 Found 55 hits with Last Name = 'sevrioukova' and Initial = 'if'
Found 55 hits with Last Name = 'sevrioukova' and Initial = 'if' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50541356
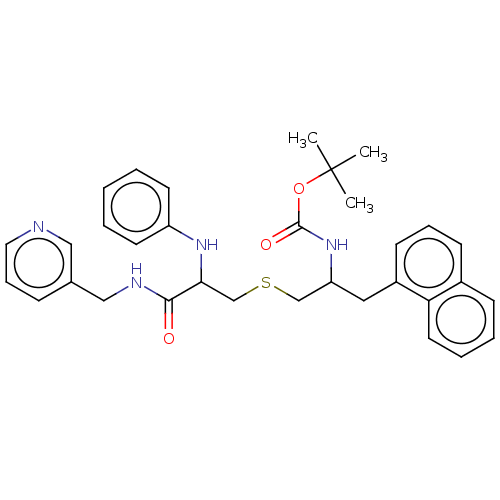
(CHEMBL4632678)Show SMILES CC(C)(C)OC(=O)NC(CSCC(Nc1ccccc1)C(=O)NCc1cccnc1)Cc1cccc2ccccc12 Show InChI InChI=1S/C33H38N4O3S/c1-33(2,3)40-32(39)37-28(19-26-14-9-13-25-12-7-8-17-29(25)26)22-41-23-30(36-27-15-5-4-6-16-27)31(38)35-21-24-11-10-18-34-20-24/h4-18,20,28,30,36H,19,21-23H2,1-3H3,(H,35,38)(H,37,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibition of human full length CYP3A4 assessed using 7-benzyloxy-4 (trifluoromethyl)coumarin as substrate preincubated for 2 mins followed by substr... |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2020.115349
BindingDB Entry DOI: 10.7270/Q20G3PPT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50541360
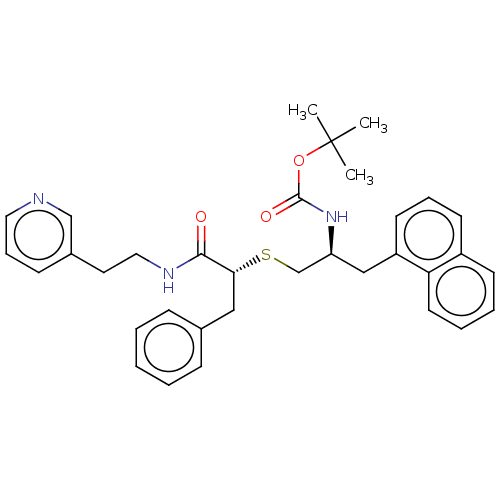
(CHEMBL4640859)Show SMILES CC(C)(C)OC(=O)N[C@H](CS[C@H](Cc1ccccc1)C(=O)NCCc1cccnc1)Cc1cccc2ccccc12 |r| Show InChI InChI=1S/C34H39N3O3S/c1-34(2,3)40-33(39)37-29(22-28-16-9-15-27-14-7-8-17-30(27)28)24-41-31(21-25-11-5-4-6-12-25)32(38)36-20-18-26-13-10-19-35-23-26/h4-17,19,23,29,31H,18,20-22,24H2,1-3H3,(H,36,38)(H,37,39)/t29-,31+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibition of human full length CYP3A4 assessed using 7-benzyloxy-4 (trifluoromethyl)coumarin as substrate preincubated for 2 mins followed by substr... |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2020.115349
BindingDB Entry DOI: 10.7270/Q20G3PPT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50541355
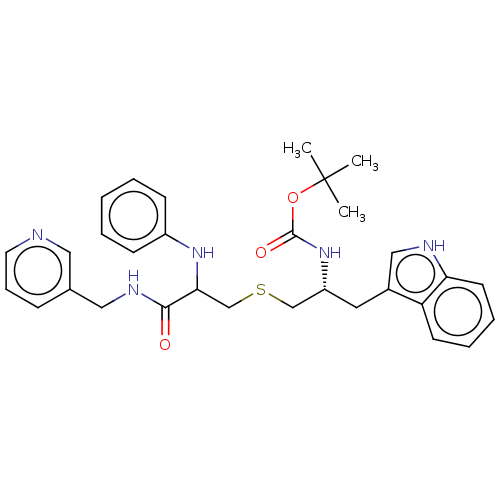
(CHEMBL4644850)Show SMILES CC(C)(C)OC(=O)N[C@@H](CSCC(Nc1ccccc1)C(=O)NCc1cccnc1)Cc1c[nH]c2ccccc12 |r| Show InChI InChI=1S/C31H37N5O3S/c1-31(2,3)39-30(38)36-25(16-23-19-33-27-14-8-7-13-26(23)27)20-40-21-28(35-24-11-5-4-6-12-24)29(37)34-18-22-10-9-15-32-17-22/h4-15,17,19,25,28,33,35H,16,18,20-21H2,1-3H3,(H,34,37)(H,36,38)/t25-,28?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 85 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibition of human full length CYP3A4 assessed using 7-benzyloxy-4 (trifluoromethyl)coumarin as substrate preincubated for 2 mins followed by substr... |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2020.115349
BindingDB Entry DOI: 10.7270/Q20G3PPT |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50541358
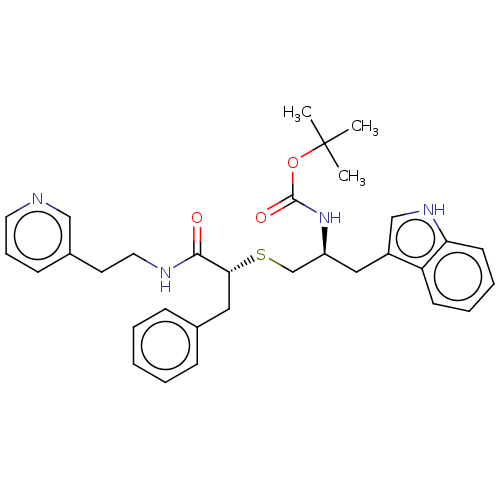
(CHEMBL4642007)Show SMILES CC(C)(C)OC(=O)N[C@H](CS[C@H](Cc1ccccc1)C(=O)NCCc1cccnc1)Cc1c[nH]c2ccccc12 |r| Show InChI InChI=1S/C32H38N4O3S/c1-32(2,3)39-31(38)36-26(19-25-21-35-28-14-8-7-13-27(25)28)22-40-29(18-23-10-5-4-6-11-23)30(37)34-17-15-24-12-9-16-33-20-24/h4-14,16,20-21,26,29,35H,15,17-19,22H2,1-3H3,(H,34,37)(H,36,38)/t26-,29+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 102 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibition of human full length CYP3A4 assessed using 7-benzyloxy-4 (trifluoromethyl)coumarin as substrate preincubated for 2 mins followed by substr... |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2020.115349
BindingDB Entry DOI: 10.7270/Q20G3PPT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50088504

