 Found 48 hits with Last Name = 'skoog' and Initial = 'm'
Found 48 hits with Last Name = 'skoog' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Gag-Pol polyprotein [489-587]
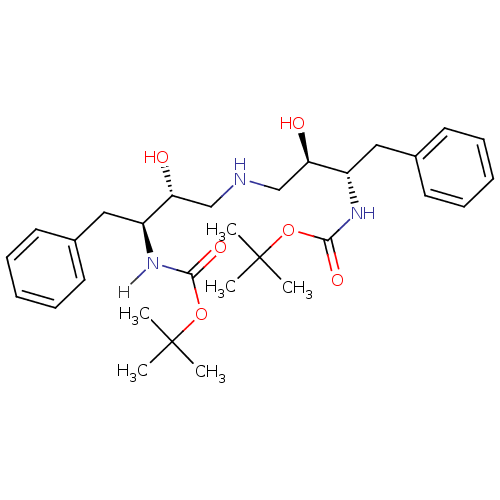
(Human immunodeficiency virus type 1) | BDBM50284925

(CHEMBL288759 | {(1S,2R)-1-Benzyl-2-hydroxy-3-[(2R,...)Show SMILES CC(C)(C)OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CNC[C@@H](O)[C@H](Cc1ccccc1)NC(=O)[C@@](C)(O)C(C)(C)C Show InChI InChI=1S/C32H49N3O6/c1-30(2,3)32(7,40)28(38)34-24(18-22-14-10-8-11-15-22)26(36)20-33-21-27(37)25(19-23-16-12-9-13-17-23)35-29(39)41-31(4,5)6/h8-17,24-27,33,36-37,40H,18-21H2,1-7H3,(H,34,38)(H,35,39)/t24-,25-,26+,27+,32+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition constant against HIV protease was determined |
Bioorg Med Chem Lett 5: 1729-1734 (1995)
Article DOI: 10.1016/0960-894X(95)00293-3
BindingDB Entry DOI: 10.7270/Q2RN37TS |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM914
(Aminodiol deriv. 9a | BMS-186318 analog 1 | [(S,R)...)Show SMILES CC(C)(C)OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CNC[C@@H](O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C |r| Show InChI InChI=1S/C30H45N3O6/c1-29(2,3)38-27(36)32-23(17-21-13-9-7-10-14-21)25(34)19-31-20-26(35)24(18-22-15-11-8-12-16-22)33-28(37)39-30(4,5)6/h7-16,23-26,31,34-35H,17-20H2,1-6H3,(H,32,36)(H,33,37)/t23-,24-,25+,26+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of HIV-1 protease. |
Bioorg Med Chem Lett 5: 1729-1734 (1995)
Article DOI: 10.1016/0960-894X(95)00293-3
BindingDB Entry DOI: 10.7270/Q2RN37TS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50284924

(((1S,2R)-1-Benzyl-2-hydroxy-3-{(2R,3S)-2-hydroxy-3...)Show SMILES CC(C)(C)OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CNC[C@@H](O)[C@H](Cc1ccccc1)NC(=O)C1(O)CCCC1(C)C Show InChI InChI=1S/C33H49N3O6/c1-31(2,3)42-30(40)36-26(20-24-15-10-7-11-16-24)28(38)22-34-21-27(37)25(19-23-13-8-6-9-14-23)35-29(39)33(41)18-12-17-32(33,4)5/h6-11,13-16,25-28,34,37-38,41H,12,17-22H2,1-5H3,(H,35,39)(H,36,40)/t25-,26-,27+,28+,33?/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of HIV-1 protease. |
Bioorg Med Chem Lett 5: 1729-1734 (1995)
Article DOI: 10.1016/0960-894X(95)00293-3
BindingDB Entry DOI: 10.7270/Q2RN37TS |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50284924

(((1S,2R)-1-Benzyl-2-hydroxy-3-{(2R,3S)-2-hydroxy-3...)Show SMILES CC(C)(C)OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CNC[C@@H](O)[C@H](Cc1ccccc1)NC(=O)C1(O)CCCC1(C)C Show InChI InChI=1S/C33H49N3O6/c1-31(2,3)42-30(40)36-26(20-24-15-10-7-11-16-24)28(38)22-34-21-27(37)25(19-23-13-8-6-9-14-23)35-29(39)33(41)18-12-17-32(33,4)5/h6-11,13-16,25-28,34,37-38,41H,12,17-22H2,1-5H3,(H,35,39)(H,36,40)/t25-,26-,27+,28+,33?/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of HIV-1 protease. |
Bioorg Med Chem Lett 5: 1729-1734 (1995)
Article DOI: 10.1016/0960-894X(95)00293-3
BindingDB Entry DOI: 10.7270/Q2RN37TS |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50286514
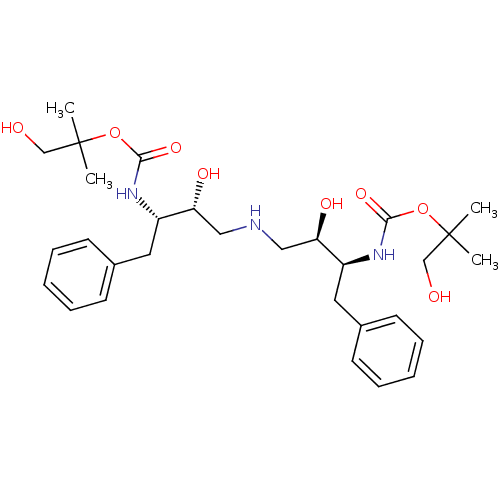
(CHEMBL357009 | {(1S,2R)-1-Benzyl-2-hydroxy-3-[(2R,...)Show SMILES CC(C)(CO)OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CNC[C@@H](O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)CO Show InChI InChI=1S/C30H45N3O8/c1-29(2,19-34)40-27(38)32-23(15-21-11-7-5-8-12-21)25(36)17-31-18-26(37)24(16-22-13-9-6-10-14-22)33-28(39)41-30(3,4)20-35/h5-14,23-26,31,34-37H,15-20H2,1-4H3,(H,32,38)(H,33,39)/t23-,24-,25+,26+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of HIV protease cleavage of V-S-Q-N-(b-naphthylalanine)-P-I-V |
Bioorg Med Chem Lett 5: 459-464 (1995)
Article DOI: 10.1016/0960-894X(95)00056-Y
BindingDB Entry DOI: 10.7270/Q2RX9C2M |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50286502

(CHEMBL143470 | {(1S,2R)-1-Benzyl-2-hydroxy-3-[(2R,...)Show SMILES CC(C)(C)OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CNC[C@@H](O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)CO Show InChI InChI=1S/C30H45N3O7/c1-29(2,3)39-27(37)32-23(16-21-12-8-6-9-13-21)25(35)18-31-19-26(36)24(17-22-14-10-7-11-15-22)33-28(38)40-30(4,5)20-34/h6-15,23-26,31,34-36H,16-20H2,1-5H3,(H,32,37)(H,33,38)/t23-,24-,25+,26+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of HIV protease cleavage of V-S-Q-N-(b-naphthylalanine)-P-I-V |
Bioorg Med Chem Lett 5: 459-464 (1995)
Article DOI: 10.1016/0960-894X(95)00056-Y
BindingDB Entry DOI: 10.7270/Q2RX9C2M |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50284937

