

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Renin (Homo sapiens (Human)) | BDBM98684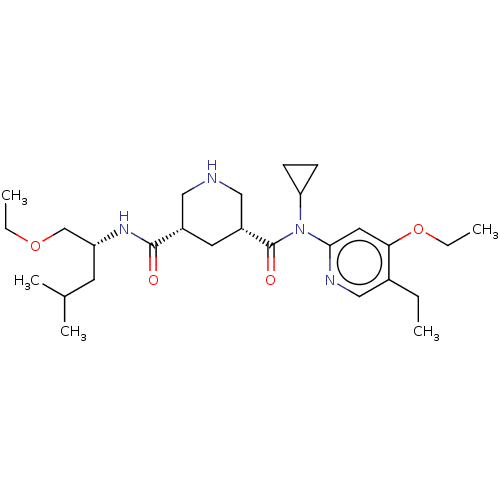 (US8497286, 160) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant renin using fluorescence-quenched RE(EDANS)IHPFHLVIHTK(Dabcyl)R substrate by fluorimetric assay | ACS Med Chem Lett 5: 787-92 (2014) Article DOI: 10.1021/ml500137b BindingDB Entry DOI: 10.7270/Q2WD4279 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM98679 (US8497286, 155) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant renin using fluorescence-quenched RE(EDANS)IHPFHLVIHTK(Dabcyl)R substrate by fluorimetric assay | ACS Med Chem Lett 5: 787-92 (2014) Article DOI: 10.1021/ml500137b BindingDB Entry DOI: 10.7270/Q2WD4279 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM98678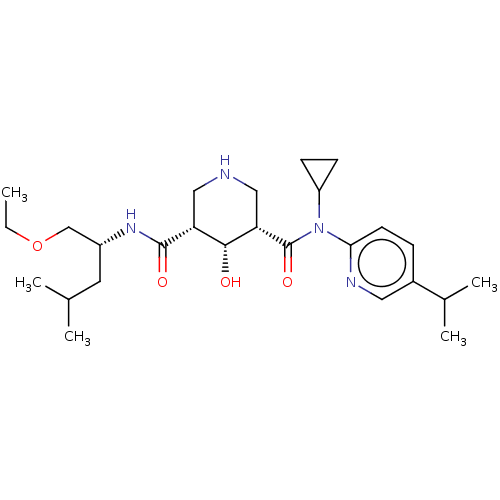 (US8497286, 154) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant renin using fluorescence-quenched RE(EDANS)IHPFHLVIHTK(Dabcyl)R substrate by fluorimetric assay | ACS Med Chem Lett 5: 787-92 (2014) Article DOI: 10.1021/ml500137b BindingDB Entry DOI: 10.7270/Q2WD4279 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM98680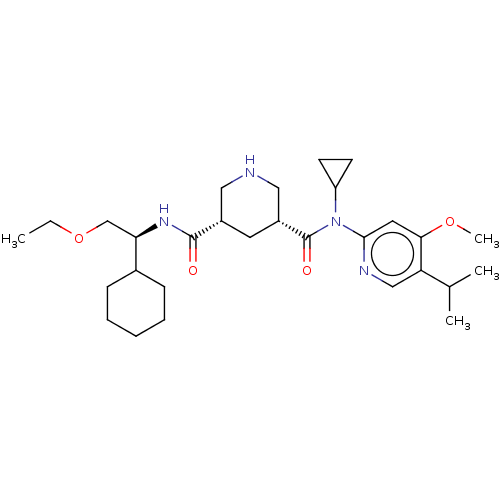 (US8497286, 156) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description Inhibition activity of renin using FRET assay. | US Patent US8497286 (2013) BindingDB Entry DOI: 10.7270/Q27P8X1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50054632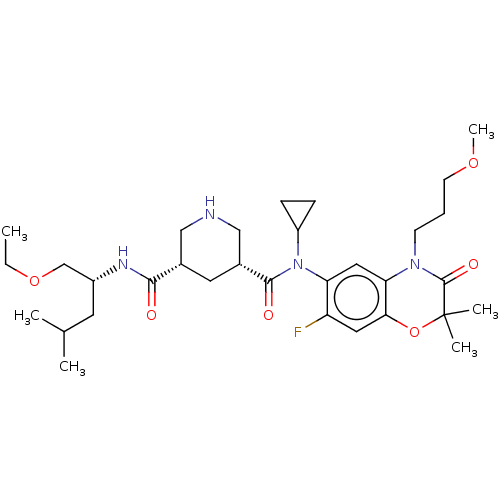 (CHEMBL3318940) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant renin using fluorescence-quenched RE(EDANS)IHPFHLVIHTK(Dabcyl)R substrate by fluorimetric assay | ACS Med Chem Lett 5: 787-92 (2014) Article DOI: 10.1021/ml500137b BindingDB Entry DOI: 10.7270/Q2WD4279 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50054540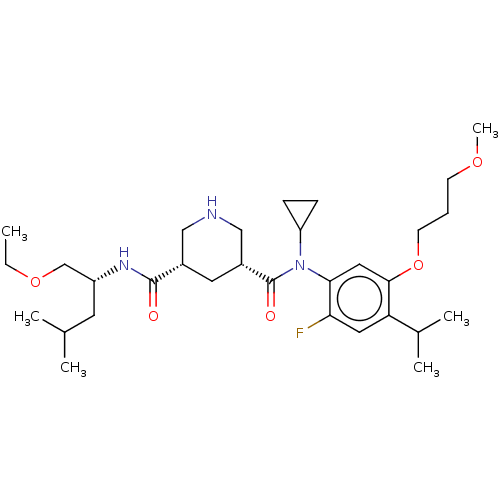 (CHEMBL3318939) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant renin using fluorescence-quenched RE(EDANS)IHPFHLVIHTK(Dabcyl)R substrate