

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50004000 ((3aS,8aR)-1,3a,8-trimethyl-1,2,3,3a,8,8a-hexahydro...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Tribhuvan University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by Ellman's method | J Nat Prod 64: 842-4 (2001) BindingDB Entry DOI: 10.7270/Q20R9P54 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50004000 ((3aS,8aR)-1,3a,8-trimethyl-1,2,3,3a,8,8a-hexahydro...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 857 | n/a | n/a | n/a | n/a | n/a | n/a |
Tribhuvan University Curated by ChEMBL | Assay Description Inhibition of horse BChE | J Nat Prod 64: 842-4 (2001) BindingDB Entry DOI: 10.7270/Q20R9P54 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50242322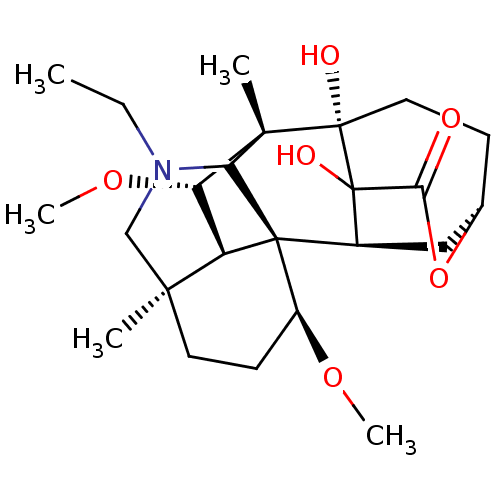 (CHEMBL4066113) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Malakand Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 15 mins followed by substrate addition by spectrophotome... | Bioorg Med Chem 25: 3368-3376 (2017) Article DOI: 10.1016/j.bmc.2017.04.022 BindingDB Entry DOI: 10.7270/Q2K64MG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50242323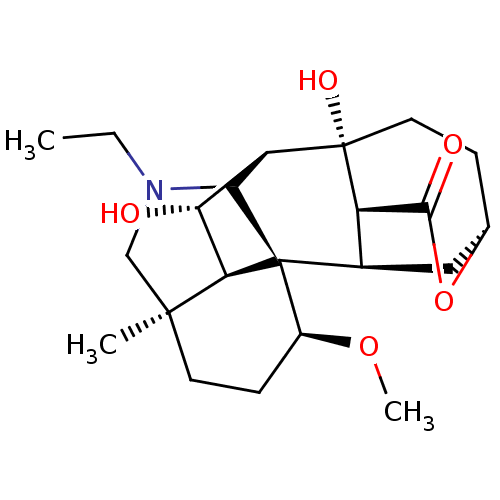 (CHEMBL4070917) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Malakand Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 15 mins followed by substrate addition by spectrophotome... | Bioorg Med Chem 25: 3368-3376 (2017) Article DOI: 10.1016/j.bmc.2017.04.022 BindingDB Entry DOI: 10.7270/Q2K64MG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50242315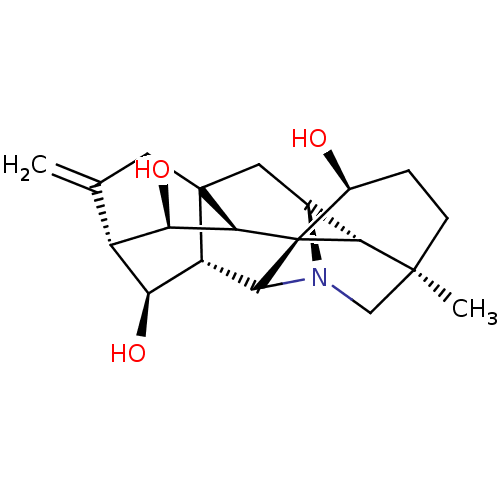 (CHEMBL4093688) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Malakand Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 15 mins followed by substrate addition by spectrophotome... | Bioorg Med Chem 25: 3368-3376 (2017) Article DOI: 10.1016/j.bmc.2017.04.022 BindingDB Entry DOI: 10.7270/Q2K64MG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM10404 ((1S,12S,14R)-9-methoxy-4-methyl-11-oxa-4-azatetrac...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Malakand Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 15 mins followed by substrate addition by spectrophotome... | Bioorg Med Chem 25: 3368-3376 (2017) Article DOI: 10.1016/j.bmc.2017.04.022 BindingDB Entry DOI: 10.7270/Q2K64MG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50242317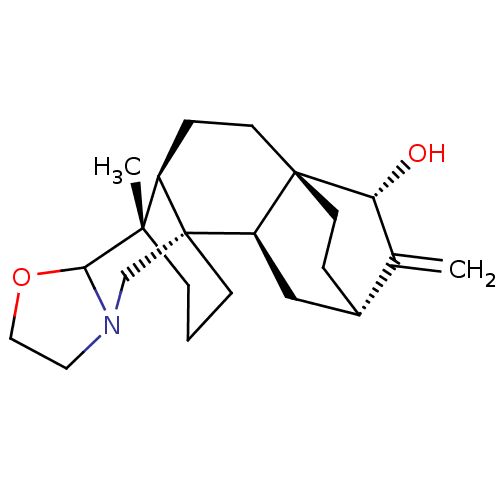 (CHEMBL4085938) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Malakand Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 15 mins followed by substrate addition by spectrophotome... | Bioorg Med Chem 25: 3368-3376 (2017) Article DOI: 10.1016/j.bmc.2017.04.022 BindingDB Entry DOI: 10.7270/Q2K64MG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50242322 (CHEMBL4066113) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Malakand Curated by ChEMBL | Assay Description Inhibition of horse serum BChE using butyrylcholine chloride as substrate preincubated for 15 mins followed by substrate addition by spectrophotometr... | Bioorg Med Chem 25: 3368-3376 (2017) Article DOI: 10.1016/j.bmc.2017.04.022 BindingDB Entry DOI: 10.7270/Q2K64MG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50242315 (CHEMBL4093688) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Malakand Curated by ChEMBL | Assay Description Inhibition of horse serum BChE using butyrylcholine chloride as substrate preincubated for 15 mins followed by substrate addition by spectrophotometr... | Bioorg Med Chem 25: 3368-3376 (2017) Article DOI: 10.1016/j.bmc.2017.04.022 BindingDB Entry DOI: 10.7270/Q2K64MG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50242345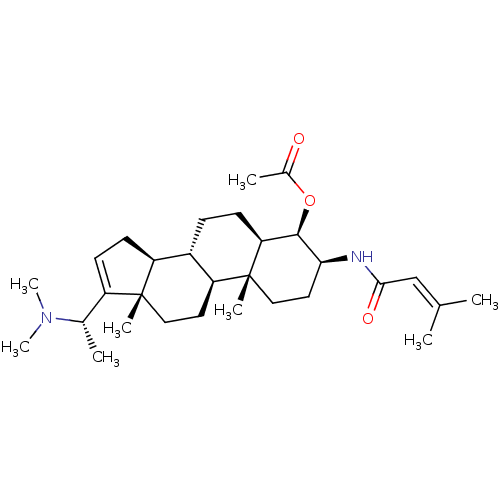 (CHEMBL460447 | [(20S)-20-(N,N-dimethylamino)-3 bet...