

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50033535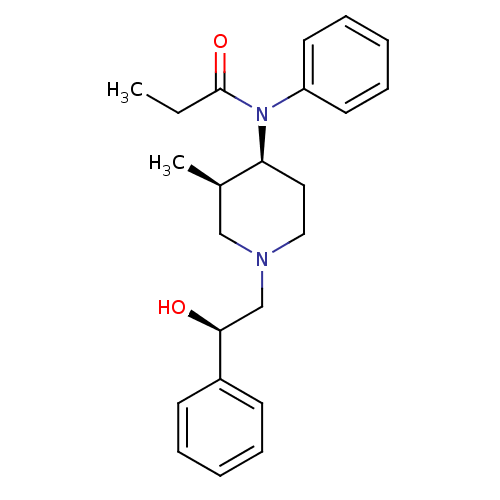 (CHEMBL331883 | N-[(3R,4S)-1-((R)-2-Hydroxy-2-pheny...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Binding affinity against Opioid receptor mu 1 | J Med Chem 43: 381-91 (2000) BindingDB Entry DOI: 10.7270/Q2DN45RB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50021347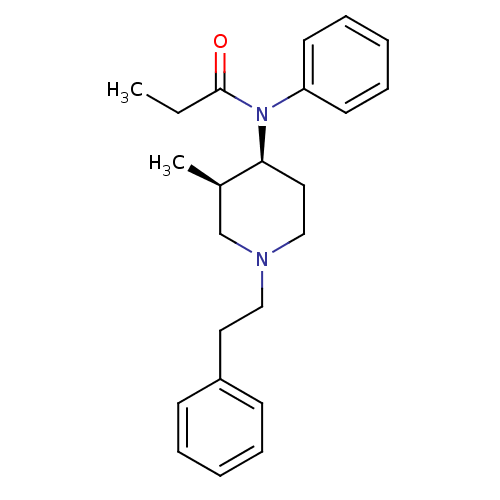 (CHEMBL12391 | N-(3-Methyl-1-phenethyl-piperidin-4-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Binding affinity against Opioid receptor mu 1 | J Med Chem 43: 381-91 (2000) BindingDB Entry DOI: 10.7270/Q2DN45RB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM50089316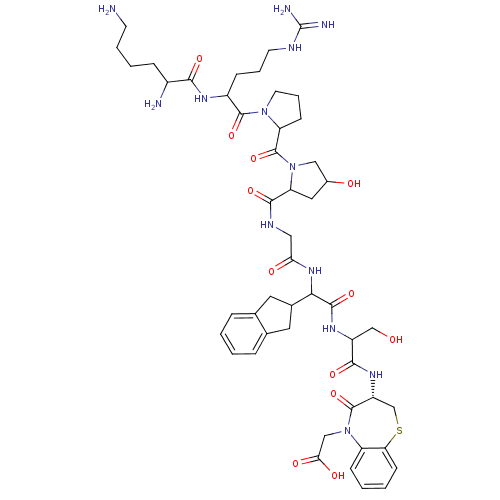 (CHEMBL410068 | H-Lys-Arg-Pro-Hyp-Gly-Igl-Ser-D-BT-...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universités Montpellier I et II Curated by ChEMBL | Assay Description Binding affinity of the compound towards human cloned B1 receptor was determined using [3H]-[des-Arg10-Leu9]-kallidin as radioligand | J Med Chem 43: 2387-94 (2000) BindingDB Entry DOI: 10.7270/Q2N87913 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50012477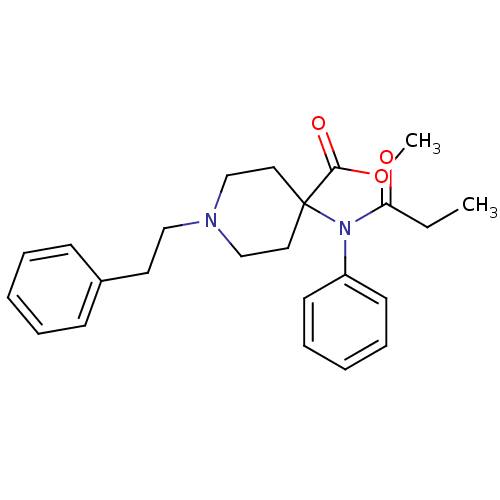 (1-Phenethyl-4-(phenyl-propionyl-amino)-piperidine-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid PDB UniChem Patents Similars | PubMed | 0.0240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Binding affinity against Opioid receptor mu 1 | J Med Chem 43: 381-91 (2000) BindingDB Entry DOI: 10.7270/Q2DN45RB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50033533 (CHEMBL121403 | N-[(3R,4R)-1-((S)-2-Hydroxy-2-pheny...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Binding affinity against Opioid receptor mu 1 | J Med Chem 43: 381-91 (2000) BindingDB Entry DOI: 10.7270/Q2DN45RB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50084768 (N-(4-Methoxymethyl-1-phenethyl-piperidin-4-yl)-N-p...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 0.0890 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Binding affinity against Opioid receptor mu 1 | J Med Chem 43: 381-91 (2000) BindingDB Entry DOI: 10.7270/Q2DN45RB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50469544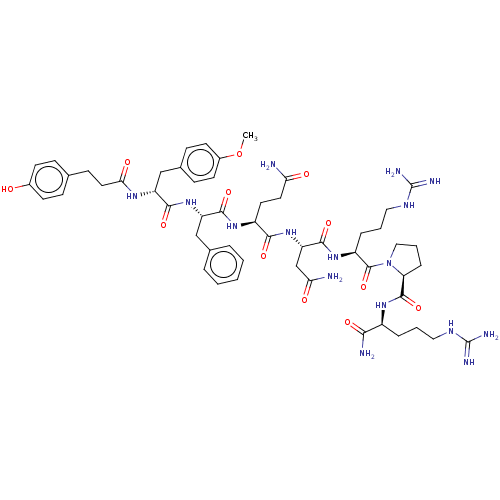 (CHEMBL4281963) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Montpellier Curated by ChEMBL | Assay Description Displacement of [Se-Se]-AVP from human V1A receptor expressed in CHO cells after 4 hrs by RP-LC-ICPMS analysis | J Med Chem 61: 10173-10184 (2018) Article DOI: 10.1021/acs.jmedchem.8b01320 BindingDB Entry DOI: 10.7270/Q2J105V6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Rattus norvegicus (rat)) | BDBM50090099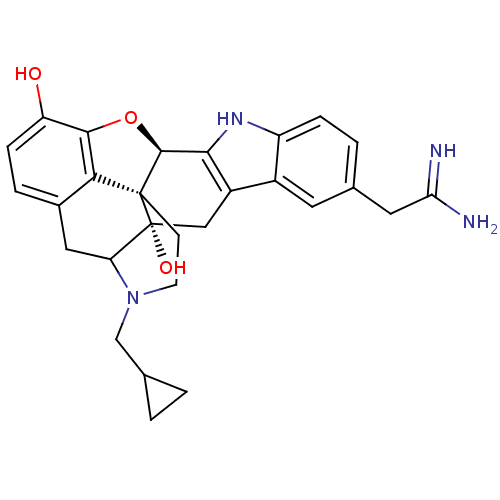 (5'-Amidinomethyl-17-cyclopropylmethyl-6,7-didehydr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.214 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Binding affinity on HEK-293 cells transiently transfected with plasmids encoding the rat Opioid receptor kappa 1 | J Med Chem 43: 2759-69 (2000) BindingDB Entry DOI: 10.7270/Q2JD4W2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM21367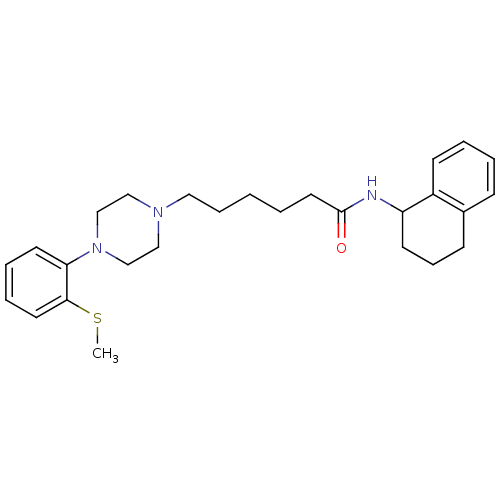 (6-{4-[2-(methylsulfanyl)phenyl]piperazin-1-yl}-N-(...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [3H]-5-CT from human 5-HT7 receptor expressed in HEK293 cells after 1 hr by beta-counting | Eur J Med Chem 78: 10-22 (2014) Article DOI: 10.1016/j.ejmech.2014.03.005 BindingDB Entry DOI: 10.7270/Q2QN6899 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Rattus norvegicus (rat)) | BDBM50090102 (5'-N-Biguanidino-17-cyclopropylmethyl-6,7-didehydr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.226 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Binding affinity on HEK-293 cells transiently transfected with plasmids encoding the rat Opioid receptor kappa 1 | J Med Chem 43: 2759-69 (2000) BindingDB Entry DOI: 10.7270/Q2JD4W2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Rattus norvegicus (rat)) | BDBM50090100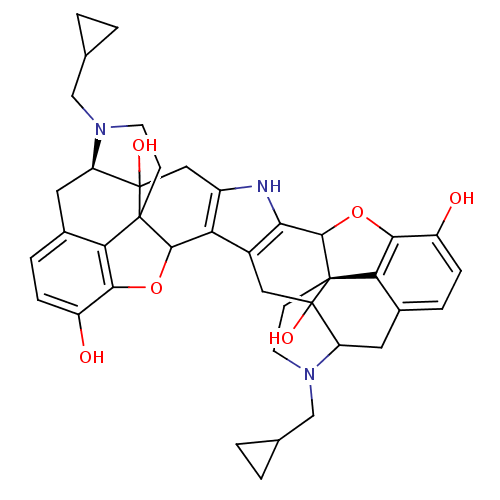 (CHEMBL319202 | Norbinaltorphimine (norBNI)) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.244 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Binding affinity on HEK-293 cells transiently transfected with plasmids encoding the rat Opioid receptor kappa 1 | J Med Chem 43: 2759-69 (2000) BindingDB Entry DOI: 10.7270/Q2JD4W2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Rattus norvegicus (rat)) | BDBM50090103 (5'-(Trimethylammonium)methyl-17-cyclopropylmethyl-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.354 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Binding affinity on HEK-293 cells transiently transfected with plasmids encoding the rat Opioid receptor kappa 1 | J Med Chem 43: 2759-69 (2000) BindingDB Entry DOI: 10.7270/Q2JD4W2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM50392788 (CHEMBL457614) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem | PDB Article PubMed | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zoetis Inc Curated by