(CHEMBL45838 | {(1S,2R)-1-Benzyl-2-hydroxy-3-[(2R,3...)Show SMILES CC(C)(C)OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CNC[C@@H](O)[C@H](Cc1ccccc1)NC(=O)C(O)C(F)(F)F Show InChI InChI=1S/C28H38F3N3O6/c1-27(2,3)40-26(39)34-21(15-19-12-8-5-9-13-19)23(36)17-32-16-22(35)20(14-18-10-6-4-7-11-18)33-25(38)24(37)28(29,30)31/h4-13,20-24,32,35-37H,14-17H2,1-3H3,(H,33,38)(H,34,39)/t20-,21-,22+,23+,24?/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of HIV-1 protease. |
Bioorg Med Chem Lett 5: 1729-1734 (1995)
Article DOI: 10.1016/0960-894X(95)00293-3
BindingDB Entry DOI: 10.7270/Q2RN37TS |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50284925

(CHEMBL288759 | {(1S,2R)-1-Benzyl-2-hydroxy-3-[(2R,...)Show SMILES CC(C)(C)OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CNC[C@@H](O)[C@H](Cc1ccccc1)NC(=O)[C@@](C)(O)C(C)(C)C Show InChI InChI=1S/C32H49N3O6/c1-30(2,3)32(7,40)28(38)34-24(18-22-14-10-8-11-15-22)26(36)20-33-21-27(37)25(19-23-16-12-9-13-17-23)35-29(39)41-31(4,5)6/h8-17,24-27,33,36-37,40H,18-21H2,1-7H3,(H,34,38)(H,35,39)/t24-,25-,26+,27+,32+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of HIV-1 protease. |
Bioorg Med Chem Lett 5: 1729-1734 (1995)
Article DOI: 10.1016/0960-894X(95)00293-3
BindingDB Entry DOI: 10.7270/Q2RN37TS |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50284937

(CHEMBL45838 | {(1S,2R)-1-Benzyl-2-hydroxy-3-[(2R,3...)Show SMILES CC(C)(C)OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CNC[C@@H](O)[C@H](Cc1ccccc1)NC(=O)C(O)C(F)(F)F Show InChI InChI=1S/C28H38F3N3O6/c1-27(2,3)40-26(39)34-21(15-19-12-8-5-9-13-19)23(36)17-32-16-22(35)20(14-18-10-6-4-7-11-18)33-25(38)24(37)28(29,30)31/h4-13,20-24,32,35-37H,14-17H2,1-3H3,(H,33,38)(H,34,39)/t20-,21-,22+,23+,24?/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of HIV-1 protease. |
Bioorg Med Chem Lett 5: 1729-1734 (1995)
Article DOI: 10.1016/0960-894X(95)00293-3
BindingDB Entry DOI: 10.7270/Q2RN37TS |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50284929

(CHEMBL48917 | {(1S,2R)-1-Benzyl-2-hydroxy-3-[(2R,3...)Show SMILES CC(C)(C)OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CNC[C@@H](O)[C@H](Cc1ccccc1)NC(=O)[C@H](O)C(C)(C)C Show InChI InChI=1S/C31H47N3O6/c1-30(2,3)27(37)28(38)33-23(17-21-13-9-7-10-14-21)25(35)19-32-20-26(36)24(18-22-15-11-8-12-16-22)34-29(39)40-31(4,5)6/h7-16,23-27,32,35-37H,17-20H2,1-6H3,(H,33,38)(H,34,39)/t23-,24-,25+,26+,27-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of HIV-1 protease. |
Bioorg Med Chem Lett 5: 1729-1734 (1995)
Article DOI: 10.1016/0960-894X(95)00293-3
BindingDB Entry DOI: 10.7270/Q2RN37TS |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50004182
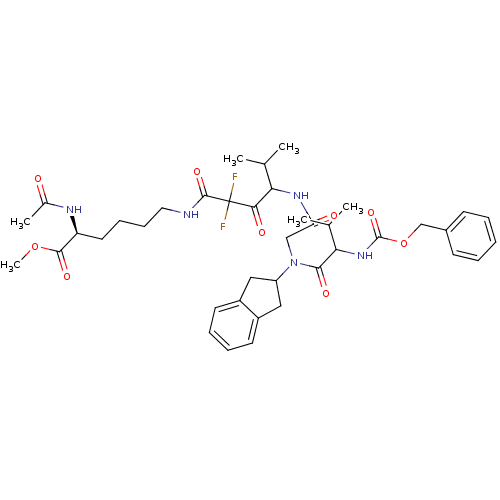
(2-Acetylamino-6-(4-{2-[(2-benzyloxycarbonylamino-3...)Show SMILES COC(=O)[C@H](CCCCNC(=O)C(F)(F)C(=O)C(NC(=O)CN(C1Cc2ccccc2C1)C(=O)C(NC(=O)OCc1ccccc1)C(C)C)C(C)C)NC(C)=O Show InChI InChI=1S/C40H53F2N5O9/c1-24(2)33(35(50)40(41,42)38(53)43-19-13-12-18-31(37(52)55-6)44-26(5)48)45-32(49)22-47(30-20-28-16-10-11-17-29(28)21-30)36(51)34(25(3)4)46-39(54)56-23-27-14-8-7-9-15-27/h7-11,14-17,24-25,30-31,33-34H,12-13,18-23H2,1-6H3,(H,43,53)(H,44,48)(H,45,49)(H,46,54)/t31-,33?,34?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 57 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against human leukocyte elastase (HLE) at pH 7.5 |
J Med Chem 35: 4795-808 (1993)
BindingDB Entry DOI: 10.7270/Q2HX1BM5 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50284930
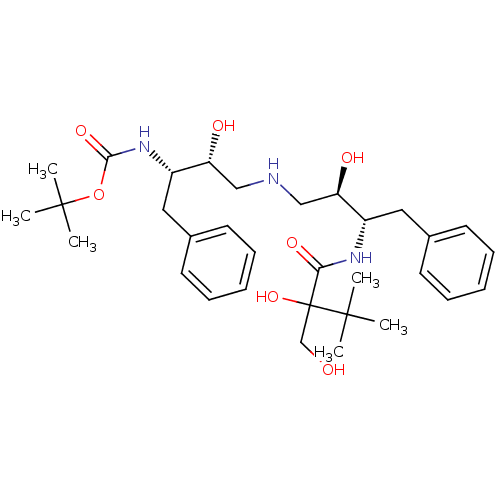
(CHEMBL49305 | {(1S,2R)-1-Benzyl-2-hydroxy-3-[(2R,3...)Show SMILES CC(C)(C)OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CNC[C@@H](O)[C@H](Cc1ccccc1)NC(=O)C(O)(CO)C(C)(C)C Show InChI InChI=1S/C32H49N3O7/c1-30(2,3)32(41,21-36)28(39)34-24(17-22-13-9-7-10-14-22)26(37)19-33-20-27(38)25(18-23-15-11-8-12-16-23)35-29(40)42-31(4,5)6/h7-16,24-27,33,36-38,41H,17-21H2,1-6H3,(H,34,39)(H,35,40)/t24-,25-,26+,27+,32?/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of HIV-1 protease. |
Bioorg Med Chem Lett 5: 1729-1734 (1995)
Article DOI: 10.1016/0960-894X(95)00293-3
BindingDB Entry DOI: 10.7270/Q2RN37TS |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50284930

