 Found 27 hits with Last Name = 'tomimoto' and Initial = 'k'
Found 27 hits with Last Name = 'tomimoto' and Initial = 'k' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Squalene synthase
(Homo sapiens (Human)) | BDBM50038096

((6R,7R)-1-((4S,5R)-4-Acetoxy-5-methyl-3-methylene-...)Show SMILES CC[C@H](C)C[C@H](C)\C=C\C(=O)O[C@@H]1[C@@H](O)C2(CCC(=C)[C@@H](OC(C)=O)[C@H](C)Cc3ccccc3)OC1(C(O)=O)C(O)(C(O2)C(O)=O)C(O)=O Show InChI InChI=1S/C35H46O14/c1-7-19(2)17-20(3)13-14-25(37)47-28-27(38)33(48-29(30(39)40)34(45,31(41)42)35(28,49-33)32(43)44)16-15-21(4)26(46-23(6)36)22(5)18-24-11-9-8-10-12-24/h8-14,19-20,22,26-29,38,45H,4,7,15-18H2,1-3,5-6H3,(H,39,40)(H,41,42)(H,43,44)/b14-13+/t19-,20+,22+,26+,27+,28+,29?,33?,34?,35?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against HepG2 Squalene Synthase (SQS) |
Bioorg Med Chem Lett 5: 1989-1994 (1995)
Article DOI: 10.1016/0960-894X(95)00339-U
BindingDB Entry DOI: 10.7270/Q2B56K6F |
More data for this
Ligand-Target Pair | |
Squalene synthase
(Homo sapiens (Human)) | BDBM50285068
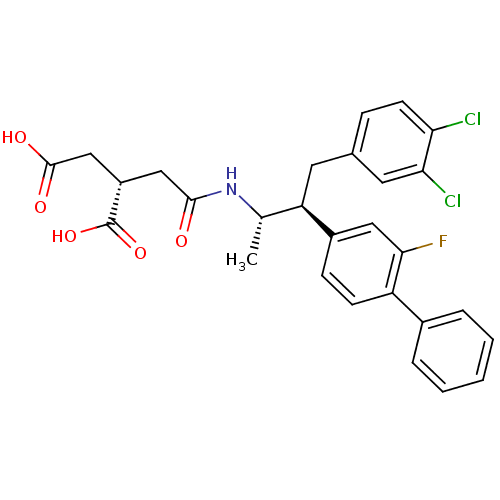
((S)-2-{[(1S,2S)-3-(3,4-Dichloro-phenyl)-2-(2-fluor...)Show SMILES C[C@H](NC(=O)C[C@@H](CC(O)=O)C(O)=O)[C@@H](Cc1ccc(Cl)c(Cl)c1)c1ccc(c(F)c1)-c1ccccc1 Show InChI InChI=1S/C28H26Cl2FNO5/c1-16(32-26(33)14-20(28(36)37)15-27(34)35)22(11-17-7-10-23(29)24(30)12-17)19-8-9-21(25(31)13-19)18-5-3-2-4-6-18/h2-10,12-13,16,20,22H,11,14-15H2,1H3,(H,32,33)(H,34,35)(H,36,37)/t16-,20-,22+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against HepG2 Squalene Synthase (SQS) |
Bioorg Med Chem Lett 5: 1989-1994 (1995)
Article DOI: 10.1016/0960-894X(95)00339-U
BindingDB Entry DOI: 10.7270/Q2B56K6F |
More data for this
Ligand-Target Pair | |
Squalene synthase
(Homo sapiens (Human)) | BDBM50285068

((S)-2-{[(1S,2S)-3-(3,4-Dichloro-phenyl)-2-(2-fluor...)Show SMILES C[C@H](NC(=O)C[C@@H](CC(O)=O)C(O)=O)[C@@H](Cc1ccc(Cl)c(Cl)c1)c1ccc(c(F)c1)-c1ccccc1 Show InChI InChI=1S/C28H26Cl2FNO5/c1-16(32-26(33)14-20(28(36)37)15-27(34)35)22(11-17-7-10-23(29)24(30)12-17)19-8-9-21(25(31)13-19)18-5-3-2-4-6-18/h2-10,12-13,16,20,22H,11,14-15H2,1H3,(H,32,33)(H,34,35)(H,36,37)/t16-,20-,22+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against squalene synthase (SQS) obtained from HepG2 cells |
Bioorg Med Chem Lett 6: 463-466 (1996)
Article DOI: 10.1016/0960-894X(96)00033-9
BindingDB Entry DOI: 10.7270/Q2MG7PG7 |
More data for this
Ligand-Target Pair | |
Squalene synthase
(Homo sapiens (Human)) | BDBM50403481
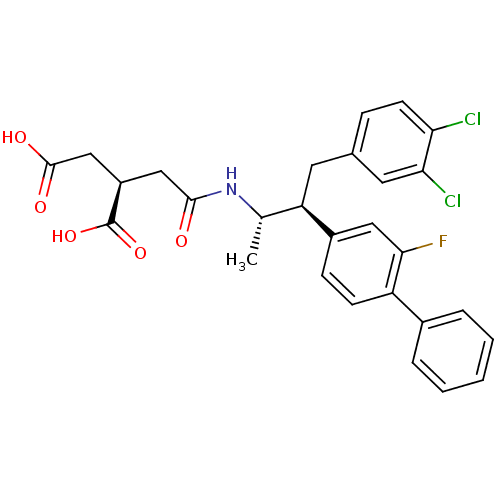