(A-84538 | ABBOTT-84538 | CHEBI:45409 | Norvir | Ri...)Show SMILES CC(C)[C@H](NC(=O)N(C)Cc1csc(n1)C(C)C)C(=O)N[C@H](C[C@H](O)[C@H](Cc1ccccc1)NC(=O)OCc1cncs1)Cc1ccccc1 |r| Show InChI InChI=1S/C37H48N6O5S2/c1-24(2)33(42-36(46)43(5)20-29-22-49-35(40-29)25(3)4)34(45)39-28(16-26-12-8-6-9-13-26)18-32(44)31(17-27-14-10-7-11-15-27)41-37(47)48-21-30-19-38-23-50-30/h6-15,19,22-25,28,31-33,44H,16-18,20-21H2,1-5H3,(H,39,45)(H,41,47)(H,42,46)/t28-,31-,32-,33-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibition of human full length CYP3A4 assessed using 7-benzyloxy-4 (trifluoromethyl)coumarin as substrate preincubated for 20 mins in presence of NA... |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2020.115349
BindingDB Entry DOI: 10.7270/Q20G3PPT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50432593
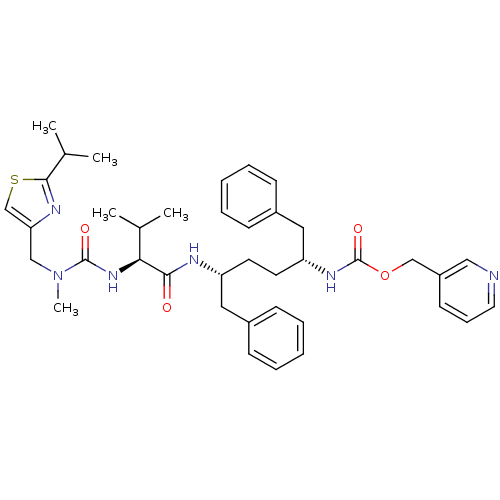
(CHEMBL2347183)Show SMILES CC(C)[C@H](NC(=O)N(C)Cc1csc(n1)C(C)C)C(=O)N[C@H](CC[C@H](Cc1ccccc1)NC(=O)OCc1cccnc1)Cc1ccccc1 |r| Show InChI InChI=1S/C39H50N6O4S/c1-27(2)35(44-38(47)45(5)24-34-26-50-37(42-34)28(3)4)36(46)41-32(21-29-13-8-6-9-14-29)18-19-33(22-30-15-10-7-11-16-30)43-39(48)49-25-31-17-12-20-40-23-31/h6-17,20,23,26-28,32-33,35H,18-19,21-22,24-25H2,1-5H3,(H,41,46)(H,43,48)(H,44,47)/t32-,33-,35+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibition of C-terminal His-tagged wild type CYP3A4 (unknown origin)-mediated hydroxylation of 7-benzyloxy-4-trifluoromethylcoumarin expressed in Es... |
J Med Chem 56: 3733-41 (2013)
Article DOI: 10.1021/jm400288z
BindingDB Entry DOI: 10.7270/Q2WM1FSZ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50541357
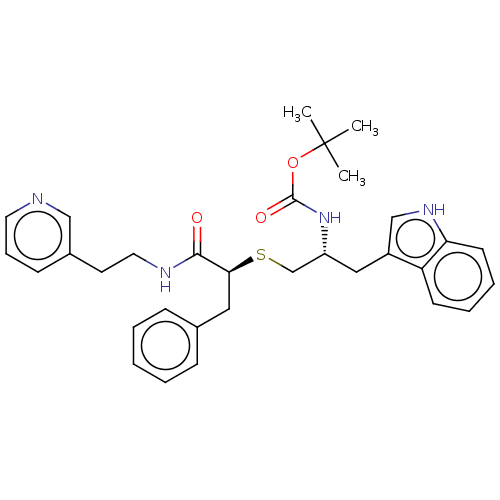
(CHEMBL4648061)Show SMILES CC(C)(C)OC(=O)N[C@@H](CS[C@@H](Cc1ccccc1)C(=O)NCCc1cccnc1)Cc1c[nH]c2ccccc12 |r| Show InChI InChI=1S/C32H38N4O3S/c1-32(2,3)39-31(38)36-26(19-25-21-35-28-14-8-7-13-27(25)28)22-40-29(18-23-10-5-4-6-11-23)30(37)34-17-15-24-12-9-16-33-20-24/h4-14,16,20-21,26,29,35H,15,17-19,22H2,1-3H3,(H,34,37)(H,36,38)/t26-,29+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 173 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibition of human full length CYP3A4 assessed using 7-benzyloxy-4 (trifluoromethyl)coumarin as substrate preincubated for 2 mins followed by substr... |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2020.115349
BindingDB Entry DOI: 10.7270/Q20G3PPT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50176903
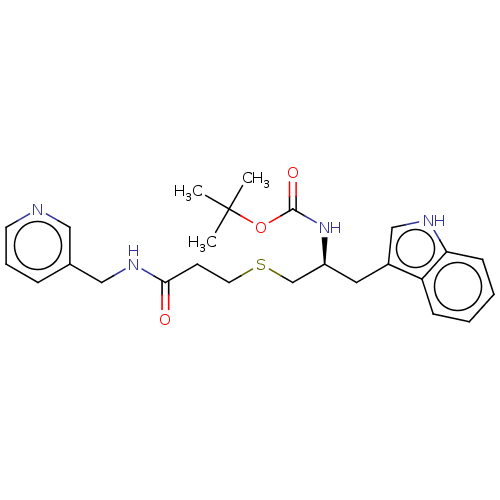
(CHEMBL3814877)Show SMILES CC(C)(C)OC(=O)N[C@H](CSCCC(=O)NCc1cccnc1)Cc1c[nH]c2ccccc12 |r| Show InChI InChI=1S/C25H32N4O3S/c1-25(2,3)32-24(31)29-20(13-19-16-27-22-9-5-4-8-21(19)22)17-33-12-10-23(30)28-15-18-7-6-11-26-14-18/h4-9,11,14,16,20,27H,10,12-13,15,17H2,1-3H3,(H,28,30)(H,29,31)/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California-Irvine
Curated by ChEMBL
| Assay Description
Inhibition of C-terminal four-histidine tagged human CYP3A4delta3 to 24 residues expressed in Escherichia coli assessed as 7-benzyloxy-4-(trifluorome... |
J Med Chem 59: 4210-20 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01146
BindingDB Entry DOI: 10.7270/Q2Q52RKB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50541362
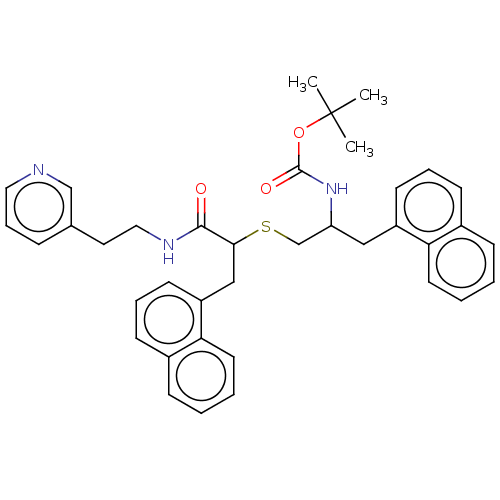
(CHEMBL4642617)Show SMILES CC(C)(C)OC(=O)NC(CSC(Cc1cccc2ccccc12)C(=O)NCCc1cccnc1)Cc1cccc2ccccc12 Show InChI InChI=1S/C38H41N3O3S/c1-38(2,3)44-37(43)41-32(23-30-16-8-14-28-12-4-6-18-33(28)30)26-45-35(36(42)40-22-20-27-11-10-21-39-25-27)24-31-17-9-15-29-13-5-7-19-34(29)31/h4-19,21,25,32,35H,20,22-24,26H2,1-3H3,(H,40,42)(H,41,43) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 218 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibition of human full length CYP3A4 assessed using 7-benzyloxy-4 (trifluoromethyl)coumarin as substrate preincubated for 2 mins followed by substr... |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2020.115349
BindingDB Entry DOI: 10.7270/Q20G3PPT |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50541361
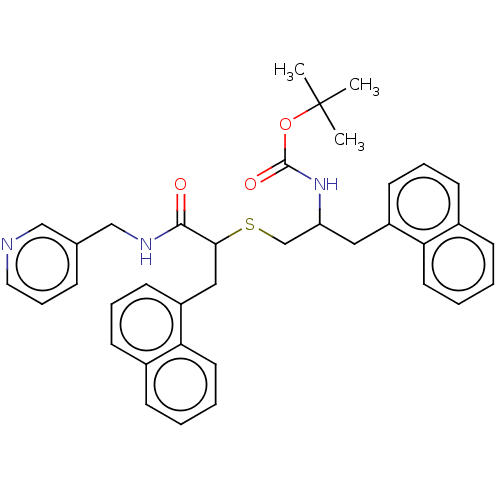
(CHEMBL4639605)Show SMILES CC(C)(C)OC(=O)NC(CSC(Cc1cccc2ccccc12)C(=O)NCc1cccnc1)Cc1cccc2ccccc12 Show InChI InChI=1S/C37H39N3O3S/c1-37(2,3)43-36(42)40-31(21-29-16-8-14-27-12-4-6-18-32(27)29)25-44-34(35(41)39-24-26-11-10-20-38-23-26)22-30-17-9-15-28-13-5-7-19-33(28)30/h4-20,23,31,34H,21-22,24-25H2,1-3H3,(H,39,41)(H,40,42) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 244 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibition of human full length CYP3A4 assessed using 7-benzyloxy-4 (trifluoromethyl)coumarin as substrate preincubated for 2 mins followed by substr... |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2020.115349
BindingDB Entry DOI: 10.7270/Q20G3PPT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50432595
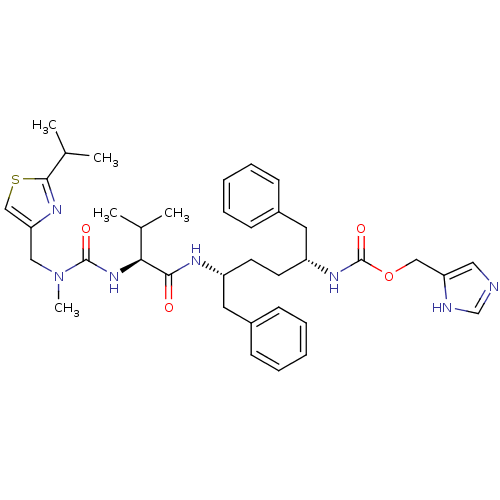
(CHEMBL2347185)Show SMILES CC(C)[C@H](NC(=O)N(C)Cc1csc(n1)C(C)C)C(=O)N[C@H](CC[C@H](Cc1ccccc1)NC(=O)OCc1cnc[nH]1)Cc1ccccc1 |r| Show InChI InChI=1S/C37H49N7O4S/c1-25(2)33(43-36(46)44(5)21-32-23-49-35(41-32)26(3)4)34(45)40-29(18-27-12-8-6-9-13-27)16-17-30(19-28-14-10-7-11-15-28)42-37(47)48-22-31-20-38-24-39-31/h6-15,20,23-26,29-30,33H,16-19,21-22H2,1-5H3,(H,38,39)(H,40,45)(H,42,47)(H,43,46)/t29-,30-,33+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibition of C-terminal His-tagged wild type CYP3A4 (unknown origin)-mediated hydroxylation of 7-benzyloxy-4-trifluoromethylcoumarin expressed in Es... |
J Med Chem 56: 3733-41 (2013)
Article DOI: 10.1021/jm400288z
BindingDB Entry DOI: 10.7270/Q2WM1FSZ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50541363
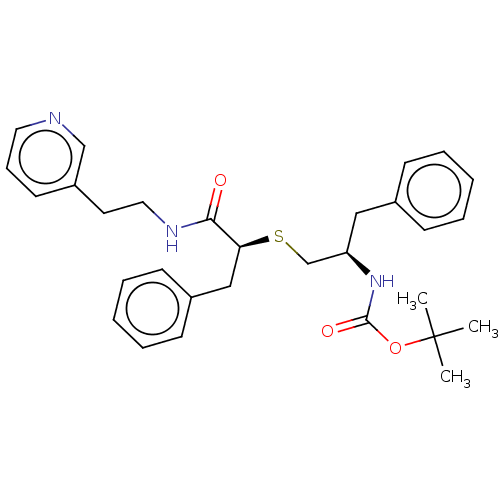
(CHEMBL4639033)Show SMILES CC(C)(C)OC(=O)N[C@@H](CS[C@@H](Cc1ccccc1)C(=O)NCCc1cccnc1)Cc1ccccc1 |r| Show InChI InChI=1S/C30H37N3O3S/c1-30(2,3)36-29(35)33-26(19-23-11-6-4-7-12-23)22-37-27(20-24-13-8-5-9-14-24)28(34)32-18-16-25-15-10-17-31-21-25/h4-15,17,21,26-27H,16,18-20,22H2,1-3H3,(H,32,34)(H,33,35)/t26-,27+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibition of human full length CYP3A4 assessed using 7-benzyloxy-4 (trifluoromethyl)coumarin as substrate preincubated for 20 mins in presence of NA... |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2020.115349
BindingDB Entry DOI: 10.7270/Q20G3PPT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50541364

