 Found 141 hits with Last Name = 'tiwari' and Initial = 'ak'
Found 141 hits with Last Name = 'tiwari' and Initial = 'ak' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50562066
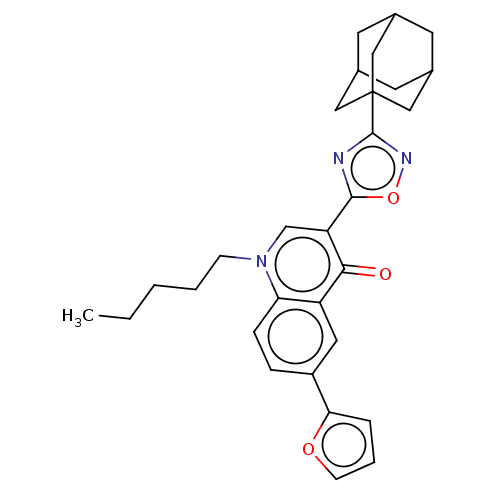
(CHEMBL4756661)Show SMILES CCCCCn1cc(-c2nc(no2)C23CC4CC(CC(C4)C2)C3)c(=O)c2cc(ccc12)-c1ccco1 |THB:18:17:14:20.19.21,18:19:16.17.22:14,21:19:16:22.13.14,21:13:16:20.18.19| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]CP-55940 from human CB2 receptor expressed in HEK cells by competitive binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00530
BindingDB Entry DOI: 10.7270/Q2W95DW9 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50562067

(CHEMBL4749288) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at human recombinant TRPV1 expressed in HEK293 cells assessed as effect on intracellular calcium level by FLOU-4 dye based fluore... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00530
BindingDB Entry DOI: 10.7270/Q2W95DW9 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50249592

(CHEMBL4061179)Show SMILES CCCCCn1cc(-c2cn(nn2)C23CC4CC(CC(C4)C2)C3)c(=O)c2cc(ccc12)-c1ccco1 |TLB:10:13:16:20.18.19,THB:18:17:14:20.19.21,18:19:16.17.22:14,21:19:16:22.13.14,21:13:16:20.18.19| Show InChI InChI=1S/C30H34N4O2/c1-2-3-4-9-33-18-25(29(35)24-14-23(7-8-27(24)33)28-6-5-10-36-28)26-19-34(32-31-26)30-15-20-11-21(16-30)13-22(12-20)17-30/h5-8,10,14,18-22H,2-4,9,11-13,15-17H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]CP-55940 from human CB2 receptor expressed in HEK cells by competitive binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00530
BindingDB Entry DOI: 10.7270/Q2W95DW9 |
More data for this
Ligand-Target Pair | |
Neprilysin
(Homo sapiens (Human)) | BDBM50006115
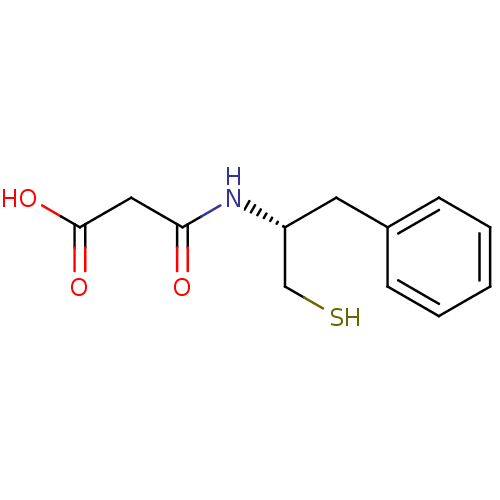
((R)-RETRO-THIORPHAN | (RETRO)N-(1-Mercaptomethyl-2...)Show InChI InChI=1S/C12H15NO3S/c14-11(7-12(15)16)13-10(8-17)6-9-4-2-1-3-5-9/h1-5,10,17H,6-8H2,(H,13,14)(H,15,16)/t10-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of NEP 24.11 (unknown origin) by fluorometric assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00530
BindingDB Entry DOI: 10.7270/Q2W95DW9 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(MOUSE) | BDBM50001465

((S)-2-[(S)-2-(2-{2-[(S)-2-Amino-3-(4-hydroxy-pheny...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccc(O)cc1)C(O)=O Show InChI InChI=1S/C28H37N5O7/c1-17(2)12-23(28(39)40)33-27(38)22(14-18-6-4-3-5-7-18)32-25(36)16-30-24(35)15-31-26(37)21(29)13-19-8-10-20(34)11-9-19/h3-11,17,21-23,34H,12-16,29H2,1-2H3,(H,30,35)(H,31,37)(H,32,36)(H,33,38)(H,39,40)/t21-,22-,23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of [3H]-Deltorphin 2 binding to mouse delta opioid receptor expressed in rat GH3 cell membranes by Cheng-Prusoff analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00530
BindingDB Entry DOI: 10.7270/Q2W95DW9 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50562065

(CHEMBL4745169)Show SMILES CCCCCn1cc(-c2nnc(o2)C23CC4CC(CC(C4)C2)C3)c(=O)c2cc(ccc12)-c1ccco1 |THB:18:17:14:20.19.21,18:19:16.17.22:14,21:19:16:22.13.14,21:13:16:20.18.19| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]CP-55940 from human CB2 receptor expressed in HEK cells by competitive binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00530
BindingDB Entry DOI: 10.7270/Q2W95DW9 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(MOUSE) | BDBM50001465