(CHEMBL2115091)Show SMILES C[C@H](NC(=O)C[C@H](CC(O)=O)C(O)=O)[C@@H](Cc1ccc(Cl)c(Cl)c1)c1ccc(c(F)c1)-c1ccccc1 Show InChI InChI=1S/C28H26Cl2FNO5/c1-16(32-26(33)14-20(28(36)37)15-27(34)35)22(11-17-7-10-23(29)24(30)12-17)19-8-9-21(25(31)13-19)18-5-3-2-4-6-18/h2-10,12-13,16,20,22H,11,14-15H2,1H3,(H,32,33)(H,34,35)(H,36,37)/t16-,20+,22+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 0.730 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against HepG2 Squalene Synthase (SQS) |
Bioorg Med Chem Lett 5: 1989-1994 (1995)
Article DOI: 10.1016/0960-894X(95)00339-U
BindingDB Entry DOI: 10.7270/Q2B56K6F |
More data for this
Ligand-Target Pair | |
Squalene synthase
(Homo sapiens (Human)) | BDBM50285065
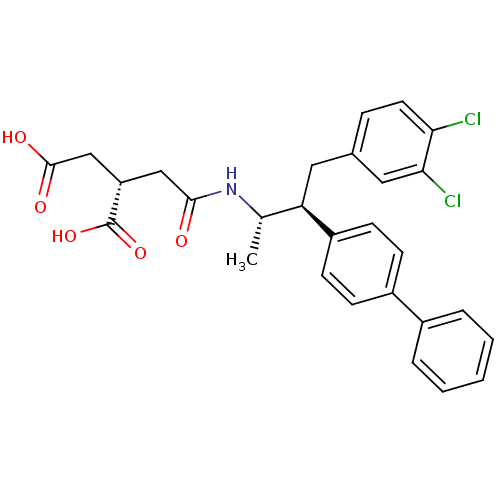
((S)-2-{[(1S,2S)-2-Biphenyl-4-yl-3-(3,4-dichloro-ph...)Show SMILES C[C@H](NC(=O)C[C@@H](CC(O)=O)C(O)=O)[C@@H](Cc1ccc(Cl)c(Cl)c1)c1ccc(cc1)-c1ccccc1 Show InChI InChI=1S/C28H27Cl2NO5/c1-17(31-26(32)15-22(28(35)36)16-27(33)34)23(13-18-7-12-24(29)25(30)14-18)21-10-8-20(9-11-21)19-5-3-2-4-6-19/h2-12,14,17,22-23H,13,15-16H2,1H3,(H,31,32)(H,33,34)(H,35,36)/t17-,22-,23+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against HepG2 Squalene Synthase (SQS) |
Bioorg Med Chem Lett 5: 1989-1994 (1995)
Article DOI: 10.1016/0960-894X(95)00339-U
BindingDB Entry DOI: 10.7270/Q2B56K6F |
More data for this
Ligand-Target Pair | |
Squalene synthase
(Homo sapiens (Human)) | BDBM50288819

((R)-4-[(E)-(1S,2S)-2-(3,4-Dichloro-benzyl)-1-methy...)Show SMILES C[C@@H](CC(O)=O)CC(=O)N[C@@H](C)[C@@H](Cc1ccc(Cl)c(Cl)c1)\C=C\c1ccc2ccccc2c1 Show InChI InChI=1S/C28H29Cl2NO3/c1-18(14-28(33)34)13-27(32)31-19(2)23(16-21-9-12-25(29)26(30)17-21)11-8-20-7-10-22-5-3-4-6-24(22)15-20/h3-12,15,17-19,23H,13-14,16H2,1-2H3,(H,31,32)(H,33,34)/b11-8+/t18-,19+,23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against squalene synthase (SQS) obtained from HepG2 cells |
Bioorg Med Chem Lett 6: 463-466 (1996)
Article DOI: 10.1016/0960-894X(96)00033-9
BindingDB Entry DOI: 10.7270/Q2MG7PG7 |
More data for this
Ligand-Target Pair | |
Squalene synthase
(Homo sapiens (Human)) | BDBM50285069
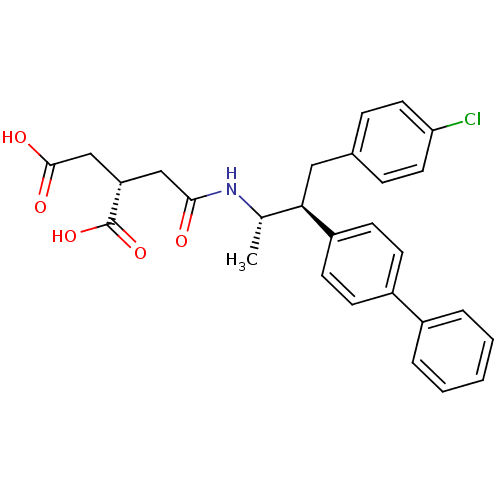
((S)-2-{[(1S,2S)-2-Biphenyl-4-yl-3-(4-chloro-phenyl...)Show SMILES C[C@H](NC(=O)C[C@@H](CC(O)=O)C(O)=O)[C@@H](Cc1ccc(Cl)cc1)c1ccc(cc1)-c1ccccc1 Show InChI InChI=1S/C28H28ClNO5/c1-18(30-26(31)16-23(28(34)35)17-27(32)33)25(15-19-7-13-24(29)14-8-19)22-11-9-21(10-12-22)20-5-3-2-4-6-20/h2-14,18,23,25H,15-17H2,1H3,(H,30,31)(H,32,33)(H,34,35)/t18-,23-,25+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against HepG2 Squalene Synthase (SQS) |
Bioorg Med Chem Lett 5: 1989-1994 (1995)
