 Found 71 hits with Last Name = 'thrall' and Initial = 'sh'
Found 71 hits with Last Name = 'thrall' and Initial = 'sh' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Peptide deformylase
(Streptococcus pneumoniae (strain ATCC BAA-255 / R6...) | BDBM92766
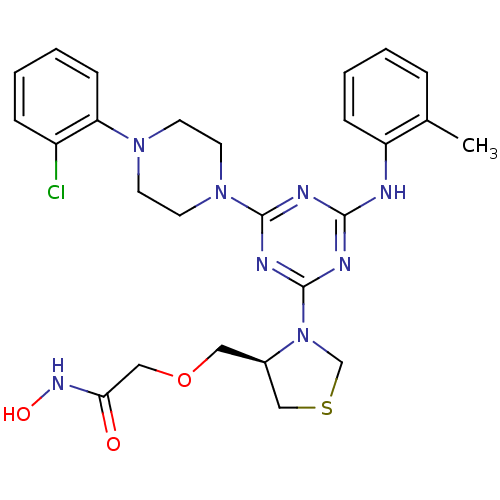
(PDF inhibitor, compound 8)Show SMILES Cc1ccccc1Nc1nc(nc(n1)N1CCN(CC1)c1ccccc1Cl)N1CSC[C@H]1COCC(=O)NO |r| Show InChI InChI=1S/C26H31ClN8O3S/c1-18-6-2-4-8-21(18)28-24-29-25(34-12-10-33(11-13-34)22-9-5-3-7-20(22)27)31-26(30-24)35-17-39-16-19(35)14-38-15-23(36)32-37/h2-9,19,37H,10-17H2,1H3,(H,32,36)(H,28,29,30,31)/t19-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 53.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
| Assay Description
Binding assay using PDF inhibitors. |
Biochemistry 50: 6642-54 (2011)
Article DOI: 10.1021/bi200655g
BindingDB Entry DOI: 10.7270/Q2MS3RBV |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Peptide deformylase
(Streptococcus pneumoniae (strain ATCC BAA-255 / R6...) | BDBM92761
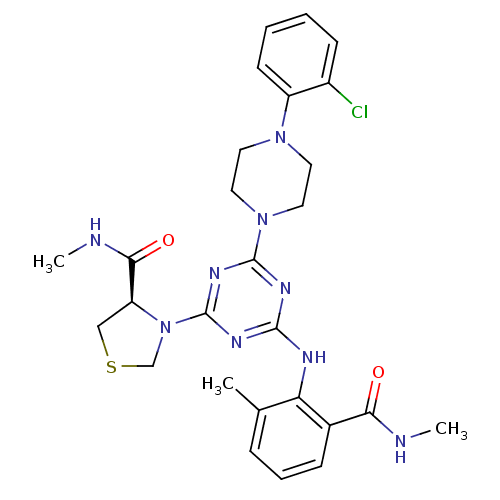
(PDF inhibitor, compound 3)Show SMILES CNC(=O)[C@@H]1CSCN1c1nc(Nc2c(C)cccc2C(=O)NC)nc(n1)N1CCN(CC1)c1ccccc1Cl |r| Show InChI InChI=1S/C27H32ClN9O2S/c1-17-7-6-8-18(23(38)29-2)22(17)31-25-32-26(34-27(33-25)37-16-40-15-21(37)24(39)30-3)36-13-11-35(12-14-36)20-10-5-4-9-19(20)28/h4-10,21H,11-16H2,1-3H3,(H,29,38)(H,30,39)(H,31,32,33,34)/t21-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 117 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
| Assay Description
Binding assay using PDF inhibitors. |
Biochemistry 50: 6642-54 (2011)
Article DOI: 10.1021/bi200655g
BindingDB Entry DOI: 10.7270/Q2MS3RBV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peptide deformylase
(Streptococcus pneumoniae (strain ATCC BAA-255 / R6...) | BDBM92764
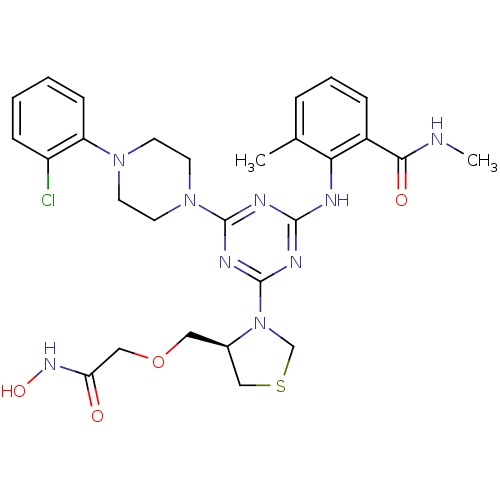
(PDF inhibitor, compound 6)Show SMILES CNC(=O)c1cccc(C)c1Nc1nc(nc(n1)N1CCN(CC1)c1ccccc1Cl)N1CSC[C@H]1COCC(=O)NO |r| Show InChI InChI=1S/C28H34ClN9O4S/c1-18-6-5-7-20(25(40)30-2)24(18)31-26-32-27(37-12-10-36(11-13-37)22-9-4-3-8-21(22)29)34-28(33-26)38-17-43-16-19(38)14-42-15-23(39)35-41/h3-9,19,41H,10-17H2,1-2H3,(H,30,40)(H,35,39)(H,31,32,33,34)/t19-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 213 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
| Assay Description
Binding assay using PDF inhibitors. |
Biochemistry 50: 6642-54 (2011)
Article DOI: 10.1021/bi200655g
BindingDB Entry DOI: 10.7270/Q2MS3RBV |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Peptide deformylase
(Streptococcus pneumoniae (strain ATCC BAA-255 / R6...) | BDBM92760
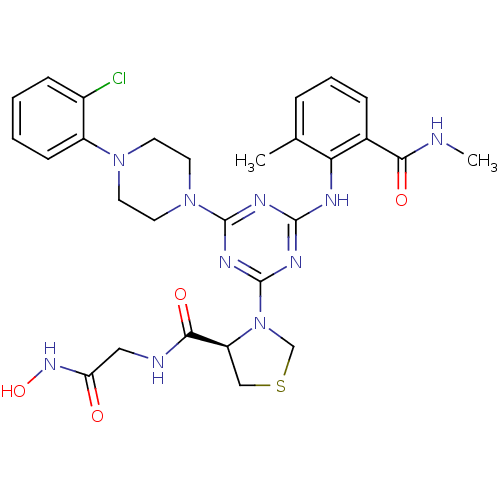
(PDF inhibitor, compound 2)Show SMILES CNC(=O)c1cccc(C)c1Nc1nc(nc(n1)N1CCN(CC1)c1ccccc1Cl)N1CSC[C@H]1C(=O)NCC(=O)NO |r| Show InChI InChI=1S/C28H33ClN10O4S/c1-17-6-5-7-18(24(41)30-2)23(17)32-26-33-27(38-12-10-37(11-13-38)20-9-4-3-8-19(20)29)35-28(34-26)39-16-44-15-21(39)25(42)31-14-22(40)36-43/h3-9,21,43H,10-16H2,1-2H3,(H,30,41)(H,31,42)(H,36,40)(H,32,33,34,35)/t21-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 334 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
| Assay Description
Binding assay using PDF inhibitors. |
Biochemistry 50: 6642-54 (2011)
Article DOI: 10.1021/bi200655g
BindingDB Entry DOI: 10.7270/Q2MS3RBV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peptide deformylase
(Streptococcus pneumoniae (strain ATCC BAA-255 / R6...) | BDBM92765
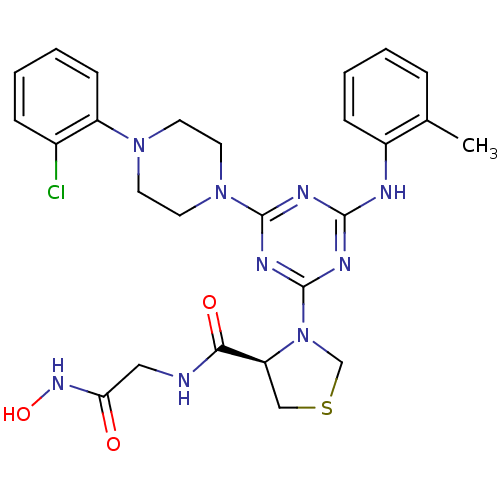
(PDF inhibitor, compound 7)Show SMILES Cc1ccccc1Nc1nc(nc(n1)N1CCN(CC1)c1ccccc1Cl)N1CSC[C@H]1C(=O)NCC(=O)NO |r| Show InChI InChI=1S/C26H30ClN9O3S/c1-17-6-2-4-8-19(17)29-24-30-25(35-12-10-34(11-13-35)20-9-5-3-7-18(20)27)32-26(31-24)36-16-40-15-21(36)23(38)28-14-22(37)33-39/h2-9,21,39H,10-16H2,1H3,(H,28,38)(H,33,37)(H,29,30,31,32)/t21-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
| Assay Description
Binding assay using PDF inhibitors. |
Biochemistry 50: 6642-54 (2011)
Article DOI: 10.1021/bi200655g
BindingDB Entry DOI: 10.7270/Q2MS3RBV |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Peptide deformylase
(Streptococcus pneumoniae (strain ATCC BAA-255 / R6...) | BDBM92759
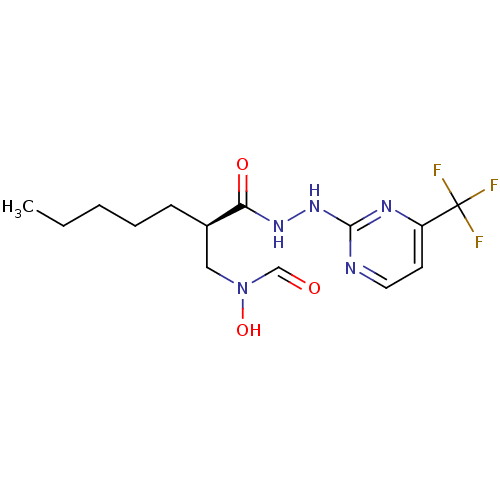
(PDF inhibitor, compound 1)Show SMILES CCCCC[C@H](CN(O)C=O)C(=O)NNc1nccc(n1)C(F)(F)F |r| Show InChI InChI=1S/C14H20F3N5O3/c1-2-3-4-5-10(8-22(25)9-23)12(24)20-21-13-18-7-6-11(19-13)14(15,16)17/h6-7,9-10,25H,2-5,8H2,1H3,(H,20,24)(H,18,19,21)/t10-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6.31E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
| Assay Description
Binding assay using PDF inhibitors. |
Biochemistry 50: 6642-54 (2011)
Article DOI: 10.1021/bi200655g
BindingDB Entry DOI: 10.7270/Q2MS3RBV |
More data for this
Ligand-Target Pair | |
Peptide deformylase
(Streptococcus pneumoniae (strain ATCC BAA-255 / R6...) | BDBM92762
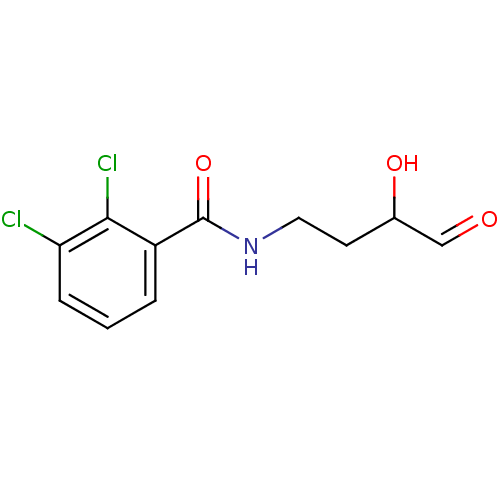
(PDF inhibitor, compound 4)Show InChI InChI=1S/C11H11Cl2NO3/c12-9-3-1-2-8(10(9)13)11(17)14-5-4-7(16)6-15/h1-3,6-7,16H,4-5H2,(H,14,17) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 2.59E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
| Assay Description
Binding assay using PDF inhibitors. |
Biochemistry 50: 6642-54 (2011)
Article DOI: 10.1021/bi200655g
BindingDB Entry DOI: 10.7270/Q2MS3RBV |