((S)-2-[(S)-2-(2-{2-[(S)-2-Amino-3-(4-hydroxy-pheny...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccc(O)cc1)C(O)=O Show InChI InChI=1S/C28H37N5O7/c1-17(2)12-23(28(39)40)33-27(38)22(14-18-6-4-3-5-7-18)32-25(36)16-30-24(35)15-31-26(37)21(29)13-19-8-10-20(34)11-9-19/h3-11,17,21-23,34H,12-16,29H2,1-2H3,(H,30,35)(H,31,37)(H,32,36)(H,33,38)(H,39,40)/t21-,22-,23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of [3H]-Deltorphin 2 binding to mouse delta opioid receptor expressed in HEK293 cell membranes incubated for 60 mins by Cheng-Prusoff anal... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00530
BindingDB Entry DOI: 10.7270/Q2W95DW9 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(MOUSE) | BDBM50562077
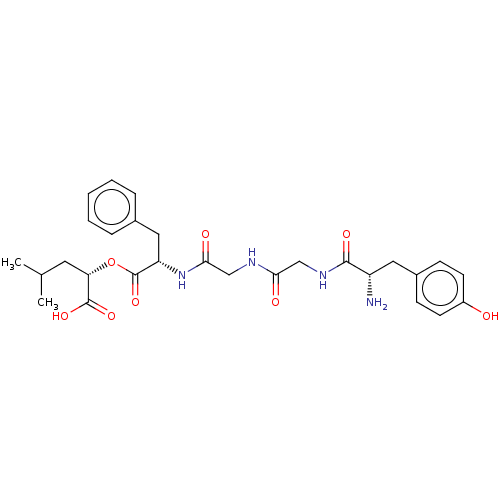
(CHEMBL4754992)Show SMILES CC(C)C[C@H](OC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccc(O)cc1)C(O)=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of [3H]-Deltorphin 2 binding to mouse delta opioid receptor expressed in rat GH3 cell membranes by Cheng-Prusoff analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00530
BindingDB Entry DOI: 10.7270/Q2W95DW9 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(MOUSE) | BDBM50562078
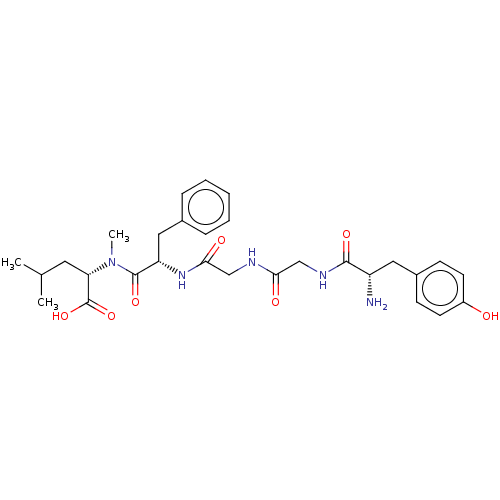
(CHEMBL98593)Show SMILES CC(C)C[C@H](N(C)C(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccc(O)cc1)C(O)=O | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of [3H]-Deltorphin 2 binding to mouse delta opioid receptor expressed in rat GH3 cell membranes by Cheng-Prusoff analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00530
BindingDB Entry DOI: 10.7270/Q2W95DW9 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50562066

(CHEMBL4756661)Show SMILES CCCCCn1cc(-c2nc(no2)C23CC4CC(CC(C4)C2)C3)c(=O)c2cc(ccc12)-c1ccco1 |THB:18:17:14:20.19.21,18:19:16.17.22:14,21:19:16:22.13.14,21:13:16:20.18.19| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]CP-55940 from human CB1 receptor expressed in HEK cells by competitive binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00530
BindingDB Entry DOI: 10.7270/Q2W95DW9 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(MOUSE) | BDBM50517920

(CHEMBL4572725)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)OC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccc(O)cc1)C(O)=O |r| Show InChI InChI=1S/C28H36N4O8/c1-17(2)12-22(28(38)39)32-27(37)23(14-18-6-4-3-5-7-18)40-25(35)16-30-24(34)15-31-26(36)21(29)13-19-8-10-20(33)11-9-19/h3-11,17,21-23,33H,12-16,29H2,1-2H3,(H,30,34)(H,31,36)(H,32,37)(H,38,39)/t21-,22-,23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of [3H]-Deltorphin 2 binding to mouse delta opioid receptor expressed in rat GH3 cell membranes by Cheng-Prusoff analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00530
BindingDB Entry DOI: 10.7270/Q2W95DW9 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(MOUSE) | BDBM50517888

(CHEMBL4442842)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)\C=C(/F)CNC(=O)CNC(=O)[C@@H](N)Cc1ccc(O)cc1)C(O)=O |r| Show InChI InChI=1S/C29H37FN4O6/c1-18(2)12-25(29(39)40)34-27(37)21(13-19-6-4-3-5-7-19)15-22(30)16-32-26(36)17-33-28(38)24(31)14-20-8-10-23(35)11-9-20/h3-11,15,18,21,24-25,35H,12-14,16-17,31H2,1-2H3,(H,32,36)(H,33,38)(H,34,37)(H,39,40)/b22-15-/t21-,24+,25+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of [3H]-Deltorphin 2 binding to mouse delta opioid receptor expressed in HEK293 cell membranes incubated for 60 mins by Cheng-Prusoff anal... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00530
BindingDB Entry DOI: 10.7270/Q2W95DW9 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(MOUSE) | BDBM50517889