Article DOI: 10.1016/0960-894X(95)00339-U
BindingDB Entry DOI: 10.7270/Q2B56K6F |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Rattus norvegicus-Rattus norvegicus (rat)) | BDBM50062250
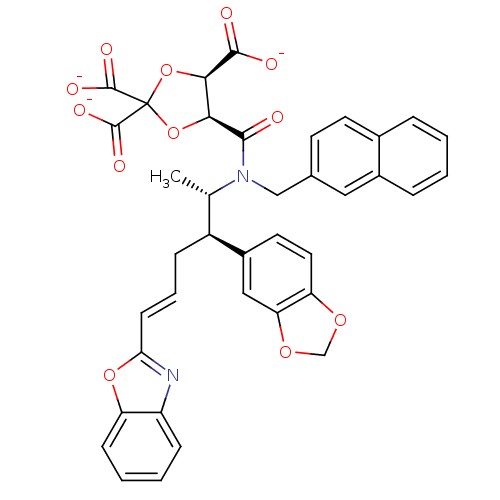
(CHEMBL36407 | trisodium 5-[2-benzo[d][1,3]dioxol-5...)Show SMILES C[C@@H]([C@H](C\C=C\c1nc2ccccc2o1)c1ccc2OCOc2c1)N(Cc1ccc2ccccc2c1)C(=O)[C@H]1OC(O[C@H]1C([O-])=O)(C([O-])=O)C([O-])=O Show InChI InChI=1S/C38H32N2O12/c1-21(26(25-15-16-29-30(18-25)49-20-48-29)9-6-12-31-39-27-10-4-5-11-28(27)50-31)40(19-22-13-14-23-7-2-3-8-24(23)17-22)34(41)32-33(35(42)43)52-38(51-32,36(44)45)37(46)47/h2-8,10-18,21,26,32-33H,9,19-20H2,1H3,(H,42,43)(H,44,45)(H,46,47)/p-3/b12-6+/t21-,26-,32-,33+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Farnesyltransferase from rat brain |
J Med Chem 41: 143-7 (1998)
Article DOI: 10.1021/jm970540f
BindingDB Entry DOI: 10.7270/Q23B5Z82 |
More data for this
Ligand-Target Pair | |
Squalene synthase
(Homo sapiens (Human)) | BDBM50288818

((R)-4-[(1S,2S)-3-(3,4-Dichloro-phenyl)-2-(2-fluoro...)Show SMILES C[C@@H](CC(O)=O)CC(=O)N[C@@H](C)[C@@H](Cc1ccc(Cl)c(Cl)c1)c1ccc(c(F)c1)-c1ccccc1 Show InChI InChI=1S/C28H28Cl2FNO3/c1-17(13-28(34)35)12-27(33)32-18(2)23(14-19-8-11-24(29)25(30)15-19)21-9-10-22(26(31)16-21)20-6-4-3-5-7-20/h3-11,15-18,23H,12-14H2,1-2H3,(H,32,33)(H,34,35)/t17-,18+,23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against squalene synthase (SQS) obtained from HepG2 cells |
Bioorg Med Chem Lett 6: 463-466 (1996)
Article DOI: 10.1016/0960-894X(96)00033-9
BindingDB Entry DOI: 10.7270/Q2MG7PG7 |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Rattus norvegicus-Rattus norvegicus (rat)) | BDBM50062251
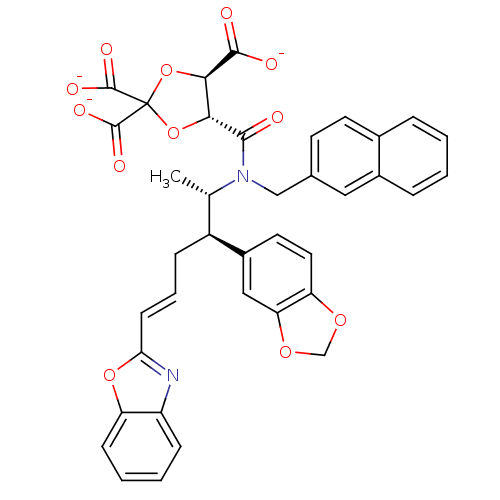
(CHEMBL285263 | trisodium 5-[2-benzo[d][1,3]dioxol-...)Show SMILES C[C@@H]([C@H](C\C=C\c1nc2ccccc2o1)c1ccc2OCOc2c1)N(Cc1ccc2ccccc2c1)C(=O)[C@@H]1OC(O[C@H]1C([O-])=O)(C([O-])=O)C([O-])=O Show InChI InChI=1S/C38H32N2O12/c1-21(26(25-15-16-29-30(18-25)49-20-48-29)9-6-12-31-39-27-10-4-5-11-28(27)50-31)40(19-22-13-14-23-7-2-3-8-24(23)17-22)34(41)32-33(35(42)43)52-38(51-32,36(44)45)37(46)47/h2-8,10-18,21,26,32-33H,9,19-20H2,1H3,(H,42,43)(H,44,45)(H,46,47)/p-3/b12-6+/t21-,26-,32+,33+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Farnesyltransferase from rat brain |
J Med Chem 41: 143-7 (1998)
Article DOI: 10.1021/jm970540f
BindingDB Entry DOI: 10.7270/Q23B5Z82 |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Rattus norvegicus-Rattus norvegicus (rat)) | BDBM50062249
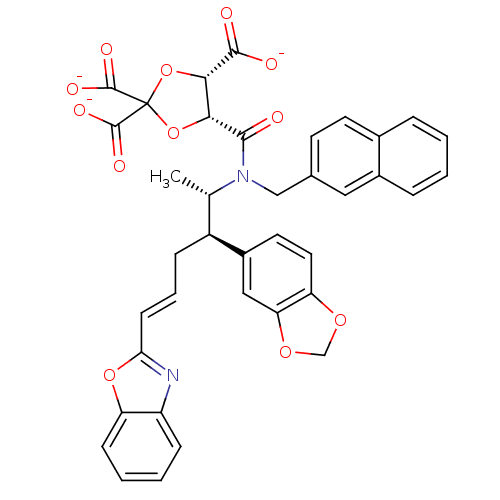
