 Found 37 hits with Last Name = 'senderowitz' and Initial = 'h'
Found 37 hits with Last Name = 'senderowitz' and Initial = 'h' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Ectonucleotide pyrophosphatase/phosphodiesterase family member 1
(Homo sapiens (Human)) | BDBM50019296
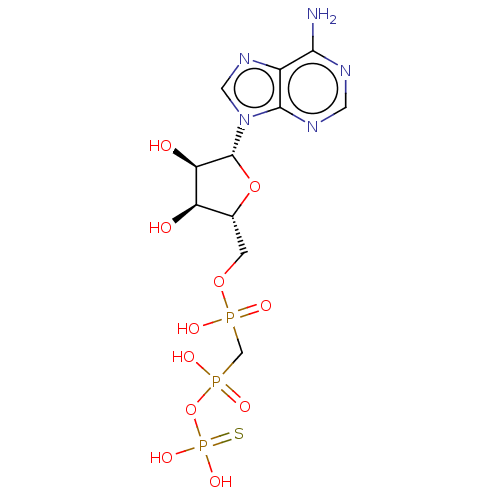
(CHEMBL3289396)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](COP(O)(=O)CP(O)(=O)OP(O)(O)=S)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C11H18N5O11P3S/c12-9-6-10(14-2-13-9)16(3-15-6)11-8(18)7(17)5(26-11)1-25-28(19,20)4-29(21,22)27-30(23,24)31/h2-3,5,7-8,11,17-18H,1,4H2,(H,19,20)(H,21,22)(H2,12,13,14)(H2,23,24,31)/t5-,7-,8-,11-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bar-Ilan University
Curated by ChEMBL
| Assay Description
Mixed type inhibition of human NPP1 using pNP-TMP as substrate after 20 mins by Dixon and Cornish-Bowden method |
J Med Chem 57: 4677-91 (2014)
Article DOI: 10.1021/jm500196c
BindingDB Entry DOI: 10.7270/Q2QN68C6 |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 1
(Homo sapiens (Human)) | BDBM50453241
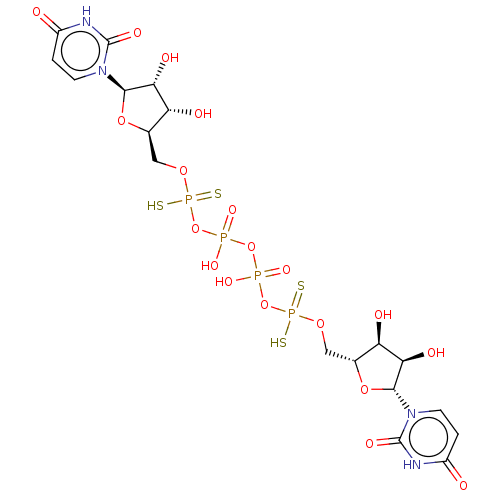
(CHEMBL4206625)Show SMILES O[C@H]1[C@@H](O)[C@@H](O[C@@H]1COP(S)(=S)OP(O)(=O)OP(O)(=O)OP(S)(=S)OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1ccc(=O)[nH]c1=O)n1ccc(=O)[nH]c1=O |r| Show InChI InChI=1S/C18H26N4O19P4S4/c23-9-1-3-21(17(29)19-9)15-13(27)11(25)7(37-15)5-35-44(46,47)40-42(31,32)39-43(33,34)41-45(48,49)36-6-8-12(26)14(28)16(38-8)22-4-2-10(24)20-18(22)30/h1-4,7-8,11-16,25-28H,5-6H2,(H,31,32)(H,33,34)(H,46,47)(H,48,49)(H,19,23,29)(H,20,24,30)/t7-,8-,11-,12-,13-,14-,15-,16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bar-Ilan University
Curated by ChEMBL
| Assay Description
Inhibition of human NPP1 using pNP-TMP as substrate preincubated for 3 mins followed by substrate addition measured after 15 mins |
J Med Chem 61: 3939-3951 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01906
BindingDB Entry DOI: 10.7270/Q2DR2Z3M |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 1
(Homo sapiens (Human)) | BDBM50453240
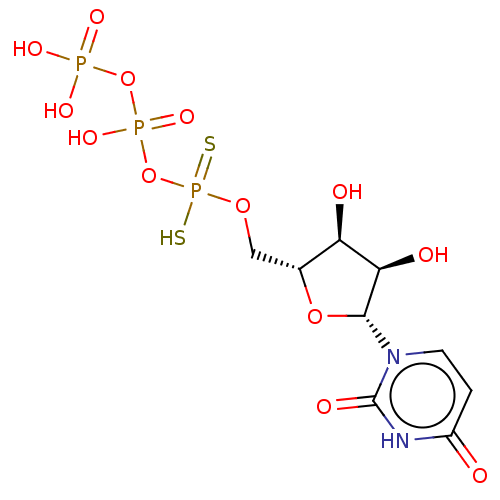
(CHEMBL4218881)Show SMILES O[C@H]1[C@@H](O)[C@@H](O[C@@H]1COP(S)(=S)OP(O)(=O)OP(O)(O)=O)n1ccc(=O)[nH]c1=O |r| Show InChI InChI=1S/C9H15N2O13P3S2/c12-5-1-2-11(9(15)10-5)8-7(14)6(13)4(22-8)3-21-27(28,29)24-26(19,20)23-25(16,17)18/h1-2,4,6-8,13-14H,3H2,(H,19,20)(H,28,29)(H,10,12,15)(H2,16,17,18)/t4-,6-,7-,8-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bar-Ilan University
Curated by ChEMBL
| Assay Description
Inhibition of human NPP1 using pNP-TMP as substrate preincubated for 3 mins followed by substrate addition measured after 15 mins |
J Med Chem 61: 3939-3951 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01906
BindingDB Entry DOI: 10.7270/Q2DR2Z3M |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 1
(Homo sapiens (Human)) | BDBM50442939
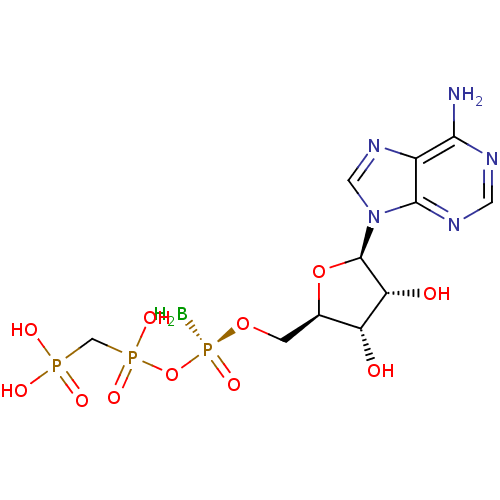
(CHEMBL3087158)Show SMILES B[P@@](=O)(OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)OP(O)(=O)CP(O)(O)=O |r| Show InChI InChI=1S/C11H19BN5O11P3/c12-31(25,28-30(23,24)4-29(20,21)22)26-1-5-7(18)8(19)11(27-5)17-3-16-6-9(13)14-2-15-10(6)17/h2-3,5,7-8,11,18-19H,1,4,12H2,(H,23,24)(H2,13,14,15)(H2,20,21,22)/t5-,7-,8-,11-,31-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université Laval
Curated by ChEMBL
| Assay Description
Mixed type inhibition of human NPP1 expressed in COS7 cells assessed as production of p-nitrophenol using pnp-TMP as the substrate preincubated for 3... |
J Med Chem 56: 8308-20 (2013)
Article DOI: 10.1021/jm400918s
BindingDB Entry DOI: 10.7270/Q2F47QMF |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 1
(Homo sapiens (Human)) | BDBM50019294
(CHEMBL3289394)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](CO[P@](O)(=S)OP(O)(=O)C(Cl)(Cl)P(O)(O)=O)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C11H16Cl2N5O11P3S/c12-11(13,30(21,22)23)31(24,25)29-32(26,33)27-1-4-6(19)7(20)10(28-4)18-3-17-5-8(14)15-2-16-9(5)18/h2-4,6-7,10,19-20H,1H2,(H,24,25)(H,26,33)(H2,14,15,16)(H2,21,22,23)/t4-,6-,7-,10-,32+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 685 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bar-Ilan University
Curated by ChEMBL
| Assay Description
Mixed type inhibition of human NPP1 using pNP-TMP as substrate after 20 mins by Dixon and Cornish-Bowden method |
J Med Chem 57: 4677-91 (2014)
Article DOI: 10.1021/jm500196c
BindingDB Entry DOI: 10.7270/Q2QN68C6 |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 1
(Homo sapiens (Human)) | BDBM50504513
(CHEMBL4465876)Show SMILES OP(O)(=O)C(Cl)(Cl)P(O)(=O)OP(S)(=S)OCCOCn1ccc(=O)[nH]c1=O Show InChI InChI=1S/C8H13Cl2N2O10P3S2/c9-8(10,23(15,16)17)24(18,19)22-25(26,27)21-4-3-20-5-12-2-1-6(13)11-7(12)14/h1-2H,3-5H2,(H,18,19)(H,26,27)(H,11,13,14)(H2,15,16,17) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 730 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bar-Ilan University
Curated by ChEMBL
| Assay Description
Inhibition of human NPP1 expressed in COS7 cell membranes using pNP-TMP as substrate preincubated for 3 mins followed by substrate addition and measu... |
Eur J Med Chem 184: (2019)
Article DOI: 10.1016/j.ejmech.2019.111754
BindingDB Entry DOI: 10.7270/Q2C250Q1 |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 1
(Homo sapiens (Human)) | BDBM50504512
(CHEMBL4443638)Show SMILES OP(O)(=O)CP(O)(=O)OP(S)(=S)OCCOCn1ccc(=O)[nH]c1=O Show InChI InChI=1S/C8H15N2O10P3S2/c11-7-1-2-10(8(12)9-7)5-18-3-4-19-23(24,25)20-22(16,17)6-21(13,14)15/h1-2H,3-6H2,(H,16,17)(H,24,25)(H,9,11,12)(H2,13,14,15) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 940 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bar-Ilan University
Curated by ChEMBL
| Assay Description
Inhibition of human NPP1 expressed in COS7 cell membranes using pNP-TMP as substrate preincubated for 3 mins followed by substrate addition and measu... |
Eur J Med Chem 184: (2019)
Article DOI: 10.1016/j.ejmech.2019.111754
BindingDB Entry DOI: 10.7270/Q2C250Q1 |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 1
(Homo sapiens (Human)) | BDBM50453242
(CHEMBL4213959)Show SMILES O[C@H]1[C@@H](O)[C@@H](O[C@@H]1COP(S)(=S)OP(O)(=O)CP(O)(O)=O)n1ccc(=O)[nH]c1=O |r| Show InChI InChI=1S/C10H17N2O12P3S2/c13-6-1-2-12(10(16)11-6)9-8(15)7(14)5(23-9)3-22-27(28,29)24-26(20,21)4-25(17,18)19/h1-2,5,7-9,14-15H,3-4H2,(H,20,21)(H,28,29)(H,11,13,16)(H2,17,18,19)/t5-,7-,8-,9-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.11E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bar-Ilan University
Curated by ChEMBL
| Assay Description
Inhibition of human NPP1 using pNP-TMP as substrate preincubated for 3 mins followed by substrate addition measured after 15 mins |
J Med Chem 61: 3939-3951 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01906
BindingDB Entry DOI: 10.7270/Q2DR2Z3M |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 1
(Homo sapiens (Human)) | BDBM50504514
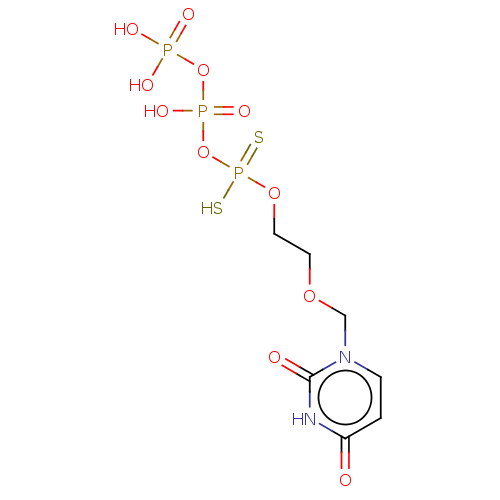
(CHEMBL4450742)Show SMILES OP(O)(=O)OP(O)(=O)OP(S)(=S)OCCOCn1ccc(=O)[nH]c1=O Show InChI InChI=1S/C7H13N2O11P3S2/c10-6-1-2-9(7(11)8-6)5-17-3-4-18-23(24,25)20-22(15,16)19-21(12,13)14/h1-2H,3-5H2,(H,15,16)(H,24,25)(H,8,10,11)(H2,12,13,14) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 1.28E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bar-Ilan University
Curated by ChEMBL
| Assay Description
Inhibition of human NPP1 expressed in COS7 cell membranes using pNP-TMP as substrate preincubated for 3 mins followed by substrate addition and measu... |
Eur J Med Chem 184: (2019)
Article DOI: 10.1016/j.ejmech.2019.111754
BindingDB Entry DOI: 10.7270/Q2C250Q1 |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 1
(Homo sapiens (Human)) | BDBM50019292
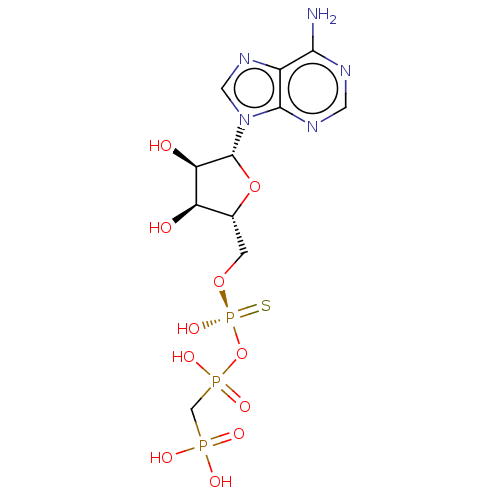
(CHEMBL3289393)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](CO[P@@](O)(=S)OP(O)(=O)CP(O)(O)=O)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C11H18N5O11P3S/c12-9-6-10(14-2-13-9)16(3-15-6)11-8(18)7(17)5(26-11)1-25-30(24,31)27-29(22,23)4-28(19,20)21/h2-3,5,7-8,11,17-18H,1,4H2,(H,22,23)(H,24,31)(H2,12,13,14)(H2,19,20,21)/t5-,7-,8-,11-,30-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bar-Ilan University
Curated by ChEMBL
| Assay Description
Mixed type inhibition of human NPP1 using pNP-TMP as substrate after 20 mins by Dixon and Cornish-Bowden method |
J Med Chem 57: 4677-91 (2014)
Article DOI: 10.1021/jm500196c
BindingDB Entry DOI: 10.7270/Q2QN68C6 |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 1
(Homo sapiens (Human)) | BDBM50504510
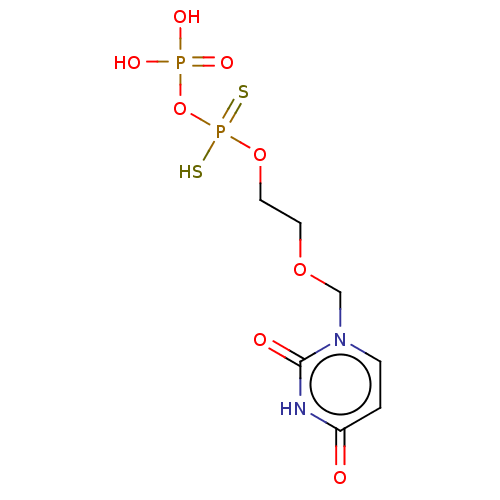
(CHEMBL4558341)Show InChI InChI=1S/C7H12N2O8P2S2/c10-6-1-2-9(7(11)8-6)5-15-3-4-16-19(20,21)17-18(12,13)14/h1-2H,3-5H2,(H,20,21)(H,8,10,11)(H2,12,13,14) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.83E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bar-Ilan University
Curated by ChEMBL
| Assay Description
Inhibition of human NPP1 expressed in COS7 cell membranes using pNP-TMP as substrate preincubated for 3 mins followed by substrate addition and measu... |
Eur J Med Chem 184: (2019)
Article DOI: 10.1016/j.ejmech.2019.111754
BindingDB Entry DOI: 10.7270/Q2C250Q1 |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 1
(Homo sapiens (Human)) | BDBM50019291
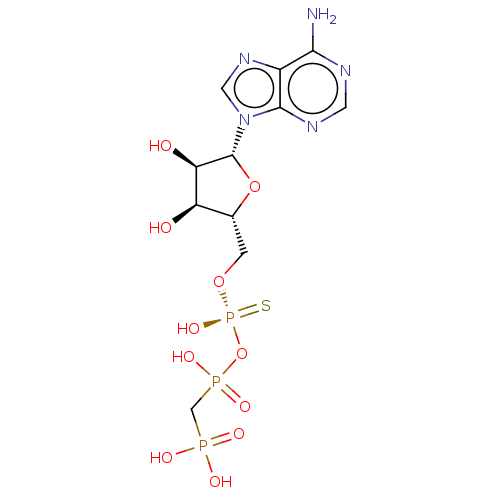
(CHEMBL3289392)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](CO[P@](O)(=S)OP(O)(=O)CP(O)(O)=O)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C11H18N5O11P3S/c12-9-6-10(14-2-13-9)16(3-15-6)11-8(18)7(17)5(26-11)1-25-30(24,31)27-29(22,23)4-28(19,20)21/h2-3,5,7-8,11,17-18H,1,4H2,(H,22,23)(H,24,31)(H2,12,13,14)(H2,19,20,21)/t5-,7-,8-,11-,30+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bar-Ilan University
Curated by ChEMBL
| Assay Description
Mixed type inhibition of human NPP1 using pNP-TMP as substrate after 20 mins by Dixon and Cornish-Bowden method |
J Med Chem 57: 4677-91 (2014)
Article DOI: 10.1021/jm500196c
BindingDB Entry DOI: 10.7270/Q2QN68C6 |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 1
(Homo sapiens (Human)) | BDBM50019291