(CHEMBL4567560)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=S)CNC(=O)CNC(=O)[C@@H](N)Cc1ccc(O)cc1)C(O)=O |r| Show InChI InChI=1S/C28H37N5O6S/c1-17(2)12-23(28(38)39)33-27(37)22(14-18-6-4-3-5-7-18)32-25(40)16-30-24(35)15-31-26(36)21(29)13-19-8-10-20(34)11-9-19/h3-11,17,21-23,34H,12-16,29H2,1-2H3,(H,30,35)(H,31,36)(H,32,40)(H,33,37)(H,38,39)/t21-,22-,23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of [3H]-Deltorphin 2 binding to mouse delta opioid receptor expressed in rat GH3 cell membranes by Cheng-Prusoff analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00530
BindingDB Entry DOI: 10.7270/Q2W95DW9 |
More data for this
Ligand-Target Pair | |
Neprilysin
(Homo sapiens (Human)) | BDBM50024095

((R) N-(1-Mercaptomethyl-2-phenyl-ethyl)-malonamic ...)Show InChI InChI=1S/C12H15NO3S/c14-11(7-12(15)16)13-10(8-17)6-9-4-2-1-3-5-9/h1-5,10,17H,6-8H2,(H,13,14)(H,15,16)/t10-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of NEP 24.11 (unknown origin) by fluorometric assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00530
BindingDB Entry DOI: 10.7270/Q2W95DW9 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50562067

(CHEMBL4749288) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-CP55940 from human recombinant cannabinoid CB1 receptor expressed in HEK cells by Cheng-Prusoff analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00530
BindingDB Entry DOI: 10.7270/Q2W95DW9 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(MOUSE) | BDBM50562079

(CHEMBL4776808)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)N\C=C\NC(=O)CNC(=O)[C@@H](N)Cc1ccc(O)cc1)C(O)=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 587 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of [3H]-Deltorphin 2 binding to mouse delta opioid receptor expressed in rat GH3 cell membranes by Cheng-Prusoff analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00530
BindingDB Entry DOI: 10.7270/Q2W95DW9 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM20461
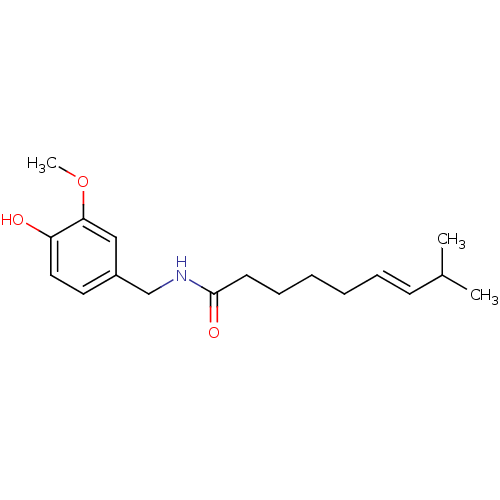
((6E)-N-[(4-hydroxy-3-methoxyphenyl)methyl]-8-methy...)Show InChI InChI=1S/C18H27NO3/c1-14(2)8-6-4-5-7-9-18(21)19-13-15-10-11-16(20)17(12-15)22-3/h6,8,10-12,14,20H,4-5,7,9,13H2,1-3H3,(H,19,21)/b8-6+ | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| >5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-CP55940 from human recombinant cannabinoid CB1 receptor expressed in HEK cells by Cheng-Prusoff analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00530
BindingDB Entry DOI: 10.7270/Q2W95DW9 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50249592

(CHEMBL4061179)Show SMILES CCCCCn1cc(-c2cn(nn2)C23CC4CC(CC(C4)C2)C3)c(=O)c2cc(ccc12)-c1ccco1 |TLB:10:13:16:20.18.19,THB:18:17:14:20.19.21,18:19:16.17.22:14,21:19:16:22.13.14,21:13:16:20.18.19| Show InChI InChI=1S/C30H34N4O2/c1-2-3-4-9-33-18-25(29(35)24-14-23(7-8-27(24)33)28-6-5-10-36-28)26-19-34(32-31-26)30-15-20-11-21(16-30)13-22(12-20)17-30/h5-8,10,14,18-22H,2-4,9,11-13,15-17H2,1H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]CP-55940 from human CB1 receptor expressed in HEK cells by competitive binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00530
BindingDB Entry DOI: 10.7270/Q2W95DW9 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50562065

(CHEMBL4745169)Show SMILES CCCCCn1cc(-c2nnc(o2)C23CC4CC(CC(C4)C2)C3)c(=O)c2cc(ccc12)-c1ccco1 |THB:18:17:14:20.19.21,18:19:16.17.22:14,21:19:16:22.13.14,21:13:16:20.18.19| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]CP-55940 from human CB1 receptor expressed in HEK cells by competitive binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00530
BindingDB Entry DOI: 10.7270/Q2W95DW9 |
More data for this
Ligand-Target Pair | |
Neprilysin
(Homo sapiens (Human)) | BDBM50562073

(CHEMBL4786514)Show SMILES OC(=O)C[C@@H](N[C@H](CS)Cc1ccccc1)C(F)(F)F |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >4.00E+9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of NEP 24.11 (unknown origin) by fluorometric assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00530
BindingDB Entry DOI: 10.7270/Q2W95DW9 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50562071

(CHEMBL319408)Show SMILES [H][C@@]1(CCOC1)OC(=O)N[C@@H](Cc1ccccc1)[C@@H](O)C[C@@H](Cc1ccccc1)C(=O)N[C@@H]1[C@H](O)Cc2ccccc12 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HIV1 protease |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00530
BindingDB Entry DOI: 10.7270/Q2W95DW9 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50562070

