

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50132022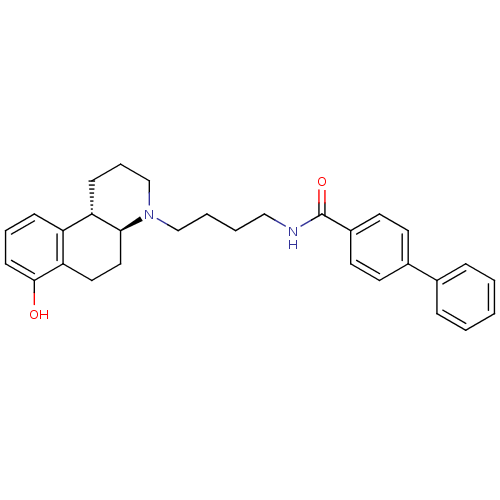 (Biphenyl-4-carboxylic acid [4-((4aS,10bS)-7-hydrox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity at Dopamine receptor D3 expressed in CHO cells by [125I]iodosulpiride displacement. | Bioorg Med Chem Lett 8: 2859-64 (1998) BindingDB Entry DOI: 10.7270/Q2319Z1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50216344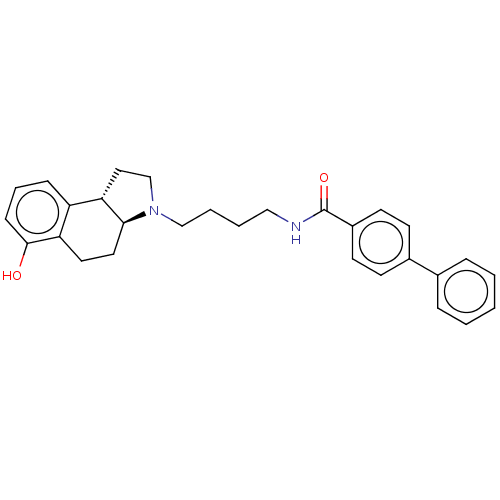 (CHEMBL319116) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity at Dopamine receptor D3 expressed in CHO cells by [125I]iodosulpiride displacement. | Bioorg Med Chem Lett 8: 2859-64 (1998) BindingDB Entry DOI: 10.7270/Q2319Z1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phenylethanolamine N-methyltransferase (Homo sapiens (Human)) | BDBM50554691 (CHEMBL4764318) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Competitive inhibition of C-terminal hexahistidine tag in human recombinant PNMT expressed in Escherichia coli assessed as inhibition constant using ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01475 BindingDB Entry DOI: 10.7270/Q2MK6HJQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phenylethanolamine N-methyltransferase (Homo sapiens (Human)) | BDBM50554692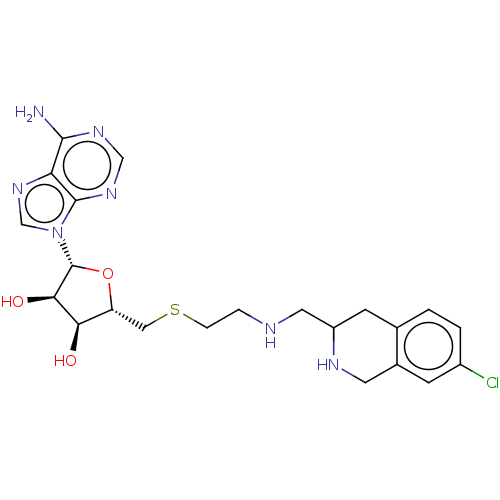 (CHEMBL4751862) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Competitive inhibition of C-terminal hexahistidine tag in human recombinant PNMT expressed in Escherichia coli assessed as inhibition constant using ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01475 BindingDB Entry DOI: 10.7270/Q2MK6HJQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phenylethanolamine N-methyltransferase (Homo sapiens (Human)) | BDBM50554690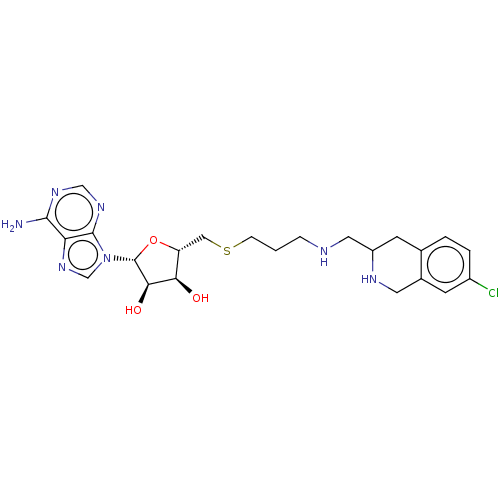 (CHEMBL4782649) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Competitive inhibition of C-terminal hexahistidine tag in human recombinant PNMT expressed in Escherichia coli assessed as inhibition constant using ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01475 BindingDB Entry DOI: 10.7270/Q2MK6HJQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phenylethanolamine N-methyltransferase (Homo sapiens (Human)) | BDBM50554695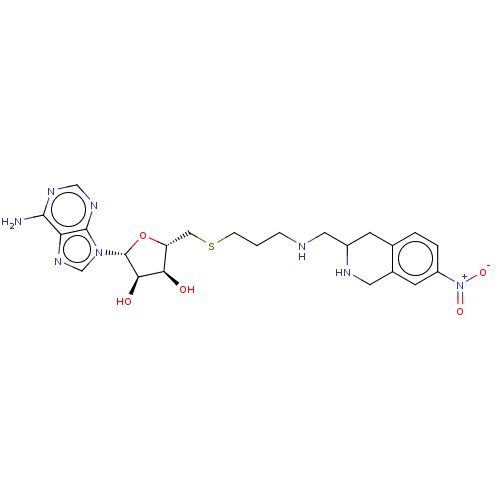 (CHEMBL4760727) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Competitive inhibition of C-terminal hexahistidine