

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dihydrofolate reductase (Escherichia coli) | BDBM18050 (2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...) | MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.00100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Inhibition of recombinant Escherichia coli DHFR | ACS Med Chem Lett 7: 692-6 (2016) Article DOI: 10.1021/acsmedchemlett.6b00120 BindingDB Entry DOI: 10.7270/Q2DJ5HKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM18050 (2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Inhibition of recombinant human DHFR assessed as reduction in NADPH oxidation using dihydrofolate as substrate by fluorescence spectrophotometric ana... | ACS Med Chem Lett 7: 692-6 (2016) Article DOI: 10.1021/acsmedchemlett.6b00120 BindingDB Entry DOI: 10.7270/Q2DJ5HKK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Enteropeptidase (Homo sapiens (Human)) | BDBM571836 ((Z)-3-((6-((4-Carbamimidoyl-2-fluorophenoxy)carbon...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of the enteropeptidase inhibitor synthesized using the purified Recombinant Human Enteropeptidase and the substrate Acetyl-As... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WD43TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Escherichia coli) | BDBM18069 (5-[(3,4,5-trimethoxyphenyl)methyl]pyrimidine-2,4-d...) | MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Inhibition of recombinant Escherichia coli DHFR expressed in Escherichia coli BL21(DE3) cells assessed as reduction in NADPH oxidation using dihydrof... | ACS Med Chem Lett 7: 692-6 (2016) Article DOI: 10.1021/acsmedchemlett.6b00120 BindingDB Entry DOI: 10.7270/Q2DJ5HKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enteropeptidase (Homo sapiens (Human)) | BDBM571793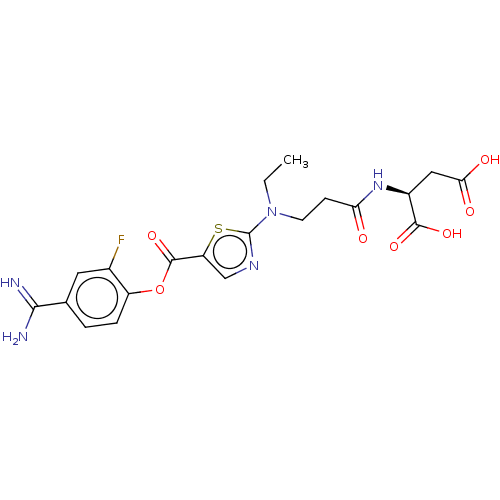 ((3-((5-((4-Carbamimidoyl-2-fluorophenoxy)carbonyl)...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of the enteropeptidase inhibitor synthesized using the purified Recombinant Human Enteropeptidase and the substrate Acetyl-As... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WD43TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enteropeptidase (Homo sapiens (Human)) | BDBM571830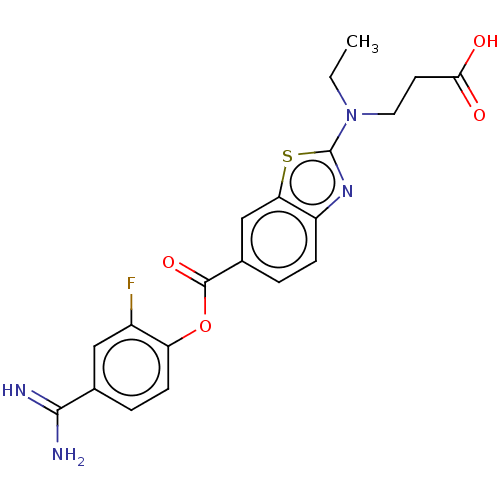 (3-((6-((4-Carbamimidoyl-2-fluorophenoxy)carbonyl)b...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of the enteropeptidase inhibitor synthesized using the purified Recombinant Human Enteropeptidase and the substrate Acetyl-As... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WD43TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enteropeptidase (Homo sapiens (Human)) | BDBM571817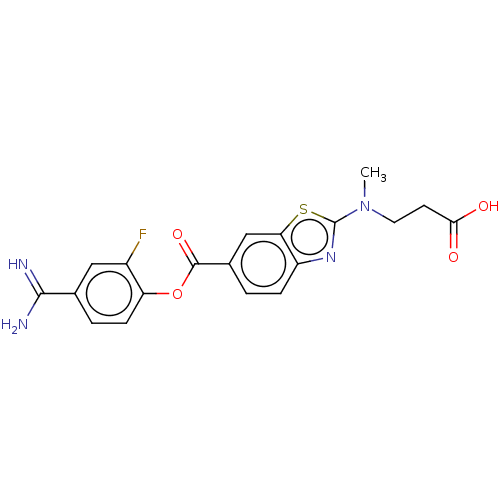 (3-((6-((4-Carbamimidoyl-2-fluorophenoxy)carbonyl)b...