(CHEMBL49305 | {(1S,2R)-1-Benzyl-2-hydroxy-3-[(2R,3...)Show SMILES CC(C)(C)OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CNC[C@@H](O)[C@H](Cc1ccccc1)NC(=O)C(O)(CO)C(C)(C)C Show InChI InChI=1S/C32H49N3O7/c1-30(2,3)32(41,21-36)28(39)34-24(17-22-13-9-7-10-14-22)26(37)19-33-20-27(38)25(18-23-15-11-8-12-16-23)35-29(40)42-31(4,5)6/h7-16,24-27,33,36-38,41H,17-21H2,1-6H3,(H,34,39)(H,35,40)/t24-,25-,26+,27+,32?/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of HIV-1 protease. |
Bioorg Med Chem Lett 5: 1729-1734 (1995)
Article DOI: 10.1016/0960-894X(95)00293-3
BindingDB Entry DOI: 10.7270/Q2RN37TS |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50004192
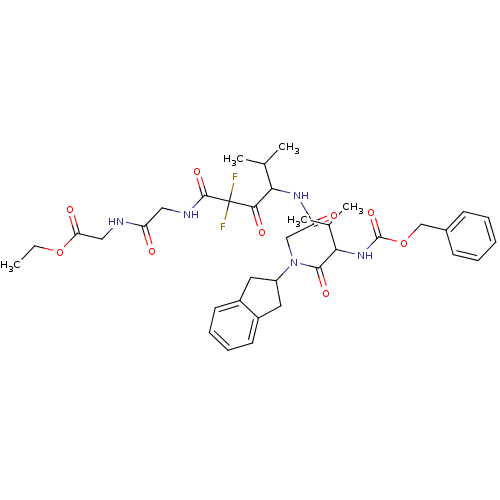
(CHEMBL344981 | [2-(4-{2-[(2-Benzyloxycarbonylamino...)Show SMILES CCOC(=O)CNC(=O)CNC(=O)C(F)(F)C(=O)C(NC(=O)CN(C1Cc2ccccc2C1)C(=O)C(NC(=O)OCc1ccccc1)C(C)C)C(C)C Show InChI InChI=1S/C37H47F2N5O9/c1-6-52-30(47)19-40-28(45)18-41-35(50)37(38,39)33(48)31(22(2)3)42-29(46)20-44(27-16-25-14-10-11-15-26(25)17-27)34(49)32(23(4)5)43-36(51)53-21-24-12-8-7-9-13-24/h7-15,22-23,27,31-32H,6,16-21H2,1-5H3,(H,40,45)(H,41,50)(H,42,46)(H,43,51) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 69 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against human leukocyte elastase (HLE) at pH 7.5 |
J Med Chem 35: 4795-808 (1993)
BindingDB Entry DOI: 10.7270/Q2HX1BM5 |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50004184
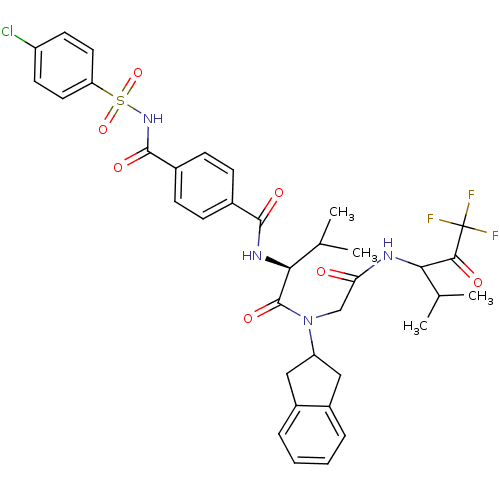
(4-(4-Chloro-benzenesulfonylaminocarbonyl)-N-((S)-1...)Show SMILES CC(C)[C@H](NC(=O)c1ccc(cc1)C(=O)NS(=O)(=O)c1ccc(Cl)cc1)C(=O)N(CC(=O)NC(C(C)C)C(=O)C(F)(F)F)C1Cc2ccccc2C1 Show InChI InChI=1S/C36H38ClF3N4O7S/c1-20(2)30(32(46)36(38,39)40)41-29(45)19-44(27-17-24-7-5-6-8-25(24)18-27)35(49)31(21(3)4)42-33(47)22-9-11-23(12-10-22)34(48)43-52(50,51)28-15-13-26(37)14-16-28/h5-16,20-21,27,30-31H,17-19H2,1-4H3,(H,41,45)(H,42,47)(H,43,48)/t30?,31-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 84 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against human leukocyte elastase (HLE) at pH 7.5 |
J Med Chem 35: 4795-808 (1993)
BindingDB Entry DOI: 10.7270/Q2HX1BM5 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50286504
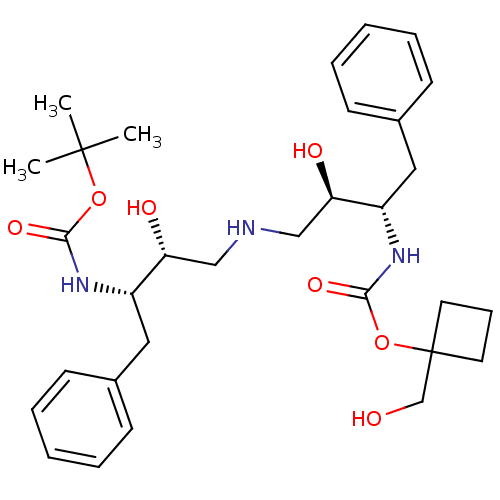
(CHEMBL435594 | {(1S,2R)-1-Benzyl-2-hydroxy-3-[(2R,...)Show SMILES CC(C)(C)OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CNC[C@@H](O)[C@H](Cc1ccccc1)NC(=O)OC1(CO)CCC1 Show InChI InChI=1S/C31H45N3O7/c1-30(2,3)40-28(38)33-24(17-22-11-6-4-7-12-22)26(36)19-32-20-27(37)25(18-23-13-8-5-9-14-23)34-29(39)41-31(21-35)15-10-16-31/h4-9,11-14,24-27,32,35-37H,10,15-21H2,1-3H3,(H,33,38)(H,34,39)/t24-,25-,26+,27+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of HIV protease cleavage of V-S-Q-N-(b-naphthylalanine)-P-I-V |
Bioorg Med Chem Lett 5: 459-464 (1995)
Article DOI: 10.1016/0960-894X(95)00056-Y
BindingDB Entry DOI: 10.7270/Q2RX9C2M |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50286513
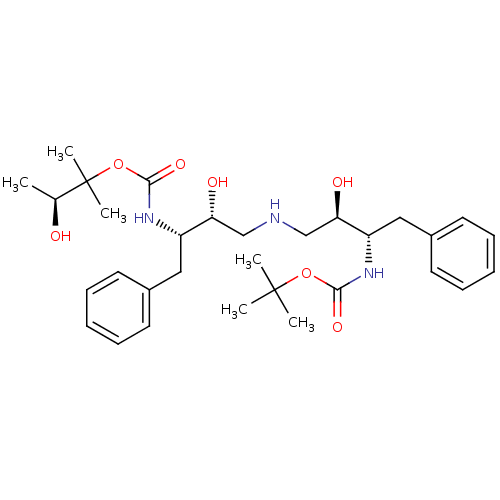
(CHEMBL343727 | {(1S,2R)-1-Benzyl-2-hydroxy-3-[(2R,...)Show SMILES C[C@H](O)C(C)(C)OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CNC[C@@H](O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C Show InChI InChI=1S/C31H47N3O7/c1-21(35)31(5,6)41-29(39)34-25(18-23-15-11-8-12-16-23)27(37)20-32-19-26(36)24(17-22-13-9-7-10-14-22)33-28(38)40-30(2,3)4/h7-16,21,24-27,32,35-37H,17-20H2,1-6H3,(H,33,38)(H,34,39)/t21-,24-,25-,26+,27+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 105 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of HIV protease cleavage of V-S-Q-N-(b-naphthylalanine)-P-I-V |
Bioorg Med Chem Lett 5: 459-464 (1995)
Article DOI: 10.1016/0960-894X(95)00056-Y
BindingDB Entry DOI: 10.7270/Q2RX9C2M |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50004190
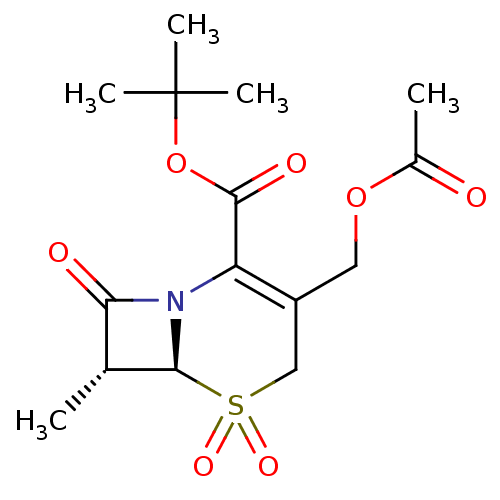
(3-Acetoxymethyl-7-methyl-5,5,8-trioxo-5lambda*6*-t...)Show SMILES C[C@H]1[C@H]2N(C1=O)C(C(=O)OC(C)(C)C)=C(COC(C)=O)CS2(=O)=O |t:14| Show InChI InChI=1S/C15H21NO7S/c1-8-12(18)16-11(14(19)23-15(3,4)5)10(6-22-9(2)17)7-24(20,21)13(8)16/h8,13H,6-7H2,1-5H3/t8-,13+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 107 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against human leukocyte elastase (HLE) at pH 7.5 |
J Med Chem 35: 4795-808 (1993)
BindingDB Entry DOI: 10.7270/Q2HX1BM5 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50284932