tag in human recombinant PNMT expressed in Escherichia coli assessed as inhibition constant using ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01475 BindingDB Entry DOI: 10.7270/Q2MK6HJQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phenylethanolamine N-methyltransferase (Homo sapiens (Human)) | BDBM50554693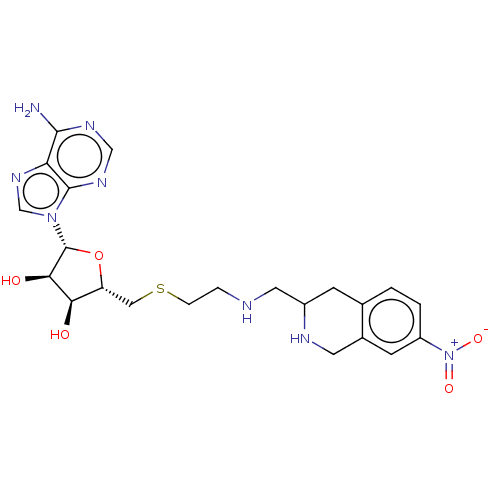 (CHEMBL4800292) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Competitive inhibition of C-terminal hexahistidine tag in human recombinant PNMT expressed in Escherichia coli assessed as inhibition constant using ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01475 BindingDB Entry DOI: 10.7270/Q2MK6HJQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phenylethanolamine N-methyltransferase (Homo sapiens (Human)) | BDBM50554689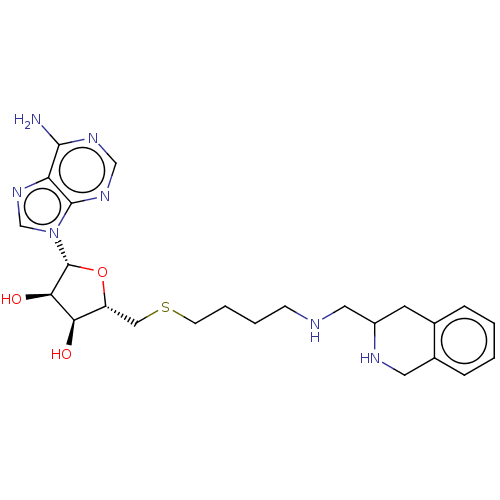 (CHEMBL4754656) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Competitive inhibition of C-terminal hexahistidine tag in human recombinant PNMT expressed in Escherichia coli assessed as inhibition constant using ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01475 BindingDB Entry DOI: 10.7270/Q2MK6HJQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM50470855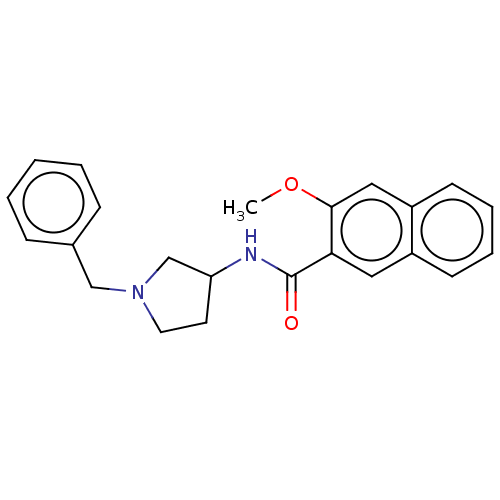 (CHEMBL60708) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Ltd Curated by ChEMBL | Assay Description Binding affinity against cloned human Dopamine receptor D4 using [3H]nemonapride as radioligand | J Med Chem 39: 1946-8 (1996) Article DOI: 10.1021/jm960017l BindingDB Entry DOI: 10.7270/Q2R49THW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50216340 (CHEMBL99876) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity at Dopamine receptor D3 expressed in CHO cells by [125I]iodosulpiride displacement. | Bioorg Med Chem Lett 8: 2859-64 (1998) BindingDB Entry DOI: 10.7270/Q2319Z1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM50470850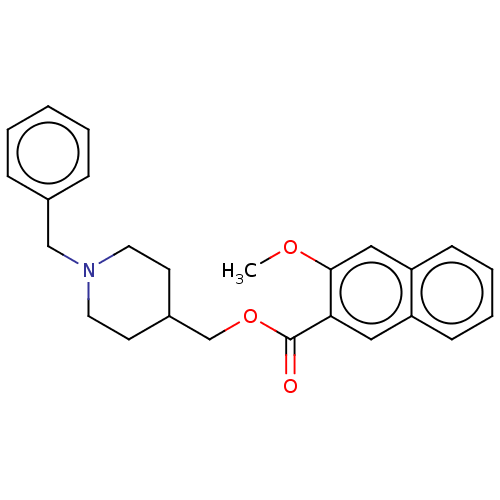 (CHEMBL59326) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Ltd Curated by ChEMBL | Assay Description Binding affinity against cloned human Dopamine receptor D4 using [3H]nemonapride as radioligand | J Med Chem 39: 1946-8 (1996) Article DOI: 10.1021/jm960017l BindingDB Entry DOI: 10.7270/Q2R49THW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM50470854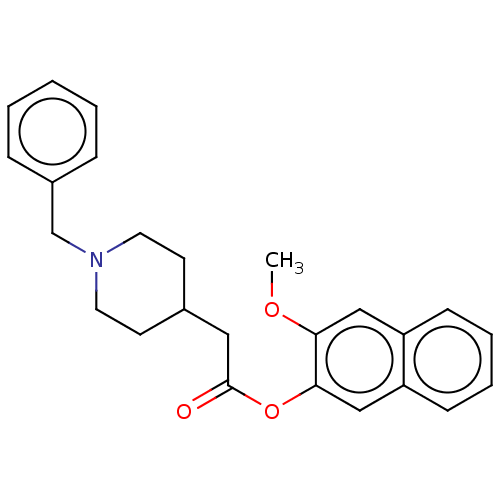 (CHEMBL433255) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Ltd Curated by ChEMBL | Assay Description Binding affinity against cloned human Dopamine receptor D4 using [3H]nemonapride as radioligand | J Med Chem 39: 1946-8 (1996) Article DOI: 10.1021/jm960017l BindingDB Entry DOI: 10.7270/Q2R49THW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50216355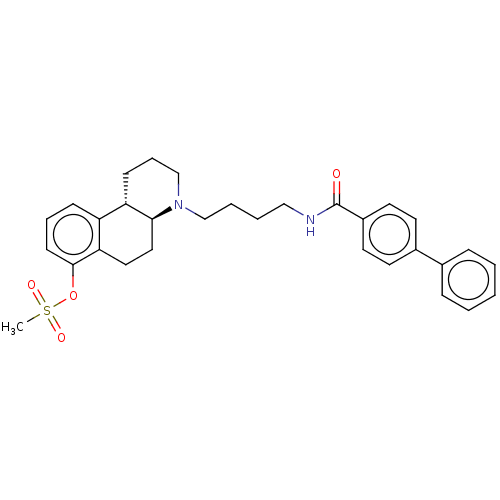 (CHEMBL318925) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity at Dopamine receptor D3 expressed in CHO cells by [125I]iodosulpiride displacement. | Bioorg Med Chem Lett 8: 2859-64 (1998) BindingDB Entry DOI: 10.7270/Q2319Z1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50216339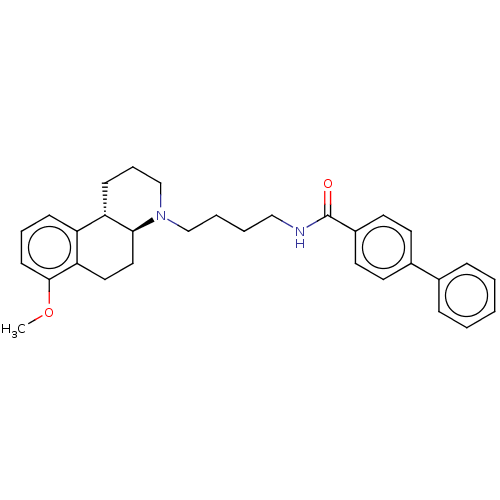 (CHEMBL2111591) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity at Dopamine receptor D3 expressed in CHO cells by [125I]iodosulpiride displacement. | Bioorg Med Chem Lett 8: 2859-64 (1998) BindingDB Entry DOI: 10.7270/Q2319Z1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50216348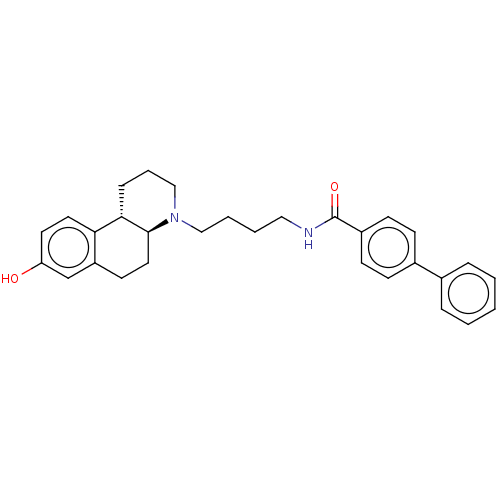 (CHEMBL95759) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity at Dopamine receptor D3 expressed in CHO cells by [125I]iodosulpiride displacement. | Bioorg Med Chem Lett 8: 2859-64 (1998) BindingDB Entry DOI: 10.7270/Q2319Z1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50216351 (CHEMBL2111592) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity at Dopamine receptor D3 expressed in CHO cells by [125I]iodosulpiride displacement. | Bioorg Med Chem Lett 8: 2859-64 (1998) BindingDB Entry DOI: 10.7270/Q2319Z1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50216360 (CHEMBL97856) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity at Dopamine receptor D3 expressed in CHO cells by [125I]iodosulpiride displacement. | Bioorg Med Chem Lett 8: 2859-64 (1998) BindingDB Entry DOI: 10.7270/Q2319Z1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50216350 (CHEMBL97118) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity at Dopamine receptor D3 expressed in CHO cells by [125I]iodosulpiride displacement. | Bioorg Med Chem Lett 8: 2859-64 (1998) BindingDB Entry DOI: 10.7270/Q2319Z1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50216346 (CHEMBL95743) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity at Dopamine receptor D3 expressed in CHO cells by [125I]iodosulpiride displacement. | Bioorg Med Chem Lett 8: 