(CHEMBL3289392)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](CO[P@](O)(=S)OP(O)(=O)CP(O)(O)=O)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C11H18N5O11P3S/c12-9-6-10(14-2-13-9)16(3-15-6)11-8(18)7(17)5(26-11)1-25-30(24,31)27-29(22,23)4-28(19,20)21/h2-3,5,7-8,11,17-18H,1,4H2,(H,22,23)(H,24,31)(H2,12,13,14)(H2,19,20,21)/t5-,7-,8-,11-,30+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bar-Ilan University
Curated by ChEMBL
| Assay Description
Mixed type inhibition of human NPP1 using pNP-TMP as substrate assessed as dissociation constant for enzyme-inhibitor-substrate complex after 20 mins... |
J Med Chem 57: 4677-91 (2014)
Article DOI: 10.1021/jm500196c
BindingDB Entry DOI: 10.7270/Q2QN68C6 |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 1
(Homo sapiens (Human)) | BDBM50442938
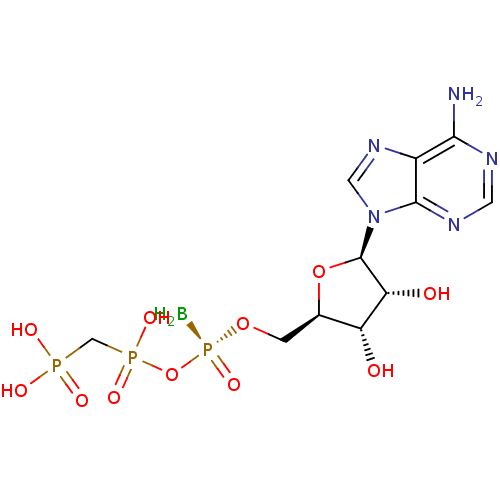
(CHEMBL3087159)Show SMILES B[P@](=O)(OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)OP(O)(=O)CP(O)(O)=O |r| Show InChI InChI=1S/C11H19BN5O11P3/c12-31(25,28-30(23,24)4-29(20,21)22)26-1-5-7(18)8(19)11(27-5)17-3-16-6-9(13)14-2-15-10(6)17/h2-3,5,7-8,11,18-19H,1,4,12H2,(H,23,24)(H2,13,14,15)(H2,20,21,22)/t5-,7-,8-,11-,31+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université Laval
Curated by ChEMBL
| Assay Description
Mixed type inhibition of human NPP1 expressed in COS7 cells assessed as production of p-nitrophenol using pnp-TMP as the substrate preincubated for 3... |
J Med Chem 56: 8308-20 (2013)
Article DOI: 10.1021/jm400918s
BindingDB Entry DOI: 10.7270/Q2F47QMF |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 1
(Homo sapiens (Human)) | BDBM50019295