ChEMBL | Assay Description Inhibition of human active TRKA assessed as decrease in substrate phosphorylation incubated for 2 hrs using CSKtide as substrate in presence of ATP m... | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.126680 BindingDB Entry DOI: 10.7270/Q2280BVB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM50557930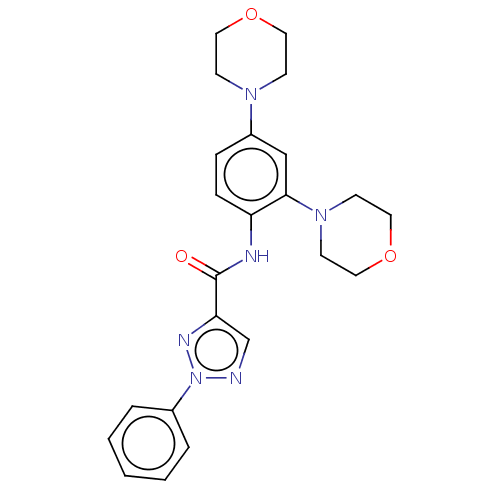 (CHEMBL4740028) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human TRKA at active state by kinetic based analysis | Citation and Details Article DOI: 10.1039/c9md00554d BindingDB Entry DOI: 10.7270/Q23J3HNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50469541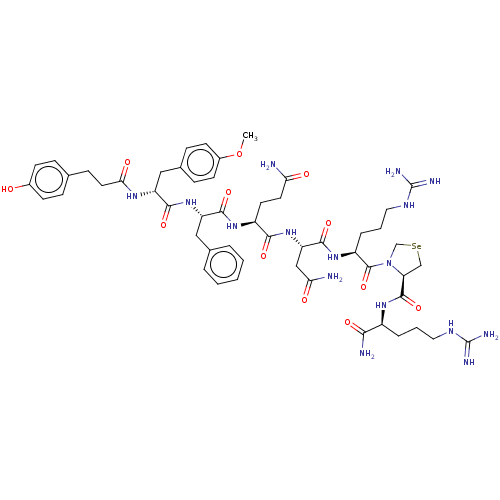 (CHEMBL4289837) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Montpellier Curated by ChEMBL | Assay Description Displacement of [Se-Se]-AVP from human V1A receptor expressed in CHO cells after 4 hrs by RP-LC-ICPMS analysis | J Med Chem 61: 10173-10184 (2018) Article DOI: 10.1021/acs.jmedchem.8b01320 BindingDB Entry DOI: 10.7270/Q2J105V6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Rattus norvegicus (rat)) | BDBM50090101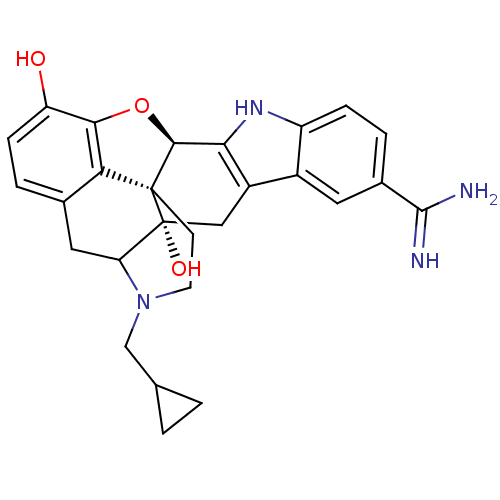 (5'-Amidino-17-cyclopropylmethyl-6,7-didehydro-4,5a...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.529 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Binding affinity on HEK-293 cells transiently transfected with plasmids encoding the rat Opioid receptor kappa 1 | J Med Chem 43: 2759-69 (2000) BindingDB Entry DOI: 10.7270/Q2JD4W2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50114031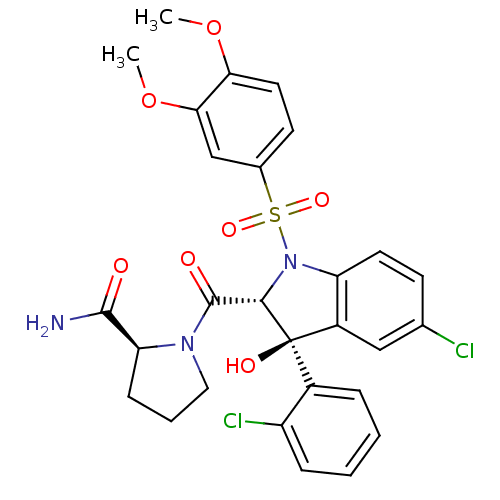 ((2S)-1-[(2R,3S)-5-chloro-3-(2-chlorophenyl)-1-(3,4...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Montpellier Curated by ChEMBL | Assay Description Displacement of [125I]OH-Phpa-LVA from human V1A receptor expressed in CHO cells after 4 hrs by gamma counter analysis | J Med Chem 61: 10173-10184 (2018) Article DOI: 10.1021/acs.jmedchem.8b01320 BindingDB Entry DOI: 10.7270/Q2J105V6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50253281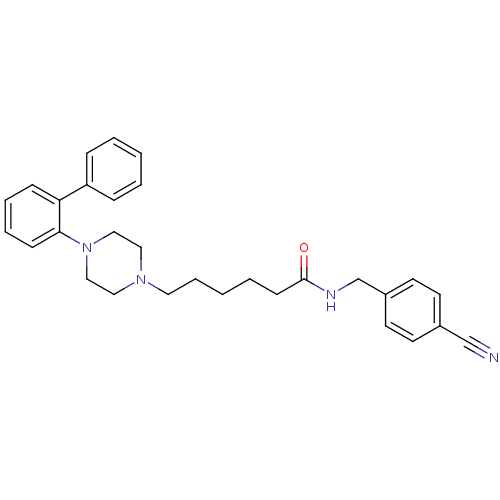 (CHEMBL522691 | N-(4-Cyanophenylmethyl)-4-(2-diphen...