

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coagulation factor VII/Tissue factor (Homo sapiens (Human)) | BDBM50189939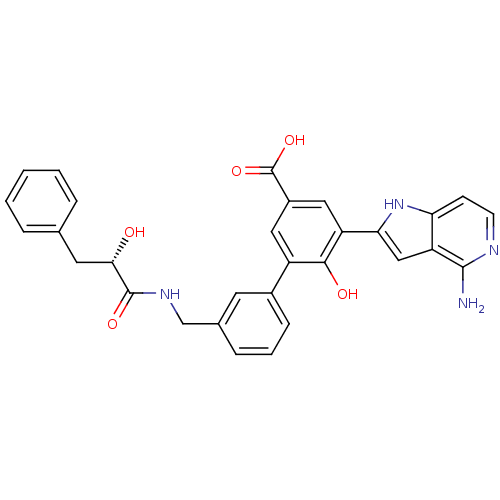 (5-(4-amino-1H-pyrrolo[3,2-c]pyridin-2-yl)-6-hydrox...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics Curated by ChEMBL | Assay Description Binding affinity to f7a/TF complex | Bioorg Med Chem Lett 16: 4567-70 (2006) Article DOI: 10.1016/j.bmcl.2006.06.016 BindingDB Entry DOI: 10.7270/Q2NG4Q67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII/Tissue factor (Homo sapiens (Human)) | BDBM50189938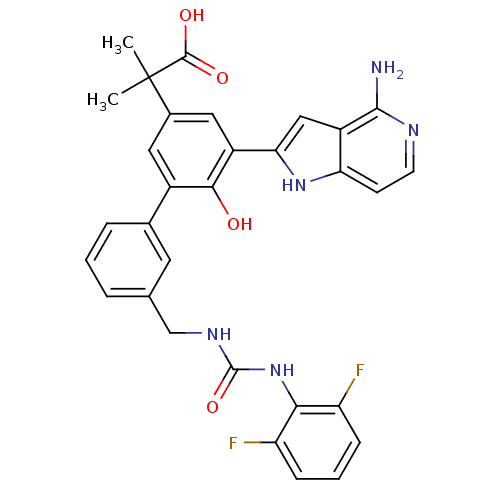 (2-{5-(4-amino-1H-pyrrolo[3,2-c]pyridin-2-yl)-3'-[3...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics Curated by ChEMBL | Assay Description Binding affinity to f7a/TF complex | Bioorg Med Chem Lett 16: 4567-70 (2006) Article DOI: 10.1016/j.bmcl.2006.06.016 BindingDB Entry DOI: 10.7270/Q2NG4Q67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM13778 (2-[3-(5-carbamimidoyl-1H-indol-2-yl)-4-hydroxy-5-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4 | -47.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Celera | Assay Description The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... | Bioorg Med Chem Lett 16: 2270-3 (2006) Article DOI: 10.1016/j.bmcl.2006.01.017 BindingDB Entry DOI: 10.7270/Q2RF5S8X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII/Tissue factor (Homo sapiens (Human)) | BDBM14863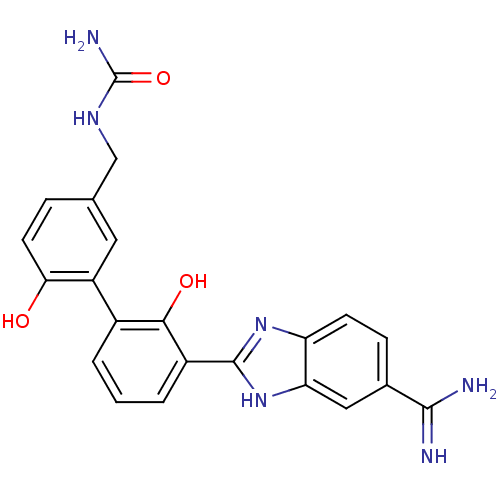 (({3-[3-(5-carbamimidoyl-1H-1,3-benzodiazol-2-yl)-2...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics Curated by ChEMBL | Assay Description Binding affinity to f7a/TF complex | Bioorg Med Chem Lett 16: 4567-70 (2006) Article DOI: 10.1016/j.bmcl.2006.06.016 BindingDB Entry DOI: 10.7270/Q2NG4Q67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM13776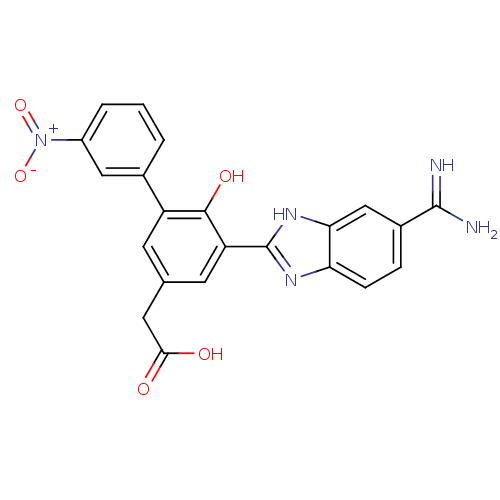 (2-[3-(5-carbamimidoyl-1H-1,3-benzodiazol-2-yl)-4-h...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 15 | -44.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Celera | Assay Description The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... | Bioorg Med Chem Lett 16: 2270-3 (2006) Article DOI: 10.1016/j.bmcl.2006.01.017 BindingDB Entry DOI: 10.7270/Q2RF5S8X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII/Tissue factor (Homo sapiens (Human)) | BDBM50189941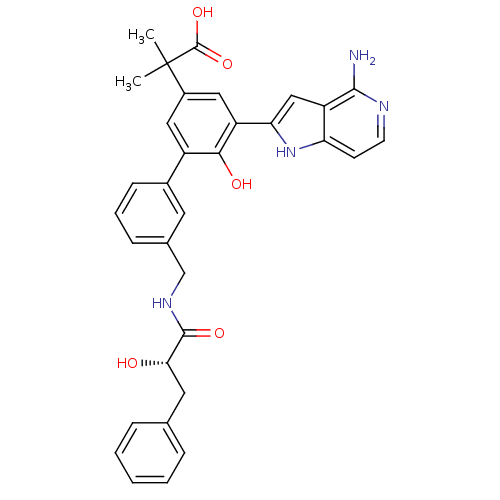 (2-{5-(4-amino-1H-pyrrolo[3,2-c]pyridin-2-yl)-6-hyd...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics Curated by ChEMBL | Assay Description Binding affinity to f7a/TF complex | Bioorg Med Chem Lett 16: 4567-70 (2006) Article DOI: 10.1016/j.bmcl.2006.06.016 BindingDB Entry DOI: 10.7270/Q2NG4Q67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII/Tissue factor (Homo sapiens (Human)) | BDBM50189942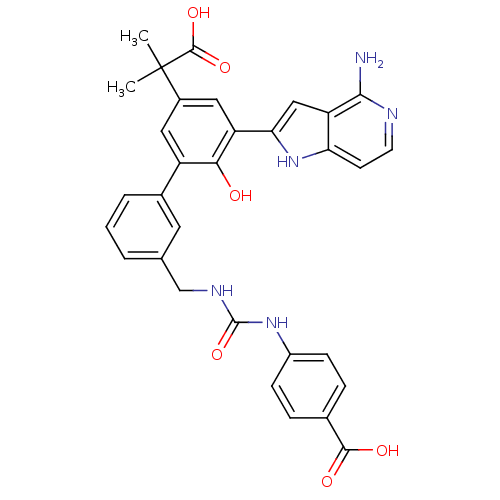 (4-{3-[3'-(4-amino-1H-pyrrolo[3,2-c]pyridin-2-yl)-5...