 Found 111 hits with Last Name = 'senturk' and Initial = 'm'
Found 111 hits with Last Name = 'senturk' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50143813
(CHEMBL3759894)Show SMILES NS(=O)(=O)c1ccc(cc1)-n1nc(ccc1=O)-c1ccc(cc1)C1CCCCC1 Show InChI InChI=1S/C22H23N3O3S/c23-29(27,28)20-12-10-19(11-13-20)25-22(26)15-14-21(24-25)18-8-6-17(7-9-18)16-4-2-1-3-5-16/h6-16H,1-5H2,(H2,23,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.980 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard (Hamdard University)
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase-2 using p-nitrophenyl acetate as substrate by esterase assay |
Bioorg Med Chem Lett 26: 1337-41 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.016
BindingDB Entry DOI: 10.7270/Q2RV0QJX |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50143808
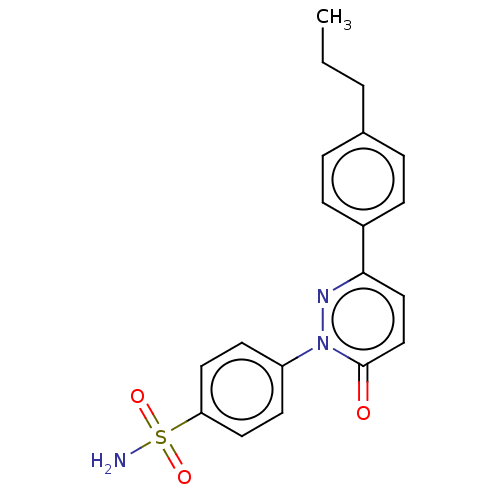
(CHEMBL3759134)Show SMILES CCCc1ccc(cc1)-c1ccc(=O)n(n1)-c1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C19H19N3O3S/c1-2-3-14-4-6-15(7-5-14)18-12-13-19(23)22(21-18)16-8-10-17(11-9-16)26(20,24)25/h4-13H,2-3H2,1H3,(H2,20,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard (Hamdard University)
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase-1 using p-nitrophenyl acetate as substrate by esterase assay |
Bioorg Med Chem Lett 26: 1337-41 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.016
BindingDB Entry DOI: 10.7270/Q2RV0QJX |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50143812
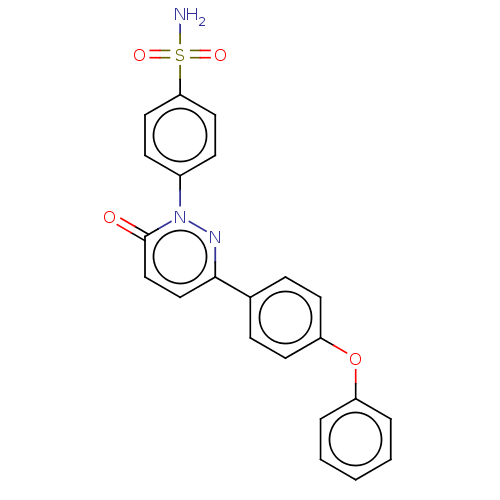
(CHEMBL3760118)Show SMILES NS(=O)(=O)c1ccc(cc1)-n1nc(ccc1=O)-c1ccc(Oc2ccccc2)cc1 Show InChI InChI=1S/C22H17N3O4S/c23-30(27,28)20-12-8-17(9-13-20)25-22(26)15-14-21(24-25)16-6-10-19(11-7-16)29-18-4-2-1-3-5-18/h1-15H,(H2,23,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard (Hamdard University)
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase-2 using p-nitrophenyl acetate as substrate by esterase assay |
Bioorg Med Chem Lett 26: 1337-41 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.016
BindingDB Entry DOI: 10.7270/Q2RV0QJX |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50143812