More data for this
Ligand-Target Pair | |
Peptide deformylase
(Streptococcus pneumoniae (strain ATCC BAA-255 / R6...) | BDBM92763
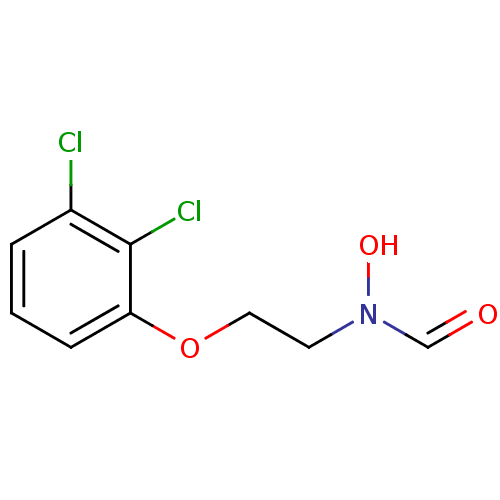
(PDF inhibitor, compound 5)Show InChI InChI=1S/C9H9Cl2NO3/c10-7-2-1-3-8(9(7)11)15-5-4-12(14)6-13/h1-3,6,14H,4-5H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.17E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
| Assay Description
Binding assay using PDF inhibitors. |
Biochemistry 50: 6642-54 (2011)
Article DOI: 10.1021/bi200655g
BindingDB Entry DOI: 10.7270/Q2MS3RBV |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM27725
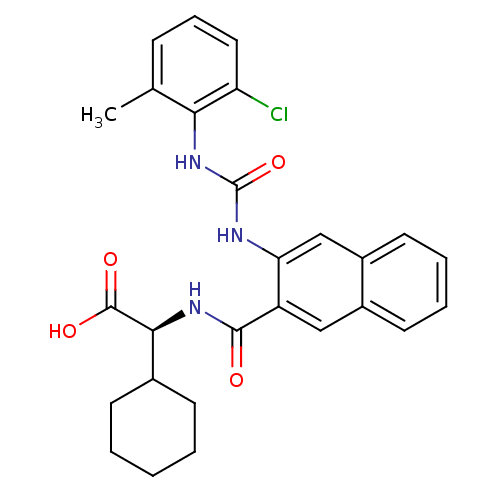
((2S)-2-[(3-{[(2-chloro-6-methylphenyl)carbamoyl]am...)Show SMILES Cc1cccc(Cl)c1NC(=O)Nc1cc2ccccc2cc1C(=O)N[C@@H](C1CCCCC1)C(O)=O |r| Show InChI InChI=1S/C27H28ClN3O4/c1-16-8-7-13-21(28)23(16)31-27(35)29-22-15-19-12-6-5-11-18(19)14-20(22)25(32)30-24(26(33)34)17-9-3-2-4-10-17/h5-8,11-15,17,24H,2-4,9-10H2,1H3,(H,30,32)(H,33,34)(H2,29,31,35)/t24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human glycogen phosphorylase alpha |
Bioorg Med Chem Lett 18: 4068-71 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.102
BindingDB Entry DOI: 10.7270/Q23R0SPM |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50243599
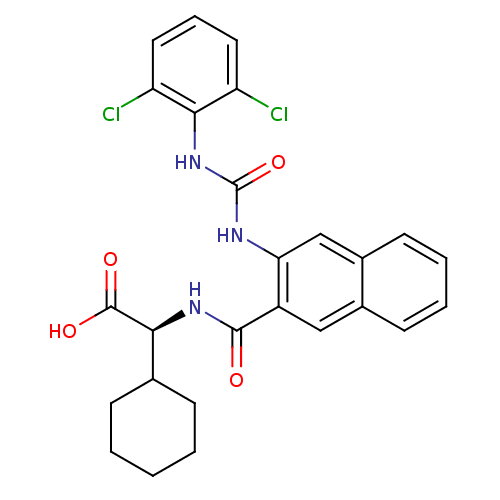
((S)-2-cyclohexyl-2-(2-(3-(2,6-dichlorophenyl)ureid...)Show SMILES OC(=O)[C@@H](NC(=O)c1cc2ccccc2cc1NC(=O)Nc1c(Cl)cccc1Cl)C1CCCCC1 |r| Show InChI InChI=1S/C26H25Cl2N3O4/c27-19-11-6-12-20(28)23(19)31-26(35)29-21-14-17-10-5-4-9-16(17)13-18(21)24(32)30-22(25(33)34)15-7-2-1-3-8-15/h4-6,9-15,22H,1-3,7-8H2,(H,30,32)(H,33,34)(H2,29,31,35)/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human glycogen phosphorylase alpha |
Bioorg Med Chem Lett 18: 4068-71 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.102
BindingDB Entry DOI: 10.7270/Q23R0SPM |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50243678
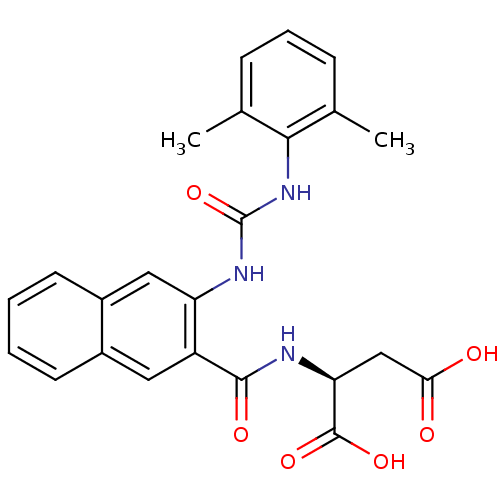
((S)-2-(2-(3-(2,6-dimethylphenyl)ureido)-2-naphtham...)Show SMILES Cc1cccc(C)c1NC(=O)Nc1cc2ccccc2cc1C(=O)N[C@@H](CC(O)=O)C(O)=O |r| Show InChI InChI=1S/C24H23N3O6/c1-13-6-5-7-14(2)21(13)27-24(33)26-18-11-16-9-4-3-8-15(16)10-17(18)22(30)25-19(23(31)32)12-20(28)29/h3-11,19H,12H2,1-2H3,(H,25,30)(H,28,29)(H,31,32)(H2,26,27,33)/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human glycogen phosphorylase alpha |
Bioorg Med Chem Lett 18: 4068-71 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.102
BindingDB Entry DOI: 10.7270/Q23R0SPM |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50243601
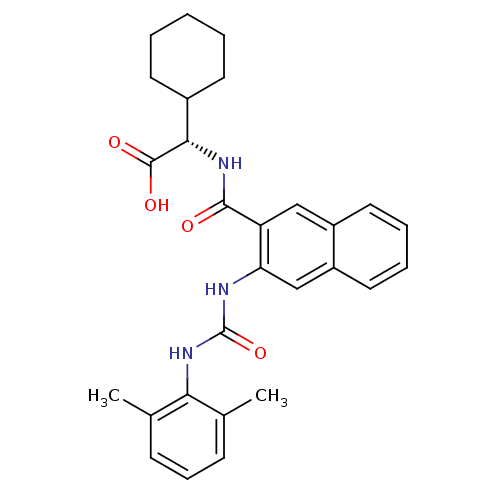
((S)-2-cyclohexyl-2-(2-(3-(2,6-dimethylphenyl)ureid...)Show SMILES Cc1cccc(C)c1NC(=O)Nc1cc2ccccc2cc1C(=O)N[C@@H](C1CCCCC1)C(O)=O |r| Show InChI InChI=1S/C28H31N3O4/c1-17-9-8-10-18(2)24(17)31-28(35)29-23-16-21-14-7-6-13-20(21)15-22(23)26(32)30-25(27(33)34)19-11-4-3-5-12-19/h6-10,13-16,19,25H,3-5,11-12H2,1-2H3,(H,30,32)(H,33,34)(H2,29,31,35)/t25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human glycogen phosphorylase alpha |
Bioorg Med Chem Lett 18: 4068-71 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.102
BindingDB Entry DOI: 10.7270/Q23R0SPM |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50243677
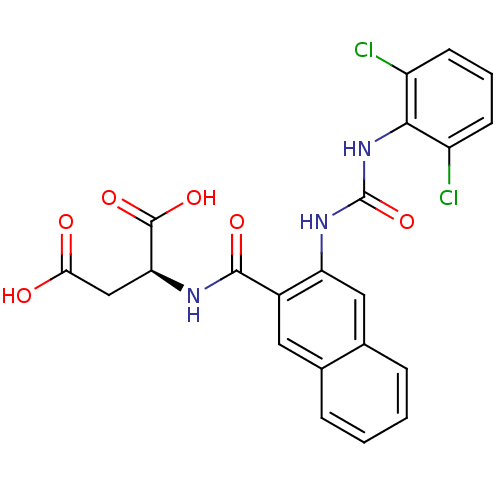
((S)-2-(2-(3-(2,6-dichlorophenyl)ureido)-2-naphtham...)Show SMILES OC(=O)C[C@H](NC(=O)c1cc2ccccc2cc1NC(=O)Nc1c(Cl)cccc1Cl)C(O)=O |r| Show InChI InChI=1S/C22H17Cl2N3O6/c23-14-6-3-7-15(24)19(14)27-22(33)26-16-9-12-5-2-1-4-11(12)8-13(16)20(30)25-17(21(31)32)10-18(28)29/h1-9,17H,10H2,(H,25,30)(H,28,29)(H,31,32)(H2,26,27,33)/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human glycogen phosphorylase alpha |
Bioorg Med Chem Lett 18: 4068-71 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.102
BindingDB Entry DOI: 10.7270/Q23R0SPM |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50243352
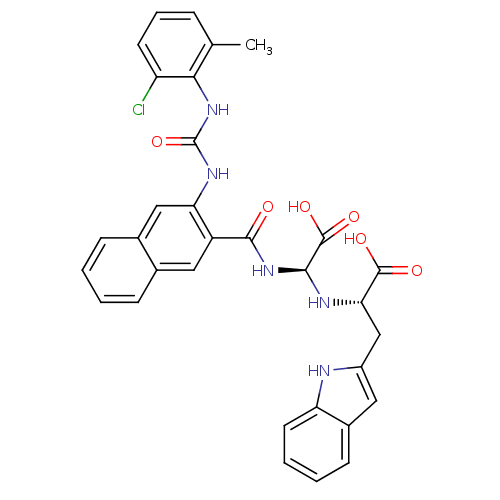
((S)-2-((S)-carboxy(2-(3-(2-chloro-6-methylphenyl)u...)Show SMILES Cc1cccc(Cl)c1NC(=O)Nc1cc2ccccc2cc1C(=O)N[C@H](N[C@@H](Cc1cc2ccccc2[nH]1)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C32H28ClN5O6/c1-17-7-6-11-23(33)27(17)37-32(44)36-25-15-19-9-3-2-8-18(19)14-22(25)29(39)38-28(31(42)43)35-26(30(40)41)16-21-13-20-10-4-5-12-24(20)34-21/h2-15,26,28,34-35H,16H2,1H3,(H,38,39)(H,40,41)(H,42,43)(H2,36,37,44)/t26-,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human glycogen phosphorylase alpha |
Bioorg Med Chem Lett 18: 4068-71 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.102
BindingDB Entry DOI: 10.7270/Q23R0SPM |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50243353
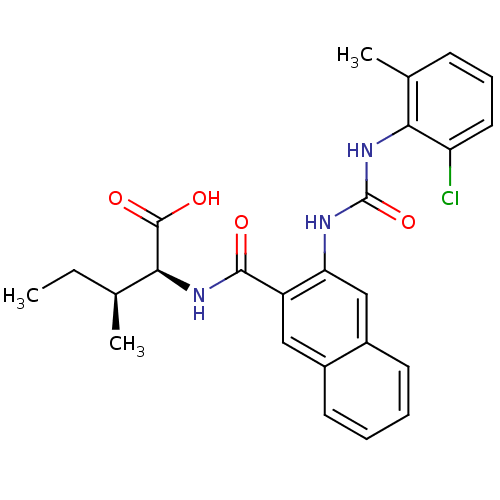
((2S,3S)-2-(2-(3-(2-chloro-6-methylphenyl)ureido)-2...)Show SMILES CC[C@H](C)[C@H](NC(=O)c1cc2ccccc2cc1NC(=O)Nc1c(C)cccc1Cl)C(O)=O |r| Show InChI InChI=1S/C25H26ClN3O4/c1-4-14(2)22(24(31)32)28-23(30)18-12-16-9-5-6-10-17(16)13-20(18)27-25(33)29-21-15(3)8-7-11-19(21)26/h5-14,22H,4H2,1-3H3,(H,28,30)(H,31,32)(H2,27,29,33)/t14-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human glycogen phosphorylase alpha |
Bioorg Med Chem Lett 18: 4068-71 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.102
BindingDB Entry DOI: 10.7270/Q23R0SPM |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50243599

