 Found 9976 hits with Last Name = 'son' and Initial = 'i'
Found 9976 hits with Last Name = 'son' and Initial = 'i' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50243536
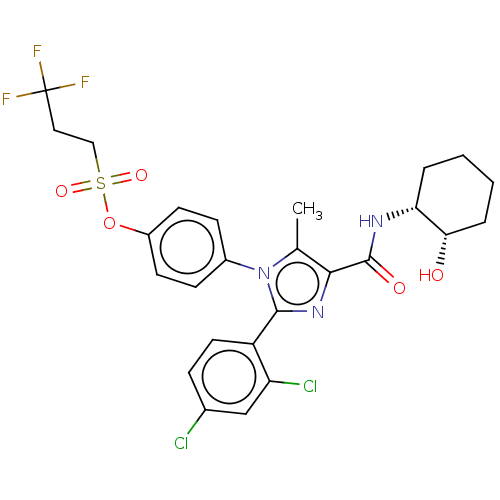
(CHEMBL4062749)Show SMILES Cc1c(nc(-c2ccc(Cl)cc2Cl)n1-c1ccc(OS(=O)(=O)CCC(F)(F)F)cc1)C(=O)N[C@@H]1CCCC[C@@H]1O |r| Show InChI InChI=1S/C26H26Cl2F3N3O5S/c1-15-23(25(36)32-21-4-2-3-5-22(21)35)33-24(19-11-6-16(27)14-20(19)28)34(15)17-7-9-18(10-8-17)39-40(37,38)13-12-26(29,30)31/h6-11,14,21-22,35H,2-5,12-13H2,1H3,(H,32,36)/t21-,22+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from recombinant human CB1 receptor expressed in beta-galactosidase expressing CHOK1 cell membranes after 60 mins by scin... |
J Med Chem 60: 9545-9564 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00861
BindingDB Entry DOI: 10.7270/Q2X92DQ7 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50243536

(CHEMBL4062749)Show SMILES Cc1c(nc(-c2ccc(Cl)cc2Cl)n1-c1ccc(OS(=O)(=O)CCC(F)(F)F)cc1)C(=O)N[C@@H]1CCCC[C@@H]1O |r| Show InChI InChI=1S/C26H26Cl2F3N3O5S/c1-15-23(25(36)32-21-4-2-3-5-22(21)35)33-24(19-11-6-16(27)14-20(19)28)34(15)17-7-9-18(10-8-17)39-40(37,38)13-12-26(29,30)31/h6-11,14,21-22,35H,2-5,12-13H2,1H3,(H,32,36)/t21-,22+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from recombinant human CB1 receptor expressed in beta-galactosidase expressing CHOK1 cell membranes after 60 mins by scin... |
J Med Chem 60: 9545-9564 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00861
BindingDB Entry DOI: 10.7270/Q2X92DQ7 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50374877
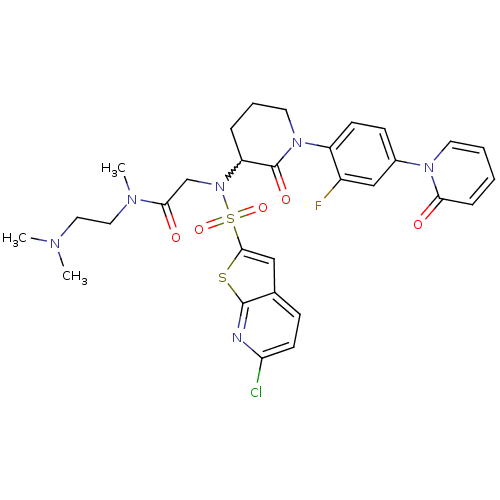
(CHEMBL270221)Show SMILES CN(C)CCN(C)C(=O)CN(C1CCCN(C1=O)c1ccc(cc1F)-n1ccccc1=O)S(=O)(=O)c1cc2ccc(Cl)nc2s1 |w:11.10| Show InChI InChI=1S/C30H32ClFN6O5S2/c1-34(2)15-16-35(3)27(40)19-38(45(42,43)28-17-20-9-12-25(31)33-29(20)44-28)24-7-6-14-37(30(24)41)23-11-10-21(18-22(23)32)36-13-5-4-8-26(36)39/h4-5,8-13,17-18,24H,6-7,14-16,19H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 2428-33 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.054
BindingDB Entry DOI: 10.7270/Q2RX9CZ8 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50374879
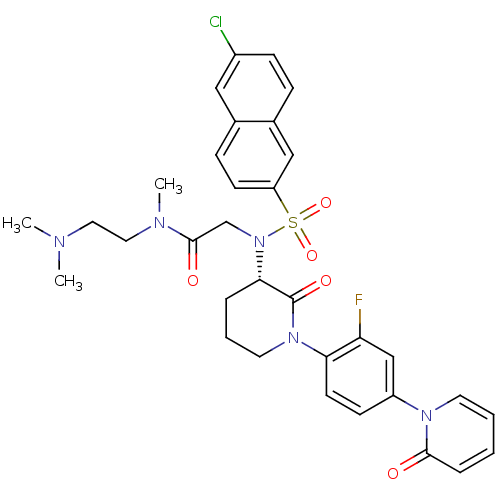
(CHEMBL401958)Show SMILES CN(C)CCN(C)C(=O)CN([C@H]1CCCN(C1=O)c1ccc(cc1F)-n1ccccc1=O)S(=O)(=O)c1ccc2cc(Cl)ccc2c1 Show InChI InChI=1S/C33H35ClFN5O5S/c1-36(2)17-18-37(3)32(42)22-40(46(44,45)27-13-10-23-19-25(34)11-9-24(23)20-27)30-7-6-16-39(33(30)43)29-14-12-26(21-28(29)35)38-15-5-4-8-31(38)41/h4-5,8-15,19-21,30H,6-7,16-18,22H2,1-3H3/t30-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 2428-33 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.054
BindingDB Entry DOI: 10.7270/Q2RX9CZ8 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50374878
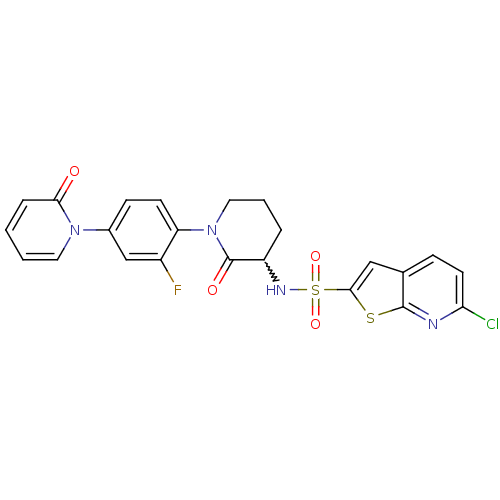
(CHEMBL270862)Show SMILES Fc1cc(ccc1N1CCCC(NS(=O)(=O)c2cc3ccc(Cl)nc3s2)C1=O)-n1ccccc1=O |w:11.12| Show InChI InChI=1S/C23H18ClFN4O4S2/c24-19-9-6-14-12-21(34-22(14)26-19)35(32,33)27-17-4-3-11-29(23(17)31)18-8-7-15(13-16(18)25)28-10-2-1-5-20(28)30/h1-2,5-10,12-13,17,27H,3-4,11H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 2428-33 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.054
BindingDB Entry DOI: 10.7270/Q2RX9CZ8 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50374879