by fluorimetric assay | ACS Med Chem Lett 5: 787-92 (2014) Article DOI: 10.1021/ml500137b BindingDB Entry DOI: 10.7270/Q2WD4279 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM98686 (US8497286, 162) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description Inhibition activity of renin using FRET assay. | US Patent US8497286 (2013) BindingDB Entry DOI: 10.7270/Q27P8X1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM98676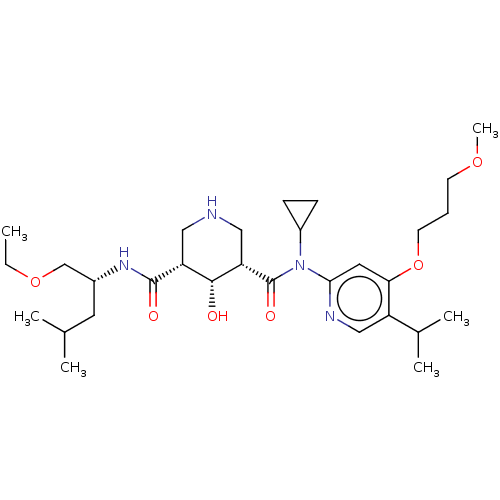 (US8497286, 152) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description Inhibition activity of renin using FRET assay. | US Patent US8497286 (2013) BindingDB Entry DOI: 10.7270/Q27P8X1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50054637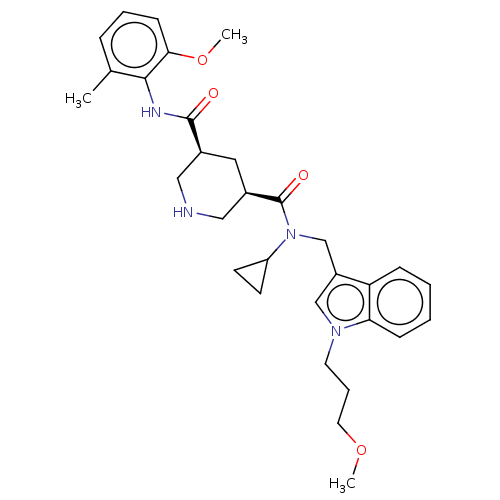 (CHEMBL3318938) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant renin using fluorescence-quenched RE(EDANS)IHPFHLVIHTK(Dabcyl)R substrate by fluorimetric assay | ACS Med Chem Lett 5: 787-92 (2014) Article DOI: 10.1021/ml500137b BindingDB Entry DOI: 10.7270/Q2WD4279 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM98679 (US8497286, 155) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant renin in presence of human plasma using Ac- IHPFHL-VIHNK-(DY-505-X5)-COOH substrate by fluorimetric assay | ACS Med Chem Lett 5: 787-92 (2014) Article DOI: 10.1021/ml500137b BindingDB Entry DOI: 10.7270/Q2WD4279 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50054633 (CHEMBL3318941) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant renin using fluorescence-quenched RE(EDANS)IHPFHLVIHTK(Dabcyl)R substrate by fluorimetric assay | ACS Med Chem Lett 5: 787-92 (2014) Article DOI: 10.1021/ml500137b BindingDB Entry DOI: 10.7270/Q2WD4279 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50054636 (CHEMBL3318937) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant renin using fluorescence-quenched RE(EDANS)IHPFHLVIHTK(Dabcyl)R substrate by fluorimetric assay | ACS Med Chem Lett 5: 787-92 (2014) Article DOI: 10.1021/ml500137b BindingDB