

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sphingosine 1-phosphate receptor 5 (Homo sapiens (Human)) | BDBM50250631 (CHEMBL4093489) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 0.574 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Displacement of [33P]-S1P from human S1P5 receptor expressed in CHO-K1 cells after 60 mins by scintillation counting method | J Med Chem 60: 9508-9530 (2017) Article DOI: 10.1021/acs.jmedchem.7b00785 BindingDB Entry DOI: 10.7270/Q2J968SK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50250631 (CHEMBL4093489) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 0.626 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Displacement of [33P]-S1P from human S1P1 receptor expressed in CHO-K1 cells after 60 mins by scintillation counting method | J Med Chem 60: 9508-9530 (2017) Article DOI: 10.1021/acs.jmedchem.7b00785 BindingDB Entry DOI: 10.7270/Q2J968SK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Rattus norvegicus) | BDBM50250631 (CHEMBL4093489) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 0.772 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Displacement of [33P]-S1P from rat S1P1 receptor after 60 mins by scintillation counting method | J Med Chem 60: 9508-9530 (2017) Article DOI: 10.1021/acs.jmedchem.7b00785 BindingDB Entry DOI: 10.7270/Q2J968SK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 4 (Homo sapiens (Human)) | BDBM50250631 (CHEMBL4093489) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Displacement of [33P]-S1P from human S1P4 receptor expressed in CHO-K1 cells after 60 mins by scintillation counting method | J Med Chem 60: 9508-9530 (2017) Article DOI: 10.1021/acs.jmedchem.7b00785 BindingDB Entry DOI: 10.7270/Q2J968SK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 2 (Homo sapiens (Human)) | BDBM50250631 (CHEMBL4093489) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | >5.45E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Displacement of [33P]-S1P from human S1P2 receptor expressed in CHO-K1 cells after 60 mins by scintillation counting method | J Med Chem 60: 9508-9530 (2017) Article DOI: 10.1021/acs.jmedchem.7b00785 BindingDB Entry DOI: 10.7270/Q2J968SK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 3 (Homo sapiens (Human)) | BDBM50250631 (CHEMBL4093489) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | >5.63E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Displacement of [33P]-S1P from human S1P3 receptor expressed in CHO-K1 cells after 60 mins by scintillation counting method | J Med Chem 60: 9508-9530 (2017) Article DOI: 10.1021/acs.jmedchem.7b00785 BindingDB Entry DOI: 10.7270/Q2J968SK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM208487 (US9266870, 3) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | 7.4 | 37 |
TAISHO PHARMACEUTICAL CO., LTD US Patent | Assay Description The antagonistic activities of the test compounds on human orexin-1 receptor (hOX1R) and orexin-2 receptor (hOX2R) were measured by modifying from th... | US Patent US9266870 (2016) BindingDB Entry DOI: 10.7270/Q2J38RCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50543836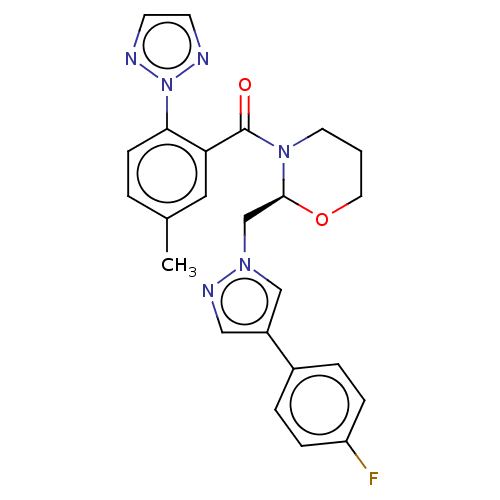 (CHEMBL4637711) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.784 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Antagonist activity at human recombinant OX2R expressed in CHOK1 cells assessed as inhibition of orexin A-induced intracellular Ca2+ release preincub... | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2020.115489 BindingDB Entry DOI: 10.7270/Q2KH0RWT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM208524 (US9266870, 40 | US9266870, 49) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | 7.4 | 37 |