(CHEMBL17612)Show SMILES [H][C@](NC(=O)c1ccc2ccccc2n1)(C1CCOC1)C(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN1C[C@@]2([H])CCCC[C@@]2([H])C[C@@]1([H])C(=O)NC(C)(C)C | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0540 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HIV1 protease using H-Val-Ser-Gln-Am-(L-b-naphthyl-alanine)-Pro-Ile-Val-OH as substrate by HPLC method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00530
BindingDB Entry DOI: 10.7270/Q2W95DW9 |
More data for this
Ligand-Target Pair | |
Integrin alpha-4/beta-1
(Homo sapiens (Human)) | BDBM50562072
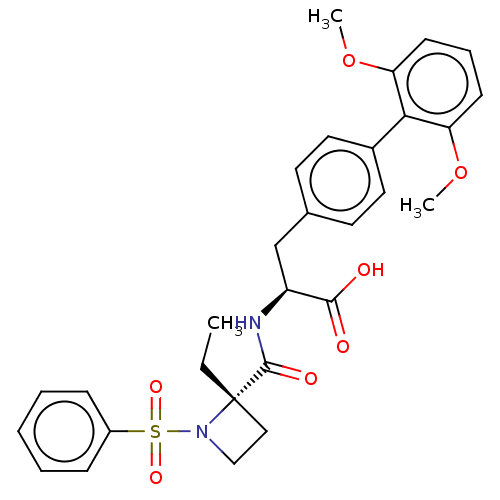
(CHEMBL4798264)Show SMILES CC[C@]1(CCN1S(=O)(=O)c1ccccc1)C(=O)N[C@@H](Cc1ccc(cc1)-c1c(OC)cccc1OC)C(O)=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of 125I-VCAM-Ig from VLA4 in human Jurkat cells incubated for 30 mins by scintillation counting method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00530
BindingDB Entry DOI: 10.7270/Q2W95DW9 |
More data for this
Ligand-Target Pair | |
Integrin alpha-4/beta-1
(Homo sapiens (Human)) | BDBM50105397
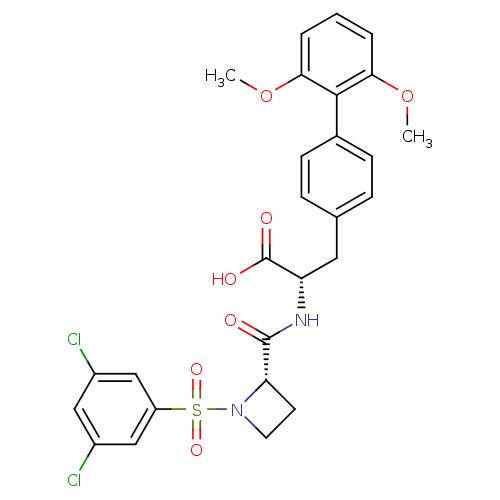
((S)-2-{[(S)-1-(3,5-Dichloro-benzenesulfonyl)-azeti...)Show SMILES COc1cccc(OC)c1-c1ccc(C[C@H](NC(=O)[C@@H]2CCN2S(=O)(=O)c2cc(Cl)cc(Cl)c2)C(O)=O)cc1 Show InChI InChI=1S/C27H26Cl2N2O7S/c1-37-23-4-3-5-24(38-2)25(23)17-8-6-16(7-9-17)12-21(27(33)34)30-26(32)22-10-11-31(22)39(35,36)20-14-18(28)13-19(29)15-20/h3-9,13-15,21-22H,10-12H2,1-2H3,(H,30,32)(H,33,34)/t21-,22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of 125I-VCAM-Ig from VLA4 in human Jurkat cells incubated for 30 mins by scintillation counting method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00530
BindingDB Entry DOI: 10.7270/Q2W95DW9 |
More data for this
Ligand-Target Pair | |
Presenilin-1
(Homo sapiens (Human)) | BDBM50241259
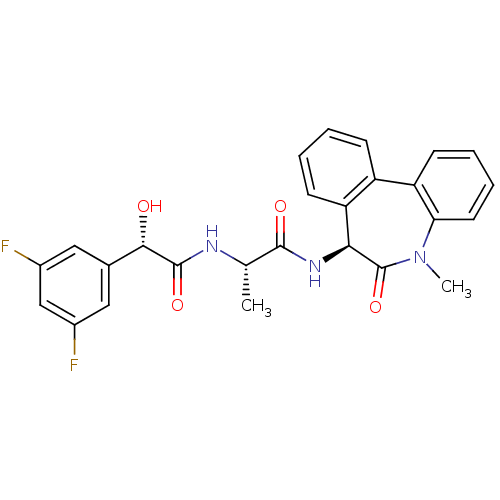
((2S)-2-((S)-2-(3,5-difluorophenyl)-2-hydroxyacetam...)Show SMILES C[C@H](NC(=O)[C@@H](O)c1cc(F)cc(F)c1)C(=O)N[C@H]1c2ccccc2-c2ccccc2N(C)C1=O |r| Show InChI InChI=1S/C26H23F2N3O4/c1-14(29-25(34)23(32)15-11-16(27)13-17(28)12-15)24(33)30-22-20-9-4-3-7-18(20)19-8-5-6-10-21(19)31(2)26(22)35/h3-14,22-23,32H,1-2H3,(H,29,34)(H,30,33)/t14-,22-,23-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0820 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of gamma secretase in HEK293 cells assessed as reduction in amyloid beta40 level incubated for 4 hrs by electrochemiluminescence detection... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00530
BindingDB Entry DOI: 10.7270/Q2W95DW9 |
More data for this
Ligand-Target Pair | |
Integrin alpha-4/beta-1
(Homo sapiens (Human)) | BDBM50144749
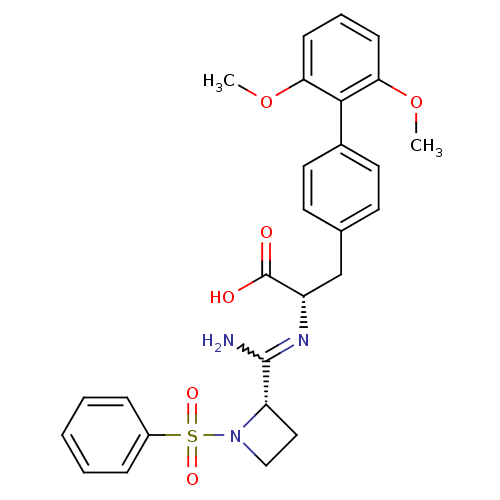
((S)-2-[((S)-1-Benzenesulfonyl-azetidine-2-carboxim...)Show SMILES COc1cccc(OC)c1-c1ccc(C[C@H](N=C(N)[C@@H]2CCN2S(=O)(=O)c2ccccc2)C(O)=O)cc1 |w:17.18| Show InChI InChI=1S/C27H29N3O6S/c1-35-23-9-6-10-24(36-2)25(23)19-13-11-18(12-14-19)17-21(27(31)32)29-26(28)22-15-16-30(22)37(33,34)20-7-4-3-5-8-20/h3-14,21-22H,15-17H2,1-2H3,(H2,28,29)(H,31,32)/t21-,22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of 125I-VCAM-Ig from VLA4 in human Jurkat cells incubated for 30 mins by scintillation counting method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00530
BindingDB Entry DOI: 10.7270/Q2W95DW9 |
More data for this
Ligand-Target Pair | |
Integrin alpha-4/beta-1
(Homo sapiens (Human)) | BDBM50144760

