

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3-oxo-5-alpha-steroid 4-dehydrogenase 2 (Homo sapiens (Human)) | BDBM50366682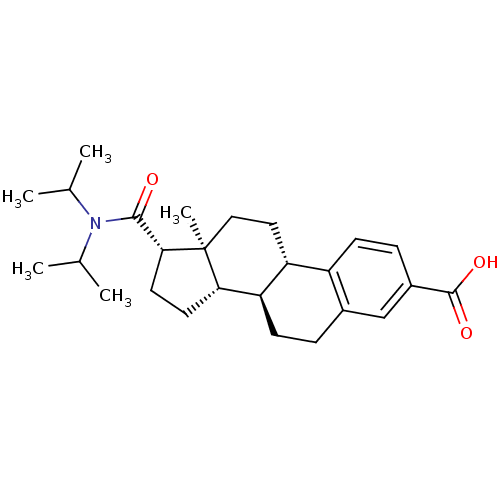 (CHEMBL1627395) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Curated by ChEMBL | Assay Description Compound was tested for the inhibition of type 2 5-alpha-reductase of human prostates | Bioorg Med Chem Lett 11: 1713-6 (2001) BindingDB Entry DOI: 10.7270/Q2ZC83C9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50471264 (CHEMBL1627893) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Unit� 439 Curated by ChEMBL | Assay Description Compound concentration required to induce transcriptional activation in MVLN cells equal to 50% of 0.1 nM estradiol response | J Med Chem 40: 2217-27 (1997) Article DOI: 10.1021/jm970019l BindingDB Entry DOI: 10.7270/Q20G3NVN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50471259 (CHEMBL1627458) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Unit� 439 Curated by ChEMBL | Assay Description Compound concentration required to induce transcriptional activation in MVLN cells equal to 50% of 0.1 nM estradiol response | J Med Chem 40: 2217-27 (1997) Article DOI: 10.1021/jm970019l BindingDB Entry DOI: 10.7270/Q20G3NVN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50471260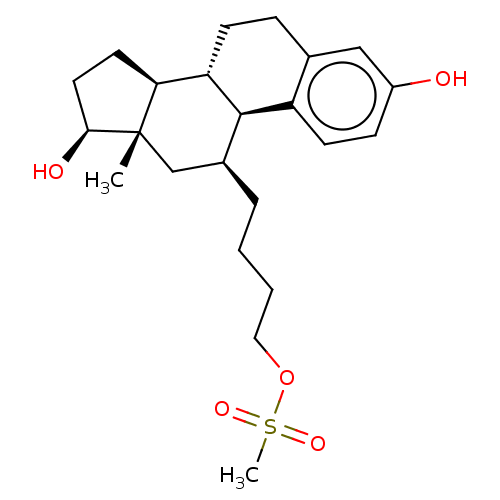 (CHEMBL1628165) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Unit� 439 Curated by ChEMBL | Assay Description Compound concentration required to induce transcriptional activation in MVLN cells equal to 50% of 0.1 nM estradiol response | J Med Chem 40: 2217-27 (1997) Article DOI: 10.1021/jm970019l BindingDB Entry DOI: 10.7270/Q20G3NVN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50471266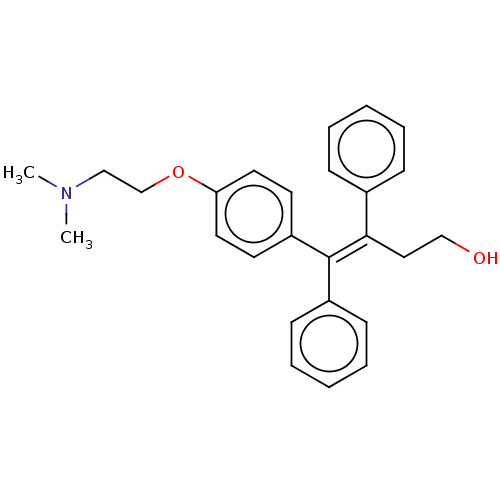 (CHEMBL303196) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Unit� 439 Curated by ChEMBL | Assay Description Compound concentration required to induce transcriptional activation in MVLN cells equal to 50% of 0.1 nM estradiol response | J Med Chem 40: 2217-27 (1997) Article DOI: 10.1021/jm970019l BindingDB Entry DOI: 10.7270/Q20G3NVN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 2 (Homo sapiens (Human)) | BDBM50101143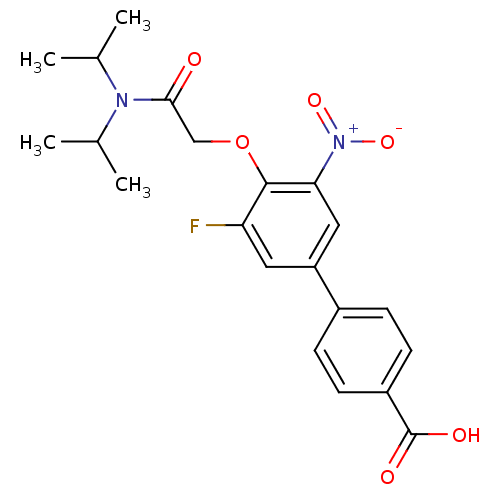 (4'-[(Diisopropylcarbamoyl)-methoxy]-5'-fluoro-3'-n...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 9.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Curated by ChEMBL | Assay Description Compound was tested for the inhibition of type 2 5-alpha-reductase of human prostates | Bioorg Med Chem Lett 11: 1713-6 (2001) BindingDB Entry DOI: 10.7270/Q2ZC83C9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM10045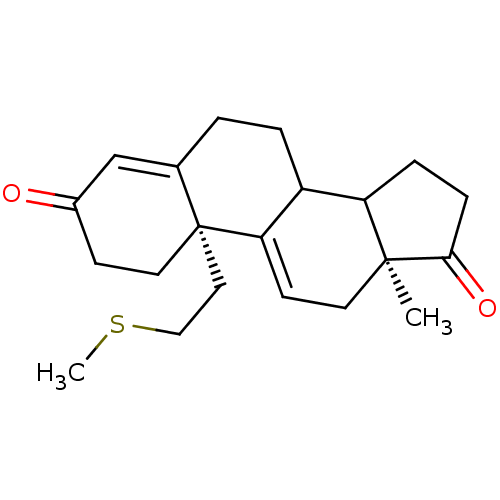 ((2R,15S)-15-methyl-2-[2-(methylsulfanyl)ethyl]tetr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | 7.2 | 37 |