Entry DOI: 10.7270/Q2WD4279 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM98686 (US8497286, 162) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant renin using fluorescence-quenched RE(EDANS)IHPFHLVIHTK(Dabcyl)R substrate by fluorimetric assay | ACS Med Chem Lett 5: 787-92 (2014) Article DOI: 10.1021/ml500137b BindingDB Entry DOI: 10.7270/Q2WD4279 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM98684 (US8497286, 160) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant renin in presence of human plasma using Ac- IHPFHL-VIHNK-(DY-505-X5)-COOH substrate by fluorimetric assay | ACS Med Chem Lett 5: 787-92 (2014) Article DOI: 10.1021/ml500137b BindingDB Entry DOI: 10.7270/Q2WD4279 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM98690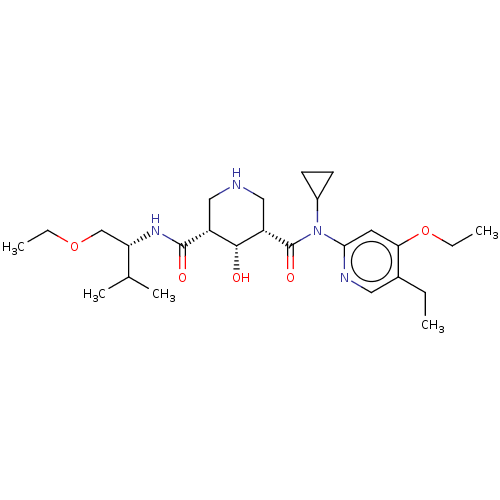 (US8497286, 166) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description Inhibition activity of renin using FRET assay. | US Patent US8497286 (2013) BindingDB Entry DOI: 10.7270/Q27P8X1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM98675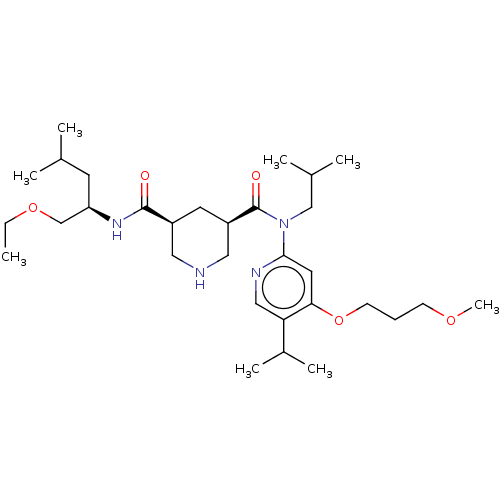 (US8497286, 151) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description Inhibition activity of renin using FRET assay. | US Patent US8497286 (2013) BindingDB Entry DOI: 10.7270/Q27P8X1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM98684 (US8497286, 160) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description Inhibition activity of renin using FRET assay. | US Patent US8497286 (2013) BindingDB Entry DOI: 10.7270/Q27P8X1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM98678 (US8497286, 154) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant renin in presence of human plasma using Ac- IHPFHL-VIHNK-(DY-505-X5)-COOH substrate by fluorimetric assay | ACS Med Chem Lett 5: 787-92 (2014) Article DOI: 10.1021/ml500137b BindingDB Entry DOI: 10.7270/Q2WD4279 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM98685 (US8497286, 161) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant renin using