) | BDBM50104712
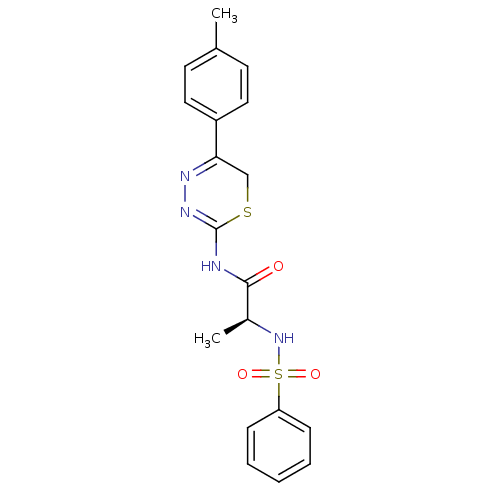
((S)-2-(phenylsulfonamido)-N-(5-p-tolyl-6H-1,3,4-th...)Show SMILES C[C@H](NS(=O)(=O)c1ccccc1)C(=O)NC1=NN=C(CS1)c1ccc(C)cc1 |c:18,t:16| Show InChI InChI=1S/C19H20N4O3S2/c1-13-8-10-15(11-9-13)17-12-27-19(22-21-17)20-18(24)14(2)23-28(25,26)16-6-4-3-5-7-16/h3-11,14,23H,12H2,1-2H3,(H,20,22,24)/t14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universität Bielefeld
Curated by ChEMBL
| Assay Description
In Vitro inhibitory activity against the catalytic domain of the matrix metalloprotease-12 |
J Med Chem 44: 3231-43 (2001)
BindingDB Entry DOI: 10.7270/Q2RN3758 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-14
(Homo sapiens (Human)) | BDBM50104723

(2-Benzenesulfonylamino-N-[5-(4-chloro-phenyl)-6H-[...)Show SMILES CC(C)(NS(=O)(=O)c1ccccc1)C(=O)NC1=NN=C(CS1)c1ccc(Cl)cc1 |c:19,t:17| Show InChI InChI=1S/C19H19ClN4O3S2/c1-19(2,24-29(26,27)15-6-4-3-5-7-15)17(25)21-18-23-22-16(12-28-18)13-8-10-14(20)11-9-13/h3-11,24H,12H2,1-2H3,(H,21,23,25) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universität Bielefeld
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against the ectodomain of the human matrix metalloproteinase-14 |
J Med Chem 44: 3231-43 (2001)
BindingDB Entry DOI: 10.7270/Q2RN3758 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50104706

(CHEMBL111179 | N-[5-(4-Chloro-phenyl)-6H-[1,3,4]th...)Show SMILES C[C@H](NS(=O)(=O)Cc1ccccc1)C(=O)NC1=NN=C(CS1)c1ccc(Cl)cc1 |c:19,t:17| Show InChI InChI=1S/C19H19ClN4O3S2/c1-13(24-29(26,27)12-14-5-3-2-4-6-14)18(25)21-19-23-22-17(11-28-19)15-7-9-16(20)10-8-15/h2-10,13,24H,11-12H2,1H3,(H,21,23,25)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universität Bielefeld
Curated by ChEMBL
| Assay Description
In Vitro inhibitory activity against the catalytic domain of the matrix metalloprotease-13 |
J Med Chem 44: 3231-43 (2001)
BindingDB Entry DOI: 10.7270/Q2RN3758 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50104718