Centre de Recherche de Roussel Uclaf | Assay Description The enzyme activity was assayed by measuring the formation of tritiated water from [1beta, 2beta-3H ]androstenedione in the presence of increasing co... | J Med Chem 39: 757-72 (1996) Article DOI: 10.1021/jm950539l BindingDB Entry DOI: 10.7270/Q28C9TGH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM10046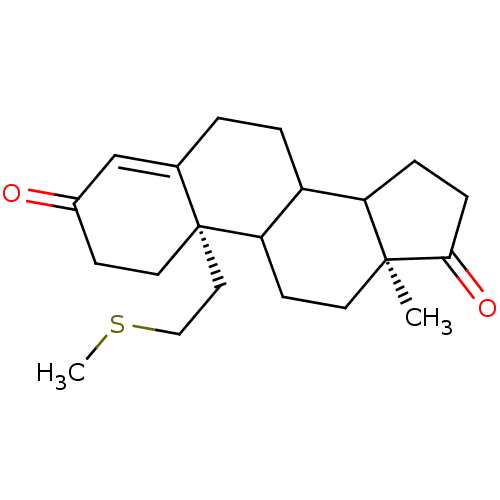 ((2S,15S)-15-methyl-2-[2-(methylsulfanyl)ethyl]tetr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | 7.2 | 37 |
Centre de Recherche de Roussel Uclaf | Assay Description The enzyme activity was assayed by measuring the formation of tritiated water from [1beta, 2beta-3H ]androstenedione in the presence of increasing co... | J Med Chem 39: 757-72 (1996) Article DOI: 10.1021/jm950539l BindingDB Entry DOI: 10.7270/Q28C9TGH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 2 (Homo sapiens (Human)) | BDBM50101145 ((4aR,6aS,7S,11aR)-4a,6a-Dimethyl-2-oxo-hexadecahyd...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Curated by ChEMBL | Assay Description Compound was tested for the inhibition of type 2 5-alpha-reductase of human prostates | Bioorg Med Chem Lett 11: 1713-6 (2001) BindingDB Entry DOI: 10.7270/Q2ZC83C9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM10044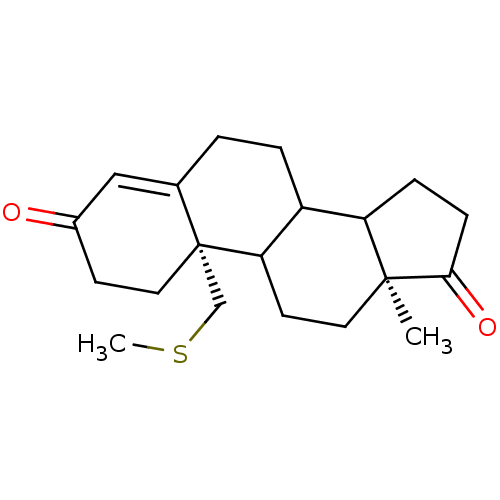 ((2S,15S)-15-methyl-2-[(methylsulfanyl)methyl]tetra...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 42 | n/a | n/a | n/a | n/a | 7.2 | 37 |
Centre de Recherche de Roussel Uclaf | Assay Description The enzyme activity was assayed by measuring the formation of tritiated water from [1beta, 2beta-3H ]androstenedione in the presence of increasing co... | J Med Chem 39: 757-72 (1996) Article DOI: 10.1021/jm950539l BindingDB Entry DOI: 10.7270/Q28C9TGH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM10064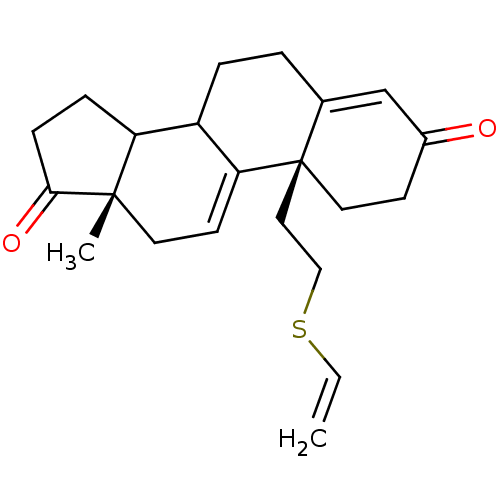 ((2R,15S)-2-[2-(ethenylsulfanyl)ethyl]-15-methyltet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche de Roussel Uclaf | Assay Description The enzyme activity was assayed by measuring the formation of tritiated water from [1beta, 2beta-3H ]androstenedione in the presence of increasing co... | J Med Chem 39: 757-72 (1996) Article DOI: 10.1021/jm950539l BindingDB Entry DOI: 10.7270/Q28C9TGH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM10069 ((2R,15S)-2-{2-[(fluoromethyl)sulfanyl]ethyl}-15-me...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche de Roussel Uclaf | Assay Description The enzyme activity was assayed by measuring the formation of tritiated water from [1beta, 2beta-3H ]androstenedione in the presence of increasing co... | J Med Chem 39: 757-72 (1996) Article DOI: 10.1021/jm950539l BindingDB Entry DOI: 10.7270/Q28C9TGH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 2 (Homo sapiens (Human)) | BDBM50101155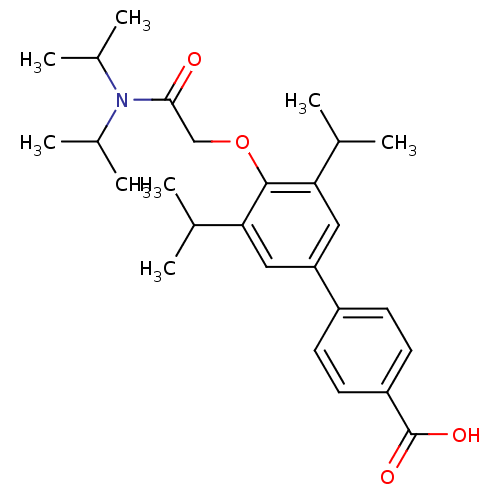 (4'-[(Diisopropylcarbamoyl)-methoxy]-3',5'-diisopro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 71 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Curated by ChEMBL | Assay Description Compound was tested for the inhibition of Steroid 5-alpha-reductase