(CHEMBL3760118)Show SMILES NS(=O)(=O)c1ccc(cc1)-n1nc(ccc1=O)-c1ccc(Oc2ccccc2)cc1 Show InChI InChI=1S/C22H17N3O4S/c23-30(27,28)20-12-8-17(9-13-20)25-22(26)15-14-21(24-25)16-6-10-19(11-7-16)29-18-4-2-1-3-5-18/h1-15H,(H2,23,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard (Hamdard University)
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase-1 using p-nitrophenyl acetate as substrate by esterase assay |
Bioorg Med Chem Lett 26: 1337-41 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.016
BindingDB Entry DOI: 10.7270/Q2RV0QJX |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50143814
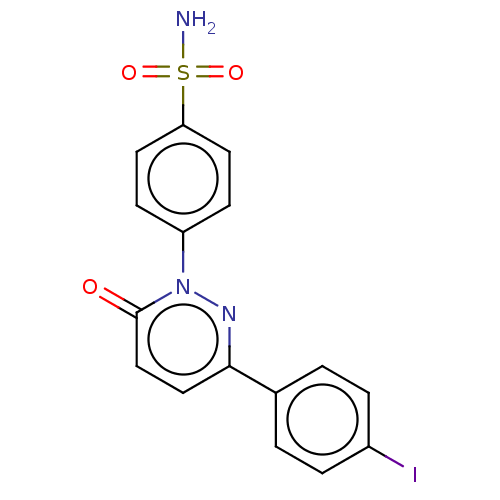
(CHEMBL3758784)Show SMILES NS(=O)(=O)c1ccc(cc1)-n1nc(ccc1=O)-c1ccc(I)cc1 Show InChI InChI=1S/C16H12IN3O3S/c17-12-3-1-11(2-4-12)15-9-10-16(21)20(19-15)13-5-7-14(8-6-13)24(18,22)23/h1-10H,(H2,18,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard (Hamdard University)
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase-2 using p-nitrophenyl acetate as substrate by esterase assay |
Bioorg Med Chem Lett 26: 1337-41 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.016
BindingDB Entry DOI: 10.7270/Q2RV0QJX |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50143807
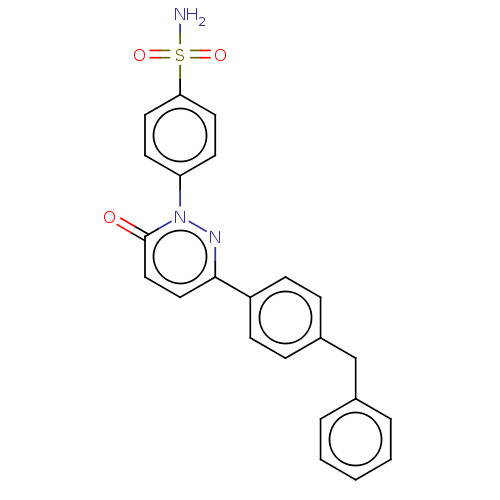
(CHEMBL3758698)Show SMILES NS(=O)(=O)c1ccc(cc1)-n1nc(ccc1=O)-c1ccc(Cc2ccccc2)cc1 Show InChI InChI=1S/C23H19N3O3S/c24-30(28,29)21-12-10-20(11-13-21)26-23(27)15-14-22(25-26)19-8-6-18(7-9-19)16-17-4-2-1-3-5-17/h1-15H,16H2,(H2,24,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard (Hamdard University)
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase-2 using p-nitrophenyl acetate as substrate by esterase assay |
Bioorg Med Chem Lett 26: 1337-41 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.016
BindingDB Entry DOI: 10.7270/Q2RV0QJX |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50143806
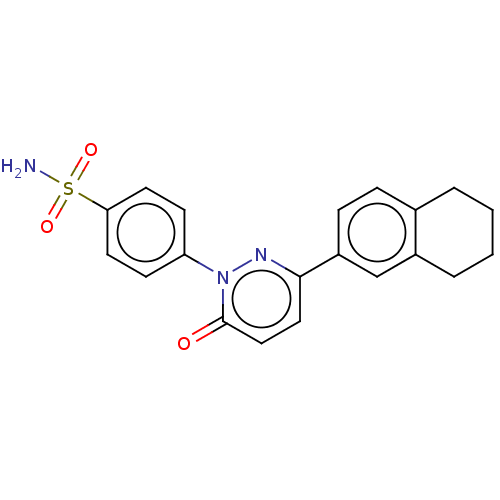
(CHEMBL3760111)Show SMILES NS(=O)(=O)c1ccc(cc1)-n1nc(ccc1=O)-c1ccc2CCCCc2c1 Show InChI InChI=1S/C20H19N3O3S/c21-27(25,26)18-9-7-17(8-10-18)23-20(24)12-11-19(22-23)16-6-5-14-3-1-2-4-15(14)13-16/h5-13H,1-4H2,(H2,21,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard (Hamdard University)
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase-2 using p-nitrophenyl acetate as substrate by esterase assay |
Bioorg Med Chem Lett 26: 1337-41 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.016
BindingDB Entry DOI: 10.7270/Q2RV0QJX |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50143810
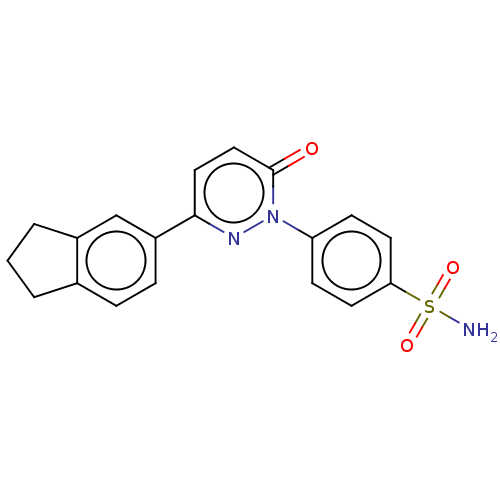
(CHEMBL3758504)Show SMILES NS(=O)(=O)c1ccc(cc1)-n1nc(ccc1=O)-c1ccc2CCCc2c1 Show InChI InChI=1S/C19H17N3O3S/c20-26(24,25)17-8-6-16(7-9-17)22-19(23)11-10-18(21-22)15-5-4-13-2-1-3-14(13)12-15/h4-12H,1-3H2,(H2,20,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard (Hamdard University)
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase-2 using p-nitrophenyl acetate as substrate by esterase assay |
Bioorg Med Chem Lett 26: 1337-41 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.016
BindingDB Entry DOI: 10.7270/Q2RV0QJX |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50143811
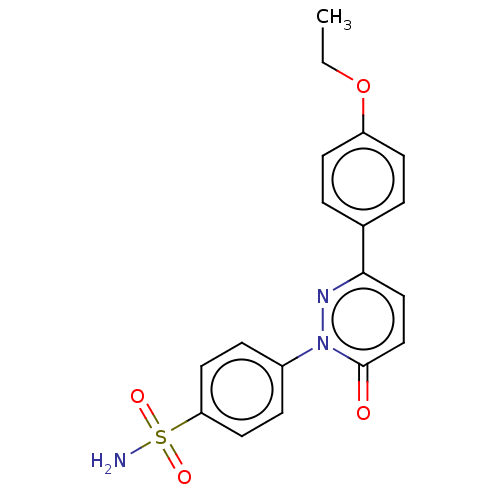
(CHEMBL3759464)Show SMILES CCOc1ccc(cc1)-c1ccc(=O)n(n1)-c1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C18H17N3O4S/c1-2-25-15-7-3-13(4-8-15)17-11-12-18(22)21(20-17)14-5-9-16(10-6-14)26(19,23)24/h3-12H,2H2,1H3,(H2,19,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard (Hamdard University)
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase-1 using p-nitrophenyl acetate as substrate by esterase assay |
Bioorg Med Chem Lett 26: 1337-41 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.016
BindingDB Entry DOI: 10.7270/Q2RV0QJX |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50143815
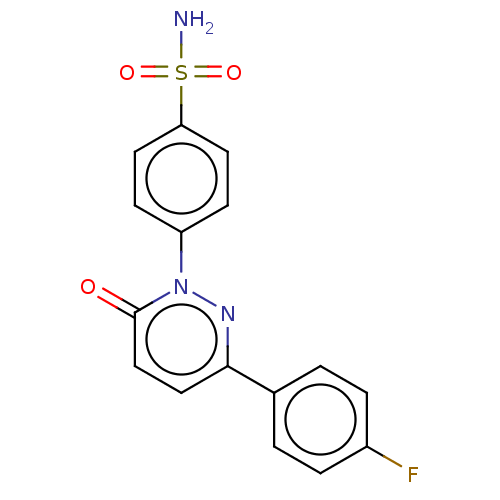
(CHEMBL3759883)Show SMILES NS(=O)(=O)c1ccc(cc1)-n1nc(ccc1=O)-c1ccc(F)cc1 Show InChI InChI=1S/C16H12FN3O3S/c17-12-3-1-11(2-4-12)15-9-10-16(21)20(19-15)13-5-7-14(8-6-13)24(18,22)23/h1-10H,(H2,18,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard (Hamdard University)
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase-2 using p-nitrophenyl acetate as substrate by esterase assay |
Bioorg Med Chem Lett 26: 1337-41 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.016
BindingDB Entry DOI: 10.7270/Q2RV0QJX |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50143809
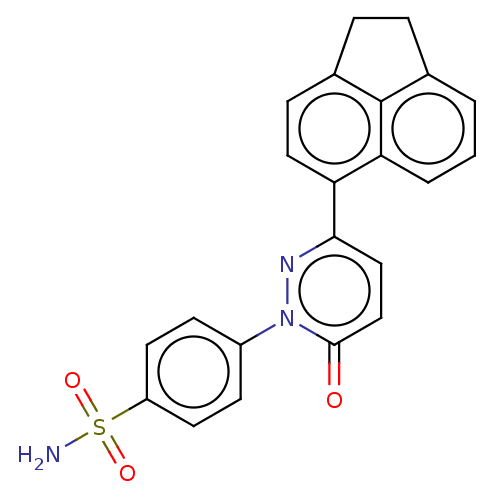
(CHEMBL3759757)Show SMILES NS(=O)(=O)c1ccc(cc1)-n1nc(ccc1=O)-c1ccc2CCc3cccc1c23 Show InChI InChI=1S/C22H17N3O3S/c23-29(27,28)17-9-7-16(8-10-17)25-21(26)13-12-20(24-25)18-11-6-15-5-4-14-2-1-3-19(18)22(14)15/h1-3,6-13H,4-5H2,(H2,23,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard (Hamdard University)
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase-1 using p-nitrophenyl acetate as substrate by esterase assay |
Bioorg Med Chem Lett 26: 1337-41 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.016
BindingDB Entry DOI: 10.7270/Q2RV0QJX |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50143811

