

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM50156455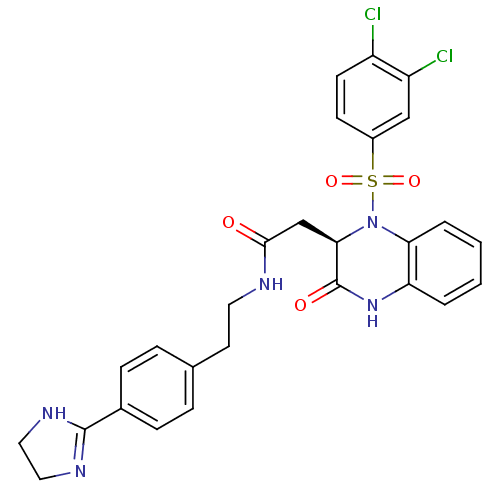 ((R)-N-(4-(4,5-dihydro-1H-imidazol-2-yl)phenethyl)-...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.00780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to human BK1 receptor E273 mutant | Bioorg Med Chem Lett 16: 2791-5 (2006) Article DOI: 10.1016/j.bmcl.2006.01.112 BindingDB Entry DOI: 10.7270/Q28915GX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM50156455 ((R)-N-(4-(4,5-dihydro-1H-imidazol-2-yl)phenethyl)-...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to human BK1 receptor | Bioorg Med Chem Lett 16: 2791-5 (2006) Article DOI: 10.1016/j.bmcl.2006.01.112 BindingDB Entry DOI: 10.7270/Q28915GX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM50156455 ((R)-N-(4-(4,5-dihydro-1H-imidazol-2-yl)phenethyl)-...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to human BK1 receptor N298 mutant | Bioorg Med Chem Lett 16: 2791-5 (2006) Article DOI: 10.1016/j.bmcl.2006.01.112 BindingDB Entry DOI: 10.7270/Q28915GX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM50184183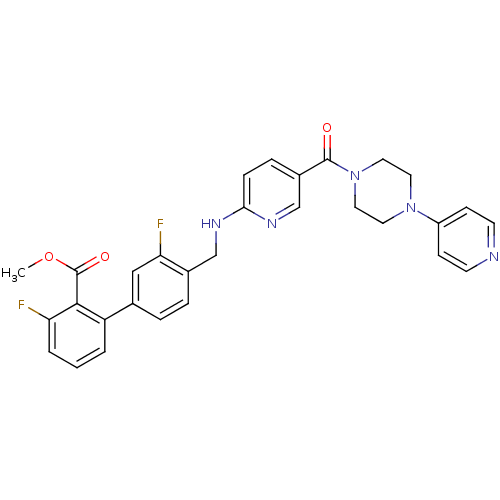 (3,3'-difluoro-4'-{[5-(4-pyridin-4-yl-piperazine-1-...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to human BK1 receptor | Bioorg Med Chem Lett 16: 2791-5 (2006) Article DOI: 10.1016/j.bmcl.2006.01.112 BindingDB Entry DOI: 10.7270/Q28915GX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM50156455 ((R)-N-(4-(4,5-dihydro-1H-imidazol-2-yl)phenethyl)-...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to human BK1 receptor D291 mutant | Bioorg Med Chem Lett 16: 2791-5 (2006) Article DOI: 10.1016/j.bmcl.2006.01.112 BindingDB Entry DOI: 10.7270/Q28915GX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM50184183 (3,3'-difluoro-4'-{[5-(4-pyridin-4-yl-piperazine-1-...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to human BK1 receptor E273 mutant | Bioorg Med Chem Lett 16: 2791-5 (2006) Article DOI: 10.1016/j.bmcl.2006.01.112 BindingDB Entry DOI: 10.7270/Q28915GX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM50184183 (3,3'-difluoro-4'-{[5-(4-pyridin-4-yl-piperazine-1-...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to human BK1 receptor D291 mutant | Bioorg Med Chem Lett 16: 2791-5 (2006) Article DOI: 10.1016/j.bmcl.2006.01.112 BindingDB Entry DOI: 10.7270/Q28915GX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Wee1-like protein kinase (Homo sapiens (Human)) | BDBM50522503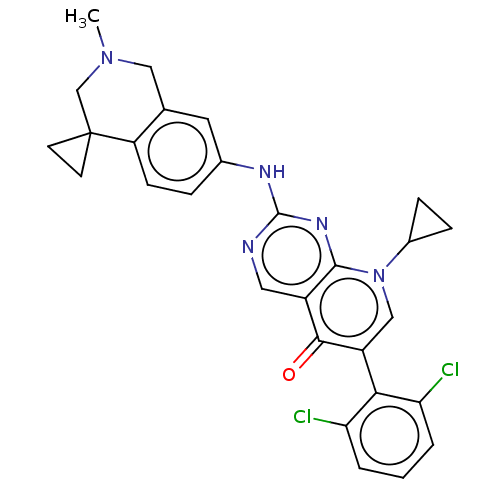 (CHEMBL4454675) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Global Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity to wild type N-terminal GST-tagged