 Found 196 hits with Last Name = 'sheng' and Initial = 'w'
Found 196 hits with Last Name = 'sheng' and Initial = 'w' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50000092

((-)-(etorphine) | (-)-morphine | (1S,5R,13R,14S)-1...)Show SMILES CN1CC[C@@]23[C@H]4Oc5c2c(C[C@@H]1[C@@H]3C=C[C@@H]4O)ccc5O |r,c:16,TLB:13:12:8.9.10:3.2.1| Show InChI InChI=1S/C17H19NO3/c1-18-7-6-17-10-3-5-13(20)16(17)21-15-12(19)4-2-9(14(15)17)8-11(10)18/h2-5,10-11,13,16,19-20H,6-8H2,1H3/t10-,11+,13-,16-,17-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 6.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University
Curated by ChEMBL
| Assay Description
Displacement of [3H]diprenorphine from human kappa receptor expressed in CHO cells by scintillation counting |
Bioorg Med Chem Lett 20: 418-21 (2010)
Article DOI: 10.1016/j.bmcl.2009.07.119
BindingDB Entry DOI: 10.7270/Q2BG2P4C |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50305087
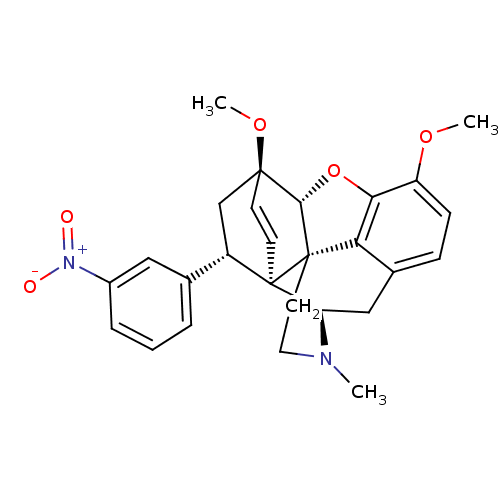
(21-nitro-8alpha-phenyl-6alpha,14alpha-endo-Ethenot...)Show SMILES COc1ccc2C[C@H]3N(C)CC[C@@]45[C@@H](Oc1c24)[C@]1(C[C@@H](c2cccc(c2)[N+]([O-])=O)[C@]35C=C1)OC |r,wU:7.7,17.38,wD:12.18,29.35,19.22,13.13,c:36,(-7.11,.52,;-5.77,1.29,;-4.43,.52,;-3.1,1.29,;-1.76,.53,;-1.76,-1.02,;-.42,-1.79,;-.42,-3.34,;.91,-4.11,;2.06,-5.14,;-.23,-2.55,;-1.77,-2.55,;-3.1,-3.33,;-4.43,-4.1,;-5.21,-2.35,;-4.43,-1.02,;-3.1,-1.8,;-4.44,-5.64,;-3.09,-6.42,;-1.76,-5.65,;-.43,-6.42,;-.43,-7.96,;.9,-8.73,;2.23,-7.96,;2.23,-6.41,;.9,-5.65,;3.56,-5.63,;4.9,-6.39,;3.56,-4.09,;-1.76,-4.11,;-3.07,-4.1,;-3.07,-5.66,;-5.91,-6.09,;-6.26,-7.59,)| Show InChI InChI=1S/C27H28N2O5/c1-28-12-11-27-22-17-7-8-20(32-2)23(22)34-24(27)25(33-3)9-10-26(27,21(28)14-17)19(15-25)16-5-4-6-18(13-16)29(30)31/h4-10,13,19,21,24H,11-12,14-15H2,1-3H3/t19-,21+,24-,25-,26+,27-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University
Curated by ChEMBL
| Assay Description
Displacement of [3H]diprenorphine from human kappa receptor expressed in CHO cells by scintillation counting |
Bioorg Med Chem Lett 20: 418-21 (2010)
Article DOI: 10.1016/j.bmcl.2009.07.119
BindingDB Entry DOI: 10.7270/Q2BG2P4C |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50000092

((-)-(etorphine) | (-)-morphine | (1S,5R,13R,14S)-1...)Show SMILES CN1CC[C@@]23[C@H]4Oc5c2c(C[C@@H]1[C@@H]3C=C[C@@H]4O)ccc5O |r,c:16,TLB:13:12:8.9.10:3.2.1| Show InChI InChI=1S/C17H19NO3/c1-18-7-6-17-10-3-5-13(20)16(17)21-15-12(19)4-2-9(14(15)17)8-11(10)18/h2-5,10-11,13,16,19-20H,6-8H2,1H3/t10-,11+,13-,16-,17-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 76.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University
Curated by ChEMBL
| Assay Description
Displacement of [3H]diprenorphine from rat delta receptor expressed in CHO cells by scintillation counting |
Bioorg Med Chem Lett 20: 418-21 (2010)
Article DOI: 10.1016/j.bmcl.2009.07.119
BindingDB Entry DOI: 10.7270/Q2BG2P4C |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50000092