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of the enteropeptidase inhibitor synthesized using the purified Recombinant Human Enteropeptidase and the substrate Acetyl-As... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WD43TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enteropeptidase (Homo sapiens (Human)) | BDBM571815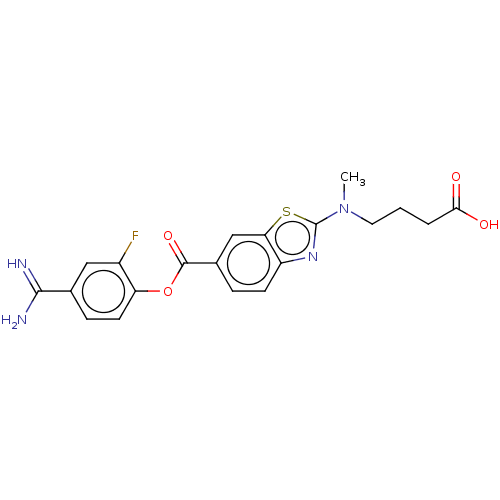 (4-((6-((4-Carbamimidoyl-2-fluorophenoxy)carbonyl)b...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of the enteropeptidase inhibitor synthesized using the purified Recombinant Human Enteropeptidase and the substrate Acetyl-As... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WD43TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enteropeptidase (Homo sapiens (Human)) | BDBM571802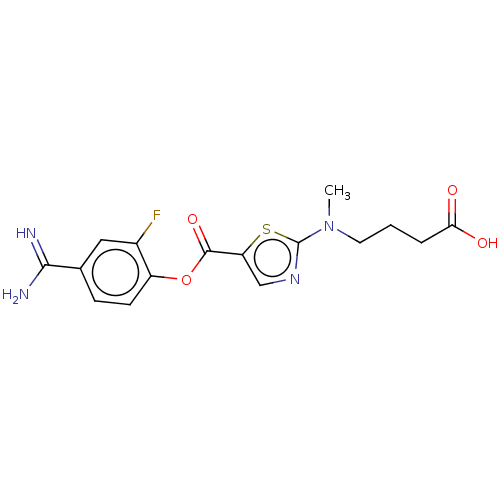 (4-((5-((4-Carbamimidoyl-2-fluorophenoxy)carbonyl)t...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of the enteropeptidase inhibitor synthesized using the purified Recombinant Human Enteropeptidase and the substrate Acetyl-As... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WD43TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enteropeptidase (Homo sapiens (Human)) | BDBM571797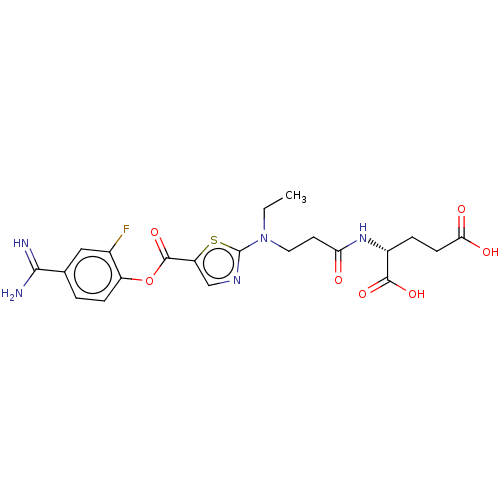 ((3-((5-((4-Carbamimidoyl-2-fluorophenoxy)carbonyl)...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of the enteropeptidase inhibitor synthesized using the purified Recombinant Human Enteropeptidase and the substrate Acetyl-As... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WD43TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enteropeptidase (Homo sapiens (Human)) | BDBM571814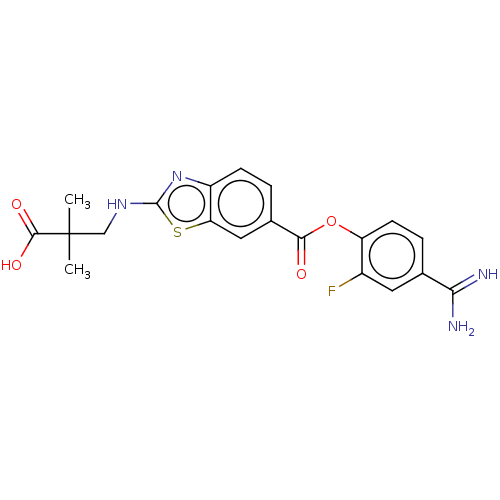 (3-((6-((4-Carbamimidoyl-2-fluorophenoxy)carbonyl)b...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of the enteropeptidase inhibitor synthesized using the purified Recombinant Human Enteropeptidase and the substrate Acetyl-As... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WD43TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enteropeptidase (Homo sapiens (Human)) | BDBM571772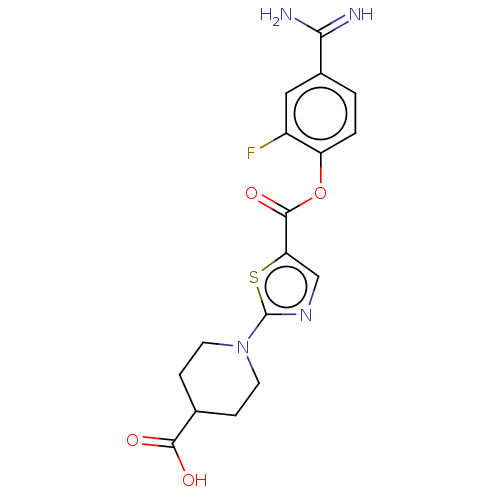 (1-(5-((4-Carbamimidoyl-2-fluorophenoxy)carbonyl)th...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of the enteropeptidase inhibitor synthesized using the purified Recombinant Human Enteropeptidase and the substrate Acetyl-As... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WD43TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enteropeptidase (Homo sapiens (Human)) | BDBM571760 (3-((5-((4-carbamimidoyl-2-fluorophenoxy)carbonyl)t...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of the enteropeptidase inhibitor synthesized using the purified Recombinant Human Enteropeptidase and the substrate Acetyl-As... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WD43TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enteropeptidase (Homo sapiens (Human)) | BDBM571843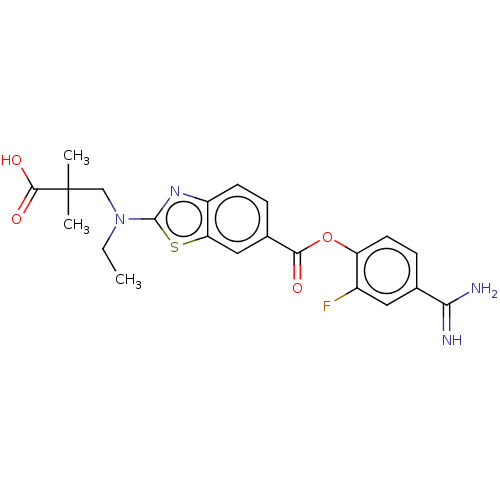 (3-((6-((4-Carbamimidoyl-2-fluorophenoxy)carbonyl)b...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of the enteropeptidase inhibitor synthesized using the purified Recombinant Human Enteropeptidase and the substrate Acetyl-As... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WD43TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enteropeptidase (Homo sapiens (Human)) | BDBM571841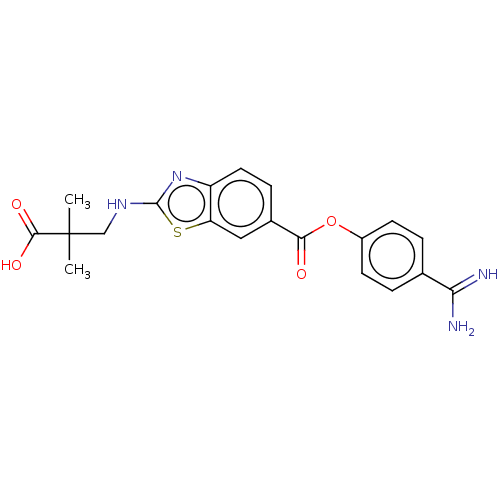 (3-((6-((4-Carbamimidoylphenoxy)carbonyl)benzo[d]th...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.890 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of the enteropeptidase inhibitor synthesized using the purified Recombinant Human Enteropeptidase and the substrate Acetyl-As... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WD43TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Escherichia coli) | BDBM210930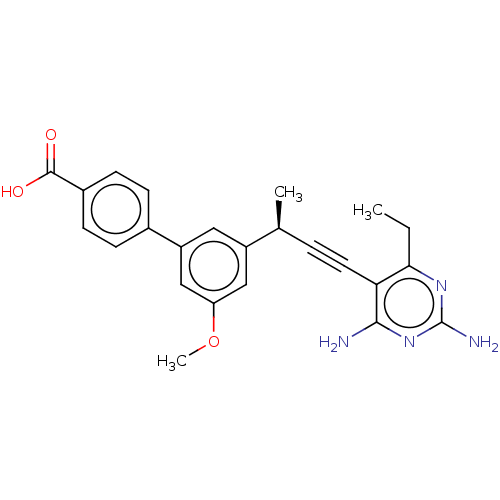 (UCP1173) | MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 0.910 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Inhibition of recombinant Escherichia coli DHFR expressed in Escherichia coli BL21(DE3) cells assessed as reduction in NADPH oxidation using dihydrof... | ACS Med Chem Lett 7: 692-6 (2016) Article DOI: 10.1021/acsmedchemlett.6b00120 BindingDB Entry DOI: 10.7270/Q2DJ5HKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enteropeptidase (Homo sapiens (Human)) | BDBM571766 (4-Carbamimidoyl-2-fluorophenyl 2-(4-(methoxycarbon...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.920 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of the enteropeptidase inhibitor synthesized using the purified Recombinant Human Enteropeptidase and the substrate Acetyl-As... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WD43TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Escherichia coli) | BDBM50190621 (CHEMBL3827532 | US10870625, Compound 15) | MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 0.980 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Inhibition of recombinant Escherichia coli DHFR expressed in Escherichia coli BL21(DE3) cells assessed as reduction in NADPH oxidation using dihydrof... | ACS Med Chem Lett 7: 692-6 (2016) Article DOI: 10.1021/acsmedchemlett.6b00120 BindingDB Entry DOI: 10.7270/Q2DJ5HKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50264380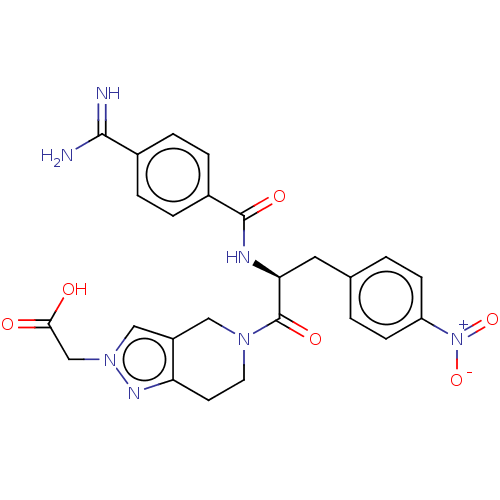 (CHEMBL4078717) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Strathclyde Curated