TAISHO PHARMACEUTICAL CO., LTD US Patent | Assay Description The antagonistic activities of the test compounds on human orexin-1 receptor (hOX1R) and orexin-2 receptor (hOX2R) were measured by modifying from th... | US Patent US9266870 (2016) BindingDB Entry DOI: 10.7270/Q2J38RCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM208519 (US9266870, 35) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | 7.4 | 37 |
TAISHO PHARMACEUTICAL CO., LTD US Patent | Assay Description The antagonistic activities of the test compounds on human orexin-1 receptor (hOX1R) and orexin-2 receptor (hOX2R) were measured by modifying from th... | US Patent US9266870 (2016) BindingDB Entry DOI: 10.7270/Q2J38RCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM208511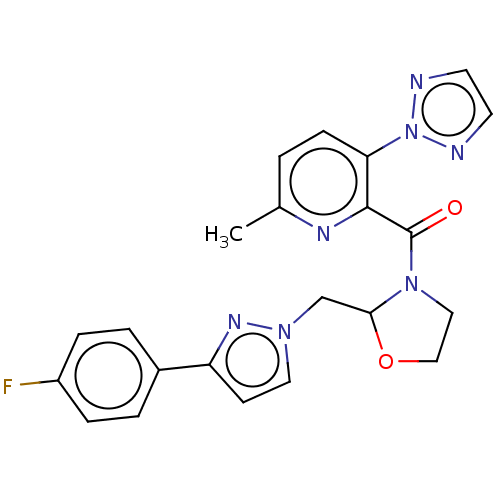 (US9266870, 27) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | 7.4 | 37 |
TAISHO PHARMACEUTICAL CO., LTD US Patent | Assay Description The antagonistic activities of the test compounds on human orexin-1 receptor (hOX1R) and orexin-2 receptor (hOX2R) were measured by modifying from th... | US Patent US9266870 (2016) BindingDB Entry DOI: 10.7270/Q2J38RCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1/2 (RAT) | BDBM50472632 (CHEMBL65140) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory effect on production of prostaglandin E2 (PGE2) in rat synovial cells. | J Med Chem 43: 2040-8 (2000) Article DOI: 10.1021/jm9906015 BindingDB Entry DOI: 10.7270/Q2H134R8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1/2 (RAT) | BDBM50472623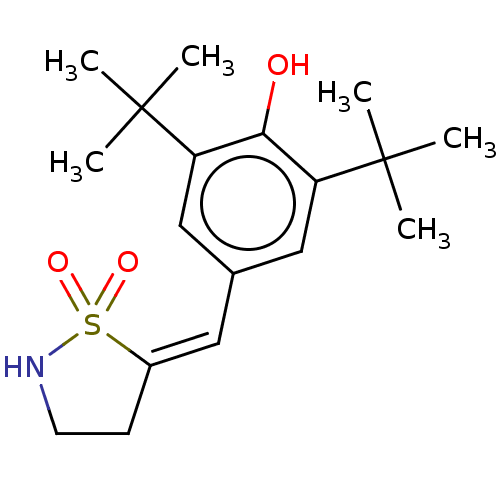 (CHEMBL294100) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory effect on production of prostaglandin E2 (PGE2) in rat synovial cells. | J Med Chem 43: 2040-8 (2000) Article DOI: 10.1021/jm9906015 BindingDB Entry DOI: 10.7270/Q2H134R8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1/2 (RAT) | BDBM50472624 (CHEMBL62439) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory effect on production of prostaglandin E2 (PGE2) in rat synovial cells. | J Med Chem 43: 2040-8 (2000) Article DOI: 10.1021/jm9906015 BindingDB Entry DOI: 10.7270/Q2H134R8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM208525 (US9266870, 41) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.4 | 37 |
TAISHO PHARMACEUTICAL CO., LTD US Patent | Assay Description The antagonistic activities of the test compounds on human orexin-1 receptor (hOX1R) and orexin-2 receptor (hOX2R) were measured by modifying from th... | US Patent US9266870 (2016) BindingDB Entry DOI: 10.7270/Q2J38RCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1/2 (RAT) | BDBM50472627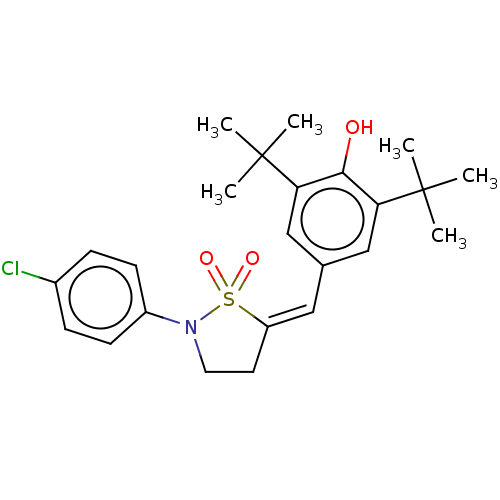 (CHEMBL304489) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory effect on production of prostaglandin E2 (PGE2) in rat synovial cells. | J Med Chem 43: 2040-8 (2000) Article DOI: 10.1021/jm9906015 BindingDB Entry DOI: 10.7270/Q2H134R8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1/2 (RAT) | BDBM50472618 (CHEMBL303092) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory effect on production of prostaglandin E2 (PGE2) in rat synovial cells. | J Med Chem 43: 2040-8 (2000) Article DOI: 10.1021/jm9906015 BindingDB Entry DOI: 10.7270/Q2H134R8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1/2 (RAT) | BDBM50472616 (CHEMBL65325) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory effect on production of prostaglandin E2 (PGE2) in rat synovial cells. | J Med Chem 43: 2040-8 (2000) Article DOI: 10.1021/jm9906015 BindingDB Entry DOI: 10.7270/Q2H134R8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM208525 (US9266870, 41) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.4 | 37 |