((-)-(etorphine) | (-)-morphine | (1S,5R,13R,14S)-1...)Show SMILES CN1CC[C@@]23[C@H]4Oc5c2c(C[C@@H]1[C@@H]3C=C[C@@H]4O)ccc5O |r,c:16,TLB:13:12:8.9.10:3.2.1| Show InChI InChI=1S/C17H19NO3/c1-18-7-6-17-10-3-5-13(20)16(17)21-15-12(19)4-2-9(14(15)17)8-11(10)18/h2-5,10-11,13,16,19-20H,6-8H2,1H3/t10-,11+,13-,16-,17-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 117 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University
Curated by ChEMBL
| Assay Description
Displacement of [3H]diprenorphine from rat mu receptor expressed in CHO cells by scintillation counting |
Bioorg Med Chem Lett 20: 418-21 (2010)
Article DOI: 10.1016/j.bmcl.2009.07.119
BindingDB Entry DOI: 10.7270/Q2BG2P4C |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50305089
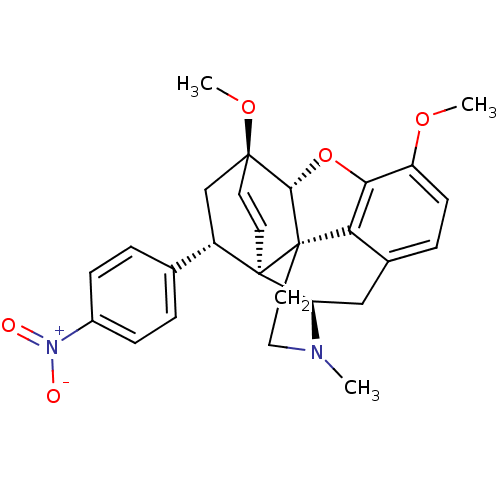
(22-nitro-8alpha-phenyl-6alpha,14alpha-endo-Ethenot...)Show SMILES COc1ccc2C[C@H]3N(C)CC[C@@]45[C@@H](Oc1c24)[C@]1(C[C@@H](c2ccc(cc2)[N+]([O-])=O)[C@]35C=C1)OC |r,wU:7.7,17.38,wD:12.18,29.35,19.22,13.13,c:36,(-7.11,.52,;-5.77,1.29,;-4.43,.52,;-3.1,1.29,;-1.76,.53,;-1.76,-1.02,;-.42,-1.79,;-.42,-3.34,;.91,-4.11,;2.06,-5.14,;-.23,-2.55,;-1.77,-2.55,;-3.1,-3.33,;-4.43,-4.1,;-5.21,-2.35,;-4.43,-1.02,;-3.1,-1.8,;-4.44,-5.64,;-3.09,-6.42,;-1.76,-5.65,;-.43,-6.42,;-.43,-7.96,;.9,-8.73,;2.23,-7.96,;2.23,-6.41,;.9,-5.65,;3.57,-8.73,;3.58,-10.27,;4.91,-7.96,;-1.76,-4.11,;-3.01,-4.1,;-3.01,-5.72,;-5.91,-6.09,;-6.26,-7.59,)| Show InChI InChI=1S/C27H28N2O5/c1-28-13-12-27-22-17-6-9-20(32-2)23(22)34-24(27)25(33-3)10-11-26(27,21(28)14-17)19(15-25)16-4-7-18(8-5-16)29(30)31/h4-11,19,21,24H,12-15H2,1-3H3/t19-,21+,24-,25-,26+,27-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 674 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University
Curated by ChEMBL
| Assay Description
Displacement of [3H]diprenorphine from human kappa receptor expressed in CHO cells by scintillation counting |
Bioorg Med Chem Lett 20: 418-21 (2010)
Article DOI: 10.1016/j.bmcl.2009.07.119
BindingDB Entry DOI: 10.7270/Q2BG2P4C |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50305085
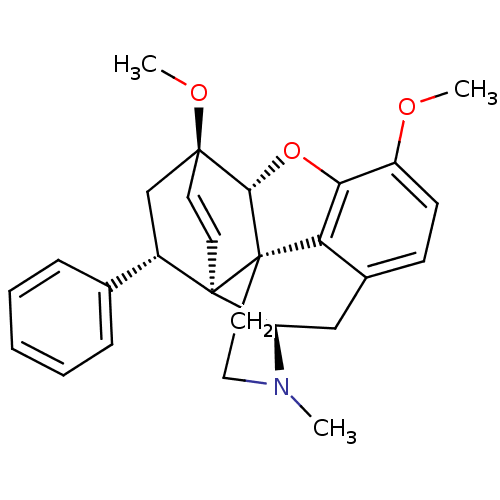
(8alpha-phenyl-6alpha,14alpha-endo-Ethenotetrahydro...)Show SMILES COc1ccc2C[C@H]3N(C)CC[C@@]45[C@@H](Oc1c24)[C@]1(C[C@@H](c2ccccc2)[C@]35C=C1)OC |r,wU:7.7,17.35,wD:13.13,12.18,26.32,19.22,c:33,(-7.92,.41,;-6.59,1.18,;-5.24,.4,;-3.92,1.17,;-2.58,.41,;-2.58,-1.14,;-1.24,-1.9,;-1.24,-3.45,;.09,-4.22,;1.24,-5.25,;-1.05,-2.67,;-2.59,-2.67,;-3.91,-3.45,;-5.25,-4.22,;-6.03,-2.47,;-5.25,-1.14,;-3.91,-1.91,;-5.25,-5.76,;-3.91,-6.53,;-2.58,-5.76,;-1.25,-6.53,;-1.25,-8.07,;.08,-8.84,;1.42,-8.07,;1.41,-6.53,;.08,-5.76,;-2.58,-4.23,;-3.89,-4.21,;-3.89,-5.84,;-6.73,-6.21,;-7.07,-7.71,)| Show InChI InChI=1S/C27H29NO3/c1-28-14-13-27-22-18-9-10-20(29-2)23(22)31-24(27)25(30-3)11-12-26(27,21(28)15-18)19(16-25)17-7-5-4-6-8-17/h4-12,19,21,24H,13-16H2,1-3H3/t19-,21+,24-,25-,26+,27-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University
Curated by ChEMBL
| Assay Description
Displacement of [3H]diprenorphine from human kappa receptor expressed in CHO cells by scintillation counting |
Bioorg Med Chem Lett 20: 418-21 (2010)
Article DOI: 10.1016/j.bmcl.2009.07.119
BindingDB Entry DOI: 10.7270/Q2BG2P4C |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50305084
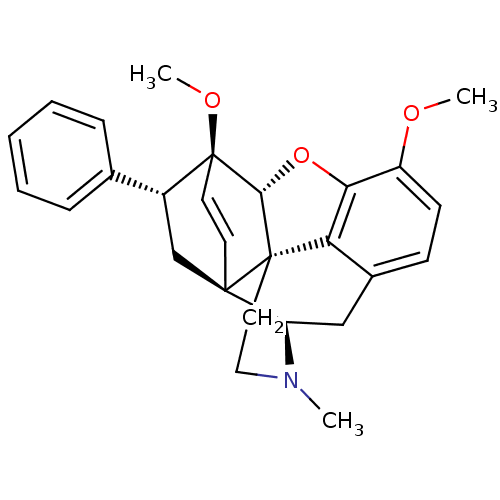
(7alpha-phenyl-6alpha,14alpha-endo-Ethenotetrahydro...)Show SMILES COc1ccc2C[C@H]3N(C)CC[C@@]45[C@@H](Oc1c24)[C@]1(OC)C=C[C@@]35C[C@@H]1c1ccccc1 |r,wU:7.7,17.20,wD:13.13,12.18,22.24,24.30,c:23,(-7.92,.41,;-6.59,1.18,;-5.24,.4,;-3.92,1.18,;-2.58,.41,;-2.58,-1.14,;-1.24,-1.9,;-1.24,-3.45,;.09,-4.23,;1.24,-5.25,;-1.05,-2.67,;-2.59,-2.67,;-3.91,-3.45,;-5.25,-4.22,;-6.03,-2.47,;-5.25,-1.14,;-3.92,-1.91,;-5.25,-5.76,;-6.73,-6.21,;-7.08,-7.71,;-3.83,-5.78,;-3.95,-4.22,;-2.58,-4.23,;-2.58,-5.76,;-3.91,-6.54,;-3.91,-8.08,;-5.25,-8.84,;-5.25,-10.38,;-3.91,-11.15,;-2.57,-10.37,;-2.58,-8.83,)| Show InChI InChI=1S/C27H29NO3/c1-28-14-13-26-22-18-9-10-20(29-2)23(22)31-24(26)27(30-3)12-11-25(26,21(28)15-18)16-19(27)17-7-5-4-6-8-17/h4-12,19,21,24H,13-16H2,1-3H3/t19-,21-,24-,25-,26+,27+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University
Curated by ChEMBL
| Assay Description
Displacement of [3H]diprenorphine from human kappa receptor expressed in CHO cells by scintillation counting |
Bioorg Med Chem Lett 20: 418-21 (2010)
Article DOI: 10.1016/j.bmcl.2009.07.119
BindingDB Entry DOI: 10.7270/Q2BG2P4C |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50305084

(7alpha-phenyl-6alpha,14alpha-endo-Ethenotetrahydro...)Show SMILES COc1ccc2C[C@H]3N(C)CC[C@@]45[C@@H](Oc1c24)[C@]1(OC)C=C[C@@]35C[C@@H]1c1ccccc1 |r,wU:7.7,17.20,wD:13.13,12.18,22.24,24.30,c:23,(-7.92,.41,;-6.59,1.18,;-5.24,.4,;-3.92,1.18,;-2.58,.41,;-2.58,-1.14,;-1.24,-1.9,;-1.24,-3.45,;.09,-4.23,;1.24,-5.25,;-1.05,-2.67,;-2.59,-2.67,;-3.91,-3.45,;-5.25,-4.22,;-6.03,-2.47,;-5.25,-1.14,;-3.92,-1.91,;-5.25,-5.76,;-6.73,-6.21,;-7.08,-7.71,;-3.83,-5.78,;-3.95,-4.22,;-2.58,-4.23,;-2.58,-5.76,;-3.91,-6.54,;-3.91,-8.08,;-5.25,-8.84,;-5.25,-10.38,;-3.91,-11.15,;-2.57,-10.37,;-2.58,-8.83,)| Show InChI InChI=1S/C27H29NO3/c1-28-14-13-26-22-18-9-10-20(29-2)23(22)31-24(26)27(30-3)12-11-25(26,21(28)15-18)16-19(27)17-7-5-4-6-8-17/h4-12,19,21,24H,13-16H2,1-3H3/t19-,21-,24-,25-,26+,27+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University
Curated by ChEMBL
| Assay Description
Displacement of [3H]diprenorphine from rat delta receptor expressed in CHO cells by scintillation counting |
Bioorg Med Chem Lett 20: 418-21 (2010)
Article DOI: 10.1016/j.bmcl.2009.07.119
BindingDB Entry DOI: 10.7270/Q2BG2P4C |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50305085