((R)-N-(5-(4-bromophenyl)-6H-1,3,4-thiadiazin-2-yl)...)Show SMILES C[C@@H](NS(=O)(=O)c1ccccc1)C(=O)NC1=NN=C(CS1)c1ccc(Br)cc1 |c:18,t:16| Show InChI InChI=1S/C18H17BrN4O3S2/c1-12(23-28(25,26)15-5-3-2-4-6-15)17(24)20-18-22-21-16(11-27-18)13-7-9-14(19)10-8-13/h2-10,12,23H,11H2,1H3,(H,20,22,24)/t12-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universität Bielefeld
Curated by ChEMBL
| Assay Description
In Vitro inhibitory activity against the catalytic domain of the matrix metalloprotease-13 |
J Med Chem 44: 3231-43 (2001)
BindingDB Entry DOI: 10.7270/Q2RN3758 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50104713
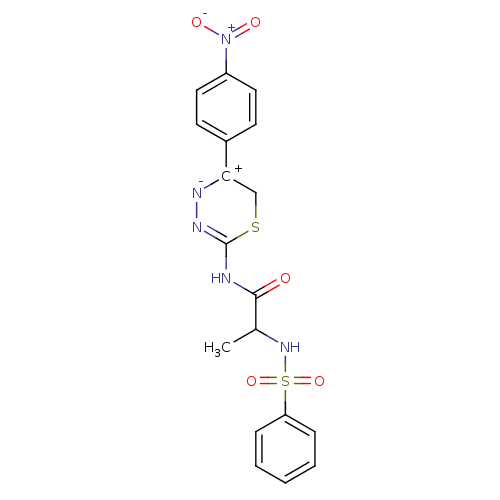
((S)-N-(5-(4-nitrophenyl)-6H-1,3,4-thiadiazin-2-yl)...)Show SMILES CC(NS(=O)(=O)c1ccccc1)C(=O)NC1=N[N-][C+](CS1)c1ccc(cc1)[N+]([O-])=O |t:16| Show InChI InChI=1S/C18H17N5O5S2/c1-12(22-30(27,28)15-5-3-2-4-6-15)17(24)19-18-21-20-16(11-29-18)13-7-9-14(10-8-13)23(25)26/h2-10,12,22H,11H2,1H3,(H,19,21,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universität Bielefeld
Curated by ChEMBL
| Assay Description
In vitro inhibition of human matrix metalloprotease-9 |
J Med Chem 44: 3231-43 (2001)
BindingDB Entry DOI: 10.7270/Q2RN3758 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50104710

((R)-N-(5-(4-chlorophenyl)-6H-1,3,4-thiadiazin-2-yl...)Show SMILES C[C@@H](NS(=O)(=O)c1ccccc1)C(=O)NC1=NN=C(CS1)c1ccc(Cl)cc1 |c:18,t:16| Show InChI InChI=1S/C18H17ClN4O3S2/c1-12(23-28(25,26)15-5-3-2-4-6-15)17(24)20-18-22-21-16(11-27-18)13-7-9-14(19)10-8-13/h2-10,12,23H,11H2,1H3,(H,20,22,24)/t12-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universität Bielefeld
Curated by ChEMBL
| Assay Description
In Vitro inhibitory activity against the catalytic domain of the matrix metalloprotease-13 |
J Med Chem 44: 3231-43 (2001)
BindingDB Entry DOI: 10.7270/Q2RN3758 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50104721

((S)-N-(5-(4-chlorophenyl)-6H-1,3,4-thiadiazin-2-yl...)Show SMILES C[C@H](NS(=O)(=O)c1ccccc1)C(=O)NC1=NN=C(CS1)c1ccc(Cl)cc1 |c:18,t:16| Show InChI InChI=1S/C18H17ClN4O3S2/c1-12(23-28(25,26)15-5-3-2-4-6-15)17(24)20-18-22-21-16(11-27-18)13-7-9-14(19)10-8-13/h2-10,12,23H,11H2,1H3,(H,20,22,24)/t12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universität Bielefeld
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against the catalytic domain of the human matrix metalloprotease-2 |
J Med Chem 44: 3231-43 (2001)
BindingDB Entry DOI: 10.7270/Q2RN3758 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-14
(Homo sapiens (Human)) | BDBM50104716

((R)-2-(phenylsulfonamido)-N-(5-p-tolyl-6H-1,3,4-th...)Show SMILES C[C@@H](NS(=O)(=O)c1ccccc1)C(=O)NC1=NN=C(CS1)c1ccc(C)cc1 |c:18,t:16| Show InChI InChI=1S/C19H20N4O3S2/c1-13-8-10-15(11-9-13)17-12-27-19(22-21-17)20-18(24)14(2)23-28(25,26)16-6-4-3-5-7-16/h3-11,14,23H,12H2,1-2H3,(H,20,22,24)/t14-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universität Bielefeld
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against the ectodomain of the human matrix metalloproteinase-14 |
J Med Chem 44: 3231-43 (2001)
BindingDB Entry DOI: 10.7270/Q2RN3758 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-14
(Homo sapiens (Human)) | BDBM50104723