(CHEMBL3289395)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](CO[P@@](O)(=S)OP(O)(=O)C(Cl)(Cl)P(O)(O)=O)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C11H16Cl2N5O11P3S/c12-11(13,30(21,22)23)31(24,25)29-32(26,33)27-1-4-6(19)7(20)10(28-4)18-3-17-5-8(14)15-2-16-9(5)18/h2-4,6-7,10,19-20H,1H2,(H,24,25)(H,26,33)(H2,14,15,16)(H2,21,22,23)/t4-,6-,7-,10-,32-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.52E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bar-Ilan University
Curated by ChEMBL
| Assay Description
Mixed type inhibition of human NPP1 using pNP-TMP as substrate after 20 mins by Dixon and Cornish-Bowden method |
J Med Chem 57: 4677-91 (2014)
Article DOI: 10.1021/jm500196c
BindingDB Entry DOI: 10.7270/Q2QN68C6 |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 1
(Homo sapiens (Human)) | BDBM50504511
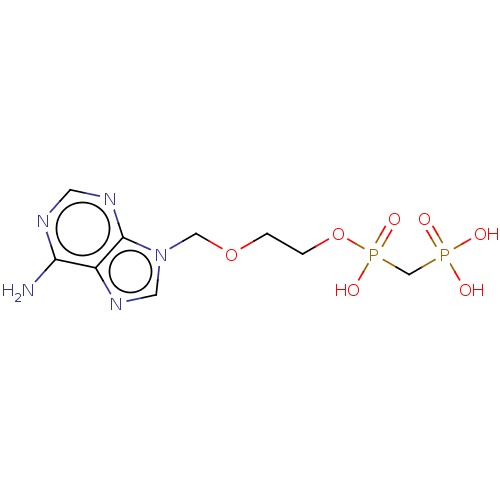
(CHEMBL4545695)Show InChI InChI=1S/C9H15N5O7P2/c10-8-7-9(12-3-11-8)14(4-13-7)5-20-1-2-21-23(18,19)6-22(15,16)17/h3-4H,1-2,5-6H2,(H,18,19)(H2,10,11,12)(H2,15,16,17) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.63E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bar-Ilan University
Curated by ChEMBL
| Assay Description
Inhibition of full-length human NPP-1 expressed in baculovirus infected Sf9 cells using p-Nph5-TMP as substrate after 60 mins by colorimetric assay |
Eur J Med Chem 184: (2019)
Article DOI: 10.1016/j.ejmech.2019.111754
BindingDB Entry DOI: 10.7270/Q2C250Q1 |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 1
(Homo sapiens (Human)) | BDBM50442936
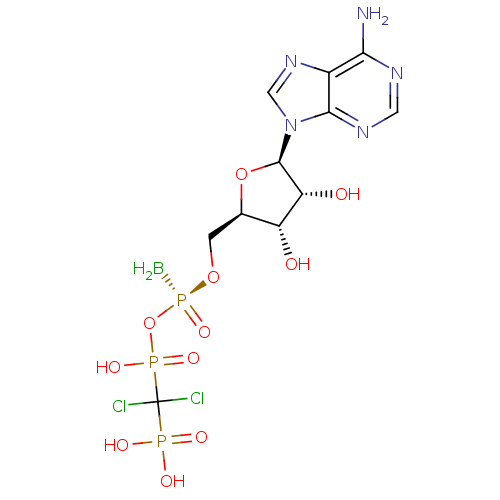
(CHEMBL3087166)Show SMILES B[P@@](=O)(OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)OP(O)(=O)C(Cl)(Cl)P(O)(O)=O |r| Show InChI InChI=1S/C11H17BCl2N5O11P3/c12-33(27,30-32(25,26)11(13,14)31(22,23)24)28-1-4-6(20)7(21)10(29-4)19-3-18-5-8(15)16-2-17-9(5)19/h2-4,6-7,10,20-21H,1,12H2,(H,25,26)(H2,15,16,17)(H2,22,23,24)/t4-,6-,7-,10-,33-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université Laval
Curated by ChEMBL
| Assay Description
Mixed type inhibition of human NPP1 expressed in COS7 cells assessed as production of p-nitrophenol using pnp-TMP as the substrate preincubated for 3... |
J Med Chem 56: 8308-20 (2013)
Article DOI: 10.1021/jm400918s
BindingDB Entry DOI: 10.7270/Q2F47QMF |
More data for this
Ligand-Target Pair | |
Ectonucleoside triphosphate diphosphohydrolase 2
(Homo sapiens (Human)) | BDBM50048062
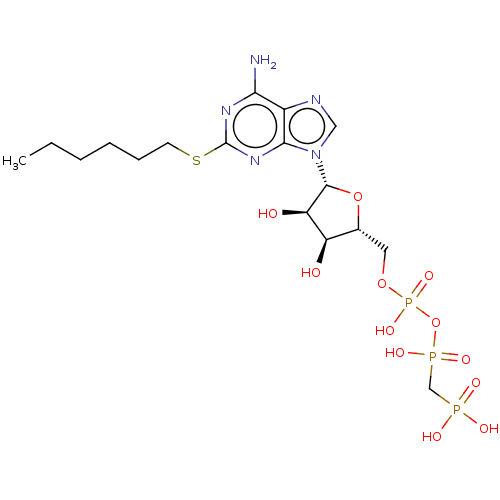
(CHEMBL3314932)Show SMILES CCCCCCSc1nc(N)c2ncn([C@@H]3O[C@H](COP(O)(=O)OP(O)(=O)CP(O)(O)=O)[C@@H](O)[C@H]3O)c2n1 |r| Show InChI InChI=1S/C17H30N5O12P3S/c1-2-3-4-5-6-38-17-20-14(18)11-15(21-17)22(8-19-11)16-13(24)12(23)10(33-16)7-32-37(30,31)34-36(28,29)9-35(25,26)27/h8,10,12-13,16,23-24H,2-7,9H2,1H3,(H,28,29)(H,30,31)(H2,18,20,21)(H2,25,26,27)/t10-,12-,13-,16-/m1/s1 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bar-Ilan University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human NTPDase2 transfected in COS-7 cells using ATP substrate |
J Med Chem 57: 5919-34 (2014)
Article DOI: 10.1021/jm401933c
BindingDB Entry DOI: 10.7270/Q2B27WXX |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 1
(Homo sapiens (Human)) | BDBM50315826