(CHEMBL401958)Show SMILES CN(C)CCN(C)C(=O)CN([C@H]1CCCN(C1=O)c1ccc(cc1F)-n1ccccc1=O)S(=O)(=O)c1ccc2cc(Cl)ccc2c1 Show InChI InChI=1S/C33H35ClFN5O5S/c1-36(2)17-18-37(3)32(42)22-40(46(44,45)27-13-10-23-19-25(34)11-9-24(23)20-27)30-7-6-16-39(33(30)43)29-14-12-26(21-28(29)35)38-15-5-4-8-31(38)41/h4-5,8-15,19-21,30H,6-7,16-18,22H2,1-3H3/t30-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 2428-33 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.054
BindingDB Entry DOI: 10.7270/Q2RX9CZ8 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50097624
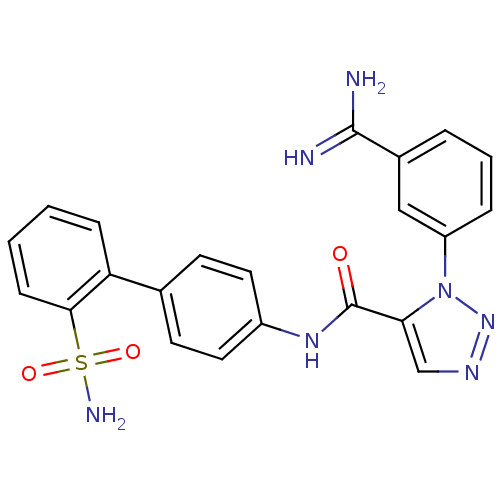
(3-(3-Carbamimidoyl-phenyl)-3H-[1,2,3]triazole-4-ca...)Show SMILES NC(=N)c1cccc(c1)-n1nncc1C(=O)Nc1ccc(cc1)-c1ccccc1S(N)(=O)=O Show InChI InChI=1S/C22H19N7O3S/c23-21(24)15-4-3-5-17(12-15)29-19(13-26-28-29)22(30)27-16-10-8-14(9-11-16)18-6-1-2-7-20(18)33(25,31)32/h1-13H,(H3,23,24)(H,27,30)(H2,25,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 0.0230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
Binding affinity towards human Serine protease FXa |
Bioorg Med Chem Lett 11: 641-5 (2001)
BindingDB Entry DOI: 10.7270/Q2RV0MZ2 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Oryctolagus cuniculus) | BDBM12751

(1-(3-carbamimidoylphenyl)-3-methyl-N-[4-(2-sulfamo...)Show SMILES Cc1cc(C(=O)Nc2ccc(cc2)-c2ccccc2S(N)(=O)=O)n(n1)-c1cccc(c1)C(N)=N Show InChI InChI=1S/C24H22N6O3S/c1-15-13-21(30(29-15)19-6-4-5-17(14-19)23(25)26)24(31)28-18-11-9-16(10-12-18)20-7-2-3-8-22(20)34(27,32)33/h2-14H,1H3,(H3,25,26)(H,28,31)(H2,27,32,33) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
Binding affinity towards human trypsin |
Bioorg Med Chem Lett 11: 641-5 (2001)
BindingDB Entry DOI: 10.7270/Q2RV0MZ2 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM12751