(CHEMBL4635548)Show SMILES CC(C)(C)OC(=O)N[C@H](CS[C@H](Cc1ccccc1)C(=O)NCCc1cccnc1)Cc1ccccc1 |r| Show InChI InChI=1S/C30H37N3O3S/c1-30(2,3)36-29(35)33-26(19-23-11-6-4-7-12-23)22-37-27(20-24-13-8-5-9-14-24)28(34)32-18-16-25-15-10-17-31-21-25/h4-15,17,21,26-27H,16,18-20,22H2,1-3H3,(H,32,34)(H,33,35)/t26-,27+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibition of human full length CYP3A4 assessed using 7-benzyloxy-4 (trifluoromethyl)coumarin as substrate preincubated for 20 mins in presence of NA... |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2020.115349
BindingDB Entry DOI: 10.7270/Q20G3PPT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50541366

(CHEMBL4645102)Show SMILES CC(C)(C)OC(=O)N[C@H](CS[C@@H](Cc1ccccc1)C(=O)NCCc1cccnc1)Cc1ccccc1 |r| Show InChI InChI=1S/C30H37N3O3S/c1-30(2,3)36-29(35)33-26(19-23-11-6-4-7-12-23)22-37-27(20-24-13-8-5-9-14-24)28(34)32-18-16-25-15-10-17-31-21-25/h4-15,17,21,26-27H,16,18-20,22H2,1-3H3,(H,32,34)(H,33,35)/t26-,27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibition of human full length CYP3A4 assessed using 7-benzyloxy-4 (trifluoromethyl)coumarin as substrate preincubated for 20 mins in presence of NA... |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2020.115349
BindingDB Entry DOI: 10.7270/Q20G3PPT |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50541365