((S)-2-cyclohexyl-2-(2-(3-(2,6-dichlorophenyl)ureid...)Show SMILES OC(=O)[C@@H](NC(=O)c1cc2ccccc2cc1NC(=O)Nc1c(Cl)cccc1Cl)C1CCCCC1 |r| Show InChI InChI=1S/C26H25Cl2N3O4/c27-19-11-6-12-20(28)23(19)31-26(35)29-21-14-17-10-5-4-9-16(17)13-18(21)24(32)30-22(25(33)34)15-7-2-1-3-8-15/h4-6,9-15,22H,1-3,7-8H2,(H,30,32)(H,33,34)(H2,29,31,35)/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human glycogen phosphorylase alpha in HepG2 cells assessed as inhibition of forskolin-induced glycogenolysis after 60 mins |
Bioorg Med Chem Lett 18: 4068-71 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.102
BindingDB Entry DOI: 10.7270/Q23R0SPM |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50243354
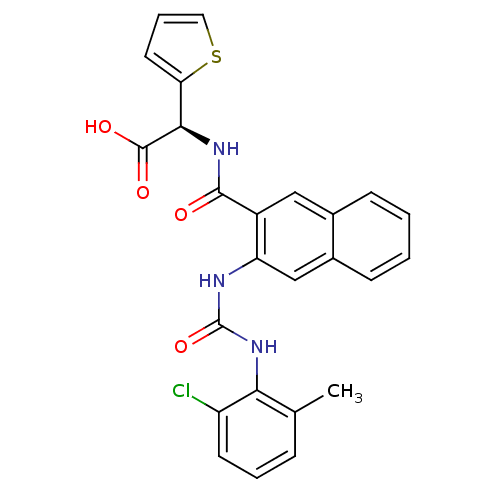
((S)-2-(2-(3-(2-chloro-6-methylphenyl)ureido)-2-nap...)Show SMILES Cc1cccc(Cl)c1NC(=O)Nc1cc2ccccc2cc1C(=O)N[C@@H](C(O)=O)c1cccs1 |r| Show InChI InChI=1S/C25H20ClN3O4S/c1-14-6-4-9-18(26)21(14)29-25(33)27-19-13-16-8-3-2-7-15(16)12-17(19)23(30)28-22(24(31)32)20-10-5-11-34-20/h2-13,22H,1H3,(H,28,30)(H,31,32)(H2,27,29,33)/t22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human glycogen phosphorylase alpha |
Bioorg Med Chem Lett 18: 4068-71 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.102
BindingDB Entry DOI: 10.7270/Q23R0SPM |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50243399
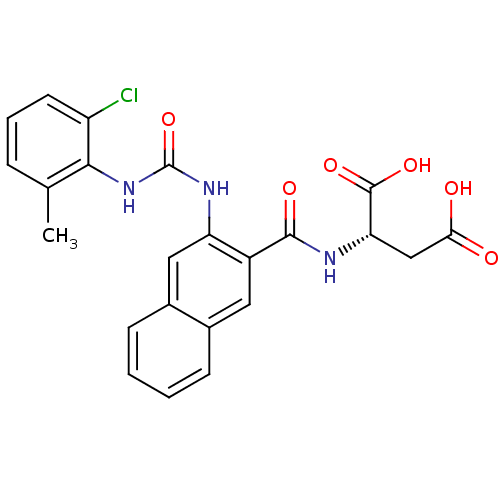
((S)-2-(2-(3-(2-chloro-6-methylphenyl)ureido)-2-nap...)Show SMILES Cc1cccc(Cl)c1NC(=O)Nc1cc2ccccc2cc1C(=O)N[C@@H](CC(O)=O)C(O)=O |r| Show InChI InChI=1S/C23H20ClN3O6/c1-12-5-4-8-16(24)20(12)27-23(33)26-17-10-14-7-3-2-6-13(14)9-15(17)21(30)25-18(22(31)32)11-19(28)29/h2-10,18H,11H2,1H3,(H,25,30)(H,28,29)(H,31,32)(H2,26,27,33)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human glycogen phosphorylase alpha |
Bioorg Med Chem Lett 18: 4068-71 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.102
BindingDB Entry DOI: 10.7270/Q23R0SPM |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50243399