(1-(3-carbamimidoylphenyl)-3-methyl-N-[4-(2-sulfamo...)Show SMILES Cc1cc(C(=O)Nc2ccc(cc2)-c2ccccc2S(N)(=O)=O)n(n1)-c1cccc(c1)C(N)=N Show InChI InChI=1S/C24H22N6O3S/c1-15-13-21(30(29-15)19-6-4-5-17(14-19)23(25)26)24(31)28-18-11-9-16(10-12-18)20-7-2-3-8-22(20)34(27,32)33/h2-14H,1H3,(H3,25,26)(H,28,31)(H2,27,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
Binding affinity towards Rabbit Coagulation factor X in a rabbit arterio-venous (A-V) shunt model |
Bioorg Med Chem Lett 11: 641-5 (2001)
BindingDB Entry DOI: 10.7270/Q2RV0MZ2 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50374876
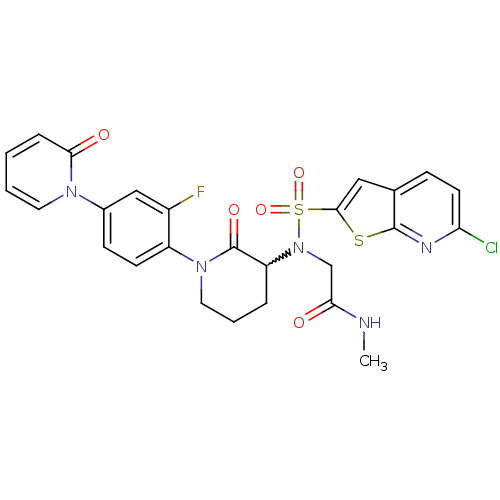
(CHEMBL270034)Show SMILES CNC(=O)CN(C1CCCN(C1=O)c1ccc(cc1F)-n1ccccc1=O)S(=O)(=O)c1cc2ccc(Cl)nc2s1 |w:6.5| Show InChI InChI=1S/C26H23ClFN5O5S2/c1-29-22(34)15-33(40(37,38)24-13-16-7-10-21(27)30-25(16)39-24)20-5-4-12-32(26(20)36)19-9-8-17(14-18(19)28)31-11-3-2-6-23(31)35/h2-3,6-11,13-14,20H,4-5,12,15H2,1H3,(H,29,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 2428-33 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.054
BindingDB Entry DOI: 10.7270/Q2RX9CZ8 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50097626
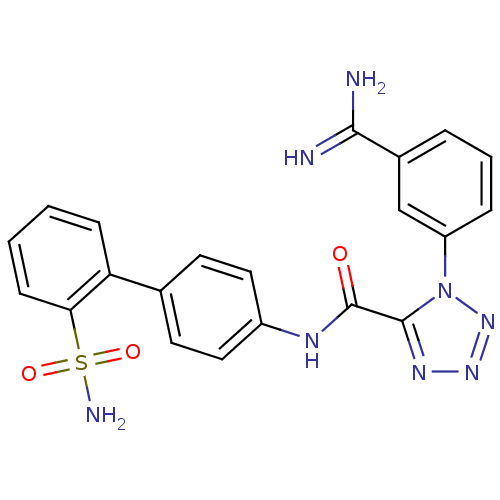
(1-(3-Carbamimidoyl-phenyl)-1H-tetrazole-5-carboxyl...)Show SMILES NC(=N)c1cccc(c1)-n1nnnc1C(=O)Nc1ccc(cc1)-c1ccccc1S(N)(=O)=O Show InChI InChI=1S/C21H18N8O3S/c22-19(23)14-4-3-5-16(12-14)29-20(26-27-28-29)21(30)25-15-10-8-13(9-11-15)17-6-1-2-7-18(17)33(24,31)32/h1-12H,(H3,22,23)(H,25,30)(H2,24,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
Binding affinity towards human Serine protease FXa |
Bioorg Med Chem Lett 11: 641-5 (2001)
BindingDB Entry DOI: 10.7270/Q2RV0MZ2 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50374871
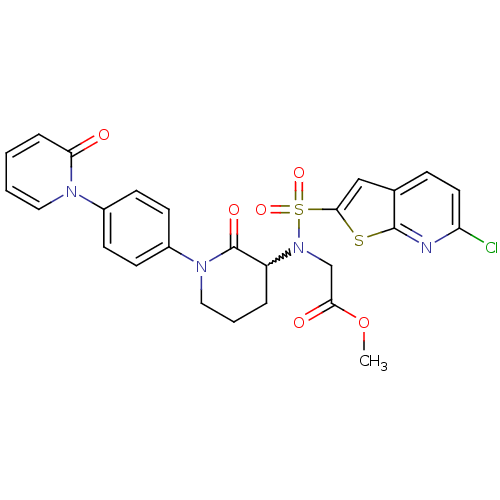
(CHEMBL258274)Show SMILES COC(=O)CN(C1CCCN(C1=O)c1ccc(cc1)-n1ccccc1=O)S(=O)(=O)c1cc2ccc(Cl)nc2s1 |w:6.5| Show InChI InChI=1S/C26H23ClN4O6S2/c1-37-23(33)16-31(39(35,36)24-15-17-7-12-21(27)28-25(17)38-24)20-5-4-14-30(26(20)34)19-10-8-18(9-11-19)29-13-3-2-6-22(29)32/h2-3,6-13,15,20H,4-5,14,16H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 2428-33 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.054
BindingDB Entry DOI: 10.7270/Q2RX9CZ8 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM16318
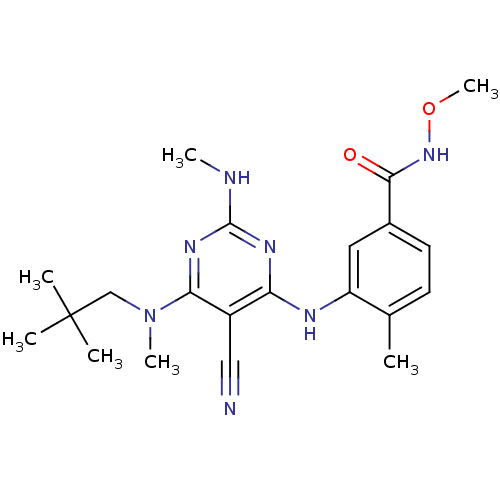
(3-({5-cyano-6-[(2,2-dimethylpropyl)(methyl)amino]-...)Show SMILES CNc1nc(Nc2cc(ccc2C)C(=O)NOC)c(C#N)c(n1)N(C)CC(C)(C)C Show InChI InChI=1S/C21H29N7O2/c1-13-8-9-14(19(29)27-30-7)10-16(13)24-17-15(11-22)18(26-20(23-5)25-17)28(6)12-21(2,3)4/h8-10H,12H2,1-7H3,(H,27,29)(H2,23,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0470 | -58.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Company
| Assay Description
The kinase activity was determined by quantitation of the amount of radioactive phosphate transferred to myelin basic protein (MBP) with or without i... |
J Med Chem 48: 6261-70 (2005)
Article DOI: 10.1021/jm0503594
BindingDB Entry DOI: 10.7270/Q25X276T |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM16319
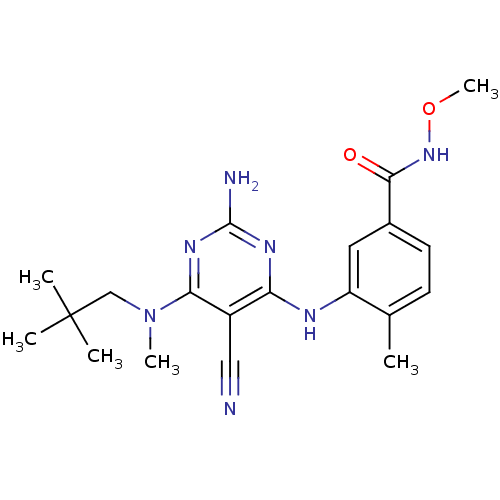
(3-({2-amino-5-cyano-6-[(2,2-dimethylpropyl)(methyl...)Show SMILES CONC(=O)c1ccc(C)c(Nc2nc(N)nc(N(C)CC(C)(C)C)c2C#N)c1 Show InChI InChI=1S/C20H27N7O2/c1-12-7-8-13(18(28)26-29-6)9-15(12)23-16-14(10-21)17(25-19(22)24-16)27(5)11-20(2,3)4/h7-9H,11H2,1-6H3,(H,26,28)(H3,22,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0500 | -58.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Company
| Assay Description
The kinase activity was determined by quantitation of the amount of radioactive phosphate transferred to myelin basic protein (MBP) with or without i... |
J Med Chem 48: 6261-70 (2005)
Article DOI: 10.1021/jm0503594
BindingDB Entry DOI: 10.7270/Q25X276T |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50374875
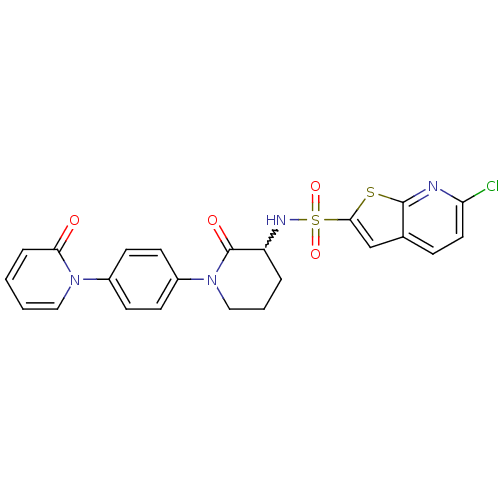
(CHEMBL269955)Show SMILES Clc1ccc2cc(sc2n1)S(=O)(=O)NC1CCCN(C1=O)c1ccc(cc1)-n1ccccc1=O |w:14.15| Show InChI InChI=1S/C23H19ClN4O4S2/c24-19-11-6-15-14-21(33-22(15)25-19)34(31,32)26-18-4-3-13-28(23(18)30)17-9-7-16(8-10-17)27-12-2-1-5-20(27)29/h1-2,5-12,14,18,26H,3-4,13H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 2428-33 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.054
BindingDB Entry DOI: 10.7270/Q2RX9CZ8 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM16329
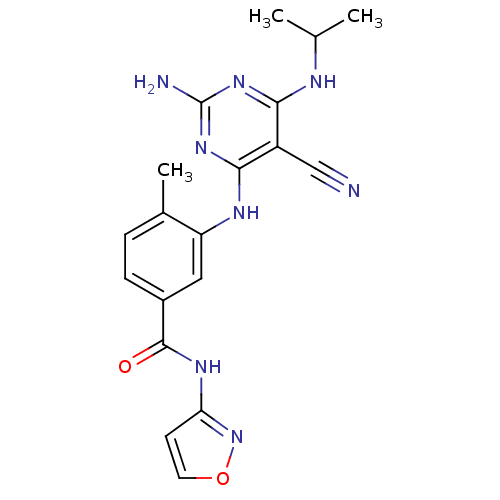
(3-({2-amino-5-cyano-6-[(1-methylethyl)amino]pyrimi...)Show SMILES CC(C)Nc1nc(N)nc(Nc2cc(ccc2C)C(=O)Nc2ccon2)c1C#N Show InChI InChI=1S/C19H20N8O2/c1-10(2)22-16-13(9-20)17(26-19(21)25-16)23-14-8-12(5-4-11(14)3)18(28)24-15-6-7-29-27-15/h4-8,10H,1-3H3,(H,24,27,28)(H4,21,22,23,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0570 | -57.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Company
| Assay Description
The kinase activity was determined by quantitation of the amount of radioactive phosphate transferred to myelin basic protein (MBP) with or without i... |
J Med Chem 48: 6261-70 (2005)
Article DOI: 10.1021/jm0503594
BindingDB Entry DOI: 10.7270/Q25X276T |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM16317
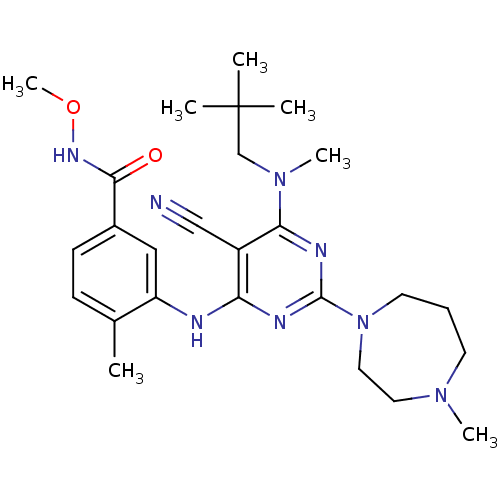
(3-({5-cyano-6-[(2,2-dimethylpropyl)(methyl)amino]-...)Show SMILES CONC(=O)c1ccc(C)c(Nc2nc(nc(N(C)CC(C)(C)C)c2C#N)N2CCCN(C)CC2)c1 Show InChI InChI=1S/C26H38N8O2/c1-18-9-10-19(24(35)31-36-7)15-21(18)28-22-20(16-27)23(33(6)17-26(2,3)4)30-25(29-22)34-12-8-11-32(5)13-14-34/h9-10,15H,8,11-14,17H2,1-7H3,(H,31,35)(H,28,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0570 | -57.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Company
| Assay Description
The kinase activity was determined by quantitation of the amount of radioactive phosphate transferred to myelin basic protein (MBP) with or without i... |
J Med Chem 48: 6261-70 (2005)
Article DOI: 10.1021/jm0503594
BindingDB Entry DOI: 10.7270/Q25X276T |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50097625
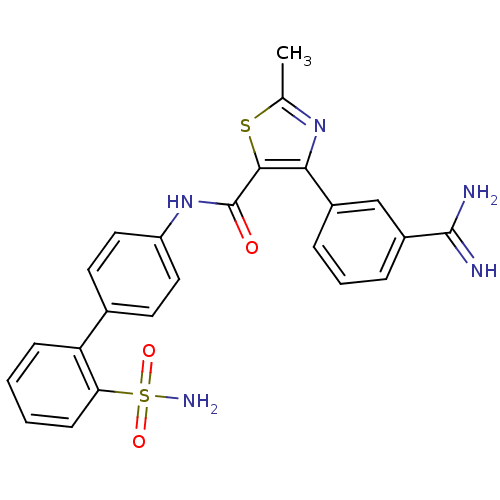
(4-(3-Carbamimidoyl-phenyl)-2-methyl-thiazole-5-car...)Show SMILES Cc1nc(c(s1)C(=O)Nc1ccc(cc1)-c1ccccc1S(N)(=O)=O)-c1cccc(c1)C(N)=N Show InChI InChI=1S/C24H21N5O3S2/c1-14-28-21(16-5-4-6-17(13-16)23(25)26)22(33-14)24(30)29-18-11-9-15(10-12-18)19-7-2-3-8-20(19)34(27,31)32/h2-13H,1H3,(H3,25,26)(H,29,30)(H2,27,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
Binding affinity towards Rabbit Coagulation factor X in a rabbit arterio-venous (A-V) shunt model |
Bioorg Med Chem Lett 11: 641-5 (2001)
BindingDB Entry DOI: 10.7270/Q2RV0MZ2 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Oryctolagus cuniculus) | BDBM50097625