((S)-2-{[(S)-1-Benzenesulfonyl-N-(2,2,2-trifluoro-e...)Show SMILES COc1cccc(OC)c1-c1ccc(C[C@H](N=C(NCC(F)(F)F)[C@@H]2CCN2S(=O)(=O)c2ccccc2)C(O)=O)cc1 |w:17.18| Show InChI InChI=1S/C29H30F3N3O6S/c1-40-24-9-6-10-25(41-2)26(24)20-13-11-19(12-14-20)17-22(28(36)37)34-27(33-18-29(30,31)32)23-15-16-35(23)42(38,39)21-7-4-3-5-8-21/h3-14,22-23H,15-18H2,1-2H3,(H,33,34)(H,36,37)/t22-,23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of 125I-VCAM-Ig from VLA4 in human Jurkat cells incubated for 30 mins by scintillation counting method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00530
BindingDB Entry DOI: 10.7270/Q2W95DW9 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50213021
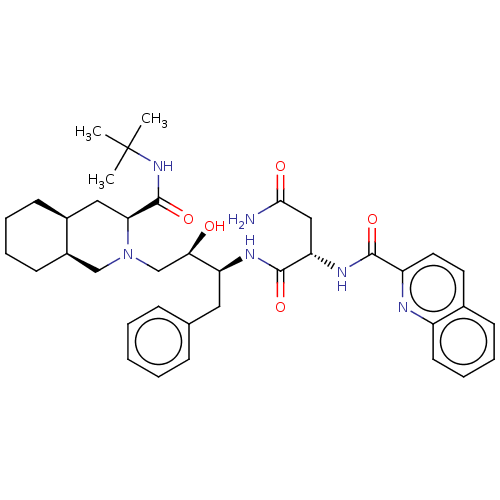
(CHEBI:63621 | Fortovase | Invirase | Ro-31-8959 | ...)Show SMILES [H][C@@]12CCCC[C@]1([H])CN(C[C@@H](O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(N)=O)NC(=O)c1ccc3ccccc3n1)[C@@H](C2)C(=O)NC(C)(C)C |r| Show InChI InChI=1S/C38H50N6O5/c1-38(2,3)43-37(49)32-20-26-14-7-8-15-27(26)22-44(32)23-33(45)30(19-24-11-5-4-6-12-24)41-36(48)31(21-34(39)46)42-35(47)29-18-17-25-13-9-10-16-28(25)40-29/h4-6,9-13,16-18,26-27,30-33,45H,7-8,14-15,19-23H2,1-3H3,(H2,39,46)(H,41,48)(H,42,47)(H,43,49)/t26-,27+,30-,31-,32-,33+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HIV1 protease using H-Val-Ser-Gln-Am-(L-b-naphthyl-alanine)-Pro-Ile-Val-OH as substrate by HPLC method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00530
BindingDB Entry DOI: 10.7270/Q2W95DW9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50562069

(CHEMBL4785595)Show SMILES CNC(=O)[C@@H](NC(=O)[C@H](CC(C)C)[C@H](CC=C)C(=O)NO)C(C)(C)C |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.850 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of TNF-induced MMP2 isolated from human HT-1080 cell medium by fluorometric assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00530
BindingDB Entry DOI: 10.7270/Q2W95DW9 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50072566

((2R,3S)-2-Allyl-N*4*-[(S)-1-benzyl-2-(1H-indol-3-y...)Show SMILES CC(C)C[C@H]([C@H](CC=C)C(=O)NO)C(=O)N[C@@H](Cc1ccccc1)C(=O)c1c[nH]c2ccccc12 Show InChI InChI=1S/C28H33N3O4/c1-4-10-21(28(34)31-35)22(15-18(2)3)27(33)30-25(16-19-11-6-5-7-12-19)26(32)23-17-29-24-14-9-8-13-20(23)24/h4-9,11-14,17-18,21-22,25,29,35H,1,10,15-16H2,2-3H3,(H,30,33)(H,31,34)/t21-,22+,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of TNF-induced MMP2 isolated from human HT-1080 cell medium by fluorometric assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00530
BindingDB Entry DOI: 10.7270/Q2W95DW9 |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50072566