TAISHO PHARMACEUTICAL CO., LTD US Patent | Assay Description The antagonistic activities of the test compounds on human orexin-1 receptor (hOX1R) and orexin-2 receptor (hOX2R) were measured by modifying from th... | US Patent US9266870 (2016) BindingDB Entry DOI: 10.7270/Q2J38RCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM208519 (US9266870, 35) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.4 | 37 |
TAISHO PHARMACEUTICAL CO., LTD US Patent | Assay Description The antagonistic activities of the test compounds on human orexin-1 receptor (hOX1R) and orexin-2 receptor (hOX2R) were measured by modifying from th... | US Patent US9266870 (2016) BindingDB Entry DOI: 10.7270/Q2J38RCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50543827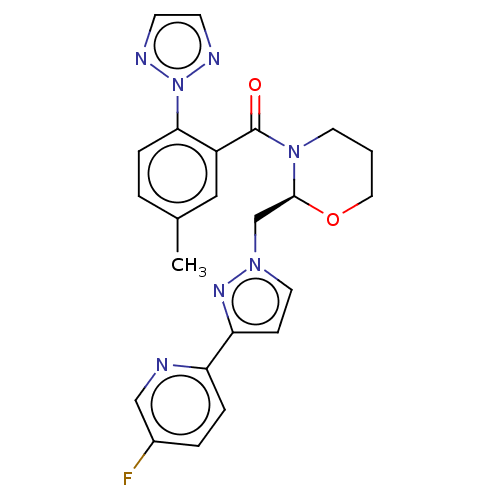 (CHEMBL4638046) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Antagonist activity at human recombinant OX1R expressed in CHOK1 cells assessed as inhibition of orexin A-induced intracellular Ca2+ release preincub... | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2020.115489 BindingDB Entry DOI: 10.7270/Q2KH0RWT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM335414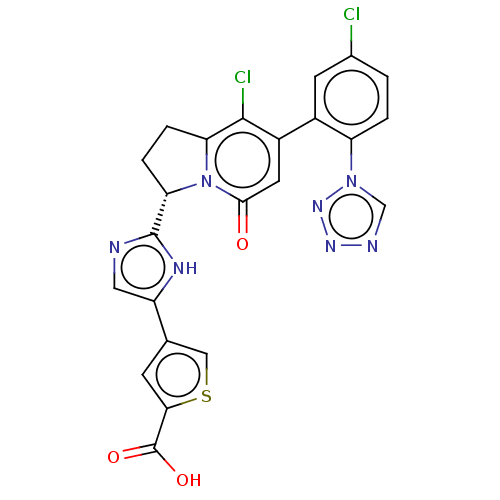 (4-(2-{(3S)-8-chloro-7-[5-chloro-2-(1H-tetrazol-1-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
ONO PHARMACEUTICAL CO., LTD. US Patent | Assay Description Human Factor XIa activity was measured at an enzyme concentration of 0.1 U/mL in 150 mM NaCl, 5 mM KCl, 1 mg/mL PEG6000, 50 mM HEPES-NaOH (pH7.4) wit... | US Patent US10717738 (2020) BindingDB Entry DOI: 10.7270/Q2X92FBQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM335416 (5-(2-{(3S)-7-[5-chloro-2-(1H-tetrazol-1-yl)phenyl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
ONO PHARMACEUTICAL CO., LTD. US Patent | Assay Description Human Factor XIa activity was measured at an enzyme concentration of 0.1 U/mL in 150 mM NaCl, 5 mM KCl, 1 mg/mL PEG6000, 50 mM HEPES-NaOH (pH7.4) wit... | US Patent US10717738 (2020) BindingDB Entry DOI: 10.7270/Q2X92FBQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM208521 (US9266870, 37) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | 7.4 | 37 |
TAISHO PHARMACEUTICAL CO., LTD US Patent | Assay Description The antagonistic activities of the test compounds on human orexin-1 receptor (hOX1R) and orexin-2 receptor (hOX2R) were measured by modifying from th... | US Patent US9266870 (2016) BindingDB Entry DOI: 10.7270/Q2J38RCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM335416 (5-(2-{(3S)-7-[5-chloro-2-(1H-tetrazol-1-yl)phenyl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
ONO PHARMACEUTICAL CO., LTD. US Patent | Assay Description Human Factor XIa (Haematologic Technologies Inc.) activity was measured at an enzyme concentration of 0.1 U/mL in 150 mM NaCl, 5 mM KCl, 1 mg/mL PEG6... | US Patent US9732085 (2017) BindingDB Entry DOI: 10.7270/Q25X2C2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM335414 (4-(2-{(3S)-8-chloro-7-[5-chloro-2-(1H-tetrazol-1-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