(4-(3-Carbamimidoyl-phenyl)-2-methyl-thiazole-5-car...)Show SMILES Cc1nc(c(s1)C(=O)Nc1ccc(cc1)-c1ccccc1S(N)(=O)=O)-c1cccc(c1)C(N)=N Show InChI InChI=1S/C24H21N5O3S2/c1-14-28-21(16-5-4-6-17(13-16)23(25)26)22(33-14)24(30)29-18-11-9-15(10-12-18)19-7-2-3-8-20(19)34(27,31)32/h2-13H,1H3,(H3,25,26)(H,29,30)(H2,27,31,32) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
Binding affinity towards human trypsin |
Bioorg Med Chem Lett 11: 641-5 (2001)
BindingDB Entry DOI: 10.7270/Q2RV0MZ2 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50095041
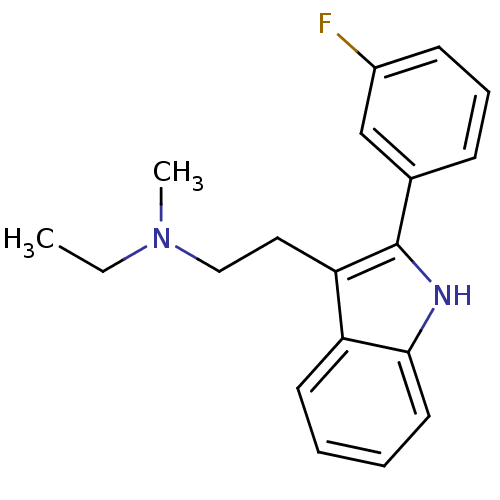
(CHEMBL328595 | Ethyl-{2-[2-(3-fluoro-phenyl)-1H-in...)Show InChI InChI=1S/C19H21FN2/c1-3-22(2)12-11-17-16-9-4-5-10-18(16)21-19(17)14-7-6-8-15(20)13-14/h4-10,13,21H,3,11-12H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories
Curated by ChEMBL
| Assay Description
Displacent of [H]-ketanserin from CHO cells expressing human 5-hydroxytryptamine 2A receptor. |
Bioorg Med Chem Lett 10: 2697-9 (2000)
BindingDB Entry DOI: 10.7270/Q27943XR |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM12659
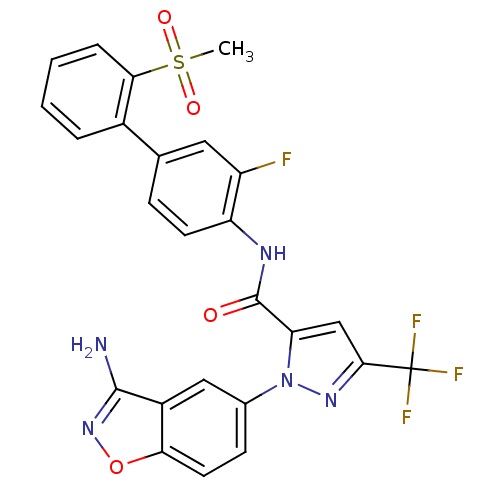
(1-(3-Aminobenzisoxazol-5-yl)-3-trifluoromethyl-5-[...)Show SMILES CS(=O)(=O)c1ccccc1-c1ccc(NC(=O)c2cc(nn2-c2ccc3onc(N)c3c2)C(F)(F)F)c(F)c1 Show InChI InChI=1S/C25H17F4N5O4S/c1-39(36,37)21-5-3-2-4-15(21)13-6-8-18(17(26)10-13)31-24(35)19-12-22(25(27,28)29)32-34(19)14-7-9-20-16(11-14)23(30)33-38-20/h2-12H,1H3,(H2,30,33)(H,31,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 17: 6481-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.091
BindingDB Entry DOI: 10.7270/Q2GX4B9Z |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50212159
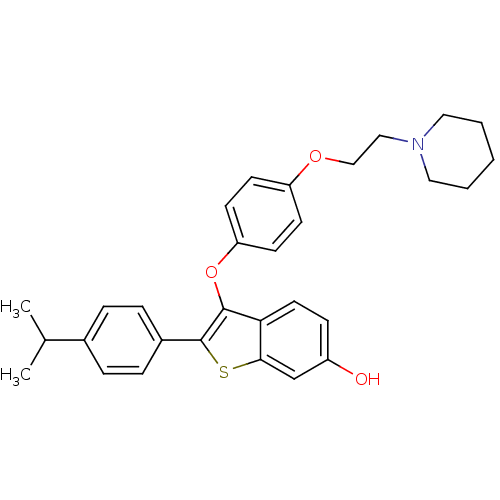
(2-(4-isopropylphenyl)-3-(4-(2-(piperidin-1-yl)etho...)Show SMILES CC(C)c1ccc(cc1)-c1sc2cc(O)ccc2c1Oc1ccc(OCCN2CCCCC2)cc1 Show InChI InChI=1S/C30H33NO3S/c1-21(2)22-6-8-23(9-7-22)30-29(27-15-10-24(32)20-28(27)35-30)34-26-13-11-25(12-14-26)33-19-18-31-16-4-3-5-17-31/h6-15,20-21,32H,3-5,16-19H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0910 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]estradiol from human recombinant ERalpha |
Bioorg Med Chem Lett 17: 3544-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.04.044
BindingDB Entry DOI: 10.7270/Q2ZS2W6F |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50243625
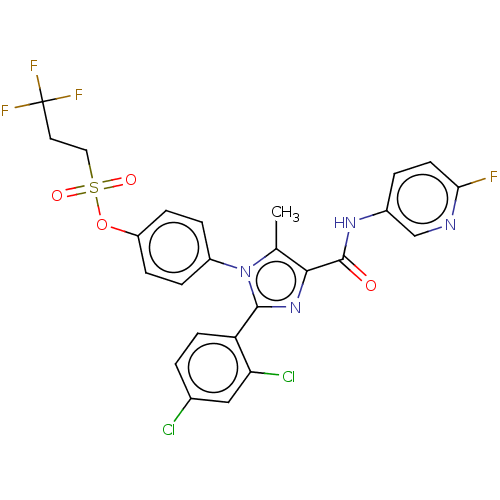
(CHEMBL4094098)Show SMILES Cc1c(nc(-c2ccc(Cl)cc2Cl)n1-c1ccc(OS(=O)(=O)CCC(F)(F)F)cc1)C(=O)Nc1ccc(F)nc1 Show InChI InChI=1S/C25H18Cl2F4N4O4S/c1-14-22(24(36)33-16-3-9-21(28)32-13-16)34-23(19-8-2-15(26)12-20(19)27)35(14)17-4-6-18(7-5-17)39-40(37,38)11-10-25(29,30)31/h2-9,12-13H,10-11H2,1H3,(H,33,36) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from recombinant human CB1 receptor expressed in beta-galactosidase expressing CHOK1 cell membranes after 60 mins by scin... |
J Med Chem 60: 9545-9564 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00861
BindingDB Entry DOI: 10.7270/Q2X92DQ7 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50243545
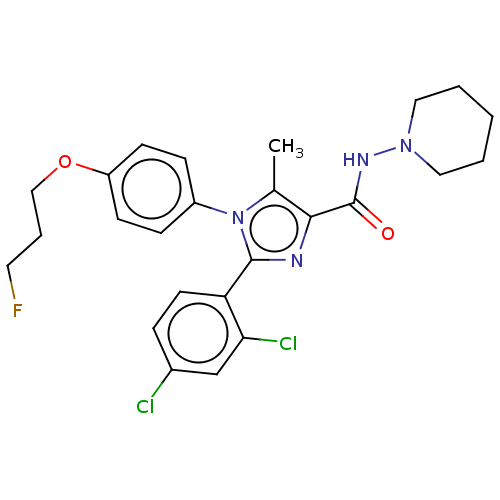
(CHEMBL4078689)Show SMILES Cc1c(nc(-c2ccc(Cl)cc2Cl)n1-c1ccc(OCCCF)cc1)C(=O)NN1CCCCC1 Show InChI InChI=1S/C25H27Cl2FN4O2/c1-17-23(25(33)30-31-13-3-2-4-14-31)29-24(21-11-6-18(26)16-22(21)27)32(17)19-7-9-20(10-8-19)34-15-5-12-28/h6-11,16H,2-5,12-15H2,1H3,(H,30,33) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from recombinant human CB1 receptor expressed in beta-galactosidase expressing CHOK1 cell membranes after 60 mins by scin... |
J Med Chem 60: 9545-9564 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00861
BindingDB Entry DOI: 10.7270/Q2X92DQ7 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50243589
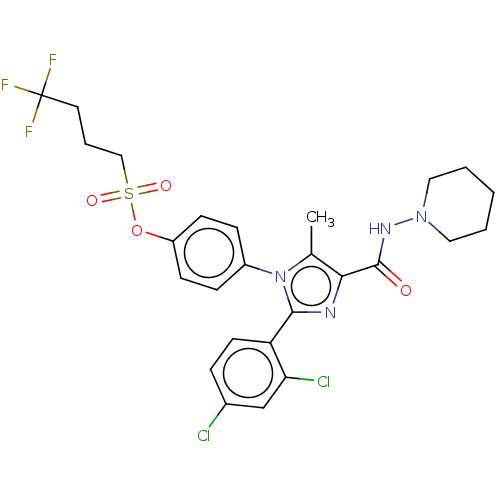
(CHEMBL4095223)Show SMILES Cc1c(nc(-c2ccc(Cl)cc2Cl)n1-c1ccc(OS(=O)(=O)CCCC(F)(F)F)cc1)C(=O)NN1CCCCC1 Show InChI InChI=1S/C26H27Cl2F3N4O4S/c1-17-23(25(36)33-34-13-3-2-4-14-34)32-24(21-11-6-18(27)16-22(21)28)35(17)19-7-9-20(10-8-19)39-40(37,38)15-5-12-26(29,30)31/h6-11,16H,2-5,12-15H2,1H3,(H,33,36) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from recombinant human CB1 receptor expressed in beta-galactosidase expressing CHOK1 cell membranes after 60 mins by scin... |
J Med Chem 60: 9545-9564 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00861
BindingDB Entry DOI: 10.7270/Q2X92DQ7 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50243608
(CHEMBL4100882)Show SMILES Cc1c(nc(-c2ccc(Cl)cc2Cl)n1-c1ccc(OS(=O)(=O)CCC(F)(F)F)cc1)C(=O)N[C@H]1CCC[C@H](O)C1 |r| Show InChI InChI=1S/C26H26Cl2F3N3O5S/c1-15-23(25(36)32-17-3-2-4-19(35)14-17)33-24(21-10-5-16(27)13-22(21)28)34(15)18-6-8-20(9-7-18)39-40(37,38)12-11-26(29,30)31/h5-10,13,17,19,35H,2-4,11-12,14H2,1H3,(H,32,36)/t17-,19-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from recombinant human CB1 receptor expressed in beta-galactosidase expressing CHOK1 cell membranes after 60 mins by scin... |
J Med Chem 60: 9545-9564 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00861
BindingDB Entry DOI: 10.7270/Q2X92DQ7 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50374874
(CHEMBL256820)Show SMILES NC(=O)CN(C1CCCN(C1=O)c1ccc(cc1)-n1ccccc1=O)S(=O)(=O)c1cc2ccc(Cl)nc2s1 |w:5.4| Show InChI InChI=1S/C25H22ClN5O5S2/c26-20-11-6-16-14-23(37-24(16)28-20)38(35,36)31(15-21(27)32)19-4-3-13-30(25(19)34)18-9-7-17(8-10-18)29-12-2-1-5-22(29)33/h1-2,5-12,14,19H,3-4,13,15H2,(H2,27,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 2428-33 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.054
BindingDB Entry DOI: 10.7270/Q2RX9CZ8 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50243545