(CHEMBL3759464)Show SMILES CCOc1ccc(cc1)-c1ccc(=O)n(n1)-c1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C18H17N3O4S/c1-2-25-15-7-3-13(4-8-15)17-11-12-18(22)21(20-17)14-5-9-16(10-6-14)26(19,23)24/h3-12H,2H2,1H3,(H2,19,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard (Hamdard University)
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase-2 using p-nitrophenyl acetate as substrate by esterase assay |
Bioorg Med Chem Lett 26: 1337-41 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.016
BindingDB Entry DOI: 10.7270/Q2RV0QJX |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50143808

(CHEMBL3759134)Show SMILES CCCc1ccc(cc1)-c1ccc(=O)n(n1)-c1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C19H19N3O3S/c1-2-3-14-4-6-15(7-5-14)18-12-13-19(23)22(21-18)16-8-10-17(11-9-16)26(20,24)25/h4-13H,2-3H2,1H3,(H2,20,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard (Hamdard University)
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase-2 using p-nitrophenyl acetate as substrate by esterase assay |
Bioorg Med Chem Lett 26: 1337-41 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.016
BindingDB Entry DOI: 10.7270/Q2RV0QJX |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50143809

(CHEMBL3759757)Show SMILES NS(=O)(=O)c1ccc(cc1)-n1nc(ccc1=O)-c1ccc2CCc3cccc1c23 Show InChI InChI=1S/C22H17N3O3S/c23-29(27,28)17-9-7-16(8-10-17)25-21(26)13-12-20(24-25)18-11-6-15-5-4-14-2-1-3-19(18)22(14)15/h1-3,6-13H,4-5H2,(H2,23,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard (Hamdard University)
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase-2 using p-nitrophenyl acetate as substrate by esterase assay |
Bioorg Med Chem Lett 26: 1337-41 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.016
BindingDB Entry DOI: 10.7270/Q2RV0QJX |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50143814

(CHEMBL3758784)Show SMILES NS(=O)(=O)c1ccc(cc1)-n1nc(ccc1=O)-c1ccc(I)cc1 Show InChI InChI=1S/C16H12IN3O3S/c17-12-3-1-11(2-4-12)15-9-10-16(21)20(19-15)13-5-7-14(8-6-13)24(18,22)23/h1-10H,(H2,18,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard (Hamdard University)
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase-1 using p-nitrophenyl acetate as substrate by esterase assay |
Bioorg Med Chem Lett 26: 1337-41 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.016
BindingDB Entry DOI: 10.7270/Q2RV0QJX |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50143816
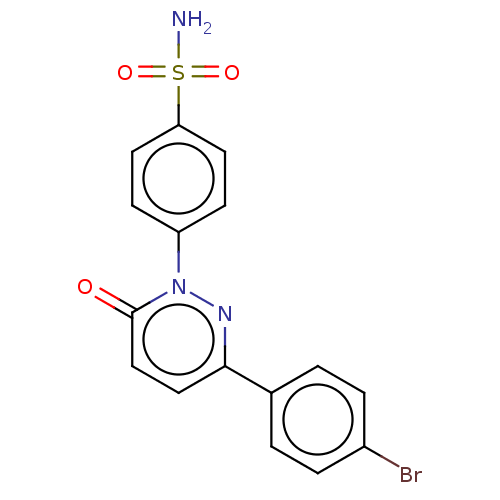
(CHEMBL3758388)Show SMILES NS(=O)(=O)c1ccc(cc1)-n1nc(ccc1=O)-c1ccc(Br)cc1 Show InChI InChI=1S/C16H12BrN3O3S/c17-12-3-1-11(2-4-12)15-9-10-16(21)20(19-15)13-5-7-14(8-6-13)24(18,22)23/h1-10H,(H2,18,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard (Hamdard University)
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase-2 using p-nitrophenyl acetate as substrate by esterase assay |
Bioorg Med Chem Lett 26: 1337-41 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.016
BindingDB Entry DOI: 10.7270/Q2RV0QJX |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50143815