ONO PHARMACEUTICAL CO., LTD. US Patent | Assay Description Human Factor XIa (Haematologic Technologies Inc.) activity was measured at an enzyme concentration of 0.1 U/mL in 150 mM NaCl, 5 mM KCl, 1 mg/mL PEG6... | US Patent US9732085 (2017) BindingDB Entry DOI: 10.7270/Q25X2C2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM208513 (US9266870, 29) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | 7.4 | 37 |
TAISHO PHARMACEUTICAL CO., LTD US Patent | Assay Description The antagonistic activities of the test compounds on human orexin-1 receptor (hOX1R) and orexin-2 receptor (hOX2R) were measured by modifying from th... | US Patent US9266870 (2016) BindingDB Entry DOI: 10.7270/Q2J38RCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM208487 (US9266870, 3) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | 7.4 | 37 |
TAISHO PHARMACEUTICAL CO., LTD US Patent | Assay Description The antagonistic activities of the test compounds on human orexin-1 receptor (hOX1R) and orexin-2 receptor (hOX2R) were measured by modifying from th... | US Patent US9266870 (2016) BindingDB Entry DOI: 10.7270/Q2J38RCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50543827 (CHEMBL4638046) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Antagonist activity at human recombinant OX2R expressed in CHOK1 cells assessed as inhibition of orexin A-induced intracellular Ca2+ release preincub... | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2020.115489 BindingDB Entry DOI: 10.7270/Q2KH0RWT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM208524 (US9266870, 40 | US9266870, 49) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | 7.4 | 37 |
TAISHO PHARMACEUTICAL CO., LTD US Patent | Assay Description The antagonistic activities of the test compounds on human orexin-1 receptor (hOX1R) and orexin-2 receptor (hOX2R) were measured by modifying from th... | US Patent US9266870 (2016) BindingDB Entry DOI: 10.7270/Q2J38RCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM335412 (4-(2-{(3S)-7-[5-chloro-2-(1H-tetrazol-1-yl)phenyl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
ONO PHARMACEUTICAL CO., LTD. US Patent | Assay Description Human Factor XIa (Haematologic Technologies Inc.) activity was measured at an enzyme concentration of 0.1 U/mL in 150 mM NaCl, 5 mM KCl, 1 mg/mL PEG6... | US Patent US9732085 (2017) BindingDB Entry DOI: 10.7270/Q25X2C2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM335412 (4-(2-{(3S)-7-[5-chloro-2-(1H-tetrazol-1-yl)phenyl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
ONO PHARMACEUTICAL CO., LTD. US Patent | Assay Description Human Factor XIa activity was measured at an enzyme concentration of 0.1 U/mL in 150 mM NaCl, 5 mM KCl, 1 mg/mL PEG6000, 50 mM HEPES-NaOH (pH7.4) wit... | US Patent US10717738 (2020) BindingDB Entry DOI: 10.7270/Q2X92FBQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM335399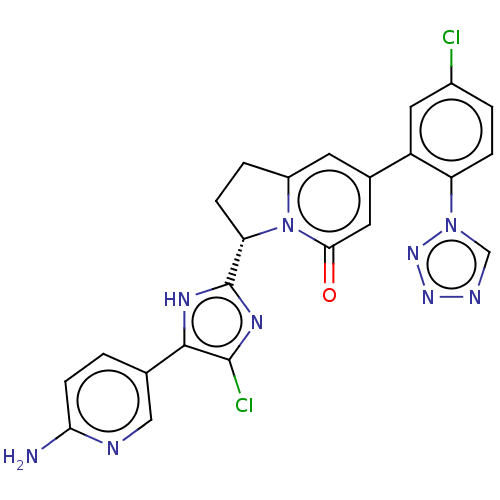 ((3S)-3-[5-(6-amino-3-pyridinyl)-4-chloro-1H-imidaz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
ONO PHARMACEUTICAL CO., LTD. US Patent | Assay Description Human Factor XIa (Haematologic Technologies Inc.) activity was measured at an enzyme concentration of 0.1 U/mL in 150 mM NaCl, 5 mM KCl, 1 mg/mL PEG6... | US Patent US9732085 (2017) BindingDB Entry DOI: 10.7270/Q25X2C2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM335399 ((3S)-3-[5-(6-amino-3-pyridinyl)-4-chloro-1H-imidaz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