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics Curated by ChEMBL | Assay Description Binding affinity to f7a/TF complex | Bioorg Med Chem Lett 16: 4567-70 (2006) Article DOI: 10.1016/j.bmcl.2006.06.016 BindingDB Entry DOI: 10.7270/Q2NG4Q67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM13786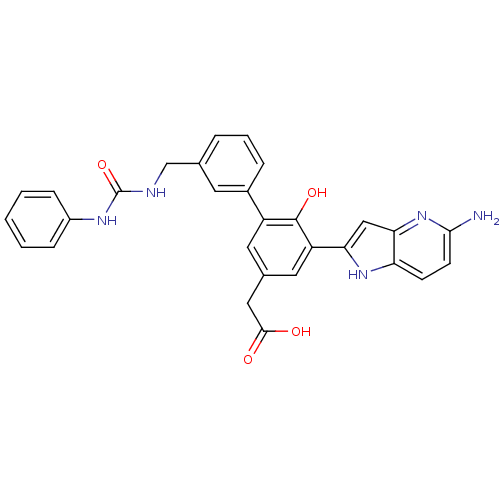 (2-(3-{5-amino-1H-pyrrolo[3,2-b]pyridin-2-yl}-4-hyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 20 | -43.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Celera | Assay Description The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... | Bioorg Med Chem Lett 16: 2270-3 (2006) Article DOI: 10.1016/j.bmcl.2006.01.017 BindingDB Entry DOI: 10.7270/Q2RF5S8X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13778 (2-[3-(5-carbamimidoyl-1H-indol-2-yl)-4-hydroxy-5-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 50 | -41.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Celera | Assay Description The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... | Bioorg Med Chem Lett 16: 2270-3 (2006) Article DOI: 10.1016/j.bmcl.2006.01.017 BindingDB Entry DOI: 10.7270/Q2RF5S8X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM13787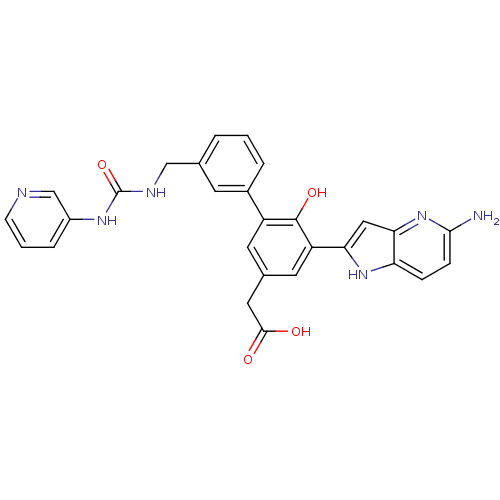 (2-(3-{5-amino-1H-pyrrolo[3,2-b]pyridin-2-yl}-4-hyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 62 | -40.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Celera | Assay Description The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... | Bioorg Med Chem Lett 16: 2270-3 (2006) Article DOI: 10.1016/j.bmcl.2006.01.017 BindingDB Entry DOI: 10.7270/Q2RF5S8X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII/Tissue factor (Homo sapiens (Human)) | BDBM50189937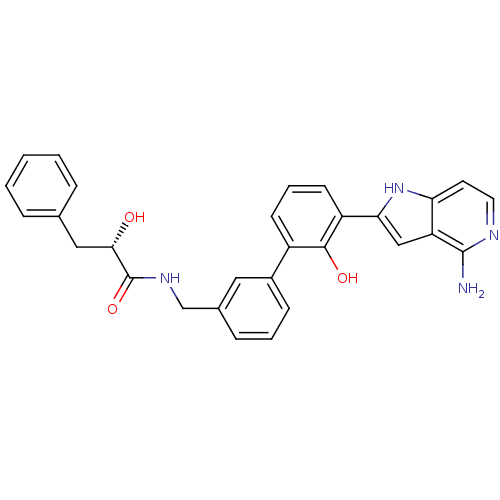 ((S)-N-[3'-(4-amino-1H-pyrrolo[3,2-c]pyridin-2-yl)-...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 81 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics Curated by ChEMBL | Assay Description Binding affinity to f7a/TF complex | Bioorg Med Chem Lett 16: 4567-70 (2006) Article DOI: 10.1016/j.bmcl.2006.06.016 BindingDB Entry DOI: 10.7270/Q2NG4Q67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII/Tissue factor (Homo sapiens (Human)) | BDBM50189940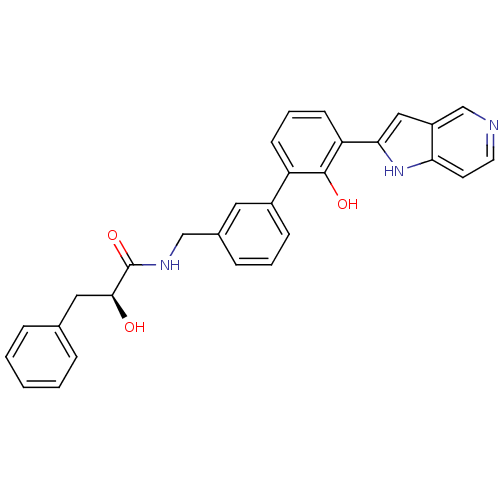 ((S)-2-hydroxy-N-[2'-hydroxy-3'-(1H-pyrrolo[3,2-c]p...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics Curated by ChEMBL | Assay Description Binding affinity to f7a/TF complex | Bioorg Med Chem Lett 16: 4567-70 (2006) Article DOI: 10.1016/j.bmcl.2006.06.016 BindingDB Entry DOI: 10.7270/Q2NG4Q67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM13781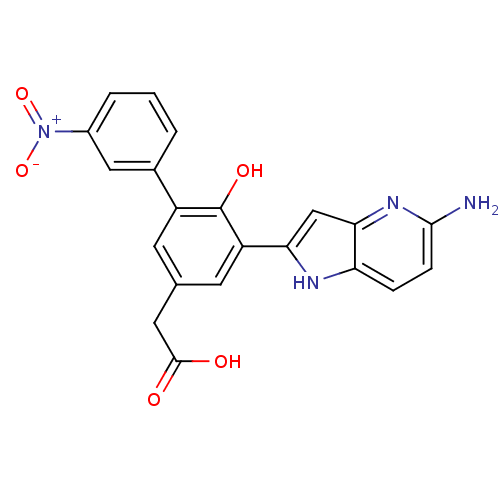 (2-(3-{5-amino-1H-pyrrolo[3,2-b]pyridin-2-yl}-4-hyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 300 | -36.