((2R,3S)-2-Allyl-N*4*-[(S)-1-benzyl-2-(1H-indol-3-y...)Show SMILES CC(C)C[C@H]([C@H](CC=C)C(=O)NO)C(=O)N[C@@H](Cc1ccccc1)C(=O)c1c[nH]c2ccccc12 Show InChI InChI=1S/C28H33N3O4/c1-4-10-21(28(34)31-35)22(15-18(2)3)27(33)30-25(16-19-11-6-5-7-12-19)26(32)23-17-29-24-14-9-8-13-20(23)24/h4-9,11-14,17-18,21-22,25,29,35H,1,10,15-16H2,2-3H3,(H,30,33)(H,31,34)/t21-,22+,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PMA-induced human skin fibroblast derived MMP1 by fluorometric assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00530
BindingDB Entry DOI: 10.7270/Q2W95DW9 |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50562069

(CHEMBL4785595)Show SMILES CNC(=O)[C@@H](NC(=O)[C@H](CC(C)C)[C@H](CC=C)C(=O)NO)C(C)(C)C |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PMA-induced human skin fibroblast derived MMP1 by fluorometric assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00530
BindingDB Entry DOI: 10.7270/Q2W95DW9 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50562068

(CHEMBL4791227)Show SMILES CNC(=O)[C@H](Cc1ccc2CCCc2c1)NC(=O)[C@H](CC(C)C)[C@H](CC=C)C(=O)NO |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of TNF-induced MMP2 isolated from human HT-1080 cell medium by fluorometric assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00530
BindingDB Entry DOI: 10.7270/Q2W95DW9 |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50562068

(CHEMBL4791227)Show SMILES CNC(=O)[C@H](Cc1ccc2CCCc2c1)NC(=O)[C@H](CC(C)C)[C@H](CC=C)C(=O)NO |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PMA-induced human skin fibroblast derived MMP1 by fluorometric assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00530
BindingDB Entry DOI: 10.7270/Q2W95DW9 |
More data for this
Ligand-Target Pair | |
Matrilysin
(Homo sapiens (Human)) | BDBM50072566

((2R,3S)-2-Allyl-N*4*-[(S)-1-benzyl-2-(1H-indol-3-y...)Show SMILES CC(C)C[C@H]([C@H](CC=C)C(=O)NO)C(=O)N[C@@H](Cc1ccccc1)C(=O)c1c[nH]c2ccccc12 Show InChI InChI=1S/C28H33N3O4/c1-4-10-21(28(34)31-35)22(15-18(2)3)27(33)30-25(16-19-11-6-5-7-12-19)26(32)23-17-29-24-14-9-8-13-20(23)24/h4-9,11-14,17-18,21-22,25,29,35H,1,10,15-16H2,2-3H3,(H,30,33)(H,31,34)/t21-,22+,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human MMP7 by fluorometric assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00530
BindingDB Entry DOI: 10.7270/Q2W95DW9 |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50072566

((2R,3S)-2-Allyl-N*4*-[(S)-1-benzyl-2-(1H-indol-3-y...)Show SMILES CC(C)C[C@H]([C@H](CC=C)C(=O)NO)C(=O)N[C@@H](Cc1ccccc1)C(=O)c1c[nH]c2ccccc12 Show InChI InChI=1S/C28H33N3O4/c1-4-10-21(28(34)31-35)22(15-18(2)3)27(33)30-25(16-19-11-6-5-7-12-19)26(32)23-17-29-24-14-9-8-13-20(23)24/h4-9,11-14,17-18,21-22,25,29,35H,1,10,15-16H2,2-3H3,(H,30,33)(H,31,34)/t21-,22+,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human MMP3 by fluorometric assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00530
BindingDB Entry DOI: 10.7270/Q2W95DW9 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50276903
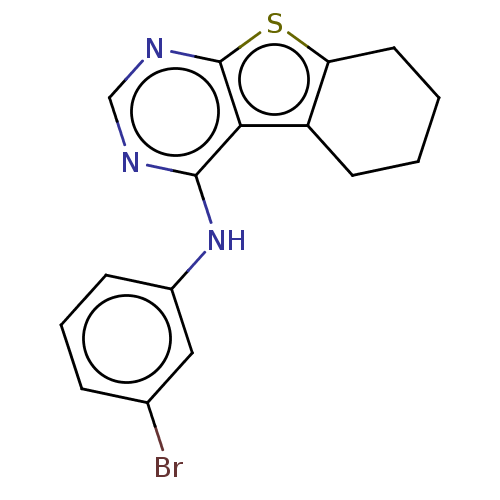
(CHEMBL4171764)Show InChI InChI=1S/C16H14BrN3S/c17-10-4-3-5-11(8-10)20-15-14-12-6-1-2-7-13(12)21-16(14)19-9-18-15/h3-5,8-9H,1-2,6-7H2,(H,18,19,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toledo
Curated by ChEMBL
| Assay Description
Inhibition of EGFR1 (unknown origin) after 1 hr in presence of adenosine 5'[gamma-33P]triphosphate by microbeta microplate counting method |
Eur J Med Chem 138: 1053-1065 (2017)
Article DOI: 10.1016/j.ejmech.2017.07.028
BindingDB Entry DOI: 10.7270/Q2794760 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50562075

(CHEMBL4758323)Show SMILES [H][C@@]1(O[C@H]1CCS(=O)(=O)N(CC)C(C)C)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(C)(C)S(C)(=O)=O)NC(=O)c1ccc2ccccc2n1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HIV-1 protease |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00530
BindingDB Entry DOI: 10.7270/Q2W95DW9 |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50562069