human WEE1 catalytic domain (215 to 646 residues) expressed in baculovirus expression system meas... | Bioorg Med Chem Lett 29: 1481-1486 (2019) Article DOI: 10.1016/j.bmcl.2019.04.017 BindingDB Entry DOI: 10.7270/Q2VX0KXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Wee1-like protein kinase (Homo sapiens (Human)) | BDBM50522508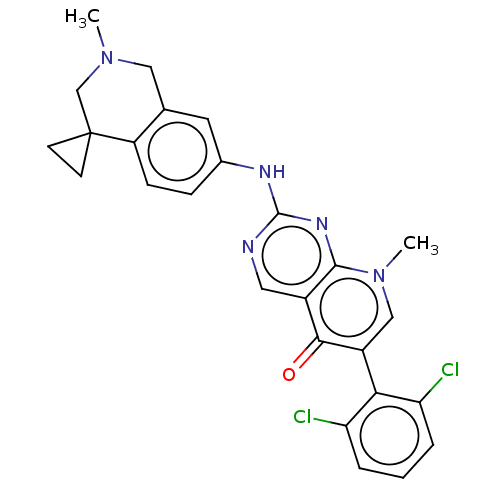 (CHEMBL4441166) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Global Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity to wild type N-terminal GST-tagged human WEE1 catalytic domain (215 to 646 residues) expressed in baculovirus expression system meas... | Bioorg Med Chem Lett 29: 1481-1486 (2019) Article DOI: 10.1016/j.bmcl.2019.04.017 BindingDB Entry DOI: 10.7270/Q2VX0KXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Wee1-like protein kinase (Homo sapiens (Human)) | BDBM50522506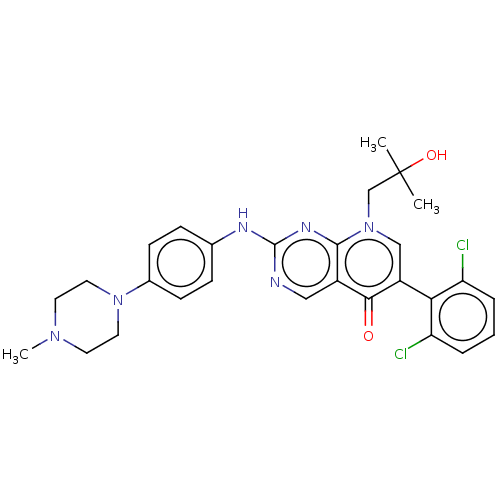 (CHEMBL4562919) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Global Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity to wild type N-terminal GST-tagged human WEE1 catalytic domain (215 to 646 residues) expressed in baculovirus expression system meas... | Bioorg Med Chem Lett 29: 1481-1486 (2019) Article DOI: 10.1016/j.bmcl.2019.04.017 BindingDB Entry DOI: 10.7270/Q2VX0KXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Wee1-like protein kinase (Homo sapiens (Human)) | BDBM50522512 (CHEMBL4544916) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Global Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity to wild type N-terminal GST-tagged human WEE1 catalytic domain (215 to 646 residues) expressed in baculovirus expression system meas... | Bioorg Med Chem Lett 29: 1481-1486 (2019) Article DOI: 10.1016/j.bmcl.2019.04.017 BindingDB Entry DOI: 10.7270/Q2VX0KXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50479471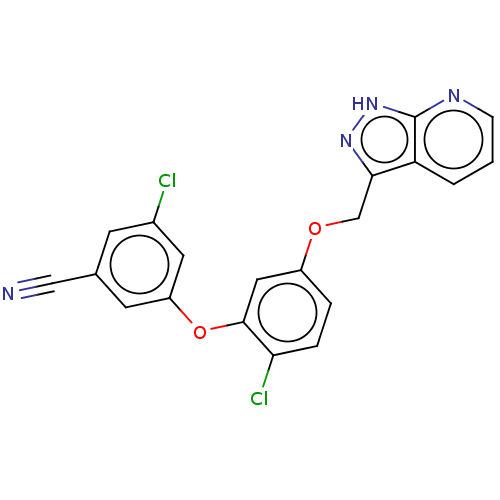 (CHEMBL491019 | MK-1107) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Co. Curated by ChEMBL | Assay Description Inhibition of Human immunodeficiency virus reverse transcriptase K103N mutant by SPA assay | J Med Chem 54: 7920-33 (2011) Article DOI: 10.1021/jm2010173 BindingDB Entry DOI: 10.7270/Q2W66PMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polycomb protein EED (Homo sapiens (Human)) | BDBM50235631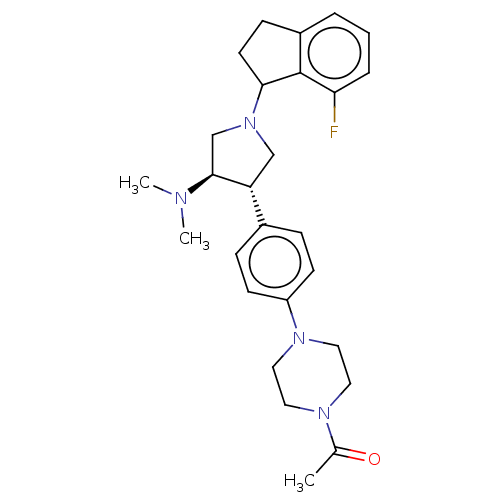 (CHEMBL4060827) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Inhibition of OG(488) labeled probe binding to GST-tagged EED (unknown origin) after 1 hr by LanthaScreen TR-FRET assay | Bioorg Med Chem Lett 27: 1576-1583 (2017) Article DOI: 10.1016/j.bmcl.2017.02.030 BindingDB Entry DOI: 