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Celera | Assay Description The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... | Bioorg Med Chem Lett 16: 2270-3 (2006) Article DOI: 10.1016/j.bmcl.2006.01.017 BindingDB Entry DOI: 10.7270/Q2RF5S8X | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13776 (2-[3-(5-carbamimidoyl-1H-1,3-benzodiazol-2-yl)-4-h...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 320 | -36.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Celera | Assay Description The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... | Bioorg Med Chem Lett 16: 2270-3 (2006) Article DOI: 10.1016/j.bmcl.2006.01.017 BindingDB Entry DOI: 10.7270/Q2RF5S8X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM13778 (2-[3-(5-carbamimidoyl-1H-indol-2-yl)-4-hydroxy-5-(...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 400 | -36.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Celera | Assay Description The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... | Bioorg Med Chem Lett 16: 2270-3 (2006) Article DOI: 10.1016/j.bmcl.2006.01.017 BindingDB Entry DOI: 10.7270/Q2RF5S8X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM50182057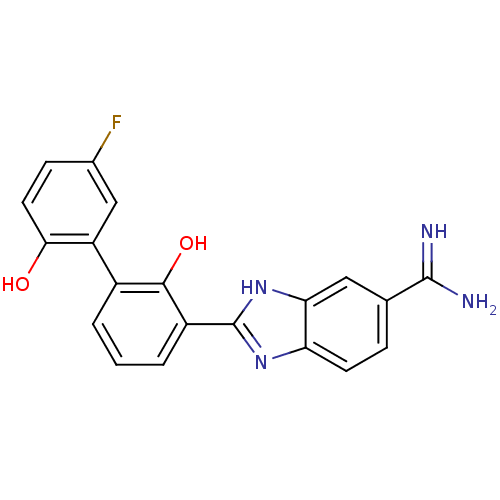 (2-(5'-fluoro-2,2'-dihydroxy-biphenyl-3-yl)-1H-benz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics Curated by ChEMBL | Assay Description Inhibition of factor7a in Sprague-Dawley rats | Bioorg Med Chem Lett 16: 2224-8 (2006) Article DOI: 10.1016/j.bmcl.2006.01.039 BindingDB Entry DOI: 10.7270/Q2TM79Q2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM13788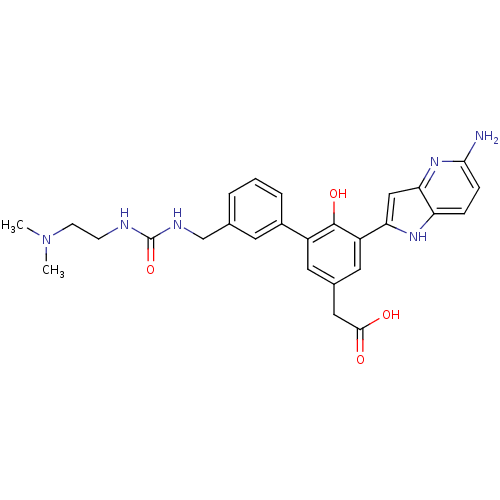 (2-(3-{5-amino-1H-pyrrolo[3,2-b]pyridin-2-yl}-5-{3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.40E+3 | -33.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Celera | Assay Description The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... | Bioorg Med Chem Lett 16: 2270-3 (2006) Article DOI: 10.1016/j.bmcl.2006.01.017 BindingDB Entry DOI: 10.7270/Q2RF5S8X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM13782 (2-(3-{5-amino-1H-pyrrolo[3,2-b]pyridin-2-yl}-4-hyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.40E+3 | -31.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Celera | Assay Description The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... | Bioorg Med Chem Lett 16: 2270-3 (2006) Article DOI: 10.1016/j.bmcl.2006.01.017 BindingDB Entry DOI: 10.7270/Q2RF5S8X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM13784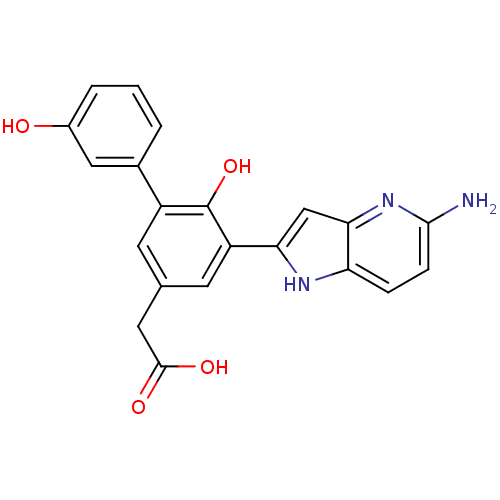 (2-(3-{5-amino-1H-pyrrolo[3,2-b]pyridin-2-yl}-4-hyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.50E+3 | -31.