(CHEMBL4644634)Show SMILES CC(C)(C)OC(=O)N[C@@H](CS[C@H](Cc1ccccc1)C(=O)NCCc1cccnc1)Cc1ccccc1 |r| Show InChI InChI=1S/C30H37N3O3S/c1-30(2,3)36-29(35)33-26(19-23-11-6-4-7-12-23)22-37-27(20-24-13-8-5-9-14-24)28(34)32-18-16-25-15-10-17-31-21-25/h4-15,17,21,26-27H,16,18-20,22H2,1-3H3,(H,32,34)(H,33,35)/t26-,27-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibition of human full length CYP3A4 assessed using 7-benzyloxy-4 (trifluoromethyl)coumarin as substrate preincubated for 20 mins in presence of NA... |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2020.115349
BindingDB Entry DOI: 10.7270/Q20G3PPT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50541354
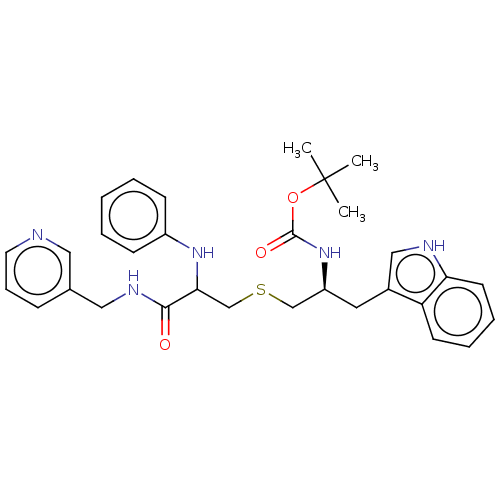
(CHEMBL4638454)Show SMILES CC(C)(C)OC(=O)N[C@H](CSCC(Nc1ccccc1)C(=O)NCc1cccnc1)Cc1c[nH]c2ccccc12 |r| Show InChI InChI=1S/C31H37N5O3S/c1-31(2,3)39-30(38)36-25(16-23-19-33-27-14-8-7-13-26(23)27)20-40-21-28(35-24-11-5-4-6-12-24)29(37)34-18-22-10-9-15-32-17-22/h4-15,17,19,25,28,33,35H,16,18,20-21H2,1-3H3,(H,34,37)(H,36,38)/t25-,28?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibition of human full length CYP3A4 assessed using 7-benzyloxy-4 (trifluoromethyl)coumarin as substrate preincubated for 2 mins followed by substr... |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2020.115349
BindingDB Entry DOI: 10.7270/Q20G3PPT |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50541359

(CHEMBL4644724)Show SMILES CC(C)(C)OC(=O)N[C@@H](CS[C@@H](Cc1ccccc1)C(=O)NCCc1cccnc1)Cc1cccc2ccccc12 |r| Show InChI InChI=1S/C34H39N3O3S/c1-34(2,3)40-33(39)37-29(22-28-16-9-15-27-14-7-8-17-30(27)28)24-41-31(21-25-11-5-4-6-12-25)32(38)36-20-18-26-13-10-19-35-23-26/h4-17,19,23,29,31H,18,20-22,24H2,1-3H3,(H,36,38)(H,37,39)/t29-,31+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 356 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibition of human full length CYP3A4 assessed using 7-benzyloxy-4 (trifluoromethyl)coumarin as substrate preincubated for 2 mins followed by substr... |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2020.115349
BindingDB Entry DOI: 10.7270/Q20G3PPT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50541354

(CHEMBL4638454)Show SMILES CC(C)(C)OC(=O)N[C@H](CSCC(Nc1ccccc1)C(=O)NCc1cccnc1)Cc1c[nH]c2ccccc12 |r| Show InChI InChI=1S/C31H37N5O3S/c1-31(2,3)39-30(38)36-25(16-23-19-33-27-14-8-7-13-26(23)27)20-40-21-28(35-24-11-5-4-6-12-24)29(37)34-18-22-10-9-15-32-17-22/h4-15,17,19,25,28,33,35H,16,18,20-21H2,1-3H3,(H,34,37)(H,36,38)/t25-,28?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 430 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibition of human full length CYP3A4 assessed using 7-benzyloxy-4 (trifluoromethyl)coumarin as substrate preincubated for 20 mins in presence of NA... |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2020.115349
BindingDB Entry DOI: 10.7270/Q20G3PPT |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50176904
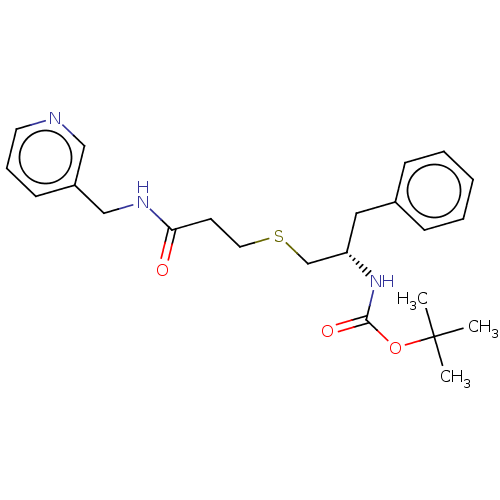
(CHEMBL3814479)Show SMILES CC(C)(C)OC(=O)N[C@H](CSCCC(=O)NCc1cccnc1)Cc1ccccc1 |r| Show InChI InChI=1S/C23H31N3O3S/c1-23(2,3)29-22(28)26-20(14-18-8-5-4-6-9-18)17-30-13-11-21(27)25-16-19-10-7-12-24-15-19/h4-10,12,15,20H,11,13-14,16-17H2,1-3H3,(H,25,27)(H,26,28)/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 520 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California-Irvine
Curated by ChEMBL
| Assay Description
Inhibition of C-terminal four-histidine tagged human CYP3A4delta3 to 24 residues expressed in Escherichia coli assessed as 7-benzyloxy-4-(trifluorome... |
J Med Chem 59: 4210-20 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01146
BindingDB Entry DOI: 10.7270/Q2Q52RKB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM520

(1,3-thiazol-5-ylmethyl N-[(2S,3S,5S)-3-hydroxy-5-[...)Show SMILES CC(C)[C@H](NC(=O)N(C)Cc1csc(n1)C(C)C)C(=O)N[C@H](C[C@H](O)[C@H](Cc1ccccc1)NC(=O)OCc1cncs1)Cc1ccccc1 |r| Show InChI InChI=1S/C37H48N6O5S2/c1-24(2)33(42-36(46)43(5)20-29-22-49-35(40-29)25(3)4)34(45)39-28(16-26-12-8-6-9-13-26)18-32(44)31(17-27-14-10-7-11-15-27)41-37(47)48-21-30-19-38-23-50-30/h6-15,19,22-25,28,31-33,44H,16-18,20-21H2,1-5H3,(H,39,45)(H,41,47)(H,42,46)/t28-,31-,32-,33-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibition of C-terminal His-tagged wild type CYP3A4 (unknown origin)-mediated hydroxylation of 7-benzyloxy-4-trifluoromethylcoumarin expressed in Es... |
J Med Chem 56: 3733-41 (2013)
Article DOI: 10.1021/jm400288z
BindingDB Entry DOI: 10.7270/Q2WM1FSZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50576969
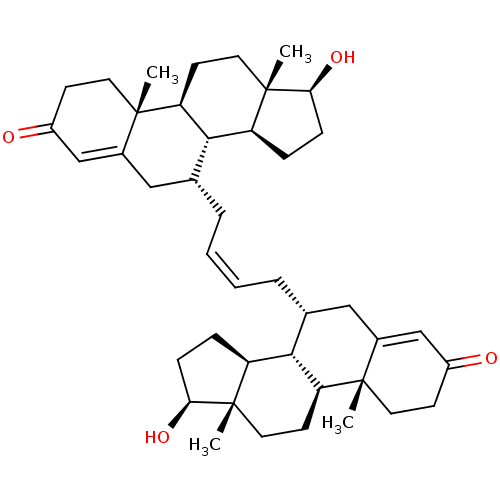
(CHEMBL4863362)Show SMILES [H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])[C@H](C\C=C/C[C@@H]2CC3=CC(=O)CC[C@]3(C)[C@@]3([H])CC[C@]4(C)[C@@H](O)CC[C@@]4([H])[C@]23[H])CC2=CC(=O)CC[C@]12C |r,t:23,50| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human CYP3A4 using BFC as substrate |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113496
BindingDB Entry DOI: 10.7270/Q2S1869N |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50576967