10.7270/Q22F7QQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Wee1-like protein kinase (Homo sapiens (Human)) | BDBM50522514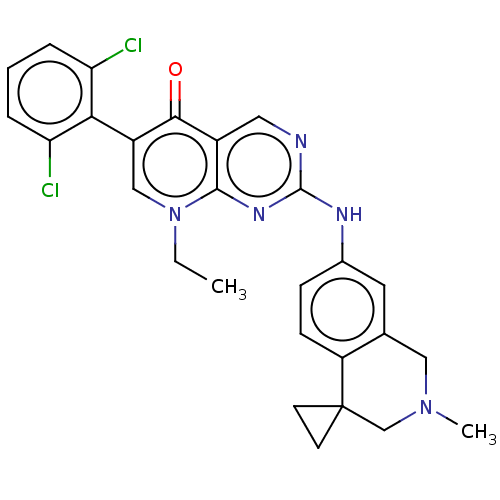 (CHEMBL4554796) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Global Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity to wild type N-terminal GST-tagged human WEE1 catalytic domain (215 to 646 residues) expressed in baculovirus expression system meas... | Bioorg Med Chem Lett 29: 1481-1486 (2019) Article DOI: 10.1016/j.bmcl.2019.04.017 BindingDB Entry DOI: 10.7270/Q2VX0KXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Wee1-like protein kinase (Homo sapiens (Human)) | BDBM50522509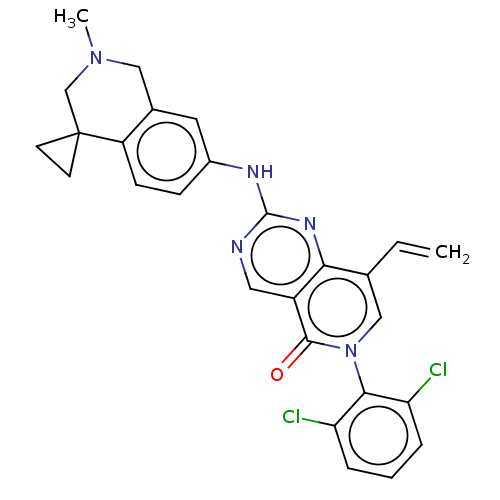 (CHEMBL4444364) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Global Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity to wild type N-terminal GST-tagged human WEE1 catalytic domain (215 to 646 residues) expressed in baculovirus expression system meas... | Bioorg Med Chem Lett 29: 1481-1486 (2019) Article DOI: 10.1016/j.bmcl.2019.04.017 BindingDB Entry DOI: 10.7270/Q2VX0KXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Wee1-like protein kinase (Homo sapiens (Human)) | BDBM50522502 (CHEMBL4529353) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Global Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity to wild type N-terminal GST-tagged human WEE1 catalytic domain (215 to 646 residues) expressed in baculovirus expression system meas... | Bioorg Med Chem Lett 29: 1481-1486 (2019) Article DOI: 10.1016/j.bmcl.2019.04.017 BindingDB Entry DOI: 10.7270/Q2VX0KXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Wee1-like protein kinase (Homo sapiens (Human)) | BDBM50522511 (CHEMBL4443172) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Global Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity to wild type N-terminal GST-tagged human WEE1 catalytic domain (215 to 646 residues) expressed in baculovirus expression system meas... | Bioorg Med Chem Lett 29: 1481-1486 (2019) Article DOI: 10.1016/j.bmcl.2019.04.017 BindingDB Entry DOI: 10.7270/Q2VX0KXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polycomb protein EED (Homo sapiens (Human)) | BDBM50235630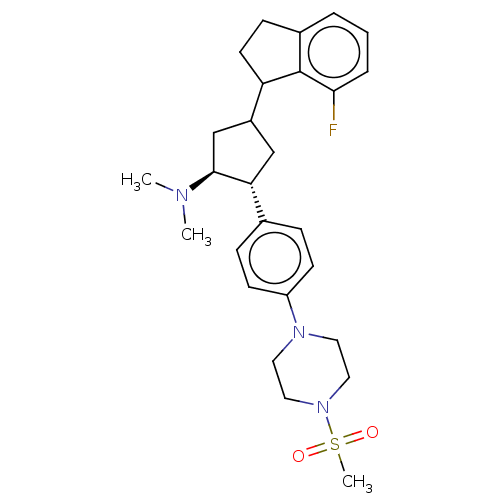 (CHEMBL4093096) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Inhibition of OG(488) labeled probe binding to GST-tagged EED (unknown origin) after 1 hr by LanthaScreen TR-FRET assay | Bioorg Med Chem Lett 27: 1576-1583 (2017) Article DOI: 10.1016/j.bmcl.2017.02.030 BindingDB Entry DOI: 10.7270/Q22F7QQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50479470 (CHEMBL489586 | MK-4965) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | MCE