(CHEMBL3759883)Show SMILES NS(=O)(=O)c1ccc(cc1)-n1nc(ccc1=O)-c1ccc(F)cc1 Show InChI InChI=1S/C16H12FN3O3S/c17-12-3-1-11(2-4-12)15-9-10-16(21)20(19-15)13-5-7-14(8-6-13)24(18,22)23/h1-10H,(H2,18,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard (Hamdard University)
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase-1 using p-nitrophenyl acetate as substrate by esterase assay |
Bioorg Med Chem Lett 26: 1337-41 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.016
BindingDB Entry DOI: 10.7270/Q2RV0QJX |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50143805
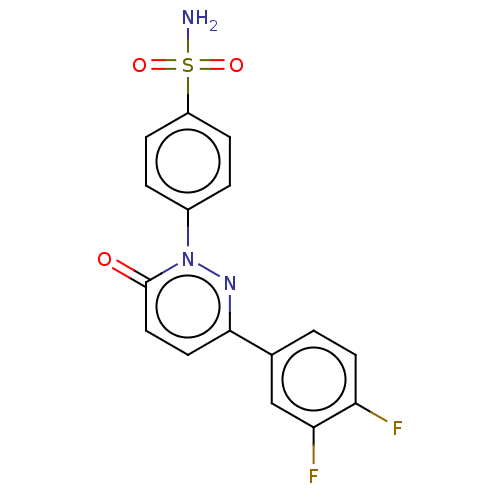
(CHEMBL3759344)Show SMILES NS(=O)(=O)c1ccc(cc1)-n1nc(ccc1=O)-c1ccc(F)c(F)c1 Show InChI InChI=1S/C16H11F2N3O3S/c17-13-6-1-10(9-14(13)18)15-7-8-16(22)21(20-15)11-2-4-12(5-3-11)25(19,23)24/h1-9H,(H2,19,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard (Hamdard University)
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase-1 using p-nitrophenyl acetate as substrate by esterase assay |
Bioorg Med Chem Lett 26: 1337-41 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.016
BindingDB Entry DOI: 10.7270/Q2RV0QJX |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50143810

(CHEMBL3758504)Show SMILES NS(=O)(=O)c1ccc(cc1)-n1nc(ccc1=O)-c1ccc2CCCc2c1 Show InChI InChI=1S/C19H17N3O3S/c20-26(24,25)17-8-6-16(7-9-17)22-19(23)11-10-18(21-22)15-5-4-13-2-1-3-14(13)12-15/h4-12H,1-3H2,(H2,20,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard (Hamdard University)
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase-1 using p-nitrophenyl acetate as substrate by esterase assay |
Bioorg Med Chem Lett 26: 1337-41 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.016
BindingDB Entry DOI: 10.7270/Q2RV0QJX |
More data for this
Ligand-Target Pair | |
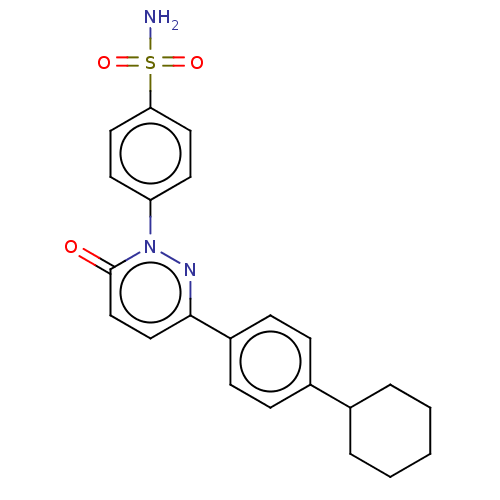
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50143813

(CHEMBL3759894)Show SMILES NS(=O)(=O)c1ccc(cc1)-n1nc(ccc1=O)-c1ccc(cc1)C1CCCCC1 Show InChI InChI=1S/C22H23N3O3S/c23-29(27,28)20-12-10-19(11-13-20)25-22(26)15-14-21(24-25)18-8-6-17(7-9-18)16-4-2-1-3-5-16/h6-16H,1-5H2,(H2,23,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard (Hamdard University)
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase-1 using p-nitrophenyl acetate as substrate by esterase assay |
Bioorg Med Chem Lett 26: 1337-41 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.016
BindingDB Entry DOI: 10.7270/Q2RV0QJX |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50143806

(CHEMBL3760111)Show SMILES NS(=O)(=O)c1ccc(cc1)-n1nc(ccc1=O)-c1ccc2CCCCc2c1 Show InChI InChI=1S/C20H19N3O3S/c21-27(25,26)18-9-7-17(8-10-18)23-20(24)12-11-19(22-23)16-6-5-14-3-1-2-4-15(14)13-16/h5-13H,1-4H2,(H2,21,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard (Hamdard University)
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase-1 using p-nitrophenyl acetate as substrate by esterase assay |
Bioorg Med Chem Lett 26: 1337-41 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.016
BindingDB Entry DOI: 10.7270/Q2RV0QJX |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50143805