(CHEMBL1094568 | [({[(2R,3S,4R,5R)-5-[6-amino-2-(me...)Show SMILES CSc1nc(N)c2ncn([C@@H]3O[C@H](COP(O)(=O)C(F)(F)P(O)(O)=O)[C@@H](O)[C@H]3O)c2n1 |r| Show InChI InChI=1S/C12H17F2N5O9P2S/c1-31-11-17-8(15)5-9(18-11)19(3-16-5)10-7(21)6(20)4(28-10)2-27-30(25,26)12(13,14)29(22,23)24/h3-4,6-7,10,20-21H,2H2,1H3,(H,25,26)(H2,15,17,18)(H2,22,23,24)/t4-,6-,7-,10-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université Laval
Curated by ChEMBL
| Assay Description
Mixed type inhibition of human NPP1 expressed in COS7 cells assessed as production of p-nitrophenol using pnp-TMP as the substrate preincubated for 3... |
J Med Chem 56: 8308-20 (2013)
Article DOI: 10.1021/jm400918s
BindingDB Entry DOI: 10.7270/Q2F47QMF |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 1
(Homo sapiens (Human)) | BDBM50442935
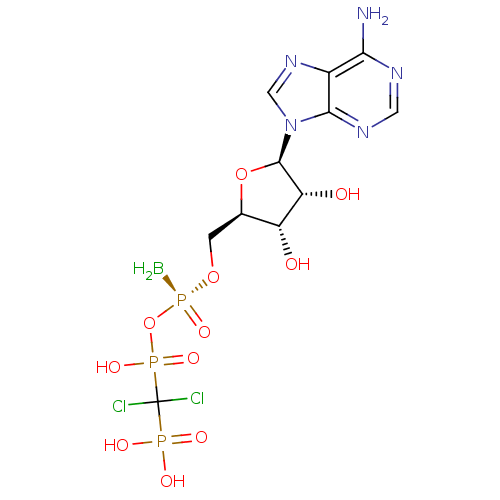
(CHEMBL3087167)Show SMILES B[P@](=O)(OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)OP(O)(=O)C(Cl)(Cl)P(O)(O)=O |r| Show InChI InChI=1S/C11H17BCl2N5O11P3/c12-33(27,30-32(25,26)11(13,14)31(22,23)24)28-1-4-6(20)7(21)10(29-4)19-3-18-5-8(15)16-2-17-9(5)19/h2-4,6-7,10,20-21H,1,12H2,(H,25,26)(H2,15,16,17)(H2,22,23,24)/t4-,6-,7-,10-,33+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université Laval
Curated by ChEMBL
| Assay Description
Mixed type inhibition of human NPP1 expressed in COS7 cells assessed as production of p-nitrophenol using pnp-TMP as the substrate preincubated for 3... |
J Med Chem 56: 8308-20 (2013)
Article DOI: 10.1021/jm400918s
BindingDB Entry DOI: 10.7270/Q2F47QMF |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 1
(Homo sapiens (Human)) | BDBM50315822