PC cid PC sid PDB UniChem | Article PubMed | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Co. Curated by ChEMBL | Assay Description Inhibition of Human immunodeficiency virus reverse transcriptase K103N mutant by SPA assay | J Med Chem 54: 7920-33 (2011) Article DOI: 10.1021/jm2010173 BindingDB Entry DOI: 10.7270/Q2W66PMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polycomb protein EED (Homo sapiens (Human)) | BDBM223987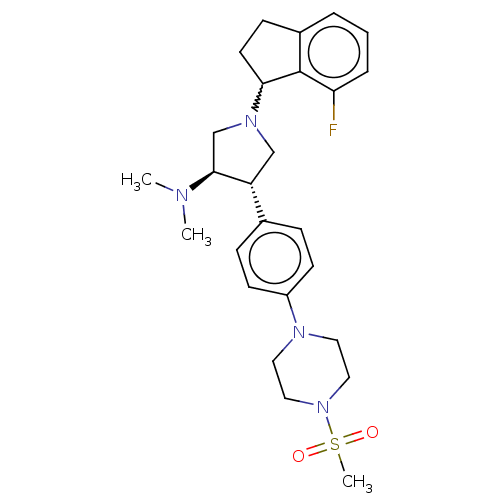 (A-395 (5) | rac-(3R,4S)-1-(7-fluoro-2,3-dihydro-1H...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 0.400 | -53.6 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
AbbVie Inc. | Assay Description For the assay, compounds were dispensed in assay-ready plates using a three-fold serial dilution from 50 μM to ~850 pM using an Echo 550 Acousti... | Nat Chem Biol 13: 389-395 (2017) Article DOI: 10.1038/nchembio.2306 BindingDB Entry DOI: 10.7270/Q2NG4PGD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50484635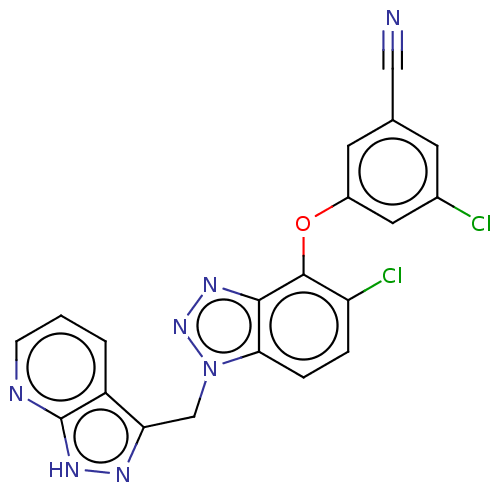 (CHEMBL1939500) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Co. Curated by ChEMBL | Assay Description Inhibition of Human immunodeficiency virus reverse transcriptase K103N mutant by SPA assay | J Med Chem 54: 7920-33 (2011) Article DOI: 10.1021/jm2010173 BindingDB Entry DOI: 10.7270/Q2W66PMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polycomb protein EED (Homo sapiens (Human)) | BDBM50235643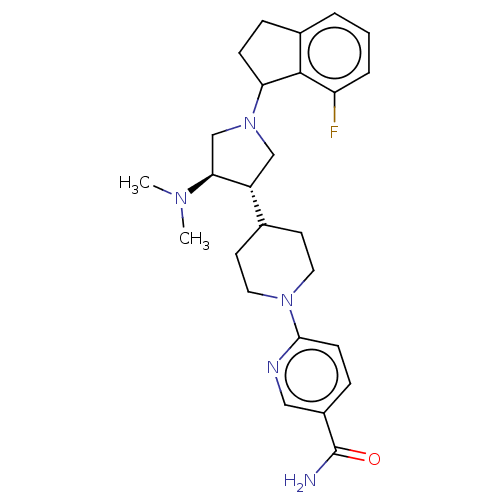 (CHEMBL4076017) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description In vitro displacement of [3H]-LY 278584 from rat cerebral cortex 5-hydroxytryptamine 3 receptor | Bioorg Med Chem Lett 27: 1576-1583 (2017) Article DOI: 10.1016/j.bmcl.2017.02.030 BindingDB Entry DOI: 10.7270/Q22F7QQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM439590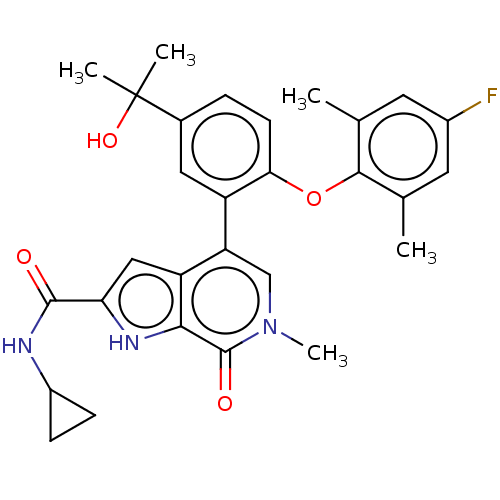 (US10633379, Example 121) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6 tagged BRD4 BD2 (352 to 457) residues expressed in Escherichia coli BL21(DE3) cells after 1 hr by TR-FRET assay | J Med Chem 63: 5585-5623 (2020) Article DOI: 10.1021/acs.jmedchem.0c00628 BindingDB Entry DOI: 10.7270/Q2057K8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50511849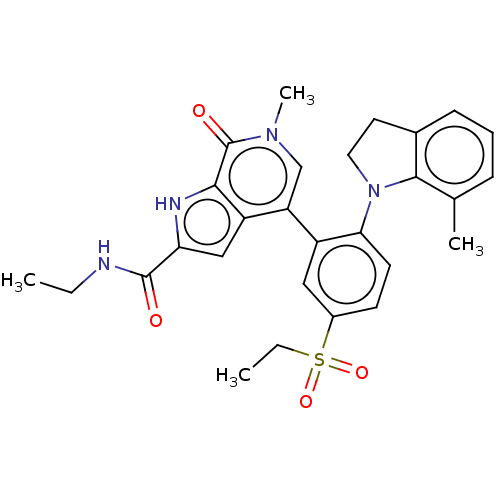 (CHEMBL4465299) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6 tagged BRD4 BD2 (352 to 457) residues expressed in Escherichia coli BL21(DE3) cells after 1 hr by TR-FRET assay | J Med Chem 63: 5585-5623 (2020) Article DOI: 10.1021/acs.jmedchem.0c00628 BindingDB Entry DOI: 10.7270/Q2057K8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Wee1-like protein kinase (Homo sapiens (Human)) | BDBM50522513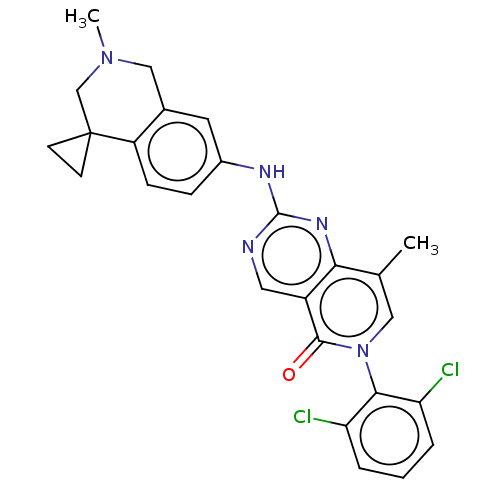 (CHEMBL4526423) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Global Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity to wild type N-terminal GST-tagged human WEE1 catalytic domain (215 to 646 residues) expressed in baculovirus expression system meas... | Bioorg Med Chem Lett 29: 1481-1486 (2019) Article DOI: 10.1016/j.bmcl.2019.04.017 BindingDB Entry DOI: 10.7270/Q2VX0KXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50511850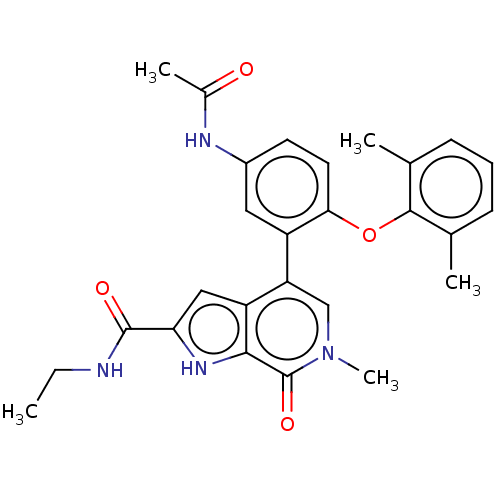 (CHEMBL4435166) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6 tagged BRD4 BD2 (352 to 457) residues expressed in Escherichia coli BL21(DE3) cells after 1 hr by TR-FRET assay | J Med Chem 63: 5585-5623 (2020) Article DOI: 10.1021/acs.jmedchem.0c00628 BindingDB Entry DOI: 10.7270/Q2057K8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50511875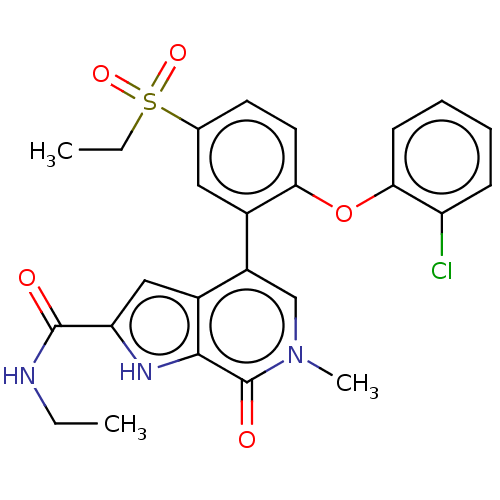 (CHEMBL4548794) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6 tagged BRD4 BD2 (352 to 457) residues expressed in Escherichia coli BL21(DE3) cells after 1 hr by TR-FRET assay | J Med Chem 63: 5585-5623 (2020) Article DOI: 10.1021/acs.jmedchem.0c00628 BindingDB Entry DOI: 10.7270/Q2057K8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polycomb protein EED (Homo sapiens (Human)) | BDBM223987 (A-395 (5) | rac-(3R,4S)-1-(7-fluoro-2,3-dihydro-1H...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 0.5 | -53.1 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
AbbVie Inc. | Assay Description For the assay, compounds were dispensed in assay-ready plates using a three-fold serial dilution from 50 μM to ~850 pM using an Echo 550 Acousti... | Nat Chem Biol 13: 389-395 (2017) Article DOI: 10.1038/nchembio.2306 BindingDB Entry DOI: 10.7270/Q2NG4PGD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50484632 (Mk-6186 | Mk6186) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Co. Curated by ChEMBL | Assay Description Inhibition of Human immunodeficiency virus reverse transcriptase K103N mutant by SPA assay | J Med Chem 54: 7920-33 (2011) Article DOI: 10.1021/jm2010173 BindingDB Entry DOI: 10.7270/Q2W66PMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50511864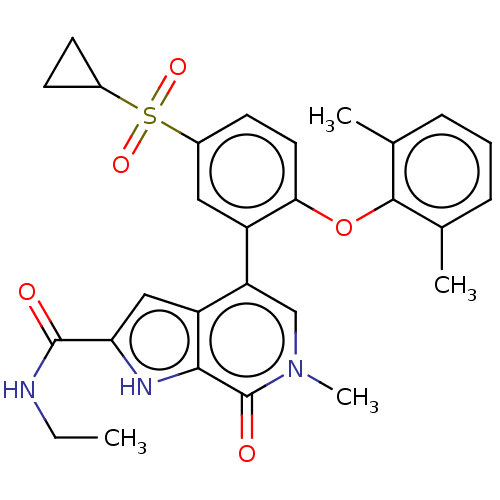 (CHEMBL4564879) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6 tagged BRD4 BD2 (352 to 457) residues expressed in Escherichia coli BL21(DE3) cells after 1 hr by TR-FRET assay | J Med Chem 63: 5585-5623 (2020) Article DOI: 10.1021/acs.jmedchem.0c00628 BindingDB Entry DOI: 10.7270/Q2057K8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM439461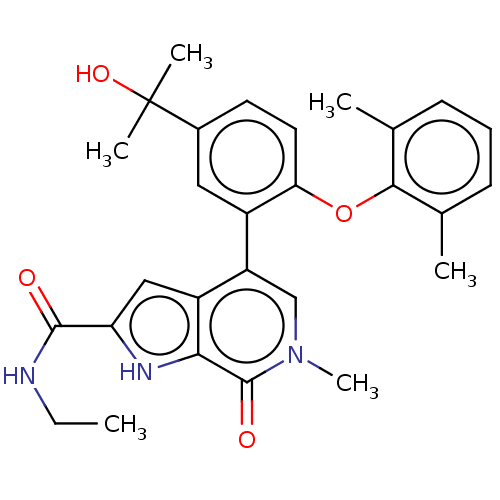 (US10633379, Example 