((S)-2-(2-(3-(2-chloro-6-methylphenyl)ureido)-2-nap...)Show SMILES Cc1cccc(Cl)c1NC(=O)Nc1cc2ccccc2cc1C(=O)N[C@@H](CC(O)=O)C(O)=O |r| Show InChI InChI=1S/C23H20ClN3O6/c1-12-5-4-8-16(24)20(12)27-23(33)26-17-10-14-7-3-2-6-13(14)9-15(17)21(30)25-18(22(31)32)11-19(28)29/h2-10,18H,11H2,1H3,(H,25,30)(H,28,29)(H,31,32)(H2,26,27,33)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human glycogen phosphorylase alpha in presence of glucose |
Bioorg Med Chem Lett 18: 4068-71 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.102
BindingDB Entry DOI: 10.7270/Q23R0SPM |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50243400
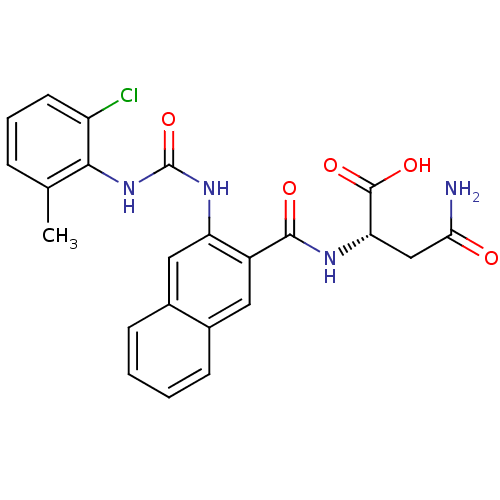
((S)-4-amino-2-(2-(3-(2-chloro-6-methylphenyl)ureid...)Show SMILES Cc1cccc(Cl)c1NC(=O)Nc1cc2ccccc2cc1C(=O)N[C@@H](CC(N)=O)C(O)=O |r| Show InChI InChI=1S/C23H21ClN4O5/c1-12-5-4-8-16(24)20(12)28-23(33)27-17-10-14-7-3-2-6-13(14)9-15(17)21(30)26-18(22(31)32)11-19(25)29/h2-10,18H,11H2,1H3,(H2,25,29)(H,26,30)(H,31,32)(H2,27,28,33)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human glycogen phosphorylase alpha |
Bioorg Med Chem Lett 18: 4068-71 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.102
BindingDB Entry DOI: 10.7270/Q23R0SPM |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50243401
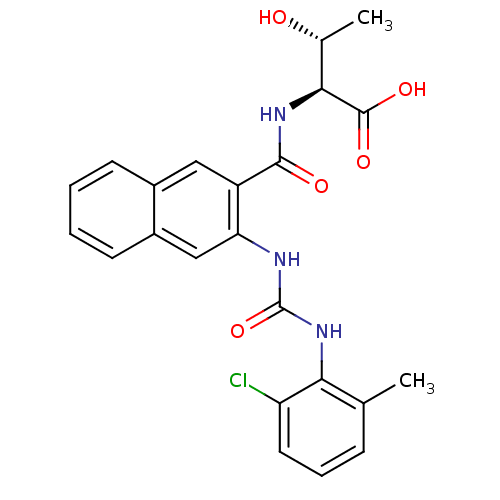
((2S,3R)-2-(2-(3-(2-chloro-6-methylphenyl)ureido)-2...)Show SMILES C[C@@H](O)[C@H](NC(=O)c1cc2ccccc2cc1NC(=O)Nc1c(C)cccc1Cl)C(O)=O |r| Show InChI InChI=1S/C23H22ClN3O5/c1-12-6-5-9-17(24)19(12)27-23(32)25-18-11-15-8-4-3-7-14(15)10-16(18)21(29)26-20(13(2)28)22(30)31/h3-11,13,20,28H,1-2H3,(H,26,29)(H,30,31)(H2,25,27,32)/t13-,20+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 430 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human glycogen phosphorylase alpha |
Bioorg Med Chem Lett 18: 4068-71 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.102
BindingDB Entry DOI: 10.7270/Q23R0SPM |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50243402
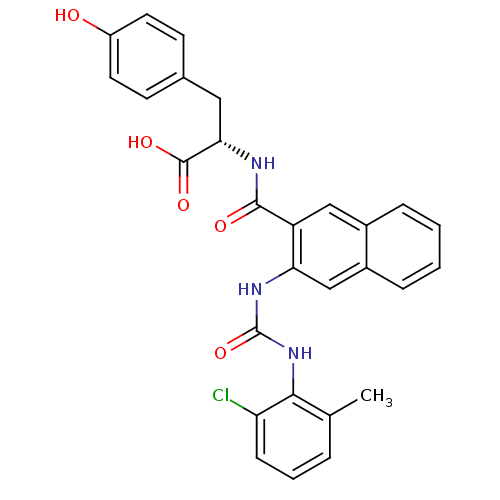
((S)-2-(2-(3-(2-chloro-6-methylphenyl)ureido)-2-nap...)Show SMILES Cc1cccc(Cl)c1NC(=O)Nc1cc2ccccc2cc1C(=O)N[C@@H](Cc1ccc(O)cc1)C(O)=O |r| Show InChI InChI=1S/C28H24ClN3O5/c1-16-5-4-8-22(29)25(16)32-28(37)31-23-15-19-7-3-2-6-18(19)14-21(23)26(34)30-24(27(35)36)13-17-9-11-20(33)12-10-17/h2-12,14-15,24,33H,13H2,1H3,(H,30,34)(H,35,36)(H2,31,32,37)/t24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human glycogen phosphorylase alpha |
Bioorg Med Chem Lett 18: 4068-71 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.102
BindingDB Entry DOI: 10.7270/Q23R0SPM |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50243444
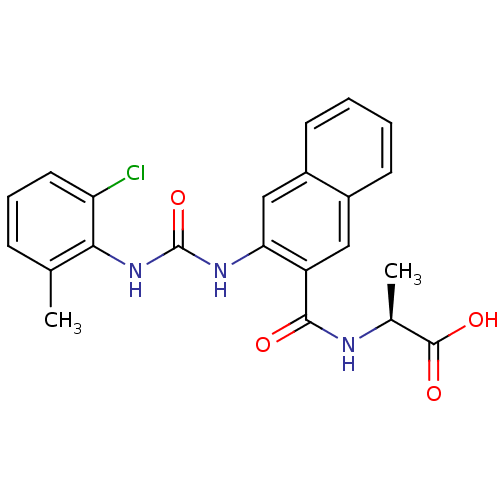
((S)-2-(2-(3-(2-chloro-6-methylphenyl)ureido)-2-nap...)Show SMILES C[C@H](NC(=O)c1cc2ccccc2cc1NC(=O)Nc1c(C)cccc1Cl)C(O)=O |r| Show InChI InChI=1S/C22H20ClN3O4/c1-12-6-5-9-17(23)19(12)26-22(30)25-18-11-15-8-4-3-7-14(15)10-16(18)20(27)24-13(2)21(28)29/h3-11,13H,1-2H3,(H,24,27)(H,28,29)(H2,25,26,30)/t13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 520 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human glycogen phosphorylase alpha |
Bioorg Med Chem Lett 18: 4068-71 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.102
BindingDB Entry DOI: 10.7270/Q23R0SPM |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50243445
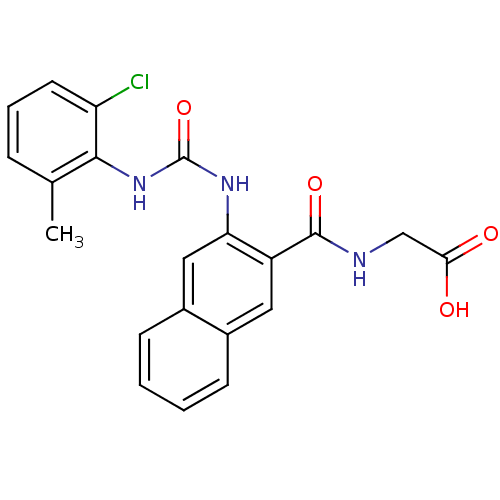
(2-(2-(3-(2-chloro-6-methylphenyl)ureido)-2-naphtha...)Show SMILES Cc1cccc(Cl)c1NC(=O)Nc1cc2ccccc2cc1C(=O)NCC(O)=O Show InChI InChI=1S/C21H18ClN3O4/c1-12-5-4-8-16(22)19(12)25-21(29)24-17-10-14-7-3-2-6-13(14)9-15(17)20(28)23-11-18(26)27/h2-10H,11H2,1H3,(H,23,28)(H,26,27)(H2,24,25,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 570 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human glycogen phosphorylase alpha |
Bioorg Med Chem Lett 18: 4068-71 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.102
BindingDB Entry DOI: 10.7270/Q23R0SPM |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50243446
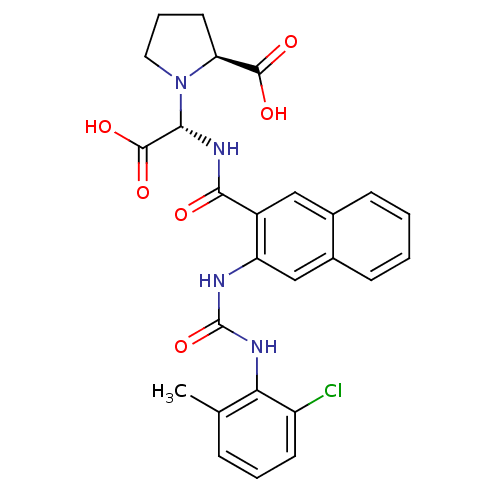
((S)-1-((S)-carboxy(2-(3-(2-chloro-6-methylphenyl)u...)Show SMILES Cc1cccc(Cl)c1NC(=O)Nc1cc2ccccc2cc1C(=O)N[C@@H](N1CCC[C@H]1C(O)=O)C(O)=O |r| Show InChI InChI=1S/C26H25ClN4O6/c1-14-6-4-9-18(27)21(14)29-26(37)28-19-13-16-8-3-2-7-15(16)12-17(19)23(32)30-22(25(35)36)31-11-5-10-20(31)24(33)34/h2-4,6-9,12-13,20,22H,5,10-11H2,1H3,(H,30,32)(H,33,34)(H,35,36)(H2,28,29,37)/t20-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 580 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human glycogen phosphorylase alpha |
Bioorg Med Chem Lett 18: 4068-71 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.102
BindingDB Entry DOI: 10.7270/Q23R0SPM |
More data for this
Ligand-Target Pair | |
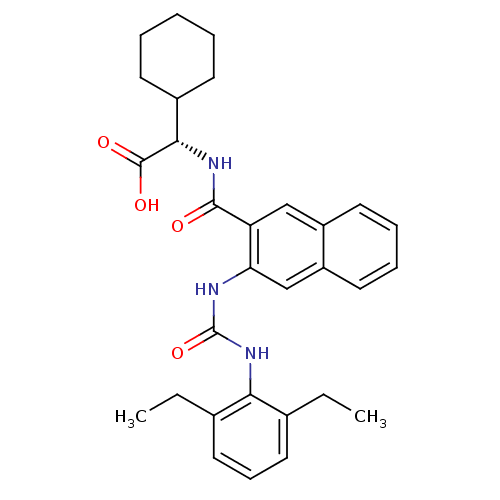
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50243640
((S)-2-cyclohexyl-2-(2-(3-(2,6-diethylphenyl)ureido...)Show SMILES CCc1cccc(CC)c1NC(=O)Nc1cc2ccccc2cc1C(=O)N[C@@H](C1CCCCC1)C(O)=O |r| Show InChI InChI=1S/C30H35N3O4/c1-3-19-15-10-16-20(4-2)26(19)33-30(37)31-25-18-23-14-9-8-13-22(23)17-24(25)28(34)32-27(29(35)36)21-11-6-5-7-12-21/h8-10,13-18,21,27H,3-7,11-12H2,1-2H3,(H,32,34)(H,35,36)(H2,31,33,37)/t27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 590 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human glycogen phosphorylase alpha |
Bioorg Med Chem Lett 18: 4068-71 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.102
BindingDB Entry DOI: 10.7270/Q23R0SPM |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50243447
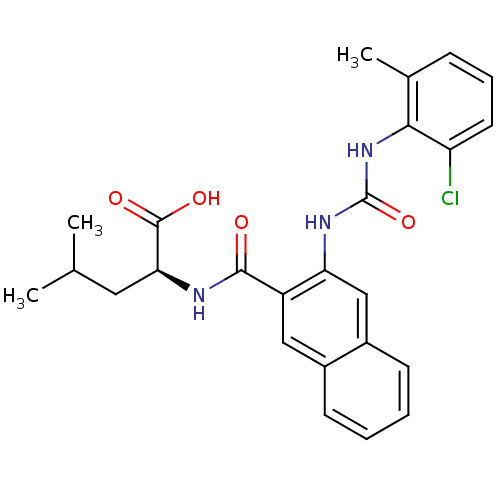
((S)-2-(2-(3-(2-chloro-6-methylphenyl)ureido)-2-nap...)Show SMILES CC(C)C[C@H](NC(=O)c1cc2ccccc2cc1NC(=O)Nc1c(C)cccc1Cl)C(O)=O |r| Show InChI InChI=1S/C25H26ClN3O4/c1-14(2)11-21(24(31)32)27-23(30)18-12-16-8-4-5-9-17(16)13-20(18)28-25(33)29-22-15(3)7-6-10-19(22)26/h4-10,12-14,21H,11H2,1-3H3,(H,27,30)(H,31,32)(H2,28,29,33)/t21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human glycogen phosphorylase alpha |
Bioorg Med Chem Lett 18: 4068-71 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.102
BindingDB Entry DOI: 10.7270/Q23R0SPM |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM27725