(CHEMBL45699 | {(1S,2R)-1-Benzyl-3-[(2R,3S)-3-(2,4-...)Show SMILES CC(C)(C)OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CNC[C@@H](O)[C@H](Cc1ccccc1)NC(=O)C(C)(O)C(C)(C)CO Show InChI InChI=1S/C32H49N3O7/c1-30(2,3)42-29(40)35-25(18-23-15-11-8-12-16-23)27(38)20-33-19-26(37)24(17-22-13-9-7-10-14-22)34-28(39)32(6,41)31(4,5)21-36/h7-16,24-27,33,36-38,41H,17-21H2,1-6H3,(H,34,39)(H,35,40)/t24-,25-,26+,27+,32?/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of HIV-1 protease. |
Bioorg Med Chem Lett 5: 1729-1734 (1995)
Article DOI: 10.1016/0960-894X(95)00293-3
BindingDB Entry DOI: 10.7270/Q2RN37TS |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM914

(Aminodiol deriv. 9a | BMS-186318 analog 1 | [(S,R)...)Show SMILES CC(C)(C)OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CNC[C@@H](O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C |r| Show InChI InChI=1S/C30H45N3O6/c1-29(2,3)38-27(36)32-23(17-21-13-9-7-10-14-21)25(34)19-31-20-26(35)24(18-22-15-11-8-12-16-22)33-28(37)39-30(4,5)6/h7-16,23-26,31,34-35H,17-20H2,1-6H3,(H,32,36)(H,33,37)/t23-,24-,25+,26+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
| n/a | n/a | 125 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of HIV-1 protease. |
Bioorg Med Chem Lett 5: 1729-1734 (1995)
Article DOI: 10.1016/0960-894X(95)00293-3
BindingDB Entry DOI: 10.7270/Q2RN37TS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM914

(Aminodiol deriv. 9a | BMS-186318 analog 1 | [(S,R)...)Show SMILES CC(C)(C)OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CNC[C@@H](O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C |r| Show InChI InChI=1S/C30H45N3O6/c1-29(2,3)38-27(36)32-23(17-21-13-9-7-10-14-21)25(34)19-31-20-26(35)24(18-22-15-11-8-12-16-22)33-28(37)39-30(4,5)6/h7-16,23-26,31,34-35H,17-20H2,1-6H3,(H,32,36)(H,33,37)/t23-,24-,25+,26+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
| n/a | n/a | 125 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of HIV protease cleavage of V-S-Q-N-(b-naphthylalanine)-P-I-V |
Bioorg Med Chem Lett 5: 459-464 (1995)
Article DOI: 10.1016/0960-894X(95)00056-Y
BindingDB Entry DOI: 10.7270/Q2RX9C2M |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50284932

(CHEMBL45699 | {(1S,2R)-1-Benzyl-3-[(2R,3S)-3-(2,4-...)Show SMILES CC(C)(C)OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CNC[C@@H](O)[C@H](Cc1ccccc1)NC(=O)C(C)(O)C(C)(C)CO Show InChI InChI=1S/C32H49N3O7/c1-30(2,3)42-29(40)35-25(18-23-15-11-8-12-16-23)27(38)20-33-19-26(37)24(17-22-13-9-7-10-14-22)34-28(39)32(6,41)31(4,5)21-36/h7-16,24-27,33,36-38,41H,17-21H2,1-6H3,(H,34,39)(H,35,40)/t24-,25-,26+,27+,32?/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of HIV-1 protease. |
Bioorg Med Chem Lett 5: 1729-1734 (1995)
Article DOI: 10.1016/0960-894X(95)00293-3
BindingDB Entry DOI: 10.7270/Q2RN37TS |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50286511
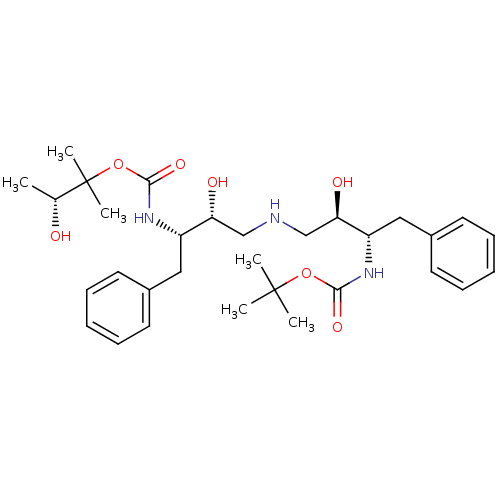
(CHEMBL140062 | {(1S,2R)-1-Benzyl-2-hydroxy-3-[(2R,...)Show SMILES C[C@@H](O)C(C)(C)OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CNC[C@@H](O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C Show InChI InChI=1S/C31H47N3O7/c1-21(35)31(5,6)41-29(39)34-25(18-23-15-11-8-12-16-23)27(37)20-32-19-26(36)24(17-22-13-9-7-10-14-22)33-28(38)40-30(2,3)4/h7-16,21,24-27,32,35-37H,17-20H2,1-6H3,(H,33,38)(H,34,39)/t21-,24+,25+,26-,27-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of HIV protease cleavage of V-S-Q-N-(b-naphthylalanine)-P-I-V |
Bioorg Med Chem Lett 5: 459-464 (1995)
Article DOI: 10.1016/0960-894X(95)00056-Y
BindingDB Entry DOI: 10.7270/Q2RX9C2M |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50286503
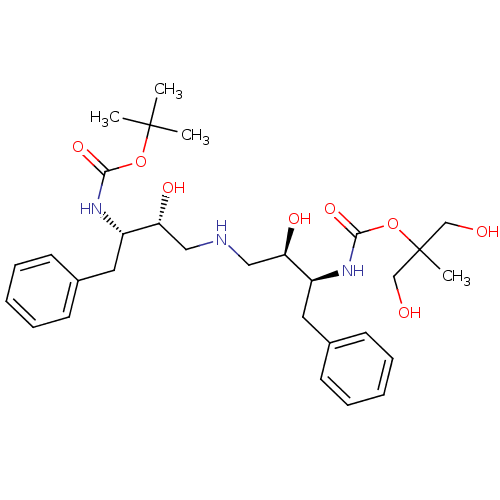
(CHEMBL142990 | {(1S,2R)-1-Benzyl-2-hydroxy-3-[(2R,...)Show SMILES CC(C)(C)OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CNC[C@@H](O)[C@H](Cc1ccccc1)NC(=O)OC(C)(CO)CO Show InChI InChI=1S/C30H45N3O8/c1-29(2,3)40-27(38)32-23(15-21-11-7-5-8-12-21)25(36)17-31-18-26(37)24(16-22-13-9-6-10-14-22)33-28(39)41-30(4,19-34)20-35/h5-14,23-26,31,34-37H,15-20H2,1-4H3,(H,32,38)(H,33,39)/t23-,24-,25+,26+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of HIV protease cleavage of V-S-Q-N-(b-naphthylalanine)-P-I-V |
Bioorg Med Chem Lett 5: 459-464 (1995)
Article DOI: 10.1016/0960-894X(95)00056-Y
BindingDB Entry DOI: 10.7270/Q2RX9C2M |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50286508
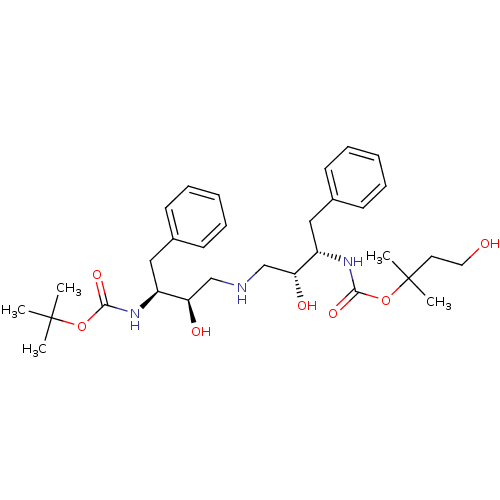
(CHEMBL356345 | {(1S,2R)-1-Benzyl-2-hydroxy-3-[(2R,...)Show SMILES CC(C)(C)OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CNC[C@@H](O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)CCO Show InChI InChI=1S/C31H47N3O7/c1-30(2,3)40-28(38)33-24(18-22-12-8-6-9-13-22)26(36)20-32-21-27(37)25(19-23-14-10-7-11-15-23)34-29(39)41-31(4,5)16-17-35/h6-15,24-27,32,35-37H,16-21H2,1-5H3,(H,33,38)(H,34,39)/t24-,25-,26+,27+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of HIV protease cleavage of V-S-Q-N-(b-naphthylalanine)-P-I-V |
Bioorg Med Chem Lett 5: 459-464 (1995)
Article DOI: 10.1016/0960-894X(95)00056-Y
BindingDB Entry DOI: 10.7270/Q2RX9C2M |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50284934