type 2 of human prostates | Bioorg Med Chem Lett 11: 1713-6 (2001) BindingDB Entry DOI: 10.7270/Q2ZC83C9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 2 (Homo sapiens (Human)) | BDBM50101154 (5'-Fluoro-3'-nitro-4'-[(trityl-carbamoyl)-methoxy]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 92 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Curated by ChEMBL | Assay Description Compound was tested for the inhibition of type 2 5-alpha-reductase of human prostates | Bioorg Med Chem Lett 11: 1713-6 (2001) BindingDB Entry DOI: 10.7270/Q2ZC83C9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM10063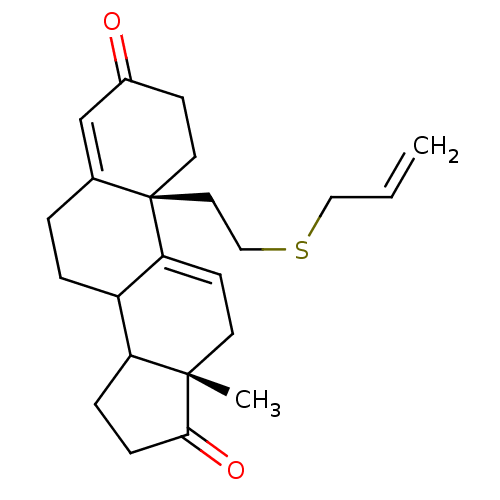 ((2R,15S)-15-methyl-2-[2-(prop-2-en-1-ylsulfanyl)et...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche de Roussel Uclaf | Assay Description The enzyme activity was assayed by measuring the formation of tritiated water from [1beta, 2beta-3H ]androstenedione in the presence of increasing co... | J Med Chem 39: 757-72 (1996) Article DOI: 10.1021/jm950539l BindingDB Entry DOI: 10.7270/Q28C9TGH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50471262 (CHEMBL1628147) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Unit� 439 Curated by ChEMBL | Assay Description Compound concentration required to induce transcriptional activation in MVLN cells equal to 50% of 0.1 nM estradiol response | J Med Chem 40: 2217-27 (1997) Article DOI: 10.1021/jm970019l BindingDB Entry DOI: 10.7270/Q20G3NVN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM10068 ((2R,15S)-2-[2-(ethynylsulfanyl)ethyl]-15-methyltet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche de Roussel Uclaf | Assay Description The enzyme activity was assayed by measuring the formation of tritiated water from [1beta, 2beta-3H ]androstenedione in the presence of increasing co... | J Med Chem 39: 757-72 (1996) Article DOI: 10.1021/jm950539l BindingDB Entry DOI: 10.7270/Q28C9TGH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 2 (Homo sapiens (Human)) | BDBM50101153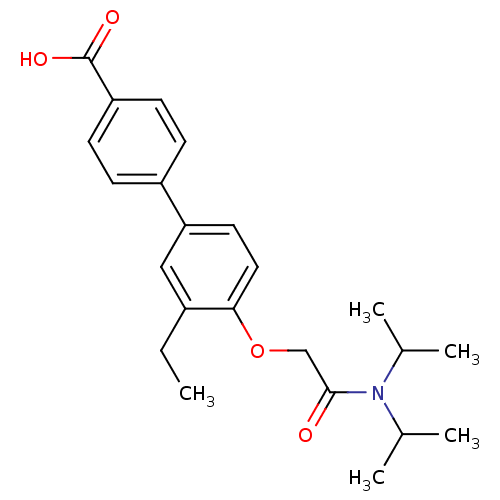 (4'-[(Diisopropylcarbamoyl)-methoxy]-3'-ethyl-biphe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Curated by ChEMBL | Assay Description Compound was tested for the inhibition of type 2 5-alpha-reductase of human prostates | Bioorg Med Chem Lett 11: 1713-6 (2001) BindingDB Entry DOI: 10.7270/Q2ZC83C9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM10054 ((2R,15S)-15-methyl-2-[2-(phenylsulfanyl)ethyl]tetr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | 7.2 | 37 |
Centre de Recherche de Roussel Uclaf | Assay Description The enzyme activity was assayed by measuring the formation of tritiated water from [1beta, 2beta-3H ]androstenedione in the presence of increasing co... | J Med Chem 39: 757-72 (1996) Article DOI: 10.1021/jm950539l BindingDB Entry DOI: 10.7270/Q28C9TGH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM10062 ((2R,15S)-15-methyl-2-(2-{[(methylsulfanyl)methyl]s...