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Celera | Assay Description The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... | Bioorg Med Chem Lett 16: 2270-3 (2006) Article DOI: 10.1016/j.bmcl.2006.01.017 BindingDB Entry DOI: 10.7270/Q2RF5S8X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM14863 (({3-[3-(5-carbamimidoyl-1H-1,3-benzodiazol-2-yl)-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics Curated by ChEMBL | Assay Description Binding affinity to f10a | Bioorg Med Chem Lett 16: 4567-70 (2006) Article DOI: 10.1016/j.bmcl.2006.06.016 BindingDB Entry DOI: 10.7270/Q2NG4Q67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM13778 (2-[3-(5-carbamimidoyl-1H-indol-2-yl)-4-hydroxy-5-(...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.90E+3 | -31.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Celera | Assay Description The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... | Bioorg Med Chem Lett 16: 2270-3 (2006) Article DOI: 10.1016/j.bmcl.2006.01.017 BindingDB Entry DOI: 10.7270/Q2RF5S8X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13781 (2-(3-{5-amino-1H-pyrrolo[3,2-b]pyridin-2-yl}-4-hyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 3.50E+3 | -30.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Celera | Assay Description The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... | Bioorg Med Chem Lett 16: 2270-3 (2006) Article DOI: 10.1016/j.bmcl.2006.01.017 BindingDB Entry DOI: 10.7270/Q2RF5S8X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM13783 (2-(3-{5-amino-1H-pyrrolo[3,2-b]pyridin-2-yl}-4-hyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 7.40E+3 | -29.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Celera | Assay Description The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... | Bioorg Med Chem Lett 16: 2270-3 (2006) Article DOI: 10.1016/j.bmcl.2006.01.017 BindingDB Entry DOI: 10.7270/Q2RF5S8X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM13776 (2-[3-(5-carbamimidoyl-1H-1,3-benzodiazol-2-yl)-4-h...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10E+4 | -28.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Celera | Assay Description The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... | Bioorg Med Chem Lett 16: 2270-3 (2006) Article DOI: 10.1016/j.bmcl.2006.01.017 BindingDB Entry DOI: 10.7270/Q2RF5S8X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM13779 (2-[3-(1H-1,3-benzodiazol-2-yl)-4-hydroxy-5-(3-nitr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.10E+4 | -28.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Celera | Assay Description The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... | Bioorg Med Chem Lett 16: 2270-3 (2006) Article DOI: 10.1016/j.bmcl.2006.01.017 BindingDB Entry DOI: 10.7270/Q2RF5S8X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM13785 (2-(3-{5-amino-1H-pyrrolo[3,2-b]pyridin-2-yl}-5-[3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.20E+4 | -27.