(((1S,2R)-1-Benzyl-2-hydroxy-3-{(2R,3S)-2-hydroxy-3...)Show SMILES CC(C)(C)OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CNC[C@@H](O)[C@H](Cc1ccccc1)NC(=O)C1(O)COCC1(C)C Show InChI InChI=1S/C32H47N3O7/c1-30(2,3)42-29(39)35-25(17-23-14-10-7-11-15-23)27(37)19-33-18-26(36)24(16-22-12-8-6-9-13-22)34-28(38)32(40)21-41-20-31(32,4)5/h6-15,24-27,33,36-37,40H,16-21H2,1-5H3,(H,34,38)(H,35,39)/t24-,25-,26+,27+,32?/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition constant against HIV protease was determined |
Bioorg Med Chem Lett 5: 1729-1734 (1995)
Article DOI: 10.1016/0960-894X(95)00293-3
BindingDB Entry DOI: 10.7270/Q2RN37TS |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50284934

(((1S,2R)-1-Benzyl-2-hydroxy-3-{(2R,3S)-2-hydroxy-3...)Show SMILES CC(C)(C)OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CNC[C@@H](O)[C@H](Cc1ccccc1)NC(=O)C1(O)COCC1(C)C Show InChI InChI=1S/C32H47N3O7/c1-30(2,3)42-29(39)35-25(17-23-14-10-7-11-15-23)27(37)19-33-18-26(36)24(16-22-12-8-6-9-13-22)34-28(38)32(40)21-41-20-31(32,4)5/h6-15,24-27,33,36-37,40H,16-21H2,1-5H3,(H,34,38)(H,35,39)/t24-,25-,26+,27+,32?/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of HIV-1 protease. |
Bioorg Med Chem Lett 5: 1729-1734 (1995)
Article DOI: 10.1016/0960-894X(95)00293-3
BindingDB Entry DOI: 10.7270/Q2RN37TS |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50286509
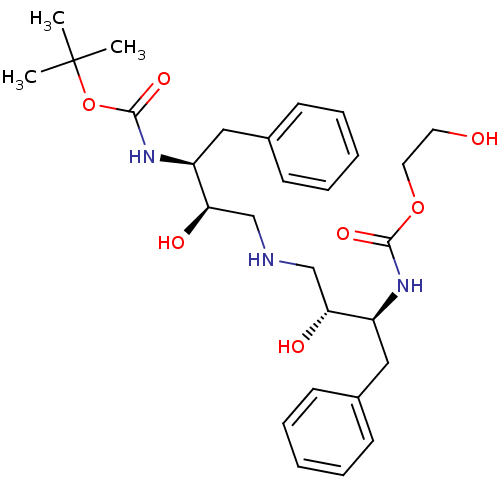
(CHEMBL143415 | {(1S,2R)-1-Benzyl-2-hydroxy-3-[(2R,...)Show SMILES CC(C)(C)OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CNC[C@@H](O)[C@H](Cc1ccccc1)NC(=O)OCCO Show InChI InChI=1S/C28H41N3O7/c1-28(2,3)38-27(36)31-23(17-21-12-8-5-9-13-21)25(34)19-29-18-24(33)22(30-26(35)37-15-14-32)16-20-10-6-4-7-11-20/h4-13,22-25,29,32-34H,14-19H2,1-3H3,(H,30,35)(H,31,36)/t22-,23-,24+,25+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of HIV protease cleavage of V-S-Q-N-(b-naphthylalanine)-P-I-V |
Bioorg Med Chem Lett 5: 459-464 (1995)
Article DOI: 10.1016/0960-894X(95)00056-Y
BindingDB Entry DOI: 10.7270/Q2RX9C2M |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50284935

(CHEMBL445886 | {(1S,2R)-1-Benzyl-2-hydroxy-3-[(2R,...)Show SMILES CC(C)(C)OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CNC[C@@H](O)[C@H](Cc1ccccc1)NC(=O)[C@@](C)(O)c1ccccc1 Show InChI InChI=1S/C34H45N3O6/c1-33(2,3)43-32(41)37-28(21-25-16-10-6-11-17-25)30(39)23-35-22-29(38)27(20-24-14-8-5-9-15-24)36-31(40)34(4,42)26-18-12-7-13-19-26/h5-19,27-30,35,38-39,42H,20-23H2,1-4H3,(H,36,40)(H,37,41)/t27-,28-,29+,30+,34-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of HIV-1 protease. |
Bioorg Med Chem Lett 5: 1729-1734 (1995)
Article DOI: 10.1016/0960-894X(95)00293-3
BindingDB Entry DOI: 10.7270/Q2RN37TS |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50284931