(CHEMBL4078689)Show SMILES Cc1c(nc(-c2ccc(Cl)cc2Cl)n1-c1ccc(OCCCF)cc1)C(=O)NN1CCCCC1 Show InChI InChI=1S/C25H27Cl2FN4O2/c1-17-23(25(33)30-31-13-3-2-4-14-31)29-24(21-11-6-18(26)16-22(21)27)32(17)19-7-9-20(10-8-19)34-15-5-12-28/h6-11,16H,2-5,12-15H2,1H3,(H,30,33) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from recombinant human CB1 receptor expressed in beta-galactosidase expressing CHOK1 cell membranes after 60 mins by scin... |
J Med Chem 60: 9545-9564 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00861
BindingDB Entry DOI: 10.7270/Q2X92DQ7 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50243589

(CHEMBL4095223)Show SMILES Cc1c(nc(-c2ccc(Cl)cc2Cl)n1-c1ccc(OS(=O)(=O)CCCC(F)(F)F)cc1)C(=O)NN1CCCCC1 Show InChI InChI=1S/C26H27Cl2F3N4O4S/c1-17-23(25(36)33-34-13-3-2-4-14-34)32-24(21-11-6-18(27)16-22(21)28)35(17)19-7-9-20(10-8-19)39-40(37,38)15-5-12-26(29,30)31/h6-11,16H,2-5,12-15H2,1H3,(H,33,36) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from recombinant human CB1 receptor expressed in beta-galactosidase expressing CHOK1 cell membranes after 60 mins by scin... |
J Med Chem 60: 9545-9564 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00861
BindingDB Entry DOI: 10.7270/Q2X92DQ7 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50243608

(CHEMBL4100882)Show SMILES Cc1c(nc(-c2ccc(Cl)cc2Cl)n1-c1ccc(OS(=O)(=O)CCC(F)(F)F)cc1)C(=O)N[C@H]1CCC[C@H](O)C1 |r| Show InChI InChI=1S/C26H26Cl2F3N3O5S/c1-15-23(25(36)32-17-3-2-4-19(35)14-17)33-24(21-10-5-16(27)13-22(21)28)34(15)18-6-8-20(9-7-18)39-40(37,38)12-11-26(29,30)31/h5-10,13,17,19,35H,2-4,11-12,14H2,1H3,(H,32,36)/t17-,19-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from recombinant human CB1 receptor expressed in beta-galactosidase expressing CHOK1 cell membranes after 60 mins by scin... |
J Med Chem 60: 9545-9564 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00861
BindingDB Entry DOI: 10.7270/Q2X92DQ7 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50243625

(CHEMBL4094098)Show SMILES Cc1c(nc(-c2ccc(Cl)cc2Cl)n1-c1ccc(OS(=O)(=O)CCC(F)(F)F)cc1)C(=O)Nc1ccc(F)nc1 Show InChI InChI=1S/C25H18Cl2F4N4O4S/c1-14-22(24(36)33-16-3-9-21(28)32-13-16)34-23(19-8-2-15(26)12-20(19)27)35(14)17-4-6-18(7-5-17)39-40(37,38)11-10-25(29,30)31/h2-9,12-13H,10-11H2,1H3,(H,33,36) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from recombinant human CB1 receptor expressed in beta-galactosidase expressing CHOK1 cell membranes after 60 mins by scin... |
J Med Chem 60: 9545-9564 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00861
BindingDB Entry DOI: 10.7270/Q2X92DQ7 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50374880