(2-Benzenesulfonylamino-N-[5-(4-chloro-phenyl)-6H-[...)Show SMILES CC(C)(NS(=O)(=O)c1ccccc1)C(=O)NC1=NN=C(CS1)c1ccc(Cl)cc1 |c:19,t:17| Show InChI InChI=1S/C19H19ClN4O3S2/c1-19(2,24-29(26,27)15-6-4-3-5-7-15)17(25)21-18-23-22-16(12-28-18)13-8-10-14(20)11-9-13/h3-11,24H,12H2,1-2H3,(H,21,23,25) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universität Bielefeld
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against the catalytic domain of human matrix metalloprotease-14 |
J Med Chem 44: 3231-43 (2001)
BindingDB Entry DOI: 10.7270/Q2RN3758 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50104718

((R)-N-(5-(4-bromophenyl)-6H-1,3,4-thiadiazin-2-yl)...)Show SMILES C[C@@H](NS(=O)(=O)c1ccccc1)C(=O)NC1=NN=C(CS1)c1ccc(Br)cc1 |c:18,t:16| Show InChI InChI=1S/C18H17BrN4O3S2/c1-12(23-28(25,26)15-5-3-2-4-6-15)17(24)20-18-22-21-16(11-27-18)13-7-9-14(19)10-8-13/h2-10,12,23H,11H2,1H3,(H,20,22,24)/t12-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universität Bielefeld
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against the catalytic domain of the human matrix metalloprotease-2 |
J Med Chem 44: 3231-43 (2001)
BindingDB Entry DOI: 10.7270/Q2RN3758 |
More data for this
Ligand-Target Pair | |
Neutrophil collagenase
(Homo sapiens (Human)) | BDBM50104710

((R)-N-(5-(4-chlorophenyl)-6H-1,3,4-thiadiazin-2-yl...)Show SMILES C[C@@H](NS(=O)(=O)c1ccccc1)C(=O)NC1=NN=C(CS1)c1ccc(Cl)cc1 |c:18,t:16| Show InChI InChI=1S/C18H17ClN4O3S2/c1-12(23-28(25,26)15-5-3-2-4-6-15)17(24)20-18-22-21-16(11-27-18)13-7-9-14(19)10-8-13/h2-10,12,23H,11H2,1H3,(H,20,22,24)/t12-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universität Bielefeld
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloprotease-8 |
J Med Chem 44: 3231-43 (2001)
BindingDB Entry DOI: 10.7270/Q2RN3758 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50104704
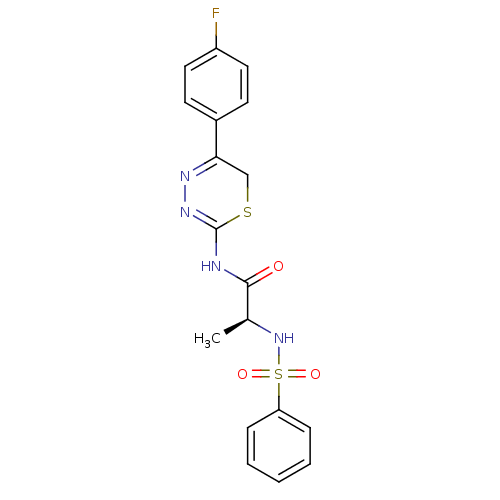
((S)-N-(5-(4-fluorophenyl)-6H-1,3,4-thiadiazin-2-yl...)Show SMILES C[C@H](NS(=O)(=O)c1ccccc1)C(=O)NC1=NN=C(CS1)c1ccc(F)cc1 |c:18,t:16| Show InChI InChI=1S/C18H17FN4O3S2/c1-12(23-28(25,26)15-5-3-2-4-6-15)17(24)20-18-22-21-16(11-27-18)13-7-9-14(19)10-8-13/h2-10,12,23H,11H2,1H3,(H,20,22,24)/t12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universität Bielefeld
Curated by ChEMBL
| Assay Description
In vitro inhibition of human matrix metalloprotease-9 |
J Med Chem 44: 3231-43 (2001)
BindingDB Entry DOI: 10.7270/Q2RN3758 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50104722