(CHEMBL49773 | {(1S,2R)-1-Benzyl-2-hydroxy-3-[(2R,3...)Show SMILES CC(C)(C)OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CNC[C@@H](O)[C@H](Cc1ccccc1)NC(=O)[C@H](O)c1ccccc1 Show InChI InChI=1S/C33H43N3O6/c1-33(2,3)42-32(41)36-27(20-24-15-9-5-10-16-24)29(38)22-34-21-28(37)26(19-23-13-7-4-8-14-23)35-31(40)30(39)25-17-11-6-12-18-25/h4-18,26-30,34,37-39H,19-22H2,1-3H3,(H,35,40)(H,36,41)/t26-,27-,28+,29+,30+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of HIV-1 protease. |
Bioorg Med Chem Lett 5: 1729-1734 (1995)
Article DOI: 10.1016/0960-894X(95)00293-3
BindingDB Entry DOI: 10.7270/Q2RN37TS |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50004187
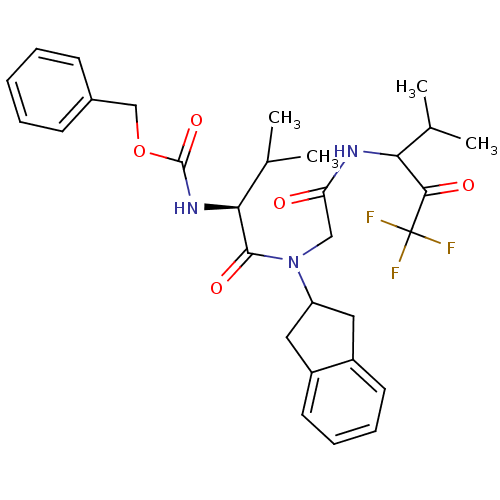
(((S)-1-{Indan-2-yl-[(3,3,3-trifluoro-1-isopropyl-2...)Show SMILES CC(C)[C@H](NC(=O)OCc1ccccc1)C(=O)N(CC(=O)NC(C(C)C)C(=O)C(F)(F)F)C1Cc2ccccc2C1 Show InChI InChI=1S/C30H36F3N3O5/c1-18(2)25(27(38)30(31,32)33)34-24(37)16-36(23-14-21-12-8-9-13-22(21)15-23)28(39)26(19(3)4)35-29(40)41-17-20-10-6-5-7-11-20/h5-13,18-19,23,25-26H,14-17H2,1-4H3,(H,34,37)(H,35,40)/t25?,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 365 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against human leukocyte elastase (HLE) at pH 7.5 |
J Med Chem 35: 4795-808 (1993)
BindingDB Entry DOI: 10.7270/Q2HX1BM5 |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50004185
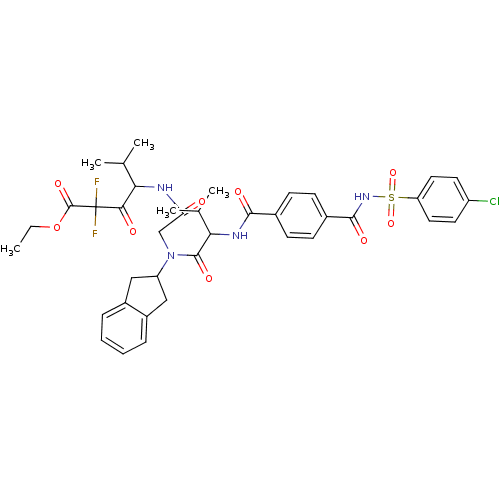
(4-[2-({2-[4-(4-Chloro-benzenesulfonylaminocarbonyl...)Show SMILES CCOC(=O)C(F)(F)C(=O)C(NC(=O)CN(C1Cc2ccccc2C1)C(=O)C(NC(=O)c1ccc(cc1)C(=O)NS(=O)(=O)c1ccc(Cl)cc1)C(C)C)C(C)C Show InChI InChI=1S/C39H43ClF2N4O9S/c1-6-55-38(52)39(41,42)34(48)32(22(2)3)43-31(47)21-46(29-19-26-9-7-8-10-27(26)20-29)37(51)33(23(4)5)44-35(49)24-11-13-25(14-12-24)36(50)45-56(53,54)30-17-15-28(40)16-18-30/h7-18,22-23,29,32-33H,6,19-21H2,1-5H3,(H,43,47)(H,44,49)(H,45,50) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 404 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against human leukocyte elastase (HLE) at pH 7.5 |
J Med Chem 35: 4795-808 (1993)
BindingDB Entry DOI: 10.7270/Q2HX1BM5 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50286506
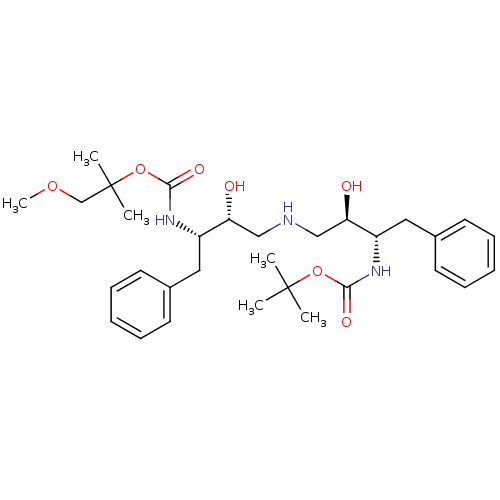
(CHEMBL344953 | {(1S,2R)-1-Benzyl-2-hydroxy-3-[(2R,...)Show SMILES COCC(C)(C)OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CNC[C@@H](O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C Show InChI InChI=1S/C31H47N3O7/c1-30(2,3)40-28(37)33-24(17-22-13-9-7-10-14-22)26(35)19-32-20-27(36)25(18-23-15-11-8-12-16-23)34-29(38)41-31(4,5)21-39-6/h7-16,24-27,32,35-36H,17-21H2,1-6H3,(H,33,37)(H,34,38)/t24-,25-,26+,27+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of HIV protease cleavage of V-S-Q-N-(b-naphthylalanine)-P-I-V |
Bioorg Med Chem Lett 5: 459-464 (1995)
Article DOI: 10.1016/0960-894X(95)00056-Y
BindingDB Entry DOI: 10.7270/Q2RX9C2M |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50284926

(CHEMBL46020 | {(1S,2R)-1-Benzyl-2-hydroxy-3-[(2R,3...)Show SMILES CC(C)(C)OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CNC[C@@H](O)[C@H](Cc1ccccc1)NC(=O)C(C)(C)O Show InChI InChI=1S/C29H43N3O6/c1-28(2,3)38-27(36)32-23(17-21-14-10-7-11-15-21)25(34)19-30-18-24(33)22(31-26(35)29(4,5)37)16-20-12-8-6-9-13-20/h6-15,22-25,30,33-34,37H,16-19H2,1-5H3,(H,31,35)(H,32,36)/t22-,23-,24+,25+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of HIV-1 protease. |
Bioorg Med Chem Lett 5: 1729-1734 (1995)
Article DOI: 10.1016/0960-894X(95)00293-3
BindingDB Entry DOI: 10.7270/Q2RN37TS |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50004183

(4-{2-[(2-Benzyloxycarbonylamino-3-methyl-butyryl)-...)Show SMILES CCOC(=O)C(F)(F)C(=O)C(NC(=O)CN(C1Cc2ccccc2C1)C(=O)C(NC(=O)OCc1ccccc1)C(C)C)C(C)C Show InChI InChI=1S/C33H41F2N3O7/c1-6-44-31(42)33(34,35)29(40)27(20(2)3)36-26(39)18-38(25-16-23-14-10-11-15-24(23)17-25)30(41)28(21(4)5)37-32(43)45-19-22-12-8-7-9-13-22/h7-15,20-21,25,27-28H,6,16-19H2,1-5H3,(H,36,39)(H,37,43) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 635 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against human leukocyte elastase (HLE) at pH 7.5 |
J Med Chem 35: 4795-808 (1993)
BindingDB Entry DOI: 10.7270/Q2HX1BM5 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50284933