fluorescence-quenched RE(EDANS)IHPFHLVIHTK(Dabcyl)R substrate by fluorimetric assay | ACS Med Chem Lett 5: 787-92 (2014) Article DOI: 10.1021/ml500137b BindingDB Entry DOI: 10.7270/Q2WD4279 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM98685 (US8497286, 161) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description Inhibition activity of renin using FRET assay. | US Patent US8497286 (2013) BindingDB Entry DOI: 10.7270/Q27P8X1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM98682 (US8497286, 158) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description Inhibition activity of renin using FRET assay. | US Patent US8497286 (2013) BindingDB Entry DOI: 10.7270/Q27P8X1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50054634 (CHEMBL3318942) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant renin using fluorescence-quenched RE(EDANS)IHPFHLVIHTK(Dabcyl)R substrate by fluorimetric assay | ACS Med Chem Lett 5: 787-92 (2014) Article DOI: 10.1021/ml500137b BindingDB Entry DOI: 10.7270/Q2WD4279 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM98677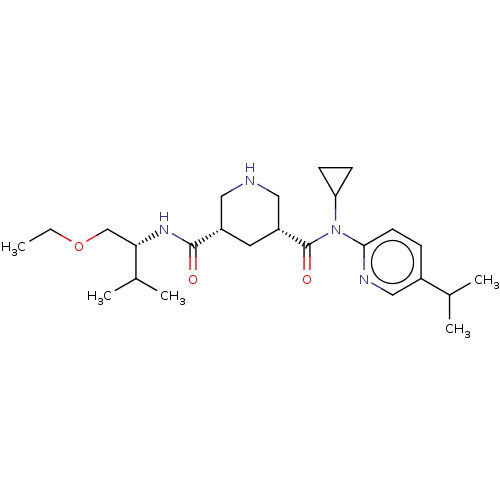 (US8497286, 153) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description Inhibition activity of renin using FRET assay. | US Patent US8497286 (2013) BindingDB Entry DOI: 10.7270/Q27P8X1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM98673 (US8497286, 149) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description Inhibition activity of renin using FRET assay. | US Patent US8497286 (2013) BindingDB Entry DOI: 10.7270/Q27P8X1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM98679 (US8497286, 155) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description Inhibition activity of renin using FRET assay. | US Patent US8497286 (2013) BindingDB Entry DOI: 10.7270/Q27P8X1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM98673 (US8497286, 149) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant renin using fluorescence-quenched RE(EDANS)IHPFHLVIHTK(Dabcyl)R substrate by