(CHEMBL4785595)Show SMILES CNC(=O)[C@@H](NC(=O)[C@H](CC(C)C)[C@H](CC=C)C(=O)NO)C(C)(C)C |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human MMP3 by fluorometric assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00530
BindingDB Entry DOI: 10.7270/Q2W95DW9 |
More data for this
Ligand-Target Pair | |
Matrilysin
(Homo sapiens (Human)) | BDBM50562068

(CHEMBL4791227)Show SMILES CNC(=O)[C@H](Cc1ccc2CCCc2c1)NC(=O)[C@H](CC(C)C)[C@H](CC=C)C(=O)NO |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human MMP7 by fluorometric assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00530
BindingDB Entry DOI: 10.7270/Q2W95DW9 |
More data for this
Ligand-Target Pair | |
Matrilysin
(Homo sapiens (Human)) | BDBM50562069

(CHEMBL4785595)Show SMILES CNC(=O)[C@@H](NC(=O)[C@H](CC(C)C)[C@H](CC=C)C(=O)NO)C(C)(C)C |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human MMP7 by fluorometric assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00530
BindingDB Entry DOI: 10.7270/Q2W95DW9 |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50562068

(CHEMBL4791227)Show SMILES CNC(=O)[C@H](Cc1ccc2CCCc2c1)NC(=O)[C@H](CC(C)C)[C@H](CC=C)C(=O)NO |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human MMP3 by fluorometric assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00530
BindingDB Entry DOI: 10.7270/Q2W95DW9 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50290520

(((R)-1-{(S)-1-[(2R,3S)-3-(2-tert-Butylsulfamoyl-et...)Show SMILES CC(C)(C)NS(=O)(=O)CC[C@@H]1O[C@@H]1[C@H](Cc1ccccc1)NC(=O)[C@@H](NC(=O)OCc1ccccc1)C(C)(C)S(C)(=O)=O Show InChI InChI=1S/C30H43N3O8S2/c1-29(2,3)33-43(38,39)18-17-24-25(41-24)23(19-21-13-9-7-10-14-21)31-27(34)26(30(4,5)42(6,36)37)32-28(35)40-20-22-15-11-8-12-16-22/h7-16,23-26,33H,17-20H2,1-6H3,(H,31,34)(H,32,35)/t23-,24-,25+,26+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HIV-1 protease |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00530
BindingDB Entry DOI: 10.7270/Q2W95DW9 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50562074
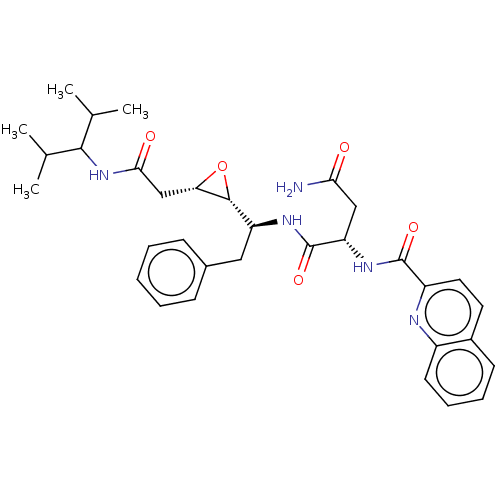
(CHEMBL312911)Show SMILES [H][C@@]1(O[C@H]1CC(=O)NC(C(C)C)C(C)C)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(N)=O)NC(=O)c1ccc2ccccc2n1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HIV-1 protease |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00530
BindingDB Entry DOI: 10.7270/Q2W95DW9 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50562076
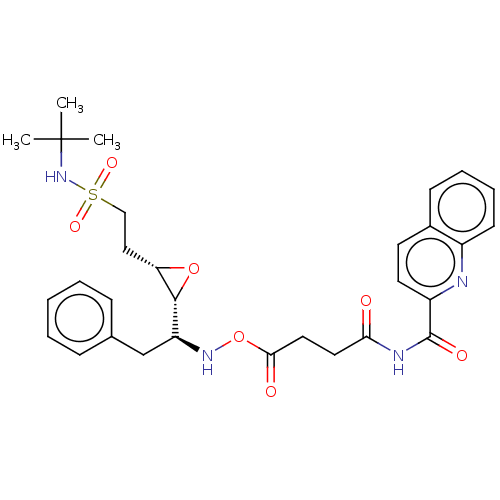
(CHEMBL4783390)Show SMILES [H][C@@]1(O[C@H]1CCS(=O)(=O)NC(C)(C)C)[C@H](Cc1ccccc1)NOC(=O)CCC(=O)NC(=O)c1ccc2ccccc2n1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HIV-1 protease |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00530
BindingDB Entry DOI: 10.7270/Q2W95DW9 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50146642
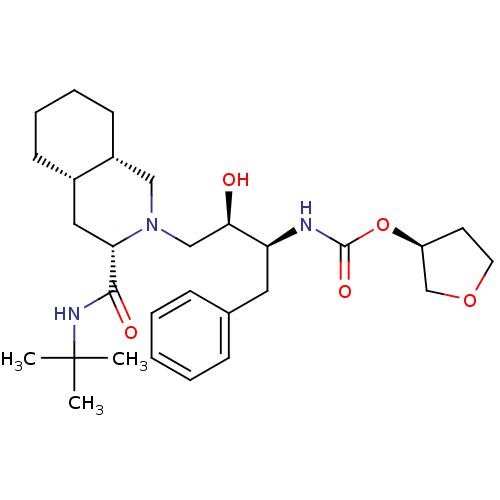
((S)-tetrahydrofuran-3-yl (2S,3R)-4-((3S,4aS,8aS)-3...)Show SMILES CC(C)(C)NC(=O)[C@@H]1C[C@@H]2CCCC[C@@H]2CN1C[C@@H](O)[C@H](Cc1ccccc1)NC(=O)O[C@H]1CCOC1 |r| Show InChI InChI=1S/C29H45N3O5/c1-29(2,3)31-27(34)25-16-21-11-7-8-12-22(21)17-32(25)18-26(33)24(15-20-9-5-4-6-10-20)30-28(35)37-23-13-14-36-19-23/h4-6,9-10,21-26,33H,7-8,11-19H2,1-3H3,(H,30,35)(H,31,34)/t21-,22+,23-,24-,25-,26+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HIV1 protease |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00530
BindingDB Entry DOI: 10.7270/Q2W95DW9 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(RAT) | BDBM50208418
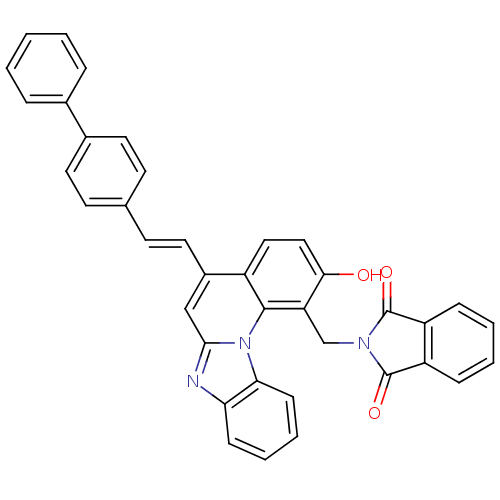
(2-[5-((E)-2-biphenyl-4-yl-vinyl)-2-hydroxy-benzo[4...)Show SMILES Oc1ccc2c(\C=C\c3ccc(cc3)-c3ccccc3)cc3nc4ccccc4n3c2c1CN1C(=O)c2ccccc2C1=O Show InChI InChI=1S/C38H25N3O3/c42-34-21-20-28-27(19-16-24-14-17-26(18-15-24)25-8-2-1-3-9-25)22-35-39-32-12-6-7-13-33(32)41(35)36(28)31(34)23-40-37(43)29-10-4-5-11-30(29)38(40)44/h1-22,42H,23H2/b19-16+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 197 | n/a | n/a | n/a | n/a | n/a | n/a |
Lucknow University
Curated by ChEMBL
| Assay Description
Displacement of [125I](BH)CCK8 from rat CCK1 after 30 mins by receptor binding assay |
Bioorg Med Chem Lett 17: 2749-55 (2007)
Article DOI: 10.1016/j.bmcl.2007.02.071
BindingDB Entry DOI: 10.7270/Q2PV6K16 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(RAT) | BDBM50208419