((2S)-2-[(3-{[(2-chloro-6-methylphenyl)carbamoyl]am...)Show SMILES Cc1cccc(Cl)c1NC(=O)Nc1cc2ccccc2cc1C(=O)N[C@@H](C1CCCCC1)C(O)=O |r| Show InChI InChI=1S/C27H28ClN3O4/c1-16-8-7-13-21(28)23(16)31-27(35)29-22-15-19-12-6-5-11-18(19)14-20(22)25(32)30-24(26(33)34)17-9-3-2-4-10-17/h5-8,11-15,17,24H,2-4,9-10H2,1H3,(H,30,32)(H,33,34)(H2,29,31,35)/t24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human glycogen phosphorylase alpha in HepG2 cells assessed as inhibition of forskolin-induced glycogenolysis after 60 mins |
Bioorg Med Chem Lett 18: 4068-71 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.102
BindingDB Entry DOI: 10.7270/Q23R0SPM |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50243677

((S)-2-(2-(3-(2,6-dichlorophenyl)ureido)-2-naphtham...)Show SMILES OC(=O)C[C@H](NC(=O)c1cc2ccccc2cc1NC(=O)Nc1c(Cl)cccc1Cl)C(O)=O |r| Show InChI InChI=1S/C22H17Cl2N3O6/c23-14-6-3-7-15(24)19(14)27-22(33)26-16-9-12-5-2-1-4-11(12)8-13(16)20(30)25-17(21(31)32)10-18(28)29/h1-9,17H,10H2,(H,25,30)(H,28,29)(H,31,32)(H2,26,27,33)/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 640 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human glycogen phosphorylase alpha in HepG2 cells assessed as inhibition of forskolin-induced glycogenolysis after 60 mins |
Bioorg Med Chem Lett 18: 4068-71 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.102
BindingDB Entry DOI: 10.7270/Q23R0SPM |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50243306
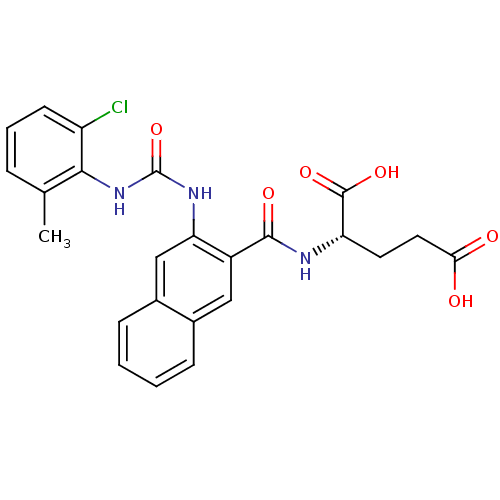
((S)-2-(2-(3-(2-chloro-6-methylphenyl)ureido)-2-nap...)Show SMILES Cc1cccc(Cl)c1NC(=O)Nc1cc2ccccc2cc1C(=O)N[C@@H](CCC(O)=O)C(O)=O |r| Show InChI InChI=1S/C24H22ClN3O6/c1-13-5-4-8-17(25)21(13)28-24(34)27-19-12-15-7-3-2-6-14(15)11-16(19)22(31)26-18(23(32)33)9-10-20(29)30/h2-8,11-12,18H,9-10H2,1H3,(H,26,31)(H,29,30)(H,32,33)(H2,27,28,34)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human glycogen phosphorylase alpha |
Bioorg Med Chem Lett 18: 4068-71 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.102
BindingDB Entry DOI: 10.7270/Q23R0SPM |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50243548
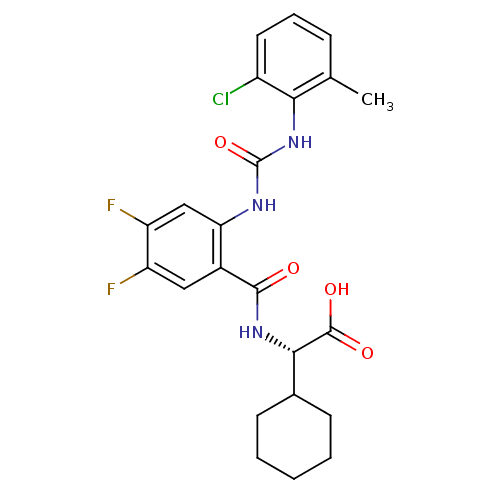
((S)-2-(2-(3-(2-chloro-6-methylphenyl)ureido)-4,5-d...)Show SMILES Cc1cccc(Cl)c1NC(=O)Nc1cc(F)c(F)cc1C(=O)N[C@@H](C1CCCCC1)C(O)=O |r| Show InChI InChI=1S/C23H24ClF2N3O4/c1-12-6-5-9-15(24)19(12)29-23(33)27-18-11-17(26)16(25)10-14(18)21(30)28-20(22(31)32)13-7-3-2-4-8-13/h5-6,9-11,13,20H,2-4,7-8H2,1H3,(H,28,30)(H,31,32)(H2,27,29,33)/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 860 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human glycogen phosphorylase alpha |
Bioorg Med Chem Lett 18: 4068-71 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.102
BindingDB Entry DOI: 10.7270/Q23R0SPM |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50243353