fluorimetric assay | ACS Med Chem Lett 5: 787-92 (2014) Article DOI: 10.1021/ml500137b BindingDB Entry DOI: 10.7270/Q2WD4279 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50054636 (CHEMBL3318937) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant renin in presence of human plasma using Ac- IHPFHL-VIHNK-(DY-505-X5)-COOH substrate by fluorimetric assay | ACS Med Chem Lett 5: 787-92 (2014) Article DOI: 10.1021/ml500137b BindingDB Entry DOI: 10.7270/Q2WD4279 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50273289 (2-Cyano-4-(1-methyl-piperidin-4-yloxy)-6-[(spiro[2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin S by fluorometric assay | Bioorg Med Chem Lett 18: 4642-6 (2008) Article DOI: 10.1016/j.bmcl.2008.07.011 BindingDB Entry DOI: 10.7270/Q2WH2PS6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50054633 (CHEMBL3318941) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant renin in presence of human plasma using Ac- IHPFHL-VIHNK-(DY-505-X5)-COOH substrate by fluorimetric assay | ACS Med Chem Lett 5: 787-92 (2014) Article DOI: 10.1021/ml500137b BindingDB Entry DOI: 10.7270/Q2WD4279 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM98673 (US8497286, 149) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant renin in presence of human plasma using Ac- IHPFHL-VIHNK-(DY-505-X5)-COOH substrate by fluorimetric assay | ACS Med Chem Lett 5: 787-92 (2014) Article DOI: 10.1021/ml500137b BindingDB Entry DOI: 10.7270/Q2WD4279 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM98685 (US8497286, 161) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant renin in presence of human plasma using Ac- IHPFHL-VIHNK-(DY-505-X5)-COOH substrate by fluorimetric assay | ACS Med Chem Lett 5: 787-92 (2014) Article DOI: 10.1021/ml500137b BindingDB Entry DOI: 10.7270/Q2WD4279 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50273288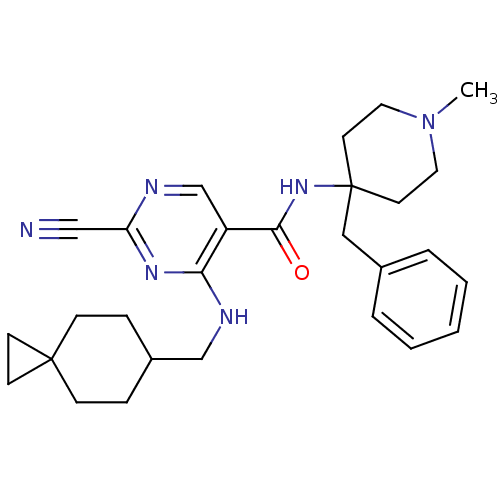 (2-Cyano-4-[(spiro[2.5]oct-6-ylmethyl)-amino]-pyrim...