ONO PHARMACEUTICAL CO., LTD. US Patent | Assay Description Human Factor XIa activity was measured at an enzyme concentration of 0.1 U/mL in 150 mM NaCl, 5 mM KCl, 1 mg/mL PEG6000, 50 mM HEPES-NaOH (pH7.4) wit... | US Patent US10717738 (2020) BindingDB Entry DOI: 10.7270/Q2X92FBQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM335418 (4-(2-{(3S)-7-[5-chloro-2-(1H-tetrazol-1-yl)phenyl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
ONO PHARMACEUTICAL CO., LTD. US Patent | Assay Description Human Factor XIa (Haematologic Technologies Inc.) activity was measured at an enzyme concentration of 0.1 U/mL in 150 mM NaCl, 5 mM KCl, 1 mg/mL PEG6... | US Patent US9732085 (2017) BindingDB Entry DOI: 10.7270/Q25X2C2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM335413 (5-(2-{(3S)-8-chloro-7-[5-chloro-2-(1H-tetrazol-1-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
ONO PHARMACEUTICAL CO., LTD. US Patent | Assay Description Human Factor XIa (Haematologic Technologies Inc.) activity was measured at an enzyme concentration of 0.1 U/mL in 150 mM NaCl, 5 mM KCl, 1 mg/mL PEG6... | US Patent US9732085 (2017) BindingDB Entry DOI: 10.7270/Q25X2C2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50543819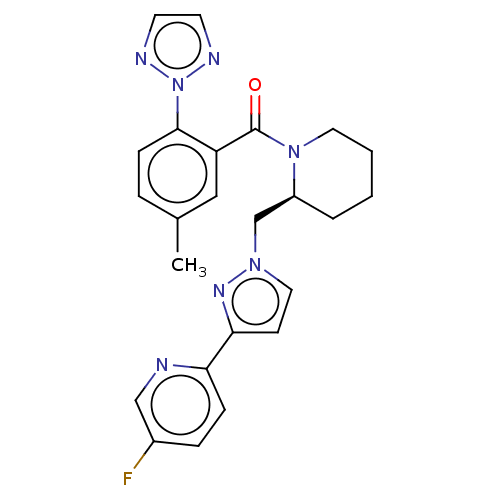 (CHEMBL4639148) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Antagonist activity at human recombinant OX2R expressed in CHOK1 cells assessed as inhibition of orexin A-induced intracellular Ca2+ release preincub... | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2020.115489 BindingDB Entry DOI: 10.7270/Q2KH0RWT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM335413 (5-(2-{(3S)-8-chloro-7-[5-chloro-2-(1H-tetrazol-1-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
ONO PHARMACEUTICAL CO., LTD. US Patent | Assay Description Human Factor XIa activity was measured at an enzyme concentration of 0.1 U/mL in 150 mM NaCl, 5 mM KCl, 1 mg/mL PEG6000, 50 mM HEPES-NaOH (pH7.4) wit... | US Patent US10717738 (2020) BindingDB Entry DOI: 10.7270/Q2X92FBQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM335418 (4-(2-{(3S)-7-[5-chloro-2-(1H-tetrazol-1-yl)phenyl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
ONO PHARMACEUTICAL CO., LTD. US Patent | Assay Description Human Factor XIa activity was measured at an enzyme concentration of 0.1 U/mL in 150 mM NaCl, 5 mM KCl, 1 mg/mL PEG6000, 50 mM HEPES-NaOH (pH7.4) wit... | US Patent US10717738 (2020) BindingDB Entry DOI: 10.7270/Q2X92FBQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM335419 (4-(5-{7-[5-chloro-2-(1H-tetrazol-1-yl)phenyl]-5-ox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
ONO PHARMACEUTICAL CO., LTD. US Patent | Assay Description Human Factor XIa (Haematologic Technologies Inc.) activity was measured at an enzyme concentration of 0.1 U/mL in 150 mM NaCl, 5 mM KCl, 1 mg/mL PEG6... | US Patent US9732085 (2017) BindingDB Entry DOI: 10.7270/Q25X2C2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM335419 (4-(5-{7-[5-chloro-2-(1H-tetrazol-1-yl)phenyl]-5-ox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
ONO PHARMACEUTICAL CO., LTD. US Patent | Assay Description Human Factor XIa activity was measured at an enzyme concentration of 0.1 U/mL in 150 mM NaCl, 5 mM KCl, 1 mg/mL PEG6000, 50 mM HEPES-NaOH (pH7.4) wit... | US Patent US10717738 (2020) BindingDB Entry DOI: 10.7270/Q2X92FBQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM208521 (US9266870, 37) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | 7.4 | 37 |