3 | US10633379, Example 68) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6 tagged BRD4 BD2 (352 to 457) residues expressed in Escherichia coli BL21(DE3) cells after 1 hr by TR-FRET assay | J Med Chem 63: 5585-5623 (2020) Article DOI: 10.1021/acs.jmedchem.0c00628 BindingDB Entry DOI: 10.7270/Q2057K8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Wee1-like protein kinase (Homo sapiens (Human)) | BDBM50522510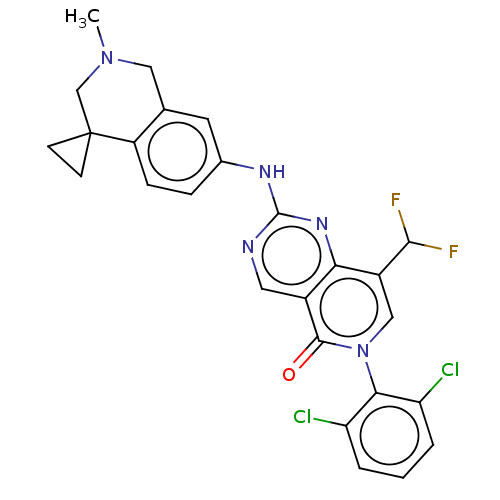 (CHEMBL4447769) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Global Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity to wild type N-terminal GST-tagged human WEE1 catalytic domain (215 to 646 residues) expressed in baculovirus expression system meas... | Bioorg Med Chem Lett 29: 1481-1486 (2019) Article DOI: 10.1016/j.bmcl.2019.04.017 BindingDB Entry DOI: 10.7270/Q2VX0KXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Wee1-like protein kinase (Homo sapiens (Human)) | BDBM50522507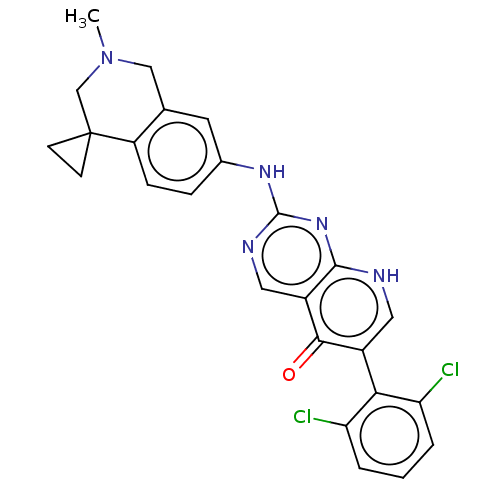 (CHEMBL4470852) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Global Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity to wild type N-terminal GST-tagged human WEE1 catalytic domain (215 to 646 residues) expressed in baculovirus expression system meas... | Bioorg Med Chem Lett 29: 1481-1486 (2019) Article DOI: 10.1016/j.bmcl.2019.04.017 BindingDB Entry DOI: 10.7270/Q2VX0KXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polycomb protein EED (Homo sapiens (Human)) | BDBM50235658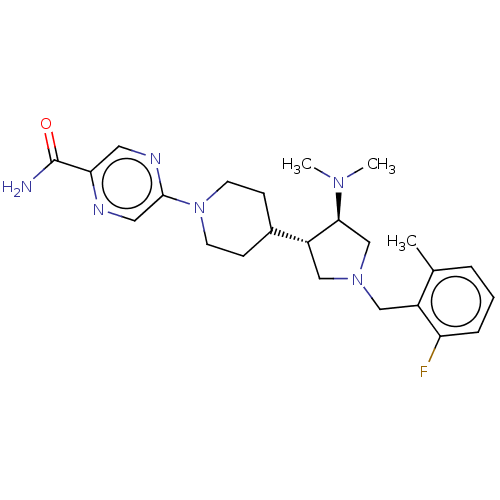 (CHEMBL4073166) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description In vitro displacement of [3H]-LY 278584 from rat cerebral cortex 5-hydroxytryptamine 3 receptor | Bioorg Med Chem Lett 27: 1576-1583 (2017) Article DOI: 10.1016/j.bmcl.2017.02.030 BindingDB Entry DOI: 10.7270/Q22F7QQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50511870 (CHEMBL4461291) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6 tagged BRD4 BD2 (352 to 457) residues expressed in Escherichia coli BL21(DE3) cells after 1 hr by TR-FRET assay | J Med Chem 63: 5585-5623 (2020) Article DOI: 10.1021/acs.jmedchem.0c00628 BindingDB Entry DOI: 10.7270/Q2057K8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50511855 (CHEMBL4454597) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6 tagged BRD4 BD2 (352 to 457) residues expressed in Escherichia coli BL21(DE3) cells after 1 hr by TR-FRET assay | J Med Chem 63: 5585-5623 (2020) Article DOI: 10.1021/acs.jmedchem.0c00628 BindingDB Entry DOI: 10.7270/Q2057K8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM50184203 (4'-{[3-(2-cyano-acetylamino)-4-methyl-pyridin-2-yl...