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [3H]-5-CT from human 5-HT7 receptor expressed in HEK293 cells after 1 hr by beta-counting | Eur J Med Chem 78: 10-22 (2014) Article DOI: 10.1016/j.ejmech.2014.03.005 BindingDB Entry DOI: 10.7270/Q2QN6899 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50469540 (CHEMBL4294901) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Montpellier Curated by ChEMBL | Assay Description Displacement of [125I]OH-Phpa-LVA from human V1A receptor expressed in CHO cells after 4 hrs by gamma counter analysis | J Med Chem 61: 10173-10184 (2018) Article DOI: 10.1021/acs.jmedchem.8b01320 BindingDB Entry DOI: 10.7270/Q2J105V6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50419052 (SB-399885) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-LSD from human 5HT6 receptor expressed in HEK293 cell membranes measured after 1 hr by microbeta counting method | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00224 BindingDB Entry DOI: 10.7270/Q2X3528B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50469544 (CHEMBL4281963) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Montpellier Curated by ChEMBL | Assay Description Displacement of [125I]OH-Phpa-LVA from human V1A receptor expressed in CHO cells after 4 hrs by gamma counter analysis | J Med Chem 61: 10173-10184 (2018) Article DOI: 10.1021/acs.jmedchem.8b01320 BindingDB Entry DOI: 10.7270/Q2J105V6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Rattus norvegicus (rat)) | BDBM50090097 (5'-(Dimethylamino)methyl-17-cyclopropylmethyl-6,7-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.823 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Binding affinity on HEK-293 cells transiently transfected with plasmids encoding the rat Opioid receptor kappa 1 | J Med Chem 43: 2759-69 (2000) BindingDB Entry DOI: 10.7270/Q2JD4W2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50114031 ((2S)-1-[(2R,3S)-5-chloro-3-(2-chlorophenyl)-1-(3,4...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Montpellier Curated by ChEMBL | Assay Description Displacement of [Se-Se]-AVP from human V1A receptor expressed in CHO cells after 4 hrs by RP-LC-ICPMS analysis | J Med Chem 61: 10173-10184 (2018) Article DOI: 10.1021/acs.jmedchem.8b01320 BindingDB Entry DOI: 10.7270/Q2J105V6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50469542 (CHEMBL4278500) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Montpellier Curated by ChEMBL | Assay Description Displacement of [125I]OH-Phpa-LVA from human V1A receptor expressed in CHO cells after 4 hrs by gamma counter analysis | J Med Chem 61: 10173-10184 (2018) Article DOI: 10.1021/acs.jmedchem.8b01320 BindingDB Entry DOI: 10.7270/Q2J105V6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50469541 (CHEMBL4289837) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Montpellier Curated by ChEMBL | Assay Description Displacement of [125I]OH-Phpa-LVA from human V1A receptor expressed in CHO cells after 4 hrs by gamma counter analysis | J Med Chem 61: 10173-10184 (2018) Article DOI: 10.1021/acs.jmedchem.8b01320 BindingDB Entry DOI: 10.7270/Q2J105V6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50579346 (CHEMBL4867565) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 0.930 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to 5HT3 receptor (unknown origin) | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00224 BindingDB Entry DOI: 10.7270/Q2X3528B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50003915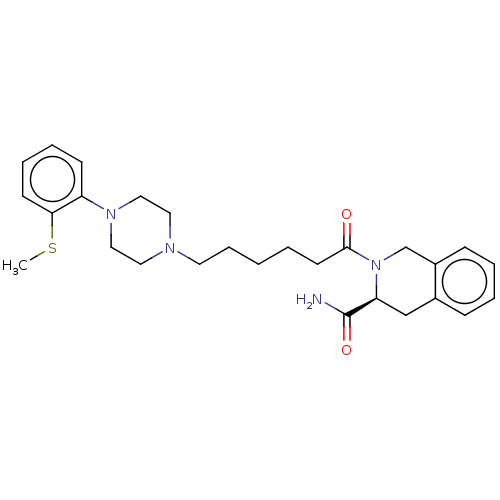 (CHEMBL3235744) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [3H]-8-OH-DPAT from human 5-HT1A receptor expressed in HEK293 cells after 1 hr by beta-counting | Eur J Med Chem 78: 10-22 (2014) Article DOI: 10.1016/j.ejmech.2014.03.005 BindingDB Entry DOI: 10.7270/Q2QN6899 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM50557930 (CHEMBL4740028) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human TRKA at inactive state by kinetic based analysis | Citation and Details Article DOI: 10.1039/c9md00554d BindingDB Entry DOI: 10.7270/Q23J3HNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50098551 ((R)-3-(2-(2-(4-methylpiperidin-1-yl)ethyl)pyrrolid...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Binding affinity to human cloned 5HT7 receptor expressed in HEK293 cells | Bioorg Med Chem Lett 19: 4827-31 (2009) Article DOI: 10.1016/j.bmcl.2009.06.038 BindingDB Entry DOI: 10.7270/Q20G3M3C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50318633 (3-benzenesulfonyl-8-piperazin-1-ylquinoline | CHEM...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-LSD from human 5HT6 receptor expressed in HEK293 cell membranes measured after 1 hr by microbeta counting method | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00224 BindingDB Entry DOI: 10.7270/Q2X3528B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50469540 (CHEMBL4294901) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Montpellier Curated by ChEMBL | Assay Description Displacement of [Se-Se]-AVP from human V1A receptor expressed in CHO cells after 4 hrs by RP-LC-ICPMS analysis | J Med Chem 61: 10173-10184 (2018) Article DOI: 10.1021/acs.jmedchem.8b01320 BindingDB Entry DOI: 10.7270/Q2J105V6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50579339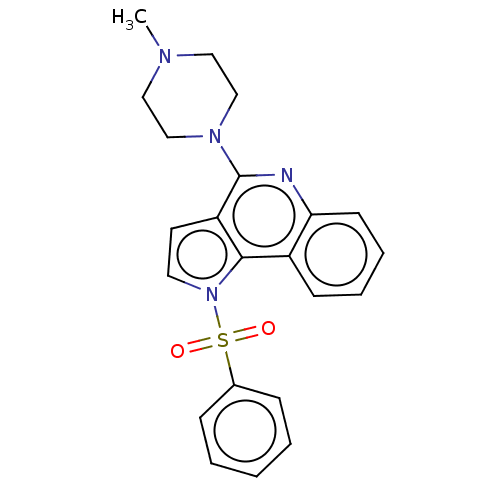 (CHEMBL4865309) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-LSD from human 5HT6 receptor