(({[({[(2R,3S,4R,5R)-5-[6-amino-2-(methylsulfanyl)-...)Show SMILES CSc1nc(N)c2ncn([C@@H]3O[C@H](COP(O)(=O)OP(O)(=O)C(F)(F)P(O)(O)=O)[C@@H](O)[C@H]3O)c2n1 |r| Show InChI InChI=1S/C12H18F2N5O12P3S/c1-35-11-17-8(15)5-9(18-11)19(3-16-5)10-7(21)6(20)4(30-10)2-29-34(27,28)31-33(25,26)12(13,14)32(22,23)24/h3-4,6-7,10,20-21H,2H2,1H3,(H,25,26)(H,27,28)(H2,15,17,18)(H2,22,23,24)/t4-,6-,7-,10-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université Laval
Curated by ChEMBL
| Assay Description
Mixed type inhibition of human NPP1 expressed in COS7 cells assessed as production of p-nitrophenol using pnp-TMP as the substrate preincubated for 3... |
J Med Chem 56: 8308-20 (2013)
Article DOI: 10.1021/jm400918s
BindingDB Entry DOI: 10.7270/Q2F47QMF |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 1
(Homo sapiens (Human)) | BDBM50442937
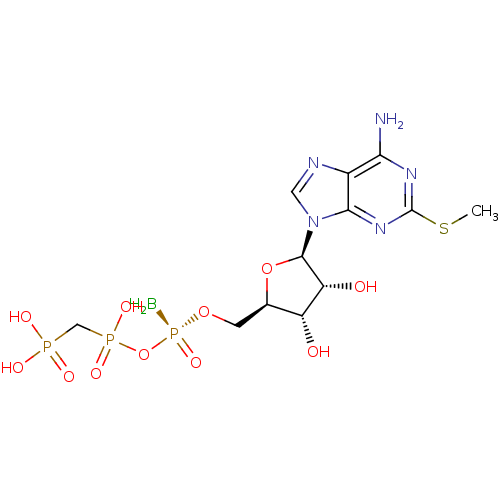
(CHEMBL3087160)Show SMILES B[P@](=O)(OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)nc(SC)nc12)OP(O)(=O)CP(O)(O)=O |r| Show InChI InChI=1S/C12H21BN5O11P3S/c1-33-12-16-9(14)6-10(17-12)18(3-15-6)11-8(20)7(19)5(28-11)2-27-32(13,26)29-31(24,25)4-30(21,22)23/h3,5,7-8,11,19-20H,2,4,13H2,1H3,(H,24,25)(H2,14,16,17)(H2,21,22,23)/t5-,7-,8-,11-,32+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université Laval
Curated by ChEMBL
| Assay Description
Mixed type inhibition of human NPP1 expressed in COS7 cells assessed as production of p-nitrophenol using pnp-TMP as the substrate preincubated for 3... |
J Med Chem 56: 8308-20 (2013)
Article DOI: 10.1021/jm400918s
BindingDB Entry DOI: 10.7270/Q2F47QMF |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 1
(Homo sapiens (Human)) | BDBM50019295