(8alpha-phenyl-6alpha,14alpha-endo-Ethenotetrahydro...)Show SMILES COc1ccc2C[C@H]3N(C)CC[C@@]45[C@@H](Oc1c24)[C@]1(C[C@@H](c2ccccc2)[C@]35C=C1)OC |r,wU:7.7,17.35,wD:13.13,12.18,26.32,19.22,c:33,(-7.92,.41,;-6.59,1.18,;-5.24,.4,;-3.92,1.17,;-2.58,.41,;-2.58,-1.14,;-1.24,-1.9,;-1.24,-3.45,;.09,-4.22,;1.24,-5.25,;-1.05,-2.67,;-2.59,-2.67,;-3.91,-3.45,;-5.25,-4.22,;-6.03,-2.47,;-5.25,-1.14,;-3.91,-1.91,;-5.25,-5.76,;-3.91,-6.53,;-2.58,-5.76,;-1.25,-6.53,;-1.25,-8.07,;.08,-8.84,;1.42,-8.07,;1.41,-6.53,;.08,-5.76,;-2.58,-4.23,;-3.89,-4.21,;-3.89,-5.84,;-6.73,-6.21,;-7.07,-7.71,)| Show InChI InChI=1S/C27H29NO3/c1-28-14-13-27-22-18-9-10-20(29-2)23(22)31-24(27)25(30-3)11-12-26(27,21(28)15-18)19(16-25)17-7-5-4-6-8-17/h4-12,19,21,24H,13-16H2,1-3H3/t19-,21+,24-,25-,26+,27-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University
Curated by ChEMBL
| Assay Description
Displacement of [3H]diprenorphine from rat delta receptor expressed in CHO cells by scintillation counting |
Bioorg Med Chem Lett 20: 418-21 (2010)
Article DOI: 10.1016/j.bmcl.2009.07.119
BindingDB Entry DOI: 10.7270/Q2BG2P4C |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50305086
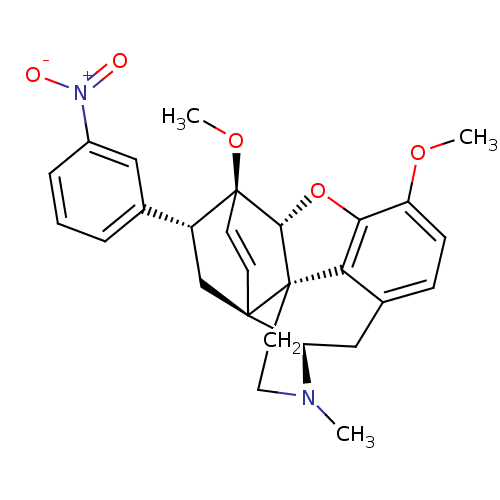
(21-nitro-7alpha-phenyl-6alpha,14alpha-endo-Ethenot...)Show SMILES COc1ccc2C[C@H]3N(C)CC[C@@]45[C@@H](Oc1c24)[C@]1(OC)C=C[C@@]35C[C@@H]1c1cccc(c1)[N+]([O-])=O |r,wU:7.7,17.20,wD:12.18,22.24,24.30,13.13,c:23,(-7.57,-14.62,;-6.24,-13.85,;-4.89,-14.63,;-3.57,-13.86,;-2.23,-14.62,;-2.23,-16.17,;-.89,-16.94,;-.89,-18.49,;.44,-19.26,;1.59,-20.28,;-.7,-17.7,;-2.24,-17.7,;-3.56,-18.48,;-4.9,-19.25,;-5.68,-17.5,;-4.9,-16.17,;-3.56,-16.94,;-4.9,-20.79,;-6.37,-21.24,;-6.72,-22.74,;-3.53,-20.81,;-3.54,-19.25,;-2.23,-19.26,;-2.23,-20.79,;-3.56,-21.57,;-3.56,-23.11,;-4.9,-23.87,;-4.89,-25.41,;-3.56,-26.18,;-2.22,-25.4,;-2.23,-23.86,;-.88,-26.16,;-.88,-27.7,;.45,-25.39,)| Show InChI InChI=1S/C27H28N2O5/c1-28-12-11-26-22-17-7-8-20(32-2)23(22)34-24(26)27(33-3)10-9-25(26,21(28)14-17)15-19(27)16-5-4-6-18(13-16)29(30)31/h4-10,13,19,21,24H,11-12,14-15H2,1-3H3/t19-,21-,24-,25-,26+,27+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University
Curated by ChEMBL
| Assay Description
Displacement of [3H]diprenorphine from rat delta receptor expressed in CHO cells by scintillation counting |
Bioorg Med Chem Lett 20: 418-21 (2010)
Article DOI: 10.1016/j.bmcl.2009.07.119
BindingDB Entry DOI: 10.7270/Q2BG2P4C |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50305087

(21-nitro-8alpha-phenyl-6alpha,14alpha-endo-Ethenot...)Show SMILES COc1ccc2C[C@H]3N(C)CC[C@@]45[C@@H](Oc1c24)[C@]1(C[C@@H](c2cccc(c2)[N+]([O-])=O)[C@]35C=C1)OC |r,wU:7.7,17.38,wD:12.18,29.35,19.22,13.13,c:36,(-7.11,.52,;-5.77,1.29,;-4.43,.52,;-3.1,1.29,;-1.76,.53,;-1.76,-1.02,;-.42,-1.79,;-.42,-3.34,;.91,-4.11,;2.06,-5.14,;-.23,-2.55,;-1.77,-2.55,;-3.1,-3.33,;-4.43,-4.1,;-5.21,-2.35,;-4.43,-1.02,;-3.1,-1.8,;-4.44,-5.64,;-3.09,-6.42,;-1.76,-5.65,;-.43,-6.42,;-.43,-7.96,;.9,-8.73,;2.23,-7.96,;2.23,-6.41,;.9,-5.65,;3.56,-5.63,;4.9,-6.39,;3.56,-4.09,;-1.76,-4.11,;-3.07,-4.1,;-3.07,-5.66,;-5.91,-6.09,;-6.26,-7.59,)| Show InChI InChI=1S/C27H28N2O5/c1-28-12-11-27-22-17-7-8-20(32-2)23(22)34-24(27)25(33-3)9-10-26(27,21(28)14-17)19(15-25)16-5-4-6-18(13-16)29(30)31/h4-10,13,19,21,24H,11-12,14-15H2,1-3H3/t19-,21+,24-,25-,26+,27-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University
Curated by ChEMBL
| Assay Description
Displacement of [3H]diprenorphine from rat delta receptor expressed in CHO cells by scintillation counting |
Bioorg Med Chem Lett 20: 418-21 (2010)
Article DOI: 10.1016/j.bmcl.2009.07.119
BindingDB Entry DOI: 10.7270/Q2BG2P4C |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50305088
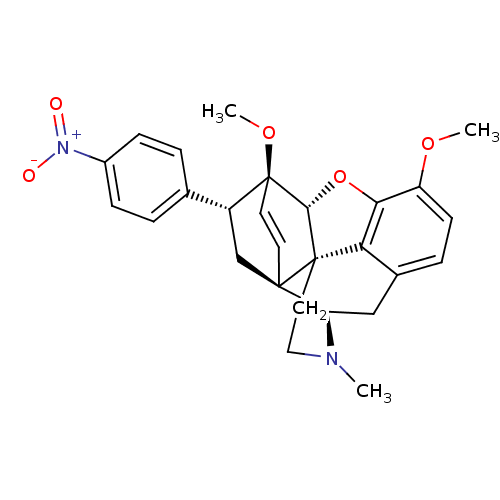
(22-nitro-7apha-phenyl-6alpha,14alpha-endo-Ethenote...)Show SMILES COc1ccc2C[C@H]3N(C)CC[C@@]45[C@@H](Oc1c24)[C@]1(OC)C=C[C@@]35C[C@@H]1c1ccc(cc1)[N+]([O-])=O |r,wU:7.7,17.20,wD:12.18,22.24,24.30,13.13,c:23,(-7.57,-14.63,;-6.24,-13.86,;-4.89,-14.63,;-3.57,-13.86,;-2.23,-14.62,;-2.23,-16.17,;-.89,-16.94,;-.89,-18.49,;.44,-19.26,;1.59,-20.29,;-.7,-17.7,;-2.24,-17.7,;-3.56,-18.48,;-4.9,-19.25,;-5.68,-17.5,;-4.9,-16.17,;-3.56,-16.95,;-4.9,-20.79,;-6.38,-21.24,;-6.72,-22.74,;-3.48,-20.81,;-3.54,-19.25,;-2.23,-19.26,;-2.23,-20.8,;-3.56,-21.57,;-3.56,-23.11,;-4.9,-23.87,;-4.9,-25.41,;-3.56,-26.18,;-2.22,-25.4,;-2.23,-23.87,;-3.55,-27.73,;-4.88,-28.5,;-2.22,-28.49,)| Show InChI InChI=1S/C27H28N2O5/c1-28-13-12-26-22-17-6-9-20(32-2)23(22)34-24(26)27(33-3)11-10-25(26,21(28)14-17)15-19(27)16-4-7-18(8-5-16)29(30)31/h4-11,19,21,24H,12-15H2,1-3H3/t19-,21-,24-,25-,26+,27+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University
Curated by ChEMBL
| Assay Description
Displacement of [3H]diprenorphine from rat delta receptor expressed in CHO cells by scintillation counting |
Bioorg Med Chem Lett 20: 418-21 (2010)
Article DOI: 10.1016/j.bmcl.2009.07.119
BindingDB Entry DOI: 10.7270/Q2BG2P4C |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50305089