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Celera | Assay Description The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... | Bioorg Med Chem Lett 16: 2270-3 (2006) Article DOI: 10.1016/j.bmcl.2006.01.017 BindingDB Entry DOI: 10.7270/Q2RF5S8X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50189940 ((S)-2-hydroxy-N-[2'-hydroxy-3'-(1H-pyrrolo[3,2-c]p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics Curated by ChEMBL | Assay Description Binding affinity to f10a | Bioorg Med Chem Lett 16: 4567-70 (2006) Article DOI: 10.1016/j.bmcl.2006.06.016 BindingDB Entry DOI: 10.7270/Q2NG4Q67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM50182056 (3-(1H-benzoimidazol-2-yl)-biphenyl-2,2'-diol | CHE...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 3.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics Curated by ChEMBL | Assay Description Inhibition of factor7a in Sprague-Dawley rats | Bioorg Med Chem Lett 16: 2224-8 (2006) Article DOI: 10.1016/j.bmcl.2006.01.039 BindingDB Entry DOI: 10.7270/Q2TM79Q2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM13780 (2-(3-{5-amino-1H-imidazo[4,5-b]pyridin-2-yl}-4-hyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.90E+4 | -24.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Celera | Assay Description The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... | Bioorg Med Chem Lett 16: 2270-3 (2006) Article DOI: 10.1016/j.bmcl.2006.01.017 BindingDB Entry DOI: 10.7270/Q2RF5S8X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM13781 (2-(3-{5-amino-1H-pyrrolo[3,2-b]pyridin-2-yl}-4-hyd...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 4.40E+4 | -24.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Celera | Assay Description The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... | Bioorg Med Chem Lett 16: 2270-3 (2006) Article DOI: 10.1016/j.bmcl.2006.01.017 BindingDB Entry DOI: 10.7270/Q2RF5S8X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50189937 ((S)-N-[3'-(4-amino-1H-pyrrolo[3,2-c]pyridin-2-yl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics Curated by ChEMBL | Assay Description Binding affinity to f10a | Bioorg Med Chem Lett 16: 4567-70 (2006) Article DOI: 10.1016/j.bmcl.2006.06.016 BindingDB Entry DOI: 10.7270/Q2NG4Q67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM14863 (({3-[3-(5-carbamimidoyl-1H-1,3-benzodiazol-2-yl)-2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 9.