2859-64 (1998) BindingDB Entry DOI: 10.7270/Q2319Z1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50216353 (CHEMBL98452) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity at Dopamine receptor D3 expressed in CHO cells by [125I]iodosulpiride displacement. | Bioorg Med Chem Lett 8: 2859-64 (1998) BindingDB Entry DOI: 10.7270/Q2319Z1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phenylethanolamine N-methyltransferase (Homo sapiens (Human)) | BDBM50554685 (CHEMBL4749263) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Competitive inhibition of C-terminal hexahistidine tag in human recombinant PNMT expressed in Escherichia coli assessed as inhibition constant using ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01475 BindingDB Entry DOI: 10.7270/Q2MK6HJQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM50470852 (CHEMBL57019) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Ltd Curated by ChEMBL | Assay Description Binding affinity against cloned human Dopamine receptor D4 using [3H]nemonapride as radioligand | J Med Chem 39: 1946-8 (1996) Article DOI: 10.1021/jm960017l BindingDB Entry DOI: 10.7270/Q2R49THW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM50203469 (3-Methoxy-naphthalene-2-carboxylic acid (1-benzyl-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Ltd Curated by ChEMBL | Assay Description Binding affinity against cloned human Dopamine receptor D4 using [3H]nemonapride as radioligand | J Med Chem 39: 1946-8 (1996) Article DOI: 10.1021/jm960017l BindingDB Entry DOI: 10.7270/Q2R49THW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM50470849 (CHEMBL59075) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Ltd Curated by ChEMBL | Assay Description Binding affinity against cloned human Dopamine receptor D4 using [3H]nemonapride as radioligand | J Med Chem 39: 1946-8 (1996) Article DOI: 10.1021/jm960017l BindingDB Entry DOI: 10.7270/Q2R49THW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50132022 (Biphenyl-4-carboxylic acid [4-((4aS,10bS)-7-hydrox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity at Dopamine receptor D2 expressed in CHO cells by [125I]iodosulpiride displacement. | Bioorg Med Chem Lett 8: 2859-64 (1998) BindingDB Entry DOI: 10.7270/Q2319Z1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM50470857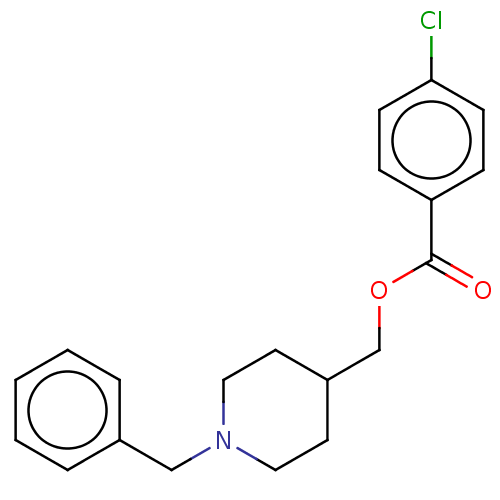 (CHEMBL299479) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Ltd