((S)-N-(5-(4-bromophenyl)-6H-1,3,4-thiadiazin-2-yl)...)Show SMILES C[C@H](NS(=O)(=O)c1ccccc1)C(=O)NC1=NN=C(CS1)c1ccc(Br)cc1 |c:18,t:16| Show InChI InChI=1S/C18H17BrN4O3S2/c1-12(23-28(25,26)15-5-3-2-4-6-15)17(24)20-18-22-21-16(11-27-18)13-7-9-14(19)10-8-13/h2-10,12,23H,11H2,1H3,(H,20,22,24)/t12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universität Bielefeld
Curated by ChEMBL
| Assay Description
In vitro inhibition of human matrix metalloprotease-9 |
J Med Chem 44: 3231-43 (2001)
BindingDB Entry DOI: 10.7270/Q2RN3758 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50104712

((S)-2-(phenylsulfonamido)-N-(5-p-tolyl-6H-1,3,4-th...)Show SMILES C[C@H](NS(=O)(=O)c1ccccc1)C(=O)NC1=NN=C(CS1)c1ccc(C)cc1 |c:18,t:16| Show InChI InChI=1S/C19H20N4O3S2/c1-13-8-10-15(11-9-13)17-12-27-19(22-21-17)20-18(24)14(2)23-28(25,26)16-6-4-3-5-7-16/h3-11,14,23H,12H2,1-2H3,(H,20,22,24)/t14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universität Bielefeld
Curated by ChEMBL
| Assay Description
In vitro inhibition of human matrix metalloprotease-9 |
J Med Chem 44: 3231-43 (2001)
BindingDB Entry DOI: 10.7270/Q2RN3758 |
More data for this
Ligand-Target Pair | |
Neutrophil collagenase
(Homo sapiens (Human)) | BDBM50104713

((S)-N-(5-(4-nitrophenyl)-6H-1,3,4-thiadiazin-2-yl)...)Show SMILES CC(NS(=O)(=O)c1ccccc1)C(=O)NC1=N[N-][C+](CS1)c1ccc(cc1)[N+]([O-])=O |t:16| Show InChI InChI=1S/C18H17N5O5S2/c1-12(22-30(27,28)15-5-3-2-4-6-15)17(24)19-18-21-20-16(11-29-18)13-7-9-14(10-8-13)23(25)26/h2-10,12,22H,11H2,1H3,(H,19,21,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universität Bielefeld
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloprotease-8 |
J Med Chem 44: 3231-43 (2001)
BindingDB Entry DOI: 10.7270/Q2RN3758 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50104711
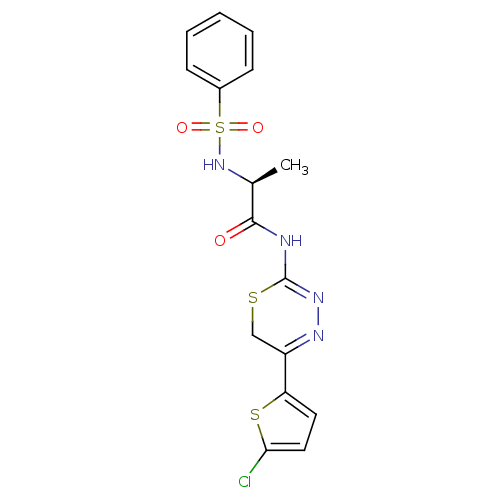
(2-Benzenesulfonylamino-N-[5-(5-chloro-thiophen-2-y...)Show SMILES C[C@H](NS(=O)(=O)c1ccccc1)C(=O)NC1=NN=C(CS1)c1ccc(Cl)s1 |c:18,t:16| Show InChI InChI=1S/C16H15ClN4O3S3/c1-10(21-27(23,24)11-5-3-2-4-6-11)15(22)18-16-20-19-12(9-25-16)13-7-8-14(17)26-13/h2-8,10,21H,9H2,1H3,(H,18,20,22)/t10-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universität Bielefeld
Curated by ChEMBL
| Assay Description
In vitro inhibition of human matrix metalloprotease-9 |
J Med Chem 44: 3231-43 (2001)
BindingDB Entry DOI: 10.7270/Q2RN3758 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50104721