by ChEMBL | Assay Description Displacement of [3H]UR-3189 from integrin alpha2b beta3 receptor in resting human platelet | J Med Chem 60: 3241-3251 (2017) Article DOI: 10.1021/acs.jmedchem.6b01711 BindingDB Entry DOI: 10.7270/Q29W0HZ4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Enteropeptidase (Homo sapiens (Human)) | BDBM571783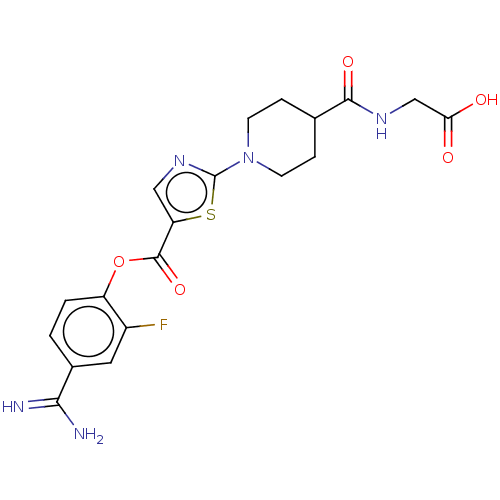 ((1-(5-((4-Carbamimidoyl-2-fluorophenoxy)carbonyl)t...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of the enteropeptidase inhibitor synthesized using the purified Recombinant Human Enteropeptidase and the substrate Acetyl-As... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WD43TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM18050 (2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus DHFR | ACS Med Chem Lett 7: 692-6 (2016) Article DOI: 10.1021/acsmedchemlett.6b00120 BindingDB Entry DOI: 10.7270/Q2DJ5HKK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Enteropeptidase (Homo sapiens (Human)) | BDBM571839 (4-Carbamimidoyl-2-fluorophenyl 2-((4-methoxy-4-oxo...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of the enteropeptidase inhibitor synthesized using the purified Recombinant Human Enteropeptidase and the substrate Acetyl-As... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WD43TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor/Kappa-type opioid receptor/Mu-type opioid receptor/Sigma non-opioid intracellular receptor 1 (Rattus norvegicus (rat)-RAT) | BDBM50223637 (Alphacemethadone | Alphacetylmethadol) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents | PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [3H]- naloxone binding to Opioid receptors in rat brain membrane in the absence of Na | J Med Chem 24: 903-6 (1981) BindingDB Entry DOI: 10.7270/Q26W9D8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM210930 (UCP1173) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Inhibition of recombinant Staphylococcus aureus DHFR expressed in Escherichia coli BL21(DE3) cells assessed as reduction in NADPH oxidation using dih... | ACS Med Chem Lett 7: 692-6 (2016) Article DOI: 10.1021/acsmedchemlett.6b00120 BindingDB Entry DOI: 10.7270/Q2DJ5HKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enteropeptidase (Homo sapiens (Human)) | BDBM571840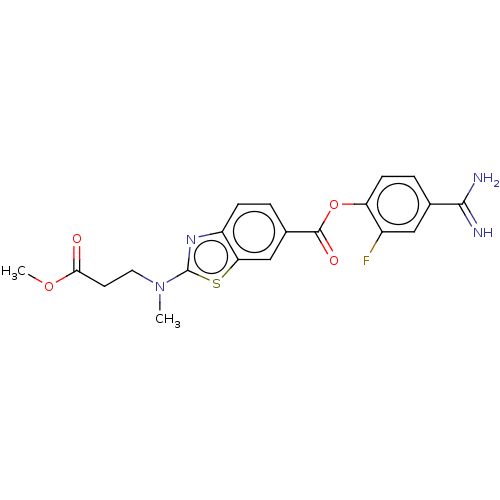 (4-Carbamimidoyl-2-fluorophenyl 2-((3-methoxy-3-oxo...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of the enteropeptidase inhibitor synthesized using the purified Recombinant Human Enteropeptidase and the substrate Acetyl-As... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WD43TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enteropeptidase (Homo sapiens (Human)) | BDBM571789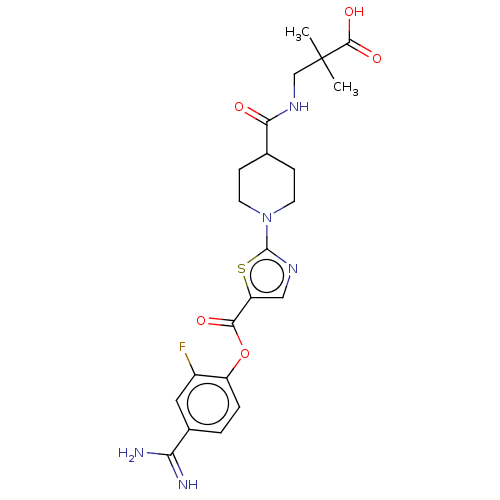 (3-(1-(5-((4-Carbamimidoyl-2-fluorophenoxy)carbonyl...