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics Curated by ChEMBL | Assay Description Binding affinity to thrombin | Bioorg Med Chem Lett 16: 4567-70 (2006) Article DOI: 10.1016/j.bmcl.2006.06.016 BindingDB Entry DOI: 10.7270/Q2NG4Q67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50189937 ((S)-N-[3'-(4-amino-1H-pyrrolo[3,2-c]pyridin-2-yl)-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics Curated by ChEMBL | Assay Description Binding affinity to thrombin | Bioorg Med Chem Lett 16: 4567-70 (2006) Article DOI: 10.1016/j.bmcl.2006.06.016 BindingDB Entry DOI: 10.7270/Q2NG4Q67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50189940 ((S)-2-hydroxy-N-[2'-hydroxy-3'-(1H-pyrrolo[3,2-c]p...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics Curated by ChEMBL | Assay Description Binding affinity to thrombin | Bioorg Med Chem Lett 16: 4567-70 (2006) Article DOI: 10.1016/j.bmcl.2006.06.016 BindingDB Entry DOI: 10.7270/Q2NG4Q67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM13781 (2-(3-{5-amino-1H-pyrrolo[3,2-b]pyridin-2-yl}-4-hyd...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 1.20E+5 | -22.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Celera | Assay Description The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... | Bioorg Med Chem Lett 16: 2270-3 (2006) Article DOI: 10.1016/j.bmcl.2006.01.017 BindingDB Entry DOI: 10.7270/Q2RF5S8X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13779 (2-[3-(1H-1,3-benzodiazol-2-yl)-4-hydroxy-5-(3-nitr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >1.50E+5 | >-21.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Celera | Assay Description The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... | Bioorg Med Chem Lett 16: 2270-3 (2006) Article DOI: 10.1016/j.bmcl.2006.01.017 BindingDB Entry DOI: 10.7270/Q2RF5S8X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13780 (2-(3-{5-amino-1H-imidazo[4,5-b]pyridin-2-yl}-4-hyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.50E+5 | >-21.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Celera | Assay Description The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... | Bioorg Med Chem Lett 16: 2270-3 (2006) Article DOI: 10.1016/j.bmcl.2006.01.017 BindingDB Entry DOI: 10.7270/Q2RF5S8X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM13776 (2-[3-(5-carbamimidoyl-1H-1,3-benzodiazol-2-yl)-4-h...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.50E+5 | >-21.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Celera | Assay Description The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... | Bioorg Med Chem Lett 16: 2270-3 (2006) Article DOI: 10.1016/j.bmcl.2006.01.017 BindingDB Entry DOI: 10.7270/Q2RF5S8X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM13780 (2-(3-{5-amino-1H-imidazo[4,5-b]pyridin-2-yl}-4-hyd...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.50E+5 | >-21.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Celera | Assay Description The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... | Bioorg Med Chem Lett 16: 2270-3 (2006) Article DOI: 10.1016/j.bmcl.2006.01.017 BindingDB Entry DOI: 10.7270/Q2RF5S8X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM13780 (2-(3-{5-amino-1H-imidazo[4,5-b]pyridin-2-yl}-4-hyd...