(CHEMBL4862783)Show SMILES [H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])[C@@H](CC2=CC(=O)CC[C@]12C)\C=C\C=C\C=C\[C@@H]1CC2=CC(=O)CC[C@]2(C)[C@@]2([H])CC[C@]3(C)[C@@H](O)CC[C@@]3([H])[C@]12[H] |r,t:18,36| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human CYP3A4 using BFC as substrate |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113496
BindingDB Entry DOI: 10.7270/Q2S1869N |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM119129

(GS8)Show SMILES CC[C@H](C)C(=O)N[C@H](CC[C@H](Cc1ccccc1)NC(=O)OCc1cncs1)Cc1ccccc1 |r| Show InChI InChI=1S/C28H35N3O3S/c1-3-21(2)27(32)30-24(16-22-10-6-4-7-11-22)14-15-25(17-23-12-8-5-9-13-23)31-28(33)34-19-26-18-29-20-35-26/h4-13,18,20-21,24-25H,3,14-17,19H2,1-2H3,(H,30,32)(H,31,33)/t21-,24+,25+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at Irvine
| Assay Description
The inhibitory potency of compounds GS4-GS8 on the 7-benzyloxy-4-(trifluoromethyl)coumarin hydroxylase activity of CYP3A4 was evaluated according to ... |
Biochemistry 52: 4474-81 (2013)
Article DOI: 10.1021/bi4005396
BindingDB Entry DOI: 10.7270/Q2PZ57GJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM119128
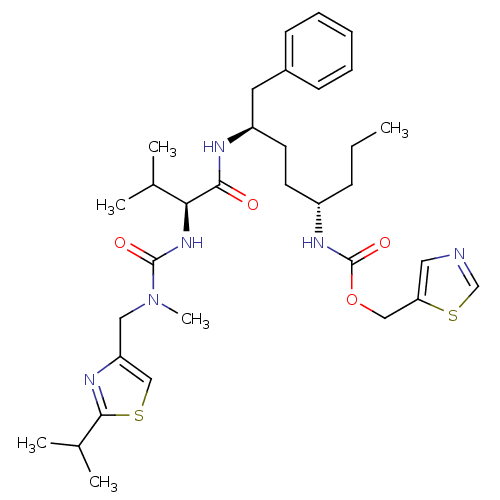
(GS7)Show SMILES CCC[C@@H](CC[C@H](Cc1ccccc1)NC(=O)[C@@H](NC(=O)N(C)Cc1csc(n1)C(C)C)C(C)C)NC(=O)OCc1cncs1 |r| Show InChI InChI=1S/C33H48N6O4S2/c1-7-11-25(37-33(42)43-19-28-17-34-21-45-28)14-15-26(16-24-12-9-8-10-13-24)35-30(40)29(22(2)3)38-32(41)39(6)18-27-20-44-31(36-27)23(4)5/h8-10,12-13,17,20-23,25-26,29H,7,11,14-16,18-19H2,1-6H3,(H,35,40)(H,37,42)(H,38,41)/t25-,26+,29-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at Irvine
| Assay Description
The inhibitory potency of compounds GS4-GS8 on the 7-benzyloxy-4-(trifluoromethyl)coumarin hydroxylase activity of CYP3A4 was evaluated according to ... |
Biochemistry 52: 4474-81 (2013)
Article DOI: 10.1021/bi4005396
BindingDB Entry DOI: 10.7270/Q2PZ57GJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50432594

(CHEMBL2347184)Show SMILES CC(C)[C@H](NC(=O)N(C)Cc1csc(n1)C(C)C)C(=O)N[C@H](CC[C@H](Cc1ccccc1)NC(=O)OCc1cnco1)Cc1ccccc1 |r| Show InChI InChI=1S/C37H48N6O5S/c1-25(2)33(42-36(45)43(5)21-31-23-49-35(40-31)26(3)4)34(44)39-29(18-27-12-8-6-9-13-27)16-17-30(19-28-14-10-7-11-15-28)41-37(46)47-22-32-20-38-24-48-32/h6-15,20,23-26,29-30,33H,16-19,21-22H2,1-5H3,(H,39,44)(H,41,46)(H,42,45)/t29-,30-,33+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibition of C-terminal His-tagged wild type CYP3A4 (unknown origin)-mediated hydroxylation of 7-benzyloxy-4-trifluoromethylcoumarin expressed in Es... |
J Med Chem 56: 3733-41 (2013)
Article DOI: 10.1021/jm400288z
BindingDB Entry DOI: 10.7270/Q2WM1FSZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50576968
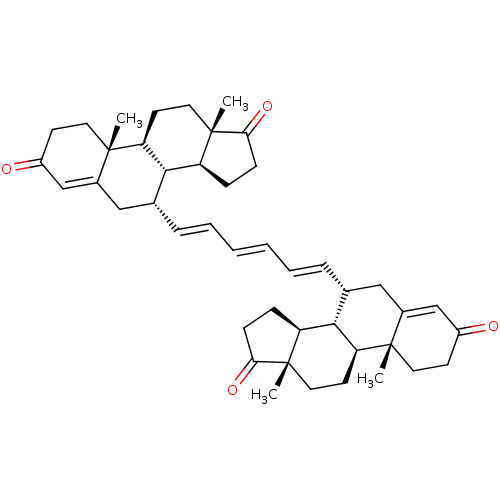
(CHEMBL4871524)Show SMILES [H][C@@]12CCC(=O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])[C@@H](CC2=CC(=O)CC[C@]12C)\C=C\C=C\C=C\[C@@H]1CC2=CC(=O)CC[C@]2(C)[C@@]2([H])CC[C@]3(C)C(=O)CC[C@@]3([H])[C@]12[H] |r,t:18,36| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human CYP3A4 using BFC as substrate |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113496
BindingDB Entry DOI: 10.7270/Q2S1869N |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50576970