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of the enteropeptidase inhibitor synthesized using the purified Recombinant Human Enteropeptidase and the substrate Acetyl-As... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WD43TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enteropeptidase (Homo sapiens (Human)) | BDBM571787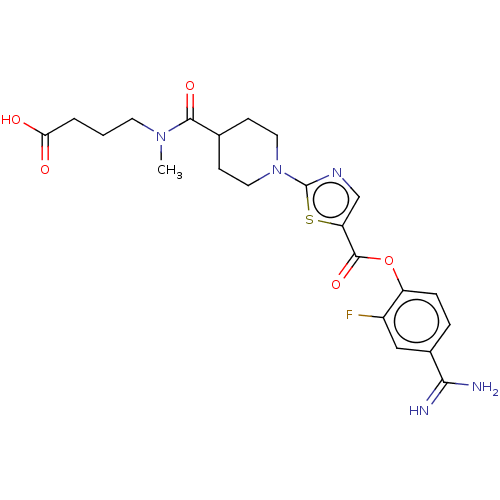 (4-(1-(5-((4-carbamimidoyl-2-fluorophenoxy)carbonyl...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of the enteropeptidase inhibitor synthesized using the purified Recombinant Human Enteropeptidase and the substrate Acetyl-As... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WD43TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM210931 (UCP1175) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Inhibition of recombinant Staphylococcus aureus DHFR expressed in Escherichia coli BL21(DE3) cells assessed as reduction in NADPH oxidation using dih... | ACS Med Chem Lett 7: 692-6 (2016) Article DOI: 10.1021/acsmedchemlett.6b00120 BindingDB Entry DOI: 10.7270/Q2DJ5HKK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Enteropeptidase (Homo sapiens (Human)) | BDBM571785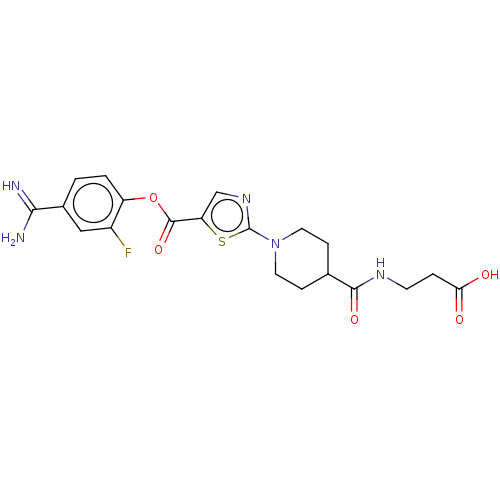 (3-(1-(5-((4-Carbamimidoyl-2-fluorophenoxy)carbonyl...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of the enteropeptidase inhibitor synthesized using the purified Recombinant Human Enteropeptidase and the substrate Acetyl-As... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WD43TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enteropeptidase (Homo sapiens (Human)) | BDBM571781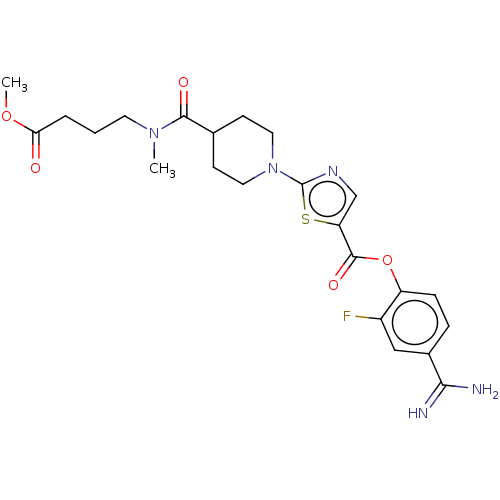 (4-Carbamimidoyl-2-fluorophenyl 2-(4-((4-methoxy-4-...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of the enteropeptidase inhibitor synthesized using the purified Recombinant Human Enteropeptidase and the substrate Acetyl-As... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WD43TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Escherichia coli) | BDBM210929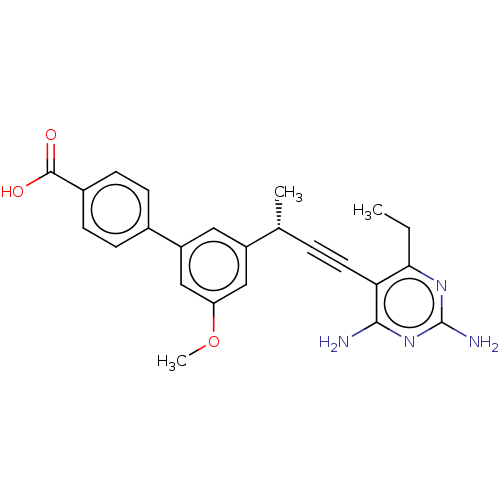 (UCP1172) | MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Inhibition of recombinant Escherichia coli DHFR expressed in Escherichia coli BL21(DE3) cells assessed as reduction in NADPH oxidation using dihydrof... | ACS Med Chem Lett 7: 692-6 (2016) Article DOI: 10.1021/acsmedchemlett.6b00120 BindingDB Entry DOI: 10.7270/Q2DJ5HKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enteropeptidase (Homo sapiens (Human)) | BDBM571773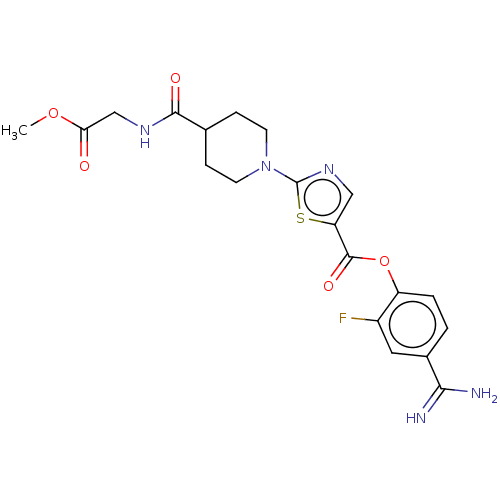 (4-Carbamimidoyl-2-fluorophenyl 2-(4-((2-methoxy-2-...