(CHEMBL3289395)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](CO[P@@](O)(=S)OP(O)(=O)C(Cl)(Cl)P(O)(O)=O)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C11H16Cl2N5O11P3S/c12-11(13,30(21,22)23)31(24,25)29-32(26,33)27-1-4-6(19)7(20)10(28-4)18-3-17-5-8(14)15-2-16-9(5)18/h2-4,6-7,10,19-20H,1H2,(H,24,25)(H,26,33)(H2,14,15,16)(H2,21,22,23)/t4-,6-,7-,10-,32-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.92E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bar-Ilan University
Curated by ChEMBL
| Assay Description
Mixed type inhibition of human NPP1 using pNP-TMP as substrate assessed as dissociation constant for enzyme-inhibitor-substrate complex after 20 mins... |
J Med Chem 57: 4677-91 (2014)
Article DOI: 10.1021/jm500196c
BindingDB Entry DOI: 10.7270/Q2QN68C6 |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 1
(Homo sapiens (Human)) | BDBM50453241

(CHEMBL4206625)Show SMILES O[C@H]1[C@@H](O)[C@@H](O[C@@H]1COP(S)(=S)OP(O)(=O)OP(O)(=O)OP(S)(=S)OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1ccc(=O)[nH]c1=O)n1ccc(=O)[nH]c1=O |r| Show InChI InChI=1S/C18H26N4O19P4S4/c23-9-1-3-21(17(29)19-9)15-13(27)11(25)7(37-15)5-35-44(46,47)40-42(31,32)39-43(33,34)41-45(48,49)36-6-8-12(26)14(28)16(38-8)22-4-2-10(24)20-18(22)30/h1-4,7-8,11-16,25-28H,5-6H2,(H,31,32)(H,33,34)(H,46,47)(H,48,49)(H,19,23,29)(H,20,24,30)/t7-,8-,11-,12-,13-,14-,15-,16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 125 | n/a | n/a | n/a | n/a | n/a | n/a |
Bar-Ilan University
Curated by ChEMBL
| Assay Description
Inhibition of human NPP1 using pNP-TMP as substrate preincubated for 3 mins followed by substrate addition measured after 15 mins |
J Med Chem 61: 3939-3951 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01906
BindingDB Entry DOI: 10.7270/Q2DR2Z3M |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 1
(Homo sapiens (Human)) | BDBM50019296

(CHEMBL3289396)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](COP(O)(=O)CP(O)(=O)OP(O)(O)=S)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C11H18N5O11P3S/c12-9-6-10(14-2-13-9)16(3-15-6)11-8(18)7(17)5(26-11)1-25-28(19,20)4-29(21,22)27-30(23,24)31/h2-3,5,7-8,11,17-18H,1,4H2,(H,19,20)(H,21,22)(H2,12,13,14)(H2,23,24,31)/t5-,7-,8-,11-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
Bar-Ilan University
Curated by ChEMBL
| Assay Description
Mixed type inhibition of human NPP1 using pNP-TMP as substrate after 20 mins by Dixon and Cornish-Bowden method |
J Med Chem 57: 4677-91 (2014)
Article DOI: 10.1021/jm500196c
BindingDB Entry DOI: 10.7270/Q2QN68C6 |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 1
(Homo sapiens (Human)) | BDBM50019294