(22-nitro-8alpha-phenyl-6alpha,14alpha-endo-Ethenot...)Show SMILES COc1ccc2C[C@H]3N(C)CC[C@@]45[C@@H](Oc1c24)[C@]1(C[C@@H](c2ccc(cc2)[N+]([O-])=O)[C@]35C=C1)OC |r,wU:7.7,17.38,wD:12.18,29.35,19.22,13.13,c:36,(-7.11,.52,;-5.77,1.29,;-4.43,.52,;-3.1,1.29,;-1.76,.53,;-1.76,-1.02,;-.42,-1.79,;-.42,-3.34,;.91,-4.11,;2.06,-5.14,;-.23,-2.55,;-1.77,-2.55,;-3.1,-3.33,;-4.43,-4.1,;-5.21,-2.35,;-4.43,-1.02,;-3.1,-1.8,;-4.44,-5.64,;-3.09,-6.42,;-1.76,-5.65,;-.43,-6.42,;-.43,-7.96,;.9,-8.73,;2.23,-7.96,;2.23,-6.41,;.9,-5.65,;3.57,-8.73,;3.58,-10.27,;4.91,-7.96,;-1.76,-4.11,;-3.01,-4.1,;-3.01,-5.72,;-5.91,-6.09,;-6.26,-7.59,)| Show InChI InChI=1S/C27H28N2O5/c1-28-13-12-27-22-17-6-9-20(32-2)23(22)34-24(27)25(33-3)10-11-26(27,21(28)14-17)19(15-25)16-4-7-18(8-5-16)29(30)31/h4-11,19,21,24H,12-15H2,1-3H3/t19-,21+,24-,25-,26+,27-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University
Curated by ChEMBL
| Assay Description
Displacement of [3H]diprenorphine from rat delta receptor expressed in CHO cells by scintillation counting |
Bioorg Med Chem Lett 20: 418-21 (2010)
Article DOI: 10.1016/j.bmcl.2009.07.119
BindingDB Entry DOI: 10.7270/Q2BG2P4C |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50305089

(22-nitro-8alpha-phenyl-6alpha,14alpha-endo-Ethenot...)Show SMILES COc1ccc2C[C@H]3N(C)CC[C@@]45[C@@H](Oc1c24)[C@]1(C[C@@H](c2ccc(cc2)[N+]([O-])=O)[C@]35C=C1)OC |r,wU:7.7,17.38,wD:12.18,29.35,19.22,13.13,c:36,(-7.11,.52,;-5.77,1.29,;-4.43,.52,;-3.1,1.29,;-1.76,.53,;-1.76,-1.02,;-.42,-1.79,;-.42,-3.34,;.91,-4.11,;2.06,-5.14,;-.23,-2.55,;-1.77,-2.55,;-3.1,-3.33,;-4.43,-4.1,;-5.21,-2.35,;-4.43,-1.02,;-3.1,-1.8,;-4.44,-5.64,;-3.09,-6.42,;-1.76,-5.65,;-.43,-6.42,;-.43,-7.96,;.9,-8.73,;2.23,-7.96,;2.23,-6.41,;.9,-5.65,;3.57,-8.73,;3.58,-10.27,;4.91,-7.96,;-1.76,-4.11,;-3.01,-4.1,;-3.01,-5.72,;-5.91,-6.09,;-6.26,-7.59,)| Show InChI InChI=1S/C27H28N2O5/c1-28-13-12-27-22-17-6-9-20(32-2)23(22)34-24(27)25(33-3)10-11-26(27,21(28)14-17)19(15-25)16-4-7-18(8-5-16)29(30)31/h4-11,19,21,24H,12-15H2,1-3H3/t19-,21+,24-,25-,26+,27-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University
Curated by ChEMBL
| Assay Description
Displacement of [3H]diprenorphine from rat mu receptor expressed in CHO cells by scintillation counting |
Bioorg Med Chem Lett 20: 418-21 (2010)
Article DOI: 10.1016/j.bmcl.2009.07.119
BindingDB Entry DOI: 10.7270/Q2BG2P4C |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50305088

(22-nitro-7apha-phenyl-6alpha,14alpha-endo-Ethenote...)Show SMILES COc1ccc2C[C@H]3N(C)CC[C@@]45[C@@H](Oc1c24)[C@]1(OC)C=C[C@@]35C[C@@H]1c1ccc(cc1)[N+]([O-])=O |r,wU:7.7,17.20,wD:12.18,22.24,24.30,13.13,c:23,(-7.57,-14.63,;-6.24,-13.86,;-4.89,-14.63,;-3.57,-13.86,;-2.23,-14.62,;-2.23,-16.17,;-.89,-16.94,;-.89,-18.49,;.44,-19.26,;1.59,-20.29,;-.7,-17.7,;-2.24,-17.7,;-3.56,-18.48,;-4.9,-19.25,;-5.68,-17.5,;-4.9,-16.17,;-3.56,-16.95,;-4.9,-20.79,;-6.38,-21.24,;-6.72,-22.74,;-3.48,-20.81,;-3.54,-19.25,;-2.23,-19.26,;-2.23,-20.8,;-3.56,-21.57,;-3.56,-23.11,;-4.9,-23.87,;-4.9,-25.41,;-3.56,-26.18,;-2.22,-25.4,;-2.23,-23.87,;-3.55,-27.73,;-4.88,-28.5,;-2.22,-28.49,)| Show InChI InChI=1S/C27H28N2O5/c1-28-13-12-26-22-17-6-9-20(32-2)23(22)34-24(26)27(33-3)11-10-25(26,21(28)14-17)15-19(27)16-4-7-18(8-5-16)29(30)31/h4-11,19,21,24H,12-15H2,1-3H3/t19-,21-,24-,25-,26+,27+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University
Curated by ChEMBL
| Assay Description
Displacement of [3H]diprenorphine from rat mu receptor expressed in CHO cells by scintillation counting |
Bioorg Med Chem Lett 20: 418-21 (2010)
Article DOI: 10.1016/j.bmcl.2009.07.119
BindingDB Entry DOI: 10.7270/Q2BG2P4C |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50305086