((S)-N-(5-(4-chlorophenyl)-6H-1,3,4-thiadiazin-2-yl...)Show SMILES C[C@H](NS(=O)(=O)c1ccccc1)C(=O)NC1=NN=C(CS1)c1ccc(Cl)cc1 |c:18,t:16| Show InChI InChI=1S/C18H17ClN4O3S2/c1-12(23-28(25,26)15-5-3-2-4-6-15)17(24)20-18-22-21-16(11-27-18)13-7-9-14(19)10-8-13/h2-10,12,23H,11H2,1H3,(H,20,22,24)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universität Bielefeld
Curated by ChEMBL
| Assay Description
In Vitro inhibitory activity against the catalytic domain of the matrix metalloprotease-13 |
J Med Chem 44: 3231-43 (2001)
BindingDB Entry DOI: 10.7270/Q2RN3758 |
More data for this
Ligand-Target Pair | |
Neutrophil collagenase
(Homo sapiens (Human)) | BDBM50104711

(2-Benzenesulfonylamino-N-[5-(5-chloro-thiophen-2-y...)Show SMILES C[C@H](NS(=O)(=O)c1ccccc1)C(=O)NC1=NN=C(CS1)c1ccc(Cl)s1 |c:18,t:16| Show InChI InChI=1S/C16H15ClN4O3S3/c1-10(21-27(23,24)11-5-3-2-4-6-11)15(22)18-16-20-19-12(9-25-16)13-7-8-14(17)26-13/h2-8,10,21H,9H2,1H3,(H,18,20,22)/t10-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universität Bielefeld
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloprotease-8 |
J Med Chem 44: 3231-43 (2001)
BindingDB Entry DOI: 10.7270/Q2RN3758 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50104723

(2-Benzenesulfonylamino-N-[5-(4-chloro-phenyl)-6H-[...)Show SMILES CC(C)(NS(=O)(=O)c1ccccc1)C(=O)NC1=NN=C(CS1)c1ccc(Cl)cc1 |c:19,t:17| Show InChI InChI=1S/C19H19ClN4O3S2/c1-19(2,24-29(26,27)15-6-4-3-5-7-15)17(25)21-18-23-22-16(12-28-18)13-8-10-14(20)11-9-13/h3-11,24H,12H2,1-2H3,(H,21,23,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universität Bielefeld
Curated by ChEMBL
| Assay Description
In Vitro inhibitory activity against the catalytic domain of the matrix metalloprotease-13 |
J Med Chem 44: 3231-43 (2001)
BindingDB Entry DOI: 10.7270/Q2RN3758 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-14
(Homo sapiens (Human)) | BDBM50104706

(CHEMBL111179 | N-[5-(4-Chloro-phenyl)-6H-[1,3,4]th...)Show SMILES C[C@H](NS(=O)(=O)Cc1ccccc1)C(=O)NC1=NN=C(CS1)c1ccc(Cl)cc1 |c:19,t:17| Show InChI InChI=1S/C19H19ClN4O3S2/c1-13(24-29(26,27)12-14-5-3-2-4-6-14)18(25)21-19-23-22-17(11-28-19)15-7-9-16(20)10-8-15/h2-10,13,24H,11-12H2,1H3,(H,21,23,25)/t13-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universität Bielefeld
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against the ectodomain of the human matrix metalloproteinase-14 |
J Med Chem 44: 3231-43 (2001)
BindingDB Entry DOI: 10.7270/Q2RN3758 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-14
(Homo sapiens (Human)) | BDBM50104718