(CHEMBL285434 | trisodium 5-[2-benzo[d][1,3]dioxol-...)Show SMILES C[C@@H]([C@H](C\C=C\c1nc2ccccc2o1)c1ccc2OCOc2c1)N(Cc1ccc2ccccc2c1)C(=O)[C@@H]1OC(O[C@@H]1C([O-])=O)(C([O-])=O)C([O-])=O Show InChI InChI=1S/C38H32N2O12/c1-21(26(25-15-16-29-30(18-25)49-20-48-29)9-6-12-31-39-27-10-4-5-11-28(27)50-31)40(19-22-13-14-23-7-2-3-8-24(23)17-22)34(41)32-33(35(42)43)52-38(51-32,36(44)45)37(46)47/h2-8,10-18,21,26,32-33H,9,19-20H2,1H3,(H,42,43)(H,44,45)(H,46,47)/p-3/b12-6+/t21-,26-,32+,33-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Farnesyltransferase from rat brain |
J Med Chem 41: 143-7 (1998)
Article DOI: 10.1021/jm970540f
BindingDB Entry DOI: 10.7270/Q23B5Z82 |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Rattus norvegicus-Rattus norvegicus (rat)) | BDBM50062249

(CHEMBL285434 | trisodium 5-[2-benzo[d][1,3]dioxol-...)Show SMILES C[C@@H]([C@H](C\C=C\c1nc2ccccc2o1)c1ccc2OCOc2c1)N(Cc1ccc2ccccc2c1)C(=O)[C@@H]1OC(O[C@@H]1C([O-])=O)(C([O-])=O)C([O-])=O Show InChI InChI=1S/C38H32N2O12/c1-21(26(25-15-16-29-30(18-25)49-20-48-29)9-6-12-31-39-27-10-4-5-11-28(27)50-31)40(19-22-13-14-23-7-2-3-8-24(23)17-22)34(41)32-33(35(42)43)52-38(51-32,36(44)45)37(46)47/h2-8,10-18,21,26,32-33H,9,19-20H2,1H3,(H,42,43)(H,44,45)(H,46,47)/p-3/b12-6+/t21-,26-,32+,33-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Farnesyltransferase in competitive manner with respect to FPP (farnesyl diphosphate) at 0.6 microM FPP and 0.36 microM Ras peptide |
J Med Chem 41: 143-7 (1998)
Article DOI: 10.1021/jm970540f
BindingDB Entry DOI: 10.7270/Q23B5Z82 |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Rattus norvegicus-Rattus norvegicus (rat)) | BDBM50062252
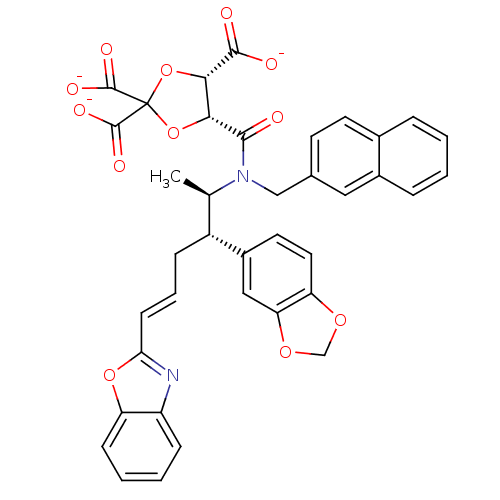
(CHEMBL36339 | trisodium 5-[2-benzo[d][1,3]dioxol-5...)Show SMILES C[C@H]([C@@H](C\C=C\c1nc2ccccc2o1)c1ccc2OCOc2c1)N(Cc1ccc2ccccc2c1)C(=O)[C@@H]1OC(O[C@@H]1C([O-])=O)(C([O-])=O)C([O-])=O Show InChI InChI=1S/C38H32N2O12/c1-21(26(25-15-16-29-30(18-25)49-20-48-29)9-6-12-31-39-27-10-4-5-11-28(27)50-31)40(19-22-13-14-23-7-2-3-8-24(23)17-22)34(41)32-33(35(42)43)52-38(51-32,36(44)45)37(46)47/h2-8,10-18,21,26,32-33H,9,19-20H2,1H3,(H,42,43)(H,44,45)(H,46,47)/p-3/b12-6+/t21-,26-,32-,33+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Farnesyltransferase from rat brain |
J Med Chem 41: 143-7 (1998)
Article DOI: 10.1021/jm970540f
BindingDB Entry DOI: 10.7270/Q23B5Z82 |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Rattus norvegicus-Rattus norvegicus (rat)) | BDBM50062249