(CHEMBL48921 | {(1S,2R)-1-Benzyl-2-hydroxy-3-[(2R,3...)Show SMILES CC(C)(C)OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CNC[C@@H](O)[C@H](Cc1ccccc1)NC(=O)[C@](C)(O)C(C)(C)C Show InChI InChI=1S/C32H49N3O6/c1-30(2,3)32(7,40)28(38)34-24(18-22-14-10-8-11-15-22)26(36)20-33-21-27(37)25(19-23-16-12-9-13-17-23)35-29(39)41-31(4,5)6/h8-17,24-27,33,36-37,40H,18-21H2,1-7H3,(H,34,38)(H,35,39)/t24-,25-,26+,27+,32-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of HIV-1 protease. |
Bioorg Med Chem Lett 5: 1729-1734 (1995)
Article DOI: 10.1016/0960-894X(95)00293-3
BindingDB Entry DOI: 10.7270/Q2RN37TS |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50286507
(CHEMBL348226 | {(1S,2R)-1-Benzyl-2-hydroxy-3-[(2R,...)Show SMILES CC(C)(C)OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CNC[C@@H](O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)CCCO Show InChI InChI=1S/C32H49N3O7/c1-31(2,3)41-29(39)34-25(19-23-13-8-6-9-14-23)27(37)21-33-22-28(38)26(20-24-15-10-7-11-16-24)35-30(40)42-32(4,5)17-12-18-36/h6-11,13-16,25-28,33,36-38H,12,17-22H2,1-5H3,(H,34,39)(H,35,40)/t25-,26-,27+,28+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of HIV protease cleavage of V-S-Q-N-(b-naphthylalanine)-P-I-V |
Bioorg Med Chem Lett 5: 459-464 (1995)
Article DOI: 10.1016/0960-894X(95)00056-Y
BindingDB Entry DOI: 10.7270/Q2RX9C2M |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50284927
(CHEMBL297829 | {(1S,2R)-1-Benzyl-2-hydroxy-3-[(2R,...)Show SMILES CC(C)(C)OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CNC[C@@H](O)[C@H](Cc1ccccc1)NC(=O)[C@@H](O)C(C)(C)C Show InChI InChI=1S/C31H47N3O6/c1-30(2,3)27(37)28(38)33-23(17-21-13-9-7-10-14-21)25(35)19-32-20-26(36)24(18-22-15-11-8-12-16-22)34-29(39)40-31(4,5)6/h7-16,23-27,32,35-37H,17-20H2,1-6H3,(H,33,38)(H,34,39)/t23-,24-,25+,26+,27+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of HIV-1 protease. |
Bioorg Med Chem Lett 5: 1729-1734 (1995)
Article DOI: 10.1016/0960-894X(95)00293-3
BindingDB Entry DOI: 10.7270/Q2RN37TS |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50286512
(CHEMBL346860 | {(1S,2R)-1-Benzyl-3-[(2R,3S)-3-(2-f...)Show SMILES CC(C)(C)OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CNC[C@@H](O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)CNC=O Show InChI InChI=1S/C31H46N4O7/c1-30(2,3)41-28(39)34-24(16-22-12-8-6-9-13-22)26(37)18-32-19-27(38)25(17-23-14-10-7-11-15-23)35-29(40)42-31(4,5)20-33-21-36/h6-15,21,24-27,32,37-38H,16-20H2,1-5H3,(H,33,36)(H,34,39)(H,35,40)/t24-,25-,26+,27+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of HIV protease cleavage of V-S-Q-N-(b-naphthylalanine)-P-I-V |
Bioorg Med Chem Lett 5: 459-464 (1995)
Article DOI: 10.1016/0960-894X(95)00056-Y
BindingDB Entry DOI: 10.7270/Q2RX9C2M |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM924

((1S-(1R*,2S*(2S*,3R*)))-[3-[[3-[(3,3-Dimethyl-1-ox...)Show SMILES CC(C)(C)CC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CNC[C@@H](O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C |r| Show InChI InChI=1S/C31H47N3O5/c1-30(2,3)19-28(37)33-24(17-22-13-9-7-10-14-22)26(35)20-32-21-27(36)25(18-23-15-11-8-12-16-23)34-29(38)39-31(4,5)6/h7-16,24-27,32,35-36H,17-21H2,1-6H3,(H,33,37)(H,34,38)/t24-,25-,26+,27+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of HIV-1 protease. |
Bioorg Med Chem Lett 5: 1729-1734 (1995)
Article DOI: 10.1016/0960-894X(95)00293-3
BindingDB Entry DOI: 10.7270/Q2RN37TS |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50004189

(4-[2-({2-[4-(4-Chloro-benzenesulfonylaminocarbonyl...)Show SMILES CCOC(=O)C(F)(F)C(=O)C(CC(C)C)NC(=O)CN(C1Cc2ccccc2C1)C(=O)C(NC(=O)c1ccc(cc1)C(=O)NS(=O)(=O)c1ccc(Cl)cc1)C(C)C Show InChI InChI=1S/C40H45ClF2N4O9S/c1-6-56-39(53)40(42,43)35(49)32(19-23(2)3)44-33(48)22-47(30-20-27-9-7-8-10-28(27)21-30)38(52)34(24(4)5)45-36(50)25-11-13-26(14-12-25)37(51)46-57(54,55)31-17-15-29(41)16-18-31/h7-18,23-24,30,32,34H,6,19-22H2,1-5H3,(H,44,48)(H,45,50)(H,46,51) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.90E+3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against human leukocyte elastase (HLE) at pH 7.5 |
J Med Chem 35: 4795-808 (1993)
BindingDB Entry DOI: 10.7270/Q2HX1BM5 |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50004188
(4-[2-({2-[4-(4-Chloro-benzenesulfonylaminocarbonyl...)Show SMILES CCOC(=O)C(F)(F)C(=O)C(Cc1ccccc1)NC(=O)CN(C1Cc2ccccc2C1)C(=O)C(NC(=O)c1ccc(cc1)C(=O)NS(=O)(=O)c1ccc(Cl)cc1)C(C)C Show InChI InChI=1S/C43H43ClF2N4O9S/c1-4-59-42(56)43(45,46)38(52)35(22-27-10-6-5-7-11-27)47-36(51)25-50(33-23-30-12-8-9-13-31(30)24-33)41(55)37(26(2)3)48-39(53)28-14-16-29(17-15-28)40(54)49-60(57,58)34-20-18-32(44)19-21-34/h5-21,26,33,35,37H,4,22-25H2,1-3H3,(H,47,51)(H,48,53)(H,49,54) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against human leukocyte elastase (HLE) at pH 7.5 |
J Med Chem 35: 4795-808 (1993)
BindingDB Entry DOI: 10.7270/Q2HX1BM5 |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50004186
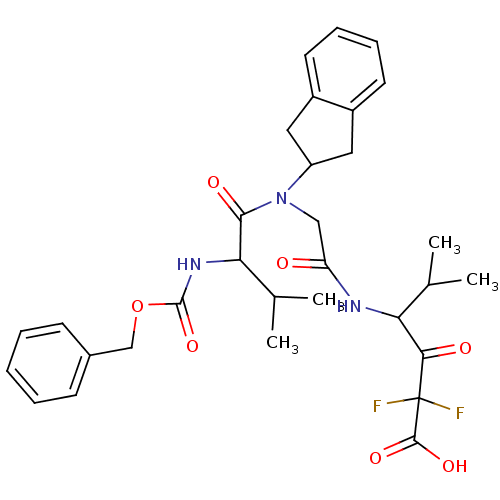
(4-{2-[(2-Benzyloxycarbonylamino-3-methyl-butyryl)-...)Show SMILES CC(C)C(NC(=O)OCc1ccccc1)C(=O)N(CC(=O)NC(C(C)C)C(=O)C(F)(F)C(O)=O)C1Cc2ccccc2C1 Show InChI InChI=1S/C31H37F2N3O7/c1-18(2)25(27(38)31(32,33)29(40)41)34-24(37)16-36(23-14-21-12-8-9-13-22(21)15-23)28(39)26(19(3)4)35-30(42)43-17-20-10-6-5-7-11-20/h5-13,18-19,23,25-26H,14-17H2,1-4H3,(H,34,37)(H,35,42)(H,40,41) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against human leukocyte elastase (HLE) at pH 7.5 |
J Med Chem 35: 4795-808 (1993)
BindingDB Entry DOI: 10.7270/Q2HX1BM5 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50284936