((2S,3S)-2-(2-(3-(2-chloro-6-methylphenyl)ureido)-2...)Show SMILES CC[C@H](C)[C@H](NC(=O)c1cc2ccccc2cc1NC(=O)Nc1c(C)cccc1Cl)C(O)=O |r| Show InChI InChI=1S/C25H26ClN3O4/c1-4-14(2)22(24(31)32)28-23(30)18-12-16-9-5-6-10-17(16)13-20(18)27-25(33)29-21-15(3)8-7-11-19(21)26/h5-14,22H,4H2,1-3H3,(H,28,30)(H,31,32)(H2,27,29,33)/t14-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human glycogen phosphorylase alpha in HepG2 cells assessed as inhibition of forskolin-induced glycogenolysis after 60 mins |
Bioorg Med Chem Lett 18: 4068-71 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.102
BindingDB Entry DOI: 10.7270/Q23R0SPM |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50243601

((S)-2-cyclohexyl-2-(2-(3-(2,6-dimethylphenyl)ureid...)Show SMILES Cc1cccc(C)c1NC(=O)Nc1cc2ccccc2cc1C(=O)N[C@@H](C1CCCCC1)C(O)=O |r| Show InChI InChI=1S/C28H31N3O4/c1-17-9-8-10-18(2)24(17)31-28(35)29-23-16-21-14-7-6-13-20(21)15-22(23)26(32)30-25(27(33)34)19-11-4-3-5-12-19/h6-10,13-16,19,25H,3-5,11-12H2,1-2H3,(H,30,32)(H,33,34)(H2,29,31,35)/t25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human glycogen phosphorylase alpha in HepG2 cells assessed as inhibition of forskolin-induced glycogenolysis after 60 mins |
Bioorg Med Chem Lett 18: 4068-71 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.102
BindingDB Entry DOI: 10.7270/Q23R0SPM |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50243678