(CHEMBL285434 | trisodium 5-[2-benzo[d][1,3]dioxol-...)Show SMILES C[C@@H]([C@H](C\C=C\c1nc2ccccc2o1)c1ccc2OCOc2c1)N(Cc1ccc2ccccc2c1)C(=O)[C@@H]1OC(O[C@@H]1C([O-])=O)(C([O-])=O)C([O-])=O Show InChI InChI=1S/C38H32N2O12/c1-21(26(25-15-16-29-30(18-25)49-20-48-29)9-6-12-31-39-27-10-4-5-11-28(27)50-31)40(19-22-13-14-23-7-2-3-8-24(23)17-22)34(41)32-33(35(42)43)52-38(51-32,36(44)45)37(46)47/h2-8,10-18,21,26,32-33H,9,19-20H2,1H3,(H,42,43)(H,44,45)(H,46,47)/p-3/b12-6+/t21-,26-,32+,33-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Farnesyltransferase in competitive manner with respect to FPP (farnesyl diphosphate) at 0.6 microM FPP and 3.6 microM Ras peptide. |
J Med Chem 41: 143-7 (1998)
Article DOI: 10.1021/jm970540f
BindingDB Entry DOI: 10.7270/Q23B5Z82 |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Rattus norvegicus-Rattus norvegicus (rat)) | BDBM50062248
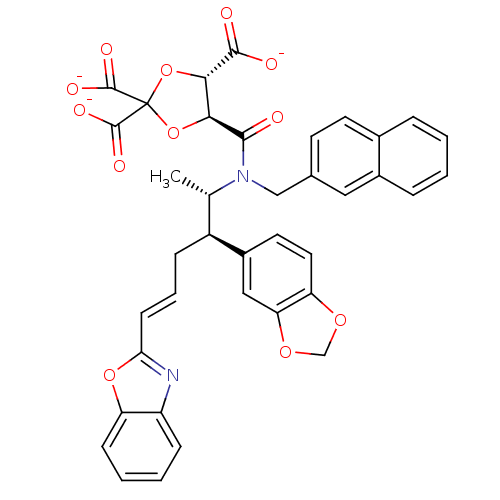
(CHEMBL36515 | trisodium 5-[2-benzo[d][1,3]dioxol-5...)Show SMILES C[C@@H]([C@H](C\C=C\c1nc2ccccc2o1)c1ccc2OCOc2c1)N(Cc1ccc2ccccc2c1)C(=O)[C@H]1OC(O[C@@H]1C([O-])=O)(C([O-])=O)C([O-])=O Show InChI InChI=1S/C38H32N2O12/c1-21(26(25-15-16-29-30(18-25)49-20-48-29)9-6-12-31-39-27-10-4-5-11-28(27)50-31)40(19-22-13-14-23-7-2-3-8-24(23)17-22)34(41)32-33(35(42)43)52-38(51-32,36(44)45)37(46)47/h2-8,10-18,21,26,32-33H,9,19-20H2,1H3,(H,42,43)(H,44,45)(H,46,47)/p-3/b12-6+/t21-,26-,32-,33-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Farnesyltransferase from rat brain |
J Med Chem 41: 143-7 (1998)
Article DOI: 10.1021/jm970540f
BindingDB Entry DOI: 10.7270/Q23B5Z82 |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Rattus norvegicus-Rattus norvegicus (rat)) | BDBM50062256
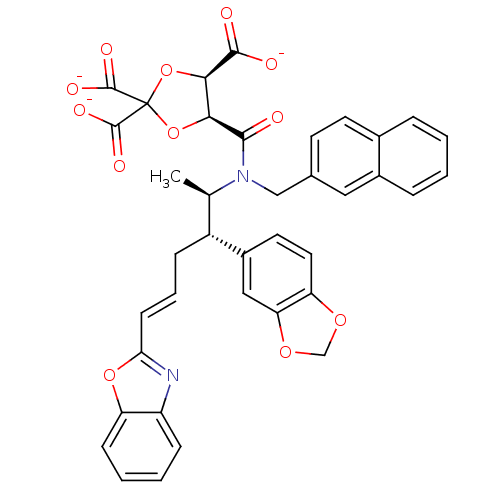
(CHEMBL284073 | trisodium 5-[2-benzo[d][1,3]dioxol-...)Show SMILES C[C@H]([C@@H](C\C=C\c1nc2ccccc2o1)c1ccc2OCOc2c1)N(Cc1ccc2ccccc2c1)C(=O)[C@H]1OC(O[C@H]1C([O-])=O)(C([O-])=O)C([O-])=O Show InChI InChI=1S/C38H32N2O12/c1-21(26(25-15-16-29-30(18-25)49-20-48-29)9-6-12-31-39-27-10-4-5-11-28(27)50-31)40(19-22-13-14-23-7-2-3-8-24(23)17-22)34(41)32-33(35(42)43)52-38(51-32,36(44)45)37(46)47/h2-8,10-18,21,26,32-33H,9,19-20H2,1H3,(H,42,43)(H,44,45)(H,46,47)/p-3/b12-6+/t21-,26-,32+,33-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Farnesyltransferase from rat brain |
J Med Chem 41: 143-7 (1998)
Article DOI: 10.1021/jm970540f
BindingDB Entry DOI: 10.7270/Q23B5Z82 |
More data for this
Ligand-Target Pair | |
Squalene synthase
(Homo sapiens (Human)) | BDBM50285066