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tribhuvan University Curated by ChEMBL | Assay Description Inhibition of horse BChE | J Nat Prod 64: 842-4 (2001) BindingDB Entry DOI: 10.7270/Q20R9P54 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50242318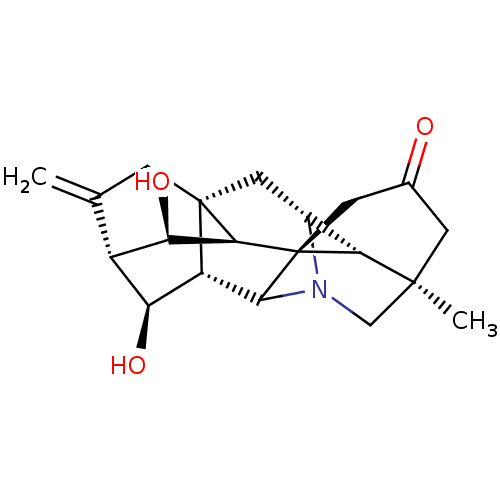 (CHEMBL4098012) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.01E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Malakand Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 15 mins followed by substrate addition by spectrophotome... | Bioorg Med Chem 25: 3368-3376 (2017) Article DOI: 10.1016/j.bmc.2017.04.022 BindingDB Entry DOI: 10.7270/Q2K64MG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50242324 (CHEMBL4072441) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.03E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Malakand Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 15 mins followed by substrate addition by spectrophotome... | Bioorg Med Chem 25: 3368-3376 (2017) Article DOI: 10.1016/j.bmc.2017.04.022 BindingDB Entry DOI: 10.7270/Q2K64MG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50242323 (CHEMBL4070917) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.04E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Malakand Curated by ChEMBL | Assay Description Inhibition of horse serum BChE using butyrylcholine chloride as substrate preincubated for 15 mins followed by substrate addition by spectrophotometr... | Bioorg Med Chem 25: 3368-3376 (2017) Article DOI: 10.1016/j.bmc.2017.04.022 BindingDB Entry DOI: 10.7270/Q2K64MG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM10404 ((1S,12S,14R)-9-methoxy-4-methyl-11-oxa-4-azatetrac...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Malakand Curated by ChEMBL | Assay Description Inhibition of horse serum BChE using butyrylcholine chloride as substrate preincubated for 15 mins followed by substrate addition by spectrophotometr... | Bioorg Med Chem 25: 3368-3376 (2017) Article DOI: 10.1016/j.bmc.2017.04.022 BindingDB Entry DOI: 10.7270/Q2K64MG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50242316 (CHEMBL4103864) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.28E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Malakand Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 15 mins followed by substrate addition by spectrophotome... | Bioorg Med Chem 25: 3368-3376 (2017) Article DOI: 10.1016/j.bmc.2017.04.022 BindingDB Entry DOI: 10.7270/Q2K64MG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50242317 (CHEMBL4085938) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.31E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Malakand Curated by ChEMBL | Assay Description Concentration required to displace 50% of [3H]oxytocin from rat uterine receptor. | Bioorg Med Chem 25: 3368-3376 (2017) Article DOI: 10.1016/j.bmc.2017.04.022 BindingDB Entry DOI: 10.7270/Q2K64MG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50242320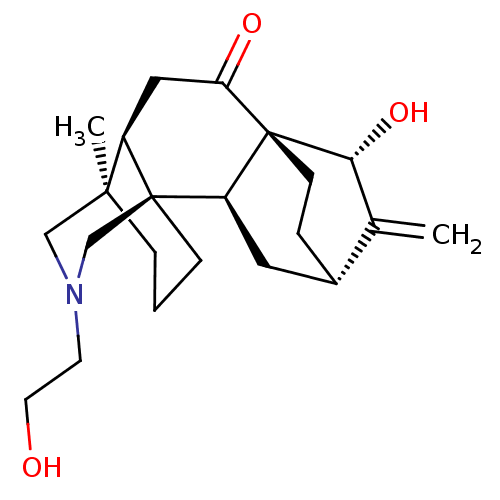 (CHEMBL4065019) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.46E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Malakand Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 