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of the enteropeptidase inhibitor synthesized using the purified Recombinant Human Enteropeptidase and the substrate Acetyl-As... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WD43TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Escherichia coli) | BDBM50190622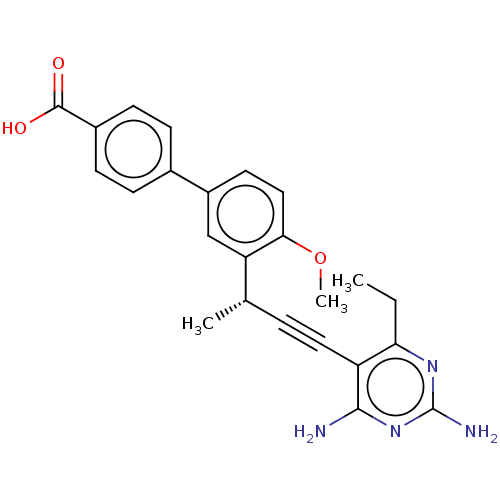 (CHEMBL3827326) | MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Inhibition of recombinant Escherichia coli DHFR expressed in Escherichia coli BL21(DE3) cells assessed as reduction in NADPH oxidation using dihydrof... | ACS Med Chem Lett 7: 692-6 (2016) Article DOI: 10.1021/acsmedchemlett.6b00120 BindingDB Entry DOI: 10.7270/Q2DJ5HKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enteropeptidase (Homo sapiens (Human)) | BDBM571824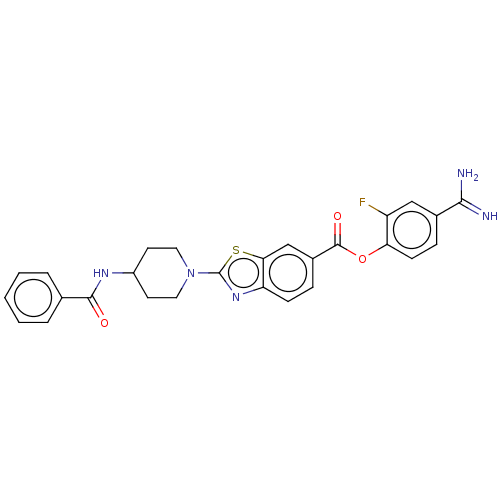 (4-Carbamimidoyl-2-fluorophenyl 2-(4-benzoamidopipe...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of the enteropeptidase inhibitor synthesized using the purified Recombinant Human Enteropeptidase and the substrate Acetyl-As... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WD43TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor/Kappa-type opioid receptor/Mu-type opioid receptor/Sigma non-opioid intracellular receptor 1 (Rattus norvegicus (rat)-RAT) | BDBM50223633 (Levomethadone) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [3H]- naloxone binding to Opioid receptors in rat brain membrane in the absence of Na | J Med Chem 24: 903-6 (1981) BindingDB Entry DOI: 10.7270/Q26W9D8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Escherichia coli) | BDBM50190619 (CHEMBL3827086 | US10870625, Compound 14) | MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Inhibition of recombinant Escherichia coli DHFR expressed in Escherichia coli BL21(DE3) cells assessed as reduction in NADPH oxidation using dihydrof... | ACS Med Chem Lett 7: 692-6 (2016) Article DOI: 10.1021/acsmedchemlett.6b00120 BindingDB Entry DOI: 10.7270/Q2DJ5HKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM210929 (UCP1172) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Inhibition of recombinant Staphylococcus aureus DHFR expressed in Escherichia coli BL21(DE3) cells assessed as reduction in NADPH oxidation using dih... | ACS Med Chem Lett 7: 692-6 (2016) Article DOI: 10.1021/acsmedchemlett.6b00120 BindingDB Entry DOI: 10.7270/Q2DJ5HKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enteropeptidase (Homo sapiens (Human)) | BDBM571828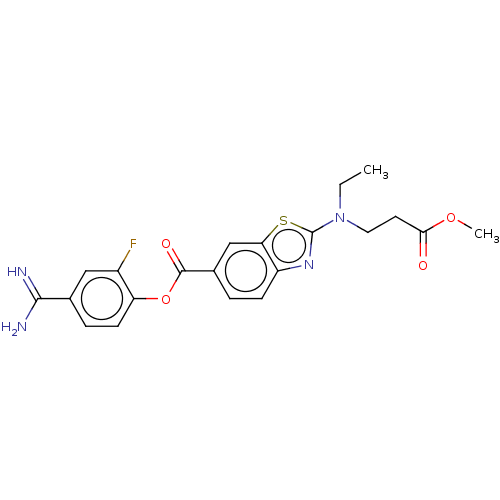 (4-Carbamimidoyl-2-fluorophenyl 2-(ethyl(3-methoxy-...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of the enteropeptidase inhibitor synthesized using the purified Recombinant Human Enteropeptidase and the substrate Acetyl-As... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WD43TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enteropeptidase (Homo sapiens (Human)) | BDBM571806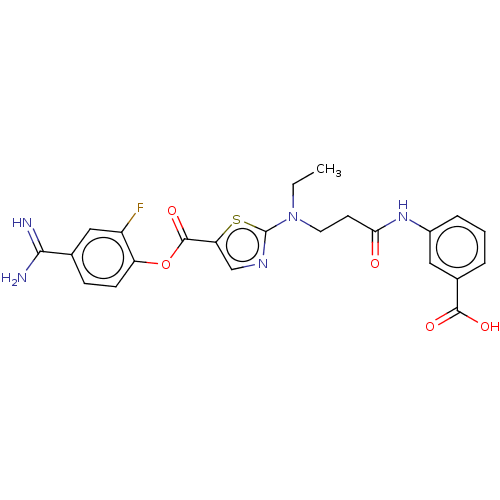 (3-(3-((5-((4-Carbamimidoyl-2-fluorophenoxy)carbony...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of the enteropeptidase inhibitor synthesized using the purified Recombinant Human Enteropeptidase and the substrate Acetyl-As... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WD43TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enteropeptidase (Homo sapiens (Human)) | BDBM571798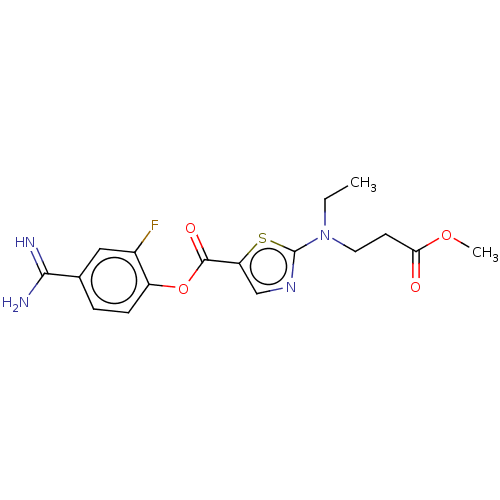 (4-Carbamimidoyl-2-fluorophenyl 2-(ethyl(3-methoxy-...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of the enteropeptidase inhibitor synthesized using the purified Recombinant Human Enteropeptidase and the substrate Acetyl-As... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WD43TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enteropeptidase (Homo sapiens (Human)) | BDBM571777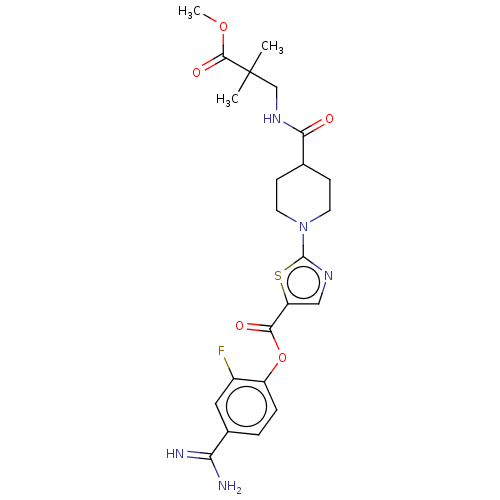 (4-Carbamimidoyl-2-fluorophenyl 2-(4-((3-methoxy-2,...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of the enteropeptidase inhibitor synthesized using the purified Recombinant Human Enteropeptidase and the substrate Acetyl-As... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WD43TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enteropeptidase (Homo sapiens (Human)) | BDBM571775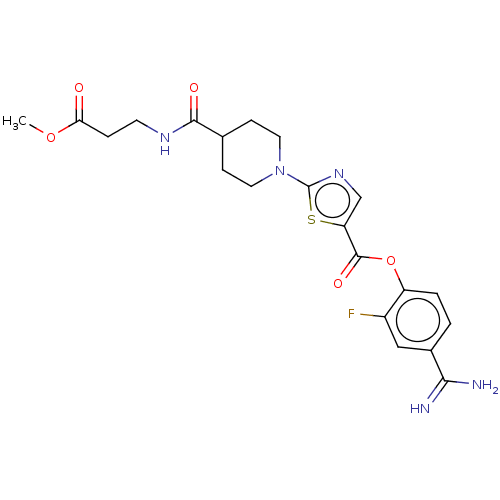 (4-Carbamimidoyl-2-fluorophenyl 2-(4-((3-methoxy-3-...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of the enteropeptidase inhibitor synthesized using the purified Recombinant Human Enteropeptidase and the substrate Acetyl-As... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WD43TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enteropeptidase (Homo sapiens (Human)) | BDBM571800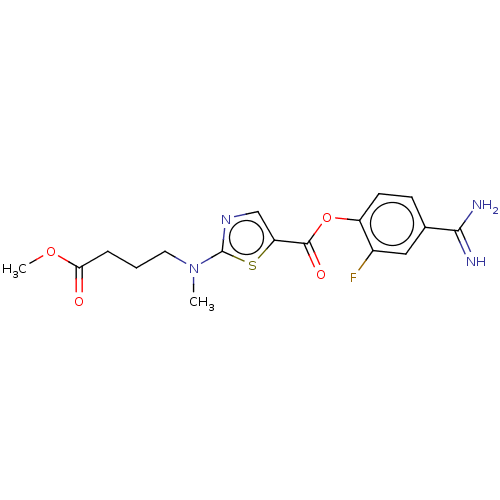 (4-Carbamimidoyl-2-fluorophenyl 2-((4-methoxy-4-oxo...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of the enteropeptidase inhibitor synthesized using the purified Recombinant Human Enteropeptidase and the substrate Acetyl-As... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WD43TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Escherichia coli) | BDBM210928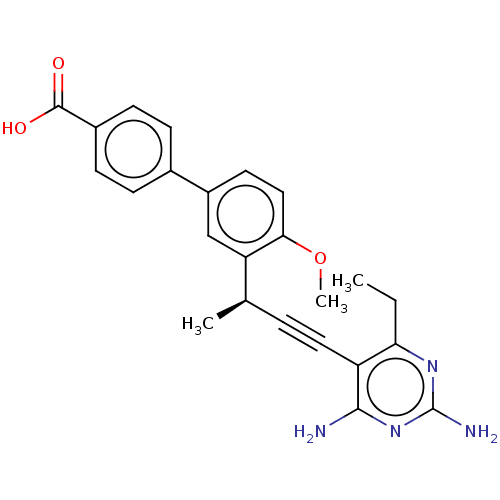 (UCP1164) | MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Inhibition of recombinant Escherichia coli DHFR expressed in Escherichia coli BL21(DE3) cells assessed as reduction in NADPH oxidation using dihydrof... | ACS Med Chem Lett 7: 692-6 (2016) Article DOI: 10.1021/acsmedchemlett.6b00120 BindingDB Entry DOI: 