(21-nitro-7alpha-phenyl-6alpha,14alpha-endo-Ethenot...)Show SMILES COc1ccc2C[C@H]3N(C)CC[C@@]45[C@@H](Oc1c24)[C@]1(OC)C=C[C@@]35C[C@@H]1c1cccc(c1)[N+]([O-])=O |r,wU:7.7,17.20,wD:12.18,22.24,24.30,13.13,c:23,(-7.57,-14.62,;-6.24,-13.85,;-4.89,-14.63,;-3.57,-13.86,;-2.23,-14.62,;-2.23,-16.17,;-.89,-16.94,;-.89,-18.49,;.44,-19.26,;1.59,-20.28,;-.7,-17.7,;-2.24,-17.7,;-3.56,-18.48,;-4.9,-19.25,;-5.68,-17.5,;-4.9,-16.17,;-3.56,-16.94,;-4.9,-20.79,;-6.37,-21.24,;-6.72,-22.74,;-3.53,-20.81,;-3.54,-19.25,;-2.23,-19.26,;-2.23,-20.79,;-3.56,-21.57,;-3.56,-23.11,;-4.9,-23.87,;-4.89,-25.41,;-3.56,-26.18,;-2.22,-25.4,;-2.23,-23.86,;-.88,-26.16,;-.88,-27.7,;.45,-25.39,)| Show InChI InChI=1S/C27H28N2O5/c1-28-12-11-26-22-17-7-8-20(32-2)23(22)34-24(26)27(33-3)10-9-25(26,21(28)14-17)15-19(27)16-5-4-6-18(13-16)29(30)31/h4-10,13,19,21,24H,11-12,14-15H2,1-3H3/t19-,21-,24-,25-,26+,27+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University
Curated by ChEMBL
| Assay Description
Displacement of [3H]diprenorphine from rat mu receptor expressed in CHO cells by scintillation counting |
Bioorg Med Chem Lett 20: 418-21 (2010)
Article DOI: 10.1016/j.bmcl.2009.07.119
BindingDB Entry DOI: 10.7270/Q2BG2P4C |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50305085

(8alpha-phenyl-6alpha,14alpha-endo-Ethenotetrahydro...)Show SMILES COc1ccc2C[C@H]3N(C)CC[C@@]45[C@@H](Oc1c24)[C@]1(C[C@@H](c2ccccc2)[C@]35C=C1)OC |r,wU:7.7,17.35,wD:13.13,12.18,26.32,19.22,c:33,(-7.92,.41,;-6.59,1.18,;-5.24,.4,;-3.92,1.17,;-2.58,.41,;-2.58,-1.14,;-1.24,-1.9,;-1.24,-3.45,;.09,-4.22,;1.24,-5.25,;-1.05,-2.67,;-2.59,-2.67,;-3.91,-3.45,;-5.25,-4.22,;-6.03,-2.47,;-5.25,-1.14,;-3.91,-1.91,;-5.25,-5.76,;-3.91,-6.53,;-2.58,-5.76,;-1.25,-6.53,;-1.25,-8.07,;.08,-8.84,;1.42,-8.07,;1.41,-6.53,;.08,-5.76,;-2.58,-4.23,;-3.89,-4.21,;-3.89,-5.84,;-6.73,-6.21,;-7.07,-7.71,)| Show InChI InChI=1S/C27H29NO3/c1-28-14-13-27-22-18-9-10-20(29-2)23(22)31-24(27)25(30-3)11-12-26(27,21(28)15-18)19(16-25)17-7-5-4-6-8-17/h4-12,19,21,24H,13-16H2,1-3H3/t19-,21+,24-,25-,26+,27-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University
Curated by ChEMBL
| Assay Description
Displacement of [3H]diprenorphine from rat mu receptor expressed in CHO cells by scintillation counting |
Bioorg Med Chem Lett 20: 418-21 (2010)
Article DOI: 10.1016/j.bmcl.2009.07.119
BindingDB Entry DOI: 10.7270/Q2BG2P4C |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50305084

(7alpha-phenyl-6alpha,14alpha-endo-Ethenotetrahydro...)Show SMILES COc1ccc2C[C@H]3N(C)CC[C@@]45[C@@H](Oc1c24)[C@]1(OC)C=C[C@@]35C[C@@H]1c1ccccc1 |r,wU:7.7,17.20,wD:13.13,12.18,22.24,24.30,c:23,(-7.92,.41,;-6.59,1.18,;-5.24,.4,;-3.92,1.18,;-2.58,.41,;-2.58,-1.14,;-1.24,-1.9,;-1.24,-3.45,;.09,-4.23,;1.24,-5.25,;-1.05,-2.67,;-2.59,-2.67,;-3.91,-3.45,;-5.25,-4.22,;-6.03,-2.47,;-5.25,-1.14,;-3.92,-1.91,;-5.25,-5.76,;-6.73,-6.21,;-7.08,-7.71,;-3.83,-5.78,;-3.95,-4.22,;-2.58,-4.23,;-2.58,-5.76,;-3.91,-6.54,;-3.91,-8.08,;-5.25,-8.84,;-5.25,-10.38,;-3.91,-11.15,;-2.57,-10.37,;-2.58,-8.83,)| Show InChI InChI=1S/C27H29NO3/c1-28-14-13-26-22-18-9-10-20(29-2)23(22)31-24(26)27(30-3)12-11-25(26,21(28)15-18)16-19(27)17-7-5-4-6-8-17/h4-12,19,21,24H,13-16H2,1-3H3/t19-,21-,24-,25-,26+,27+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University
Curated by ChEMBL
| Assay Description
Displacement of [3H]diprenorphine from rat mu receptor expressed in CHO cells by scintillation counting |
Bioorg Med Chem Lett 20: 418-21 (2010)
Article DOI: 10.1016/j.bmcl.2009.07.119
BindingDB Entry DOI: 10.7270/Q2BG2P4C |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50305088

(22-nitro-7apha-phenyl-6alpha,14alpha-endo-Ethenote...)Show SMILES COc1ccc2C[C@H]3N(C)CC[C@@]45[C@@H](Oc1c24)[C@]1(OC)C=C[C@@]35C[C@@H]1c1ccc(cc1)[N+]([O-])=O |r,wU:7.7,17.20,wD:12.18,22.24,24.30,13.13,c:23,(-7.57,-14.63,;-6.24,-13.86,;-4.89,-14.63,;-3.57,-13.86,;-2.23,-14.62,;-2.23,-16.17,;-.89,-16.94,;-.89,-18.49,;.44,-19.26,;1.59,-20.29,;-.7,-17.7,;-2.24,-17.7,;-3.56,-18.48,;-4.9,-19.25,;-5.68,-17.5,;-4.9,-16.17,;-3.56,-16.95,;-4.9,-20.79,;-6.38,-21.24,;-6.72,-22.74,;-3.48,-20.81,;-3.54,-19.25,;-2.23,-19.26,;-2.23,-20.8,;-3.56,-21.57,;-3.56,-23.11,;-4.9,-23.87,;-4.9,-25.41,;-3.56,-26.18,;-2.22,-25.4,;-2.23,-23.87,;-3.55,-27.73,;-4.88,-28.5,;-2.22,-28.49,)| Show InChI InChI=1S/C27H28N2O5/c1-28-13-12-26-22-17-6-9-20(32-2)23(22)34-24(26)27(33-3)11-10-25(26,21(28)14-17)15-19(27)16-4-7-18(8-5-16)29(30)31/h4-11,19,21,24H,12-15H2,1-3H3/t19-,21-,24-,25-,26+,27+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University
Curated by ChEMBL
| Assay Description
Displacement of [3H]diprenorphine from human kappa receptor expressed in CHO cells by scintillation counting |
Bioorg Med Chem Lett 20: 418-21 (2010)
Article DOI: 10.1016/j.bmcl.2009.07.119
BindingDB Entry DOI: 10.7270/Q2BG2P4C |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50305086