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.50E+5 | >-21.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Celera | Assay Description The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... | Bioorg Med Chem Lett 16: 2270-3 (2006) Article DOI: 10.1016/j.bmcl.2006.01.017 BindingDB Entry DOI: 10.7270/Q2RF5S8X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM13779 (2-[3-(1H-1,3-benzodiazol-2-yl)-4-hydroxy-5-(3-nitr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >1.50E+5 | >-21.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Celera | Assay Description The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... | Bioorg Med Chem Lett 16: 2270-3 (2006) Article DOI: 10.1016/j.bmcl.2006.01.017 BindingDB Entry DOI: 10.7270/Q2RF5S8X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM13779 (2-[3-(1H-1,3-benzodiazol-2-yl)-4-hydroxy-5-(3-nitr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >1.50E+5 | >-21.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Celera | Assay Description The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... | Bioorg Med Chem Lett 16: 2270-3 (2006) Article DOI: 10.1016/j.bmcl.2006.01.017 BindingDB Entry DOI: 10.7270/Q2RF5S8X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inosine-5'-monophosphate dehydrogenase 2 (Homo sapiens (Human)) | BDBM50054042 ((E)-6-(4-Hydroxy-7-methyl-3-oxo-6-vinyl-1,3-dihydr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.51 | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Research Curated by ChEMBL | Assay Description Inhibition of human recombinant inosine monophosphate dehydrogenase type II . | J Med Chem 39: 4181-96 (1996) Article DOI: 10.1021/jm9603633 BindingDB Entry DOI: 10.7270/Q2668C8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-inhibited 3',5'-cyclic phosphodiesterase 3A/3B (Homo sapiens (Human)) | BDBM50000871 (CHEMBL69139 | N-Cyclohexyl-N-methyl-4-(2-oxo-1,2,3...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Research Curated by ChEMBL | Assay Description Inhibition of platelet cAMP phosphodiesterase | J Med Chem 31: 2136-45 (1988) BindingDB Entry DOI: 10.7270/Q2VX0JQQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inosine-5'-monophosphate dehydrogenase 2 (Homo sapiens (Human)) | BDBM50054021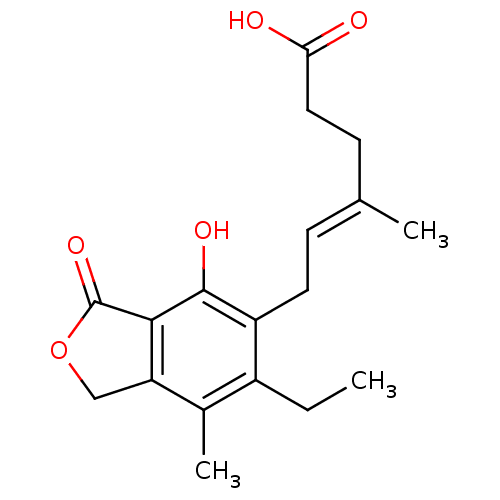 ((E)-6-(6-Ethyl-4-hydroxy-7-methyl-3-oxo-1,3-dihydr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 12.6 | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Research Curated by ChEMBL | Assay Description Inhibition of human recombinant inosine monophosphate dehydrogenase type II . | J Med Chem 39: 4181-96 (1996) Article DOI: 10.1021/jm9603633 BindingDB Entry DOI: 10.7270/Q2668C8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inosine-5'-monophosphate dehydrogenase 2 (Homo sapiens (Human)) | BDBM50054019 ((E)-6-(4-Hydroxy-6,7-dimethyl-3-oxo-1,3-dihydro-is...