(CHEMBL4863066)Show SMILES [H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])[C@H](C\C=C\C[C@@H]2CC3=CC(=O)CC[C@]3(C)[C@@]3([H])CC[C@]4(C)[C@@H](O)CC[C@@]4([H])[C@]23[H])CC2=CC(=O)CC[C@]12C |r,t:23,50| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human CYP3A4 using BFC as substrate |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113496
BindingDB Entry DOI: 10.7270/Q2S1869N |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM119127
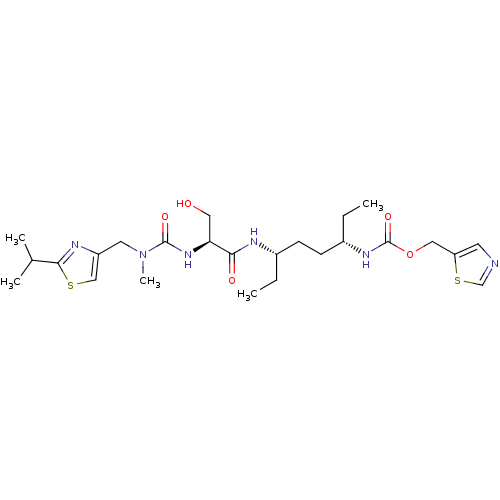
(GS6)Show SMILES CC[C@@H](CC[C@H](CC)NC(=O)[C@H](CO)NC(=O)N(C)Cc1csc(n1)C(C)C)NC(=O)OCc1cncs1 |r| Show InChI InChI=1S/C25H40N6O5S2/c1-6-17(8-9-18(7-2)29-25(35)36-13-20-10-26-15-38-20)27-22(33)21(12-32)30-24(34)31(5)11-19-14-37-23(28-19)16(3)4/h10,14-18,21,32H,6-9,11-13H2,1-5H3,(H,27,33)(H,29,35)(H,30,34)/t17-,18-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 9.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at Irvine
| Assay Description
The inhibitory potency of compounds GS4-GS8 on the 7-benzyloxy-4-(trifluoromethyl)coumarin hydroxylase activity of CYP3A4 was evaluated according to ... |
Biochemistry 52: 4474-81 (2013)
Article DOI: 10.1021/bi4005396
BindingDB Entry DOI: 10.7270/Q2PZ57GJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM119126
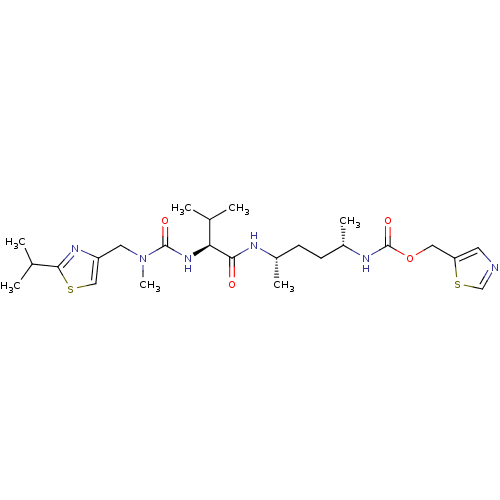
(GS5)Show SMILES C[C@@H](CC[C@H](C)NC(=O)[C@@H](NC(=O)N(C)Cc1csc(n1)C(C)C)C(C)C)NC(=O)OCc1cncs1 |r| Show InChI InChI=1S/C25H40N6O4S2/c1-15(2)21(30-24(33)31(7)11-19-13-36-23(29-19)16(3)4)22(32)27-17(5)8-9-18(6)28-25(34)35-12-20-10-26-14-37-20/h10,13-18,21H,8-9,11-12H2,1-7H3,(H,27,32)(H,28,34)(H,30,33)/t17-,18-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at Irvine
| Assay Description
The inhibitory potency of compounds GS4-GS8 on the 7-benzyloxy-4-(trifluoromethyl)coumarin hydroxylase activity of CYP3A4 was evaluated according to ... |
Biochemistry 52: 4474-81 (2013)
Article DOI: 10.1021/bi4005396
BindingDB Entry DOI: 10.7270/Q2PZ57GJ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM119125

(GS4)Show SMILES CC(C)[C@H](NC(=O)N(C)Cc1csc(n1)C(C)C)C(=O)NCCCCNC(=O)OCc1cncs1 |r| Show InChI InChI=1S/C23H36N6O4S2/c1-15(2)19(28-22(31)29(5)11-17-13-34-21(27-17)16(3)4)20(30)25-8-6-7-9-26-23(32)33-12-18-10-24-14-35-18/h10,13-16,19H,6-9,11-12H2,1-5H3,(H,25,30)(H,26,32)(H,28,31)/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at Irvine
| Assay Description
The inhibitory potency of compounds GS4-GS8 on the 7-benzyloxy-4-(trifluoromethyl)coumarin hydroxylase activity of CYP3A4 was evaluated according to ... |
Biochemistry 52: 4474-81 (2013)
Article DOI: 10.1021/bi4005396
BindingDB Entry DOI: 10.7270/Q2PZ57GJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50576966

(CHEMBL4864434)Show SMILES [H][C@@]12CC[C@@H](O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])[C@@H](CC2=CC(=O)CC[C@]12C)\C=C\C=C\C=C\[C@@H]1CC2=CC(=O)CC[C@]2(C)[C@@]2([H])CC[C@]3(C)[C@H](O)CC[C@@]3([H])[C@]12[H] |r,t:18,36| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human CYP3A4 using BFC as substrate |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113496
BindingDB Entry DOI: 10.7270/Q2S1869N |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50176905
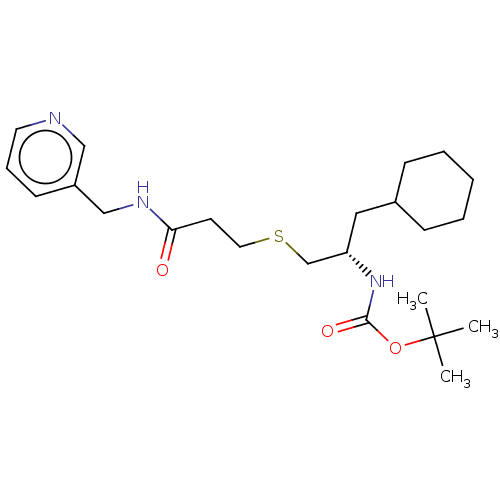
(CHEMBL3814345)Show SMILES CC(C)(C)OC(=O)N[C@H](CSCCC(=O)NCc1cccnc1)CC1CCCCC1 |r| Show InChI InChI=1S/C23H37N3O3S/c1-23(2,3)29-22(28)26-20(14-18-8-5-4-6-9-18)17-30-13-11-21(27)25-16-19-10-7-12-24-15-19/h7,10,12,15,18,20H,4-6,8-9,11,13-14,16-17H2,1-3H3,(H,25,27)(H,26,28)/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California-Irvine
Curated by ChEMBL
| Assay Description
Inhibition of C-terminal four-histidine tagged human CYP3A4delta3 to 24 residues expressed in Escherichia coli assessed as 7-benzyloxy-4-(trifluorome... |
J Med Chem 59: 4210-20 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01146
BindingDB Entry DOI: 10.7270/Q2Q52RKB |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50176908
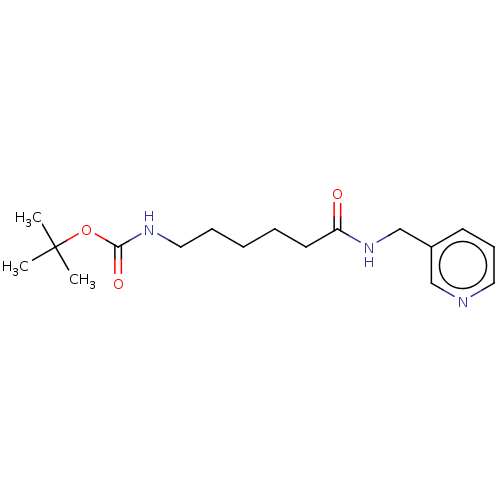
(CHEMBL3814075)Show InChI InChI=1S/C17H27N3O3/c1-17(2,3)23-16(22)19-11-6-4-5-9-15(21)20-13-14-8-7-10-18-12-14/h7-8,10,12H,4-6,9,11,13H2,1-3H3,(H,19,22)(H,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 7.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California-Irvine
Curated by ChEMBL
| Assay Description
Inhibition of C-terminal four-histidine tagged human CYP3A4delta3 to 24 residues expressed in Escherichia coli assessed as 7-benzyloxy-4-(trifluorome... |
J Med Chem 59: 4210-20 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01146
BindingDB Entry DOI: 10.7270/Q2Q52RKB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50176907
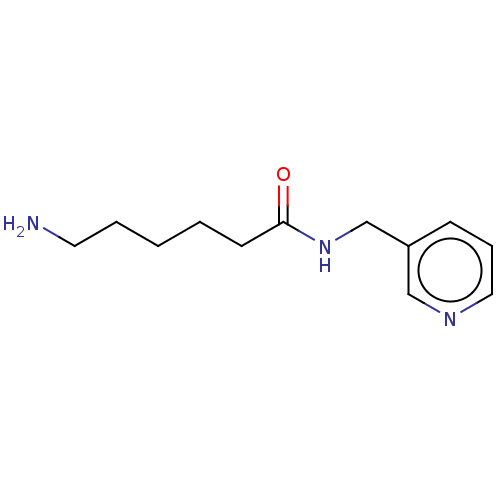
(CHEMBL3814729)Show InChI InChI=1S/C12H19N3O/c13-7-3-1-2-6-12(16)15-10-11-5-4-8-14-9-11/h4-5,8-9H,1-3,6-7,10,13H2,(H,15,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California-Irvine
Curated by ChEMBL
| Assay Description
Inhibition of C-terminal four-histidine tagged human CYP3A4delta3 to 24 residues expressed in Escherichia coli assessed as 7-benzyloxy-4-(trifluorome... |
J Med Chem 59: 4210-20 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01146
BindingDB Entry DOI: 10.7270/Q2Q52RKB |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50176909