(21-nitro-7alpha-phenyl-6alpha,14alpha-endo-Ethenot...)Show SMILES COc1ccc2C[C@H]3N(C)CC[C@@]45[C@@H](Oc1c24)[C@]1(OC)C=C[C@@]35C[C@@H]1c1cccc(c1)[N+]([O-])=O |r,wU:7.7,17.20,wD:12.18,22.24,24.30,13.13,c:23,(-7.57,-14.62,;-6.24,-13.85,;-4.89,-14.63,;-3.57,-13.86,;-2.23,-14.62,;-2.23,-16.17,;-.89,-16.94,;-.89,-18.49,;.44,-19.26,;1.59,-20.28,;-.7,-17.7,;-2.24,-17.7,;-3.56,-18.48,;-4.9,-19.25,;-5.68,-17.5,;-4.9,-16.17,;-3.56,-16.94,;-4.9,-20.79,;-6.37,-21.24,;-6.72,-22.74,;-3.53,-20.81,;-3.54,-19.25,;-2.23,-19.26,;-2.23,-20.79,;-3.56,-21.57,;-3.56,-23.11,;-4.9,-23.87,;-4.89,-25.41,;-3.56,-26.18,;-2.22,-25.4,;-2.23,-23.86,;-.88,-26.16,;-.88,-27.7,;.45,-25.39,)| Show InChI InChI=1S/C27H28N2O5/c1-28-12-11-26-22-17-7-8-20(32-2)23(22)34-24(26)27(33-3)10-9-25(26,21(28)14-17)15-19(27)16-5-4-6-18(13-16)29(30)31/h4-10,13,19,21,24H,11-12,14-15H2,1-3H3/t19-,21-,24-,25-,26+,27+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University
Curated by ChEMBL
| Assay Description
Displacement of [3H]diprenorphine from human kappa receptor expressed in CHO cells by scintillation counting |
Bioorg Med Chem Lett 20: 418-21 (2010)
Article DOI: 10.1016/j.bmcl.2009.07.119
BindingDB Entry DOI: 10.7270/Q2BG2P4C |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50305087

(21-nitro-8alpha-phenyl-6alpha,14alpha-endo-Ethenot...)Show SMILES COc1ccc2C[C@H]3N(C)CC[C@@]45[C@@H](Oc1c24)[C@]1(C[C@@H](c2cccc(c2)[N+]([O-])=O)[C@]35C=C1)OC |r,wU:7.7,17.38,wD:12.18,29.35,19.22,13.13,c:36,(-7.11,.52,;-5.77,1.29,;-4.43,.52,;-3.1,1.29,;-1.76,.53,;-1.76,-1.02,;-.42,-1.79,;-.42,-3.34,;.91,-4.11,;2.06,-5.14,;-.23,-2.55,;-1.77,-2.55,;-3.1,-3.33,;-4.43,-4.1,;-5.21,-2.35,;-4.43,-1.02,;-3.1,-1.8,;-4.44,-5.64,;-3.09,-6.42,;-1.76,-5.65,;-.43,-6.42,;-.43,-7.96,;.9,-8.73,;2.23,-7.96,;2.23,-6.41,;.9,-5.65,;3.56,-5.63,;4.9,-6.39,;3.56,-4.09,;-1.76,-4.11,;-3.07,-4.1,;-3.07,-5.66,;-5.91,-6.09,;-6.26,-7.59,)| Show InChI InChI=1S/C27H28N2O5/c1-28-12-11-27-22-17-7-8-20(32-2)23(22)34-24(27)25(33-3)9-10-26(27,21(28)14-17)19(15-25)16-5-4-6-18(13-16)29(30)31/h4-10,13,19,21,24H,11-12,14-15H2,1-3H3/t19-,21+,24-,25-,26+,27-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University
Curated by ChEMBL
| Assay Description
Displacement of [3H]diprenorphine from rat mu receptor expressed in CHO cells by scintillation counting |
Bioorg Med Chem Lett 20: 418-21 (2010)
Article DOI: 10.1016/j.bmcl.2009.07.119
BindingDB Entry DOI: 10.7270/Q2BG2P4C |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50611953

(CHEMBL5289716) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50611950
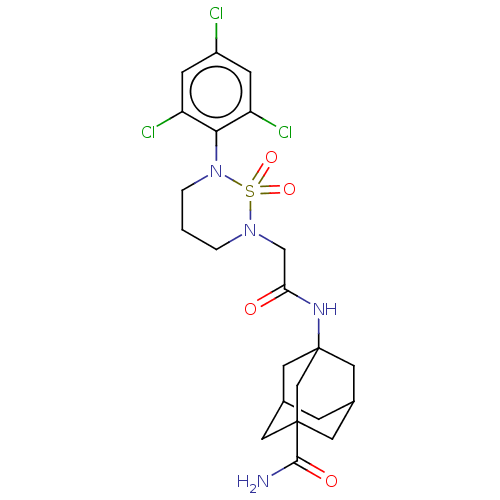
(CHEMBL5280689) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50353386

(CHEMBL1829763 | US8592410, 88 | US8592410, Compara...)Show SMILES C[C@H](N1CC[C@@](CCO)(OC1=O)c1ccc(F)cc1)c1ccc(cc1)-c1ccc(F)cc1F |r| Show InChI InChI=1S/C26H24F3NO3/c1-17(18-2-4-19(5-3-18)23-11-10-22(28)16-24(23)29)30-14-12-26(13-15-31,33-25(30)32)20-6-8-21(27)9-7-20/h2-11,16-17,31H,12-15H2,1H3/t17-,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50611946

(HSD-016 | Hsd-016) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50611950

(CHEMBL5280689) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Hydroxysteroid 11-beta dehydrogenase 1
(Macaca mulatta) | BDBM50611953

(CHEMBL5289716) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50611953

(CHEMBL5289716) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Polyamine deacetylase HDAC10
(Homo sapiens (Human)) | BDBM50188961

(CHEMBL3622533)Show SMILES COc1ccc(cn1)-c1nc(N2CCOCC2)c2sc(CN(C)c3ncc(cn3)C(=O)NO)cc2n1 Show InChI InChI=1S/C23H24N8O4S/c1-30(23-25-11-15(12-26-23)22(32)29-33)13-16-9-17-19(36-16)21(31-5-7-35-8-6-31)28-20(27-17)14-3-4-18(34-2)24-10-14/h3-4,9-12,33H,5-8,13H2,1-2H3,(H,29,32) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Hunan University of Chinese Medicine
Curated by ChEMBL
| Assay Description
Inhibition of HDAC10 (unknown origin) using Color de lys as substrate by HTS assay |
Eur J Med Chem 136: 195-211 (2017)
Article DOI: 10.1016/j.ejmech.2017.05.016
BindingDB Entry DOI: 10.7270/Q2D79DX7 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50611951
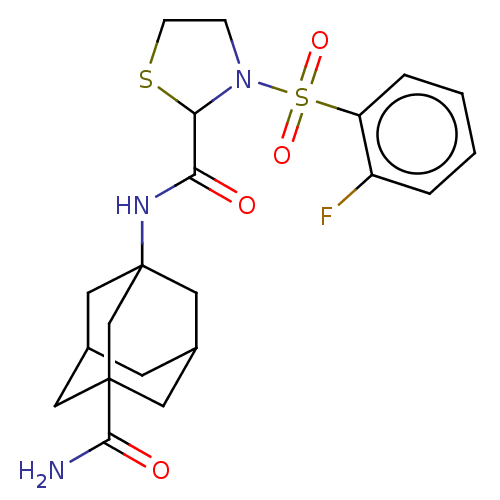
(CHEMBL5289930) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Mus musculus (mouse)) | BDBM50373109
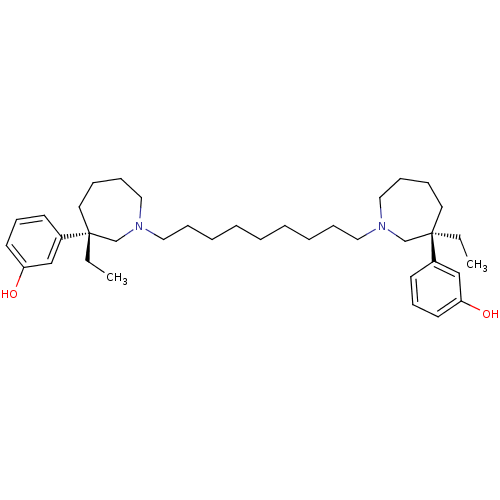
(CHEMBL406645)Show SMILES CC[C@]1(CCCCN(CCCCCCCCCN2CCCC[C@](CC)(C2)c2cccc(O)c2)C1)c1cccc(O)c1 Show InChI InChI=1S/C37H58N2O2/c1-3-36(32-18-16-20-34(40)28-32)22-10-14-26-38(30-36)24-12-8-6-5-7-9-13-25-39-27-15-11-23-37(4-2,31-39)33-19-17-21-35(41)29-33/h16-21,28-29,40-41H,3-15,22-27,30-31H2,1-2H3/t36-,37-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University
Curated by ChEMBL
| Assay Description
Inhibition of mouse brain AChE |
J Med Chem 51: 2027-36 (2008)
Article DOI: 10.1021/jm070154q
BindingDB Entry DOI: 10.7270/Q2C82B4G |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM107664
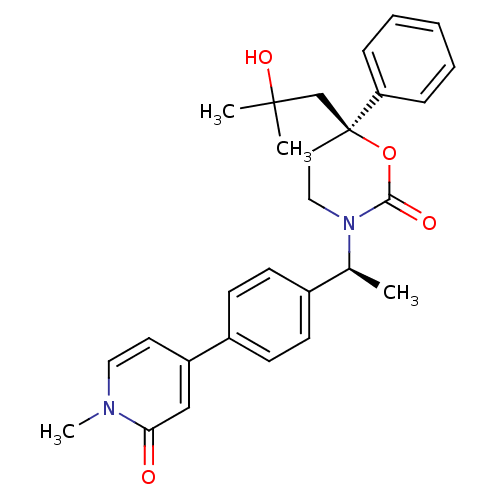
(US8575157, 48)Show SMILES C[C@H](N1CC[C@@](CC(C)(C)O)(OC1=O)c1ccccc1)c1ccc(cc1)-c1ccn(C)c(=O)c1 |r| Show InChI InChI=1S/C28H32N2O4/c1-20(21-10-12-22(13-11-21)23-14-16-29(4)25(31)18-23)30-17-15-28(34-26(30)32,19-27(2,3)33)24-8-6-5-7-9-24/h5-14,16,18,20,33H,15,17,19H2,1-4H3/t20-,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50188961