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to human BK1 receptor | Bioorg Med Chem Lett 16: 2791-5 (2006) Article DOI: 10.1016/j.bmcl.2006.01.112 BindingDB Entry DOI: 10.7270/Q28915GX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polycomb protein EED (Homo sapiens (Human)) | BDBM50235644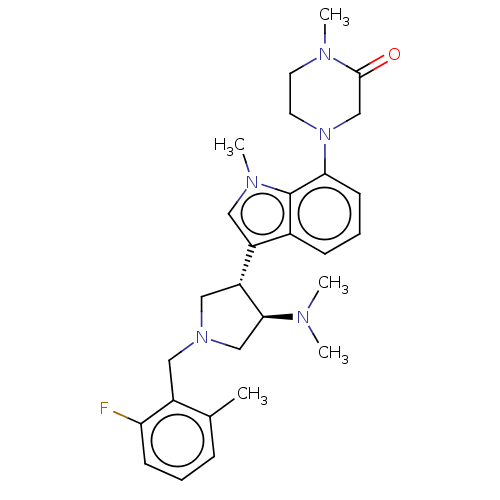 (CHEMBL4065766) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description In vitro displacement of [3H]-LY 278584 from rat cerebral cortex 5-hydroxytryptamine 3 receptor | Bioorg Med Chem Lett 27: 1576-1583 (2017) Article DOI: 10.1016/j.bmcl.2017.02.030 BindingDB Entry DOI: 10.7270/Q22F7QQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50511859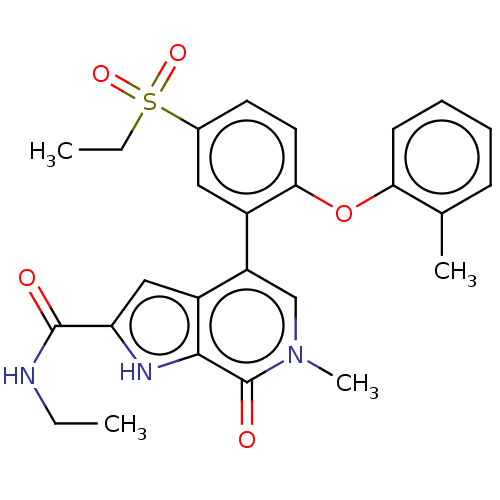 (CHEMBL4470856) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6 tagged BRD4 BD2 (352 to 457) residues expressed in Escherichia coli BL21(DE3) cells after 1 hr by TR-FRET assay | J Med Chem 63: 5585-5623 (2020) Article DOI: 10.1021/acs.jmedchem.0c00628 BindingDB Entry DOI: 10.7270/Q2057K8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50511861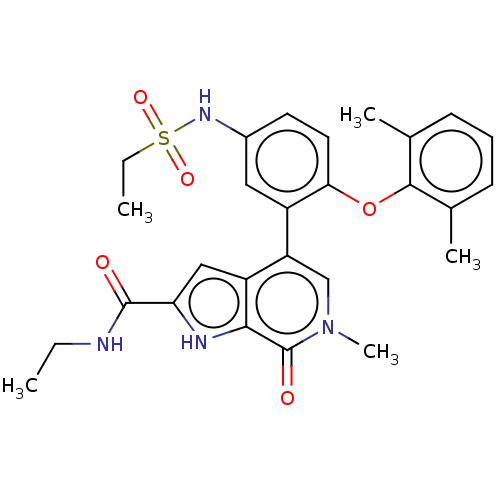 (CHEMBL4529861) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6 tagged BRD4 BD2 (352 to 457) residues expressed in Escherichia coli BL21(DE3) cells after 1 hr by TR-FRET assay | J Med Chem 63: 5585-5623 (2020) Article DOI: 10.1021/acs.jmedchem.0c00628 BindingDB Entry DOI: 10.7270/Q2057K8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50511869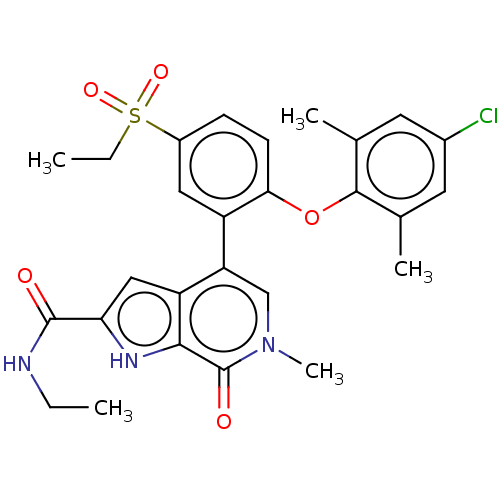 (CHEMBL4548127) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6 tagged BRD4 BD2 (352 to 457) residues expressed in Escherichia coli BL21(DE3) cells after 1 hr by TR-FRET assay | J Med Chem 63: 5585-5623 (2020) Article DOI: 10.1021/acs.jmedchem.0c00628 BindingDB Entry DOI: 10.7270/Q2057K8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM50184183 (3,3'-difluoro-4'-{[5-(4-pyridin-4-yl-piperazine-1-...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.830 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to human BK1 receptor N298 mutant | Bioorg Med Chem Lett 16: 2791-5 (2006) Article DOI: 10.1016/j.bmcl.2006.01.112 BindingDB Entry DOI: 10.7270/Q28915GX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM50184209 (4'-{[5-(4-cyclopropylmethyl-piperazine-1-carbonyl)...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to human BK1 receptor | Bioorg Med Chem Lett 16: 2791-5 (2006) Article DOI: 10.1016/j.bmcl.2006.01.112 BindingDB Entry DOI: 10.7270/Q28915GX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM50156455 ((R)-N-(4-(4,5-dihydro-1H-imidazol-2-yl)phenethyl)-...