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin S by fluorometric assay | Bioorg Med Chem Lett 18: 4642-6 (2008) Article DOI: 10.1016/j.bmcl.2008.07.011 BindingDB Entry DOI: 10.7270/Q2WH2PS6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50263668 (2-Cyano-4-[2-(1-methyl-piperidin-4-yl)-ethoxy]-6-[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin S by fluorometric assay | Bioorg Med Chem Lett 18: 4642-6 (2008) Article DOI: 10.1016/j.bmcl.2008.07.011 BindingDB Entry DOI: 10.7270/Q2WH2PS6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM98678 (US8497286, 154) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description Inhibition activity of renin using FRET assay. | US Patent US8497286 (2013) BindingDB Entry DOI: 10.7270/Q27P8X1B | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM98683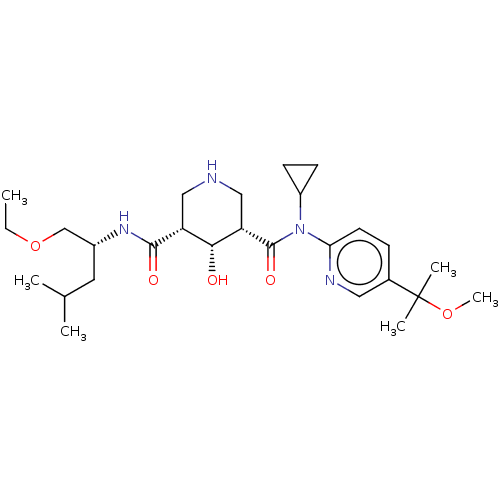 (US8497286, 159) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description Inhibition activity of renin using FRET assay. | US Patent US8497286 (2013) BindingDB Entry DOI: 10.7270/Q27P8X1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50272786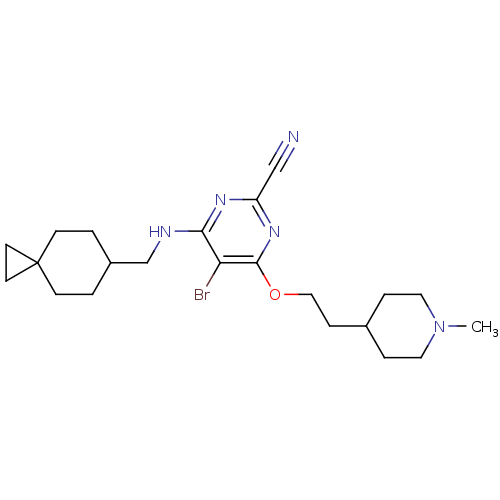 (5-Bromo-4-[2-(1-methyl-piperidin-4-yl)-ethoxy]-6-[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin S by fluorometric assay | Bioorg Med Chem Lett 18: 4642-6 (2008) Article DOI: 10.1016/j.bmcl.2008.07.011 BindingDB Entry DOI: 10.7270/Q2WH2PS6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50054637 (CHEMBL3318938) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant renin in presence of human plasma using Ac- IHPFHL-VIHNK-(DY-505-X5)-COOH substrate by fluorimetric assay | ACS Med Chem Lett 5: 787-92 (2014) Article DOI: 10.1021/ml500137b BindingDB Entry DOI: 10.7270/Q2WD4279 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50054540 (CHEMBL3318939) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant renin in presence of human plasma using Ac- IHPFHL-VIHNK-(DY-505-X5)-COOH substrate by fluorimetric assay | ACS Med Chem Lett 5: 787-92 (2014) Article DOI: 10.1021/ml500137b BindingDB Entry DOI: 10.7270/Q2WD4279 