(CHEMBL3759344)Show SMILES NS(=O)(=O)c1ccc(cc1)-n1nc(ccc1=O)-c1ccc(F)c(F)c1 Show InChI InChI=1S/C16H11F2N3O3S/c17-13-6-1-10(9-14(13)18)15-7-8-16(22)21(20-15)11-2-4-12(5-3-11)25(19,23)24/h1-9H,(H2,19,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard (Hamdard University)
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase-2 using p-nitrophenyl acetate as substrate by esterase assay |
Bioorg Med Chem Lett 26: 1337-41 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.016
BindingDB Entry DOI: 10.7270/Q2RV0QJX |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50143816

(CHEMBL3758388)Show SMILES NS(=O)(=O)c1ccc(cc1)-n1nc(ccc1=O)-c1ccc(Br)cc1 Show InChI InChI=1S/C16H12BrN3O3S/c17-12-3-1-11(2-4-12)15-9-10-16(21)20(19-15)13-5-7-14(8-6-13)24(18,22)23/h1-10H,(H2,18,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard (Hamdard University)
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase-1 using p-nitrophenyl acetate as substrate by esterase assay |
Bioorg Med Chem Lett 26: 1337-41 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.016
BindingDB Entry DOI: 10.7270/Q2RV0QJX |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50143807

(CHEMBL3758698)Show SMILES NS(=O)(=O)c1ccc(cc1)-n1nc(ccc1=O)-c1ccc(Cc2ccccc2)cc1 Show InChI InChI=1S/C23H19N3O3S/c24-30(28,29)21-12-10-20(11-13-21)26-23(27)15-14-22(25-26)19-8-6-18(7-9-19)16-17-4-2-1-3-5-17/h1-15H,16H2,(H2,24,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard (Hamdard University)
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase-1 using p-nitrophenyl acetate as substrate by esterase assay |
Bioorg Med Chem Lett 26: 1337-41 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.016
BindingDB Entry DOI: 10.7270/Q2RV0QJX |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM10880

(AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...)Show InChI InChI=1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard (Hamdard University)
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase-2 using p-nitrophenyl acetate as substrate by esterase assay |
Bioorg Med Chem Lett 26: 1337-41 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.016
BindingDB Entry DOI: 10.7270/Q2RV0QJX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM10890

(1-N-(3-chloro-1H-indol-7-yl)benzene-1,4-disulfonam...)Show SMILES NS(=O)(=O)c1ccc(cc1)S(=O)(=O)Nc1cccc2c(Cl)c[nH]c12 Show InChI InChI=1S/C14H12ClN3O4S2/c15-12-8-17-14-11(12)2-1-3-13(14)18-24(21,22)10-6-4-9(5-7-10)23(16,19)20/h1-8,17-18H,(H2,16,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard (Hamdard University)
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase-2 using p-nitrophenyl acetate as substrate by esterase assay |
Bioorg Med Chem Lett 26: 1337-41 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.016
BindingDB Entry DOI: 10.7270/Q2RV0QJX |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM10890

(1-N-(3-chloro-1H-indol-7-yl)benzene-1,4-disulfonam...)Show SMILES NS(=O)(=O)c1ccc(cc1)S(=O)(=O)Nc1cccc2c(Cl)c[nH]c12 Show InChI InChI=1S/C14H12ClN3O4S2/c15-12-8-17-14-11(12)2-1-3-13(14)18-24(21,22)10-6-4-9(5-7-10)23(16,19)20/h1-8,17-18H,(H2,16,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard (Hamdard University)
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase-1 using p-nitrophenyl acetate as substrate by esterase assay |
Bioorg Med Chem Lett 26: 1337-41 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.016
BindingDB Entry DOI: 10.7270/Q2RV0QJX |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM10880

(AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...)Show InChI InChI=1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard (Hamdard University)
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase-1 using p-nitrophenyl acetate as substrate by esterase assay |
Bioorg Med Chem Lett 26: 1337-41 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.016
BindingDB Entry DOI: 10.7270/Q2RV0QJX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM10882

(6-ethoxy-1,3-benzothiazole-2-sulfonamide | CHEMBL1...)Show InChI InChI=1S/C9H10N2O3S2/c1-2-14-6-3-4-7-8(5-6)15-9(11-7)16(10,12)13/h3-5H,2H2,1H3,(H2,10,12,13) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 320 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Ondokuz Mayis University
| Assay Description
Carbonic anhydrase activity was assayed by following the change in absorbance at 348 nm of 4-nitrophenylacetate (NPA) to 4-nitrophenylate ion over a ... |
J Enzyme Inhib Med Chem 28: 283-8 (2013)
Article DOI: 10.3109/14756366.2011.643303
BindingDB Entry DOI: 10.7270/Q2QR4W1F |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM10880

(AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...)Show InChI InChI=1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| 370 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Ondokuz Mayis University
| Assay Description
Carbonic anhydrase activity was assayed by following the change in absorbance at 348 nm of 4-nitrophenylacetate (NPA) to 4-nitrophenylate ion over a ... |
J Enzyme Inhib Med Chem 28: 283-8 (2013)
Article DOI: 10.3109/14756366.2011.643303
BindingDB Entry DOI: 10.7270/Q2QR4W1F |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 4
(Homo sapiens (Human)) | BDBM10880

(AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...)Show InChI InChI=1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 580 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Ondokuz Mayis University
| Assay Description
Carbonic anhydrase activity was assayed by following the change in absorbance at 348 nm of 4-nitrophenylacetate (NPA) to 4-nitrophenylate ion over a ... |
J Enzyme Inhib Med Chem 28: 283-8 (2013)
Article DOI: 10.3109/14756366.2011.643303
BindingDB Entry DOI: 10.7270/Q2QR4W1F |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM7459