Curated by ChEMBL | Assay Description Binding affinity against cloned human Dopamine receptor D4 using [3H]nemonapride as radioligand | J Med Chem 39: 1946-8 (1996) Article DOI: 10.1021/jm960017l BindingDB Entry DOI: 10.7270/Q2R49THW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM50470851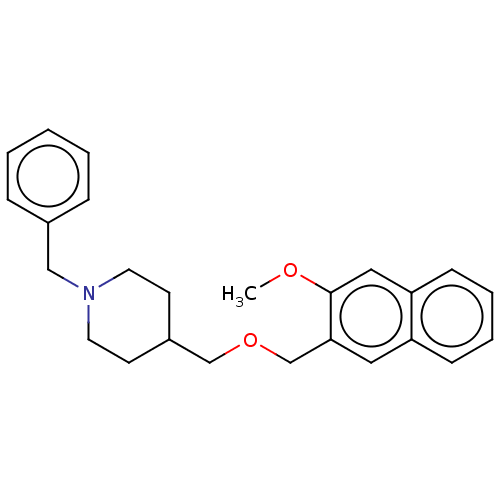 (CHEMBL57407) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Ltd Curated by ChEMBL | Assay Description Binding affinity against cloned human Dopamine receptor D4 using [3H]nemonapride as radioligand | J Med Chem 39: 1946-8 (1996) Article DOI: 10.1021/jm960017l BindingDB Entry DOI: 10.7270/Q2R49THW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM50470856 (CHEMBL292186) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Ltd Curated by ChEMBL | Assay Description Binding affinity against cloned human Dopamine receptor D4 using [3H]nemonapride as radioligand | J Med Chem 39: 1946-8 (1996) Article DOI: 10.1021/jm960017l BindingDB Entry DOI: 10.7270/Q2R49THW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phenylethanolamine N-methyltransferase (Homo sapiens (Human)) | BDBM50554694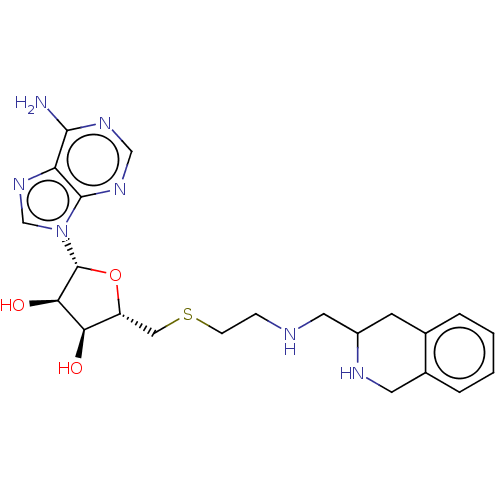 (CHEMBL4740168) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Competitive inhibition of C-terminal hexahistidine tag in human recombinant PNMT expressed in Escherichia coli assessed as inhibition constant using ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01475 BindingDB Entry DOI: 10.7270/Q2MK6HJQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50470855 (CHEMBL60708) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Ltd Curated by ChEMBL | Assay Description Binding affinity against cloned human Dopamine receptor D2 (long) using [125I]iodosulpiride as radioligand | J Med Chem 39: 1946-8 (1996) Article DOI: 10.1021/jm960017l BindingDB Entry DOI: 10.7270/Q2R49THW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2A13 (Homo sapiens (Human)) | BDBM50041234 (6-hydroxy-7-methoxy-5-benzofuranacrylic acid delta...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Mixed inhibition of CYP2A13 (unknown origin) | Drug Metab Dispos 40: 1797-802 (2012) Article DOI: 10.1124/dmd.112.045161 BindingDB Entry DOI: 10.7270/Q2BK1F3P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Phenylethanolamine N-methyltransferase (Homo sapiens (Human)) | BDBM50554681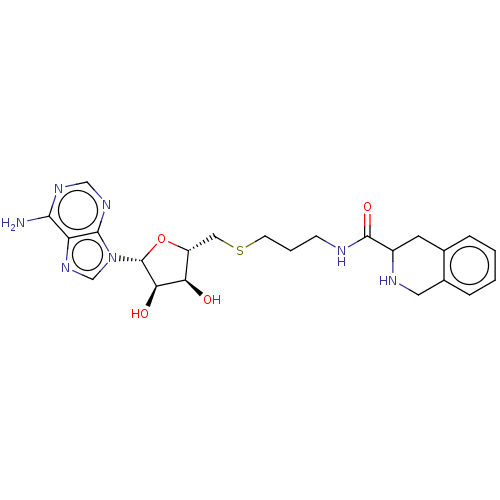 (CHEMBL4795252) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Competitive inhibition of C-terminal hexahistidine tag in human recombinant PNMT expressed in Escherichia coli assessed as inhibition constant using ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01475 BindingDB Entry DOI: 10.7270/Q2MK6HJQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50216357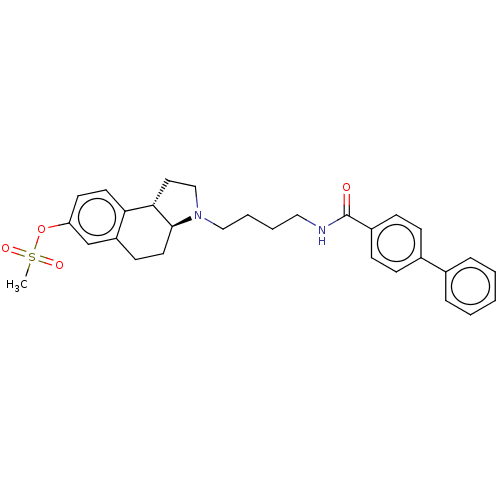 (CHEMBL329702) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity at Dopamine receptor D3 expressed in CHO cells by [125I]iodosulpiride displacement. | Bioorg Med Chem Lett 8: 2859-64 (1998) BindingDB Entry DOI: 10.7270/Q2319Z1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50216359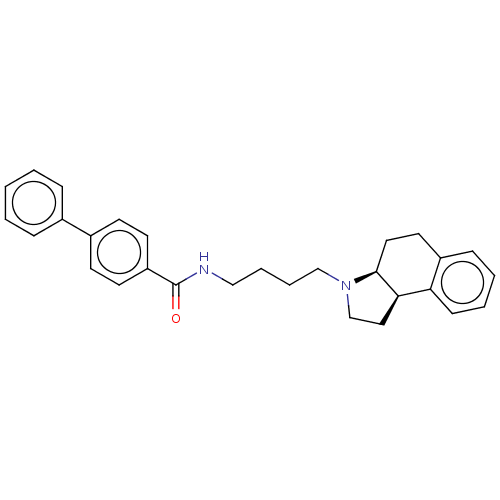 (CHEMBL97107) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity at Dopamine receptor D3 expressed in CHO cells by [125I]iodosulpiride displacement. | Bioorg Med Chem Lett 8: 2859-64 (1998) BindingDB Entry DOI: 10.7270/Q2319Z1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phenylethanolamine N-methyltransferase (Homo sapiens (Human)) | BDBM50554684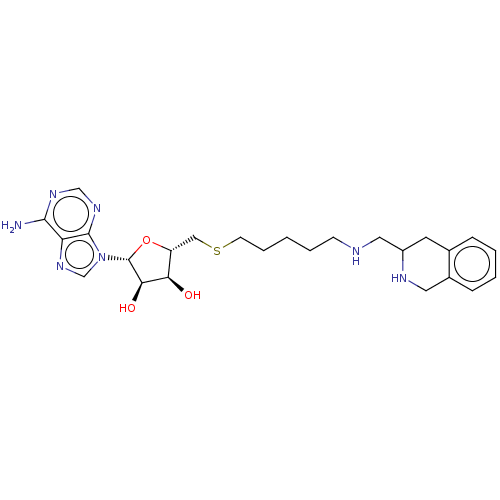 (CHEMBL4796664) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 57 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Competitive inhibition of C-terminal hexahistidine tag in human recombinant PNMT expressed in Escherichia coli assessed as inhibition constant using ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01475 BindingDB Entry DOI: 10.7270/Q2MK6HJQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50216344 (CHEMBL319116) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 63 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity at Dopamine receptor D2 expressed in CHO cells by [125I]iodosulpiride displacement. | Bioorg Med Chem Lett 8: 2859-64 (1998) BindingDB Entry DOI: 10.7270/Q2319Z1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phenylethanolamine N-methyltransferase (Homo sapiens (Human)) | BDBM50554682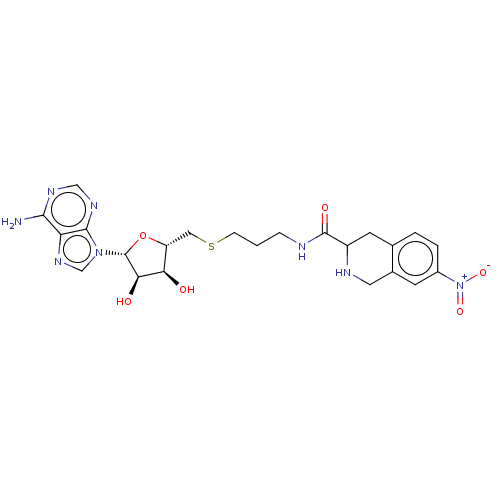 (CHEMBL4789060) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Competitive inhibition of C-terminal hexahistidine tag in human recombinant PNMT expressed in Escherichia coli assessed as inhibition constant using ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01475 BindingDB Entry DOI: 10.7270/Q2MK6HJQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phenylethanolamine