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 570 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche de Roussel Uclaf | Assay Description The enzyme activity was assayed by measuring the formation of tritiated water from [1beta, 2beta-3H ]androstenedione in the presence of increasing co... | J Med Chem 39: 757-72 (1996) Article DOI: 10.1021/jm950539l BindingDB Entry DOI: 10.7270/Q28C9TGH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 2 (Homo sapiens (Human)) | BDBM50101146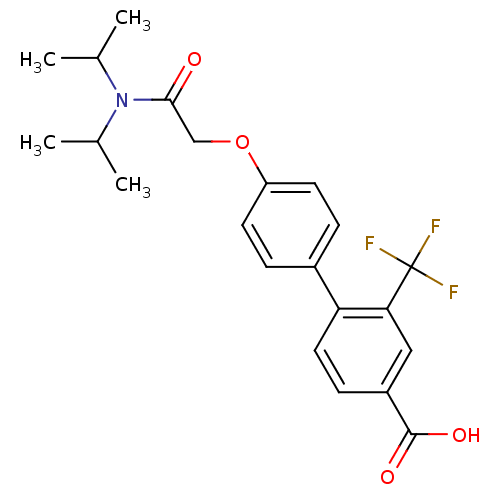 (4'-[(Diisopropylcarbamoyl)-methoxy]-2-trifluoromet...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 570 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Curated by ChEMBL | Assay Description Compound was tested for the inhibition of Steroid 5-alpha-reductase type 2 of human prostates | Bioorg Med Chem Lett 11: 1713-6 (2001) BindingDB Entry DOI: 10.7270/Q2ZC83C9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 2 (Homo sapiens (Human)) | BDBM50101149 (4'-[(Diisopropylcarbamoyl)-methoxy]-biphenyl-4-car...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 830 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Curated by ChEMBL | Assay Description Compound was tested for the inhibition of Steroid 5-alpha-reductase type 2 of human prostates | Bioorg Med Chem Lett 11: 1713-6 (2001) BindingDB Entry DOI: 10.7270/Q2ZC83C9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 2 (Homo sapiens (Human)) | BDBM50101156 (2-Chloro-4'-[(diisopropylcarbamoyl)-methoxy]-biphe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 870 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Curated by ChEMBL | Assay Description Compound was tested for the inhibition of Steroid 5-alpha-reductase type 2 of human prostates | Bioorg Med Chem Lett 11: 1713-6 (2001) BindingDB Entry DOI: 10.7270/Q2ZC83C9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM10047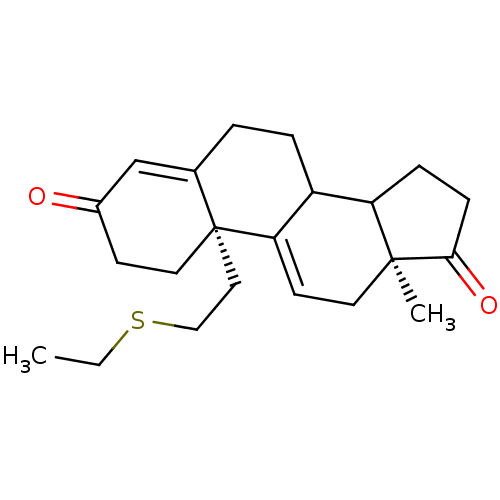 ((2R,15S)-2-[2-(ethylsulfanyl)ethyl]-15-methyltetra...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 870 | n/a | n/a | n/a | n/a | 7.2 | 37 |
Centre de Recherche de Roussel Uclaf | Assay Description The enzyme activity was assayed by measuring the formation of tritiated water from [1beta, 2beta-3H ]androstenedione in the presence of increasing co... | J Med Chem 39: 757-72 (1996) Article DOI: 10.1021/jm950539l BindingDB Entry DOI: 10.7270/Q28C9TGH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM10071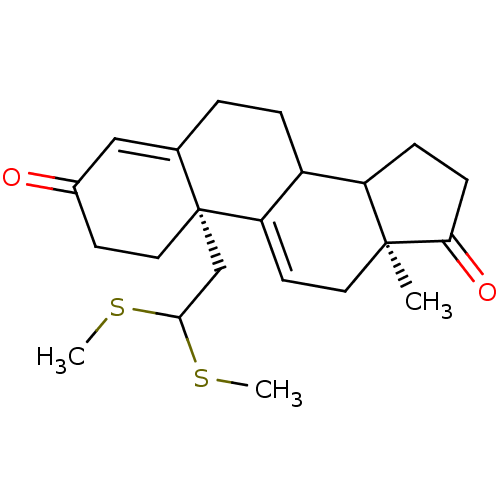 ((2R,15S)-2-[2,2-bis(methylsulfanyl)ethyl]-15-methy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche de Roussel Uclaf | Assay Description The enzyme activity was assayed by measuring the formation of tritiated water from [1beta, 2beta-3H ]androstenedione in the presence of increasing co... | J Med Chem 39: 757-72 (1996) Article DOI: 10.1021/jm950539l BindingDB Entry DOI: 10.7270/Q28C9TGH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM10072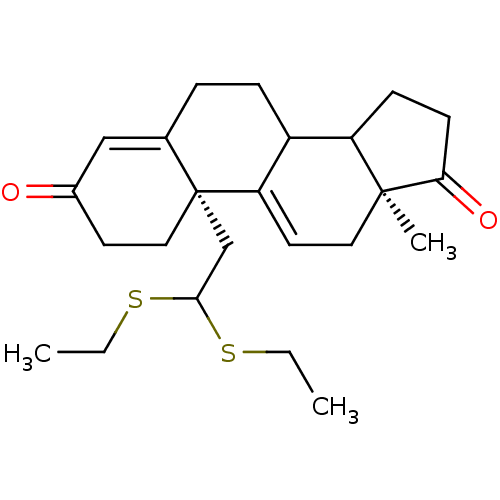 ((2R,15S)-2-[2,2-bis(ethylsulfanyl)ethyl]-15-methyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche de Roussel Uclaf | Assay Description The enzyme activity was assayed by measuring the formation of tritiated water from [1beta, 2beta-3H ]androstenedione in the presence of increasing co... | J Med Chem 39: 757-72 (1996) Article DOI: 10.1021/jm950539l BindingDB Entry DOI: 10.7270/Q28C9TGH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM10053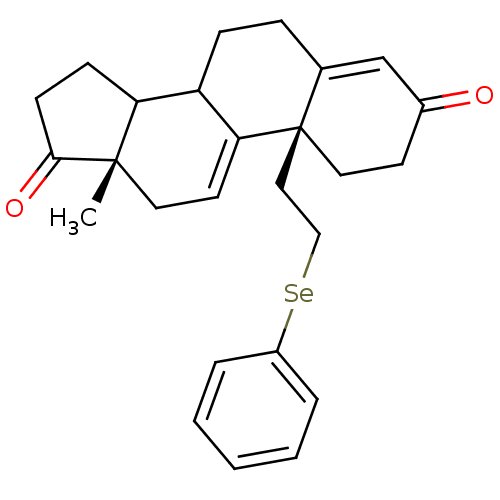 ((2R,15S)-15-methyl-2-[2-(phenylselanyl)ethyl]tetra...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | 7.2 | 37 |
Centre de Recherche de Roussel Uclaf | Assay Description The enzyme activity was assayed by measuring the formation of tritiated water from [1beta, 2beta-3H ]androstenedione in the presence of increasing co... | J Med Chem 39: 757-72 (1996) Article DOI: 10.1021/jm950539l BindingDB Entry DOI: 10.7270/Q28C9TGH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM10059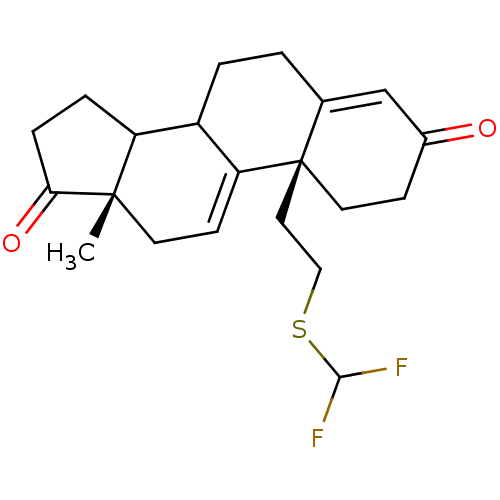 ((2R,15S)-2-{2-[(difluoromethyl)sulfanyl]ethyl}-15-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche de Roussel Uclaf | Assay Description The enzyme activity was assayed by measuring the formation of tritiated water from [1beta, 2beta-3H ]androstenedione in the presence of increasing co... | J Med Chem 39: 757-72 (1996) Article DOI: 10.1021/jm950539l BindingDB Entry DOI: 10.7270/Q28C9TGH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM10061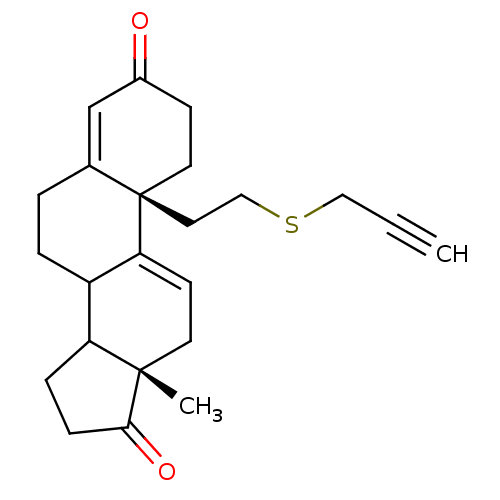 ((2R,15S)-15-methyl-2-[2-(prop-2-yn-1-ylsulfanyl)et...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche de Roussel Uclaf | Assay Description The enzyme activity was assayed by measuring the formation of tritiated water from [1beta, 2beta-3H ]androstenedione in the presence of increasing co... | J Med Chem 39: 757-72 (1996) Article DOI: 10.1021/jm950539l BindingDB Entry DOI: 10.7270/Q28C9TGH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM10058 ((2R,15S)-15-methyl-2-{2-[(2-sulfanylethyl)sulfanyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | 7.2 | 37 |
Centre de Recherche de Roussel Uclaf | Assay Description The enzyme activity was assayed by measuring the formation of tritiated water from [1beta, 2beta-3H ]androstenedione in the presence of increasing co... | J Med Chem 39: 757-72 (1996) Article DOI: 10.1021/jm950539l BindingDB Entry DOI: 10.7270/Q28C9TGH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM10060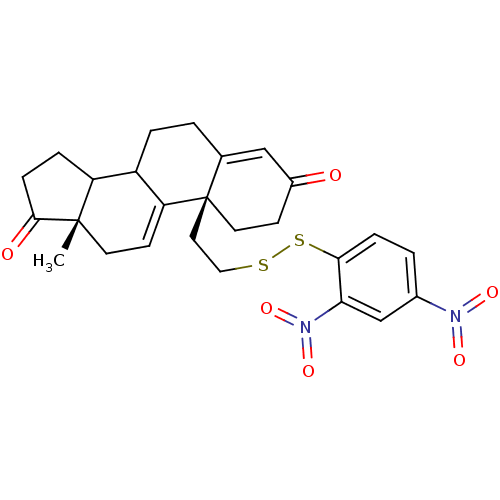 ((2R,15S)-2-{2-[(2,4-dinitrophenyl)disulfanyl]ethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche de Roussel Uclaf | Assay Description The enzyme activity was assayed by measuring the formation of tritiated water from [1beta, 2beta-3H ]androstenedione in the presence of increasing co... | J Med Chem 39: 757-72 (1996) Article DOI: 10.1021/jm950539l BindingDB Entry DOI: 10.7270/Q28C9TGH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM10057 ((2R,15S)-2-{2-[(2-chloroethyl)sulfanyl]ethyl}-15-m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | 7.2 | 37 |