(CHEMBL271904)Show SMILES Fc1cc(ccc1N1CCCC(NS(=O)(=O)c2ccc3cc(Cl)ccc3c2)C1=O)-n1ccccc1=O |w:11.12| Show InChI InChI=1S/C26H21ClFN3O4S/c27-19-8-6-18-15-21(10-7-17(18)14-19)36(34,35)29-23-4-3-13-31(26(23)33)24-11-9-20(16-22(24)28)30-12-2-1-5-25(30)32/h1-2,5-12,14-16,23,29H,3-4,13H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 2428-33 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.054
BindingDB Entry DOI: 10.7270/Q2RX9CZ8 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50005120
(CHEMBL274491 | N-((2S,3S)-1-Benzyl-2-methyl-pyrrol...)Show SMILES CNc1cc(OC)c(cc1Cl)C(=O)N[C@H]1CCN(Cc2ccccc2)[C@H]1C Show InChI InChI=1S/C21H26ClN3O2/c1-14-18(9-10-25(14)13-15-7-5-4-6-8-15)24-21(26)16-11-17(22)19(23-2)12-20(16)27-3/h4-8,11-12,14,18,23H,9-10,13H2,1-3H3,(H,24,26)/t14-,18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Lund
Curated by ChEMBL
| Assay Description
Compound was evaluated for binding affinity towards DA D-2 receptor using radioligand [3H]SPI |
J Med Chem 35: 2355-63 (1992)
BindingDB Entry DOI: 10.7270/Q2R2120B |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50243645
(CHEMBL4087520)Show SMILES Cc1c(nc(-c2ccc(Cl)cc2Cl)n1-c1ccc(OS(=O)(=O)CCC(F)(F)F)cc1)C(=O)NN1CCCCC1 Show InChI InChI=1S/C25H25Cl2F3N4O4S/c1-16-22(24(35)32-33-12-3-2-4-13-33)31-23(20-10-5-17(26)15-21(20)27)34(16)18-6-8-19(9-7-18)38-39(36,37)14-11-25(28,29)30/h5-10,15H,2-4,11-14H2,1H3,(H,32,35) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from recombinant human CB1 receptor expressed in beta-galactosidase expressing CHOK1 cell membranes after 60 mins by scin... |
J Med Chem 60: 9545-9564 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00861
BindingDB Entry DOI: 10.7270/Q2X92DQ7 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50243627
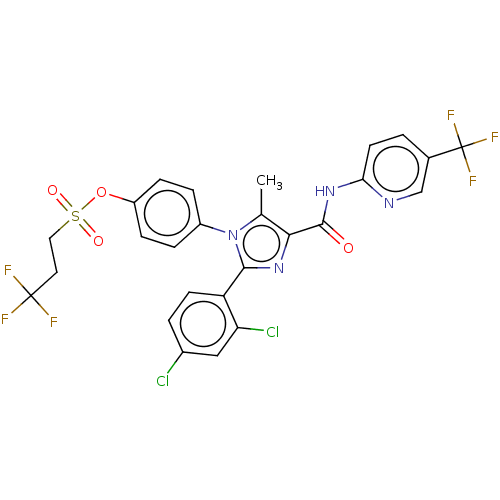
(CHEMBL4068708)Show SMILES Cc1c(nc(-c2ccc(Cl)cc2Cl)n1-c1ccc(OS(=O)(=O)CCC(F)(F)F)cc1)C(=O)Nc1ccc(cn1)C(F)(F)F Show InChI InChI=1S/C26H18Cl2F6N4O4S/c1-14-22(24(39)36-21-9-2-15(13-35-21)26(32,33)34)37-23(19-8-3-16(27)12-20(19)28)38(14)17-4-6-18(7-5-17)42-43(40,41)11-10-25(29,30)31/h2-9,12-13H,10-11H2,1H3,(H,35,36,39) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from recombinant human CB1 receptor expressed in beta-galactosidase expressing CHOK1 cell membranes after 60 mins by scin... |
J Med Chem 60: 9545-9564 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00861
BindingDB Entry DOI: 10.7270/Q2X92DQ7 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50243626
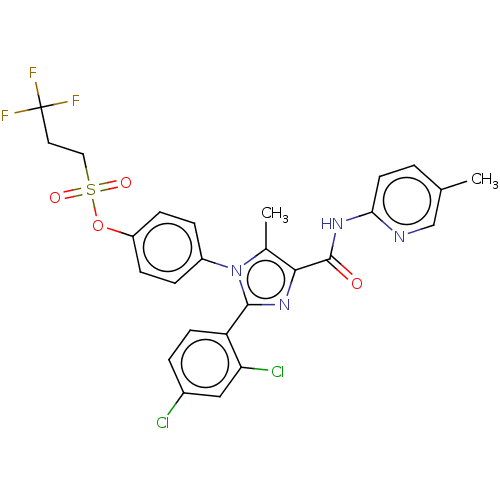
(CHEMBL4089821)Show SMILES Cc1c(nc(-c2ccc(Cl)cc2Cl)n1-c1ccc(OS(=O)(=O)CCC(F)(F)F)cc1)C(=O)Nc1ccc(C)cn1 Show InChI InChI=1S/C26H21Cl2F3N4O4S/c1-15-3-10-22(32-14-15)33-25(36)23-16(2)35(24(34-23)20-9-4-17(27)13-21(20)28)18-5-7-19(8-6-18)39-40(37,38)12-11-26(29,30)31/h3-10,13-14H,11-12H2,1-2H3,(H,32,33,36) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from recombinant human CB1 receptor expressed in beta-galactosidase expressing CHOK1 cell membranes after 60 mins by scin... |
J Med Chem 60: 9545-9564 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00861
BindingDB Entry DOI: 10.7270/Q2X92DQ7 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50243588
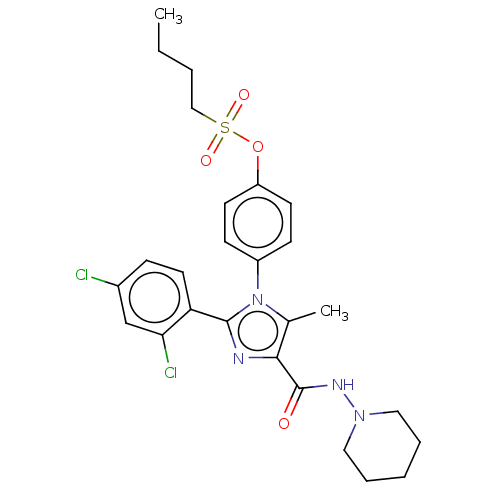
(CHEMBL4067800)Show SMILES CCCCS(=O)(=O)Oc1ccc(cc1)-n1c(C)c(nc1-c1ccc(Cl)cc1Cl)C(=O)NN1CCCCC1 Show InChI InChI=1S/C26H30Cl2N4O4S/c1-3-4-16-37(34,35)36-21-11-9-20(10-12-21)32-18(2)24(26(33)30-31-14-6-5-7-15-31)29-25(32)22-13-8-19(27)17-23(22)28/h8-13,17H,3-7,14-16H2,1-2H3,(H,30,33) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from recombinant human CB1 receptor expressed in beta-galactosidase expressing CHOK1 cell membranes after 60 mins by scin... |
J Med Chem 60: 9545-9564 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00861
BindingDB Entry DOI: 10.7270/Q2X92DQ7 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50243645