((R)-2-{[(1R,2R)-3-(3,4-Dichloro-phenyl)-2-(2-fluor...)Show SMILES C[C@@H](NC(=O)C[C@H](CC(O)=O)C(O)=O)[C@H](Cc1ccc(Cl)c(Cl)c1)c1ccc(c(F)c1)-c1ccccc1 Show InChI InChI=1S/C28H26Cl2FNO5/c1-16(32-26(33)14-20(28(36)37)15-27(34)35)22(11-17-7-10-23(29)24(30)12-17)19-8-9-21(25(31)13-19)18-5-3-2-4-6-18/h2-10,12-13,16,20,22H,11,14-15H2,1H3,(H,32,33)(H,34,35)(H,36,37)/t16-,20-,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against HepG2 Squalene Synthase (SQS) |
Bioorg Med Chem Lett 5: 1989-1994 (1995)
Article DOI: 10.1016/0960-894X(95)00339-U
BindingDB Entry DOI: 10.7270/Q2B56K6F |
More data for this
Ligand-Target Pair | |
Squalene synthase
(Homo sapiens (Human)) | BDBM50285067
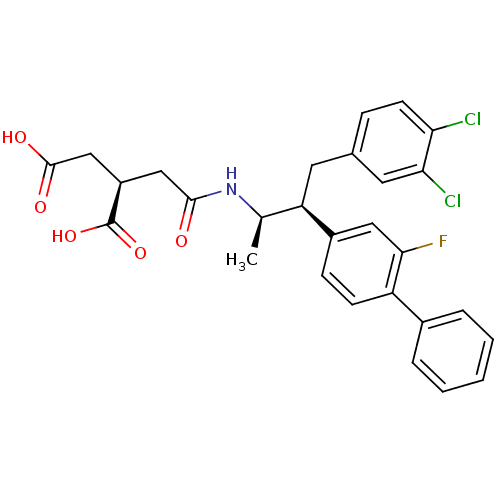
((R)-2-{[(1R,2S)-3-(3,4-Dichloro-phenyl)-2-(2-fluor...)Show SMILES C[C@@H](NC(=O)C[C@H](CC(O)=O)C(O)=O)[C@@H](Cc1ccc(Cl)c(Cl)c1)c1ccc(c(F)c1)-c1ccccc1 Show InChI InChI=1S/C28H26Cl2FNO5/c1-16(32-26(33)14-20(28(36)37)15-27(34)35)22(11-17-7-10-23(29)24(30)12-17)19-8-9-21(25(31)13-19)18-5-3-2-4-6-18/h2-10,12-13,16,20,22H,11,14-15H2,1H3,(H,32,33)(H,34,35)(H,36,37)/t16-,20-,22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against HepG2 Squalene Synthase (SQS) |
Bioorg Med Chem Lett 5: 1989-1994 (1995)
Article DOI: 10.1016/0960-894X(95)00339-U
BindingDB Entry DOI: 10.7270/Q2B56K6F |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Rattus norvegicus-Rattus norvegicus (rat)) | BDBM50062258
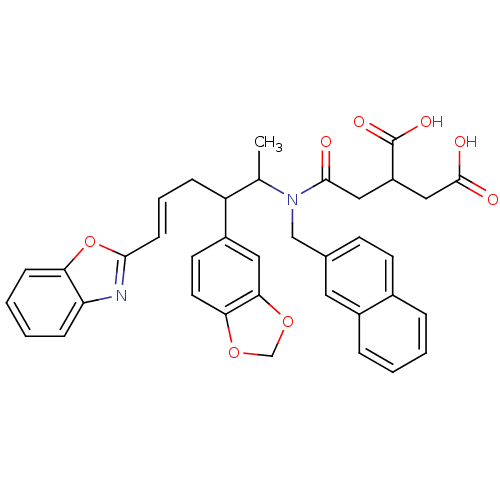
(2-{[((E)-2-Benzo[1,3]dioxol-5-yl-5-benzooxazol-2-y...)Show SMILES CC(C(C\C=C\c1nc2ccccc2o1)c1ccc2OCOc2c1)N(Cc1ccc2ccccc2c1)C(=O)CC(CC(O)=O)C(O)=O Show InChI InChI=1S/C37H34N2O8/c1-23(39(35(40)19-28(37(43)44)20-36(41)42)21-24-13-14-25-7-2-3-8-26(25)17-24)29(27-15-16-32-33(18-27)46-22-45-32)9-6-12-34-38-30-10-4-5-11-31(30)47-34/h2-8,10-18,23,28-29H,9,19-22H2,1H3,(H,41,42)(H,43,44)/b12-6+ | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Farnesyltransferase from rat brain |
J Med Chem 41: 143-7 (1998)
Article DOI: 10.1021/jm970540f
BindingDB Entry DOI: 10.7270/Q23B5Z82 |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Rattus norvegicus-Rattus norvegicus (rat)) | BDBM50062257
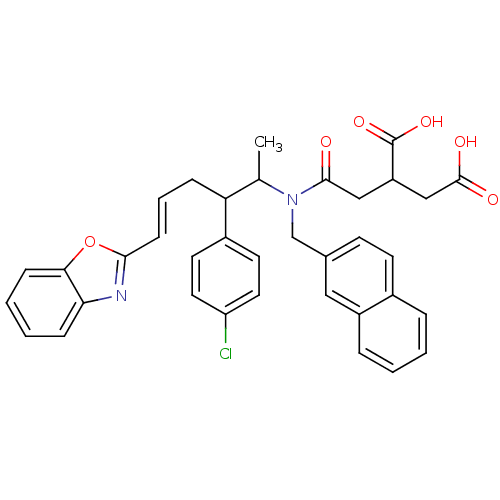
(2-({[(E)-5-Benzooxazol-2-yl-2-(4-chloro-phenyl)-1-...)Show SMILES CC(C(C\C=C\c1nc2ccccc2o1)c1ccc(Cl)cc1)N(Cc1ccc2ccccc2c1)C(=O)CC(CC(O)=O)C(O)=O Show InChI InChI=1S/C36H33ClN2O6/c1-23(30(26-15-17-29(37)18-16-26)9-6-12-33-38-31-10-4-5-11-32(31)45-33)39(34(40)20-28(36(43)44)21-35(41)42)22-24-13-14-25-7-2-3-8-27(25)19-24/h2-8,10-19,23,28,30H,9,20-22H2,1H3,(H,41,42)(H,43,44)/b12-6+ | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Farnesyltransferase from rat brain |
J Med Chem 41: 143-7 (1998)
Article DOI: 10.1021/jm970540f