Centre de Recherche de Roussel Uclaf | Assay Description The enzyme activity was assayed by measuring the formation of tritiated water from [1beta, 2beta-3H ]androstenedione in the presence of increasing co... | J Med Chem 39: 757-72 (1996) Article DOI: 10.1021/jm950539l BindingDB Entry DOI: 10.7270/Q28C9TGH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM10065 ((2R,15S)-15-methyl-2-[2-(methyldisulfanyl)ethyl]te...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche de Roussel Uclaf | Assay Description The enzyme activity was assayed by measuring the formation of tritiated water from [1beta, 2beta-3H ]androstenedione in the presence of increasing co... | J Med Chem 39: 757-72 (1996) Article DOI: 10.1021/jm950539l BindingDB Entry DOI: 10.7270/Q28C9TGH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM10066 ((2R,15S)-15-methyl-2-[2-(phenyldisulfanyl)ethyl]te...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche de Roussel Uclaf | Assay Description The enzyme activity was assayed by measuring the formation of tritiated water from [1beta, 2beta-3H ]androstenedione in the presence of increasing co... | J Med Chem 39: 757-72 (1996) Article DOI: 10.1021/jm950539l BindingDB Entry DOI: 10.7270/Q28C9TGH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM10067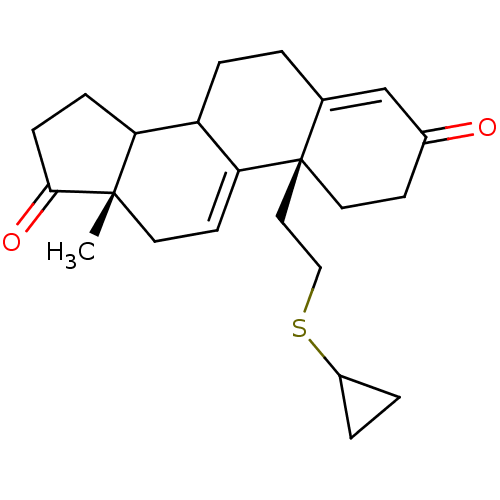 ((2R,15S)-2-[2-(cyclopropylsulfanyl)ethyl]-15-methy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche de Roussel Uclaf | Assay Description The enzyme activity was assayed by measuring the formation of tritiated water from [1beta, 2beta-3H ]androstenedione in the presence of increasing co... | J Med Chem 39: 757-72 (1996) Article DOI: 10.1021/jm950539l BindingDB Entry DOI: 10.7270/Q28C9TGH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM10056 ((2R,15S)-2-{2-[(2-hydroxyethyl)sulfanyl]ethyl}-15-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | 7.2 | 37 |
Centre de Recherche de Roussel Uclaf | Assay Description The enzyme activity was assayed by measuring the formation of tritiated water from [1beta, 2beta-3H ]androstenedione in the presence of increasing co... | J Med Chem 39: 757-72 (1996) Article DOI: 10.1021/jm950539l BindingDB Entry DOI: 10.7270/Q28C9TGH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM10070 (({2-[(2R,15S)-15-methyl-5,14-dioxotetracyclo[8.7.0...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche de Roussel Uclaf | Assay Description The enzyme activity was assayed by measuring the formation of tritiated water from [1beta, 2beta-3H ]androstenedione in the presence of increasing co... | J Med Chem 39: 757-72 (1996) Article DOI: 10.1021/jm950539l BindingDB Entry DOI: 10.7270/Q28C9TGH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 2 (Homo sapiens (Human)) | BDBM50101144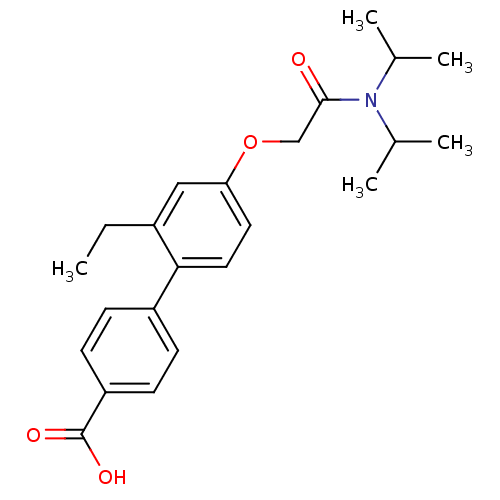 (4'-[(Diisopropylcarbamoyl)-methoxy]-2'-ethyl-biphe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Curated by ChEMBL | Assay Description Compound was tested for the inhibition of type 2 5-alpha-reductase of human prostates | Bioorg Med Chem Lett 11: 1713-6 (2001) BindingDB Entry DOI: 10.7270/Q2ZC83C9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM10043 ((2R,15S)-15-methyl-2-(methylsulfanyl)tetracyclo[8....) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.40E+3 | n/a | n/a | n/a | n/a | 7.2 | 37 |