expressed in HEK293 cell membranes measured after 1 hr by microbeta counting method | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00224 BindingDB Entry DOI: 10.7270/Q2X3528B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50003916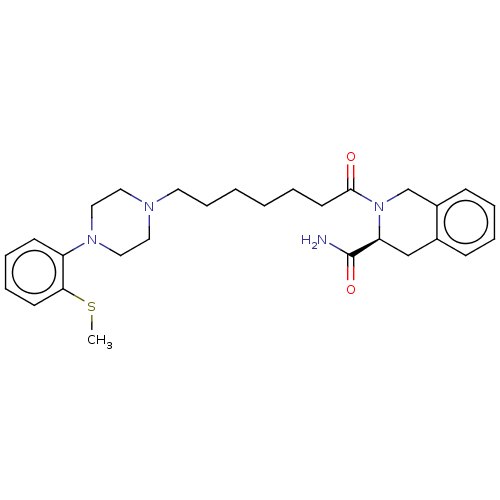 (CHEMBL3235745) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [3H]-8-OH-DPAT from human 5-HT1A receptor expressed in HEK293 cells after 1 hr by beta-counting | Eur J Med Chem 78: 10-22 (2014) Article DOI: 10.1016/j.ejmech.2014.03.005 BindingDB Entry DOI: 10.7270/Q2QN6899 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50579342 (CHEMBL4872586) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-LSD from human 5HT6 receptor expressed in HEK293 cell membranes measured after 1 hr by microbeta counting method | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00224 BindingDB Entry DOI: 10.7270/Q2X3528B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50579341 (CHEMBL4860809) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-LSD from human 5HT6 receptor expressed in HEK293 cell membranes measured after 1 hr by microbeta counting method | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00224 BindingDB Entry DOI: 10.7270/Q2X3528B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Rattus norvegicus (rat)) | BDBM50090098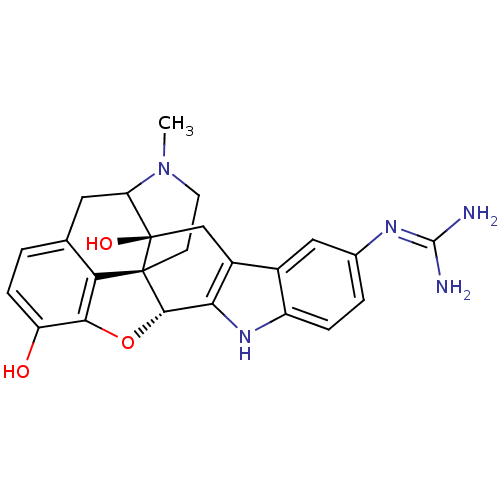 (6,7-Dihydro-4,5alpha-epoxy-5'-guanidinyl-17-methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.83 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Binding affinity on HEK-293 cells transiently transfected with plasmids encoding the rat Opioid receptor kappa 1 | J Med Chem 43: 2759-69 (2000) BindingDB Entry DOI: 10.7270/Q2JD4W2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50579348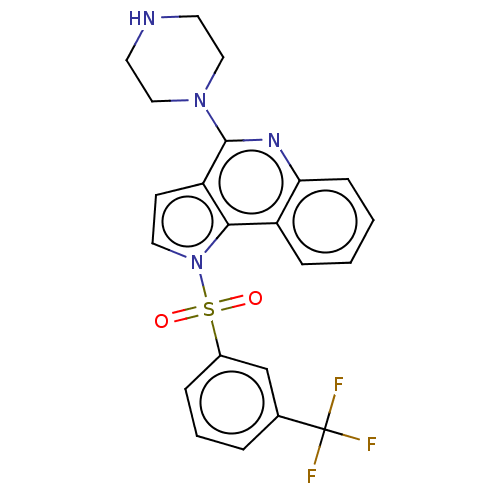 (CHEMBL4862354) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-LSD from human 5HT6 receptor expressed in HEK293 cell membranes measured after 1 hr by microbeta counting method | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00224 BindingDB Entry DOI: 10.7270/Q2X3528B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50186245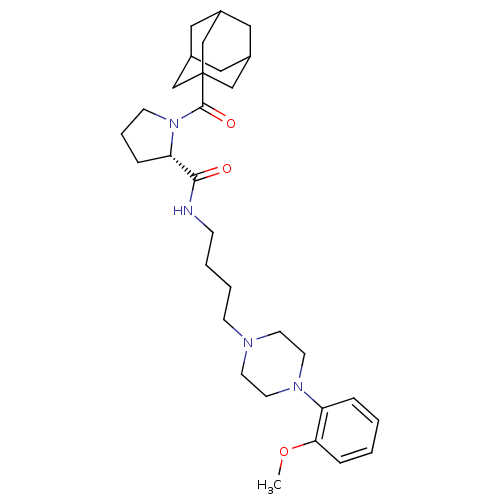 ((2S)-1-[(adamantan-1-yl)carbonyl]-N-{4-[4-(2-metho...