(2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-4H-chromen-4...)Show InChI InChI=1S/C15H10O6/c16-8-4-11(19)15-12(20)6-13(21-14(15)5-8)7-1-2-9(17)10(18)3-7/h1-6,16-19H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 740 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Ondokuz Mayis University
| Assay Description
Carbonic anhydrase activity was assayed by following the change in absorbance at 348 nm of 4-nitrophenylacetate (NPA) to 4-nitrophenylate ion over a ... |
J Enzyme Inhib Med Chem 28: 283-8 (2013)
Article DOI: 10.3109/14756366.2011.643303
BindingDB Entry DOI: 10.7270/Q2QR4W1F |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 4
(Homo sapiens (Human)) | BDBM10882

(6-ethoxy-1,3-benzothiazole-2-sulfonamide | CHEMBL1...)Show InChI InChI=1S/C9H10N2O3S2/c1-2-14-6-3-4-7-8(5-6)15-9(11-7)16(10,12)13/h3-5H,2H2,1H3,(H2,10,12,13) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 840 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Ondokuz Mayis University
| Assay Description
Carbonic anhydrase activity was assayed by following the change in absorbance at 348 nm of 4-nitrophenylacetate (NPA) to 4-nitrophenylate ion over a ... |
J Enzyme Inhib Med Chem 28: 283-8 (2013)
Article DOI: 10.3109/14756366.2011.643303
BindingDB Entry DOI: 10.7270/Q2QR4W1F |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM10888
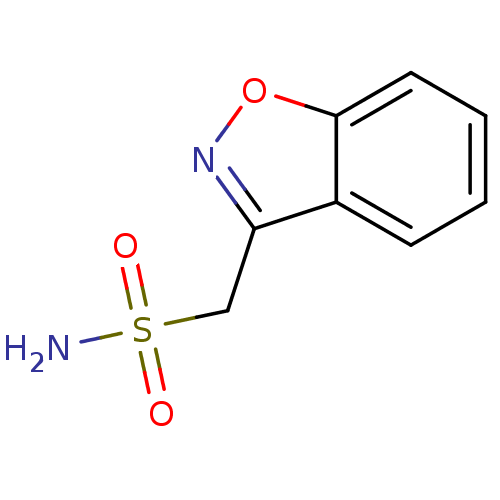
(1,2-benzoxazol-3-ylmethanesulfonamide | CHEMBL750 ...)Show InChI InChI=1S/C8H8N2O3S/c9-14(11,12)5-7-6-3-1-2-4-8(6)13-10-7/h1-4H,5H2,(H2,9,11,12) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Ondokuz Mayis University
| Assay Description
Carbonic anhydrase activity was assayed by following the change in absorbance at 348 nm of 4-nitrophenylacetate (NPA) to 4-nitrophenylate ion over a ... |
J Enzyme Inhib Med Chem 28: 283-8 (2013)
Article DOI: 10.3109/14756366.2011.643303
BindingDB Entry DOI: 10.7270/Q2QR4W1F |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50240969
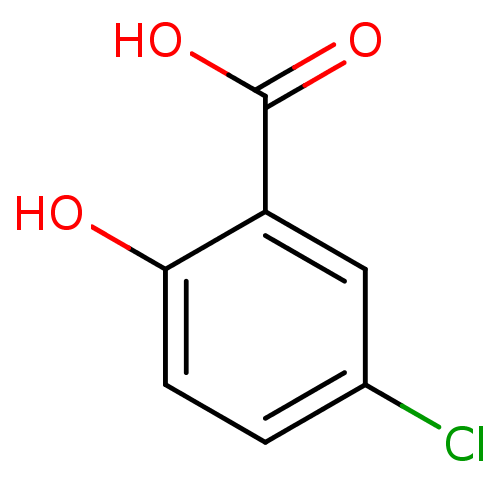
(5-Chloro-2-hydroxy-benzoic acid | 5-Chlorosalicyli...)Show InChI InChI=1S/C7H5ClO3/c8-4-1-2-6(9)5(3-4)7(10)11/h1-3,9H,(H,10,11) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.27E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ataturk University
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 2 esterase activity by uncompetitive Lineweaver-Burk plot |
Bioorg Med Chem 16: 9101-5 (2008)
Article DOI: 10.1016/j.bmc.2008.09.028
BindingDB Entry DOI: 10.7270/Q2GB23W2 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM7459

(2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-4H-chromen-4...)Show InChI InChI=1S/C15H10O6/c16-8-4-11(19)15-12(20)6-13(21-14(15)5-8)7-1-2-9(17)10(18)3-7/h1-6,16-19H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Ondokuz Mayis University
| Assay Description
Carbonic anhydrase activity was assayed by following the change in absorbance at 348 nm of 4-nitrophenylacetate (NPA) to 4-nitrophenylate ion over a ... |
J Enzyme Inhib Med Chem 28: 283-8 (2013)
Article DOI: 10.3109/14756366.2011.643303
BindingDB Entry DOI: 10.7270/Q2QR4W1F |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 3
(Bos taurus (Cattle)) | BDBM23416

(α-CA inhibitor, 3 | (+)-Catechin | (2R,3S)-2-...)Show SMILES O[C@H]1Cc2c(O)cc(O)cc2O[C@@H]1c1ccc(O)c(O)c1 |r| Show InChI InChI=1S/C15H14O6/c16-8-4-11(18)9-6-13(20)15(21-14(9)5-8)7-1-2-10(17)12(19)3-7/h1-5,13,15-20H,6H2/t13-,15+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Ondokuz Mayis University
| Assay Description
Carbonic anhydrase activity was assayed by following the change in absorbance at 348 nm of 4-nitrophenylacetate (NPA) to 4-nitrophenylate ion over a ... |
J Enzyme Inhib Med Chem 28: 283-8 (2013)
Article DOI: 10.3109/14756366.2011.643303
BindingDB Entry DOI: 10.7270/Q2QR4W1F |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM7460

(2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-4H-chrome...)Show InChI InChI=1S/C15H10O7/c16-7-4-10(19)12-11(5-7)22-15(14(21)13(12)20)6-1-2-8(17)9(18)3-6/h1-5,16-19,21H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Ondokuz Mayis University
| Assay Description
Carbonic anhydrase activity was assayed by following the change in absorbance at 348 nm of 4-nitrophenylacetate (NPA) to 4-nitrophenylate ion over a ... |
J Enzyme Inhib Med Chem 28: 283-8 (2013)
Article DOI: 10.3109/14756366.2011.643303
BindingDB Entry DOI: 10.7270/Q2QR4W1F |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM7458