TAISHO PHARMACEUTICAL CO., LTD US Patent | Assay Description The antagonistic activities of the test compounds on human orexin-1 receptor (hOX1R) and orexin-2 receptor (hOX2R) were measured by modifying from th... | US Patent US9266870 (2016) BindingDB Entry DOI: 10.7270/Q2J38RCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50543833 (CHEMBL4634034) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Antagonist activity at human recombinant OX2R expressed in CHOK1 cells assessed as inhibition of orexin A-induced intracellular Ca2+ release preincub... | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2020.115489 BindingDB Entry DOI: 10.7270/Q2KH0RWT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50543831 (CHEMBL4637497) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Antagonist activity at human recombinant OX2R expressed in CHOK1 cells assessed as inhibition of orexin A-induced intracellular Ca2+ release preincub... | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2020.115489 BindingDB Entry DOI: 10.7270/Q2KH0RWT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50543837 (CHEMBL4641191) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Antagonist activity at human recombinant OX2R expressed in CHOK1 cells assessed as inhibition of orexin A-induced intracellular Ca2+ release preincub... | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2020.115489 BindingDB Entry DOI: 10.7270/Q2KH0RWT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM335415 (4-(2-{(6S)-2-[5-chloro-2-(1H-tetrazol-1-yl)phenyl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
ONO PHARMACEUTICAL CO., LTD. US Patent | Assay Description Human Factor XIa (Haematologic Technologies Inc.) activity was measured at an enzyme concentration of 0.1 U/mL in 150 mM NaCl, 5 mM KCl, 1 mg/mL PEG6... | US Patent US9732085 (2017) BindingDB Entry DOI: 10.7270/Q25X2C2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM208522 (US9266870, 38) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | 7.4 | 37 |
TAISHO PHARMACEUTICAL CO., LTD US Patent | Assay Description The antagonistic activities of the test compounds on human orexin-1 receptor (hOX1R) and orexin-2 receptor (hOX2R) were measured by modifying from th... | US Patent US9266870 (2016) BindingDB Entry DOI: 10.7270/Q2J38RCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1/2 (RAT) | BDBM50472613 (CHEMBL305076) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory effect on production of prostaglandin E2 (PGE2) in rat synovial cells. | J Med Chem 43: 2040-8 (2000) Article DOI: 10.1021/jm9906015 BindingDB Entry DOI: 10.7270/Q2H134R8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM335415 (4-(2-{(6S)-2-[5-chloro-2-(1H-tetrazol-1-yl)phenyl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
ONO PHARMACEUTICAL CO., LTD. US Patent | Assay Description Human Factor XIa activity was measured at an enzyme concentration of 0.1 U/mL in 150 mM NaCl, 5 mM KCl, 1 mg/mL PEG6000, 50 mM HEPES-NaOH (pH7.4) wit... | US Patent US10717738 (2020) BindingDB Entry DOI: 10.7270/Q2X92FBQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM208518 (US9266870, 34) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | 7.4 | 37 |
TAISHO PHARMACEUTICAL CO., LTD US Patent | Assay Description The antagonistic activities of the test compounds on human orexin-1 receptor (hOX1R) and orexin-2 receptor (hOX2R) were measured by modifying from th... | US Patent US9266870 (2016) BindingDB Entry DOI: 10.7270/Q2J38RCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 406 total ) | Next | Last >> |