BindingDB Entry DOI: 10.7270/Q23B5Z82 |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Rattus norvegicus-Rattus norvegicus (rat)) | BDBM50062249

(CHEMBL285434 | trisodium 5-[2-benzo[d][1,3]dioxol-...)Show SMILES C[C@@H]([C@H](C\C=C\c1nc2ccccc2o1)c1ccc2OCOc2c1)N(Cc1ccc2ccccc2c1)C(=O)[C@@H]1OC(O[C@@H]1C([O-])=O)(C([O-])=O)C([O-])=O Show InChI InChI=1S/C38H32N2O12/c1-21(26(25-15-16-29-30(18-25)49-20-48-29)9-6-12-31-39-27-10-4-5-11-28(27)50-31)40(19-22-13-14-23-7-2-3-8-24(23)17-22)34(41)32-33(35(42)43)52-38(51-32,36(44)45)37(46)47/h2-8,10-18,21,26,32-33H,9,19-20H2,1H3,(H,42,43)(H,44,45)(H,46,47)/p-3/b12-6+/t21-,26-,32+,33-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of FTase in competitive manner with respect to FPP (farnesyl diphosphate) at 6.0 microM FPP and 0.36 microM Ras peptide. |
J Med Chem 41: 143-7 (1998)
Article DOI: 10.1021/jm970540f
BindingDB Entry DOI: 10.7270/Q23B5Z82 |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Rattus norvegicus-Rattus norvegicus (rat)) | BDBM50062253
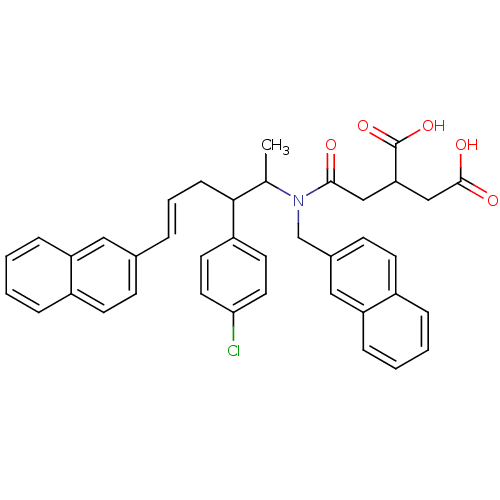
(2-({[(E)-2-(4-Chloro-phenyl)-1-methyl-5-naphthalen...)Show SMILES CC(C(C\C=C\c1ccc2ccccc2c1)c1ccc(Cl)cc1)N(Cc1ccc2ccccc2c1)C(=O)CC(CC(O)=O)C(O)=O Show InChI InChI=1S/C39H36ClNO5/c1-26(36(31-17-19-35(40)20-18-31)12-6-7-27-13-15-29-8-2-4-10-32(29)21-27)41(37(42)23-34(39(45)46)24-38(43)44)25-28-14-16-30-9-3-5-11-33(30)22-28/h2-11,13-22,26,34,36H,12,23-25H2,1H3,(H,43,44)(H,45,46)/b7-6+ | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Farnesyltransferase from rat brain |
J Med Chem 41: 143-7 (1998)
Article DOI: 10.1021/jm970540f
BindingDB Entry DOI: 10.7270/Q23B5Z82 |
More data for this
Ligand-Target Pair | |
Squalene synthase
(Homo sapiens (Human)) | BDBM50285066

((R)-2-{[(1R,2R)-3-(3,4-Dichloro-phenyl)-2-(2-fluor...)Show SMILES C[C@@H](NC(=O)C[C@H](CC(O)=O)C(O)=O)[C@H](Cc1ccc(Cl)c(Cl)c1)c1ccc(c(F)c1)-c1ccccc1 Show InChI InChI=1S/C28H26Cl2FNO5/c1-16(32-26(33)14-20(28(36)37)15-27(34)35)22(11-17-7-10-23(29)24(30)12-17)19-8-9-21(25(31)13-19)18-5-3-2-4-6-18/h2-10,12-13,16,20,22H,11,14-15H2,1H3,(H,32,33)(H,34,35)(H,36,37)/t16-,20-,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | >300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against HepG2 Squalene Synthase (SQS) |
Bioorg Med Chem Lett 5: 1989-1994 (1995)
Article DOI: 10.1016/0960-894X(95)00339-U
BindingDB Entry DOI: 10.7270/Q2B56K6F |
More data for this
Ligand-Target Pair | |
Squalene synthase
(Homo sapiens (Human)) | BDBM50285068