((R)-N-(5-(4-bromophenyl)-6H-1,3,4-thiadiazin-2-yl)...)Show SMILES C[C@@H](NS(=O)(=O)c1ccccc1)C(=O)NC1=NN=C(CS1)c1ccc(Br)cc1 |c:18,t:16| Show InChI InChI=1S/C18H17BrN4O3S2/c1-12(23-28(25,26)15-5-3-2-4-6-15)17(24)20-18-22-21-16(11-27-18)13-7-9-14(19)10-8-13/h2-10,12,23H,11H2,1H3,(H,20,22,24)/t12-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universität Bielefeld
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against the ectodomain of the human matrix metalloproteinase-14 |
J Med Chem 44: 3231-43 (2001)
BindingDB Entry DOI: 10.7270/Q2RN3758 |
More data for this
Ligand-Target Pair | |
Neutrophil collagenase
(Homo sapiens (Human)) | BDBM50104708
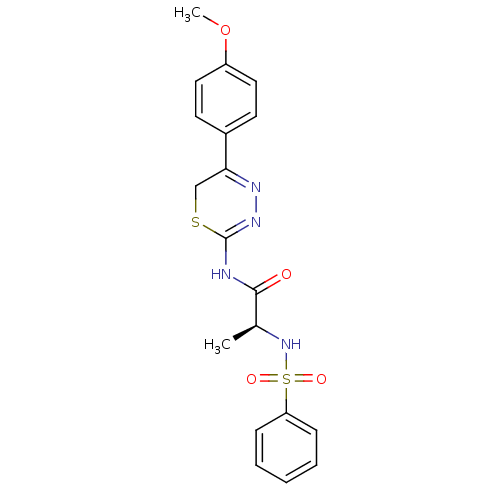
((S)-N-(5-(4-methoxyphenyl)-6H-1,3,4-thiadiazin-2-y...)Show SMILES COc1ccc(cc1)C1=NN=C(NC(=O)[C@H](C)NS(=O)(=O)c2ccccc2)SC1 |t:9,11| Show InChI InChI=1S/C19H20N4O4S2/c1-13(23-29(25,26)16-6-4-3-5-7-16)18(24)20-19-22-21-17(12-28-19)14-8-10-15(27-2)11-9-14/h3-11,13,23H,12H2,1-2H3,(H,20,22,24)/t13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universität Bielefeld
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloprotease-8 |
J Med Chem 44: 3231-43 (2001)
BindingDB Entry DOI: 10.7270/Q2RN3758 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-14
(Homo sapiens (Human)) | BDBM50104706

(CHEMBL111179 | N-[5-(4-Chloro-phenyl)-6H-[1,3,4]th...)Show SMILES C[C@H](NS(=O)(=O)Cc1ccccc1)C(=O)NC1=NN=C(CS1)c1ccc(Cl)cc1 |c:19,t:17| Show InChI InChI=1S/C19H19ClN4O3S2/c1-13(24-29(26,27)12-14-5-3-2-4-6-14)18(25)21-19-23-22-17(11-28-19)15-7-9-16(20)10-8-15/h2-10,13,24H,11-12H2,1H3,(H,21,23,25)/t13-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universität Bielefeld
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against the catalytic domain of human matrix metalloprotease-14 |
J Med Chem 44: 3231-43 (2001)
BindingDB Entry DOI: 10.7270/Q2RN3758 |
More data for this
Ligand-Target Pair | |
Neutrophil collagenase
(Homo sapiens (Human)) | BDBM50104704

((S)-N-(5-(4-fluorophenyl)-6H-1,3,4-thiadiazin-2-yl...)Show SMILES C[C@H](NS(=O)(=O)c1ccccc1)C(=O)NC1=NN=C(CS1)c1ccc(F)cc1 |c:18,t:16| Show InChI InChI=1S/C18H17FN4O3S2/c1-12(23-28(25,26)15-5-3-2-4-6-15)17(24)20-18-22-21-16(11-27-18)13-7-9-14(19)10-8-13/h2-10,12,23H,11H2,1H3,(H,20,22,24)/t12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universität Bielefeld
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloprotease-8 |
J Med Chem 44: 3231-43 (2001)
BindingDB Entry DOI: 10.7270/Q2RN3758 |
More data for this
Ligand-Target Pair | |
Neutrophil collagenase
(Homo sapiens (Human)) | BDBM50104706

(CHEMBL111179 | N-[5-(4-Chloro-phenyl)-6H-[1,3,4]th...)Show SMILES C[C@H](NS(=O)(=O)Cc1ccccc1)C(=O)NC1=NN=C(CS1)c1ccc(Cl)cc1 |c:19,t:17| Show InChI InChI=1S/C19H19ClN4O3S2/c1-13(24-29(26,27)12-14-5-3-2-4-6-14)18(25)21-19-23-22-17(11-28-19)15-7-9-16(20)10-8-15/h2-10,13,24H,11-12H2,1H3,(H,21,23,25)/t13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universität Bielefeld
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloprotease-8 |
J Med Chem 44: 3231-43 (2001)
BindingDB Entry DOI: 10.7270/Q2RN3758 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data