(CHEBI:16227 | Pyridine) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California-Irvine
Curated by ChEMBL
| Assay Description
Inhibition of C-terminal four-histidine tagged human CYP3A4delta3 to 24 residues expressed in Escherichia coli assessed as 7-benzyloxy-4-(trifluorome... |
J Med Chem 59: 4210-20 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01146
BindingDB Entry DOI: 10.7270/Q2Q52RKB |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50417104
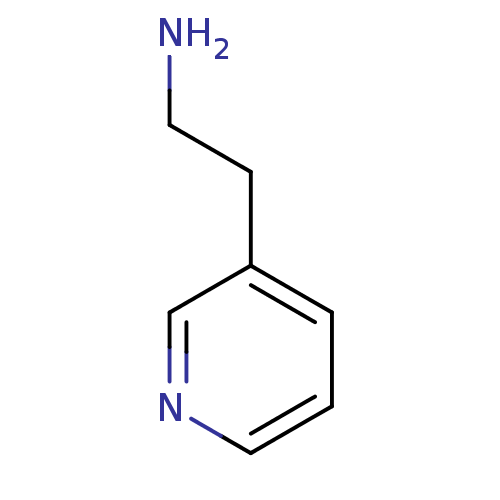
(CHEMBL1256004)Show InChI InChI=1S/C7H10N2/c8-4-3-7-2-1-5-9-6-7/h1-2,5-6H,3-4,8H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California-Irvine
Curated by ChEMBL
| Assay Description
Inhibition of C-terminal four-histidine tagged human CYP3A4delta3 to 24 residues expressed in Escherichia coli assessed as 7-benzyloxy-4-(trifluorome... |
J Med Chem 59: 4210-20 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01146
BindingDB Entry DOI: 10.7270/Q2Q52RKB |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM119125

(GS4)Show SMILES CC(C)[C@H](NC(=O)N(C)Cc1csc(n1)C(C)C)C(=O)NCCCCNC(=O)OCc1cncs1 |r| Show InChI InChI=1S/C23H36N6O4S2/c1-15(2)19(28-22(31)29(5)11-17-13-34-21(27-17)16(3)4)20(30)25-8-6-7-9-26-23(32)33-12-18-10-24-14-35-18/h10,13-16,19H,6-9,11-12H2,1-5H3,(H,25,30)(H,26,32)(H,28,31)/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | 1.31E+4 | n/a | n/a | n/a | 7.4 | n/a |
University of California at Irvine
| Assay Description
Ligand binidng to CYP3A4 was monitored in 50 mM phosphate (pH 7.4) containing 20% glycerol and 1 mM DTT. The protein was titrated with small aliquots... |
Biochemistry 52: 4474-81 (2013)
Article DOI: 10.1021/bi4005396
BindingDB Entry DOI: 10.7270/Q2PZ57GJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM119125

(GS4)Show SMILES CC(C)[C@H](NC(=O)N(C)Cc1csc(n1)C(C)C)C(=O)NCCCCNC(=O)OCc1cncs1 |r| Show InChI InChI=1S/C23H36N6O4S2/c1-15(2)19(28-22(31)29(5)11-17-13-34-21(27-17)16(3)4)20(30)25-8-6-7-9-26-23(32)33-12-18-10-24-14-35-18/h10,13-16,19H,6-9,11-12H2,1-5H3,(H,25,30)(H,26,32)(H,28,31)/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | 7.4 | n/a |
University of California at Irvine
| Assay Description
Ligand binidng to CYP3A4 was monitored in 50 mM phosphate (pH 7.4) containing 20% glycerol and 1 mM DTT. The protein was titrated with small aliquots... |
Biochemistry 52: 4474-81 (2013)
Article DOI: 10.1021/bi4005396
BindingDB Entry DOI: 10.7270/Q2PZ57GJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50576966

(CHEMBL4864434)Show SMILES [H][C@@]12CC[C@@H](O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])[C@@H](CC2=CC(=O)CC[C@]12C)\C=C\C=C\C=C\[C@@H]1CC2=CC(=O)CC[C@]2(C)[C@@]2([H])CC[C@]3(C)[C@H](O)CC[C@@]3([H])[C@]12[H] |r,t:18,36| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human CYP3A4 assessed as dissociation constant |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113496
BindingDB Entry DOI: 10.7270/Q2S1869N |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50576967

(CHEMBL4862783)Show SMILES [H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])[C@@H](CC2=CC(=O)CC[C@]12C)\C=C\C=C\C=C\[C@@H]1CC2=CC(=O)CC[C@]2(C)[C@@]2([H])CC[C@]3(C)[C@@H](O)CC[C@@]3([H])[C@]12[H] |r,t:18,36| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 5.50E+3 | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human CYP3A4 assessed as dissociation constant |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113496
BindingDB Entry DOI: 10.7270/Q2S1869N |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50576968

(CHEMBL4871524)Show SMILES [H][C@@]12CCC(=O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])[C@@H](CC2=CC(=O)CC[C@]12C)\C=C\C=C\C=C\[C@@H]1CC2=CC(=O)CC[C@]2(C)[C@@]2([H])CC[C@]3(C)C(=O)CC[C@@]3([H])[C@]12[H] |r,t:18,36| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human CYP3A4 assessed as dissociation constant |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113496
BindingDB Entry DOI: 10.7270/Q2S1869N |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50576969

(CHEMBL4863362)Show SMILES [H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])[C@H](C\C=C/C[C@@H]2CC3=CC(=O)CC[C@]3(C)[C@@]3([H])CC[C@]4(C)[C@@H](O)CC[C@@]4([H])[C@]23[H])CC2=CC(=O)CC[C@]12C |r,t:23,50| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human CYP3A4 assessed as dissociation constant |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113496
BindingDB Entry DOI: 10.7270/Q2S1869N |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50576970