(2-{2-hydroxy-5-[(E)-2-(4-hydroxy-phenyl)-vinyl]-be...)Show SMILES Oc1ccc(\C=C\c2cc3nc4ccccc4n3c3c(CN4C(=O)c5ccccc5C4=O)c(O)ccc23)cc1 Show InChI InChI=1S/C32H21N3O4/c36-21-13-10-19(11-14-21)9-12-20-17-29-33-26-7-3-4-8-27(26)35(29)30-22(20)15-16-28(37)25(30)18-34-31(38)23-5-1-2-6-24(23)32(34)39/h1-17,36-37H,18H2/b12-9+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 665 | n/a | n/a | n/a | n/a | n/a | n/a |
Lucknow University
Curated by ChEMBL
| Assay Description
Displacement of [125I](BH)CCK8 from rat CCK1 after 30 mins by receptor binding assay |
Bioorg Med Chem Lett 17: 2749-55 (2007)
Article DOI: 10.1016/j.bmcl.2007.02.071
BindingDB Entry DOI: 10.7270/Q2PV6K16 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(RAT) | BDBM50208420

(2-[2-hydroxy-5-((E)-styryl)-benzo[4,5]imidazo[1,2-...)Show SMILES Oc1ccc2c(\C=C\c3ccccc3)cc3nc4ccccc4n3c2c1CN1C(=O)c2ccccc2C1=O Show InChI InChI=1S/C32H21N3O3/c36-28-17-16-22-21(15-14-20-8-2-1-3-9-20)18-29-33-26-12-6-7-13-27(26)35(29)30(22)25(28)19-34-31(37)23-10-4-5-11-24(23)32(34)38/h1-18,36H,19H2/b15-14+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 942 | n/a | n/a | n/a | n/a | n/a | n/a |
Lucknow University
Curated by ChEMBL
| Assay Description
Displacement of [125I](BH)CCK8 from rat CCK1 after 30 mins by receptor binding assay |
Bioorg Med Chem Lett 17: 2749-55 (2007)
Article DOI: 10.1016/j.bmcl.2007.02.071
BindingDB Entry DOI: 10.7270/Q2PV6K16 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50072064

(5-Chloro-3-(4-methanesulfonyl-phenyl)-6''-methyl-[...)Show SMILES Cc1ccc(cn1)-c1ncc(Cl)cc1-c1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C18H15ClN2O2S/c1-12-3-4-14(10-20-12)18-17(9-15(19)11-21-18)13-5-7-16(8-6-13)24(2,22)23/h3-11H,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Delhi
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 using arachidonic acid as substrate preincubated for 5 mins followed by substrate addition and measured after 2 ... |
Eur J Med Chem 151: 520-532 (2018)
Article DOI: 10.1016/j.ejmech.2018.04.007
BindingDB Entry DOI: 10.7270/Q2HT2RXH |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data