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from 5HT1A receptor in rat hippocampal membrane | Eur J Med Chem 44: 800-8 (2009) Article DOI: 10.1016/j.ejmech.2008.05.021 BindingDB Entry DOI: 10.7270/Q20Z7468 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50579346 (CHEMBL4867565) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-LSD from human 5HT6 receptor expressed in HEK293 cell membranes measured after 1 hr by microbeta counting method | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00224 BindingDB Entry DOI: 10.7270/Q2X3528B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50579349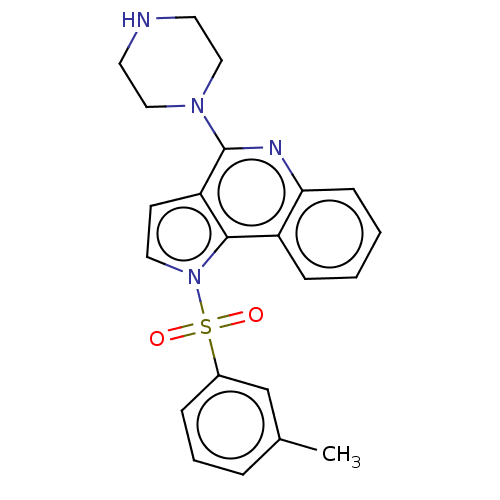 (CHEMBL4870374) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-LSD from human 5HT6 receptor expressed in HEK293 cell membranes measured after 1 hr by microbeta counting method | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00224 BindingDB Entry DOI: 10.7270/Q2X3528B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50206353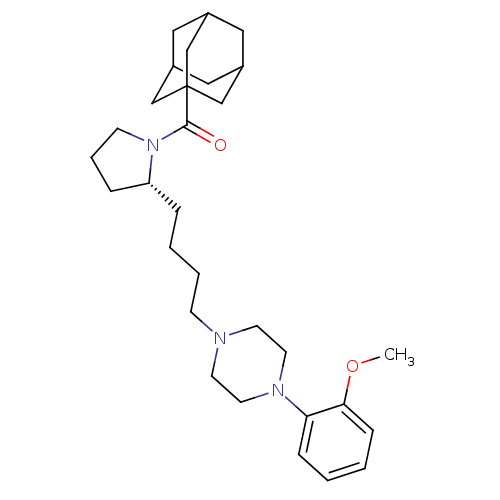 (1-(adamantylcarbonyl)-N-{4-[4-(2-methoxyphenyl)pip...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université Montpellier I et II Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from serotonin 5HT1A receptor in rat brain hippocampus | Bioorg Med Chem 15: 2907-19 (2007) Article DOI: 10.1016/j.bmc.2007.02.018 BindingDB Entry DOI: 10.7270/Q20G3JTZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50579347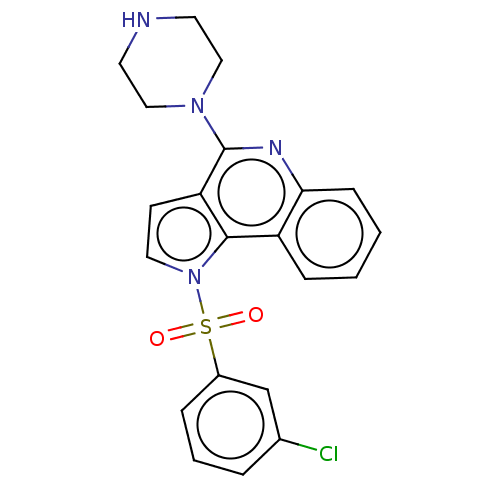 (CHEMBL4846153) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-LSD from human 5HT6 receptor expressed in HEK293 cell membranes measured after 1 hr by microbeta counting method | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00224 BindingDB Entry DOI: 10.7270/Q2X3528B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50274767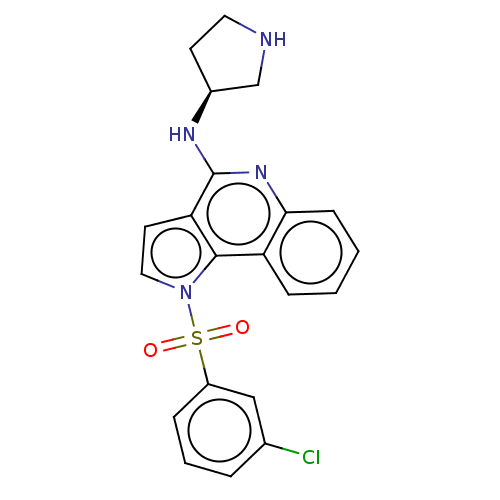 (CHEMBL4125735) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-LSD from human 5HT6 receptor expressed in HEK293 cell membranes measured after 1 hr by microbeta counting method | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00224 BindingDB Entry DOI: 10.7270/Q2X3528B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50012477 (1-Phenethyl-4-(phenyl-propionyl-amino)-piperidine-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid PDB UniChem Patents Similars | PubMed | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Binding affinity against Opioid receptor delta 1 | J Med Chem 43: 381-91 (2000) BindingDB Entry DOI: 10.7270/Q2DN45RB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50469539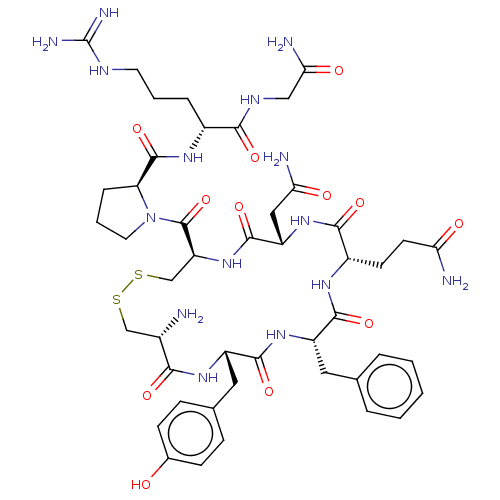 (CHEMBL562961) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem | Article PubMed | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Montpellier Curated by ChEMBL | Assay Description