(CHEMBL4863066)Show SMILES [H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])[C@H](C\C=C\C[C@@H]2CC3=CC(=O)CC[C@]3(C)[C@@]3([H])CC[C@]4(C)[C@@H](O)CC[C@@]4([H])[C@]23[H])CC2=CC(=O)CC[C@]12C |r,t:23,50| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 7.10E+3 | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human CYP3A4 assessed as dissociation constant |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113496
BindingDB Entry DOI: 10.7270/Q2S1869N |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM119126

(GS5)Show SMILES C[C@@H](CC[C@H](C)NC(=O)[C@@H](NC(=O)N(C)Cc1csc(n1)C(C)C)C(C)C)NC(=O)OCc1cncs1 |r| Show InChI InChI=1S/C25H40N6O4S2/c1-15(2)21(30-24(33)31(7)11-19-13-36-23(29-19)16(3)4)22(32)27-17(5)8-9-18(6)28-25(34)35-12-20-10-26-14-37-20/h10,13-18,21H,8-9,11-12H2,1-7H3,(H,27,32)(H,28,34)(H,30,33)/t17-,18-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 400 | n/a | n/a | n/a | 7.4 | n/a |
University of California at Irvine
| Assay Description
Ligand binidng to CYP3A4 was monitored in 50 mM phosphate (pH 7.4) containing 20% glycerol and 1 mM DTT. The protein was titrated with small aliquots... |
Biochemistry 52: 4474-81 (2013)
Article DOI: 10.1021/bi4005396
BindingDB Entry DOI: 10.7270/Q2PZ57GJ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM119126

(GS5)Show SMILES C[C@@H](CC[C@H](C)NC(=O)[C@@H](NC(=O)N(C)Cc1csc(n1)C(C)C)C(C)C)NC(=O)OCc1cncs1 |r| Show InChI InChI=1S/C25H40N6O4S2/c1-15(2)21(30-24(33)31(7)11-19-13-36-23(29-19)16(3)4)22(32)27-17(5)8-9-18(6)28-25(34)35-12-20-10-26-14-37-20/h10,13-18,21H,8-9,11-12H2,1-7H3,(H,27,32)(H,28,34)(H,30,33)/t17-,18-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 7.30E+3 | n/a | n/a | n/a | 7.4 | n/a |
University of California at Irvine
| Assay Description
Ligand binidng to CYP3A4 was monitored in 50 mM phosphate (pH 7.4) containing 20% glycerol and 1 mM DTT. The protein was titrated with small aliquots... |
Biochemistry 52: 4474-81 (2013)
Article DOI: 10.1021/bi4005396
BindingDB Entry DOI: 10.7270/Q2PZ57GJ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM119127

(GS6)Show SMILES CC[C@@H](CC[C@H](CC)NC(=O)[C@H](CO)NC(=O)N(C)Cc1csc(n1)C(C)C)NC(=O)OCc1cncs1 |r| Show InChI InChI=1S/C25H40N6O5S2/c1-6-17(8-9-18(7-2)29-25(35)36-13-20-10-26-15-38-20)27-22(33)21(12-32)30-24(34)31(5)11-19-14-37-23(28-19)16(3)4/h10,14-18,21,32H,6-9,11-13H2,1-5H3,(H,27,33)(H,29,35)(H,30,34)/t17-,18-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | 7.4 | n/a |
University of California at Irvine
| Assay Description
Ligand binidng to CYP3A4 was monitored in 50 mM phosphate (pH 7.4) containing 20% glycerol and 1 mM DTT. The protein was titrated with small aliquots... |
Biochemistry 52: 4474-81 (2013)
Article DOI: 10.1021/bi4005396
BindingDB Entry DOI: 10.7270/Q2PZ57GJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM119128

(GS7)Show SMILES CCC[C@@H](CC[C@H](Cc1ccccc1)NC(=O)[C@@H](NC(=O)N(C)Cc1csc(n1)C(C)C)C(C)C)NC(=O)OCc1cncs1 |r| Show InChI InChI=1S/C33H48N6O4S2/c1-7-11-25(37-33(42)43-19-28-17-34-21-45-28)14-15-26(16-24-12-9-8-10-13-24)35-30(40)29(22(2)3)38-32(41)39(6)18-27-20-44-31(36-27)23(4)5/h8-10,12-13,17,20-23,25-26,29H,7,11,14-16,18-19H2,1-6H3,(H,35,40)(H,37,42)(H,38,41)/t25-,26+,29-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | 40 | n/a | n/a | n/a | 7.4 | n/a |
University of California at Irvine
| Assay Description
Ligand binidng to CYP3A4 was monitored in 50 mM phosphate (pH 7.4) containing 20% glycerol and 1 mM DTT. The protein was titrated with small aliquots... |
Biochemistry 52: 4474-81 (2013)
Article DOI: 10.1021/bi4005396
BindingDB Entry DOI: 10.7270/Q2PZ57GJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM119129

(GS8)Show SMILES CC[C@H](C)C(=O)N[C@H](CC[C@H](Cc1ccccc1)NC(=O)OCc1cncs1)Cc1ccccc1 |r| Show InChI InChI=1S/C28H35N3O3S/c1-3-21(2)27(32)30-24(16-22-10-6-4-7-11-22)14-15-25(17-23-12-8-5-9-13-23)31-28(33)34-19-26-18-29-20-35-26/h4-13,18,20-21,24-25H,3,14-17,19H2,1-2H3,(H,30,32)(H,31,33)/t21-,24+,25+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | n/a | 160 | n/a | n/a | n/a | 7.4 | n/a |
University of California at Irvine
| Assay Description
Ligand binidng to CYP3A4 was monitored in 50 mM phosphate (pH 7.4) containing 20% glycerol and 1 mM DTT. The protein was titrated with small aliquots... |
Biochemistry 52: 4474-81 (2013)
Article DOI: 10.1021/bi4005396
BindingDB Entry DOI: 10.7270/Q2PZ57GJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50176903

(CHEMBL3814877)Show SMILES CC(C)(C)OC(=O)N[C@H](CSCCC(=O)NCc1cccnc1)Cc1c[nH]c2ccccc12 |r| Show InChI InChI=1S/C25H32N4O3S/c1-25(2,3)32-24(31)29-20(13-19-16-27-22-9-5-4-8-21(19)22)17-33-12-10-23(30)28-15-18-7-6-11-26-14-18/h4-9,11,14,16,20,27H,10,12-13,15,17H2,1-3H3,(H,28,30)(H,29,31)/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a |
University of California-Irvine
Curated by ChEMBL
| Assay Description
Binding affinity to C-terminal four-histidine tagged human CYP3A4delta3 to 24 residues expressed in Escherichia coli assessed as spectral dissociatio... |
J Med Chem 59: 4210-20 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01146
BindingDB Entry DOI: 10.7270/Q2Q52RKB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50176904

(CHEMBL3814479)Show SMILES CC(C)(C)OC(=O)N[C@H](CSCCC(=O)NCc1cccnc1)Cc1ccccc1 |r| Show InChI InChI=1S/C23H31N3O3S/c1-23(2,3)29-22(28)26-20(14-18-8-5-4-6-9-18)17-30-13-11-21(27)25-16-19-10-7-12-24-15-19/h4-10,12,15,20H,11,13-14,16-17H2,1-3H3,(H,25,27)(H,26,28)/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a |
University of California-Irvine
Curated by ChEMBL
| Assay Description
Binding affinity to C-terminal four-histidine tagged human CYP3A4delta3 to 24 residues expressed in Escherichia coli assessed as spectral dissociatio... |
J Med Chem 59: 4210-20 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01146
BindingDB Entry DOI: 10.7270/Q2Q52RKB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data