10.7270/Q2DJ5HKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor/Kappa-type opioid receptor/Mu-type opioid receptor/Sigma non-opioid intracellular receptor 1 (Rattus norvegicus (rat)-RAT) | BDBM50223637 (Alphacemethadone | Alphacetylmethadol) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents | PubMed | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of [3H]- naloxone binding to Opioid receptors in rat brain membrane in the presence of Na | J Med Chem 24: 903-6 (1981) BindingDB Entry DOI: 10.7270/Q26W9D8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM18069 (5-[(3,4,5-trimethoxyphenyl)methyl]pyrimidine-2,4-d...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Inhibition of recombinant Staphylococcus aureus DHFR expressed in Escherichia coli BL21(DE3) cells assessed as reduction in NADPH oxidation using dih... | ACS Med Chem Lett 7: 692-6 (2016) Article DOI: 10.1021/acsmedchemlett.6b00120 BindingDB Entry DOI: 10.7270/Q2DJ5HKK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Enteropeptidase (Homo sapiens (Human)) | BDBM571838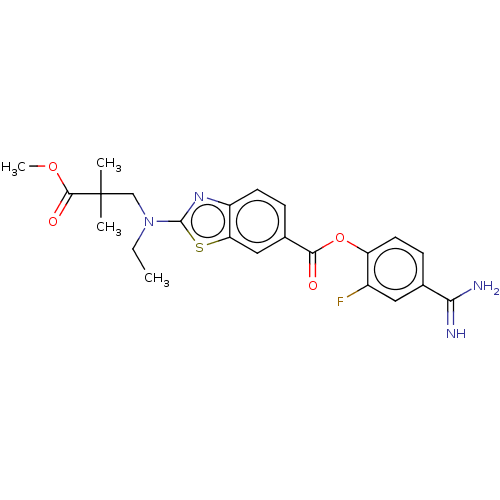 (4-Carbamimidoyl-2-fluorophenyl 2-(ethyl(3-methoxy-...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of the enteropeptidase inhibitor synthesized using the purified Recombinant Human Enteropeptidase and the substrate Acetyl-As... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WD43TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enteropeptidase (Homo sapiens (Human)) | BDBM571834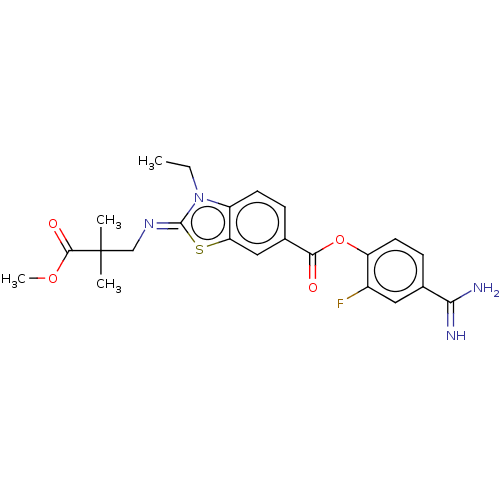 (4-Carbamimidoyl-2-fluorophenyl (Z)-3-ethyl-2-((3-m...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of the enteropeptidase inhibitor synthesized using the purified Recombinant Human Enteropeptidase and the substrate Acetyl-As... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WD43TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enteropeptidase (Homo sapiens (Human)) | BDBM571823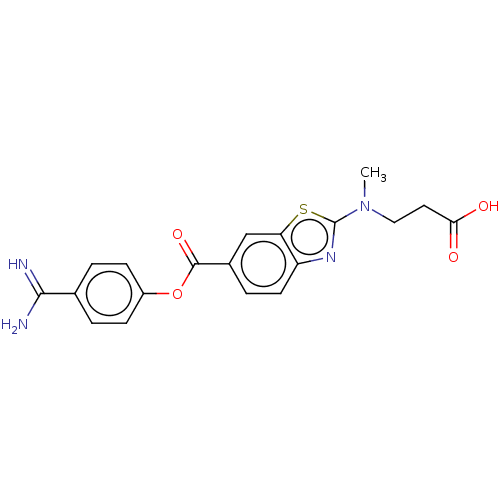 (3-((6-((4-Carbamimidoylphenoxy)carbonyl)benzo[d]th...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of the enteropeptidase inhibitor synthesized using the purified Recombinant Human Enteropeptidase and the substrate Acetyl-As... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WD43TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enteropeptidase (Homo sapiens (Human)) | BDBM571807 ((1-(5-((4-Carbamimidoyl)phenoxy)carbonyl)thiazol-2...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of the enteropeptidase inhibitor synthesized using the purified Recombinant Human Enteropeptidase and the substrate Acetyl-As... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WD43TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 2944 total ) | Next | Last >> |