15 mins followed by substrate addition by spectrophotome... | Bioorg Med Chem 25: 3368-3376 (2017) Article DOI: 10.1016/j.bmc.2017.04.022 BindingDB Entry DOI: 10.7270/Q2K64MG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50242324 (CHEMBL4072441) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.47E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Malakand Curated by ChEMBL | Assay Description Inhibition of horse serum BChE using butyrylcholine chloride as substrate preincubated for 15 mins followed by substrate addition by spectrophotometr... | Bioorg Med Chem 25: 3368-3376 (2017) Article DOI: 10.1016/j.bmc.2017.04.022 BindingDB Entry DOI: 10.7270/Q2K64MG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50242318 (CHEMBL4098012) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.56E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Malakand Curated by ChEMBL | Assay Description Inhibition of horse serum BChE using butyrylcholine chloride as substrate preincubated for 15 mins followed by substrate addition by spectrophotometr... | Bioorg Med Chem 25: 3368-3376 (2017) Article DOI: 10.1016/j.bmc.2017.04.022 BindingDB Entry DOI: 10.7270/Q2K64MG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50242316 (CHEMBL4103864) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.62E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Malakand Curated by ChEMBL | Assay Description Inhibition of horse serum BChE using butyrylcholine chloride as substrate preincubated for 15 mins followed by substrate addition by spectrophotometr... | Bioorg Med Chem 25: 3368-3376 (2017) Article DOI: 10.1016/j.bmc.2017.04.022 BindingDB Entry DOI: 10.7270/Q2K64MG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50242320 (CHEMBL4065019) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.81E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Malakand Curated by ChEMBL | Assay Description Inhibition of horse serum BChE using butyrylcholine chloride as substrate preincubated for 15 mins followed by substrate addition by spectrophotometr... | Bioorg Med Chem 25: 3368-3376 (2017) Article DOI: 10.1016/j.bmc.2017.04.022 BindingDB Entry DOI: 10.7270/Q2K64MG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50242346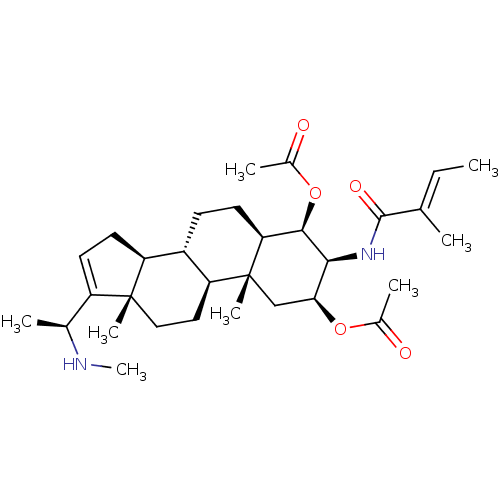 (CHEMBL500603 | [(20S)-20-(N-methylamino)-3beta-(ti...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tribhuvan University Curated by ChEMBL | Assay Description Inhibition of horse BChE | J Nat Prod 64: 842-4 (2001) BindingDB Entry DOI: 10.7270/Q20R9P54 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50242345 (CHEMBL460447 | [(20S)-20-(N,N-dimethylamino)-3 bet...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.69E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tribhuvan University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by Ellman's method | J Nat Prod 64: 842-4 (2001) BindingDB Entry DOI: 10.7270/Q20R9P54 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50242346 (CHEMBL500603 | [(20S)-20-(N-methylamino)-3beta-(ti...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.01E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tribhuvan University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by Ellman's method | J Nat Prod 64: 842-4 (2001) BindingDB Entry DOI: 10.7270/Q20R9P54 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||