(CHEMBL3622533)Show SMILES COc1ccc(cn1)-c1nc(N2CCOCC2)c2sc(CN(C)c3ncc(cn3)C(=O)NO)cc2n1 Show InChI InChI=1S/C23H24N8O4S/c1-30(23-25-11-15(12-26-23)22(32)29-33)13-16-9-17-19(36-16)21(31-5-7-35-8-6-31)28-20(27-17)14-3-4-18(34-2)24-10-14/h3-4,9-12,33H,5-8,13H2,1-2H3,(H,29,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hunan University of Chinese Medicine
Curated by ChEMBL
| Assay Description
Inhibition of HDAC2 (unknown origin) using Color de lys as substrate by HTS assay |
Eur J Med Chem 136: 195-211 (2017)
Article DOI: 10.1016/j.ejmech.2017.05.016
BindingDB Entry DOI: 10.7270/Q2D79DX7 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50611951

(CHEMBL5289930) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM107590

(US8575157, 31)Show SMILES C[C@H](N1CC[C@@](CCO)(OC1=O)c1ccc(F)cc1)c1ccc(cc1)-c1ccc(=O)n(C)c1 |r| Show InChI InChI=1S/C26H27FN2O4/c1-18(19-3-5-20(6-4-19)21-7-12-24(31)28(2)17-21)29-15-13-26(14-16-30,33-25(29)32)22-8-10-23(27)11-9-22/h3-12,17-18,30H,13-16H2,1-2H3/t18-,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM5447

(CHEMBL939 | GEFITINIB | Iressa | N-(3-Chloro-4-flu...)Show SMILES COc1cc2ncnc(Nc3ccc(F)c(Cl)c3)c2cc1OCCCN1CCOCC1 Show InChI InChI=1S/C22H24ClFN4O3/c1-29-20-13-19-16(12-21(20)31-8-2-5-28-6-9-30-10-7-28)22(26-14-25-19)27-15-3-4-18(24)17(23)11-15/h3-4,11-14H,2,5-10H2,1H3,(H,25,26,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
US Patent
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
BEIJING SCITECH-MQ PHARMACEUTICALS LIMITED
US Patent
| Assay Description
The sample compounds were dissolved in DMSO and diluted it to 500 μM concentration with DMSO and transferred to a dose plate. The compounds were... |
US Patent US10106508 (2018)
BindingDB Entry DOI: 10.7270/Q26M38VB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50611946

(HSD-016 | Hsd-016) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Rattus norvegicus (rat)) | BDBM50611946

(HSD-016 | Hsd-016) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Mus musculus (mouse)) | BDBM50373108

(CHEMBL259523)Show SMILES CC[C@]1(CCCCN(CCCCCCCCCCN2CCCC[C@](CC)(C2)c2cccc(O)c2)C1)c1cccc(O)c1 Show InChI InChI=1S/C38H60N2O2/c1-3-37(33-19-17-21-35(41)29-33)23-11-15-27-39(31-37)25-13-9-7-5-6-8-10-14-26-40-28-16-12-24-38(4-2,32-40)34-20-18-22-36(42)30-34/h17-22,29-30,41-42H,3-16,23-28,31-32H2,1-2H3/t37-,38-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University
Curated by ChEMBL
| Assay Description
Inhibition of mouse brain AChE |
J Med Chem 51: 2027-36 (2008)
Article DOI: 10.1021/jm070154q
BindingDB Entry DOI: 10.7270/Q2C82B4G |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50611946

(HSD-016 | Hsd-016) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cholinesterase
(Mus musculus (Mouse)) | BDBM50373109

(CHEMBL406645)Show SMILES CC[C@]1(CCCCN(CCCCCCCCCN2CCCC[C@](CC)(C2)c2cccc(O)c2)C1)c1cccc(O)c1 Show InChI InChI=1S/C37H58N2O2/c1-3-36(32-18-16-20-34(40)28-32)22-10-14-26-38(30-36)24-12-8-6-5-7-9-13-25-39-27-15-11-23-37(4-2,31-39)33-19-17-21-35(41)29-33/h16-21,28-29,40-41H,3-15,22-27,30-31H2,1-2H3/t36-,37-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University
Curated by ChEMBL
| Assay Description
Inhibition of mouse serum BChE |
J Med Chem 51: 2027-36 (2008)
Article DOI: 10.1021/jm070154q
BindingDB Entry DOI: 10.7270/Q2C82B4G |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Mus musculus (Mouse)) | BDBM50373108

(CHEMBL259523)Show SMILES CC[C@]1(CCCCN(CCCCCCCCCCN2CCCC[C@](CC)(C2)c2cccc(O)c2)C1)c1cccc(O)c1 Show InChI InChI=1S/C38H60N2O2/c1-3-37(33-19-17-21-35(41)29-33)23-11-15-27-39(31-37)25-13-9-7-5-6-8-10-14-26-40-28-16-12-24-38(4-2,32-40)34-20-18-22-36(42)30-34/h17-22,29-30,41-42H,3-16,23-28,31-32H2,1-2H3/t37-,38-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University
Curated by ChEMBL
| Assay Description
Inhibition of mouse serum BChE |
J Med Chem 51: 2027-36 (2008)
Article DOI: 10.1021/jm070154q
BindingDB Entry DOI: 10.7270/Q2C82B4G |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50188961

(CHEMBL3622533)Show SMILES COc1ccc(cn1)-c1nc(N2CCOCC2)c2sc(CN(C)c3ncc(cn3)C(=O)NO)cc2n1 Show InChI InChI=1S/C23H24N8O4S/c1-30(23-25-11-15(12-26-23)22(32)29-33)13-16-9-17-19(36-16)21(31-5-7-35-8-6-31)28-20(27-17)14-3-4-18(34-2)24-10-14/h3-4,9-12,33H,5-8,13H2,1-2H3,(H,29,32) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Hunan University of Chinese Medicine
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length human N-terminal GST-tagged PI3K p110alpha/untagged p85alpha expressed in baculovirus infected insect Sf9 cells... |
Eur J Med Chem 136: 195-211 (2017)
Article DOI: 10.1016/j.ejmech.2017.05.016
BindingDB Entry DOI: 10.7270/Q2D79DX7 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM25028