((S)-2-(2-(3-(2,6-dimethylphenyl)ureido)-2-naphtham...)Show SMILES Cc1cccc(C)c1NC(=O)Nc1cc2ccccc2cc1C(=O)N[C@@H](CC(O)=O)C(O)=O |r| Show InChI InChI=1S/C24H23N3O6/c1-13-6-5-7-14(2)21(13)27-24(33)26-18-11-16-9-4-3-8-15(16)10-17(18)22(30)25-19(23(31)32)12-20(28)29/h3-11,19H,12H2,1-2H3,(H,25,30)(H,28,29)(H,31,32)(H2,26,27,33)/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human glycogen phosphorylase alpha in HepG2 cells assessed as inhibition of forskolin-induced glycogenolysis after 60 mins |
Bioorg Med Chem Lett 18: 4068-71 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.102
BindingDB Entry DOI: 10.7270/Q23R0SPM |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50243680
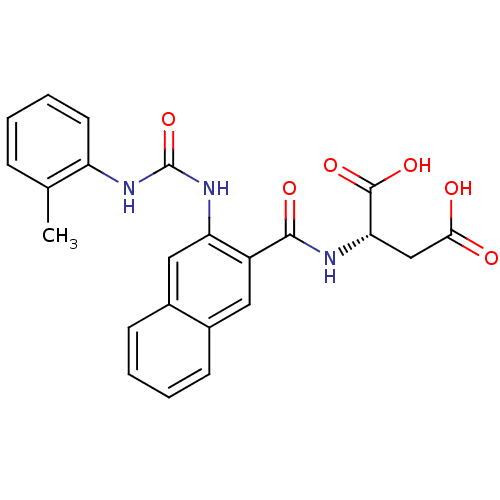
((S)-2-(2-(3-o-tolylureido)-2-naphthamido)succinic ...)Show SMILES Cc1ccccc1NC(=O)Nc1cc2ccccc2cc1C(=O)N[C@@H](CC(O)=O)C(O)=O |r| Show InChI InChI=1S/C23H21N3O6/c1-13-6-2-5-9-17(13)25-23(32)26-18-11-15-8-4-3-7-14(15)10-16(18)21(29)24-19(22(30)31)12-20(27)28/h2-11,19H,12H2,1H3,(H,24,29)(H,27,28)(H,30,31)(H2,25,26,32)/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human glycogen phosphorylase alpha |
Bioorg Med Chem Lett 18: 4068-71 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.102
BindingDB Entry DOI: 10.7270/Q23R0SPM |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50243488
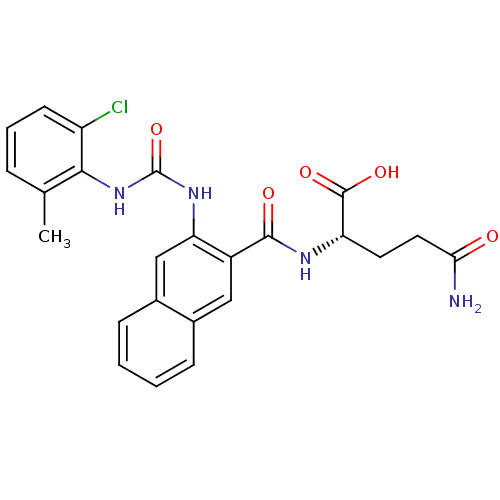
((S)-5-amino-2-(2-(3-(2-chloro-6-methylphenyl)ureid...)Show SMILES Cc1cccc(Cl)c1NC(=O)Nc1cc2ccccc2cc1C(=O)N[C@@H](CCC(N)=O)C(O)=O |r| Show InChI InChI=1S/C24H23ClN4O5/c1-13-5-4-8-17(25)21(13)29-24(34)28-19-12-15-7-3-2-6-14(15)11-16(19)22(31)27-18(23(32)33)9-10-20(26)30/h2-8,11-12,18H,9-10H2,1H3,(H2,26,30)(H,27,31)(H,32,33)(H2,28,29,34)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human glycogen phosphorylase alpha |
Bioorg Med Chem Lett 18: 4068-71 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.102
BindingDB Entry DOI: 10.7270/Q23R0SPM |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50242847
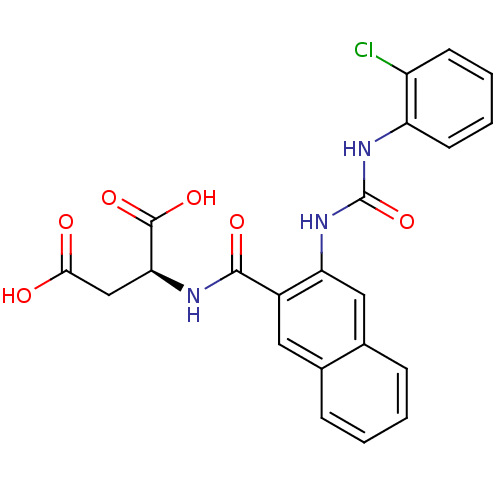
((S)-2-(2-(3-(2-chlorophenyl)ureido)-2-naphthamido)...)Show SMILES OC(=O)C[C@H](NC(=O)c1cc2ccccc2cc1NC(=O)Nc1ccccc1Cl)C(O)=O |r| Show InChI InChI=1S/C22H18ClN3O6/c23-15-7-3-4-8-16(15)25-22(32)26-17-10-13-6-2-1-5-12(13)9-14(17)20(29)24-18(21(30)31)11-19(27)28/h1-10,18H,11H2,(H,24,29)(H,27,28)(H,30,31)(H2,25,26,32)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human glycogen phosphorylase alpha |
Bioorg Med Chem Lett 18: 4068-71 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.102
BindingDB Entry DOI: 10.7270/Q23R0SPM |
More data for this
Ligand-Target Pair | |
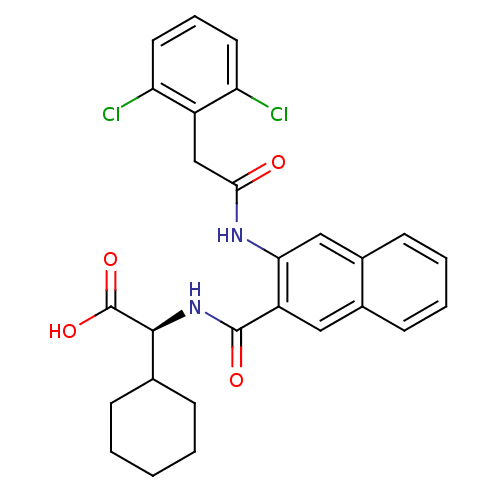
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50243600
((S)-2-cyclohexyl-2-(2-(2-(2,6-dichlorophenyl)aceta...)Show SMILES OC(=O)[C@@H](NC(=O)c1cc2ccccc2cc1NC(=O)Cc1c(Cl)cccc1Cl)C1CCCCC1 |r| Show InChI InChI=1S/C27H26Cl2N2O4/c28-21-11-6-12-22(29)19(21)15-24(32)30-23-14-18-10-5-4-9-17(18)13-20(23)26(33)31-25(27(34)35)16-7-2-1-3-8-16/h4-6,9-14,16,25H,1-3,7-8,15H2,(H,30,32)(H,31,33)(H,34,35)/t25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human glycogen phosphorylase alpha in HepG2 cells assessed as inhibition of forskolin-induced glycogenolysis after 60 mins |
Bioorg Med Chem Lett 18: 4068-71 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.102
BindingDB Entry DOI: 10.7270/Q23R0SPM |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50243399

((S)-2-(2-(3-(2-chloro-6-methylphenyl)ureido)-2-nap...)Show SMILES Cc1cccc(Cl)c1NC(=O)Nc1cc2ccccc2cc1C(=O)N[C@@H](CC(O)=O)C(O)=O |r| Show InChI InChI=1S/C23H20ClN3O6/c1-12-5-4-8-16(24)20(12)27-23(33)26-17-10-14-7-3-2-6-13(14)9-15(17)21(30)25-18(22(31)32)11-19(28)29/h2-10,18H,11H2,1H3,(H,25,30)(H,28,29)(H,31,32)(H2,26,27,33)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human glycogen phosphorylase alpha in absence of glucose |
Bioorg Med Chem Lett 18: 4068-71 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.102
BindingDB Entry DOI: 10.7270/Q23R0SPM |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50243354

((S)-2-(2-(3-(2-chloro-6-methylphenyl)ureido)-2-nap...)Show SMILES Cc1cccc(Cl)c1NC(=O)Nc1cc2ccccc2cc1C(=O)N[C@@H](C(O)=O)c1cccs1 |r| Show InChI InChI=1S/C25H20ClN3O4S/c1-14-6-4-9-18(26)21(14)29-25(33)27-19-13-16-8-3-2-7-15(16)12-17(19)23(30)28-22(24(31)32)20-10-5-11-34-20/h2-13,22H,1H3,(H,28,30)(H,31,32)(H2,27,29,33)/t22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human glycogen phosphorylase alpha in HepG2 cells assessed as inhibition of forskolin-induced glycogenolysis after 60 mins |
Bioorg Med Chem Lett 18: 4068-71 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.102
BindingDB Entry DOI: 10.7270/Q23R0SPM |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50243401

((2S,3R)-2-(2-(3-(2-chloro-6-methylphenyl)ureido)-2...)Show SMILES C[C@@H](O)[C@H](NC(=O)c1cc2ccccc2cc1NC(=O)Nc1c(C)cccc1Cl)C(O)=O |r| Show InChI InChI=1S/C23H22ClN3O5/c1-12-6-5-9-17(24)19(12)27-23(32)25-18-11-15-8-4-3-7-14(15)10-16(18)21(29)26-20(13(2)28)22(30)31/h3-11,13,20,28H,1-2H3,(H,26,29)(H,30,31)(H2,25,27,32)/t13-,20+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human glycogen phosphorylase alpha in HepG2 cells assessed as inhibition of forskolin-induced glycogenolysis after 60 mins |
Bioorg Med Chem Lett 18: 4068-71 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.102
BindingDB Entry DOI: 10.7270/Q23R0SPM |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50243305
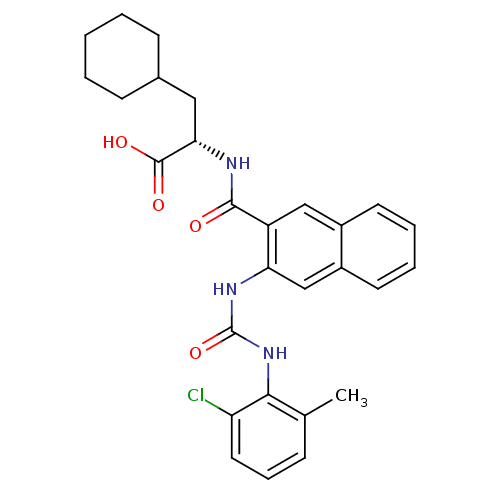
((S)-2-(2-(3-(2-chloro-6-methylphenyl)ureido)-2-nap...)Show SMILES Cc1cccc(Cl)c1NC(=O)Nc1cc2ccccc2cc1C(=O)N[C@@H](CC1CCCCC1)C(O)=O |r| Show InChI InChI=1S/C28H30ClN3O4/c1-17-8-7-13-22(29)25(17)32-28(36)31-23-16-20-12-6-5-11-19(20)15-21(23)26(33)30-24(27(34)35)14-18-9-3-2-4-10-18/h5-8,11-13,15-16,18,24H,2-4,9-10,14H2,1H3,(H,30,33)(H,34,35)(H2,31,32,36)/t24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human glycogen phosphorylase alpha |
Bioorg Med Chem Lett 18: 4068-71 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.102
BindingDB Entry DOI: 10.7270/Q23R0SPM |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50243305