(CHEMBL4087520)Show SMILES Cc1c(nc(-c2ccc(Cl)cc2Cl)n1-c1ccc(OS(=O)(=O)CCC(F)(F)F)cc1)C(=O)NN1CCCCC1 Show InChI InChI=1S/C25H25Cl2F3N4O4S/c1-16-22(24(35)32-33-12-3-2-4-13-33)31-23(20-10-5-17(26)15-21(20)27)34(16)18-6-8-19(9-7-18)38-39(36,37)14-11-25(28,29)30/h5-10,15H,2-4,11-14H2,1H3,(H,32,35) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from recombinant human CB1 receptor expressed in beta-galactosidase expressing CHOK1 cell membranes after 60 mins by scin... |
J Med Chem 60: 9545-9564 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00861
BindingDB Entry DOI: 10.7270/Q2X92DQ7 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50243627

(CHEMBL4068708)Show SMILES Cc1c(nc(-c2ccc(Cl)cc2Cl)n1-c1ccc(OS(=O)(=O)CCC(F)(F)F)cc1)C(=O)Nc1ccc(cn1)C(F)(F)F Show InChI InChI=1S/C26H18Cl2F6N4O4S/c1-14-22(24(39)36-21-9-2-15(13-35-21)26(32,33)34)37-23(19-8-3-16(27)12-20(19)28)38(14)17-4-6-18(7-5-17)42-43(40,41)11-10-25(29,30)31/h2-9,12-13H,10-11H2,1H3,(H,35,36,39) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from recombinant human CB1 receptor expressed in beta-galactosidase expressing CHOK1 cell membranes after 60 mins by scin... |
J Med Chem 60: 9545-9564 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00861
BindingDB Entry DOI: 10.7270/Q2X92DQ7 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50243588

(CHEMBL4067800)Show SMILES CCCCS(=O)(=O)Oc1ccc(cc1)-n1c(C)c(nc1-c1ccc(Cl)cc1Cl)C(=O)NN1CCCCC1 Show InChI InChI=1S/C26H30Cl2N4O4S/c1-3-4-16-37(34,35)36-21-11-9-20(10-12-21)32-18(2)24(26(33)30-31-14-6-5-7-15-31)29-25(32)22-13-8-19(27)17-23(22)28/h8-13,17H,3-7,14-16H2,1-2H3,(H,30,33) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from recombinant human CB1 receptor expressed in beta-galactosidase expressing CHOK1 cell membranes after 60 mins by scin... |
J Med Chem 60: 9545-9564 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00861
BindingDB Entry DOI: 10.7270/Q2X92DQ7 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50243626

(CHEMBL4089821)Show SMILES Cc1c(nc(-c2ccc(Cl)cc2Cl)n1-c1ccc(OS(=O)(=O)CCC(F)(F)F)cc1)C(=O)Nc1ccc(C)cn1 Show InChI InChI=1S/C26H21Cl2F3N4O4S/c1-15-3-10-22(32-14-15)33-25(36)23-16(2)35(24(34-23)20-9-4-17(27)13-21(20)28)18-5-7-19(8-6-18)39-40(37,38)12-11-26(29,30)31/h3-10,13-14H,11-12H2,1-2H3,(H,32,33,36) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from recombinant human CB1 receptor expressed in beta-galactosidase expressing CHOK1 cell membranes after 60 mins by scin... |
J Med Chem 60: 9545-9564 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00861
BindingDB Entry DOI: 10.7270/Q2X92DQ7 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50374873