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM98681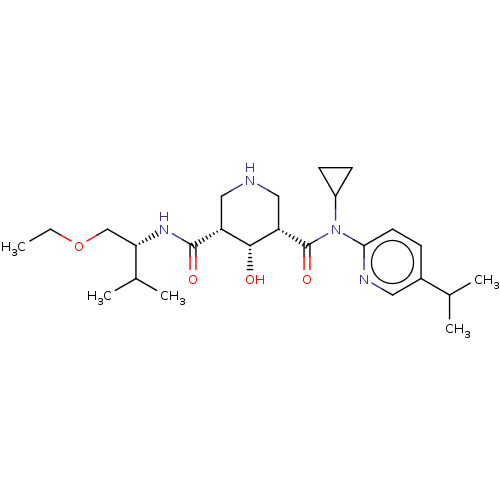 (US8497286, 157 | US8497286, 164) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description Inhibition activity of renin using FRET assay. | US Patent US8497286 (2013) BindingDB Entry DOI: 10.7270/Q27P8X1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM98686 (US8497286, 162) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant renin in presence of human plasma using Ac- IHPFHL-VIHNK-(DY-505-X5)-COOH substrate by fluorimetric assay | ACS Med Chem Lett 5: 787-92 (2014) Article DOI: 10.1021/ml500137b BindingDB Entry DOI: 10.7270/Q2WD4279 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM98681 (US8497286, 157 | US8497286, 164) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description Inhibition activity of renin using FRET assay. | US Patent US8497286 (2013) BindingDB Entry DOI: 10.7270/Q27P8X1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50054632 (CHEMBL3318940) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant renin in presence of human plasma using Ac- IHPFHL-VIHNK-(DY-505-X5)-COOH substrate by fluorimetric assay | ACS Med Chem Lett 5: 787-92 (2014) Article DOI: 10.1021/ml500137b BindingDB Entry DOI: 10.7270/Q2WD4279 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50054635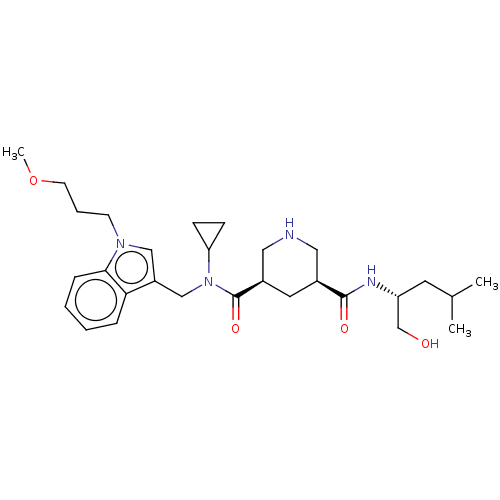 (CHEMBL3318936) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant renin using fluorescence-quenched RE(EDANS)IHPFHLVIHTK(Dabcyl)R substrate by fluorimetric assay | ACS Med Chem Lett 5: 787-92 (2014) Article DOI: 10.1021/ml500137b BindingDB Entry DOI: 10.7270/Q2WD4279 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM98674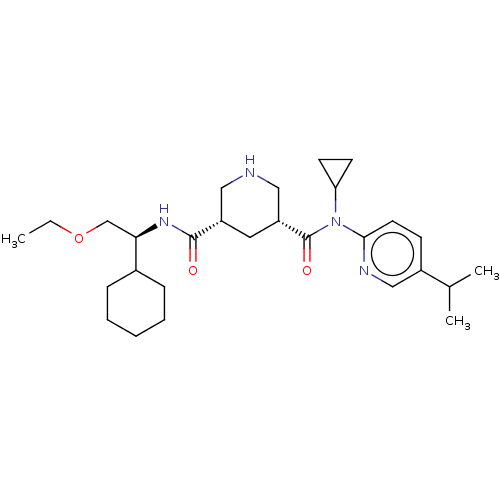 (US8497286, 150) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description Inhibition activity of renin using FRET assay. | US Patent US8497286 (2013) BindingDB Entry DOI: 10.7270/Q27P8X1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50263609 (2-cyano-4-((1-(2-hydroxyethyl)piperidin-4-yl)metho...