((S)-2-(2-(3-(2-chloro-6-methylphenyl)ureido)-2-nap...)Show SMILES Cc1cccc(Cl)c1NC(=O)Nc1cc2ccccc2cc1C(=O)N[C@@H](CC1CCCCC1)C(O)=O |r| Show InChI InChI=1S/C28H30ClN3O4/c1-17-8-7-13-22(29)25(17)32-28(36)31-23-16-20-12-6-5-11-19(20)15-21(23)26(33)30-24(27(34)35)14-18-9-3-2-4-10-18/h5-8,11-13,15-16,18,24H,2-4,9-10,14H2,1H3,(H,30,33)(H,34,35)(H2,31,32,36)/t24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human glycogen phosphorylase alpha in presence of glucose |
Bioorg Med Chem Lett 18: 4068-71 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.102
BindingDB Entry DOI: 10.7270/Q23R0SPM |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50243352

((S)-2-((S)-carboxy(2-(3-(2-chloro-6-methylphenyl)u...)Show SMILES Cc1cccc(Cl)c1NC(=O)Nc1cc2ccccc2cc1C(=O)N[C@H](N[C@@H](Cc1cc2ccccc2[nH]1)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C32H28ClN5O6/c1-17-7-6-11-23(33)27(17)37-32(44)36-25-15-19-9-3-2-8-18(19)14-22(25)29(39)38-28(31(42)43)35-26(30(40)41)16-21-13-20-10-4-5-12-24(20)34-21/h2-15,26,28,34-35H,16H2,1H3,(H,38,39)(H,40,41)(H,42,43)(H2,36,37,44)/t26-,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human glycogen phosphorylase alpha in HepG2 cells assessed as inhibition of forskolin-induced glycogenolysis after 60 mins |
Bioorg Med Chem Lett 18: 4068-71 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.102
BindingDB Entry DOI: 10.7270/Q23R0SPM |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50243446

((S)-1-((S)-carboxy(2-(3-(2-chloro-6-methylphenyl)u...)Show SMILES Cc1cccc(Cl)c1NC(=O)Nc1cc2ccccc2cc1C(=O)N[C@@H](N1CCC[C@H]1C(O)=O)C(O)=O |r| Show InChI InChI=1S/C26H25ClN4O6/c1-14-6-4-9-18(27)21(14)29-26(37)28-19-13-16-8-3-2-7-15(16)12-17(19)23(32)30-22(25(35)36)31-11-5-10-20(31)24(33)34/h2-4,6-9,12-13,20,22H,5,10-11H2,1H3,(H,30,32)(H,33,34)(H,35,36)(H2,28,29,37)/t20-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human glycogen phosphorylase alpha in HepG2 cells assessed as inhibition of forskolin-induced glycogenolysis after 60 mins |
Bioorg Med Chem Lett 18: 4068-71 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.102
BindingDB Entry DOI: 10.7270/Q23R0SPM |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50243402

((S)-2-(2-(3-(2-chloro-6-methylphenyl)ureido)-2-nap...)Show SMILES Cc1cccc(Cl)c1NC(=O)Nc1cc2ccccc2cc1C(=O)N[C@@H](Cc1ccc(O)cc1)C(O)=O |r| Show InChI InChI=1S/C28H24ClN3O5/c1-16-5-4-8-22(29)25(16)32-28(37)31-23-15-19-7-3-2-6-18(19)14-21(23)26(34)30-24(27(35)36)13-17-9-11-20(33)12-10-17/h2-12,14-15,24,33H,13H2,1H3,(H,30,34)(H,35,36)(H2,31,32,37)/t24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human glycogen phosphorylase alpha in HepG2 cells assessed as inhibition of forskolin-induced glycogenolysis after 60 mins |
Bioorg Med Chem Lett 18: 4068-71 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.102
BindingDB Entry DOI: 10.7270/Q23R0SPM |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50243489
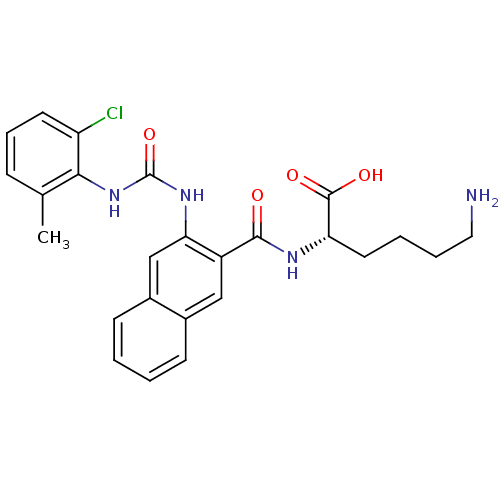
((S)-6-amino-2-(2-(3-(2-chloro-6-methylphenyl)ureid...)Show SMILES Cc1cccc(Cl)c1NC(=O)Nc1cc2ccccc2cc1C(=O)N[C@@H](CCCCN)C(O)=O |r| Show InChI InChI=1S/C25H27ClN4O4/c1-15-7-6-10-19(26)22(15)30-25(34)29-21-14-17-9-3-2-8-16(17)13-18(21)23(31)28-20(24(32)33)11-4-5-12-27/h2-3,6-10,13-14,20H,4-5,11-12,27H2,1H3,(H,28,31)(H,32,33)(H2,29,30,34)/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human glycogen phosphorylase alpha |
Bioorg Med Chem Lett 18: 4068-71 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.102
BindingDB Entry DOI: 10.7270/Q23R0SPM |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50243447

((S)-2-(2-(3-(2-chloro-6-methylphenyl)ureido)-2-nap...)Show SMILES CC(C)C[C@H](NC(=O)c1cc2ccccc2cc1NC(=O)Nc1c(C)cccc1Cl)C(O)=O |r| Show InChI InChI=1S/C25H26ClN3O4/c1-14(2)11-21(24(31)32)27-23(30)18-12-16-8-4-5-9-17(16)13-20(18)28-25(33)29-22-15(3)7-6-10-19(22)26/h4-10,12-14,21H,11H2,1-3H3,(H,27,30)(H,31,32)(H2,28,29,33)/t21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human glycogen phosphorylase alpha in HepG2 cells assessed as inhibition of forskolin-induced glycogenolysis after 60 mins |
Bioorg Med Chem Lett 18: 4068-71 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.102
BindingDB Entry DOI: 10.7270/Q23R0SPM |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50243351
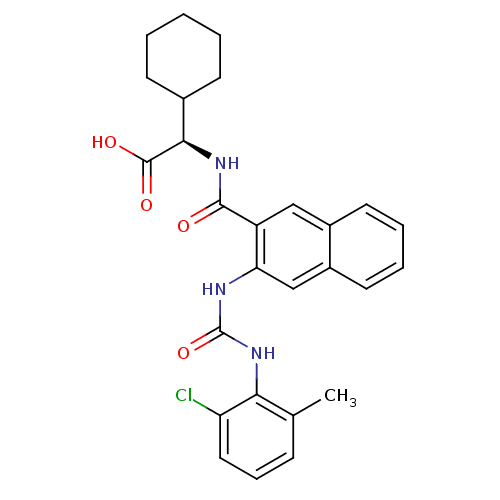
((R)-2-(2-(3-(2-chloro-6-methylphenyl)ureido)-2-nap...)Show SMILES Cc1cccc(Cl)c1NC(=O)Nc1cc2ccccc2cc1C(=O)N[C@H](C1CCCCC1)C(O)=O |r| Show InChI InChI=1S/C27H28ClN3O4/c1-16-8-7-13-21(28)23(16)31-27(35)29-22-15-19-12-6-5-11-18(19)14-20(22)25(32)30-24(26(33)34)17-9-3-2-4-10-17/h5-8,11-15,17,24H,2-4,9-10H2,1H3,(H,30,32)(H,33,34)(H2,29,31,35)/t24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human glycogen phosphorylase alpha |
Bioorg Med Chem Lett 18: 4068-71 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.102
BindingDB Entry DOI: 10.7270/Q23R0SPM |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50243640

((S)-2-cyclohexyl-2-(2-(3-(2,6-diethylphenyl)ureido...)Show SMILES CCc1cccc(CC)c1NC(=O)Nc1cc2ccccc2cc1C(=O)N[C@@H](C1CCCCC1)C(O)=O |r| Show InChI InChI=1S/C30H35N3O4/c1-3-19-15-10-16-20(4-2)26(19)33-30(37)31-25-18-23-14-9-8-13-22(23)17-24(25)28(34)32-27(29(35)36)21-11-6-5-7-12-21/h8-10,13-18,21,27H,3-7,11-12H2,1-2H3,(H,32,34)(H,35,36)(H2,31,33,37)/t27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human glycogen phosphorylase alpha in HepG2 cells assessed as inhibition of forskolin-induced glycogenolysis after 60 mins |
Bioorg Med Chem Lett 18: 4068-71 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.102
BindingDB Entry DOI: 10.7270/Q23R0SPM |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data