(5,7-dihydroxy-2-(4-hydroxyphenyl)-4H-chromen-4-one...)Show InChI InChI=1S/C15H10O5/c16-9-3-1-8(2-4-9)13-7-12(19)15-11(18)5-10(17)6-14(15)20-13/h1-7,16-18H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Ondokuz Mayis University
| Assay Description
Carbonic anhydrase activity was assayed by following the change in absorbance at 348 nm of 4-nitrophenylacetate (NPA) to 4-nitrophenylate ion over a ... |
J Enzyme Inhib Med Chem 28: 283-8 (2013)
Article DOI: 10.3109/14756366.2011.643303
BindingDB Entry DOI: 10.7270/Q2QR4W1F |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM7460

(2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-4H-chrome...)Show InChI InChI=1S/C15H10O7/c16-7-4-10(19)12-11(5-7)22-15(14(21)13(12)20)6-1-2-8(17)9(18)3-6/h1-5,16-19,21H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Ondokuz Mayis University
| Assay Description
Carbonic anhydrase activity was assayed by following the change in absorbance at 348 nm of 4-nitrophenylacetate (NPA) to 4-nitrophenylate ion over a ... |
J Enzyme Inhib Med Chem 28: 283-8 (2013)
Article DOI: 10.3109/14756366.2011.643303
BindingDB Entry DOI: 10.7270/Q2QR4W1F |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM10882

(6-ethoxy-1,3-benzothiazole-2-sulfonamide | CHEMBL1...)Show InChI InChI=1S/C9H10N2O3S2/c1-2-14-6-3-4-7-8(5-6)15-9(11-7)16(10,12)13/h3-5H,2H2,1H3,(H2,10,12,13) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Ondokuz Mayis University
| Assay Description
Carbonic anhydrase activity was assayed by following the change in absorbance at 348 nm of 4-nitrophenylacetate (NPA) to 4-nitrophenylate ion over a ... |
J Enzyme Inhib Med Chem 28: 283-8 (2013)
Article DOI: 10.3109/14756366.2011.643303
BindingDB Entry DOI: 10.7270/Q2QR4W1F |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM7458

(5,7-dihydroxy-2-(4-hydroxyphenyl)-4H-chromen-4-one...)Show InChI InChI=1S/C15H10O5/c16-9-3-1-8(2-4-9)13-7-12(19)15-11(18)5-10(17)6-14(15)20-13/h1-7,16-18H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Ondokuz Mayis University
| Assay Description
Carbonic anhydrase activity was assayed by following the change in absorbance at 348 nm of 4-nitrophenylacetate (NPA) to 4-nitrophenylate ion over a ... |
J Enzyme Inhib Med Chem 28: 283-8 (2013)
Article DOI: 10.3109/14756366.2011.643303
BindingDB Entry DOI: 10.7270/Q2QR4W1F |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50240969

(5-Chloro-2-hydroxy-benzoic acid | 5-Chlorosalicyli...)Show InChI InChI=1S/C7H5ClO3/c8-4-1-2-6(9)5(3-4)7(10)11/h1-3,9H,(H,10,11) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ataturk University
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 1 esterase activity by uncompetitive Lineweaver-Burk plot |
Bioorg Med Chem 16: 9101-5 (2008)
Article DOI: 10.1016/j.bmc.2008.09.028
BindingDB Entry DOI: 10.7270/Q2GB23W2 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 4
(Homo sapiens (Human)) | BDBM7459

(2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-4H-chromen-4...)Show InChI InChI=1S/C15H10O6/c16-8-4-11(19)15-12(20)6-13(21-14(15)5-8)7-1-2-9(17)10(18)3-7/h1-6,16-19H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Ondokuz Mayis University
| Assay Description
Carbonic anhydrase activity was assayed by following the change in absorbance at 348 nm of 4-nitrophenylacetate (NPA) to 4-nitrophenylate ion over a ... |
J Enzyme Inhib Med Chem 28: 283-8 (2013)
Article DOI: 10.3109/14756366.2011.643303
BindingDB Entry DOI: 10.7270/Q2QR4W1F |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM26658
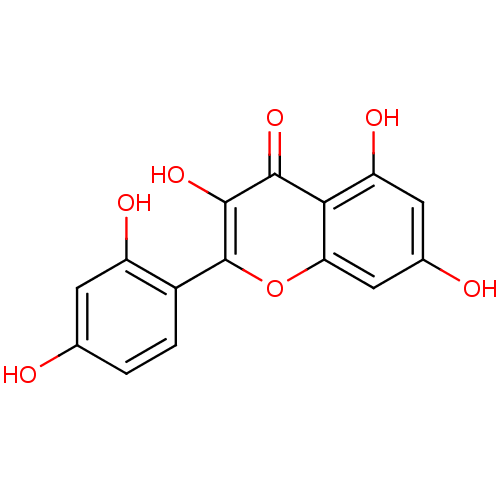
(2-(2,4-dihydroxyphenyl)-3,5,7-trihydroxy-1-benzopy...)Show InChI InChI=1S/C15H10O7/c16-6-1-2-8(9(18)3-6)15-14(21)13(20)12-10(19)4-7(17)5-11(12)22-15/h1-5,16-19,21H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Ondokuz Mayis University
| Assay Description
Carbonic anhydrase activity was assayed by following the change in absorbance at 348 nm of 4-nitrophenylacetate (NPA) to 4-nitrophenylate ion over a ... |
J Enzyme Inhib Med Chem 28: 283-8 (2013)
Article DOI: 10.3109/14756366.2011.643303
BindingDB Entry DOI: 10.7270/Q2QR4W1F |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 3
(Bos taurus (Cattle)) | BDBM26187
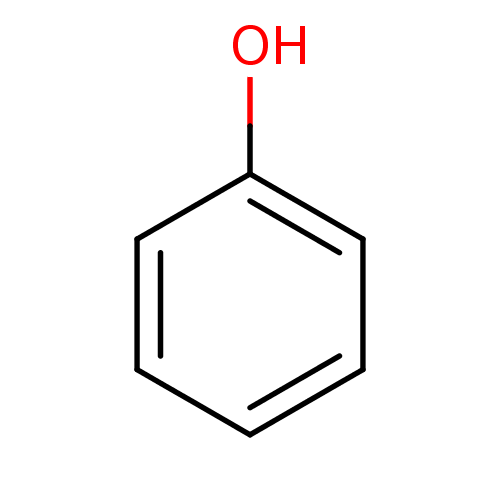
(α-CA inhibitor, 11 | CHEMBL14060 | US9688816,...)Show InChI InChI=1S/C6H6O/c7-6-4-2-1-3-5-6/h1-5,7H | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Ondokuz Mayis University
| Assay Description
Carbonic anhydrase activity was assayed by following the change in absorbance at 348 nm of 4-nitrophenylacetate (NPA) to 4-nitrophenylate ion over a ... |
J Enzyme Inhib Med Chem 28: 283-8 (2013)
Article DOI: 10.3109/14756366.2011.643303
BindingDB Entry DOI: 10.7270/Q2QR4W1F |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 3
(Bos taurus (Cattle)) | BDBM7459