(CHEMBL48993 | {(1S,2R)-1-Benzyl-2-hydroxy-3-[(2R,3...)Show SMILES CC(C)(C)OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CNC[C@@H](O)[C@H](Cc1ccccc1)NC(=O)[C@](C)(O)c1ccccc1 Show InChI InChI=1S/C34H45N3O6/c1-33(2,3)43-32(41)37-28(21-25-16-10-6-11-17-25)30(39)23-35-22-29(38)27(20-24-14-8-5-9-15-24)36-31(40)34(4,42)26-18-12-7-13-19-26/h5-19,27-30,35,38-39,42H,20-23H2,1-4H3,(H,36,40)(H,37,41)/t27-,28-,29+,30+,34+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 6.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of HIV-1 protease. |
Bioorg Med Chem Lett 5: 1729-1734 (1995)
Article DOI: 10.1016/0960-894X(95)00293-3
BindingDB Entry DOI: 10.7270/Q2RN37TS |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50286510
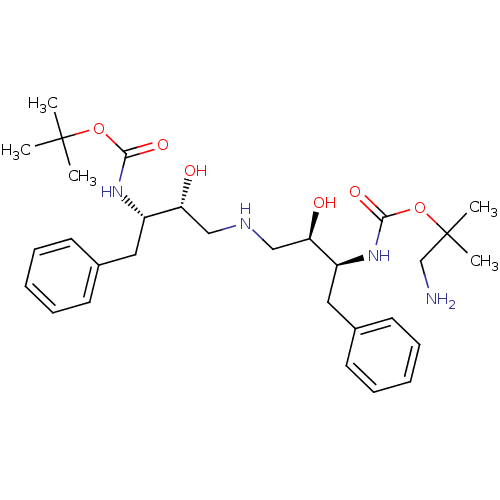
(CHEMBL141252 | {(1S,2R)-3-[(2R,3S)-3-(2-Amino-1,1-...)Show SMILES CC(C)(C)OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CNC[C@@H](O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)CN Show InChI InChI=1S/C30H46N4O6/c1-29(2,3)39-27(37)33-23(16-21-12-8-6-9-13-21)25(35)18-32-19-26(36)24(17-22-14-10-7-11-15-22)34-28(38)40-30(4,5)20-31/h6-15,23-26,32,35-36H,16-20,31H2,1-5H3,(H,33,37)(H,34,38)/t23-,24-,25+,26+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of HIV protease cleavage of V-S-Q-N-(b-naphthylalanine)-P-I-V |
Bioorg Med Chem Lett 5: 459-464 (1995)
Article DOI: 10.1016/0960-894X(95)00056-Y
BindingDB Entry DOI: 10.7270/Q2RX9C2M |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50284928

(CHEMBL301382 | {(1S,2R)-1-Benzyl-2-hydroxy-3-[(2R,...)Show SMILES CC(C)(C)OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CNC[C@@H](O)[C@H](Cc1ccccc1)NC(=O)[C@@H](O)c1ccccc1 Show InChI InChI=1S/C33H43N3O6/c1-33(2,3)42-32(41)36-27(20-24-15-9-5-10-16-24)29(38)22-34-21-28(37)26(19-23-13-7-4-8-14-23)35-31(40)30(39)25-17-11-6-12-18-25/h4-18,26-30,34,37-39H,19-22H2,1-3H3,(H,35,40)(H,36,41)/t26-,27-,28+,29+,30-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of HIV-1 protease. |
Bioorg Med Chem Lett 5: 1729-1734 (1995)
Article DOI: 10.1016/0960-894X(95)00293-3
BindingDB Entry DOI: 10.7270/Q2RN37TS |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50286505
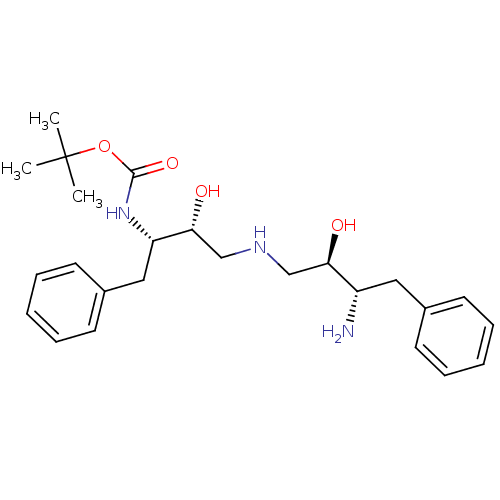
(CHEMBL140755 | [(1S,2R)-3-((2R,3S)-3-Amino-2-hydro...)Show SMILES CC(C)(C)OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CNC[C@@H](O)[C@@H](N)Cc1ccccc1 Show InChI InChI=1S/C25H37N3O4/c1-25(2,3)32-24(31)28-21(15-19-12-8-5-9-13-19)23(30)17-27-16-22(29)20(26)14-18-10-6-4-7-11-18/h4-13,20-23,27,29-30H,14-17,26H2,1-3H3,(H,28,31)/t20-,21-,22+,23+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of HIV protease cleavage of V-S-Q-N-(b-naphthylalanine)-P-I-V |
Bioorg Med Chem Lett 5: 459-464 (1995)
Article DOI: 10.1016/0960-894X(95)00056-Y
BindingDB Entry DOI: 10.7270/Q2RX9C2M |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50004191
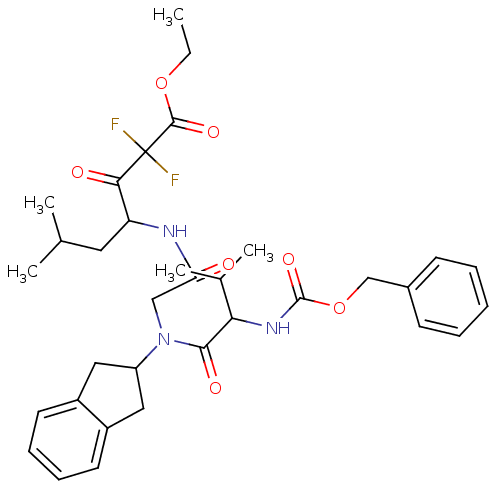
(4-{2-[(2-Benzyloxycarbonylamino-3-methyl-butyryl)-...)Show SMILES CCOC(=O)C(F)(F)C(=O)C(CC(C)C)NC(=O)CN(C1Cc2ccccc2C1)C(=O)C(NC(=O)OCc1ccccc1)C(C)C Show InChI InChI=1S/C34H43F2N3O7/c1-6-45-32(43)34(35,36)30(41)27(16-21(2)3)37-28(40)19-39(26-17-24-14-10-11-15-25(24)18-26)31(42)29(22(4)5)38-33(44)46-20-23-12-8-7-9-13-23/h7-15,21-22,26-27,29H,6,16-20H2,1-5H3,(H,37,40)(H,38,44) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.11E+4 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against human leukocyte elastase (HLE) at pH 7.5 |
J Med Chem 35: 4795-808 (1993)
BindingDB Entry DOI: 10.7270/Q2HX1BM5 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data