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 18.6 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Syntex Research Curated by ChEMBL | Assay Description Inhibition of human recombinant type II Inosine Monophosphate Dehydrogenase. at pH 8.0 | J Med Chem 39: 4181-96 (1996) Article DOI: 10.1021/jm9603633 BindingDB Entry DOI: 10.7270/Q2668C8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inosine-5'-monophosphate dehydrogenase 2 (Homo sapiens (Human)) | BDBM19264 ((4E)-6-(4-hydroxy-6-methoxy-7-methyl-3-oxo-1,3-dih...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 24.8 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Syntex Research Curated by ChEMBL | Assay Description Compound was tested for inhibition of human recombinant type II Inosine Monophosphate Dehydrogenase at pH 8.0 | J Med Chem 39: 4181-96 (1996) Article DOI: 10.1021/jm9603633 BindingDB Entry DOI: 10.7270/Q2668C8Q | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Inosine-5'-monophosphate dehydrogenase 2 (Homo sapiens (Human)) | BDBM19264 ((4E)-6-(4-hydroxy-6-methoxy-7-methyl-3-oxo-1,3-dih...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Syntex Research Curated by ChEMBL | Assay Description Compound was tested for inhibition of human recombinant type II Inosine Monophosphate Dehydrogenase at pH 8.0 | J Med Chem 39: 4181-96 (1996) Article DOI: 10.1021/jm9603633 BindingDB Entry DOI: 10.7270/Q2668C8Q | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Inosine-5'-monophosphate dehydrogenase 2 (Homo sapiens (Human)) | BDBM50053997 ((E)-6-(3-Chloro-2-hydroxy-6-methoxy-4,5-dimethyl-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 33.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Research Curated by ChEMBL | Assay Description Inhibition of human recombinant inosine monophosphate dehydrogenase type II . | J Med Chem 39: 4181-96 (1996) Article DOI: 10.1021/jm9603633 BindingDB Entry DOI: 10.7270/Q2668C8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inosine-5'-monophosphate dehydrogenase 2 (Homo sapiens (Human)) | BDBM50054004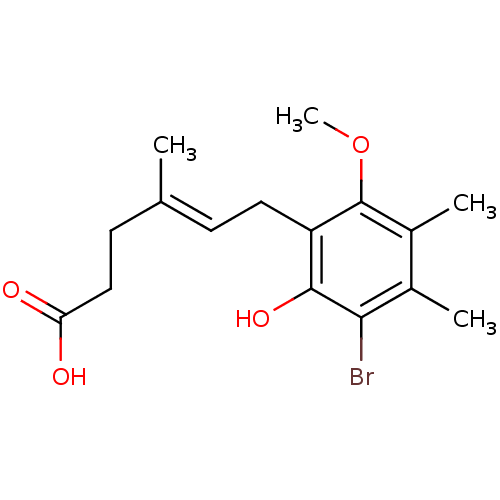 ((E)-6-(3-Bromo-2-hydroxy-6-methoxy-4,5-dimethyl-ph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 36.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Research Curated by ChEMBL | Assay Description Inhibition of human recombinant inosine monophosphate dehydrogenase type II . | J Med Chem 39: 4181-96 (1996) Article DOI: 10.1021/jm9603633 BindingDB Entry DOI: 10.7270/Q2668C8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 150 total ) | Next | Last >> |