(2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-4H-chromen-4...)Show InChI InChI=1S/C15H10O6/c16-8-4-11(19)15-12(20)6-13(21-14(15)5-8)7-1-2-9(17)10(18)3-7/h1-6,16-19H | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 5.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Ondokuz Mayis University
| Assay Description
Carbonic anhydrase activity was assayed by following the change in absorbance at 348 nm of 4-nitrophenylacetate (NPA) to 4-nitrophenylate ion over a ... |
J Enzyme Inhib Med Chem 28: 283-8 (2013)
Article DOI: 10.3109/14756366.2011.643303
BindingDB Entry DOI: 10.7270/Q2QR4W1F |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50275340
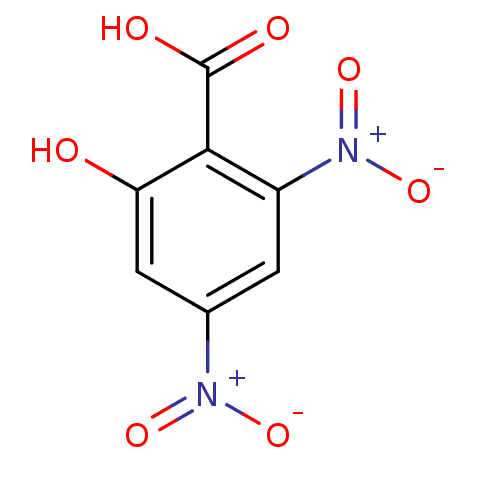
(4,6-Dinitro salicylic acid | CHEMBL447810)Show SMILES OC(=O)c1c(O)cc(cc1[N+]([O-])=O)[N+]([O-])=O Show InChI InChI=1S/C7H4N2O7/c10-5-2-3(8(13)14)1-4(9(15)16)6(5)7(11)12/h1-2,10H,(H,11,12) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 5.45E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ataturk University
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 2 esterase activity by non-competitive Lineweaver-Burk plot |
Bioorg Med Chem 16: 9101-5 (2008)
Article DOI: 10.1016/j.bmc.2008.09.028
BindingDB Entry DOI: 10.7270/Q2GB23W2 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 4
(Homo sapiens (Human)) | BDBM23416

(α-CA inhibitor, 3 | (+)-Catechin | (2R,3S)-2-...)Show SMILES O[C@H]1Cc2c(O)cc(O)cc2O[C@@H]1c1ccc(O)c(O)c1 |r| Show InChI InChI=1S/C15H14O6/c16-8-4-11(18)9-6-13(20)15(21-14(9)5-8)7-1-2-10(17)12(19)3-7/h1-5,13,15-20H,6H2/t13-,15+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Ondokuz Mayis University
| Assay Description
Carbonic anhydrase activity was assayed by following the change in absorbance at 348 nm of 4-nitrophenylacetate (NPA) to 4-nitrophenylate ion over a ... |
J Enzyme Inhib Med Chem 28: 283-8 (2013)
Article DOI: 10.3109/14756366.2011.643303
BindingDB Entry DOI: 10.7270/Q2QR4W1F |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 3
(Bos taurus (Cattle)) | BDBM26188
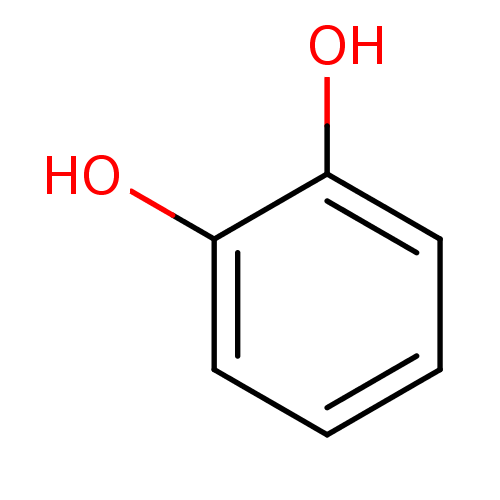
(α-CA inhibitor, 12 | 1,2-Dihydroxybenzene, XI...)Show InChI InChI=1S/C6H6O2/c7-5-3-1-2-4-6(5)8/h1-4,7-8H | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 6.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Ondokuz Mayis University
| Assay Description
Carbonic anhydrase activity was assayed by following the change in absorbance at 348 nm of 4-nitrophenylacetate (NPA) to 4-nitrophenylate ion over a ... |
J Enzyme Inhib Med Chem 28: 283-8 (2013)
Article DOI: 10.3109/14756366.2011.643303
BindingDB Entry DOI: 10.7270/Q2QR4W1F |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data