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.890 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to human BK1 receptor Q295 mutant | Bioorg Med Chem Lett 16: 2791-5 (2006) Article DOI: 10.1016/j.bmcl.2006.01.112 BindingDB Entry DOI: 10.7270/Q28915GX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50511868 (CHEMBL4560574) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6 tagged BRD4 BD2 (352 to 457) residues expressed in Escherichia coli BL21(DE3) cells after 1 hr by TR-FRET assay | J Med Chem 63: 5585-5623 (2020) Article DOI: 10.1021/acs.jmedchem.0c00628 BindingDB Entry DOI: 10.7270/Q2057K8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50511880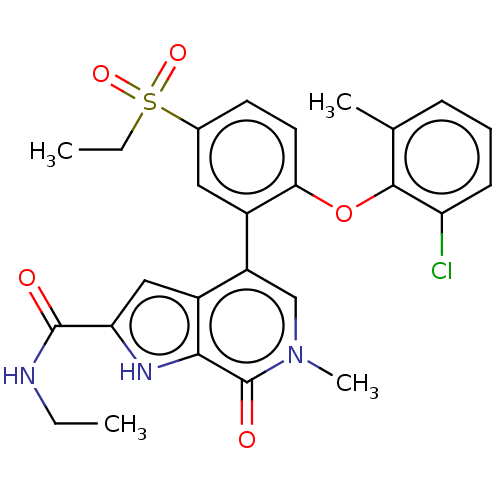 (CHEMBL4469571) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6 tagged BRD4 BD2 (352 to 457) residues expressed in Escherichia coli BL21(DE3) cells after 1 hr by TR-FRET assay | J Med Chem 63: 5585-5623 (2020) Article DOI: 10.1021/acs.jmedchem.0c00628 BindingDB Entry DOI: 10.7270/Q2057K8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50511871 (CHEMBL4454614) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6 tagged BRD4 BD2 (352 to 457) residues expressed in Escherichia coli BL21(DE3) cells after 1 hr by TR-FRET assay | J Med Chem 63: 5585-5623 (2020) Article DOI: 10.1021/acs.jmedchem.0c00628 BindingDB Entry DOI: 10.7270/Q2057K8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50484629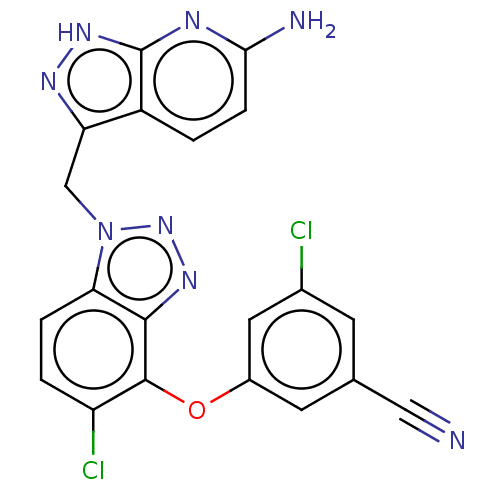 (CHEMBL1939503) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.940 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Co. Curated by ChEMBL | Assay Description Inhibition of Human immunodeficiency virus reverse transcriptase K103N mutant by SPA assay | J Med Chem 54: 7920-33 (2011) Article DOI: 10.1021/jm2010173 BindingDB Entry DOI: 10.7270/Q2W66PMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Wee1-like protein kinase (Homo sapiens (Human)) | BDBM50043648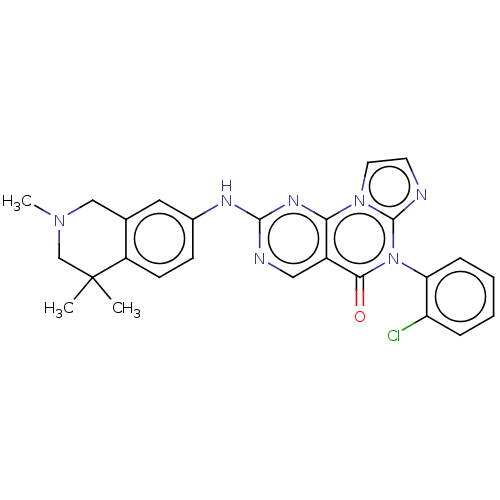 (CHEMBL3355533) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Curated by ChEMBL | Assay Description Inhibition of GST-tagged human Wee1 | ACS Med Chem Lett 6: 58-62 (2015) Article DOI: 10.1021/ml5002745 BindingDB Entry DOI: 10.7270/Q2NZ8972 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polycomb protein EED (Homo sapiens (Human)) | BDBM50235632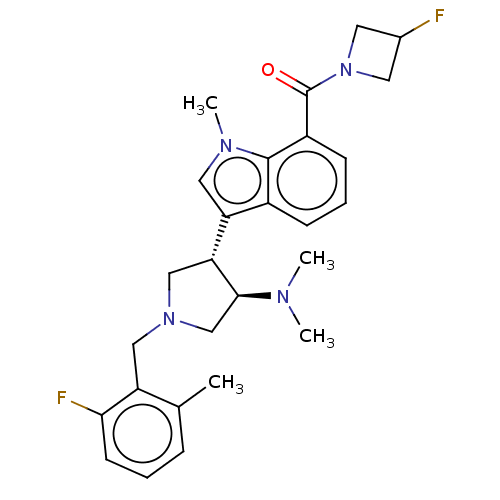 (CHEMBL4077363) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description In vitro displacement of [3H]-LY 278584 from rat cerebral cortex 5-hydroxytryptamine 3 receptor | Bioorg Med Chem Lett 27: 1576-1583 (2017) Article DOI: 10.1016/j.bmcl.2017.02.030 BindingDB Entry DOI: 10.7270/Q22F7QQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 646 total ) | Next | Last >> |