N-methyltransferase (Homo sapiens (Human)) | BDBM50554688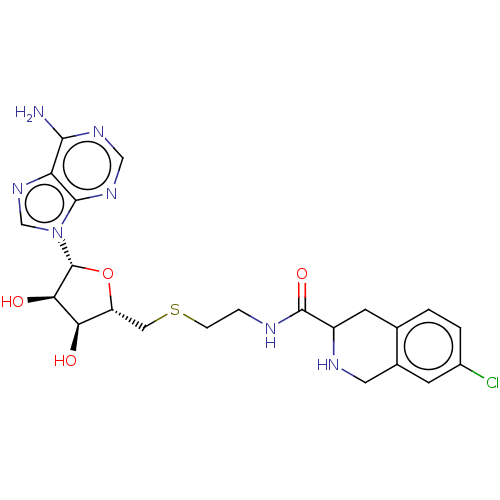 (CHEMBL4791266) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 71 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Competitive inhibition of C-terminal hexahistidine tag in human recombinant PNMT expressed in Escherichia coli assessed as inhibition constant using ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01475 BindingDB Entry DOI: 10.7270/Q2MK6HJQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phenylethanolamine N-methyltransferase (Homo sapiens (Human)) | BDBM50554680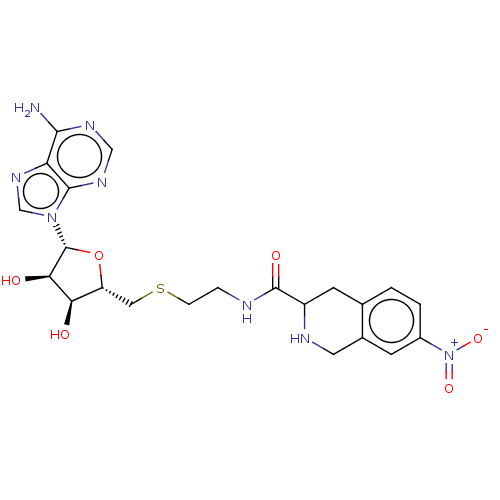 (CHEMBL4791373) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 86 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Competitive inhibition of C-terminal hexahistidine tag in human recombinant PNMT expressed in Escherichia coli assessed as inhibition constant using ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01475 BindingDB Entry DOI: 10.7270/Q2MK6HJQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50203469 (3-Methoxy-naphthalene-2-carboxylic acid (1-benzyl-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Ltd Curated by ChEMBL | Assay Description Binding affinity against cloned human Dopamine receptor D2 (long) using [125I]iodosulpiride as radioligand | J Med Chem 39: 1946-8 (1996) Article DOI: 10.1021/jm960017l BindingDB Entry DOI: 10.7270/Q2R49THW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2A6 (Homo sapiens (Human)) | BDBM50240772 ((1R,2S)-(-)-2-phenylcyclopropylamine | (1R,2S)-2-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Mixed inhibition of CYP2A6 (unknown origin) | Drug Metab Dispos 40: 1797-802 (2012) Article DOI: 10.1124/dmd.112.045161 BindingDB Entry DOI: 10.7270/Q2BK1F3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM50470853 (CHEMBL61090) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Ltd Curated by ChEMBL | Assay Description Binding affinity against cloned human Dopamine receptor D4 using [3H]nemonapride as radioligand | J Med Chem 39: 1946-8 (1996) Article DOI: 10.1021/jm960017l BindingDB Entry DOI: 10.7270/Q2R49THW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50216345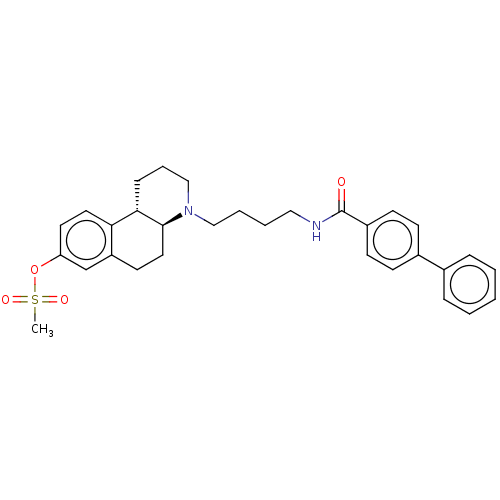 (CHEMBL97440) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity at Dopamine receptor D3 expressed in CHO cells by [125I]iodosulpiride displacement. | Bioorg Med Chem Lett 8: 2859-64 (1998) BindingDB Entry DOI: 10.7270/Q2319Z1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2A6 (Homo sapiens (Human)) | BDBM50041234 (6-hydroxy-7-methoxy-5-benzofuranacrylic acid delta...