Displacement of [125I]OH-LVA from human V1A receptor expressed in African green monkey COS7 cell membranes after 60 mins | J Med Chem 61: 10173-10184 (2018) Article DOI: 10.1021/acs.jmedchem.8b01320 BindingDB Entry DOI: 10.7270/Q2J105V6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM50392788 (CHEMBL457614) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem | PDB Article PubMed | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zoetis Inc Curated by ChEMBL | Assay Description Inhibition of human inactive TRKA assessed as decrease in substrate phosphorylation incubated for 2 hrs using CSKtide as substrate in presence of ATP... | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.126680 BindingDB Entry DOI: 10.7270/Q2280BVB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50469539 (CHEMBL562961) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem | Article PubMed | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Montpellier Curated by ChEMBL | Assay Description Displacement of [125I]OH-Phpa-LVA from human V1A receptor expressed in CHO cells after 4 hrs by gamma counter analysis | J Med Chem 61: 10173-10184 (2018) Article DOI: 10.1021/acs.jmedchem.8b01320 BindingDB Entry DOI: 10.7270/Q2J105V6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50469541 (CHEMBL4289837) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Montpellier Curated by ChEMBL | Assay Description Displacement of [Sez6]-HO-Phpa-LVA from human V1A receptor expressed in CHO cells after 4 hrs by RP-LC-ICPMS analysis | J Med Chem 61: 10173-10184 (2018) Article DOI: 10.1021/acs.jmedchem.8b01320 BindingDB Entry DOI: 10.7270/Q2J105V6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50469546 (CHEMBL4286411) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Montpellier Curated by ChEMBL | Assay Description Displacement of non-sulfated CCK-8 from CCKB receptor (unknown origin) expressed in HEK cells after 4 hrs by RP-LC-ICPMS analysis | J Med Chem 61: 10173-10184 (2018) Article DOI: 10.1021/acs.jmedchem.8b01320 BindingDB Entry DOI: 10.7270/Q2J105V6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50186254 (2-(bicyclo[2.2.1]heptan-2-yl)-N-((R)-1-(4-(4-(2-me...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université Montpellier I et II Curated by ChEMBL | Assay Description Affinity for 5HT1A receptor | Bioorg Med Chem Lett 16: 3406-10 (2006) Article DOI: 10.1016/j.bmcl.2006.04.035 BindingDB Entry DOI: 10.7270/Q2542N6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1114 total ) | Next | Last >> |