(CHEMBL442457)Show SMILES OC(=O)CN(C1CCCN(C1=O)c1ccc(cc1)-n1ccccc1=O)S(=O)(=O)c1cc2ccc(Cl)nc2s1 |w:5.4| Show InChI InChI=1S/C25H21ClN4O6S2/c26-20-11-6-16-14-23(37-24(16)27-20)38(35,36)30(15-22(32)33)19-4-3-13-29(25(19)34)18-9-7-17(8-10-18)28-12-2-1-5-21(28)31/h1-2,5-12,14,19H,3-4,13,15H2,(H,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 2428-33 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.054
BindingDB Entry DOI: 10.7270/Q2RX9CZ8 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50095043
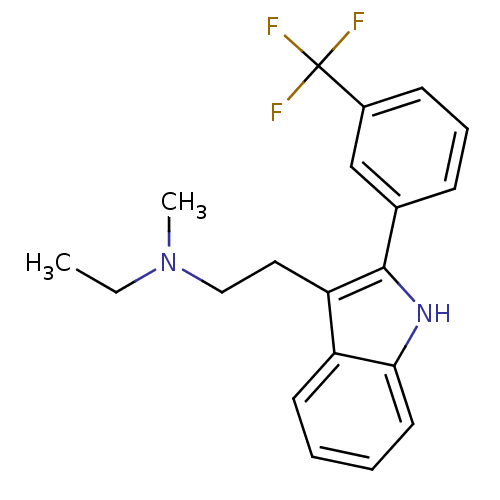
(CHEMBL90911 | Ethyl-methyl-{2-[2-(3-trifluoromethy...)Show SMILES CCN(C)CCc1c([nH]c2ccccc12)-c1cccc(c1)C(F)(F)F Show InChI InChI=1S/C20H21F3N2/c1-3-25(2)12-11-17-16-9-4-5-10-18(16)24-19(17)14-7-6-8-15(13-14)20(21,22)23/h4-10,13,24H,3,11-12H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories
Curated by ChEMBL
| Assay Description
Displacent of [H]-ketanserin from CHO cells expressing human 5-hydroxytryptamine 2A receptor. |
Bioorg Med Chem Lett 10: 2697-9 (2000)
BindingDB Entry DOI: 10.7270/Q27943XR |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50095035
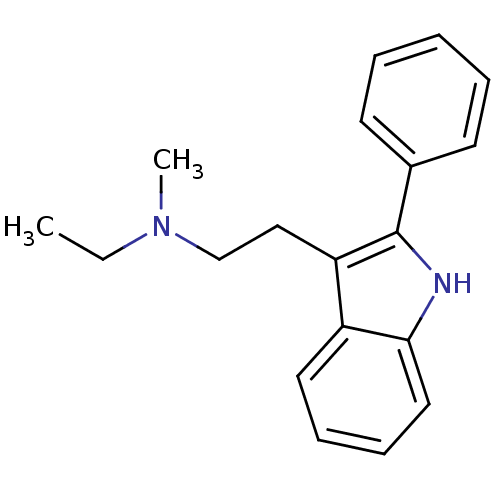
(CHEMBL91549 | Ethyl-methyl-[2-(2-phenyl-1H-indol-3...)Show InChI InChI=1S/C19H22N2/c1-3-21(2)14-13-17-16-11-7-8-12-18(16)20-19(17)15-9-5-4-6-10-15/h4-12,20H,3,13-14H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories
Curated by ChEMBL
| Assay Description
Displacent of [H]-ketanserin from CHO cells expressing human 5-hydroxytryptamine 2A receptor. |
Bioorg Med Chem Lett 10: 2697-9 (2000)
BindingDB Entry DOI: 10.7270/Q27943XR |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50095038
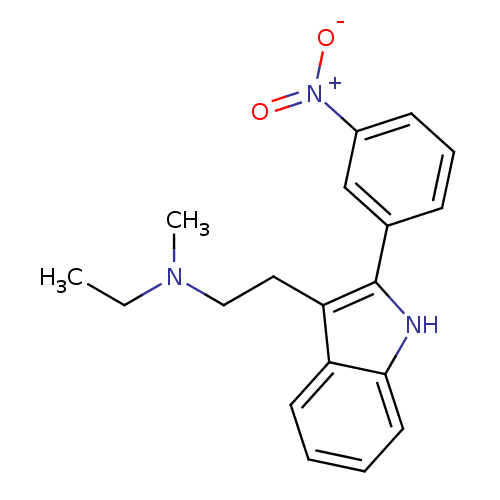
(CHEMBL90837 | Ethyl-methyl-{2-[2-(3-nitro-phenyl)-...)Show SMILES CCN(C)CCc1c([nH]c2ccccc12)-c1cccc(c1)[N+]([O-])=O Show InChI InChI=1S/C19H21N3O2/c1-3-21(2)12-11-17-16-9-4-5-10-18(16)20-19(17)14-7-6-8-15(13-14)22(23)24/h4-10,13,20H,3,11-12H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories
Curated by ChEMBL
| Assay Description
Displacent of [H]-ketanserin from CHO cells expressing human 5-hydroxytryptamine 2A receptor. |
Bioorg Med Chem Lett 10: 2697-9 (2000)
BindingDB Entry DOI: 10.7270/Q27943XR |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM16320
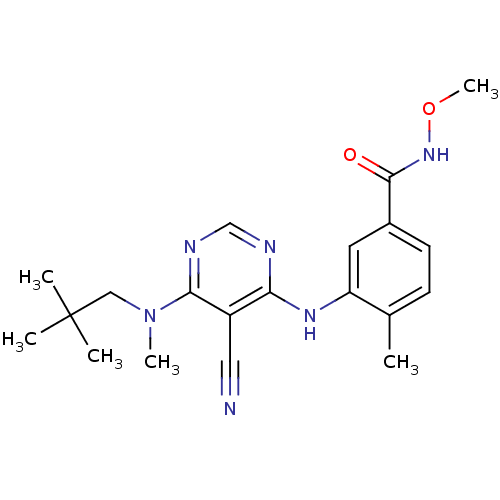
(3-({5-cyano-6-[(2,2-dimethylpropyl)(methyl)amino]p...)Show SMILES CONC(=O)c1ccc(C)c(Nc2ncnc(N(C)CC(C)(C)C)c2C#N)c1 Show InChI InChI=1S/C20H26N6O2/c1-13-7-8-14(19(27)25-28-6)9-16(13)24-17-15(10-21)18(23-12-22-17)26(5)11-20(2,3)4/h7-9,12H,11H2,1-6H3,(H,25,27)(H,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.150 | -55.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Company
| Assay Description
The kinase activity was determined by quantitation of the amount of radioactive phosphate transferred to myelin basic protein (MBP) with or without i... |
J Med Chem 48: 6261-70 (2005)
Article DOI: 10.1021/jm0503594
BindingDB Entry DOI: 10.7270/Q25X276T |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50087533
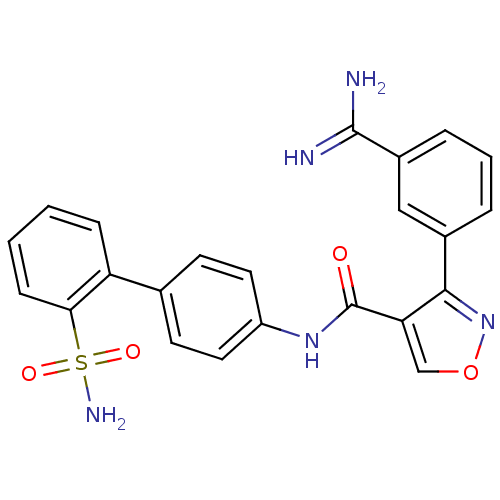
(3-(3-Carbamimidoyl-phenyl)-isoxazole-4-carboxylic ...)Show SMILES NC(=N)c1cccc(c1)-c1nocc1C(=O)Nc1ccc(cc1)-c1ccccc1S(N)(=O)=O Show InChI InChI=1S/C23H19N5O4S/c24-22(25)16-5-3-4-15(12-16)21-19(13-32-28-21)23(29)27-17-10-8-14(9-11-17)18-6-1-2-7-20(18)33(26,30)31/h1-13H,(H3,24,25)(H,27,29)(H2,26,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
Binding affinity towards human Serine protease FXa |
Bioorg Med Chem Lett 11: 641-5 (2001)
BindingDB Entry DOI: 10.7270/Q2RV0MZ2 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50096095
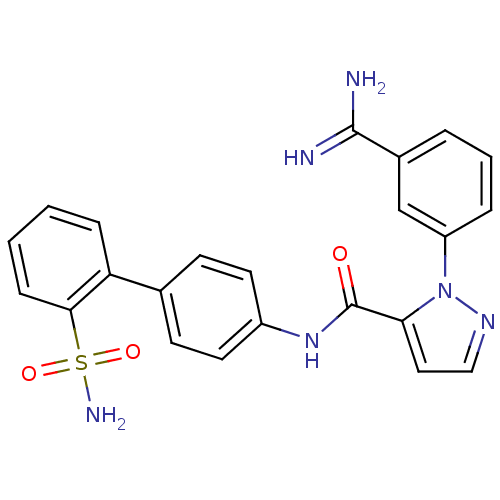
(2-(3-Carbamimidoyl-phenyl)-2H-pyrazole-3-carboxyli...)Show SMILES NC(=N)c1cccc(c1)-n1nccc1C(=O)Nc1ccc(cc1)-c1ccccc1S(N)(=O)=O Show InChI InChI=1S/C23H20N6O3S/c24-22(25)16-4-3-5-18(14-16)29-20(12-13-27-29)23(30)28-17-10-8-15(9-11-17)19-6-1-2-7-21(19)33(26,31)32/h1-14H,(H3,24,25)(H,28,30)(H2,26,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
Binding affinity towards human Serine protease FXa |
Bioorg Med Chem Lett 11: 641-5 (2001)
BindingDB Entry DOI: 10.7270/Q2RV0MZ2 |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50212148
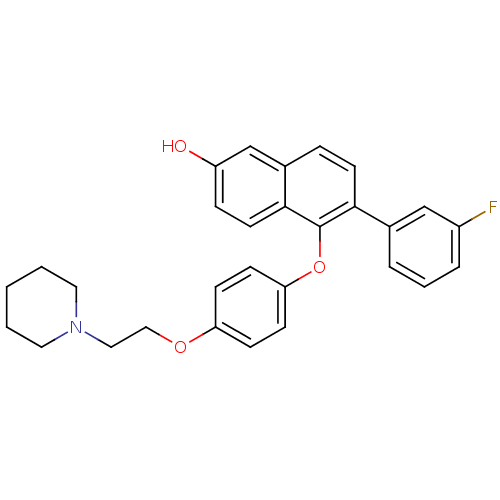
(6-(3-fluorophenyl)-5-(4-(2-(piperidin-1-yl)ethoxy)...)Show SMILES Oc1ccc2c(Oc3ccc(OCCN4CCCCC4)cc3)c(ccc2c1)-c1cccc(F)c1 Show InChI InChI=1S/C29H28FNO3/c30-23-6-4-5-21(19-23)27-13-7-22-20-24(32)8-14-28(22)29(27)34-26-11-9-25(10-12-26)33-18-17-31-15-2-1-3-16-31/h4-14,19-20,32H,1-3,15-18H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]estradiol from human recombinant ERbeta |
Bioorg Med Chem Lett 17: 3544-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.04.044
BindingDB Entry DOI: 10.7270/Q2ZS2W6F |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM16330
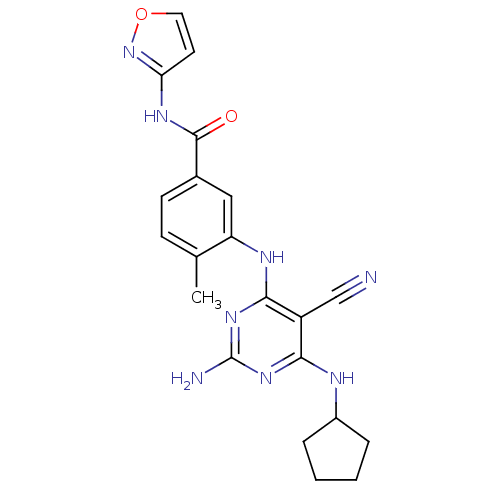
(3-{[2-amino-5-cyano-6-(cyclopentylamino)pyrimidin-...)Show SMILES Cc1ccc(cc1Nc1nc(N)nc(NC2CCCC2)c1C#N)C(=O)Nc1ccon1 Show InChI InChI=1S/C21H22N8O2/c1-12-6-7-13(20(30)26-17-8-9-31-29-17)10-16(12)25-19-15(11-22)18(27-21(23)28-19)24-14-4-2-3-5-14/h6-10,14H,2-5H2,1H3,(H,26,29,30)(H4,23,24,25,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.160 | -55.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Company
| Assay Description
The kinase activity was determined by quantitation of the amount of radioactive phosphate transferred to myelin basic protein (MBP) with or without i... |
J Med Chem 48: 6261-70 (2005)
Article DOI: 10.1021/jm0503594
BindingDB Entry DOI: 10.7270/Q25X276T |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data