Centre de Recherche de Roussel Uclaf | Assay Description The enzyme activity was assayed by measuring the formation of tritiated water from [1beta, 2beta-3H ]androstenedione in the presence of increasing co... | J Med Chem 39: 757-72 (1996) Article DOI: 10.1021/jm950539l BindingDB Entry DOI: 10.7270/Q28C9TGH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM10048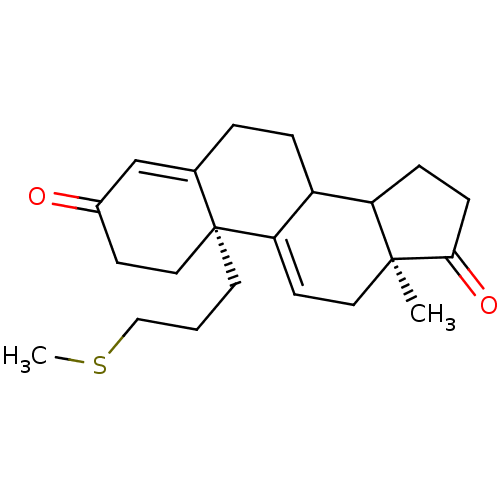 ((2R,15S)-15-methyl-2-[3-(methylsulfanyl)propyl]tet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | 7.2 | 37 |
Centre de Recherche de Roussel Uclaf | Assay Description The enzyme activity was assayed by measuring the formation of tritiated water from [1beta, 2beta-3H ]androstenedione in the presence of increasing co... | J Med Chem 39: 757-72 (1996) Article DOI: 10.1021/jm950539l BindingDB Entry DOI: 10.7270/Q28C9TGH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM10049 ((2R,15S)-2-[2-(dimethylamino)ethyl]-15-methyltetra...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | 7.2 | 37 |
Centre de Recherche de Roussel Uclaf | Assay Description The enzyme activity was assayed by measuring the formation of tritiated water from [1beta, 2beta-3H ]androstenedione in the presence of increasing co... | J Med Chem 39: 757-72 (1996) Article DOI: 10.1021/jm950539l BindingDB Entry DOI: 10.7270/Q28C9TGH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM10050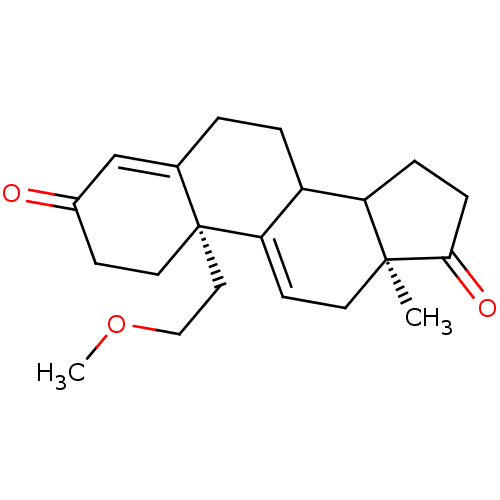 ((2R,15S)-2-(2-methoxyethyl)-15-methyltetracyclo[8....) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | 7.2 | 37 |
Centre de Recherche de Roussel Uclaf | Assay Description The enzyme activity was assayed by measuring the formation of tritiated water from [1beta, 2beta-3H ]androstenedione in the presence of increasing co... | J Med Chem 39: 757-72 (1996) Article DOI: 10.1021/jm950539l BindingDB Entry DOI: 10.7270/Q28C9TGH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM10051 ((2R,15S)-2-(2-iodoethyl)-15-methyltetracyclo[8.7.0...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | 7.2 | 37 |
Centre de Recherche de Roussel Uclaf | Assay Description The enzyme activity was assayed by measuring the formation of tritiated water from [1beta, 2beta-3H ]androstenedione in the presence of increasing co... | J Med Chem 39: 757-72 (1996) Article DOI: 10.1021/jm950539l BindingDB Entry DOI: 10.7270/Q28C9TGH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM10052 (19-Heteromethyl-Substituted Androstenedione Analog...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | 7.2 | 37 |