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Mixed inhibition of CYP2A6 (unknown origin) | Drug Metab Dispos 40: 1797-802 (2012) Article DOI: 10.1124/dmd.112.045161 BindingDB Entry DOI: 10.7270/Q2BK1F3P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50216358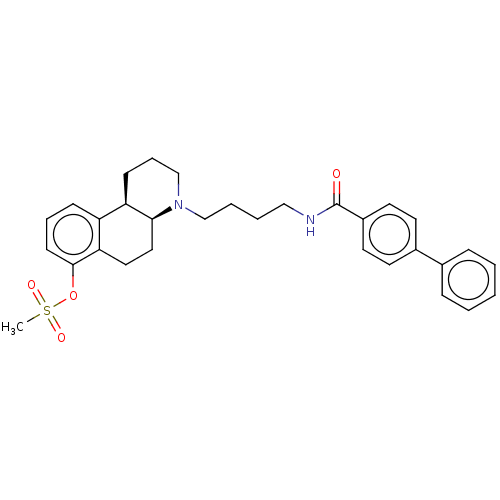 (CHEMBL317996) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity at Dopamine receptor D3 expressed in CHO cells by [125I]iodosulpiride displacement. | Bioorg Med Chem Lett 8: 2859-64 (1998) BindingDB Entry DOI: 10.7270/Q2319Z1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50216356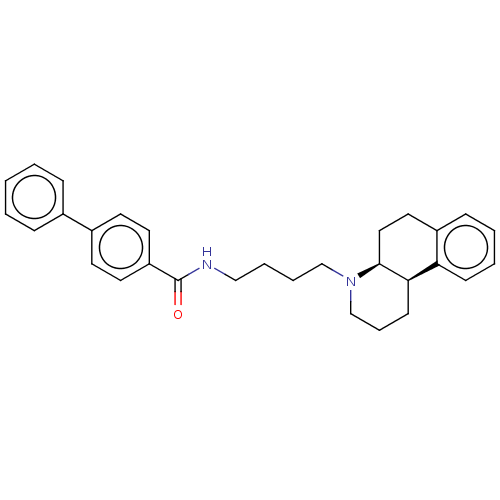 (CHEMBL97840) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity at Dopamine receptor D3 expressed in CHO cells by [125I]iodosulpiride displacement. | Bioorg Med Chem Lett 8: 2859-64 (1998) BindingDB Entry DOI: 10.7270/Q2319Z1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50216355 (CHEMBL318925) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity at Dopamine receptor D2 expressed in CHO cells by [125I]iodosulpiride displacement. | Bioorg Med Chem Lett 8: 2859-64 (1998) BindingDB Entry DOI: 10.7270/Q2319Z1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50216340 (CHEMBL99876) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity at Dopamine receptor D2 expressed in CHO cells by [125I]iodosulpiride displacement. | Bioorg Med Chem Lett 8: 2859-64 (1998) BindingDB Entry DOI: 10.7270/Q2319Z1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50216360 (CHEMBL97856) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity at Dopamine receptor D2 expressed in CHO cells by [125I]iodosulpiride displacement. | Bioorg Med Chem Lett 8: 2859-64 (1998) BindingDB Entry DOI: 10.7270/Q2319Z1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50216351 (CHEMBL2111592) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity at Dopamine receptor D2 expressed in CHO cells by [125I]iodosulpiride displacement. | Bioorg Med Chem Lett 8: 2859-64 (1998) BindingDB Entry DOI: 10.7270/Q2319Z1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 176 total ) | Next | Last >> |