((S)-2-{[(1S,2S)-3-(3,4-Dichloro-phenyl)-2-(2-fluor...)Show SMILES C[C@H](NC(=O)C[C@@H](CC(O)=O)C(O)=O)[C@@H](Cc1ccc(Cl)c(Cl)c1)c1ccc(c(F)c1)-c1ccccc1 Show InChI InChI=1S/C28H26Cl2FNO5/c1-16(32-26(33)14-20(28(36)37)15-27(34)35)22(11-17-7-10-23(29)24(30)12-17)19-8-9-21(25(31)13-19)18-5-3-2-4-6-18/h2-10,12-13,16,20,22H,11,14-15H2,1H3,(H,32,33)(H,34,35)(H,36,37)/t16-,20-,22+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | >300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against HepG2 Squalene Synthase (SQS) |
Bioorg Med Chem Lett 5: 1989-1994 (1995)
Article DOI: 10.1016/0960-894X(95)00339-U
BindingDB Entry DOI: 10.7270/Q2B56K6F |
More data for this
Ligand-Target Pair | |
Squalene synthase
(Homo sapiens (Human)) | BDBM50285064
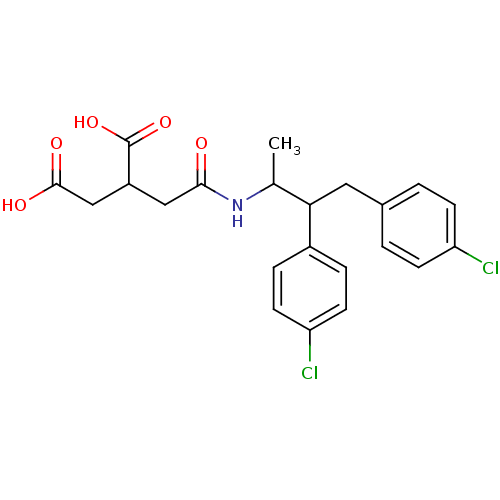
(2-{[2,3-Bis-(4-chloro-phenyl)-1-methyl-propylcarba...)Show SMILES CC(NC(=O)CC(CC(O)=O)C(O)=O)C(Cc1ccc(Cl)cc1)c1ccc(Cl)cc1 Show InChI InChI=1S/C22H23Cl2NO5/c1-13(25-20(26)11-16(22(29)30)12-21(27)28)19(15-4-8-18(24)9-5-15)10-14-2-6-17(23)7-3-14/h2-9,13,16,19H,10-12H2,1H3,(H,25,26)(H,27,28)(H,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 660 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against HepG2 Squalene Synthase (SQS) |
Bioorg Med Chem Lett 5: 1989-1994 (1995)
Article DOI: 10.1016/0960-894X(95)00339-U
BindingDB Entry DOI: 10.7270/Q2B56K6F |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Rattus norvegicus-Rattus norvegicus (rat)) | BDBM50062255
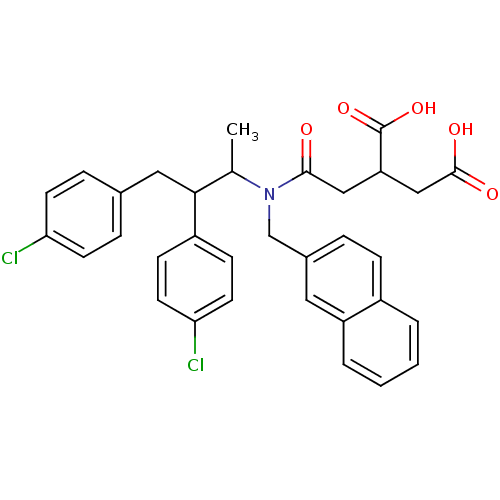
(2-({[2,3-Bis-(4-chloro-phenyl)-1-methyl-propyl]-na...)Show SMILES CC(C(Cc1ccc(Cl)cc1)c1ccc(Cl)cc1)N(Cc1ccc2ccccc2c1)C(=O)CC(CC(O)=O)C(O)=O Show InChI InChI=1S/C33H31Cl2NO5/c1-21(30(25-10-14-29(35)15-11-25)17-22-7-12-28(34)13-8-22)36(31(37)18-27(33(40)41)19-32(38)39)20-23-6-9-24-4-2-3-5-26(24)16-23/h2-16,21,27,30H,17-20H2,1H3,(H,38,39)(H,40,41) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Farnesyltransferase from rat brain |
J Med Chem 41: 143-7 (1998)
Article DOI: 10.1021/jm970540f
BindingDB Entry DOI: 10.7270/Q23B5Z82 |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Rattus norvegicus-Rattus norvegicus (rat)) | BDBM50062254
(2-({[2,3-Bis-(4-chloro-phenyl)-1-methyl-propyl]-me...)Show SMILES CC(C(Cc1ccc(Cl)cc1)c1ccc(Cl)cc1)N(C)C(=O)CC(CC(O)=O)C(O)=O Show InChI InChI=1S/C23H25Cl2NO5/c1-14(26(2)21(27)12-17(23(30)31)13-22(28)29)20(16-5-9-19(25)10-6-16)11-15-3-7-18(24)8-4-15/h3-10,14,17,20H,11-13H2,1-2H3,(H,28,29)(H,30,31) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Farnesyltransferase from rat brain |
J Med Chem 41: 143-7 (1998)
Article DOI: 10.1021/jm970540f
BindingDB Entry DOI: 10.7270/Q23B5Z82 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data