Centre de Recherche de Roussel Uclaf | Assay Description The enzyme activity was assayed by measuring the formation of tritiated water from [1beta, 2beta-3H ]androstenedione in the presence of increasing co... | J Med Chem 39: 757-72 (1996) Article DOI: 10.1021/jm950539l BindingDB Entry DOI: 10.7270/Q28C9TGH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 2 (Homo sapiens (Human)) | BDBM50101151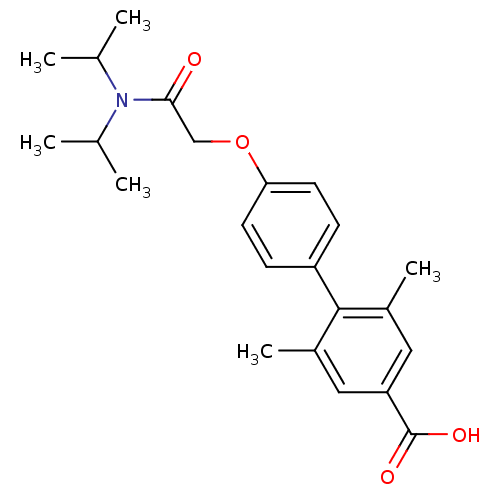 (4'-[(Diisopropylcarbamoyl)-methoxy]-2,6-dimethyl-b...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Curated by ChEMBL | Assay Description Compound was tested for the inhibition of Steroid 5-alpha-reductase type 2 of human prostates | Bioorg Med Chem Lett 11: 1713-6 (2001) BindingDB Entry DOI: 10.7270/Q2ZC83C9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 2 (Homo sapiens (Human)) | BDBM50101150 (2',6'-Dichloro-4'-[(diisopropylcarbamoyl)-methoxy]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Curated by ChEMBL | Assay Description Compound was tested for the inhibition of Steroid 5-alpha-reductase type 2 of human prostates | Bioorg Med Chem Lett 11: 1713-6 (2001) BindingDB Entry DOI: 10.7270/Q2ZC83C9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 2 (Homo sapiens (Human)) | BDBM50101148 (4'-[(Diisopropylcarbamoyl)-methoxy]-2,6-difluoro-b...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Curated by ChEMBL | Assay Description Compound was tested for the inhibition of Steroid 5-alpha-reductase type 2 of human prostates | Bioorg Med Chem Lett 11: 1713-6 (2001) BindingDB Entry DOI: 10.7270/Q2ZC83C9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM10055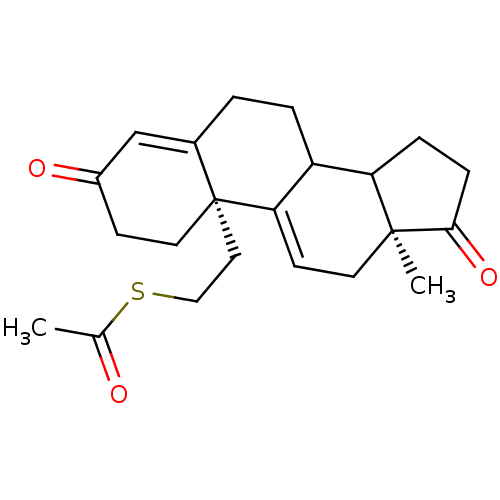 ((2R,15S)-2-[2-(acetylsulfanyl)ethyl]-15-methyltetr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | 7.2 | 37 |
Centre de Recherche de Roussel Uclaf | Assay Description The enzyme activity was assayed by measuring the formation of tritiated water from [1beta, 2beta-3H ]androstenedione in the presence of increasing co... | J Med Chem 39: 757-72 (1996) Article DOI: 10.1021/jm950539l BindingDB Entry DOI: 10.7270/Q28C9TGH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 2 (Homo sapiens (Human)) | BDBM50101147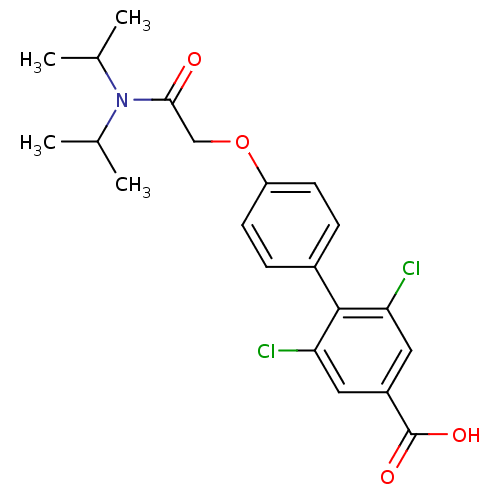 (2,6-Dichloro-4'-[(diisopropylcarbamoyl)-methoxy]-b...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Curated by ChEMBL | Assay Description Compound was tested for the inhibition of Steroid 5-alpha-reductase type 2 of human prostates | Bioorg Med Chem Lett 11: 1713-6 (2001) BindingDB Entry DOI: 10.7270/Q2ZC83C9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50471261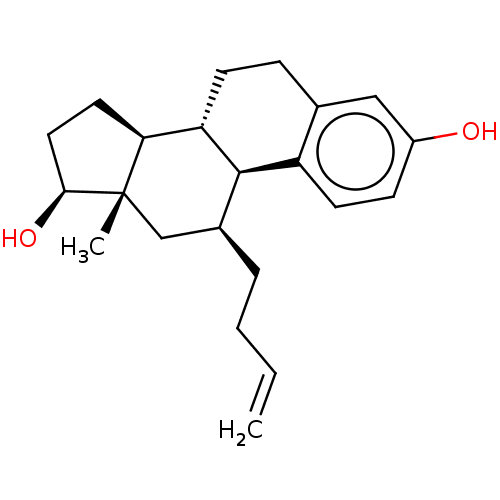 (CHEMBL1627868) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | <10 | n/a | n/a | n/a | n/a |
INSERM Unit� 439 Curated by ChEMBL | Assay Description Compound concentration required to induce transcriptional activation in MVLN cells comparable to 50% of 0.1 nM estradiol response | J Med Chem 40: 2217-27 (1997) Article DOI: 10.1021/jm970019l BindingDB Entry DOI: 10.7270/Q20G3NVN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 52 total ) | Next | Last >> |