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin S by fluorometric assay | Bioorg Med Chem Lett 18: 5280-4 (2008) Article DOI: 10.1016/j.bmcl.2008.08.067 BindingDB Entry DOI: 10.7270/Q2736QQ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50263557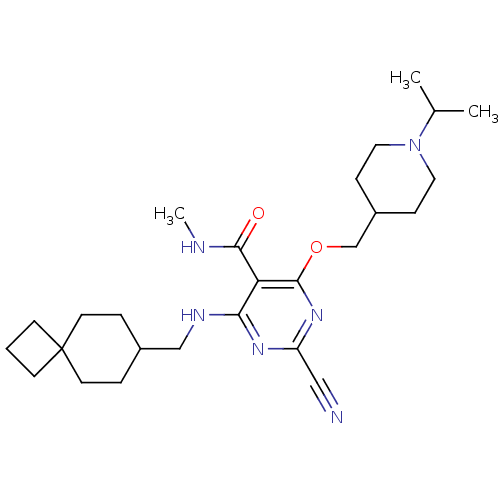 (2-cyano-4-((1-isopropylpiperidin-4-yl)methoxy)-N-m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin S by fluorometric assay | Bioorg Med Chem Lett 18: 5280-4 (2008) Article DOI: 10.1016/j.bmcl.2008.08.067 BindingDB Entry DOI: 10.7270/Q2736QQ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50263555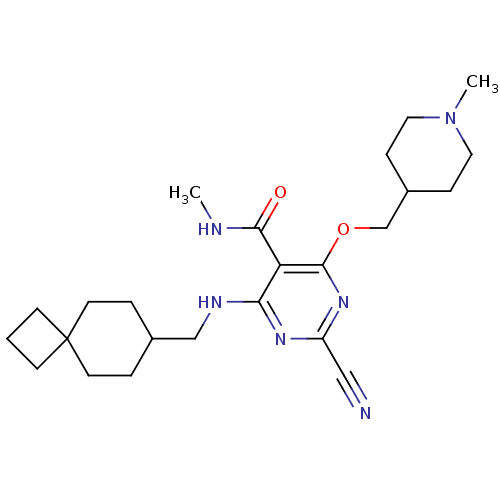 (2-cyano-N-methyl-4-((1-methylpiperidin-4-yl)methox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin S by fluorometric assay | Bioorg Med Chem Lett 18: 5280-4 (2008) Article DOI: 10.1016/j.bmcl.2008.08.067 BindingDB Entry DOI: 10.7270/Q2736QQ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50273220 (2-Cyano-4-[(spiro[2.5]oct-6-ylmethyl)-amino]-pyrim...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin S by fluorometric assay | Bioorg Med Chem Lett 18: 4642-6 (2008) Article DOI: 10.1016/j.bmcl.2008.07.011 BindingDB Entry DOI: 10.7270/Q2WH2PS6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50054642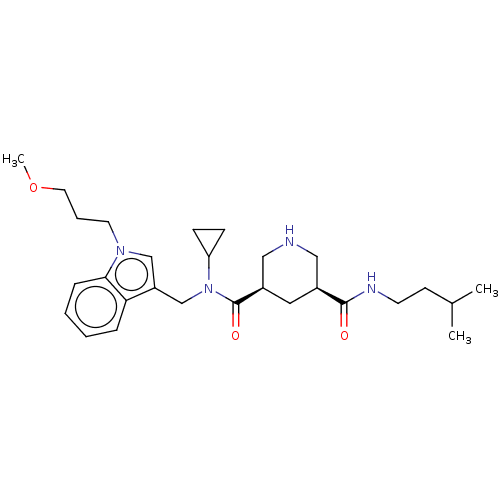 (CHEMBL3318935) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant renin using fluorescence-quenched RE(EDANS)IHPFHLVIHTK(Dabcyl)R substrate by fluorimetric assay | ACS Med Chem Lett 5: 787-92 (2014) Article DOI: 10.1021/ml500137b BindingDB Entry DOI: 10.7270/Q2WD4279 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50263554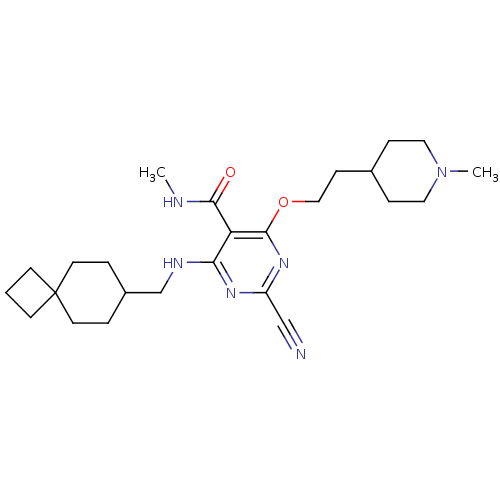 (2-cyano-N-methyl-4-(2-(1-methylpiperidin-4-yl)etho...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin S by fluorometric assay | Bioorg Med Chem Lett 18: 5280-4 (2008) Article DOI: 10.1016/j.bmcl.2008.08.067 BindingDB Entry DOI: 10.7270/Q2736QQ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 227 total ) | Next | Last >> |