(4-[2-(1H-indazol-4-yl)-6-[(4-methanesulfonylpipera...)Show SMILES CS(=O)(=O)N1CCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2cccc3[nH]ncc23)CC1 Show InChI InChI=1S/C23H27N7O3S2/c1-35(31,32)30-7-5-28(6-8-30)15-16-13-20-21(34-16)23(29-9-11-33-12-10-29)26-22(25-20)17-3-2-4-19-18(17)14-24-27-19/h2-4,13-14H,5-12,15H2,1H3,(H,24,27) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Hunan University of Chinese Medicine
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length human N-terminal GST-tagged PI3K p110alpha/untagged p85alpha expressed in baculovirus infected insect Sf9 cells... |
Eur J Med Chem 136: 195-211 (2017)
Article DOI: 10.1016/j.ejmech.2017.05.016
BindingDB Entry DOI: 10.7270/Q2D79DX7 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor [T790M]
(Homo sapiens (Human)) | BDBM5447

(CHEMBL939 | GEFITINIB | Iressa | N-(3-Chloro-4-flu...)Show SMILES COc1cc2ncnc(Nc3ccc(F)c(Cl)c3)c2cc1OCCCN1CCOCC1 Show InChI InChI=1S/C22H24ClFN4O3/c1-29-20-13-19-16(12-21(20)31-8-2-5-28-6-9-30-10-7-28)22(26-14-25-19)27-15-3-4-18(24)17(23)11-15/h3-4,11-14H,2,5-10H2,1H3,(H,25,26,27) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
US Patent
| n/a | n/a | 22.5 | n/a | n/a | n/a | n/a | n/a | n/a |
BEIJING SCITECH-MQ PHARMACEUTICALS LIMITED
US Patent
| Assay Description
The sample compounds were dissolved in DMSO and diluted it to 500 μM concentration with DMSO and transferred to a dose plate. The compounds were... |
US Patent US10106508 (2018)
BindingDB Entry DOI: 10.7270/Q26M38VB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Mus musculus (mouse)) | BDBM50373118
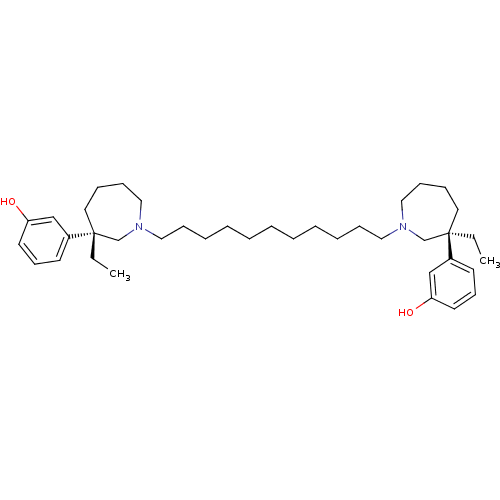
(CHEMBL406885)Show SMILES CC[C@]1(CCCCN(CCCCCCCCCCCN2CCCC[C@](CC)(C2)c2cccc(O)c2)C1)c1cccc(O)c1 Show InChI InChI=1S/C39H62N2O2/c1-3-38(34-20-18-22-36(42)30-34)24-12-16-28-40(32-38)26-14-10-8-6-5-7-9-11-15-27-41-29-17-13-25-39(4-2,33-41)35-21-19-23-37(43)31-35/h18-23,30-31,42-43H,3-17,24-29,32-33H2,1-2H3/t38-,39-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University
Curated by ChEMBL
| Assay Description
Inhibition of mouse brain AChE |
J Med Chem 51: 2027-36 (2008)
Article DOI: 10.1021/jm070154q
BindingDB Entry DOI: 10.7270/Q2C82B4G |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50188961

(CHEMBL3622533)Show SMILES COc1ccc(cn1)-c1nc(N2CCOCC2)c2sc(CN(C)c3ncc(cn3)C(=O)NO)cc2n1 Show InChI InChI=1S/C23H24N8O4S/c1-30(23-25-11-15(12-26-23)22(32)29-33)13-16-9-17-19(36-16)21(31-5-7-35-8-6-31)28-20(27-17)14-3-4-18(34-2)24-10-14/h3-4,9-12,33H,5-8,13H2,1-2H3,(H,29,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Hunan University of Chinese Medicine
Curated by ChEMBL
| Assay Description
Inhibition of HDAC6 (unknown origin) using Color de lys as substrate by HTS assay |
Eur J Med Chem 136: 195-211 (2017)
Article DOI: 10.1016/j.ejmech.2017.05.016
BindingDB Entry DOI: 10.7270/Q2D79DX7 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50309934
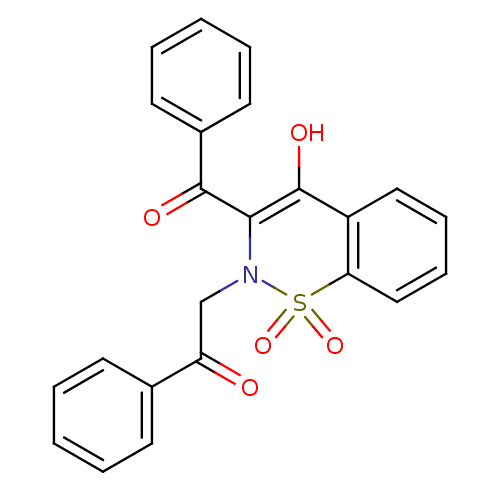
(2-(3-Benzoyl-4-hydroxy-1,1-dioxo-1H-1lambda*6*-ben...)Show SMILES OC1=C(N(CC(=O)c2ccccc2)S(=O)(=O)c2ccccc12)C(=O)c1ccccc1 |t:1| Show InChI InChI=1S/C23H17NO5S/c25-19(16-9-3-1-4-10-16)15-24-21(22(26)17-11-5-2-6-12-17)23(27)18-13-7-8-14-20(18)30(24,28)29/h1-14,27H,15H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50188961

(CHEMBL3622533)Show SMILES COc1ccc(cn1)-c1nc(N2CCOCC2)c2sc(CN(C)c3ncc(cn3)C(=O)NO)cc2n1 Show InChI InChI=1S/C23H24N8O4S/c1-30(23-25-11-15(12-26-23)22(32)29-33)13-16-9-17-19(36-16)21(31-5-7-35-8-6-31)28-20(27-17)14-3-4-18(34-2)24-10-14/h3-4,9-12,33H,5-8,13H2,1-2H3,(H,29,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Hunan University of Chinese Medicine
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length human N-terminal GST-tagged PI3K p110delta/untagged p85alpha expressed in baculovirus infected insect Sf9 cells... |
Eur J Med Chem 136: 195-211 (2017)
Article DOI: 10.1016/j.ejmech.2017.05.016
BindingDB Entry DOI: 10.7270/Q2D79DX7 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM25028

(4-[2-(1H-indazol-4-yl)-6-[(4-methanesulfonylpipera...)Show SMILES CS(=O)(=O)N1CCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2cccc3[nH]ncc23)CC1 Show InChI InChI=1S/C23H27N7O3S2/c1-35(31,32)30-7-5-28(6-8-30)15-16-13-20-21(34-16)23(29-9-11-33-12-10-29)26-22(25-20)17-3-2-4-19-18(17)14-24-27-19/h2-4,13-14H,5-12,15H2,1H3,(H,24,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Hunan University of Chinese Medicine
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length human N-terminal GST-tagged PI3K p110delta/untagged p85alpha expressed in baculovirus infected insect Sf9 cells... |
Eur J Med Chem 136: 195-211 (2017)
Article DOI: 10.1016/j.ejmech.2017.05.016
BindingDB Entry DOI: 10.7270/Q2D79DX7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data