(CHEMBL3289394)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](CO[P@](O)(=S)OP(O)(=O)C(Cl)(Cl)P(O)(O)=O)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C11H16Cl2N5O11P3S/c12-11(13,30(21,22)23)31(24,25)29-32(26,33)27-1-4-6(19)7(20)10(28-4)18-3-17-5-8(14)15-2-16-9(5)18/h2-4,6-7,10,19-20H,1H2,(H,24,25)(H,26,33)(H2,14,15,16)(H2,21,22,23)/t4-,6-,7-,10-,32+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Bar-Ilan University
Curated by ChEMBL
| Assay Description
Mixed type inhibition of human NPP1 using pNP-TMP as substrate after 20 mins by Dixon and Cornish-Bowden method |
J Med Chem 57: 4677-91 (2014)
Article DOI: 10.1021/jm500196c
BindingDB Entry DOI: 10.7270/Q2QN68C6 |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 1
(Homo sapiens (Human)) | BDBM50453240

(CHEMBL4218881)Show SMILES O[C@H]1[C@@H](O)[C@@H](O[C@@H]1COP(S)(=S)OP(O)(=O)OP(O)(O)=O)n1ccc(=O)[nH]c1=O |r| Show InChI InChI=1S/C9H15N2O13P3S2/c12-5-1-2-11(9(15)10-5)8-7(14)6(13)4(22-8)3-21-27(28,29)24-26(19,20)23-25(16,17)18/h1-2,4,6-8,13-14H,3H2,(H,19,20)(H,28,29)(H,10,12,15)(H2,16,17,18)/t4-,6-,7-,8-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bar-Ilan University
Curated by ChEMBL
| Assay Description
Inhibition of human NPP1 using pNP-TMP as substrate preincubated for 3 mins followed by substrate addition measured after 15 mins |
J Med Chem 61: 3939-3951 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01906
BindingDB Entry DOI: 10.7270/Q2DR2Z3M |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 1
(Homo sapiens (Human)) | BDBM50453242

(CHEMBL4213959)Show SMILES O[C@H]1[C@@H](O)[C@@H](O[C@@H]1COP(S)(=S)OP(O)(=O)CP(O)(O)=O)n1ccc(=O)[nH]c1=O |r| Show InChI InChI=1S/C10H17N2O12P3S2/c13-6-1-2-12(10(16)11-6)9-8(15)7(14)5(23-9)3-22-27(28,29)24-26(20,21)4-25(17,18)19/h1-2,5,7-9,14-15H,3-4H2,(H,20,21)(H,28,29)(H,11,13,16)(H2,17,18,19)/t5-,7-,8-,9-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bar-Ilan University
Curated by ChEMBL
| Assay Description
Inhibition of human NPP1 using pNP-TMP as substrate preincubated for 3 mins followed by substrate addition measured after 15 mins |
J Med Chem 61: 3939-3951 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01906
BindingDB Entry DOI: 10.7270/Q2DR2Z3M |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 1
(Homo sapiens (Human)) | BDBM50019291

(CHEMBL3289392)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](CO[P@](O)(=S)OP(O)(=O)CP(O)(O)=O)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C11H18N5O11P3S/c12-9-6-10(14-2-13-9)16(3-15-6)11-8(18)7(17)5(26-11)1-25-30(24,31)27-29(22,23)4-28(19,20)21/h2-3,5,7-8,11,17-18H,1,4H2,(H,22,23)(H,24,31)(H2,12,13,14)(H2,19,20,21)/t5-,7-,8-,11-,30+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.63E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bar-Ilan University
Curated by ChEMBL
| Assay Description
Mixed type inhibition of human NPP1 using pNP-TMP as substrate after 20 mins by Dixon and Cornish-Bowden method |
J Med Chem 57: 4677-91 (2014)
Article DOI: 10.1021/jm500196c
BindingDB Entry DOI: 10.7270/Q2QN68C6 |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 1
(Homo sapiens (Human)) | BDBM50019292

(CHEMBL3289393)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](CO[P@@](O)(=S)OP(O)(=O)CP(O)(O)=O)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C11H18N5O11P3S/c12-9-6-10(14-2-13-9)16(3-15-6)11-8(18)7(17)5(26-11)1-25-30(24,31)27-29(22,23)4-28(19,20)21/h2-3,5,7-8,11,17-18H,1,4H2,(H,22,23)(H,24,31)(H2,12,13,14)(H2,19,20,21)/t5-,7-,8-,11-,30-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.87E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bar-Ilan University
Curated by ChEMBL
| Assay Description
Mixed type inhibition of human NPP1 using pNP-TMP as substrate after 20 mins by Dixon and Cornish-Bowden method |
J Med Chem 57: 4677-91 (2014)
Article DOI: 10.1021/jm500196c
BindingDB Entry DOI: 10.7270/Q2QN68C6 |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 1
(Homo sapiens (Human)) | BDBM50019295

(CHEMBL3289395)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](CO[P@@](O)(=S)OP(O)(=O)C(Cl)(Cl)P(O)(O)=O)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C11H16Cl2N5O11P3S/c12-11(13,30(21,22)23)31(24,25)29-32(26,33)27-1-4-6(19)7(20)10(28-4)18-3-17-5-8(14)15-2-16-9(5)18/h2-4,6-7,10,19-20H,1H2,(H,24,25)(H,26,33)(H2,14,15,16)(H2,21,22,23)/t4-,6-,7-,10-,32-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.12E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bar-Ilan University
Curated by ChEMBL
| Assay Description
Mixed type inhibition of human NPP1 using pNP-TMP as substrate after 20 mins by Dixon and Cornish-Bowden method |
J Med Chem 57: 4677-91 (2014)
Article DOI: 10.1021/jm500196c
BindingDB Entry DOI: 10.7270/Q2QN68C6 |
More data for this
Ligand-Target Pair | |
Ectonucleoside triphosphate diphosphohydrolase 2
(Homo sapiens (Human)) | BDBM50048062

(CHEMBL3314932)Show SMILES CCCCCCSc1nc(N)c2ncn([C@@H]3O[C@H](COP(O)(=O)OP(O)(=O)CP(O)(O)=O)[C@@H](O)[C@H]3O)c2n1 |r| Show InChI InChI=1S/C17H30N5O12P3S/c1-2-3-4-5-6-38-17-20-14(18)11-15(21-17)22(8-19-11)16-13(24)12(23)10(33-16)7-32-37(30,31)34-36(28,29)9-35(25,26)27/h8,10,12-13,16,23-24H,2-7,9H2,1H3,(H,28,29)(H,30,31)(H2,18,20,21)(H2,25,26,27)/t10-,12-,13-,16-/m1/s1 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bar-Ilan University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human NTPDase2 transfected in COS-7 cells using ATP substrate |
J Med Chem 57: 5919-34 (2014)
Article DOI: 10.1021/jm401933c
BindingDB Entry DOI: 10.7270/Q2B27WXX |
More data for this
Ligand-Target Pair | |
5'-AMP-activated protein kinase catalytic subunit alpha-2
(Rattus norvegicus) | BDBM50436624
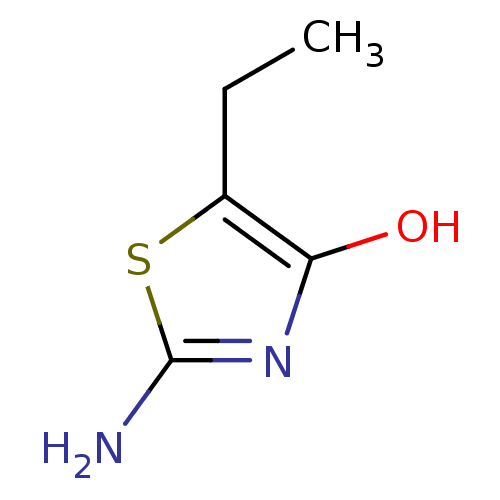
(CHEMBL1522339)Show InChI InChI=1S/C5H8N2OS/c1-2-3-4(8)7-5(6)9-3/h8H,2H2,1H3,(H2,6,7) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 6.15E+4 | n/a | n/a | n/a | n/a |
Bar Ilan University
Curated by ChEMBL
| Assay Description
Activation of AMPK in rat L6 cells assessed as increase of [3H]dGlc uptake after 5 hrs |
J Med Chem 56: 5335-50 (2014)
Article DOI: 10.1021/jm4001488
BindingDB Entry DOI: 10.7270/Q2222W5P |
More data for this
Ligand-Target Pair | |
5'-AMP-activated protein kinase catalytic subunit alpha-2
(Rattus norvegicus) | BDBM50436623
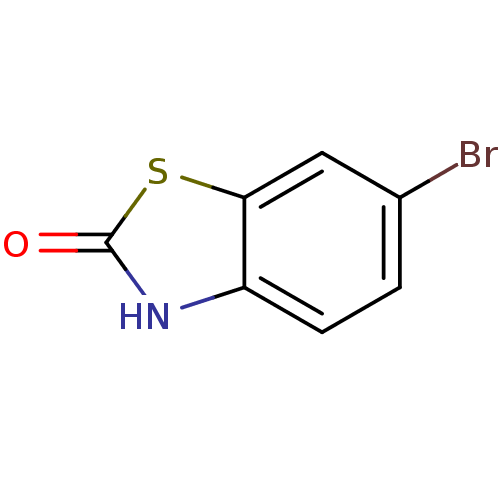
(CHEMBL2398100)Show InChI InChI=1S/C7H4BrNOS/c8-4-1-2-5-6(3-4)11-7(10)9-5/h1-3H,(H,9,10) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 7.29E+4 | n/a | n/a | n/a | n/a |
Bar Ilan University
Curated by ChEMBL
| Assay Description
Activation of AMPK in rat L6 cells assessed as increase of [3H]dGlc uptake after 5 hrs |
J Med Chem 56: 5335-50 (2014)
Article DOI: 10.1021/jm4001488
BindingDB Entry DOI: 10.7270/Q2222W5P |
More data for this
Ligand-Target Pair | |
5'-AMP-activated protein kinase catalytic subunit alpha-2
(Rattus norvegicus) | BDBM50436622
(CHEMBL568708)Show InChI InChI=1S/C9H9NOS2/c1-2-11-6-3-4-7-8(5-6)13-9(12)10-7/h3-5H,2H2,1H3,(H,10,12) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 7.61E+4 | n/a | n/a | n/a | n/a |
Bar Ilan University
Curated by ChEMBL
| Assay Description
Activation of AMPK in rat L6 cells assessed as increase of [3H]dGlc uptake after 5 hrs |
J Med Chem 56: 5335-50 (2014)
Article DOI: 10.1021/jm4001488
BindingDB Entry DOI: 10.7270/Q2222W5P |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 11
(Homo sapiens (Human)) | BDBM50019292

(CHEMBL3289393)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](CO[P@@](O)(=S)OP(O)(=O)CP(O)(O)=O)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C11H18N5O11P3S/c12-9-6-10(14-2-13-9)16(3-15-6)11-8(18)7(17)5(26-11)1-25-30(24,31)27-29(22,23)4-28(19,20)21/h2-3,5,7-8,11,17-18H,1,4H2,(H,22,23)(H,24,31)(H2,12,13,14)(H2,19,20,21)/t5-,7-,8-,11-,30-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 900 | n/a | n/a | n/a | n/a |
Bar-Ilan University
Curated by ChEMBL
| Assay Description
Agonist activity at human P2Y11 receptor expressed in human 1321N1 cells assessed as increase of intracellular calcium level after 30 mins using fura... |
J Med Chem 57: 4677-91 (2014)
Article DOI: 10.1021/jm500196c
BindingDB Entry DOI: 10.7270/Q2QN68C6 |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 1
(Homo sapiens (Human)) | BDBM50366480
(ADENOSINE TRIPHOSPHATE | ATP)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](CO[P@](O)(=O)O[P@@](O)(=O)OP(O)(O)=O)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C10H16N5O13P3/c11-8-5-9(13-2-12-8)15(3-14-5)10-7(17)6(16)4(26-10)1-25-30(21,22)28-31(23,24)27-29(18,19)20/h2-4,6-7,10,16-17H,1H2,(H,21,22)(H,23,24)(H2,11,12,13)(H2,18,19,20)/t4-,6-,7-,10-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 850 | n/a | n/a | n/a | n/a |
Bar-Ilan University
Curated by ChEMBL
| Assay Description
Agonist activity at human P2Y1 receptor expressed in human 1321N1 cells assessed as increase of intracellular calcium level after 30 mins using fura-... |
J Med Chem 57: 4677-91 (2014)
Article DOI: 10.1021/jm500196c
BindingDB Entry DOI: 10.7270/Q2QN68C6 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data